Abstract
The underlying mechanism of vascular endothelial hyperpermeability caused by decrease of endothelial junctions occurring in atherosclerosis remains elusive. Our findings identified that plasma exosomes from patients with stable coronary artery disease (ExoSCAD) contain differentially expressed miRNAs that are clustered with genes related to cell junctions, prompting us to investigate the role of ExoSCAD in regulating vascular endothelial junctions and to elucidate the underlying mechanisms. Here, we show that ExoSCAD markedly impair vascular endothelial junctions via suppressing VE-Cadherin and ZO-1 in endothelial cells in vitro and in vivo, consequently increases endothelial permeability. Critically, exosomal miR-140-3p plays a crucial role in ExoSCAD-induced inhibition of ZO-1, and may be an important causative factor in the development of endothelial hyperpermeability during atherosclerosis. Additionally, exosomal miR-140–3p level coordinates with severity of SCAD. Targeting miR-140-3p in circulating exosomes might open novel options for treatment of atherosclerosis.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-81352-8.
Keywords: Stable coronary artery disease, Plasma exosomes, miR-140-3p, Atherosclerosis, Endothelial cell junctions
Subject terms: Cardiovascular biology, Cardiovascular diseases, Non-coding RNAs, Post-translational modifications
Introduction
Coronary artery disease (CAD), which can be classified into stable coronary artery disease (SCAD) and acute coronary syndrome (ACS), poses great threat to human health due to its high morbidity and mortality1. It is noteworthy that SCAD will develop to ACS if it cannot be timely and effectively controlled, resulting in an enhanced risk of severe and death of patients2. Atherosclerosis, the pathogenesis of which includes endothelial damage, lipid accumulation, inflammatory cell adhesion, plaque formation and rupture, is the pathological basis of CAD3. However, the methods to prevent and treat atherosclerosis are still limited due to the lack of an in-depth understanding of its pathogenesis. Consequently, it is of great significance to explore the pathogenesis of atherosclerosis in order to determine novel and effective therapeutic targets.
Exosomes, a type of nanoscale extracellular vesicles secreted from various cell types, can regulate various physiological and pathological processes through mediating intercellular communication4. Mounting evidences in recent years have found that miRNAs, as a main component of exosomes5, are crucial mediators for exosomes to perform biological functions6,7. Exosomal miRNAs have been demonstrated to be involved in the occurrence and development of various vascular diseases, including atherosclerosis8. For example, small cell lung cancer cells-secreted exosomal miR-141 and hypoxic papillary thyroid cancer cells-derived exosomal miR-181a aggravate tumor angiogenesis and growth via promoting endothelial cells (ECs) proliferation, invasion, migration and tube formation9,10. Exosomal miRNA-9-3p derived from the vitreous humor of proliferative diabetic retinopathy patients promotes proliferation, migration and tube formation of primary human retinal ECs11. M1-like macrophage-derived exosomal miR-155 reportedly leads to inhibition of angiogenesis and exacerbation of cardiac dysfunction via modulating its target genes12. Notably, vascular smooth muscle cells-derived exosomal miR-221/222 from diabetic sources were confirmed to promote vascular inflammation and atherogenesis13.
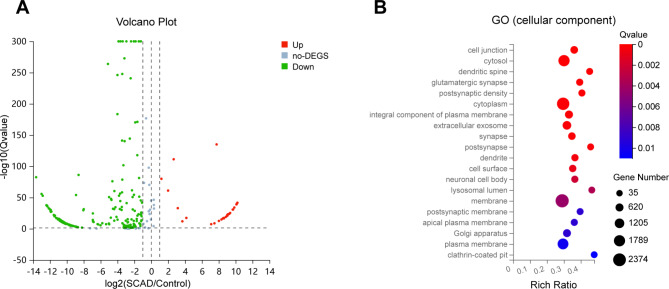
In recent years, the roles of exosomal miRNAs in circulation have been widely studied and found to be function crucially in atherosclerosis14,15. As an illustration, serum exosomal miR-143 and miR-126-3p from patients with myocardial infarction promote atherosclerosis through IGF-IR and TSC1/mTORC1/HIF-1α pathway, respectively16,17. Based on our previous small RNA sequencing (RNA-Seq) results, a total of 244 miRNAs (29 up-regulated and 215 down-regulated) were identified to be potentially differentially expressed in plasma exosomes of SCAD patients (ExoSCAD) in comparison with control subjects (Fig. 1A). Functional enrichment analyses identified these differentially expressed miRNAs (DEmiRNAs) to be clustered with genes associated with cell junctions, cytosol, dendritic spine, glutamatergic synapse, postsynaptic density, among which cell junctions that are critical in regulating vascular endothelial permeability18–20 showed relatively most significant abundant (Fig. 1B). More importantly, the increased vascular endothelial permeability mediated by impaired endothelial cell junctions is the key step for lipid and leukocyte to cross the vascular endothelial barrier and deposit in the subendothelial layer in early atherosclerosis21–23.
Fig. 1.
Plasma exosomal DEmiRNAs identified by small RNA-Seq in patients with SCAD and the corresponding GO (cellular component) enrichment analysis. (A) Plasma exosomal statistically significant DEmiRNAs in patients with SCAD (n = 3) in comparison with healthy controls (n = 3). DEmiRNAs up-regulated and down-regulated with statistical significance are represented by the red and green points, respectively. (B) Enrichment analysis of GO (cellular component) presenting the most abundant cellular components associated with these DEmiRNAs target genes. no-DEGS: not differentially expressed genes; SCAD: stable coronary artery disease; GO: Gene Ontology; DEmiRNAs: differentially expressed miRNAs.
It was reported that exosomal miRNAs secreted from highly metastatic cancer cells increase endothelial permeability and facilitate tumor metastasis by targeting endothelial junctional proteins24,25. Besides, pericyte-derived exosomal miR-210-5p regulates endothelial permeability through suppressing JAK1/STAT3 signaling26. These prompted us to suggest that the altered ExoSCAD-contained miRNAs may deteriorate endothelial permeability and promote atherogenesis through regulation of endothelial cell junctions. In a bid to determine plasma derived exosomal miRNAs that facilitate atherosclerosis via disrupting endothelial junctions, we identified that miR-140-3p highly expressed in ExoSCAD could be transferred into ECs and induced endothelial hyperpermeability through suppressing the expression of zonula occludens 1 (ZO-1). Significantly, our findings illustrate that plasma exosomal miRNAs may play crucial roles in atherosclerotic pathophysiology.
