Abstract
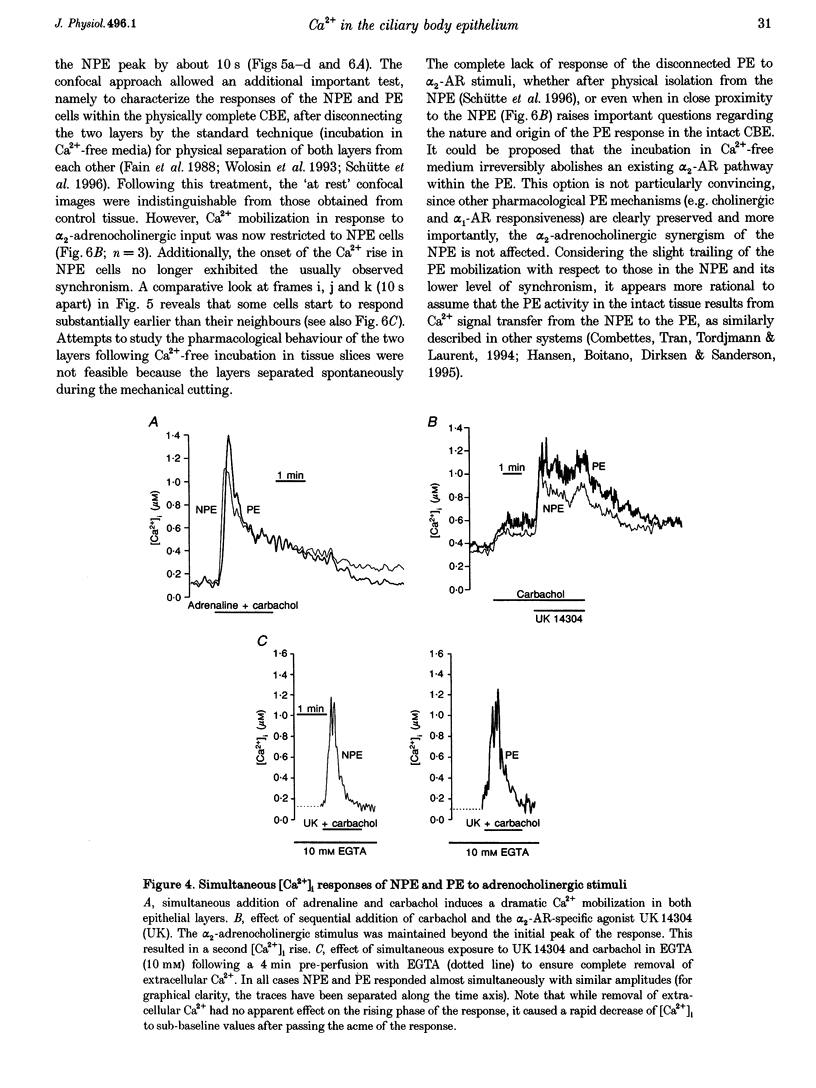
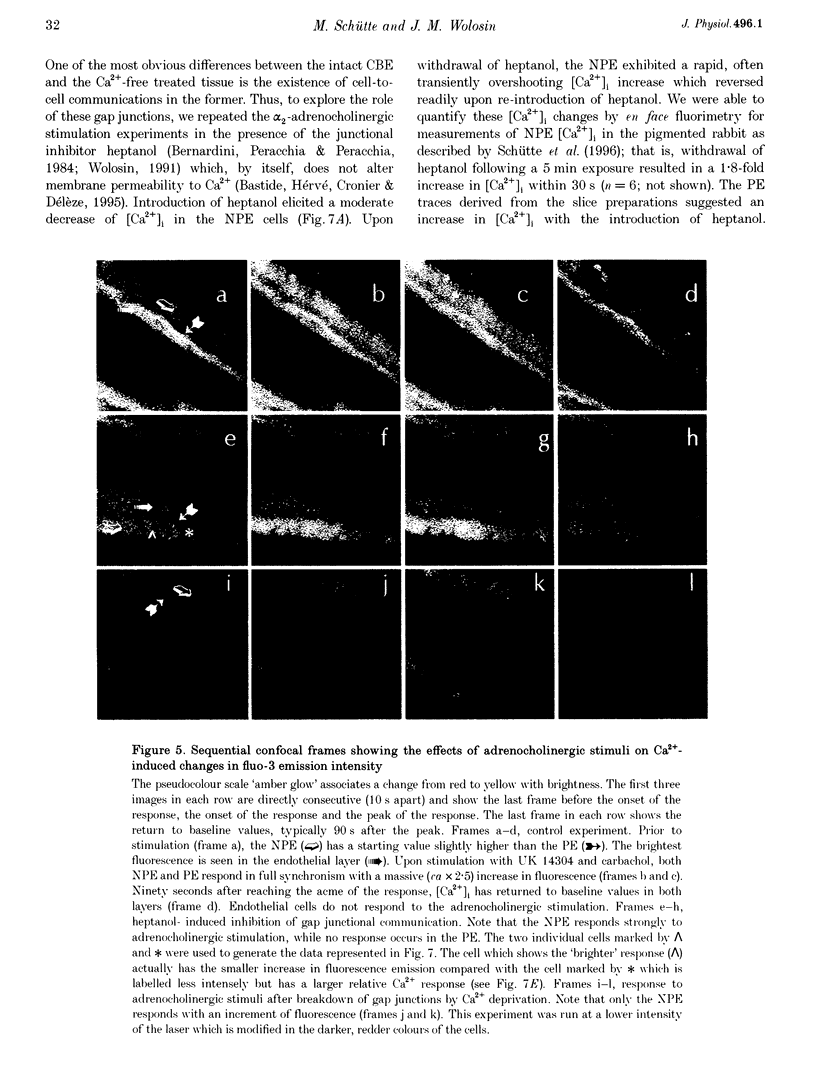
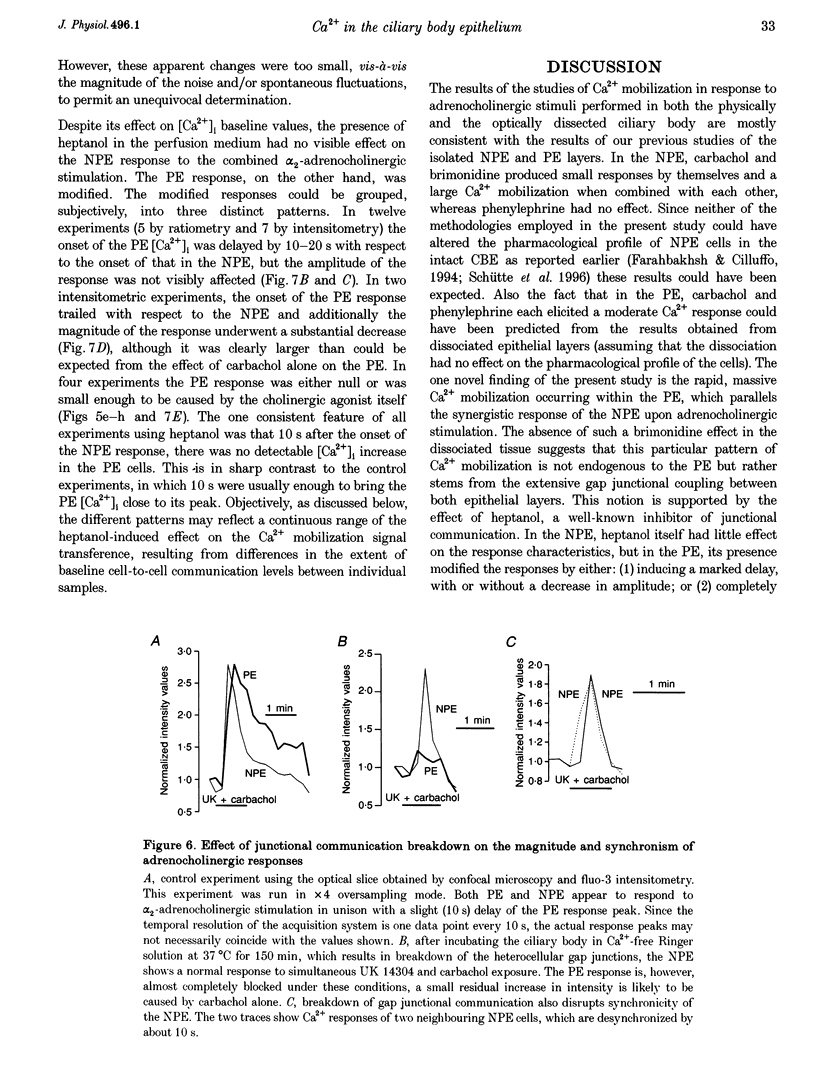
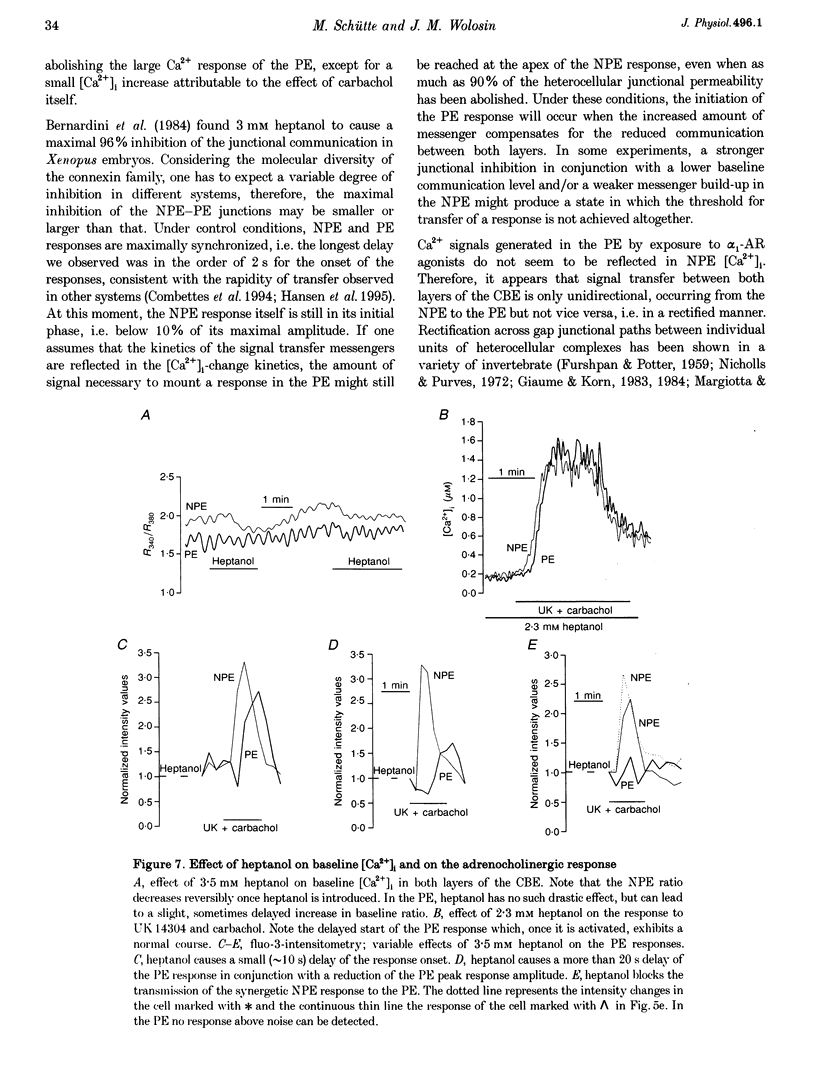
1. 'Ratiometric' fura-2 methodology in slice preparations and 'intensitometric' fluo-3 measurements of confocal images were used to simultaneously monitor Ca2+ mobilization in the two distinct, apically joined cell layers which constitute the ciliary body epithelium (CBE): the non-pigmented (NPE) and pigmented (PE) epithelia. 2. Both methods yielded comparable results regarding Ca2+ responses in the syncytium upon stimulation with adrenergic and cholinergic agonists. 