Abstract
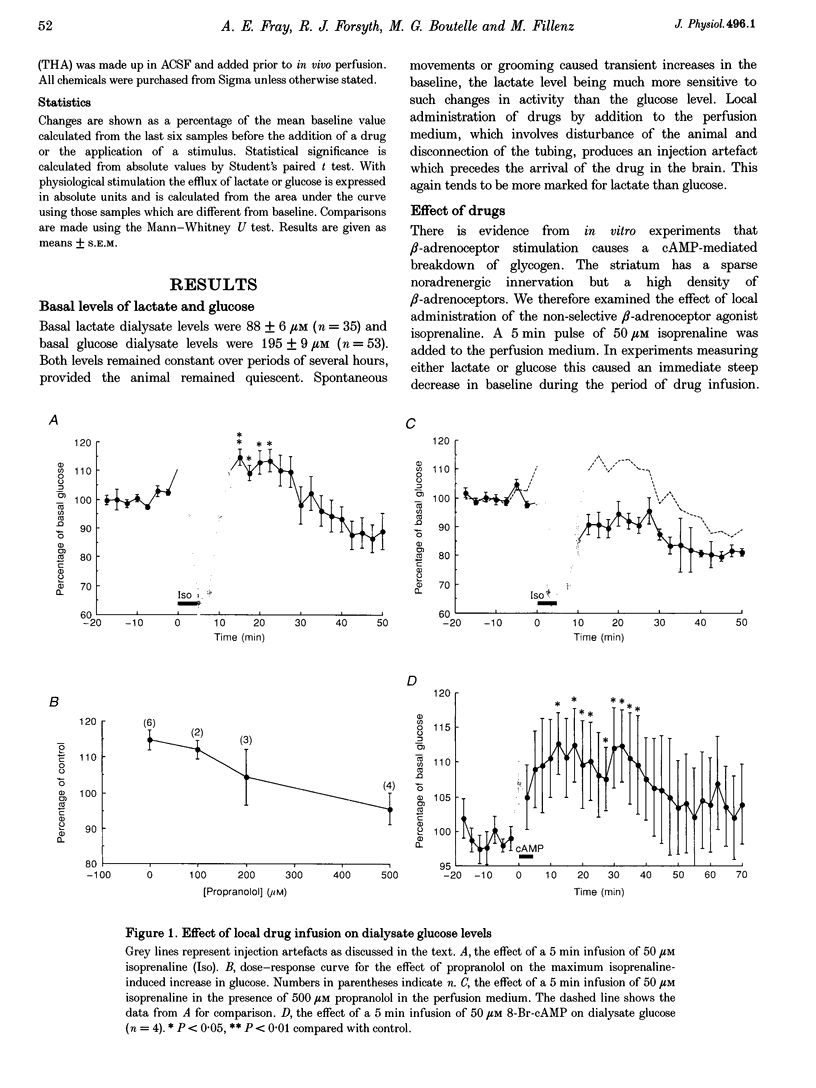
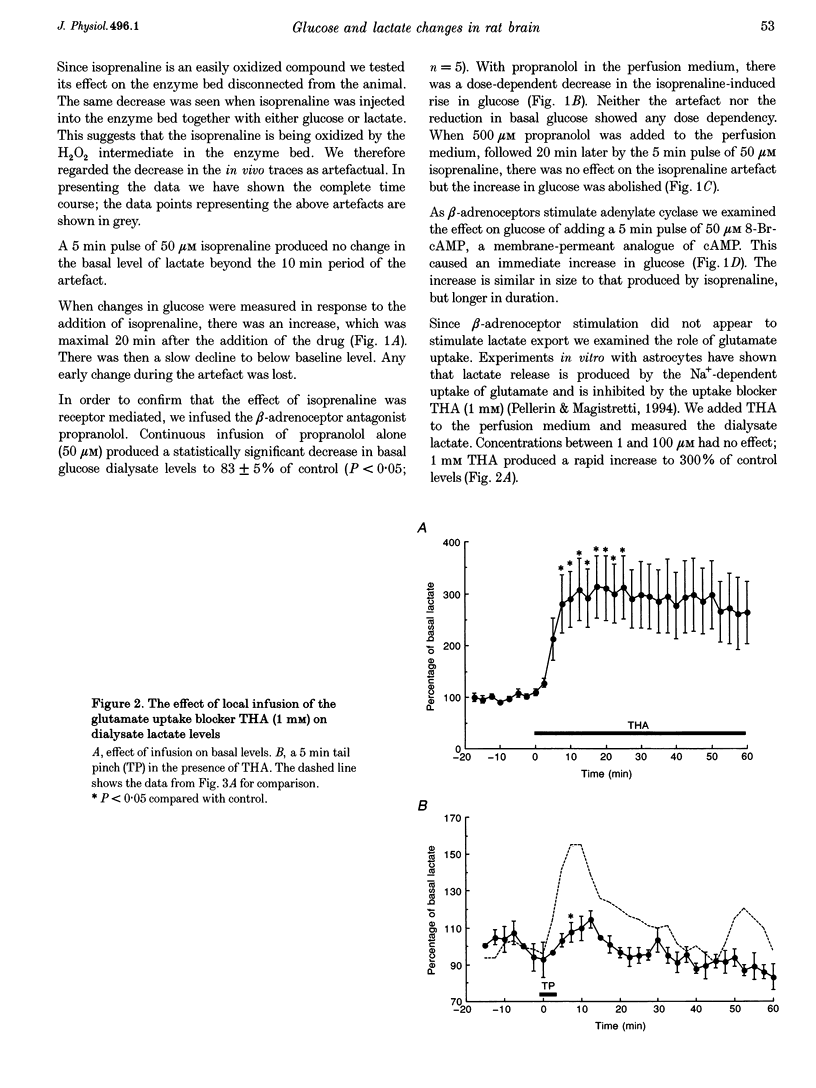
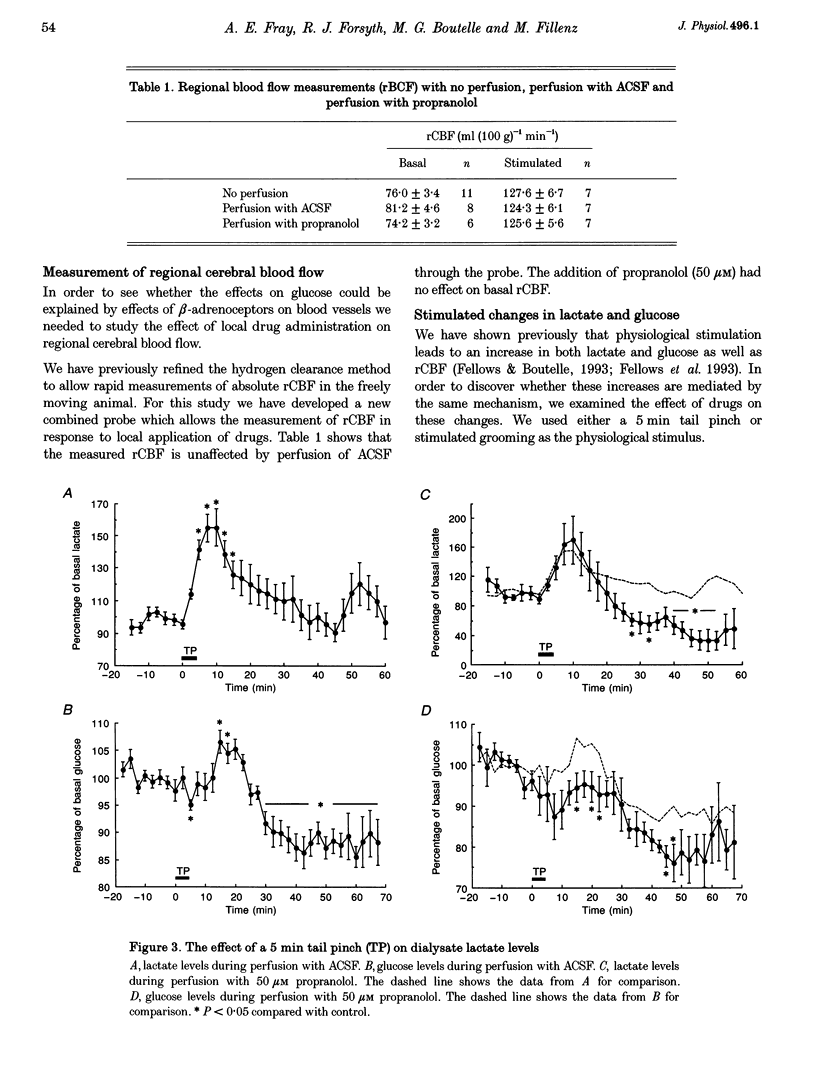
1. This study is concerned with the supply of metabolic substrates for neuronal metabolism. Experiments were carried out to investigate whether mechanisms demonstrated in cultured astrocytes also occurred in vivo; these were cAMP-mediated breakdown of glycogen and glutamate uptake-stimulated release of lactate. 2. In vivo microdialysis was used in freely moving rats. Lactate and glucose in the dialysate were assayed using enzyme-based on-line assays. Drugs were given locally through the dialysis probe. Regional cerebral blood flow was measured using the hydrogen clearance method. 3. There was an increase in dialysate glucose in response to the beta-adrenoceptor agonist isoprenaline and to 8-bromo-cAMP, an analogue of cAMP, the second messenger of beta-adrenoceptor stimulation. The effect of isoprenaline was blocked by the antagonist propranolol. Isoprenaline had no effect on dialysate lactate, which was increased by the glutamate uptake blocker beta-D,L-threohydroxyaspartate (THA). 4. Physiological stimulation of neuronal activity produced an increase in both lactate and glucose. The increase in lactate was depressed in the presence of THA but was unaffected by propranolol. The increase in glucose was blocked by propranolol. Regional cerebral blood flow was increased by physiological stimulation but was unaffected by propranolol. 5. These results demonstrate that physiologically stimulated increases in glucose and lactate in the brain are mediated by different mechanisms.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bell J. E., Hume R., Busuttil A., Burchell A. Immunocytochemical detection of the microsomal glucose-6-phosphatase in human brain astrocytes. Neuropathol Appl Neurobiol. 1993 Oct;19(5):429–435. doi: 10.1111/j.1365-2990.1993.tb00465.x. [DOI] [PubMed] [Google Scholar]
- Berners M. O., Boutelle M. G., Fillenz M. On-line measurement of brain glutamate with an enzyme/polymer-coated tubular electrode. Anal Chem. 1994 Jul 1;66(13):2017–2021. doi: 10.1021/ac00085a016. [DOI] [PubMed] [Google Scholar]