Materials and methods
Collection of plasma samples
Plasma samples from 39 patients with SCAD and 39 paired healthy controls were prospectively collected (clinical characteristics are summarized in Table S1) from January to June 2022 at the Second Hospital of Shanxi Medical University in Taiyuan, China. The following are the primary inclusion criteria for the SCAD group27: (1) A luminal stenosis of at least 50% in the left main stem or at least 70% in no less than one major coronary arteries. (2) The pain or discomfort in the chest persists for less than 10 min. Healthy controls were selected based on age- and sex-matched being adults with no clinical signs of CAD and normal ECG results. Subjects with inflammation, infection, autoimmune diseases, severe hepatic or renal dysfunction, or a history of malignancy diseases were eliminated. All experiments were approved by the Ethics Committee of The Second Hospital of Shanxi Medical University, and performed following the Declaration of Helsinki. Informed consent was obtained from all the participants.
Extraction, identification and quantification of plasma exosomes
To remove cells and cell debris, plasma underwent a series of centrifugation (300 ×g for 10 min, 2000×g for 10 min and 10,000 ×g for 30 min). After being filtered by a 0.22 μm filter membrane (Millipore), the supernatant underwent additional ultracentrifugation for 5 h at 110,000×g (CP100NX, HITACHI, Tokyo, Japan). The precipitates were washed 1 time with PBS by ultracentrifugation at 110,000×g for another 5 h. Exosome samples were resuspended by adding 200 µL PBS. The exosome suspension (10 µL) was then added onto a copper grid coated with formvar for 15 min before adding a 2% phosphotungstate solution (10 µL) for 1 min. After blotting the residual liquid by filter paper, the copper grids were then observed under an transmission electron microscope (HT-7800, HITACHI, Tokyo, Japan) at 100 kV. The exosome suspension (30 µL) was analyzed for nanoparticle tracking analysis using NanoFCM (Flow NanoAnalyzer, Xiamen, China). To measure the protein concentration of the isolated exosomes, a bicinchoninic acid protein assay kit (Solarbio, Beijing, China) was utilized. The cholesterol content of the exosome preparations was measured using the Amplex Red Cholesterol Assay Kit (Invitrogen, USA) according to the manufacturer’s instructions. For cell treatment, an addition of exosomes (approximately 2 µg) to 2 × 105 recipient cells was performed, as described in a previous report28. For in vivo administration, recipient mice were intravenously injected with 100 µg of exosomes every 3 days for 4 weeks as previously reported29.
Cell culture
Human aortic endothelial cells (HAECs; Sciencell, USA) were grown in an endothelial cell medium supplemented with 10% exosome-depleted FBS (prepared through ultracentrifugation for 8 h at 110,000×g), ECs growth supplement (ScienCell, USA), 100 µg/mL streptomycin sulphate and 100 U/mL penicillin sodium (Hyclone, USA), at 37°C in humidified air containing 5% CO2.
Uptake of exosomes by HAECs
The exosome preparation was labeled by PKH26 (Sigma-Aldrich, USA). Briefly, exosome preparation and 2 µL of PKH26 were resuspended in 1 ml Diluent C, respectively. The exosome suspension was then added to the PKH26 solution and incubated for 5 min before adding 0.5% bovine serum albumin to stop the staining. The labeled exosomes were ultracentrifuged at 110,000 g at 4°C for 70 min and then resuspended by adding 200 µL PBS before filtering by a 0.22 μm filter membrane (Millipore, USA). The HAECs at 80% confluence were exposed to the labeled exosomes for 12 h. The nuclei were stained by using DAPI (Beyotime, Shanghai, China) for 5 min before imaging under a confocal microscope (LSM710, Carl Zeiss, Oberkochen, Germany).
Cell and exosome transfection
The miR-140-3p agomir (agomir-140-3p), miR-140-3p antagomir (antagomir-140-3p), and their negative controls were synthesized by RiboBio (Guangzhou, China). HAECs were transfected with RNA duplex (50 nM) or miRNA antagomir (100 nM) using lipofectamine 3000 (Invitrogen, USA) following the manufacturer’s guidelines. Briefly, the lipofectamine 3000/RNA complexes were prepared in OptiMEM (Invitrogen, USA) and then incubated with HAECs for 8 h before replacing the medium with fresh medium supplemented with 10% exosome-deleted FBS for further treatment. Transfection of antagomir-140-3p/NC into exosomes was performed using Exo-Fect Exosome Transfection Kit (System Biosciences, USA) according to the manufacturer’s guidelines.
Endothelial permeability assay
For endothelial permeability analysis, HAECs (2 × 104) were grown on the upper chamber of a 24-well transwell inserts (0.4 μm pore size; Corning, USA) and allowed to reach a monolayer cell barrier at 100% confluence, followed by addition of PBS, ExoSACD, ExoHC or Lipopolysaccharide (10 µg/ml) and continued culture for 48 h. Rhodamine B isothiocyanate dextran (rhodamine-dextran, average MW 70,000; Sigma, USA) at a concentration of 20 mg/mL was added into the upper chamber. The amount of rhodamine-dextran in the bottom well was quantified to assess the endothelial permeability. Briefly, 40 µL medium in the bottom well was taken out at 30 min, 60 min and 90 min and analyzed by using a SpectraMax microplate reader (excitation at 488 nm, emission at 525 nm; SpectraMax M5, Molecular Devices, USA).