3. The alpha 1-adrenoceptor agonist phenylephrine elicited a moderate [Ca2+]i increase in the PE, whereas NPE [Ca2+]i remained unchanged or exhibited a slight diminution. 4. In combination with carbachol, the alpha 2-adrenoceptor agonist brimonidine elicited large Ca2+ increases (> 10-fold) in both the NPE and PE cell layers, even though previous studies indicated the absence of an alpha 2-adrenergic effect on [Ca2+]i in the PE. The onset, as well as the peak of the Ca2+ responses in PE cells frequently exhibited a small delay with respect to adjacent NPE cells. No such time difference was observed between adjacent NPE cells. 5. Pre-incubation of the ciliary body in Ca(2+)-free solution under conditions known to elicit overt NPE-PE separation abolished the alpha 2-adrenocholinergic response in the PE. 6. Addition of heptanol to the perfusate, to block gap-junctional communication, caused a small [Ca2+]i decrease in the NPE and a slight increase in PE[Ca2+]i. Subsequently, the Ca2+ mobilization in the Pe in response to the brimonidine and carbachol combination was either blocked or showed a substantial delay. The Ca2+ mobilization in the NPE, in contrast, remained unchanged. 7. We conclude that the heterocellular syncytium exhibits rectificatory behaviour with respect to Ca2+ mobilization; responses originating within the NPE are easily transferred to the PE, while the reverse does not occur.
