Abstract
A 49-year-old man was admitted with peritonitis nine months after starting continuous ambulatory peritoneal dialysis (CAPD) for kidney failure. Ceftazidime and cefazolin were started. Peritoneal dialysate culture was negative for bacteria, but antibiotic treatment was continued because peritonitis improved. Twenty days later, the patient was discharged with no signs of peritonitis. However, 40-day culture of the original peritoneal dialysate detected Mycobacterium tuberculosis, and peritonitis recurred, leading to readmission. A T-SPOT test was performed and was positive in 4 days. Anti-tuberculosis therapy was started, which cured the peritonitis. The T-SPOT test may enable early diagnosis of tuberculosis.
Keywords: Tuberculous peritonitis, Peritoneal dialysis, T-SPOT test
Introduction
Peritonitis is a serious complication in patients on peritoneal dialysis. In most cases, the peritonitis is bacterial and the causative organism is identified within a few days. Guidelines recommend that tuberculous peritonitis should be considered when the organism is resistant to antibiotics or when bacteria are undetectable [1]. Tuberculous peritonitis is a troublesome form of peritonitis because it is not easily detected in peritoneal dialysis drainage by the Ziehl–Neelsen stain, and culture of Mycobacterium tuberculosis takes a considerable amount of time [1]. Therefore, tuberculous peritonitis has a low-detection rate, even though it is reported to account for 1% to 2% or about 4% of cases of peritonitis. Some articles recommend that peritoneal biopsy should also be performed if drainage-fluid culture fails to detect the organism [2, 3]. In the United States, tuberculous peritonitis is most commonly found in patients with HIV, followed by patients on peritoneal dialysis. An evocation against tuberculous peritonitis has also been made [4].
We experienced a case of peritonitis in which it took 40 days to obtain a positive M tuberculosis culture result from peritoneal dialysate, but in which the T-SPOT test was positive within 4 days of the recurrence of peritonitis. We report on the difficulty of diagnosing tuberculous peritonitis and the effectiveness of the T-SPOT test for early diagnosis.
Case presentation
A 49-year-old Japanese man was started on continuous ambulatory peritoneal dialysis (CAPD) for kidney failure due to autosomal dominant polycystic kidney disease. Nine months later, he developed mild abdominal pain and cloudiness of peritoneal dialysate and was diagnosed with peritonitis and hospitalized. He had no history of tuberculosis.
On admission, he was 176 cm tall and weighed 80 kg. His temperature was 36.6 °C; pulse, 105 beats/min; and blood pressure, 119/90 mmHg. He had tenderness and recurrent pain throughout the abdomen.
Laboratory-test results were as follows: white blood cell count, 6800/µL; red blood cell count, 454 × 104/µL; hemoglobin, 13.4 g/dL; albumin, 2.3 g/dL; serum urea nitrogen, 70 mg/dL; serum creatinine, 12.1 mg/dL; C-reactive protein (CRP), 9.1 mg/dL; and peritoneal dialysate cell count, 2305/dL (segment neutrophils, 64.5%; lymphocytes, 6.5%) (Table 1).
Table 1.