- Boutelle M. G., Fellows L. K., Cook C. Enzyme packed bed system for the on-line measurement of glucose, glutamate, and lactate in brain microdialysate. Anal Chem. 1992 Sep 1;64(17):1790–1794. doi: 10.1021/ac00041a010. [DOI] [PubMed] [Google Scholar]
- Bungay P. M., Morrison P. F., Dedrick R. L. Steady-state theory for quantitative microdialysis of solutes and water in vivo and in vitro. Life Sci. 1990;46(2):105–119. doi: 10.1016/0024-3205(90)90043-q. [DOI] [PubMed] [Google Scholar]
- Derouiche A., Frotscher M. Astroglial processes around identified glutamatergic synapses contain glutamine synthetase: evidence for transmitter degradation. Brain Res. 1991 Jun 28;552(2):346–350. doi: 10.1016/0006-8993(91)90103-3. [DOI] [PubMed] [Google Scholar]
- Dringen R., Gebhardt R., Hamprecht B. Glycogen in astrocytes: possible function as lactate supply for neighboring cells. Brain Res. 1993 Oct 1;623(2):208–214. doi: 10.1016/0006-8993(93)91429-v. [DOI] [PubMed] [Google Scholar]
- Fellows L. K., Boutelle M. G., Fillenz M. Physiological stimulation increases nonoxidative glucose metabolism in the brain of the freely moving rat. J Neurochem. 1993 Apr;60(4):1258–1263. doi: 10.1111/j.1471-4159.1993.tb03285.x. [DOI] [PubMed] [Google Scholar]
- Fellows L. K., Boutelle M. G. Rapid changes in extracellular glucose levels and blood flow in the striatum of the freely moving rat. Brain Res. 1993 Feb 26;604(1-2):225–231. doi: 10.1016/0006-8993(93)90373-u. [DOI] [PubMed] [Google Scholar]
- Forsyth R. J. Astrocytes and the delivery of glucose from plasma to neurons. Neurochem Int. 1996 Mar;28(3):231–241. doi: 10.1016/0197-0186(95)00094-1. [DOI] [PubMed] [Google Scholar]
- Forsyth R. J., Bartlett K., Burchell A., Scott H. M., Eyre J. A. Astrocytic glucose-6-phosphatase and the permeability of brain microsomes to glucose 6-phosphate. Biochem J. 1993 Aug 15;294(Pt 1):145–151. doi: 10.1042/bj2940145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsyth R. J., Bartlett K., Eyre J. Dephosphorylation of 2-deoxyglucose 6-phosphate and 2-deoxyglucose export from cultured astrocytes. Neurochem Int. 1996 Mar;28(3):243–250. doi: 10.1016/0197-0186(95)00095-x. [DOI] [PubMed] [Google Scholar]