Western blot assay
The exosome suspension or cells were lysed on ice using RIPA buffer (Solarbio, Beijing, China) containing PMSF, and the protein concentrations were determined using a bicinchoninic acid protein assay kit (Solarbio, Beijing, China). Protein lysate was separated by SDS-PAGE and transferred onto polyvinylidene fluoride membranes, which were subsequently blocked for 2 h at 4°C and incubated with primary antibodies overnight at 4°C, then incubated with the peroxidase-conjugated secondary antibodies (1:5000 dilution; Bioss, Beijing, China) for 1 h at 37°C. Protein bands were visualized using a chemiluminescence reagent (Abbkine, Wuhan, China). First antibodies used in the experiment were as follows: anti-ZO-1 (1:1000 dilution; Abcam, ab276131), anti-VE-Cadherin (1:1000 dilution; Abcam, ab33168), anti-CD81 (1:1000 dilution; Abcam, ab109201), anti-CD63 (1:1000 dilution; Abcam, ab134045), anti-CD9 (1:1000 dilution; Abcam, ab236630), anti-Calnexin (1:1000 dilution; Abcam, ab133615), and anti-Apolipoprotein B (1:1000 dilution; Abcam, ab312318).
Reverse transcription quantitative real-time polymerase chain reaction
Total RNA from exosomes or cells/tissues was extracted using exoRNeasy Serum/Plasma Midi Kit (Qiagen, Hilden, Germany) or Trizol (Invitrogen, USA), respectively. After purification and quantification, the reverse transcription of RNA into cDNA was performed using a miRcute Plus miRNA First-Strand cDNA Kit (Tiangen, Beijing, China). A miRcute Plus miRNA qPCR Kit (SYBR Green) (Tiangen, Beijing, China) was used to perform real-time reactions following the manufacturer’s instructions. The relative expression levels of miRNAs were normalized to U6 and calculated using the 2−∆∆CT method. The primer sequences are presented in Table S2.
Luciferase reporter assay
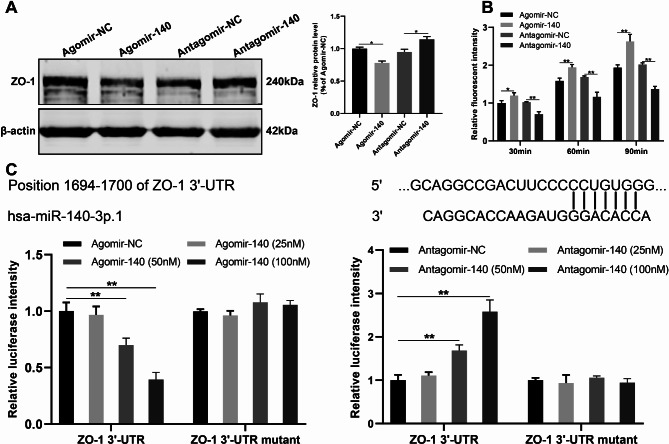
The predicted miR-140-3p binding site in the 3’-UTR of human ZO-1 sequence and its mutant were cloned into a luciferase reporter vector. Next, reporter vectors (wild-type or mutant) with either the agomir-140-3p/antagomir-140-3p or their negative controls at three different concentrations (25 nM, 50 nM, 100 nM) were co-transfected into HEK293T cells using lipofectamine 3000 (Invitrogen, USA) and cultured for 48 h. Firefly luciferase activity normalized to Renilla luciferase activity was analyzed using a dual-luciferase reporter assay system (Promega, USA) following the manufacturer’s protocol. The luciferase activities were measured using a SpectraMax microplate reader (SpectraMax M5, Molecular Devices, USA).
Animal models
Male ApoE−/− mice aged 8 weeks were obtained from the GemPharmatech Co., Ltd (Beijing, China), and given a high-fat diet (HFD) for 12 weeks under a specific pathogen-free environment with a 12 h:12 h light-dark cycle. Sample size determination was based on previous experience with similar studies. Four weeks after HFD feeding, mice were randomly treated with plasma exosomes (100 µg each time) or PBS via tail vein every 3 days for 4 weeks. After 12 weeks of HFD, mice were either euthanized for tissue collection and assessment, or subjected to the injection of Evans blue. All animal experiments were performed under the rules approved by the Institutional Animal Care and Use Committee of Shanxi Medical University.
In vivo vascular permeability assay
For in vivo permeability assay, 100 µL Evans blue (1% in PBS; Sigma-Aldrich, USA) was injected i.v. into the recipient mice via tail vein and sacrificed after 1 h. After a transcardiac perfusion with 10 mL PBS, aortas were then removed and imaged under a stereomicroscope after washing with PBS. Extraction of Evans blue dye from the aortas was performed by incubating the aortas in formamide at 55°C for 24 h before subjecting to centrifugation. Absorbance at 610 nm was measured to evaluate the concentration of the Evans blue in supernatant. The amount of Evans blue extravasated per milligram of artery represented vascular permeability.
Analyses of atherosclerotic lesion
The hearts with entire length of the aorta were harvested and fixed in 4% paraformaldehyde. The thoracoabdominal aortas were unfolded longitudinally and pinned on a black wax surface, followed by staining with Oil Red O (Solarbio, Beijing, China). Images were obtained with a digital camera (Canon, Tokyo, Japan) and quantifications for percent lesion area were then analyzed using ImageJ software. Hearts with aortic root were embedded in OCT and cryosectioned into 7 μm slices, followed by staining with Oil Red O. Images were obtained using a microscope (Zeiss, Oberkochen, Germany) and were quantified for percent lesion area using Image J software. Data were averaged in 3–5 sections per mouse.
Immunostaining
For immunohistochemistry staining, the sections of aorta were incubated with 3% H2O2 for 15 min to eliminate endogenous peroxidase. After blocking with 5% goat serum for 2 h, the samples were immunostained with anti-ZO-1 antibodies (1:250 dilution; Abcam, ab276131) or anti-VE-Cad antibodies (1:50 dilution; Abcam, ab282277) overnight at 4 °C prior to incubation with the corresponding secondary antibodies (1:200, Life Technologies, USA) for 1 h. Nuclei were visualized with hematoxylin (Beyotime, Shanghai, China). Images were recorded using a microscope (Zeiss, Oberkochen, Germany) equipped with a camera.
Statistical analyses
Data are expressed as mean ± standard error of the mean or numbers and percentages for continuous variables and categorical variables, respectively. At least three replicates were conducted in every trial group. Data normalization was examined by the Kolmogorov-Smirnov test. Statistical analyses were performed with unpaired Student’s t-test or non-parametric Mann-Whitney U-test for 2-group comparisons using GraphPad Prism 8.0 (GraphPad Software Inc). Categorical variables were assessed using the Chi-square test or Fisher’s exact test. Relationships between continuous variables were analyzed by using Spearman’s correlation analysis. p values < 0.05 were considered statistically significant.
