Abstract
Lymphocytes respond to antigen receptor engagement with tyrosine phosphorylation of many cellular proteins, some of which have been identified and functionally characterized. Here we describe SH3P7, a novel substrate protein for Src and Syk family kinases. SH3P7 migrates in sodium dodecyl sulfate-polyacrylamide gel electrophoresis as a 55-kDa protein that is preferentially expressed in brain, thymus, and spleen. It contains multiple amino acid sequence motifs, including two consensus tyrosine phosphorylation sites of the YXXP type and one SH3 domain. A region of sequence similarity, which we named SCAD, was found in SH3P7 and three actin-binding proteins. The SCAD region may represent a new type of protein-protein interaction domain that mediates binding to actin. Consistent with this possibility, SH3P7 colocalizes with actin filaments of the cytoskeleton. Altogether, our data implicate SH3P7 as an adapter protein which links antigen receptor signaling to components of the cytoskeleton.
Stimulation of the B-cell antigen receptor (BCR) triggers a series of cellular responses, such as altered gene transcription, changes in cell metabolism, and internalization of BCR-antigen complexes (8). These events require the activation of different signal transduction pathways. A few have been identified but are only partly understood. Biochemical and genetic evidence indicates a critical role for the early activation of protein tyrosine kinases (PTKs) of the Src and Syk family (16). Upon BCR aggregation, one or more of these PTKs phosphorylates immunoreceptor tyrosine-based activation motifs (ITAMs) in the cytoplasmic tail of the BCR signaling components Ig-α and Ig-β (29). An important function of phosphorylated ITAMs is the binding of SH2 domains of cytoplasmic signaling proteins. SH2-mediated binding of Src and Syk family kinases to phosphorylated ITAMs leads to increased PTK activity and enhanced substrate phosphorylation (8, 16, 29).
Some PTK substrate proteins in activated B cells have been identified, providing important insight into the molecular mechanisms of the functional coupling of the activated BCR to certain signaling cascades. Upon tyrosine phosphorylation, phospholipase C-γ becomes activated and hydrolyzes phosphoinositides. The resulting second messengers induce elevation of intracellular Ca2+ and activation of protein kinase C (8, 16). Other PTK substrates, such as HS1 and p120GAP, are implicated in the regulation of gene transcription and activation of the ras/mitogen-activated protein kinase pathway, respectively (14, 46). The reorganization of the actin cytoskeleton in antigen-stimulated B and T cells is ITAM dependent (6, 19) and requires tyrosine phosphorylation of Vav (12, 15), a guanine nucleotide exchange factor for the Rho family of small G proteins (7). The importance of actin-dependent signaling pathways has been demonstrated by the recent analysis of Vav-deficient mice. TCR-stimulated T cells from these mice show severe defects in cap formation, cytokine production, Ca2+ mobilization, and cell cycle progression (12, 15, 37, 47). However, with the exception of Vav, little is known about the intracellular effector molecules that could link antigen receptor stimulation to components of the cytoskeleton.
Although it is likely that BCR-regulated effector proteins have a defined subcellular localization, the structural organization of signaling cascades within the living cell is largely unknown. We have suggested that the resting BCR recruits and organizes its intracellular effector proteins (such as PTKs and their substrates) into a multimeric signaling complex (29, 42). This could allow the rapid and selective activation of BCR-specific signaling pathways, even when downstream elements are common to the BCR and other receptors. The formation of signaling complexes seems to depend on the function of adapter proteins (26, 39, 43). Adapter proteins do not possess enzymatic activity but contain conserved sequence motifs of 60 to 140 amino acids which mediate specific protein-protein interactions (25). These motifs were initially identified as regions of similarity among different proteins. It is now known that many of the amino acid motifs fold into a compact domain structure which functions as a protein module that is largely independent of the surrounding sequences (25). Two of the best-studied protein modules are SH2 and PTB domains, which recognize phosphotyrosine-containing peptide ligands (2, 22). SH3 and WW domains bind to proline-rich peptides (3, 21), while binding of PH domains to phosphoinositides can tether cytoplasmic proteins to the plasma membrane (18).
In this report, we identify a 55-kDa phosphoprotein from activated B cells, SH3P7, whose cDNA was previously isolated in a screen for novel SH3 domain-containing proteins (35). Further analysis showed that SH3P7 contains multiple sequence motifs for protein-protein interactions. A region of sequence similarity to actin-binding proteins, which we called SCAD, could represent a novel protein module. Finally, confocal microscopy reveals an association of SH3P7 with components of the actin cytoskeleton.
MATERIALS AND METHODS
Materials.
The phenotype and culture conditions of the lymphoid cell lines and the mouse macrophage cell line S388 have been described previously (42). NIH 3T3 fibroblasts were maintained in Dulbecco modified Eagle high-glucose medium supplemented with 10% fetal calf serum, 2 mM l-glutamine, 50 U of penicillin per ml, and 50 mg of streptomycin per ml. Mouse splenic B cells were enriched from freshly isolated spleens by depletion of CD43+ cells by using the MACS system (Miltenyi Biotec, Cologne, Germany). Immobilized PT66 antiphosphotyrosine-agarose beads (Sigma-Aldrich, Deisenhofen, Germany) and soluble 4G10 antiphosphotyrosine antibodies (Upstate Biotechnology, Lake Placid, N.Y.) were used for precipitation and immunoblot analysis, respectively. Anti-Flag antibodies were purchased from Integra Biosciences, Fernwald, Germany. Polyclonal anti-SH3P7 antibodies were produced by immunizing rabbits with KLH-coupled peptides corresponding to amino acids 334 to 352 (EPTYEVPPEQDTLYEEPPL) and 260 to 273 (APHPREIFKQKERAM) of the SH3P7 protein. The antibodies obtained were (i) 85-B2 and 86-B2 and (ii) 87-B2 and 88-B2, respectively.
Protein biochemistry.
Stimulation of cells via their antigen receptors or with pervanadate-H2O2, immunoprecipitations, and Western blot analysis were described previously (1, 42). Large-scale purification of tyrosine-phosphorylated proteins and amino acid sequence analysis of their peptides have been described previously (43). Briefly, a total of 1010 J558Lδm7.1 cells were stimulated in aliquots of 3 × 107 cells/ml for 3 min with 50 μM pervanadate-H2O2, and precleared lysates were subjected to immunoprecipitation with agarose-conjugated antiphosphotyrosine antibodies. Bound proteins were specifically eluted with 50 mM phenyl phosphate-200 mM NaCl, concentrated, separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and stained with Coomassie blue. The 55-kDa protein band was excised and subjected to in-gel digestion with lysyl endopeptidase C (Lys-C), and the resulting, high-pressure liquid chromatography-purified peptides were sequenced (TOPLAB, Munich, Germany).
In vitro kinase assay.
Twenty micrograms of glutathione S-transferase (GST) or GST fusion proteins was incubated with baculovirus-expressed Lyn, Blk, or Syk for 15 min at 37°C in 500 μl of kinase buffer containing 50 mM Tris (pH 7.4), 5 mM MnCl2, 0.1 mM NaVa3, and 200 μM ATP. Phosphorylated proteins were detected by 4G10 immunoblotting.
DNA constructs, purification of recombinant fusion proteins, and transfections.