Laboratory findings
| First admission | Second admission | Normal range | ||
|---|---|---|---|---|
| Blood | Total protein | 5.9 | 5.6 | 6.6–8.1 |
| Alb (g/dL) | 2.3 | 2.5 | 4.1–5.1 | |
| AST (U/L) | 15 | 12 | 13–30 | |
| ALT (U/L) | 11 | 5 | 10–42 | |
| LD (U/L) | 206 | 203 | 124–222 | |
| GGT (U/L) | 76 | 89 | 13–64 | |
| Cre (mg/dL) | 12.1 | 10.8 | 0.65–1.07 | |
| eGFR (mL/min/1.73 m2) | 4.1 | 4.7 | > 90 | |
| UA (mg/dL) | 4.6 | 4.7 | 3.7–7.0 | |
| UN (mg/dL) | 72 | 61 | 8.0–20.0 | |
| Na (mmol/L) | 137 | 139 | 138–145 | |
| K (mmol/L) | 3.9 | 3.5 | 3.6–4.8 | |
| Cl (mmol/L) | 96 | 101 | 101–108 | |
| Ca (mg/dL) | 8.3 | 7.9 | 8.8–10.1 | |
| P (mg/dL) | 5.1 | 5.5 | 2.7–4.6 | |
| CRP (mg/dL) | 9.05 | 1.11 | < 0.04 | |
| WBC (/µL) | 6800 | 5000 | 3300–8600 | |
| RBC (× 106/µL) | 4.54 | 4.61 | 4.35–5.55 | |
| Hb (g/dL) | 13.4 | 13.9 | 13.7–16.8 | |
| Plt (× 103/µL) | 245 | 169 | 158–348 | |
| T-SPOT test | Not enforced | Positive | Negative | |
| CAPD drainage effluent | White blood cells in the peritoneal dialysis effluent (/μL) | 2305 | 1493 | < 50 |
| Segmented neutrophil (%) | 64,5 | 52 | ||
| Lymphocite (%) | 6.5 | 13 | ||
| Culture for Mycobacterium tuberculosis | Positive after 40-day culture | Positive after 30-days culture |
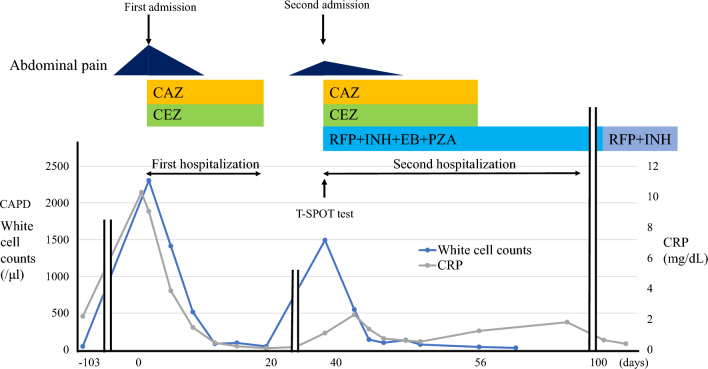
At first, we assumed that the patient had bacterial peritonitis and therefore decided to start treatment with antibiotics and reexamine treatment after culture results were obtained. As antibiotic treatment, 1 g/day of ceftazidime and 2 g/day of cefazolin were administered intravenously and 0.75 g each of ceftazidime and cefazolin were administered in each 5-changs CAPD bag. Results of bacterial and fungal culture of the peritoneal dialysate and blood culture on admission were negative, but nevertheless, antibiotics were continued for 20 days because the peritonitis was improving. After confirming that the CAPD effluent cell count and CRP had normalized, we discontinued the antibiotics and discharged the patient from the hospital. However, 40-day culture of the CAPD drainage effluent taken at admission detected M tuberculosis, even though antimicrobial staining by the Ziehl–Neelsen method was negative. The peritonitis recurred, and the patient was readmitted to the hospital.
At the time of the second admission, the white blood cell count was 5.0 × 103/μL, CRP was 1.11 mg/dL, the CAPD effluent was cloudy again, and the peritoneal dialysate cell count was 1493 cells/dL (segment neutrophils, 52.0%; lymphocytes, 13.0%) (Table 1). A T-SPOT test performed on a serum specimen taken at the second admission yielded a positive result four days later. Furthermore, a culture of peritoneal dialysate taken at the time of second admission was positive for M tuberculosis 30 days later, even though antimicrobial staining by the Ziehl–Neelsen method was negative. Sputum culture performed for three consecutive days did not detect M tuberculosis, and computed tomography scan and upper and lower gastrointestinal endoscopy did not show any abnormalities.