Results
Plasma exosomes are taken up by HAECs
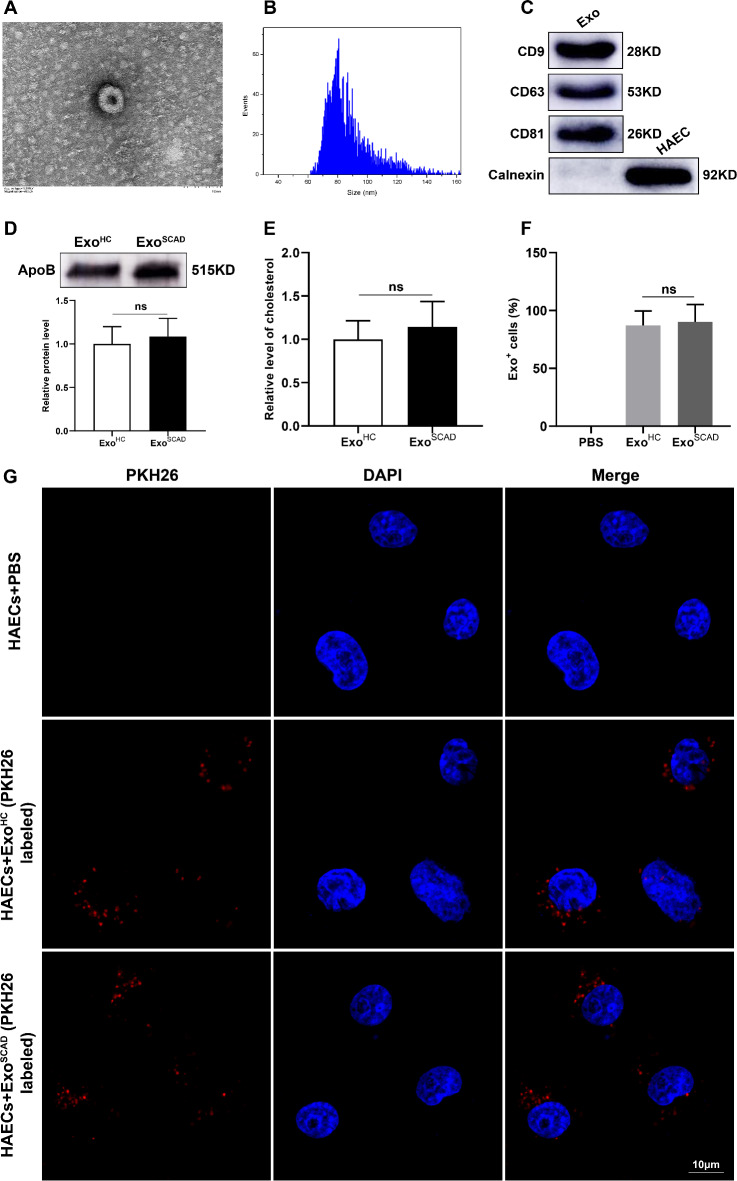
Vesicles isolated from plasma exhibited a bilayer membrane structure under transmission electron microscope (Fig. 2A) and expressed the positive markers CD9, CD63 and CD81, but not the negative marker Calnexin (Fig. 2C). Nanoparticle tracking analysis revealed that the diameters of the isolated vesicles were between 60 and 150 nm, with a average size of approximately 80 nm (Fig. 2B). To analyze the expression levels of apoB in exosomes, western blot was performed in the same protein amount of exosome preparations, which showed that the expression levels of apoB did not differ significantly between ExoHC and ExoSCAD (Fig. 2D). Meanwhile, no significant differences in cholesterol contents were observed between ExoHC and ExoSCAD (Fig. 2E). Additionally, exosomes could be internalized by HAECs under confocal microscopy, as evidenced by the images that red fluorescence was observed around the nucleus and in the cytoplasm of HAECs (Fig. 2G). Quantification of exosome distribution identified that more than 85% of HAECs were positive for PKH26 fluorescence, with no difference between the uptake of ExoHC and ExoSCAD (Fig. 2F).
Fig. 2.
Plasma exosomes identification and uptake by HAECs. (A) Morphological structure of the isolated vesicles by transmission electron microscope. (B) Particle sizes of the extracted vesicles by nanoparticle tracking analysis. (C) Western blot presenting the positive expressions of CD9, CD63 and CD81 in the extracted vesicles. Calnexin was negatively expressed. (D) Western blot presenting the expression levels of apoB (up panel) and the corresponding quantification results (down panel) in the same protein amount of exosome preparations. (E) Quantification of cholesterol content in ExoHC and ExoSCAD. (F and G) Quantification of exosome distribution by exosome-positive HAECs and representative microphotographs of HAECs incubated with PKH26-labeled plasma exosomes (red). An average of five random fields per sample were counted. Exo: exosome; HAEC: human aortic endothelial cell. ExoHC: plasma exosomes of healthy controls; ExoSCAD: plasma exosomes of patients with stable coronary artery disease.