The mouse SH3P7 cDNA was amplified from poly(A)+ RNA of J558L cells by reverse transcription-PCR with the primer pair 5′-GCCAGGTCTCGGCCTCAC-3′ and 5′-GGACCGTGGGGCGTGCCA-3′. The PCR product was cloned into pTZ19 via the SmaI restriction site (pTZ-SH3P7) and sequenced. To produce constructs encoding fusion proteins between the GST and SH3P7, a KpnI/BamHI restriction fragment of pTZ-SH3P7 was isolated, and the single-stranded 5′ and 3′ ends were removed by using the exonuclease and polymerase activities of the Klenow enzyme, respectively. Subsequently, the blunt-end fragment was cloned in reverse orientation into SmaI-digested pUC19 (p19-SH3P7). To obtain pRP-SH3P7, a KpnI/HindIII fragment of p19-SH3P7 encompassing the complete SH3P7 coding sequence was ligated via the same restriction sites into pRP261, a derivative of pGEX-3X (Pharmacia, Freiburg, Germany). Constructs coding for GST fusion proteins that contain either amino acids 1 to 337 (pRP-SCAD) or 303 to 433 (pRP-SH3pp) were generated by deleting the NdeI/XbaI fragment or the KpnI/EagI fragment of pRP-SH3P7, respectively. All constructs were transformed in E. coli DH10B, and the expression of in-frame GST-SH3P7 fusion proteins of the predicted molecular weight was confirmed by SDS-PAGE and immunoblot analysis with anti-SH3P7 antibodies (data not shown). Induction and purification of the fusion proteins was performed according to the manufacturer’s instructions, except that a French press was used to break up the bacteria.
Expression vectors for SH3P7-Flag.
The BstXI/BamHI fragment of p19-SH3P7 was replaced with annealed synthetic oligonucleotides 5′-GTGGAACTCATAGAGGACTACAAGGACGACGATGACAAGTGAG-3′ and 5′-GATCCTCACTTGTCATCGTCGTCCTTGTAGTCCTCTATGAGTTCCACGTAG-3′ (p19-SH3P7-Flag), and a KpnI/XbaI fragment coding for the SH3P7-Flag protein was inserted 3′ of the cytomegalovirus promoter into pCDNA3 (Invitrogen, Carlsbad, Calif.). The Quick Change system (Stratagene, La Jolla, Calif.) was used for site-directed mutagenesis of single and double Y→F substitutions in p19-SH3P7-Flag. Primer combinations are 5′-GAAGAAGAACCTACATTTGAAGTACCCCCAG-3′ and 5′-CTGGGGGTACTTCAAATGTAGGTTCTTCTTC-3′ for Y337F and 5′-CCAGAGCAGGACACCCTCTTCGAAGAACCACCACTGG-3′ and 5′-CCAGTGGTGGTTCTTCGAAGAGGGTGTCCTGCTCTGG-3′ for Y347F. Mutations were confirmed by sequencing, and the KpnI/XbaI fragment of each vector was cloned into pCDNA3. For transient expression of SH3P7-Flag proteins, 3 × 107 K46 cells in 300 μl of RPMI were mixed with 15 μg of plasmid DNA and electroporated (320 V at 950 μF) with a Bio-Rad gene pulser. Analysis was performed after 36 h. The expression construct encoding a fusion protein between the enhanced green fluorescent protein EGFP and SH3P7 was created by inserting the EcoRI/BamHI fragment of p19-SH3P7 into pEGFPC1 (Clontech, Palo Alto, Calif.) digested with the same enzymes.
Analysis of GFP-SH3P7 by confocal laser scanning microscopy.
NIH 3T3 cells were seeded on glass coverslides and transfected with constructs for EGFP and EGFP-SH3P7 by using the CaPO4 method. Forty-eight hours after transfection, the slides were rinsed with ice-cold phosphate-buffered saline (PBS)–0.1% NaN3 and cells were fixed with 3% paraformaldehyde on ice for 15 min. Slides were blocked on ice for 1 h with 10% fetal calf serum–PBS with gentle agitation. To stain the actin cytoskeleton, the slides were overlaid with a 1:400 dilution of tetramethyl rhodamine isocyanate (TRITC)-labelled phalloidin (Sigma-Aldrich, Deisenhofen, Germany) for 1 h on ice. After being washed three times for 10 min each) with 0.1% PBS, slides were mounted in Fluoromount G (Southern Biotechnologies, Birmingham, Ala.) and sealed. Confocal laser scanning microscopy was performed with a Leica Fluovert microscope equipped with an argon/krypton laser by using the fluorescein isothiocyanate (FITC) and TRITC filter settings to detect GFP and phalloidin, respectively.
RESULTS
Purification of SH3P7 from activated J558Lδm7.1 cells.
Tyrosine-phosphorylated Lyn comigrates with a PTK substrate that could not be depleted with anti-Lyn antibodies (data not shown). To identify this (and other) PTK substrate(s), phosphotyrosine-containing proteins were affinity purified from pervanadate-H2O2-stimulated J558Lδm7.1 B cells with antiphosphotyrosine antibody columns and separated by SDS-PAGE, and a Coomassie-stained protein of about 55 kDa was digested with Lys-C. From two of the resulting peptide fragments, the amino acid sequences FVILNWTGEGVNDVRK (Lys C-48) and ASGANYSFHK (Lys C-15) were obtained. These sequences were compared to those in the databases and found to be identical to amino acid sequences predicted from a murine cDNA clone called SH3P7 (accession no. U58884 [35]) and a human EST sequence (accession no. AA687496). The Lys C-48 and Lys C-15 peptides corresponded to amino acids 79 to 94 and 135 to 144 of the mouse SH3P7 protein, respectively (see Fig. 1). The EST sequence entry no. AA687496 is likely to represent a partial cDNA clone of the human SH3P7 homologue. The presence of SH3P7 in antiphosphotyrosine precipitates from pervanadate-H2O2-stimulated J558Lδm7.1 cells suggested a signaling function for this protein in activated B cells.
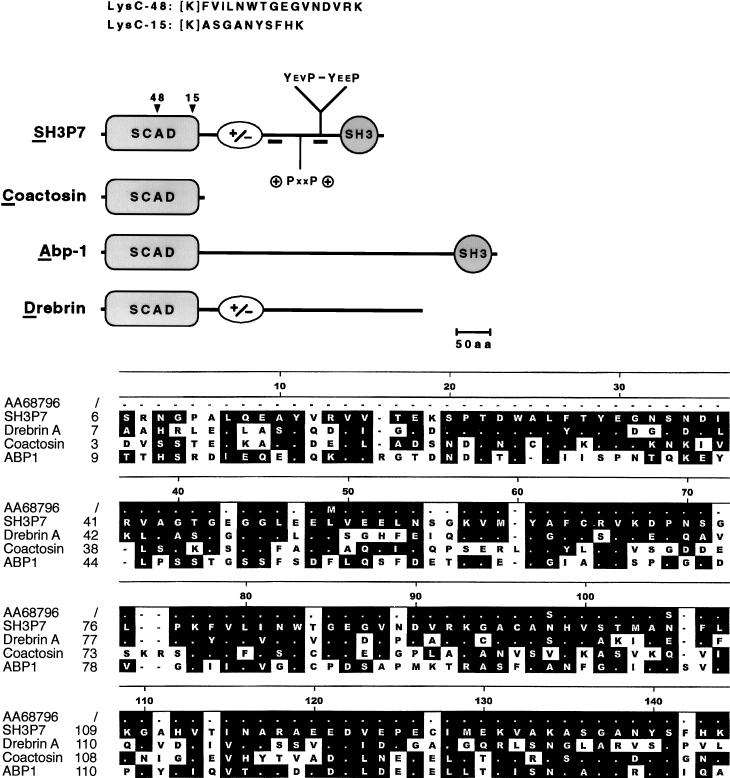
FIG. 1.