He was diagnosed with tuberculous peritonitis, and on the day of his second admission, treatment was immediately started with rifampicin 600 mg/day, isoniazid 300 mg/day, pyridoxal 20 mg/day for 9 months, ethambutol 500 mg/2 days, and pyrazinamide 1.7 g/day for 2 months. In addition, 1 g/day of ceftazidime and 2 g/day cefazolin were administered intravenously and 0.75 g each of ceftazidime and cefazolin were administered in each 5-change CAPD bag. Seven days after initiation of treatment, the abdominal pain disappeared, the white blood cell count in CAPD effluent normalized, and CRP decreased. Ceftazidime and cefazolin were discontinued after 16 days. The patient wanted to change from CAPD to hemodialysis, so an arterial venous fistula was created in the left forearm. Hemodialysis was started 23 days later, and the peritoneal catheter was removed 32 days after the second admission. The T-SPOT test was negative one week after starting treatment with tuberculosis drugs and remains negative 1 year later (Fig. 1).
Fig. 1.
Clinical course. RFP rifampicin, INH isoniazid, EB ethambutol, PZA pyrazinamide, CAZ ceftazidime, CEZ cefazolin
Discussion
Tuberculous peritonitis has been reported to be the likely cause of peritonitis when peritoneal dialysis drainage-fluid culture tests are negative for bacteria or in case of recurrent peritonitis. To examine this topic more closely, we performed a literature search to identify articles on tuberculous peritonitis in patients on peritoneal dialysis.
Hung et al. studied five patients on peritoneal dialysis in whom tuberculous peritonitis was diagnosed. Lymphocyte predominance was found in the leukocytes of the ascites fluid in two patients, and neutrophil predominance, in three. Detection of M tuberculosis took 6 weeks by culture of ascites fluid but only 1 week by laparoscopic biopsy. The authors reported that early diagnosis of tuberculous peritonitis was associated with a better prognosis [5].
Wang et al. studied 35 patients diagnosed with tuberculous peritonitis, including four patients on CAPD; 26 patients (74%) had pulmonary tuberculosis, and five of the 26 had miliary lung lesions; all patients were refractory to treatment with antibiotics [6].
Marshall et al. reported that gastrointestinal tuberculosis and tuberculous peritonitis remain common problems in impoverished areas of the world but occur relatively infrequently in the United States. Immigrants and AIDS patients are two population groups at particular risk for abdominal tuberculosis. Only 15–20% of patients have concomitant active pulmonary tuberculosis. Tuberculous peritonitis needs to be considered in all cases of unexplained exudative ascites [7].
Su et al. evaluated the validity of adenosine deaminase and NLR family pyrin domain containing 3 (NLRP3) inflammasome measurements in tuberculous peritonitis and found that they are useful as indicators for diagnosis and treatment [8].
Uzunkoy et al. studied 11 patients diagnosed with abdominal tuberculosis and reported that polymerase chain reaction of ascitic fluid obtained by ultrasound-guided fine needle aspiration is a reliable method for diagnosing the disease [9].
Yang et al. studied 18 patients receiving peritoneal dialysis who were diagnosed with tuberculosis peritonitis. Thirteen patients (72.2%) had a predominantly neutrophilic white blood cell count and acid fast stain was positive in seven patients (38.9%). After tuberculosis infection, only two patients (11.1%) remained on peritoneal dialysis and 10 (55.6%) were converted to hemodialysis; seven patients (38.9%) died within a year [10].
The T-SPOT test is a type of enzyme-linked immunosorbent spot assay used for tuberculosis diagnosis. It belongs to the group of interferon gamma release assays and provides an overall measurement of the host immune response against mycobacteria, which can reveal the presence of infection with M tuberculosis [11]. Although we found one report of a case in which the T-SPOT test was effective in diagnosing tuberculous peritonitis in a patient after liver transplantation [12], to our knowledge there are no reports in which the test assisted in the diagnosis of tuberculous peritonitis in patients on peritoneal dialysis.
A metanalysis have been recently done to assess T-SPOT test for tuberculosis peritonitis (although not PD) [13]. Fan et al. studied 317 patients with CAPD peritonitis, and examined T-spots in 9 patients diagnosed with tuberculous peritonitis and 13 patients diagnosed with other bacterial peritonitis. T-SPOT values in blood and CAPD effluent samples were significantly higher in patients with tuberculous peritonitis, leading to the conclusion that T-SPOT is useful for the adjunctive diagnosis of tuberculous peritonitis. However, they did not discuss the positive criteria for T-SPOT values [14].