ExoSCAD suppress VE-cadherin and ZO-1 expressions aggravating atherosclerosis
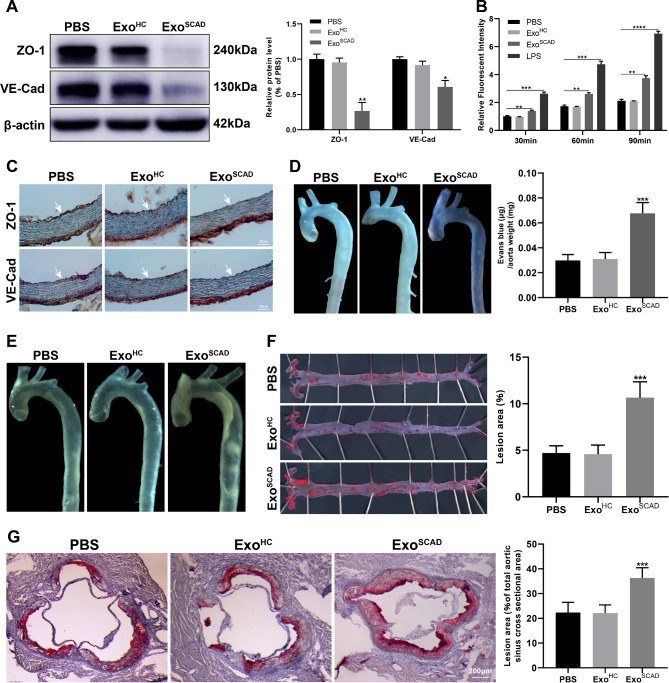
Our previous study suggested that the most enriched cellular component in the geneontology category of the target genes of DEmiRNAs expressed in ExoSCAD was cell junctions, which are crucial biomolecules in regulating vascular endothelial permeability. This prompted us to identify the potential of ExoSCAD in modulating the expressions of VE-cadherin (VE-Cad) and ZO-1 that are the main component of cell junctions between ECs. Strikingly, western blot results revealed that HAECs treated with ExoSCAD, but not ExoHC, displayed a significant decrease of VE-Cad and ZO-1 expressions (Fig. 3A). Due to the fact that increased vascular permeability mediated by disrupted endothelial cell junctions is the critical factor that facilitate atherosclerosis, we sought to determine whether ExoSCAD affect the endothelial permeability. As shown, there was significantly more dextran passed through the ExoSCAD-treated HAECs monolayer in comparison with HAECs treated with PBS or ExoHC (p < 0.01, Fig. 3B). However, endothelial permeability did not significantly differ between HAECs treated with PBS or ExoHC (p > 0.05, Fig. 3B).
Fig. 3.
ExoSCAD suppress VE-Cad and ZO-1 expressions in ECs and aggravates atherosclerotic lesions. (A) Western blot analyses of abundance of VE-Cad and ZO-1 in HAECs treated with ExoSCAD, ExoHC or PBS. Representative blots (left panel) and the corresponding quantification results (right panel) of 3 replicated independent experiments. (B) Quantification of endothelial permeability by calculating the amount of rhodamine-dextran passing through the monolayer of HAECs treated with ExoSCAD, ExoHC or PBS (n = 3 per group). LPS was used as a positive control. (C) Immunohistochemistry analyses of VE-Cad and ZO-1 expression in aortas from the 12-week HFD-fed ApoE−/− mice injected with ExoSCAD, ExoHC or PBS (n = 6–10 per group). White arrows indicate tunica intima. (D) Representative images of aortas from the 12-week HFD-fed ApoE−/− mice received different treatment after intravenous injection of Evans blue in each group (left panel). Quantitation of vascular permeability by calculating the amount of Evans blue extravasated per milligram of artery in each group (n = 7–9, right panel). (E) Representative bright field images of aortas from the 12-week HFD-fed ApoE−/− mice treated with ExoSCAD, ExoHC or PBS. (F) Representative images of the en face Oil Red O-stained aortas from the 12-week HFD-fed ApoE−/− mice in each group (left panel). Quantification of atherosclerotic lesion area as the percentage of positive stained areas to the respective whole arterial areas (n = 7–9, right panel). (G) Representative microphotographs (left panel) and quantification (right panel) of Oil Red O-stained aortic root sections from the 12-week HFD-fed ApoE−/− mice in each group (n = 7–9). Results are represented by mean ± SEM with 7–9 replicates for each group. ExoHC: plasma exosomes of healthy controls; ExoSCAD: plasma exosomes of patients with stable coronary artery disease; HAECs: human aortic endothelial cells; HFD: high-fat diet; LPS: lipopolysaccharide; ApoE: apolipoprotein E. *p < 0.05, **p < 0.01, ***p < 0.001.
We next conducted in vivo studies in a ApoE−/− mouse model (atherosclerosis-prone mouse) to further identify the above-mentioned findings. ApoE−/− mice were fed with HFD and intravenously injected with ExoSCAD, ExoHC or PBS. After 12 weeks of HFD, ExoSCAD-treated mice exhibited reduced protein levels of VE-Cad and ZO-1 in the aortic intima (Fig. 3C) and increased endothelial permeability (p < 0.001, Fig. 3D), in comparison with either ExoHC or PBS-treated controls. Notably, treatment of recipient mice with ExoSCAD but not ExoHC or PBS resulted in a significant increase in atherosclerotic lesion area in the aorta (p < 0.001, Fig. 3E and F) and aortic sinus (p < 0.001, Fig. 3G). Meanwhile, as predicted from our in vitro results, no differences in VE-Cad and ZO-1 expressions, endothelial permeability and lesion sizes in the aortas were observed between mice treated with PBS or ExoHC (p > 0.05, Fig. 3C–G).