SH3P7 is an adapter protein with multiple motifs for protein-protein interactions. (Upper panel) Schematic representation of the protein structures of SH3P7, coactosin, drebrin, and Abp-1. The N-terminal region of sequence similarity was named SCAD. Amino acid (aa) residues 170 to 250 of SH3P7 contain a high content of positively (28.5%) and negatively (27.5%) charged amino acids (indicated by +/−) and may adopt an α-helical structure. This part of the protein contains four repeats of a hexamer which are also present in drebrin and which have the consensus sequence R/KXEEXR. A putative SH3 domain binding site (+PXXP+) and two consensus tyrosine phosphorylation sites (YEVP-YEEP) are at positions 304 to 313, 337 to 340, and 347 to 350, respectively. The C-terminal SH3 domain has been reported previously (35). The positions and sequences of the Lys C-48 and Lys C-15 peptides are shown. Brackets indicate that the N-terminal lysine residue is inferred after Lys-C digestion. Locations of peptide sequences used for immunization are underlined. Protein structures are drawn to scale. (Lower panel) Amino acid sequence alignment of the SCAD regions together with the translated human EST sequence no. AA687496. Related and identical (white dot) amino acid residues are boxed. Gaps are indicated by black dots.
Sequence analysis of SH3P7.
The open reading frame in the SH3P7 cDNA predicts a protein of 433 amino acids, with a calculated molecular mass of 48.4 kDa and an isoelectric point of 4.8. We performed an amino acid sequence comparison of SH3P7 with proteins from the GenBank database and used the Hein method to align related proteins. As shown in Fig. 1 (lower panel), the N-terminal 140 amino acids of SH3P7 have significant similarity to the actin-binding proteins drebrin, coactosin, and Abp-1 (9, 10, 33). The closest similarity is found between SH3P7 from mouse and human to the brain-specific drebrin proteins from chicken, i.e., 43% sequence identity and 22% conservative changes. Coactosin and Abp-1 from Dictyostelium discoideum and Saccharomyces exiguus, respectively, show more sequence variations. In all cases, a very high degree of similarity was found between two conserved tryptophan residues, from which it is known that they can act as central elements in protein domain folding. Interestingly, coactosin consists of only 146 amino acids comprising the entire region of similarity. In that part of the protein, the mouse SH3P7 and the translated human EST sequence exhibit only four amino acid changes. We conclude that the described region has similar functions in SH3P7, coactosin, Abp-1, and drebrin. Using the initial letters of the names of the four proteins, we propose the name SCAD for this region. The SCAD regions of the four proteins may exhibit similar folding patterns and may define a new type of protein module, such as the SH2, SH3, or PH domain.
C-terminal of the SCAD region, SH3P7 is rich in positively and negatively charged amino acids (Fig. 1, upper panel) which may adopt an α-helical structure. This part of the protein contains four repeats with the consensus sequence R/KXEEXR. A putative binding motif for SH3 domains is at positions 304 to 313. It contains the consensus PXXP sequence, which is flanked on either side by positively charged amino acids. Two of 13 total tyrosine residues of SH3P7 are located within consensus phosphorylation motifs. These tyrosine residues are at positions 337 and 347 and are both followed by proline residues in the Y+3 and Y+4 positions. A C-terminal SH3 domain explains the ability of SH3P7 to bind proline-rich peptides (35). In summary, our analysis revealed that SH3P7 bears a number of amino acid sequence motifs which could mediate specific protein-protein interactions.
Expression analysis of SH3P7.
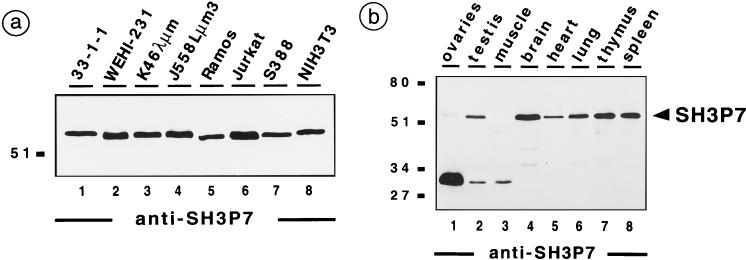
To functionally characterize SH3P7, two peptide antiserum samples were generated and used for immunoblot analysis of different mouse and human cell lines (Fig. 2a). SH3P7 is detected as a 55-kDa protein in the mouse B-cell lines 33-1-1, WEHI-231, K46λμm, and J558Lμm3 (lanes 1 to 4), which represent the pre-B, immature, mature, and plasma cell stage of B-cell development, respectively. The human SH3P7 was detected in Ramos B cells and Jurkat T cells (lanes 5 and 6). Also, the mouse macrophage cell line S388 and NIH 3T3 fibroblasts express SH3P7 (lanes 7 and 8). When lysates from different mouse tissues were analyzed (Fig. 2b), the 55-kDa protein species was detected in testis, heart, and lung (lanes 2, 5, and 6) and was most prominent in brain, thymus, and spleen (lane 4, 7, and 8). Expression in ovaries and muscle was weak or nonexistent (lanes 1 and 3). In ovaries, muscle, and testis, our antibodies reacted with an unidentified protein species of about 32 kDa which seems to be enriched in ovaries. In summary, SH3P7 migrates in SDS-PAGE with an apparent molecular mass of about 55 kDa and is expressed in all tested cell lines and in a number of tissues. The difference between the predicted molecular mass and the apparent molecular mass may result from the high content of charged amino acids, which can lead to retarded protein migration in SDS-PAGE. Using expression constructs that are based on the SH3P7 cDNA and that encode a tagged fusion protein, we confirmed that the apparent molecular mass of SH3P7 is 55 kDa (see below, Fig. 4d).
FIG. 2.
Expression of the SH3P7 protein in cell lines and different tissues. Anti-SH3P7 immunoblot analysis of cleared cellular lysates from approximately 106 cells of the indicated cell lines (a) or with 2 mg of total protein from different mouse tissues (b). In the experiments shown, the 87-B2 antibodies (see Materials and Methods) were used. Identical protein patterns were detected by the 85-B2 antibody but not with preimmune serum (data not shown). The apparent molecular masses of marker proteins are indicated in kilodaltons.
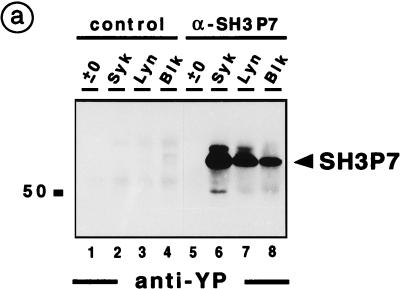
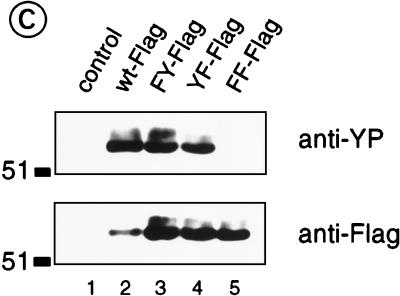
FIG. 4.