There are recent studies of using molecular assay such as the Xpert MTB/RIF assay and PDF neutrophil–lymphocyte ratio (NLR), a readily available result on admission, as alternative to aid diagnosis of tuberculous peritonitis or non-tuberculous peritonitis [15, 16].
PD peritonitis is a significant complication of PD. In cases of PD peritonitis, PD effluent culture influences treatment choice and prognosis. While some effluent cultures may be negative, a diagnosis of bacterial peritonitis is made if there is a response to empirical antibiotic treatment. However, if the effluent culture is negative and there is no response to conventional antibiotic therapy, non-bacterial peritonitis should be suspected. It is exceedingly rare to diagnose tuberculous peritonitis, as in this case report, due to the very low incidence of tuberculous peritonitis, the lengthy time required for culturing mycobacteria (about 1 month), and because symptoms are often non-specific, such as fever and abdominal pain. Interferon-γ release assay (IGRA) includes quantiferon (QFT) and T-SPOT test. Initially, the former was used, but the latter has become more commonly used in recent years because of its higher sensitivity. The T-SPOT test is convenient and has high sensitivity and specificity, making it useful as an adjunctive diagnostic test for tuberculosis, and it is widely used in clinical practice. T-SPOT may remain positive even after tuberculosis is presumed cured, which has been reported to be influenced by the bacterial load, suggesting that the bacteria have not been completely eradicated from the body. Indeed, it is known that tuberculosis can recur with aging or immunosuppressive therapy due to decreased host immunity. In this case, the T-SPOT test became negative after treatment, suggesting a cure, but it will likely remain important as a marker for recurrence. Antimycobacterial genetic testing (TaqManPCR method) is one of the ancillary tests used when tuberculosis is suspected, but this alone does not lead to a definitive diagnosis of tuberculosis. Ultimately, identification of Mycobacterium tuberculosis by culture may be the final diagnosis. Adenosine deaminase activity has been considered in demand for the diagnosis of tuberculosis, but it is also elevated in liver disease, malignancy, and infection other than tuberculosis, and reflects tissue inflammation, necrosis, and lymphocyte activity. Therefore, the test has little diagnostic value for tuberculosis alone. We first performed this test because T-SPOT has been reported to be effective as an adjunctive diagnosis of tuberculosis. Since culture of Mycobacterium tuberculosis takes approximately 1 month, the T-SPOT test can easily provide results in a few days if a bacterial culture of drainage is not immediately detectable, and is considered important in terms of early diagnosis and intervention in treatment.
In conclusion, we experienced a patient with peritonitis who appeared to be cured after antibiotic therapy (because the CAPD drainage cell count decreased and the CRP level decreased) but who later showed recurrence of peritonitis. Bacteria were never detected as the causative agent, and a 40-day culture of the CAPD effluent collected at the time of the first admission detected M tuberculosis. The T-SPOT test performed at the time of recurrence of peritonitis was positive after four days, but a culture of the CAPD effluent was not positive for tuberculosis until after 30 days. This case indicates that the T-SPOT test is effective in the early diagnosis of tuberculous peritonitis.
Author contributions
Dr. Okubo: study concept and design and data acquisition; Drs. Sawa, Suwabe, Takaya: data analysis and interpretation; Drs. Ikuma, Mizuno, Oba, Yamanouchi: study supervision; Drs. Nakamura, Miki, Yokoyama, Ishii: surgery; Dr. Ubara: critical revision of the manuscript for important intellectual content; All authors: approval of the final version of the manuscript.
Declarations
Conflict of interest
The authors have declared that no Conflict of interest exists.
Ethics approval
The present report was produced in conformity with the Declaration of Helsinki, and the patient gave his written consent for this case report to be published.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Naoto Okubo, Email: onaoto28@gmail.com.
Yoshifumi Ubara, Email: ubara@toranomon.gr.jp.