ExoSCAD inhibit ZO-1 expression to increase endothelial permeability via transferring highly expressed mir-140-3p into HAECs
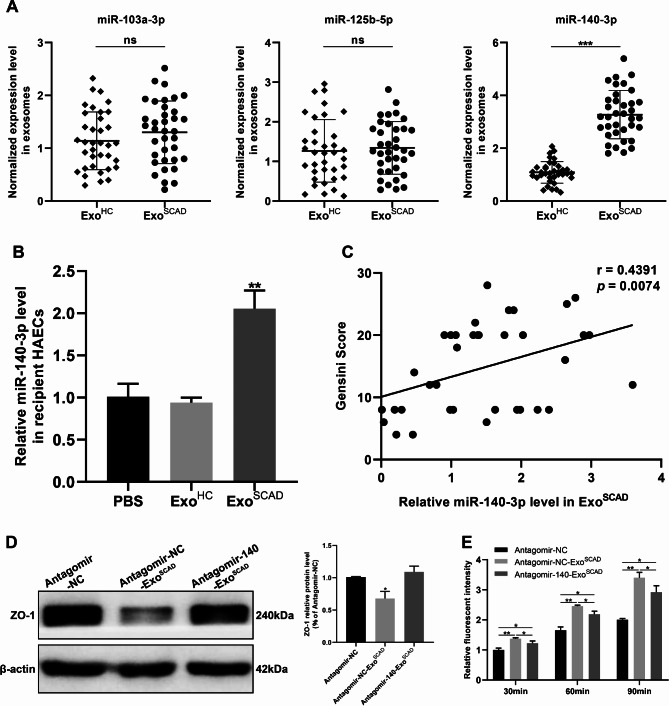
To identify the DEmiRNAs in ExoSCAD that are potentially associated with downregulation of VE-Cad and ZO−1, we sought to investigate the potential target DEmiRNAs of VE-Cad and ZO−1. Considering the inhibitory effect of miRNA on mRNA translation, we selected the 29 up-regulated exosomal miRNAs from small RNA-Seq as candidate miRNAs for further analysis. Bioinformatics analyses showed that miR−103a−3p exists the binding site of both VE-Cad and ZO−1 mRNA 3’-UTR, miR−125b−5p and miR−140−3p exist the binding site of VE-Cad and ZO−1 mRNA 3’-UTR, respectively. To further establish the expression levels of miR−103a−3p, miR−125b−5p and miR−140−3p expressed in ExoSCAD, we performed reverse transcription quantitative real-time polymerase chain reaction (RT-qPCR) in a larger clinical plasma samples of subjects (including 36 SCAD patients and 36 healthy controls), which showed that miR−140−3p level was marked elevated (p < 0.001, Fig. 4A) in ExoSCAD compared to ExoHC, while miR−103a−3p and miR−125b−5p levels were unaltered (p ≥ 0.05, Fig. 4A). Beyond that, miR-140-3p expression was significantly increased in HAECs following treatment with ExoSCAD, but not ExoHC (p < 0.01, Fig. 4B), suggesting that the highly expressed miR−140−3p can be transfered from ExoSCAD into ECs. Critically, the levels of plasma exosomal miR-140-3p were positively correlated with Gensini Scores representing the severity of coronary artery stenosis of the SCAD patients (r = 0.4391, p = 0.0074, Fig. 4C).
Fig. 4.
ExoSCAD inhibit ZO-1 expression to increase endothelial permeability via transferring highly expressed miR-140-3p into HAECs. (A) RT-qPCR analyses of miR-140-3p and miR-125b-5p expression in ExoSCAD and ExoHC. (B) RT-qPCR analyses of miR-140-3p expression in recipient HAECs after treatment with ExoSCAD, ExoHC or PBS. (C) Correlation of plasma exosomal miR-140-3p levels with Gensini scores in patients with SCAD. (D) Western blot analyses of ZO-1 expression in HAECs treated with antagomir-NC, antagomir-NC-ExoSCAD or antagomir-140-ExoSCAD. Representative blots (left panel) and quantification (right panel) of 3 replicated independent experiments. (E) Quantification of endothelial permeability by calculating the amount of rhodamine-dextran passing through the monolayer of HAECs after 30, 60, and 90 min, respectively (n = 3 per group). ExoHC: plasma exosomes of healthy controls; ExoSCAD: plasma exosomes of patients with stable coronary artery disease; HAECs: human aortic endothelial cells; agomir-140: miR-140-3p agomir; agomir-NC: agomir-140 negative control; antagomir-140: miR-140-3p antagomir; antagomir-NC: antagomir-140 negative control; antagomir-NC-ExoSCAD: ExoSCAD transfected with antagomir-NC; antagomir-140-ExoSCAD: ExoSCAD transfected with antagomir-140. *p < 0.05, **p < 0.01, ***p < 0.001. ns, no significance.
To examine whether ExoSCAD-induced downregulation of ZO−1 is through the highly expressed miR−140−3p, ExoSCAD were transfected with antagomir−140−3p before adding to the HAECs. Strikingly, blockade of miR−140−3p reversed the effect of ExoSCAD to inhibit ZO−1 (p < 0.05, Fig. 4D) and attenuated the enhanced effect of ExoSCAD on endothelial permeability (p < 0.05, Fig. 4E). Collectively, these findings reveal that miR−140−3p highly expressed in ExoSCAD is responsible for the suppressive effect of ExoSCAD on ZO−1, thereby increasing endothelial permeability.
Mir-140-3p inhibits ZO-1 to increase endothelial permeability via targeting the 3’-UTR of ZO-1 mRNA
To further determine the contribution of miR-140-3p to the regulation of ZO-1 and endothelial permeability by ExoSCAD, HAECs were transfected with either a agomir-140-3p or antagomir-140-3p. As shown, overexpression of miR-140-3p resulted in a down-regulated expression of ZO-1 (p < 0.05, Fig. 5A) and enhanced endothelial permeability (p < 0.01, Fig. 5B) in HAECs. Conversely, antagonism of miR-140-3p enhanced the ZO-1 expression (p < 0.05, Fig. 5A) and decreased endothelial permeability (p < 0.01, Fig. 5B) in HAECs, suggesting that miR-140-3p may regulate ZO-1 expression and endothelial permeability in ECs. Dual-luciferase reporter assay was then performed to investigate how miR-140-3p expressed in ExoSCAD inhibits ZO-1 in HAECs. As shown in Fig. 5C, the activity of firefly luciferase reporter carrying wild-type 3’-UTR of ZO-1 was significantly inhibited by agomir-140-3p in a dose-dependent matter (p < 0.01), whereas the mutation of the predicted binding site in 3’-UTR abrogated this repressive effect. These findings suggest that miR-140-3p may regulate ZO-1 expression by directly targeting the 3’-UTR of ZO-1 mRNA.
Fig. 5.
miR-140-3p inhibits the expression of ZO-1 via targeting the 3’-UTR of ZO-1 mRNA to increase endothelial permeability in HAECs. (A) Western blot analyses of ZO-1 expression in HAECs treated with agomir-140, antagomir-140, or their NCs. Representative blots (left panel) and quantification (right panel) of 3 replicated independent experiments. (B) Quantification of endothelial permeability by calculating the amount of rhodamine-dextran passing through the monolayer of HAECs treated with agomir-140, antagomir-140, or their NCs (n = 3 per group). (C) miR-140-3p modulated the luciferase activity of reporter containing wild-type but not mutant 3’-UTR of ZO-1. miR-140-3p and its predicted binding sequence in the 3’-UTR of ZO-1 (upper panel). The activity of firefly luciferase reporter carrying wild-type or mutant 3’-UTR of ZO-1 after transfection with the indicated concentrations of miR-140-agomir/antagomir or their NCs (lower panel). The firefly luciferase activity was normalized to renilla luciferase activity. HAECs: human aortic endothelial cells; agomir-140: miR-140-3p agomir; agomir-NC: agomir-140 negative control; antagomir-140: miR-140-3p antagomir; antagomir-NC: antagomir-140 negative control. *p < 0.05, **p < 0.01. ns, no significance.