Full text
PDF


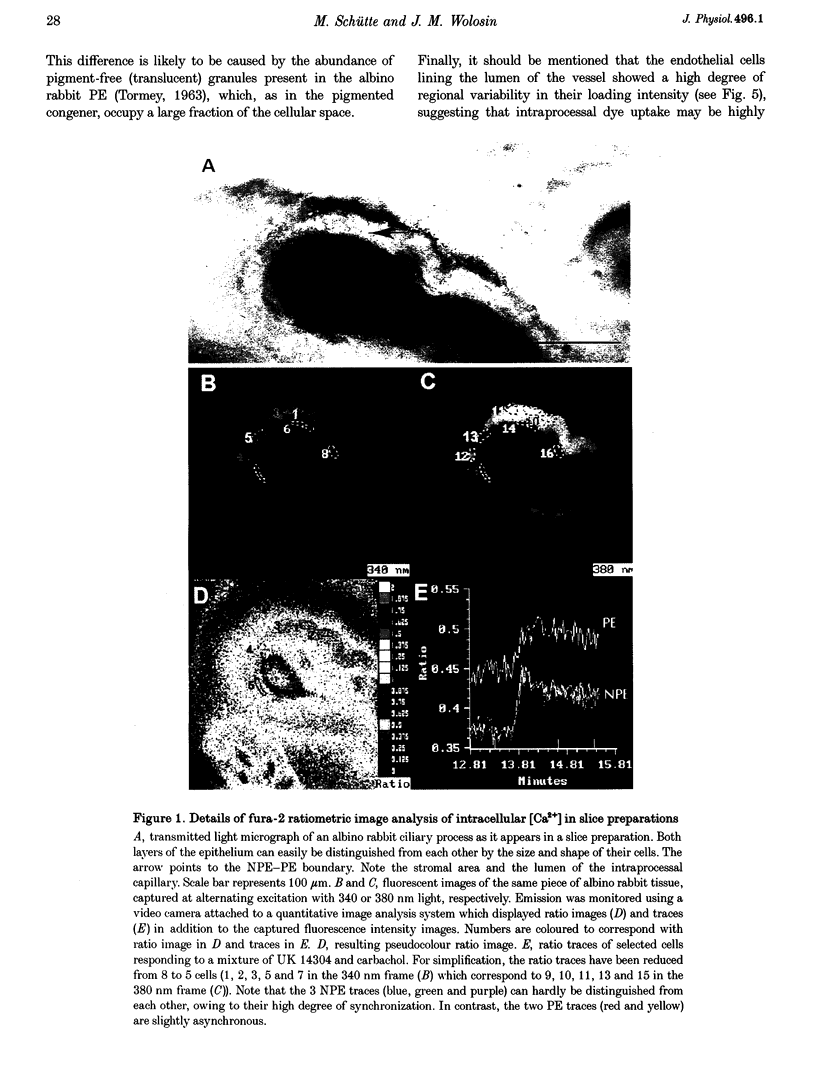
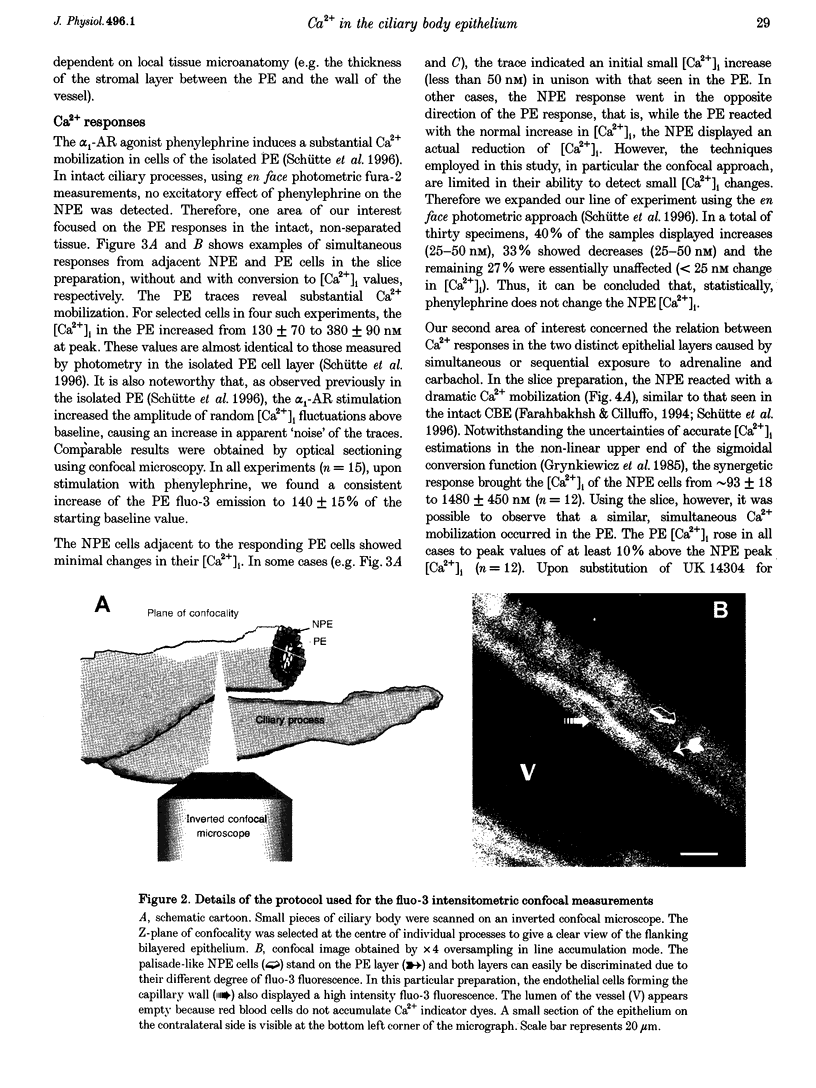
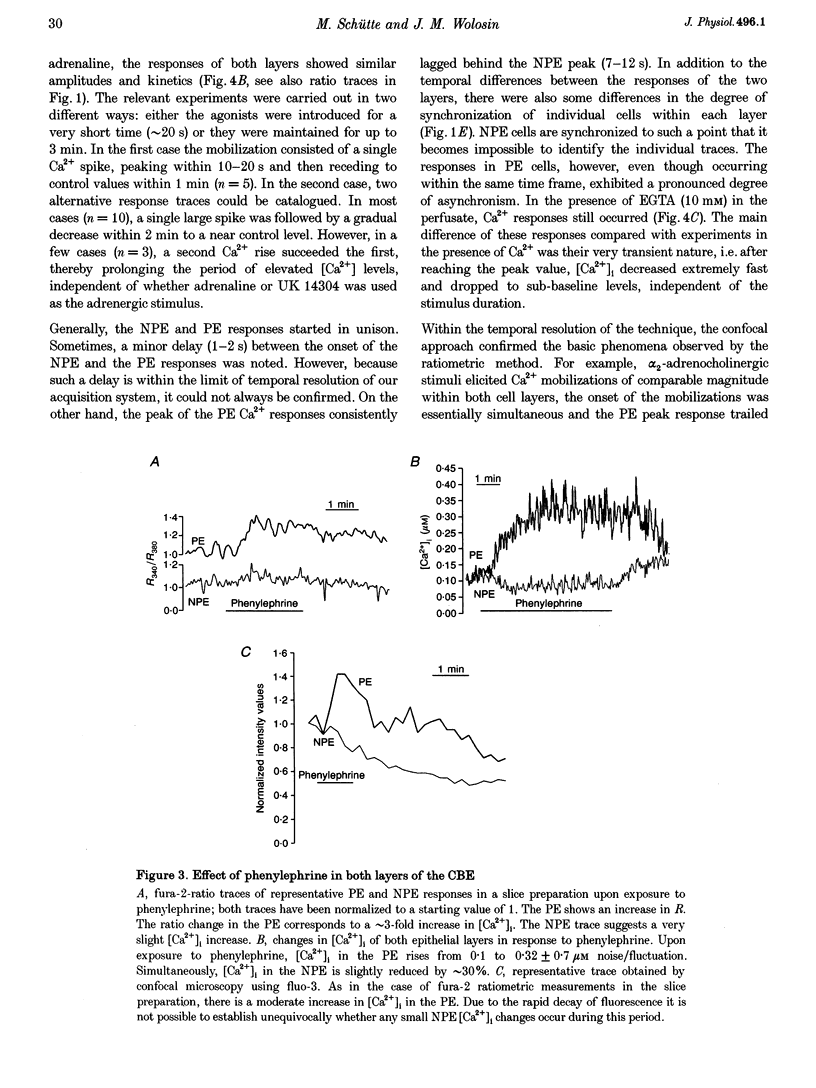



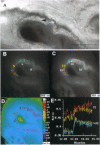
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Assmann S. M., Wu W. H. Inhibition of guard-cell inward K+ channels by abscisic acid: links and gaps in the signal transduction chain. Symp Soc Exp Biol. 1994;48:193–202. [PubMed] [Google Scholar]
- Bastide B., Hervé J. C., Cronier L., Délèze J. Rapid onset and calcium independence of the gap junction uncoupling induced by heptanol in cultured heart cells. Pflugers Arch. 1995 Jan;429(3):386–393. doi: 10.1007/BF00374154. [DOI] [PubMed] [Google Scholar]
- Bernardini G., Peracchia C., Peracchia L. L. Reversible effects of heptanol on gap junction structure and cell-to-cell electrical coupling. Eur J Cell Biol. 1984 Jul;34(2):307–312. [PubMed] [Google Scholar]
- Berridge M. J. The Albert Lasker Medical Awards. Inositol trisphosphate, calcium, lithium, and cell signaling. JAMA. 1989 Oct 6;262(13):1834–1841. [PubMed] [Google Scholar]
- Bill A. Blood circulation and fluid dynamics in the eye. Physiol Rev. 1975 Jul;55(3):383–417. doi: 10.1152/physrev.1975.55.3.383. [DOI] [PubMed] [Google Scholar]
- Brubaker R. F. Flow of aqueous humor in humans [The Friedenwald Lecture]. Invest Ophthalmol Vis Sci. 1991 Dec;32(13):3145–3166. [PubMed] [Google Scholar]
- Butler G. A., Chen M., Stegman Z., Wolosin J. M. Na(+-) Cl(-)- and HCO3(-)-dependent base uptake in the ciliary body pigment pigment epithelium. Exp Eye Res. 1994 Sep;59(3):343–349. doi: 10.1006/exer.1994.1116. [DOI] [PubMed] [Google Scholar]
- Candia O. A., Shi X. P., Chu T. C. Ascorbate-stimulated active Na+ transport in rabbit ciliary epithelium. Curr Eye Res. 1991 Mar;10(3):197–203. doi: 10.3109/02713689109003441. [DOI] [PubMed] [Google Scholar]