- Fox P. T., Raichle M. E., Mintun M. A., Dence C. Nonoxidative glucose consumption during focal physiologic neural activity. Science. 1988 Jul 22;241(4864):462–464. doi: 10.1126/science.3260686. [DOI] [PubMed] [Google Scholar]
- JENKINS W. T. Glutamic-aspartic transaminase. VI. The reaction with certain beta-substituted aspartic acid analogues. J Biol Chem. 1961 Apr;236:1121–1125. [PubMed] [Google Scholar]
- Kuhr W. G., van den Berg C. J., Korf J. In vivo identification and quantitative evaluation of carrier-mediated transport of lactate at the cellular level in the striatum of conscious, freely moving rats. J Cereb Blood Flow Metab. 1988 Dec;8(6):848–856. doi: 10.1038/jcbfm.1988.142. [DOI] [PubMed] [Google Scholar]
- Lear J. L., Kasliwal R. K. Autoradiographic measurement of cerebral lactate transport rate constants in normal and activated conditions. J Cereb Blood Flow Metab. 1991 Jul;11(4):576–580. doi: 10.1038/jcbfm.1991.106. [DOI] [PubMed] [Google Scholar]
- Morton D. B., Griffiths P. H. Guidelines on the recognition of pain, distress and discomfort in experimental animals and an hypothesis for assessment. Vet Rec. 1985 Apr 20;116(16):431–436. doi: 10.1136/vr.116.16.431. [DOI] [PubMed] [Google Scholar]
- Ogawa S., Tank D. W., Menon R., Ellermann J. M., Kim S. G., Merkle H., Ugurbil K. Intrinsic signal changes accompanying sensory stimulation: functional brain mapping with magnetic resonance imaging. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):5951–5955. doi: 10.1073/pnas.89.13.5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei Q., Zetterström T., Fillenz M. Tail pinch-induced changes in the turnover and release of dopamine and 5-hydroxytryptamine in different brain regions of the rat. Neuroscience. 1990;35(1):133–138. doi: 10.1016/0306-4522(90)90127-p. [DOI] [PubMed] [Google Scholar]
- Pellerin L., Magistretti P. J. Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proc Natl Acad Sci U S A. 1994 Oct 25;91(22):10625–10629. doi: 10.1073/pnas.91.22.10625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petroff O. A., Novotny E. J., Avison M., Rothman D. L., Alger J. R., Ogino T., Shulman G. I., Prichard J. W. Cerebral lactate turnover after electroshock: in vivo measurements by 1H/13C magnetic resonance spectroscopy. J Cereb Blood Flow Metab. 1992 Nov;12(6):1022–1029. doi: 10.1038/jcbfm.1992.139. [DOI] [PubMed] [Google Scholar]