Exosomal miR-140-3p plays a significant role in ExoSCAD-induced regulatory effects on ZO-1 and atherosclerotic lesion formation in a ApoE-/- mouse model.
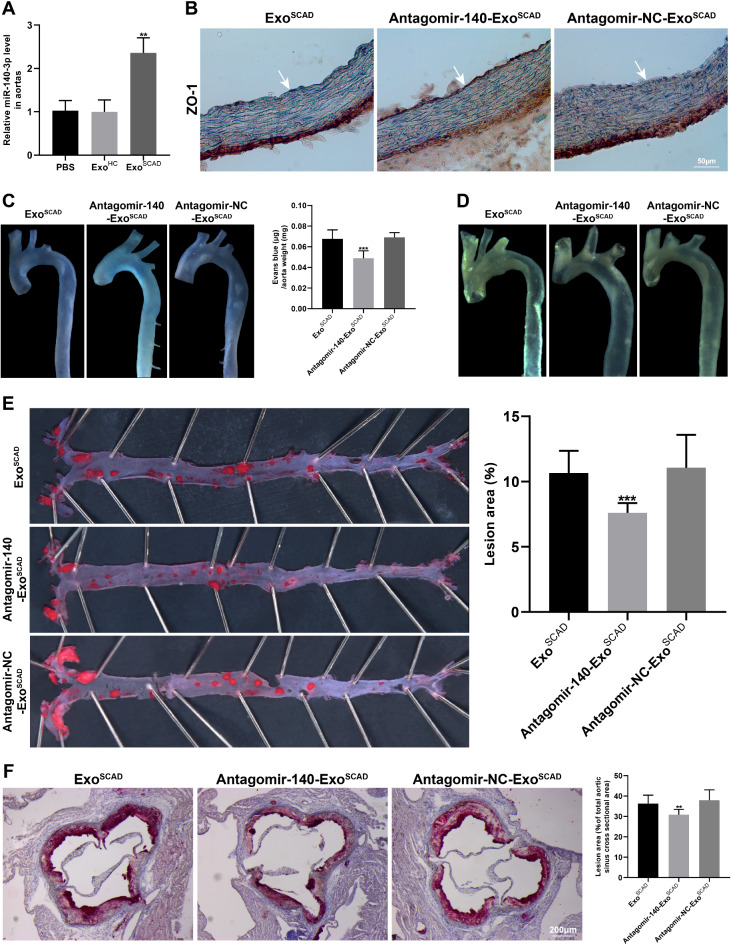
Given the marked in vitro effects of miR-140-3p highly expressed in ExoSCAD on ZO-1 expression and endothelial permeability in HAECs, we conducted in vivo studies in HFD-fed ApoE−/− mice. Firstly, RT-qPCR analyses of miR-140-3p expression were performed in the aortas from recipient mice treated with ExoSCAD, ExoHC or PBS. As a result, treatment of recipient mice with ExoSCAD but not ExoHC or PBS led to a robust increase in miR-140-3p expression in the aorta (p < 0.01, Fig. 6A). Subsequently, ExoSCAD transfected with antagomir−140−3p were intravenously injected into recipient mice every 3 days for 4 weeks in the presence of continued HFD for 12 weeks. As expected, antagonism of miR−140−3p in ExoSCAD reversed the effect of ExoSCAD to inhibit ZO−1 (Fig. 6B). Meanwhile, the increase effect of ExoSCAD on endothelial permeability was attenuated after blockade of exosomal miR-140-3p (p < 0.001, Fig. 6C). Significantly, transfection with antagomir−140−3p ameliorated the effect of ExoSCAD to accelerate atherosclerotic lesion formation (p < 0.001, Fig. 6D–F). Collectively, these data suggest that the suppressive effect of ExoSCAD on ZO−1 expression is mediated by overexpressed exosomal miR-140-3p. In addition, miR-140-3p plays a significant role in ExoSCAD-induced endothelial hyperpermeability and atherosclerotic lesion formation.
Fig. 6.
Exosomal miR-140-3p plays a significant role in ExoSCAD-induced regulatory effects on ZO-1 and atherosclerotic lesion formation in vivo. (A) RT-qPCR analyses of miR-140-3p expression in the aortas from recipient mice treated with ExoSCAD, ExoHC or PBS. (B) Immunohistochemistry analyses of ZO-1 expression in aortas from the 12-week HFD-fed ApoE−/− mice injected with ExoSCAD, antagomir-140-ExoSCAD or antagomir-NC-ExoSCAD (n = 7–9 per group)). White arrows indicate tunica intima. (C) Representative images of aortas from the 12-week HFD-fed ApoE−/− mice received different treatment after intravenous injection of Evans blue in each group (left panel). Quantitation of vascular permeability by calculating the amount of Evans blue extravasated per milligram of artery in each group (n = 7–9, right panel). (D) Representative bright field images of aortas from the 12-week HFD-fed ApoE−/− mice treated with ExoSCAD, antagomir-140-ExoSCAD or antagomir-NC-ExoSCAD. (E) Representative images of the en face Oil Red O-stained aortas from the 12-week HFD-fed ApoE−/− mice in each group (left panel). Quantification of atherosclerotic lesion area as the percentage of positive stained areas to the respective whole arterial areas (n = 7–9, right panel). (F) Representative microphotographs and quantification of Oil Red O stained aortic root sections from the 12-week HFD-fed ApoE−/− mice injected with ExoSCAD, antagomir-140-ExoSCAD or antagomir-NC-ExoSCAD (n = 7–9). Results are represented by mean ± SEM with 7–9 replicates for each group. ExoHC: plasma exosomes of healthy controls; ExoSCAD: plasma exosomes of patients with stable coronary artery disease; antagomir-140: miR-140-3p antagomir; antagomir-NC: antagomir-140 negative control; antagomir-NC-ExoSCAD: ExoSCAD transfected with antagomir-NC; antagomir-140-ExoSCAD: ExoSCAD transfected with antagomir-140; HFD: high-fat diet; ApoE: apolipoprotein E. *p < 0.05, **p < 0.01, ***p < 0.001.