- Cole D. F. Secretion of the aqueous humour. Exp Eye Res. 1977;25 (Suppl):161–176. doi: 10.1016/s0014-4835(77)80015-8. [DOI] [PubMed] [Google Scholar]
- Combettes L., Tran D., Tordjmann T., Laurent M., Berthon B., Claret M. Ca(2+)-mobilizing hormones induce sequentially ordered Ca2+ signals in multicellular systems of rat hepatocytes. Biochem J. 1994 Dec 1;304(Pt 2):585–594. doi: 10.1042/bj3040585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danoff S. K., Ross C. A. The inositol trisphosphate receptor gene family: implications for normal and abnormal brain function. Prog Neuropsychopharmacol Biol Psychiatry. 1994 Jan;18(1):1–16. doi: 10.1016/0278-5846(94)90021-3. [DOI] [PubMed] [Google Scholar]
- Edelman J. L., Sachs G., Adorante J. S. Ion transport asymmetry and functional coupling in bovine pigmented and nonpigmented ciliary epithelial cells. Am J Physiol. 1994 May;266(5 Pt 1):C1210–C1221. doi: 10.1152/ajpcell.1994.266.5.C1210. [DOI] [PubMed] [Google Scholar]
- Ehrlich B. E., Kaftan E., Bezprozvannaya S., Bezprozvanny I. The pharmacology of intracellular Ca(2+)-release channels. Trends Pharmacol Sci. 1994 May;15(5):145–149. doi: 10.1016/0165-6147(94)90074-4. [DOI] [PubMed] [Google Scholar]
- FURSHPAN E. J., POTTER D. D. Transmission at the giant motor synapses of the crayfish. J Physiol. 1959 Mar 3;145(2):289–325. doi: 10.1113/jphysiol.1959.sp006143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fain G. L., Cilluffo M. C., Fain M. J., Lee D. A. Isolation of non-pigmented epithelial cells from rabbit ciliary body. Invest Ophthalmol Vis Sci. 1988 May;29(5):817–821. [PubMed] [Google Scholar]
- Farahbakhsh N. A., Cilluffo M. C. Synergistic effect of adrenergic and muscarinic receptor activation on [Ca2+]i in rabbit ciliary body epithelium. J Physiol. 1994 Jun 1;477(Pt 2):215–221. doi: 10.1113/jphysiol.1994.sp020185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaume C., Korn H. Bidirectional transmission at the rectifying electrotonic synapse: a voltage-dependent process. Science. 1983 Apr 1;220(4592):84–87. doi: 10.1126/science.6298940. [DOI] [PubMed] [Google Scholar]