Src and Syk family kinases can phosphorylate SH3P7 at the YXXP motif. (a) Antiphosphotyrosine immunoblot analysis of proteins precipitated from approximately 2.5 × 106 unstimulated J558Lδm7.1 cells with either rabbit control serum (control; lanes 1 to 4) or anti-SH3P7 antibodies (α-SH3P7; lanes 5 to 8) and subsequently subjected to an in vitro kinase assay in buffer alone (lane 1 and 5) or together with baculovirus-expressed Syk (lanes 2 and 6), Lyn (lanes 3 and 7), or Blk (lanes 4 and 8). (b) Baculovirus-expressed Lyn (lanes 1 to 5), Blk (lanes 6 to 10), and Syk (lanes 11 to 15) were used in an in vitro kinase assay without additional proteins (lanes 5, 10, and 15) or together with either GST (lanes 1, 6, and 11) or GST-SH3P7 fusion proteins that contain amino acids 1 to 337 (lanes 2, 7, and 12), 1 to 433 (lanes 3, 8, and 13), or 303 to 433 (lanes 4, 9, and 14) of SH3P7. Phosphorylated proteins were visualized by antiphosphotyrosine immunoblotting. (c) K46 cells were transiently transfected with expression vectors bearing the gene encoding wild-type flagged SH3P7 (wt-Flag; lane 2) or a flagged SH3P7 mutant with Y→F substitutions at position 337 (FY-Flag; lane 3) or 347 (YF-Flag; lane 4) or positions 337 and 347 (FF-Flag; lane 5). After stimulation of untransfected (lane 1) and transfected (lanes 2 to 5) cells with 50 μM pervanadate-H2O2 for 5 min, proteins were precipitated with anti-Flag antibodies and analyzed by antiphosphotyrosine and anti-Flag immunoblotting (upper and lower panel, respectively). The apparent molecular masses of marker proteins are indicated in kilodaltons.
Tyrosine phosphorylation of SH3P7.
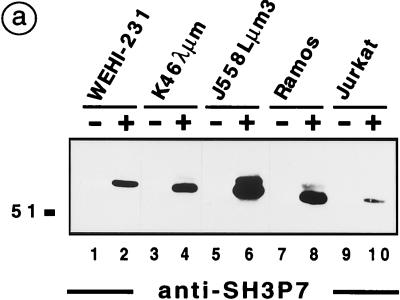
To address the role of SH3P7 in signal transduction, different B-cell lines and Jurkat T cells were stimulated through their antigen receptors or with pervanadate-H2O2. Tyrosine-phosphorylated proteins were immunoprecipitated and subjected to anti-SH3P7 immunoblotting (Fig. 3a). SH3P7 was detected in antiphosphotyrosine precipitates from anti-immunoglobulin M (IgM)-stimulated WEHI-231, K46λμm, and Ramos B cells (lanes 2, 4, and 8). As expected, a strong SH3P7 signal was observed in precipitates from pervanadate-H2O2-stimulated J558Lμm3 (lane 6) and J558Lδm7.1 (data not shown) cells. SH3P7 was also present in antiphosphotyrosine precipitates from TCR-stimulated Jurkat T cells (lane 10), although the signal was weaker than that obtained from BCR-stimulated B cells. No SH3P7 signal was detectable in the analysis of unstimulated cells (lanes 1, 3, 5, 7, and 9).
FIG. 3.
SH3P7 is tyrosine phosphorylated in antigen receptor-stimulated lymphocytes. (a) Antiphosphotyrosine precipitates were prepared from the indicated cell lines, which were either untreated (lanes 1, 3, 5, 7, and 9) or stimulated with 10 μg of anti-mouse IgM antibodies (lanes 2 and 4) per ml, 50 μM pervanadate-H2O2 (lane 6), 7.5 μg of F(ab′)2 fragments of anti-human IgM antibodies (lane 8) per ml, or 10 μg of Okt-3 antibodies (lane 10) per ml. Purified phosphoproteins obtained from 1.5 × 107 cells (lanes 1 to 4 and 7 to 10) or 5 × 106 cells (lanes 5 to 6) were separated by SDS-PAGE and subjected to immunoblot analysis with anti-SH3P7 antibodies (87-B2). (b) By using anti-SH3P7 antibodies (87-B2) coupled to protein G-Sepharose, the SH3P7 protein was precipitated from the indicated cell lines, which were treated as described in the legend for panel a. Purified proteins from 1.5 × 107 cells (lanes 1 to 4) or 5 × 106 cells (lanes 5 to 6) were analyzed by immunoblotting with antiphosphotyrosine antibodies. The position of the endogenous γ2am heavy chain of K46λμm, which is detected by the secondary anti-mouse IgG antibodies, is indicated. (c) Approximately 2 × 107 Ramos B cells (lanes 1 to 4) and 5 × 107 purified splenic B cells (lanes 5 to 8) were either left unstimulated (lanes 1, 3, 5, and 7) or stimulated through their antigen receptors with 7.5 μg of F(ab′)2 fragments from anti-human IgM antibodies (lanes 2 and 4) per ml or with 15 μg of anti-mouse κ antibodies (lanes 6 and 8) per ml, respectively. From these cells, antiphosphotyrosine precipitates (α-YP; lanes 1 to 2 and 5 to 6) and anti-SH3P7 precipitates (α-SH3P7; lanes 3 to 4 and 7 to 8) were prepared and analyzed by antiphosphotyrosine immunoblotting. The apparent molecular masses of marker proteins are indicated in kilodaltons.
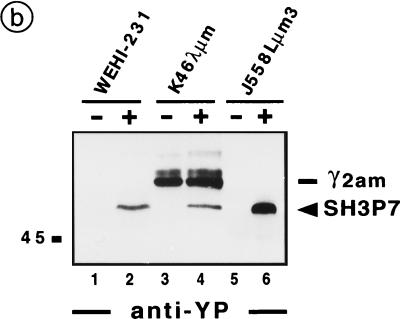
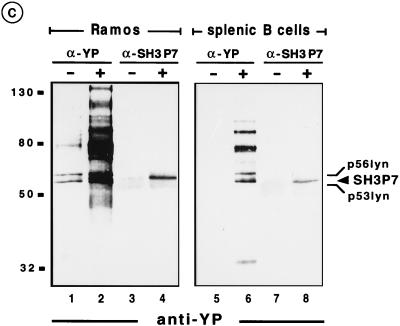
The results indicated that SH3P7 is involved not only in BCR- but also in TCR-mediated signaling. However, purification of SH3P7 with antiphosphotyrosine antibodies could be due to the association of SH3P7 with one or more phosphorylated protein(s) or to tyrosine phosphorylation of SH3P7 itself. To discriminate between the two possibilities, SH3P7 was immunoprecipitated from unstimulated and stimulated B-cell lines and analyzed by antiphosphotyrosine immunoblotting. Figure 3b shows that purified SH3P7 from stimulated, but not unstimulated, WEHI-231, K46λμm, and J558Lμm3 cells is phosphorylated (lanes 1 to 6). The 60-kDa–62-kDa protein doublet in precipitates from K46λμm cells is derived from the endogenous γ2am heavy chain (lanes 3 to 4). Control experiments with metabolically labeled cells showed that SH3P7 can be precipitated to equal amounts from unstimulated and stimulated cells (data not shown). Collectively the data show that SH3P7 is a substrate for activated PTKs in mouse B-cell lines. As shown in Fig. 3c, the same is true for the human B-cell line Ramos (lanes 3 and 4) and for purified B cells of mouse spleen (lanes 7 to 8). In this experiment, antiphosphotyrosine precipitates (lanes 1 to 2 and 5 to 6) and anti-SH3P7 precipitates (lanes 3 to 4 and 7 to 8) were analyzed in parallel. The comparison revealed that SH3P7 migrates between two phosphoproteins, which we identified as the 53- and 56-kDa isoforms of tyrosine-phosphorylated Lyn (data not shown). This finding confirms our initial observation that an unidentified phosphoprotein comigrated with Lyn and might explain why tyrosine-phosphorylated SH3P7 has been overlooked thus far.