- Poitry-Yamate C. L., Poitry S., Tsacopoulos M. Lactate released by Müller glial cells is metabolized by photoreceptors from mammalian retina. J Neurosci. 1995 Jul;15(7 Pt 2):5179–5191. doi: 10.1523/JNEUROSCI.15-07-05179.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prichard J., Rothman D., Novotny E., Petroff O., Kuwabara T., Avison M., Howseman A., Hanstock C., Shulman R. Lactate rise detected by 1H NMR in human visual cortex during physiologic stimulation. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5829–5831. doi: 10.1073/pnas.88.13.5829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selak I., Skaper S. D., Varon S. Pyruvate participation in the low molecular weight trophic activity for central nervous system neurons in glia-conditioned media. J Neurosci. 1985 Jan;5(1):23–28. doi: 10.1523/JNEUROSCI.05-01-00023.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsacopoulos M., Magistretti P. J. Metabolic coupling between glia and neurons. J Neurosci. 1996 Feb 1;16(3):877–885. doi: 10.1523/JNEUROSCI.16-03-00877.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler D., Boutelle M. G., Fillenz M. The role of N-methyl-D-aspartate receptors in the regulation of physiologically released dopamine. Neuroscience. 1995 Apr;65(3):767–774. doi: 10.1016/0306-4522(95)93905-7. [DOI] [PubMed] [Google Scholar]
- Wolff J. R. The astrocyte as link between capillary and nerve cell. Triangle. 1970;9(5):153–164. [PubMed] [Google Scholar]
- Young W. H2 clearance measurement of blood flow: a review of technique and polarographic principles. Stroke. 1980 Sep-Oct;11(5):552–564. doi: 10.1161/01.str.11.5.552. [DOI] [PubMed] [Google Scholar]
- de Boer P., Damsma G., Fibiger H. C., Timmerman W., de Vries J. B., Westerink B. H. Dopaminergic-cholinergic interactions in the striatum: the critical significance of calcium concentrations in brain microdialysis. Naunyn Schmiedebergs Arch Pharmacol. 1990 Nov;342(5):528–534. doi: 10.1007/BF00169041. [DOI] [PubMed] [Google Scholar]