- Giaume C., Korn H. Voltage-dependent dye coupling at a rectifying electrotonic synapse of the crayfish. J Physiol. 1984 Nov;356:151–167. doi: 10.1113/jphysiol.1984.sp015458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hampson E. C., Vaney D. I., Weiler R. Dopaminergic modulation of gap junction permeability between amacrine cells in mammalian retina. J Neurosci. 1992 Dec;12(12):4911–4922. doi: 10.1523/JNEUROSCI.12-12-04911.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen M., Boitano S., Dirksen E. R., Sanderson M. J. A role for phospholipase C activity but not ryanodine receptors in the initiation and propagation of intercellular calcium waves. J Cell Sci. 1995 Jul;108(Pt 7):2583–2590. doi: 10.1242/jcs.108.7.2583. [DOI] [PubMed] [Google Scholar]
- Jacob T. J. Identification of a low-threshold T-type calcium channel in bovine ciliary epithelial cells. Am J Physiol. 1991 Nov;261(5 Pt 1):C808–C813. doi: 10.1152/ajpcell.1991.261.5.C808. [DOI] [PubMed] [Google Scholar]
- Lasater E. M., Dowling J. E. Dopamine decreases conductance of the electrical junctions between cultured retinal horizontal cells. Proc Natl Acad Sci U S A. 1985 May;82(9):3025–3029. doi: 10.1073/pnas.82.9.3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margiotta J. F., Walcott B. Conductance and dye permeability of a rectifying electrical synapse. Nature. 1983 Sep 1;305(5929):52–55. doi: 10.1038/305052a0. [DOI] [PubMed] [Google Scholar]
- Mills S. L., Massey S. C. Differential properties of two gap junctional pathways made by AII amacrine cells. Nature. 1995 Oct 26;377(6551):734–737. doi: 10.1038/377734a0. [DOI] [PubMed] [Google Scholar]