SH3P7 is phosphorylated by Syk, Lyn, and Blk at tyrosines 337 and 347.
To test the ability of BCR-regulated PTKs to phosphorylate SH3P7, anti-SH3P7 precipitates were subjected to an in vitro kinase assay with baculovirus-expressed Syk, Lyn, and Blk (Fig. 4a). Strong SH3P7 phosphorylation was obtained with all three PTKs (lanes 6 to 8). After phosphorylation by Syk and Lyn, a phosphoprotein was detected which migrates slightly above the 55-kDa protein band of SH3P7 (lanes 6 and 7). This upper band is likely to be a differently phosphorylated form of SH3P7, because a similar protein doublet was recognized by anti-SH3P7 antibodies (compare Fig. 3a, lanes 6 and 8). The 50-kDa phosphoprotein seen in the Syk kinase assay (Fig. 4a, lane 6) might result from a degradation product of SH3P7 or from a Syk substrate that was copurified with SH3P7. No phosphorylated proteins were detected when the same assays were performed with rabbit control serum (lanes 1 to 4) or when recombinant PTKs were omitted (lane 5). The latter finding indicates that under our experimental conditions, no endogenous PTK coprecipitated with SH3P7.
Next, we incubated recombinant PTKs with GST fusion proteins encompassing SH3P7-derived amino acids 1 to 337, 1 to 433 (full length), and 303 to 433. Antiphosphotyrosine immunoblotting demonstrated Lyn-, Blk-, and Syk-mediated phosphorylation of GST fusion proteins encompassing either the complete or the C-terminal 101 amino acids (Fig. 4b, lanes 3, 8, and 13 and lanes 4, 9, and 14). Even longer exposure of the film did not reveal any phosphorylation of the first 337 amino acids of SH3P7 (lanes 2, 7, and 13) or of GST itself (lanes 1, 6, and 11). Thus, Lyn, Blk, and Syk are capable of phosphorylating SH3P7 in vitro only within the last 101 amino acids. This suggests that the two YXXP phosphorylation motifs found in this part of the protein contain the dominant phosphorylation sites, i.e., Y337 and Y347. To test this hypothesis, wild-type or mutated SH3P7 carrying either single or double Y→F substitutions were expressed as Flag-tagged fusion proteins in K46 cells. Following stimulation of the cells with pervanadate-H2O2, the fusion proteins were precipitated with anti-Flag antibodies and analyzed by antiphosphotyrosine and anti-Flag immunoblotting (Fig. 4c). In all transfectants, but not in mock transfected cells, the SH3P7-Flag proteins were produced and migrated in SDS-PAGE with the expected molecular mass of 55 to 56 kDa (lower panel, lanes 1 to 5). Expression of the wild-type SH3P7-Flag (lane 2) was lower than that of the mutants (lanes 3 to 5). Phosphorylation was found for wild-type and for singly mutated SH3P7-Flag (Y337F or Y347F) but not for the doubly mutated SH3P7-Flag (Y337F and Y347F) (Fig. 4c, upper panel). Considering the low expression of the wild-type fusion protein, the similar intensities of the antiphosphotyrosine-derived signals demonstrate that a single Y→F mutation leads to reduced phosphorylation. In summary, the experiments identify tyrosine residues 337 and 347 as the only phosphorylation sites in vitro and in vivo.
SH3P7 is associated with the actin cytoskeleton.
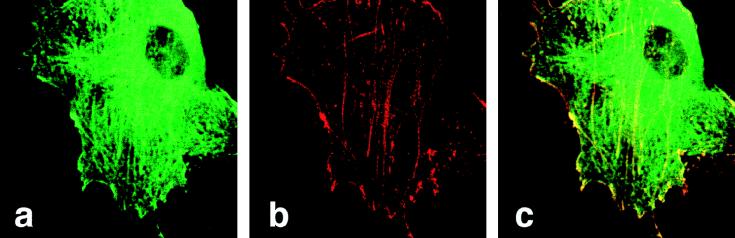
To test for a possible association of SH3P7 with the actin-containing cytoskeleton, a fusion protein between GFP and SH3P7, GFP-SH3P7, was transiently expressed in NIH 3T3 fibroblasts. Fibroblasts were chosen because lymphocytes contain only a small cytoplasmic compartment, which does not allow microscopic detection of subcellular structures. Transfectants were fixed in 3% paraformaldehyde to preserve the cytoskeleton architecture and were analyzed by confocal microscopy (Fig. 5). The GFP-SH3P7 fusion protein was found in the cytoplasm of the cells but not in the nucleus (Fig. 5a). It became organized into fiber-like structures which were also stained by phalloidin (Fig. 5b). This compound binds specifically to filamentous actin (F-actin) and hence stains the actin cytoskeleton. Figure 5c shows that GFP-SH3P7 and F-actin fibers colocalize and that both proteins are enriched in submembraneous patches of the cortical cytoplasm. The GFP-SH3P7 and F-actin-containing fibers were destroyed following treatment of the cells with cytochalasin D (data not shown), which disrupts the actin cytoskeleton. In contrast to what occurred with GFP-SH3P7 transfectants, when NIH 3T3 fibroblasts expressing large amounts of GFP only or a GFP-B-Raf fusion protein were fixed with methanol to allow diffusion of cytosolic proteins, only a very faint signal was detected in the two latter cell lines (data not shown). This confirms that GFP and GFP-B-raf, but not GFP-SH3P7, are soluble proteins and that not all GFP fusion proteins are anchored to the cytoskeleton. We conclude that SH3P7 is directly or indirectly associated with F-actin fibers and is thus a component of the cytoskeleton.
FIG. 5.
Subcellular localization of SH3P7. NIH 3T3 fibroblasts were transiently transfected with an expression vector bearing the gene encoding a GFP-SH3P7 fusion protein (a to c). Forty-eight hours after transfection, cells were fixed in 3% paraformaldehyde and stained with TRITC-labelled phalloidin to visualize the actin-containing cytoskeleton. Subsequently, confocal microscopy was performed by using the FITC and TRITC filter settings to detect either GFP-SH3P7 (a) or phalloidin–F-actin (b) conjugate, respectively. (c) Overlay of panels a and b.