References
- 1.Li PK-T, Chow KM, Cho Y, et al. ISPD peritonitis guideline recommendations: 2022 update on prevention and treatment. Peritoneal Dial Int. 2022;42(2):110–153. [DOI] [PubMed]
- 2.Chow KM, Chow VC, Szeto CC. Indication for peritoneal biopsy in tuberculous peritonitis. Am J Surg. 2003;185(6):567–73. [DOI] [PubMed] [Google Scholar]
- 3.Ram R, Swarnalatha G, Akpolat T, Dakshinamurty KV. Mycobacterium tuberculous peritonitis in CAPD patients: a report of 11 patients and review of literature. Int Urol Nephrol. 2013;45(4):1129–35. [DOI] [PubMed] [Google Scholar]
- 4.Sieloff EM, Ladzinski AT, Alcantara Lima N, Vos D, Boamah H, Melgar TA. Hospitalizations for tuberculous peritonitis in the United States: Results from the national inpatient sample database from 2002 to 2014. Int J Mycobacteriol. 2020;9(2):167–72. [DOI] [PubMed] [Google Scholar]
- 5.Hung YM, Chan HH, Chung HM. Tuberculous peritonitis in different dialysis patients in Southern Taiwan. Am J Trop Med Hyg. 2004;70(5):532–5. [PubMed] [Google Scholar]
- 6.Wang HK, Hsueh PR, Hung CC, Chang SC, Luh KT, Hsieh WC. Tuberculous peritonitis: analysis of 35 cases. J Microbiol Immunol Infect. 1998;31(2):113–8. [PubMed] [Google Scholar]
- 7.Marshall JB. Tuberculosis of the gastrointestinal tract and peritoneum. Am J Gastroenterol. 1993;88(7):989–99. [PubMed] [Google Scholar]
- 8.Su H, Yan G, Li Z, Fu L, Li L. Expression of adenosine deaminase and NLRP3 inflammasome in tuberculous peritonitis and their relationship with clinical efficacy. Dis Markers. 2022;2022:3664931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Uzunkoy A, Harma M, Harma M. Diagnosis of abdominal tuberculosis: experience from 11 cases and review of the literature. World J Gastroenterol. 2004;10(24):3647–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang TY, Tian YC, Yen TH, Chang MY, Lin CY, Liu S H. Tuberculous peritonitis in patients on peritoneal dialysis: a 35-year experience from a large medical center in Northern Taiwan. Renal Failure. 45(1): 2153064. [DOI] [PMC free article] [PubMed]
- 11.Meier T, Eulenbruch HP, Wrighton-Smith P, Enders G, Regnath T. Sensitivity of a new commercial enzyme-linked immunospot assay (T SPOT-TB) for diagnosis of tuberculosis in clinical practice. Eur J Clin Microbiol Infect Dis. 2005;24(8):529–36. [DOI] [PubMed] [Google Scholar]
- 12.Koff A, Azar MM. Diagnosing peritoneal tuberculosis. BMJ Case Rep. 2020;13(2): e233131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luo Y, Xue Y, Mao L, Lin Q, Tang G, Song H, Wang F, Sun Z. Diagnostic value of T-SPOT.TB assay for tuberculous peritonitis: a meta-analysis. Front Med (Lausanne). 2020;7:585180 [DOI] [PMC free article] [PubMed]
- 14.Fan Q, Huang X, Zhang J, Sun Y, Xiong Z, Xiong Z. Value of gamma interferon enzyme-linked immunospot assay in the diagnosis of peritoneal dialysis-associated tuberculous peritonitis. Int Urol Nephrol. 2022;54(4):843–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Edwards S, Glynn P, David MD, Kamesh L. Diagnosing tuberculous peritonitis early in patients on peritoneal dialysis: use of Xpert MTB/RIF assay. Perit Dial Int. 2016;36(4):461–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fung WW, Chow KM, Ng JK, Chan GC, Li PK, Szeto CC. The clinical utility of the neutrophil-to-lymphocyte ratio as a discriminatory test among bacterial, mycobacterium tuberculosis, and nontuberculous mycobacterium peritoneal dialysis-related peritonitis. Kidney360. 2022;3(6):1031–1038. [DOI] [PMC free article] [PubMed]