- Minta A., Kao J. P., Tsien R. Y. Fluorescent indicators for cytosolic calcium based on rhodamine and fluorescein chromophores. J Biol Chem. 1989 May 15;264(14):8171–8178. [PubMed] [Google Scholar]
- Nicholls J. G., Purves D. Monosynaptic chemical and electrical connexions between sensory and motor cells in the central nervous system of the leech. J Physiol. 1970 Aug;209(3):647–667. doi: 10.1113/jphysiol.1970.sp009184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh J., Krupin T., Tang L. Q., Sveen J., Lahlum R. A. Dye coupling of rabbit ciliary epithelial cells in vitro. Invest Ophthalmol Vis Sci. 1994 Apr;35(5):2509–2514. [PubMed] [Google Scholar]
- Peracchia C., Peracchia L. L. Gap junction dynamics: reversible effects of divalent cations. J Cell Biol. 1980 Dec;87(3 Pt 1):708–718. doi: 10.1083/jcb.87.3.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peracchia C., Peracchia L. L. Gap junction dynamics: reversible effects of hydrogen ions. J Cell Biol. 1980 Dec;87(3 Pt 1):719–727. doi: 10.1083/jcb.87.3.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccolino M., Neyton J., Gerschenfeld H. M. Decrease of gap junction permeability induced by dopamine and cyclic adenosine 3':5'-monophosphate in horizontal cells of turtle retina. J Neurosci. 1984 Oct;4(10):2477–2488. doi: 10.1523/JNEUROSCI.04-10-02477.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raviola G., Raviola E. Intercellular junctions in the ciliary epithelium. Invest Ophthalmol Vis Sci. 1978 Oct;17(10):958–981. [PubMed] [Google Scholar]
- Ringham G. L. Localization and electrical characteristics of a giant synapse in the spinal cord of the lamprey. J Physiol. 1975 Oct;251(2):395–407. doi: 10.1113/jphysiol.1975.sp011100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schütte M., Diadori A., Wang C., Wolosin J. M. Comparative adrenocholinergic control of intracellular Ca2+ in the layers of the ciliary body epithelium. Invest Ophthalmol Vis Sci. 1996 Jan;37(1):212–220. [PubMed] [Google Scholar]