DISCUSSION
A 55-kDa protein was purified with antiphosphotyrosine antibodies from pervanadate-H2O2-stimulated J558Lδm7.1 cells. Amino acid sequences of two peptides matched to a GenBank cDNA clone, called SH3P7 (35). Rabbit polyclonal antibodies were generated and employed to characterize the protein. Expression of SH3P7 was found in many cell types and seems not to be restricted to a particular stage of B-cell development. Studies with B-cell lines and splenic B cells showed that upon BCR stimulation, SH3P7 is a direct substrate for activated PTKs. Phosphorylation by Syk and Src family kinases occurs at two consensus tyrosine phosphorylation motifs of the YXXP type. Other sequence motifs in SH3P7 are the SH3 domain and the SCAD region. The SCAD region comprises about 140 amino acids which have significant sequence similarity to drebrin, Abp-1, and coactosin (9, 10, 33). As shown for the latter proteins, our data reveal an association of SH3P7 with actin fibers of the cytoskeleton. The data identify SH3P7 as a BCR-regulated effector protein with sequence motifs for constitutive and stimulation-dependent protein-protein interactions which couple BCR activation to the cytoskeleton.
The sites of phosphorylation in SH3P7 are the two YXXP motifs (YEVP and YEEP). Very similar phosphorylation motifs are also found in four other adapter proteins which are known targets for activated PTKs in B lymphocytes. These are Cbl (YDVP), p62dok (YELP and YDEP), Cas (7 × YDVP), and SLP-65 (YENP, YEPP, and YVVP). The proto-oncogene product Cbl is a reported substrate for Lyn (23, 38) and was originally discovered as the transforming gene v-cbl of the murine Casitas NS-1 retrovirus (17). The p120GAP-associated protein p62dok is tyrosine phosphorylated in response to a variety of stimuli, including BCR activation (5, 45). The Crk-associated substrate Cas contains a total of 15 YXXP motifs (32). For six of the seven YDVP sequences in Cas, the sequence similarity to the SH3P7 motifs extends to the Y+4 position in that they all have a proline in that position (YXXPP). The SLP-65 protein, which we have recently identified as the B-cell analog of the T-cell adapter protein SLP-76, is the earliest PTK substrate protein in activated B cells and may be part of a BCR transducer complex (43). Thus, the family of YXXP-containing adapter proteins has a key regulatory role in signal transduction and can participate in cellular transformation. Upon tyrosine phosphorylation, these proteins can be recognized by and bound to SH2 domain-containing proteins (25). SH2 domains bind their phosphopeptide ligands in a sequence-specific manner. When a phosphopeptide library was screened with different SH2 domains, tyrosine-phosphorylated peptides having a proline residue in the Y+3 position were selected as high-affinity ligands by the SH2 domains of Abl, Crk, and Nck (34). The SH2-mediated binding of these proteins to phosphorylated YXXP sequences may nucleate the formation of multimeric signaling complexes and may be one way all these proteins contribute to cellular activation and/or neoplastic transformation. Experiments are under way to test whether tyrosine-phosphorylated SH3P7 binds to one or more of the above-mentioned proteins during B-cell activation.
In addition to the transient protein-protein interactions mediated by SH2-phosphotyrosine binding, the SH3 domain of SH3P7 can mediate a stimulation-independent association to proline-rich proteins (11, 21, 28). SH3 domains are frequently found in proteins of the cytoskeleton. The SH3 domain of SH3P7 is most closely related to that of cortactin, HS1, and Abp-1 (data not shown). The SH3 domain is separated from the N terminus of SH3P7 by a stretch of highly charged amino acids containing four repeats of the consensus sequence R/KXEEXR. The RE-rich hexamer is found in a number of other proteins, for example, drebrins, troponin I, and caldesmon (data not shown), but not in coactosin and Abp-1. This part of SH3P7 could adopt an α-helical, rod-like structure, which may function to prevent an intramolecular interaction between the C-terminal SH3 domain and the N-terminal part of the protein, which contains the SCAD region.
Four features of the SCAD region strongly suggest that it represents a new type of protein module which mediates the specific binding to actin. First, the SCAD region is found in distantly related proteins from different organisms, demonstrating that the structural folding of the SCAD region is independent of surrounding sequences. This is particularly evident in coactosin, where the entire protein is composed of one SCAD region. Second, a very high degree of sequence similarity is clustered around two conserved tryptophan residues, a characteristic also described for SH3 and WW domains (21, 36). The crystal structure analysis of the latter two modules revealed that the bulky tryptophan residue is a central element for the three-dimensional folding of the domain. Third, despite the evolutionary distance of the organisms from which the SCAD-containing proteins were isolated, the degree of sequence similarity between their SCAD regions is even higher than that found between PH domains or between certain SH2 domains (data not shown). Finally, all four SCAD-containing proteins are linked to cellular functions that involve the actin cytoskeleton. The known isoforms of the neuron-specific drebrin proteins (A, E1, and E2) directly bind actin and regulate actin filament assembly (33). Drebrin E-actin complexes accumulate in the submembranous, cortical cytoplasm, and overexpression of drebrin E in fibroblasts results in the formation of cell processes. The yeast F-actin-binding protein Abp-1 is associated with the cortical cytoskeleton and plays a role in endocytosis (10, 41). The latter function is dependent on the Abp-1 SH3 domain, which binds to the adenylate cyclase-associated protein CAP, a positive regulator of cyclic AMP synthesis (13, 41). Most strikingly, coactosin, which does not possess amino acids in addition to the SCAD region, binds to F-actin (9). This enhances F-actin polymerization by antagonizing the binding of F-actin-capping proteins, which retard the process (30). The colocalization of SH3P7 with actin fibers and its sensitivity to cytochalasin D show that SH3P7 is also linked to the actin cytoskeleton and may participate in its reorganization.
The functional significance of the cytoskeleton for lymphocyte development and activation is now established. A stimulation-dependent and -independent association of cytoskeletal components with both B- and T-cell antigen receptors has been reported previously (4, 20, 24, 31). Antigen-mediated reorganization of the microtubule-organizing center in T cells and the F-actin assembly in B cells is ITAM dependent (6, 19). A functional actin cytoskeleton is thought to be a prerequisite for the internalization of BCR-antigen complexes leading to antigen processing and presentation to T cells. The most compelling and direct evidence that the actin cytoskeleton is required for antigen receptor signaling is provided by the analysis of mice deficient for the vav proto-oncogene (12, 15). Tyrosine-phosphorylated Vav exhibits GDP/GTP exchange activity for the Rho-like small GTPase Rac 1 (7), which regulates reorganization of the cytoskeleton (27). In the absence of Vav, T cells fail to induce antigen-mediated actin polymerization and clustering of T-cell receptors into patches and caps (12, 15). Other signaling defects include reduced Ca2+ mobilization and NFAT-mediated transcriptional activation. These observations help to explain the impaired development of T and B cells in Vav-deficient mice (12, 15, 37, 47). A physical association of Vav with components of the cytoskeleton was demonstrated by coimmunoprecipitation with talin and vinculin (12). Coupling of Vav to antigen receptors may involve its association to SLP-76 in T cells (40, 44) and to SLP-65 in B cells (43). These results indicate that in lymphocytes, the cytoskeleton plays a role not only in the organization of cell shape and morphology but also in the structural organization of early and late signaling events. The molecular mechanisms underlying these processes are unclear. Our data implicate SH3P7 as a second effector molecule that transmits signals from lymphocyte antigen receptors to the cytoskeleton. Like other adapter proteins, SH3P7 may act as regulatory element to achieve and maintain specificity within the network of signaling proteins.
ACKNOWLEDGMENTS
We thank M. Reth for generous support and many critical discussions.
This work is supported by the Deutsche Forschungsgemeinschaft through grant SFB388 and the Leibniz prize to M. Reth.
REFERENCES
- 1.Baumann G, Maier D, Freuler F, Tschopp C, Baudisch K, Wienands J. In vitro characterization of major ligands for Src homology 2 domains derived from tyrosine kinases, from the adaptor protein SHC and from GTPase-activating protein in Ramos B cells. Eur J Immunol. 1994;42:1799–1807. doi: 10.1002/eji.1830240812. [DOI] [PubMed] [Google Scholar]
- 2.Borg J-P, Margolis B. Function of PTB domains. Curr Top Microbiol Immunol. 1998;228:23–38. doi: 10.1007/978-3-642-80481-6_2. [DOI] [PubMed] [Google Scholar]
- 3.Bork P, Sudol M. The WW domain: a signalling site in dystrophin. Trends Biochem Sci. 1994;19:531–533. doi: 10.1016/0968-0004(94)90053-1. [DOI] [PubMed] [Google Scholar]
- 4.Caplan S, Zeliger S, Wang L, Baniyash M. Cell-surface-expressed T-cell antigen-receptor ζ chain is associated with the cytoskeleton. Proc Natl Acad Sci USA. 1995;92:4768–4772. doi: 10.1073/pnas.92.11.4768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carpino N, Wisniewski D, Strife A, Marshak D, Kobayashi R, Stillman B, Clarkson B. p62dok: a constitutively tyrosine-phosphorylated GAP-associated protein in chronic myelogenous leukemia progenitor cells. Cell. 1997;88:197–204. doi: 10.1016/s0092-8674(00)81840-1. [DOI] [PubMed] [Google Scholar]
- 6.Cox D, Chang P, Kurosaki T, Greenberg S. Syk tyrosine kinase is required for immunoreceptor tyrosine activation motif-dependent actin assembly. J Biol Chem. 1996;271:16597–16602. doi: 10.1074/jbc.271.28.16597. [DOI] [PubMed] [Google Scholar]
- 7.Crespo P, Schuebel K E, Ostrom A A, Gutkind J S, Bustelo X R. Phosphotyrosine-dependent activation of Rac-1 GDP/GTP exchange by the vav proto-oncogene product. Nature. 1997;385:169–172. doi: 10.1038/385169a0. [DOI] [PubMed] [Google Scholar]
- 8.DeFranco A L. The complexity of signaling pathways activated by the BCR. Curr Opin Immunol. 1997;9:296–308. doi: 10.1016/s0952-7915(97)80074-x. [DOI] [PubMed] [Google Scholar]
- 9.deHostos E L, Bradtke B, Lottspeich F, Gerisch G. Coactosin, a 17 kDa F-actin binding protein from Dictyostelium discoideum. Cell Motil Cytoskelet. 1993;26:181–191. doi: 10.1002/cm.970260302. [DOI] [PubMed] [Google Scholar]
- 10.Drubrin D G, Mulholland J, Zhu Z, Botstein D. Homology of a yeast actin-binding protein to signal transduction proteins and myosin-1. Nature. 1990;343:288–290. doi: 10.1038/343288a0. [DOI] [PubMed] [Google Scholar]
- 11.Feng S, Chen J K, Yu H, Simon J A, Schreiber S L. Two binding orientations for peptides to the Src SH3 domain: development of a general model for SH3-ligand interactions. Science. 1994;266:1241–1247. doi: 10.1126/science.7526465. [DOI] [PubMed] [Google Scholar]
- 12.Fischer K-D, Kong Y-Y, Tedford K, Marengère L E M, Kozieradzki I, Sasaki T, Starr M, Chan G, Gardener S, Nghiem M P, Bouchard D, Barbacid M, Bernstein A, Penninger J M. Vav is a regulator of the cytoskeletal reorganization mediated by the T-cell receptor. Curr Biol. 1998;8:554–562. doi: 10.1016/s0960-9822(98)70224-6. [DOI] [PubMed] [Google Scholar]
- 13.Freemann N L, Lila T, Mintzer K, Chen Z, Pahk A J, Ren R, Drubin D G, Field J. A conserved proline-rich region of the Saccharomyces cerevisiae cyclase-associated protein binds SH3 domains and modulates cytoskeletal localization. Mol Cell Biol. 1996;16:548–556. doi: 10.1128/mcb.16.2.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gold M R, Crowley M T, Martin G A, McCormick F, DeFranco A L. Targets of B lymphocyte antigen receptor signal transduction include the p21ras GTPase-activating protein (GAP) and two GAP-associated proteins. J Immunol. 1993;150:377–386. [PubMed] [Google Scholar]
- 15.Holsinger L J, Graef I A, Swat W, Chi T, Bautista D M, Davidson L, Lewis R S, Alt F W, Crabtree G R. Defects in actin-cap formation in Vav-deficient mice implicate an actin requirement for lymphocyte signal transduction. Curr Biol. 1998;8:563–572. doi: 10.1016/s0960-9822(98)70225-8. [DOI] [PubMed] [Google Scholar]
- 16.Kurosaki T. Molecular mechanisms in B cell antigen receptor signaling. Curr Opin Immunol. 1997;9:309–318. doi: 10.1016/s0952-7915(97)80075-1. [DOI] [PubMed] [Google Scholar]
- 17.Langdon W Y, Hartley J W, Klinken S P, Ruscetti S K, Morse H C D. v-cbl, an oncogene from a dual-recombinant murine retrovirus that induces early B-lineage lymphomas. Proc Natl Acad Sci USA. 1989;86:1168–1172. doi: 10.1073/pnas.86.4.1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lemmon M A, Falasca M, Ferguson K M, Schlessinger J. Regulatory recruitment of signalling molecules to the cell membrane by pleckstrin-homology domains. Trends Cell Biol. 1997;7:237–242. doi: 10.1016/S0962-8924(97)01065-9. [DOI] [PubMed] [Google Scholar]
- 19.Lowin-Kropf B, Smith Shapiro V, Weiss A. Cytoskeletal polarization of T cells is regulated by an immunoceptor tyrosine-based activation motif-dependent mechanism. J Cell Biol. 1998;140:861–971. doi: 10.1083/jcb.140.4.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marano N, Holowka D, Baird B. Bivalent binding of an anti-CD3 antibody to Jurkat cells induces association of the T cell receptor complex with cytoskeleton. J Immunol. 1989;143:931–938. [PubMed] [Google Scholar]
- 21.Mayer B J, Eck M J. Minding your p’s and q’s. Curr Biol. 1995;4:364–367. doi: 10.1016/s0960-9822(95)00073-x. [DOI] [PubMed] [Google Scholar]
- 22.Mayer B J, Gupta G. Functions of SH2 and SH3 domains. Curr Top Microbiol Immunol. 1998;228:1–22. doi: 10.1007/978-3-642-80481-6_1. [DOI] [PubMed] [Google Scholar]
- 23.Panchamoorthy G, Fukazawa T, Miyake S, Soltoff S, Reedquist K, Druker B, Shoelson S, Cantley L, Band H. p120cbl is a major substrate of tyrosine phosphorylation upon B cell antigen receptor stimulation and interacts in vivo with Fyn and Syk tyrosine kinases, Grb2 and Shc adaptors, and the p85 subunit of phosphytidylinositol 3-kinase. J Biol Chem. 1996;271:3187–3194. doi: 10.1074/jbc.271.6.3187. [DOI] [PubMed] [Google Scholar]