- Shi X. P., Zamudio A. C., Candia O. A., Wolosin J. M. Adreno-cholinergic modulation of junctional communications between the pigmented and nonpigmented layers of the ciliary body epithelium. Invest Ophthalmol Vis Sci. 1996 May;37(6):1037–1046. [PubMed] [Google Scholar]
- Sugrue M. F. The pharmacology of antiglaucoma drugs. Pharmacol Ther. 1989;43(1):91–138. doi: 10.1016/0163-7258(89)90049-1. [DOI] [PubMed] [Google Scholar]
- TORMEY J. M. Fine structure of the ciliary epithelium of the rabbit, with particular reference to "infoldedmembranes," "vesicles," and the effects of Diamox. J Cell Biol. 1963 Jun;17:641–659. doi: 10.1083/jcb.17.3.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teranishi T., Negishi K., Kato S. Dopamine modulates S-potential amplitude and dye-coupling between external horizontal cells in carp retina. Nature. 1983 Jan 20;301(5897):243–246. doi: 10.1038/301243a0. [DOI] [PubMed] [Google Scholar]
- Toris C. B., Gleason M. L., Camras C. B., Yablonski M. E. Effects of brimonidine on aqueous humor dynamics in human eyes. Arch Ophthalmol. 1995 Dec;113(12):1514–1517. doi: 10.1001/archopht.1995.01100120044006. [DOI] [PubMed] [Google Scholar]
- Wolosin J. M., Chen M., Gordon R. E., Stegman Z., Butler G. A. Separation of the rabbit ciliary body epithelial layers in viable form: identification of differences in bicarbonate transport. Exp Eye Res. 1993 Apr;56(4):401–409. doi: 10.1006/exer.1993.1054. [DOI] [PubMed] [Google Scholar]
- Wolosin J. M. Gap junctions in rabbit corneal epithelium: limited permeability and inhibition by cAMP. Am J Physiol. 1991 Nov;261(5 Pt 1):C857–C864. doi: 10.1152/ajpcell.1991.261.5.C857. [DOI] [PubMed] [Google Scholar]
- von Gersdorff H., Matthews G. Dynamics of synaptic vesicle fusion and membrane retrieval in synaptic terminals. Nature. 1994 Feb 24;367(6465):735–739. doi: 10.1038/367735a0. [DOI] [PubMed] [Google Scholar]