- 24.Park J Y, Jongstra-Bilen J. Interactions between membrane IgM and the cytoskeleton involve the cytoplasmic domain of the immunoglobulin receptor. Eur J Immunol. 1997;27:3001–3009. doi: 10.1002/eji.1830271137. [DOI] [PubMed] [Google Scholar]
- 25.Pawson T. Protein modules and signalling networks. Nature. 1995;373:573–580. doi: 10.1038/373573a0. [DOI] [PubMed] [Google Scholar]
- 26.Posas F, Saito H. Osmotic activation of the HOG MAPK pathway via Ste11p MAPKKK: scaffold role of PBS2p MAPKK. Science. 1997;276:1702–1705. doi: 10.1126/science.276.5319.1702. [DOI] [PubMed] [Google Scholar]
- 27.Reif K, Cantrell D A. Networking Rho family GTPases in lymphocytes. Immunity. 1998;8:395–401. doi: 10.1016/s1074-7613(00)80545-2. [DOI] [PubMed] [Google Scholar]
- 28.Ren R, Mayer B J, Cicchetti P, Baltimore D. Identification of a 10-amino acid proline-rich SH3 binding site. Science. 1993;259:1157–1161. doi: 10.1126/science.8438166. [DOI] [PubMed] [Google Scholar]
- 29.Reth M, Wienands J. Initiation and processing of signals from the B cell antigen receptor. Annu Rev Immunol. 1997;15:453–479. doi: 10.1146/annurev.immunol.15.1.453. [DOI] [PubMed] [Google Scholar]
- 30.Röhrig U, Gerisch G, Morozova L, Schleicher M, Wegner A. Coactosin interferes with the capping of actin filaments. FEBS Lett. 1995;374:284–286. doi: 10.1016/0014-5793(95)01130-7. [DOI] [PubMed] [Google Scholar]
- 31.Rozdzial M M, Malissen B, Finkel T H. Tyrosine-phosphorylated T cell receptor ζ chain associates with the actin cytoskeleton upon activation of mature T lymphocytes. Immunity. 1995;3:623–633. doi: 10.1016/1074-7613(95)90133-7. [DOI] [PubMed] [Google Scholar]
- 32.Sakai R, Iwamatsu A, Hirano N, Ogawa S, Tanaka T, Mano H, Yazaki Y, Hirai H. A novel signaling molecule, p130, forms stable complexes in vivo with v-Crk and v-Src in a tyrosine phosphorylation-dependent manner. EMBO J. 1994;13:3748–3756. doi: 10.1002/j.1460-2075.1994.tb06684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shirao T. The roles of microfilament-associated proteins, drebrins, in brain morphogenesis. J Biochem. 1995;117:231–236. doi: 10.1093/jb/117.2.231. [DOI] [PubMed] [Google Scholar]
- 34.Songyang Z, Shoelson S E, Chaudhuri M, Gish G, Pawson T, Haser W G, King F, Roberts T, Ratnofsky S, Lechleider R J, Neel B G, Birge R B, Fajardo J E, Chou M M, Hanafusa H, Schaffhausen B, Cantley L C. SH2 domains recognize specific phosphopeptide sequences. Cell. 1993;72:767–778. doi: 10.1016/0092-8674(93)90404-e. [DOI] [PubMed] [Google Scholar]
- 35.Sparks A B, Hoffmann N G, McConnell S J, Fowlkes D M, Kay B K. Cloning of ligand targets: systematic isolation of SH3 domain-containing proteins. Nat Biotechnol. 1996;14:741–744. doi: 10.1038/nbt0696-741. [DOI] [PubMed] [Google Scholar]
- 36.Staub O, Rotin D. WW domains. Structure. 1996;4:495–499. doi: 10.1016/s0969-2126(96)00054-8. [DOI] [PubMed] [Google Scholar]
- 37.Tarakhovsky A, Turner M, Schaal S, Mee P J, Duddy L P, Rajewsky K, Tybulewicz V L. Defective antigen receptor-mediated proliferation of B and T cells in the absence of Vav. Nature. 1995;374:467–470. doi: 10.1038/374467a0. [DOI] [PubMed] [Google Scholar]
- 38.Tezuka T, Umemori H, Fusaki N, Yagi T, Takata M, Kurosaki T, Yamamoto T. Physical and functional association of the cbl proto-oncogene product with an Src-family protein tyrosine kinase, p53/56lyn, in the B cell antigen receptor-mediated signaling. J Exp Med. 1996;183:675–680. doi: 10.1084/jem.183.2.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tsunoda S, Sierralta J, Sun Y, Bodner R, Suzuki E, Becker A, Socolich M, Zuker C S. A multivalent PDZ-domain protein assembles signalling complexes in a G-protein-coupled cascade. Nature. 1997;388:243–249. doi: 10.1038/40805. [DOI] [PubMed] [Google Scholar]
- 40.Tuosto L, Michel F, Acuto O. p95vav associates with tyrosine-phosphorylated SLP-76 in antigen-stimulated T cells. J Exp Med. 1996;184:1161–1166. doi: 10.1084/jem.184.3.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wesp A, Hicke L, Palecek J, Lombardi R, Aust T, Munn A L, Riezman H. End4p/Sla2p interacts with actin-associated proteins for endocytosis in Saccharomyces cerevisiae. Mol Biol Cell. 1997;8:2291–2306. doi: 10.1091/mbc.8.11.2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wienands J, Larbolette O, Reth M. Evidence for a preformed transducer complex organized by the B cell antigen receptor. Proc Natl Acad Sci USA. 1996;93:7865–7870. doi: 10.1073/pnas.93.15.7865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wienands J, Schweikert J, Wollscheid B, Jumaa H, Nielsen P J, Reth M. SLP-65: a new signaling component in B lymphocytes which requires expression of the antigen receptor for phosphorylation. J Exp Med. 1998;188:791–795. doi: 10.1084/jem.188.4.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu J, Motto D G, Koretzky G A, Weiss A. Vav and SLP-76 interact and functionally cooperate in IL-2 gene activation. Immunity. 1996;4:593–602. doi: 10.1016/s1074-7613(00)80485-9. [DOI] [PubMed] [Google Scholar]
- 45.Yamanashi Y, Baltimore D. Identification of the Abl- and rasGAP-associated 62 kDa protein as a docking protein, Dok. Cell. 1997;88:205–211. doi: 10.1016/s0092-8674(00)81841-3. [DOI] [PubMed] [Google Scholar]
- 46.Yamanashi Y, Okada M, Semba T, Yamori T, Umemori H, Tsunasawa S, Toyoshima K, Kitamura D, Watanabe T, Yamamoto T. Identification of HS1 protein as a major substrate of protein-tyrosine kinase(s) upon B-cell antigen receptor-mediated signaling. Proc Natl Acad Sci USA. 1993;90:3631–3635. doi: 10.1073/pnas.90.8.3631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang R, Alt F W, Davidson L, Orkin S H, Swat W. Defective signalling through the T- and B-cell antigen receptors in lymphoid cells lacking the vav proto-oncogene. Nature. 1995;374:470–473. doi: 10.1038/374470a0. [DOI] [PubMed] [Google Scholar]