Discussion
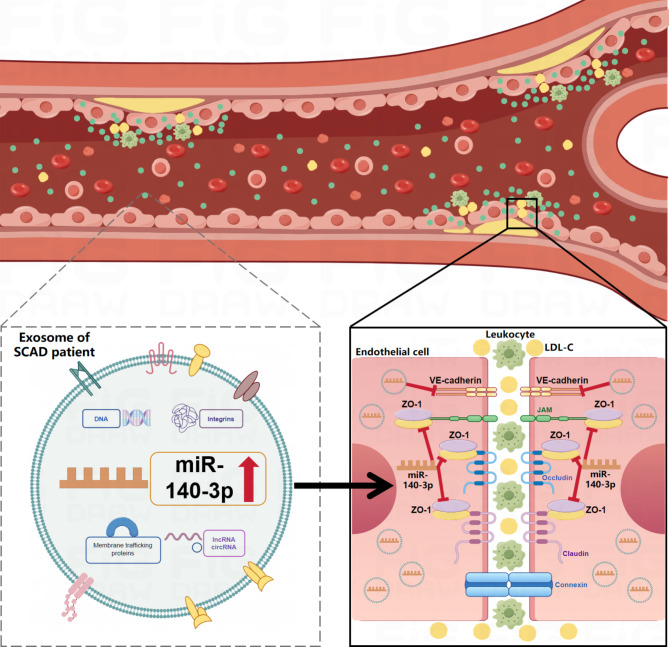
Exosomes, which are generated by all cell types and actively secreted into various body fluids, can regulate both physiological and pathological processes and mediate various biological functions via their cargos, such as nucleic acids, proteins, lipids, and metabolites30. Enormous studies have indicated the crucial roles of exosomes in the regulation of ECs function. For instance, exosomes from platelets activated by von willebrand factor under high shear stress were proved to disrupt endothelial barrier function in patients with end-stage heart failure31. Osteocalcin-overexpressed endothelial progenitor cells-secreted exosomes reportedly promote ECs proliferation, migration and tube formation32. Critically, various exosomes derived from different cell types, including monocytes/macrophages, ECs, platelets and vascular smooth muscle cells, have been demonstrated to be intimately associated with atherosclerosis33. Enrichment of exosomes induced by adipocytes-secreted adiponectin were found to improve cardiovascular disease including atherosclerosis34. As depicted in Fig. 7, our findings reveal that ExoSCAD could suppress VE-Cad and ZO-1 expressions in ECs to impair vascular endothelial junctions, leading to lipids and leukocytes to cross the endothelial barrier and deposit in the subendothelial layer, thereby deteriorating atherosclerosis. Notably, the decreased expression of ZO-1 is mediated by transferring of highly expressed miR-140-3p into ECs through ExoSCAD. In addition, exosomal miR-140-3p has a key function in ExoSCAD-induced enhancement of endothelial permeability and atherosclerotic lesion formation.
Fig. 7.
Hypothesis model for the role of ExoSCAD in atherosclerosis. ExoSCAD suppress VE-Cad and ZO-1 expressions in endothelial cells to impair vascular endothelial junctions, leading to lipids and leukocytes to cross the endothelial barrier and deposit in the subendothelial layer, thereby deteriorating atherosclerosis. Notably, overexpressed miR-140-3p is transferred from ExoSCAD into endothelial cells to inhibit ZO-1. ExoSCAD: plasma exosomes of patients with stable coronary artery disease; LDL-C: low density lipoprotein cholesterol.
Fatty streak, caused by deposition of oxidized low-density lipoproteins (Ox-LDL) beneath the intima, is the earliest morphological change in early stage of atherogenesis35. Given that Ox-LDL accumulation is primarily attributed to increased vascular permeability36,37, exploring the mechanism of endothelial hyperpermeability is therefore critical to an understanding of atherogenesis. Accumulating studies have confirmed that increased endothelial permeability resulted from impaired cell junctional proteins is the major reason for Ox-LDL to cross the endothelial barrier in early atherogenesis. The potential mechanisms underlying the endothelial hyperpermeability mediated by decreased endothelial junctions during atherosclerosis have been investigated in a limited number of studies. Researches demonstrated that Ox-LDL deposited beneath the intima and endothelial insulin-like growth factor-1 receptor deficiency enhance endothelial permeability by disrupting endothelial junctions in atherosclerosis38,39. Hemodynamics was identified to play a crucial role in the progression of atherosclerosis40,41, research found that disturbed flow may increase endothelial permeability and promote atherosclerosis throgh disrupting VE-Cad/catenin complex18. Additionally, CD163 + macrophages were validated to induce endothelial hyperpermeability via suppressing VE-Cad expression in atherosclerosis42.
Circulating exosomes have been confirmed to promote atherosclerosis by facilitating the adhesion of pro-inflammatory cells, stimulating the proliferation of vascular smooth muscle cells, and activating macrophages43,44. However, few researches have focused on the potential link between circulating exosomes and endothelial hyperpermeability during atherosclerosis. In this study, treatment with ExoSCAD significantly decreased VE-Cad and ZO-1 expressions and enhanced endothelial permeability in HAECs. Consistent with this concept, administration of mice with ExoSCAD led to downregulation of VE-Cad and ZO-1, hyperpermeability, and exacerbation of atherosclerosis in the aortas. Overall, these findings indicate an important function for ExoSCAD in impairing endothelial barrier integrity and increasing endothelial permeability in the development of atherosclerosis.
Given that the filter used during exosome preparation in our study would not remove most lipoproteins, including apoB and cholesterol that are proposed to play causative roles in the development of atherosclerosis, we characterized the exosomes for presence of apoB and cholesterol. Our data revealed that apoB and cholesterol contents were similar in ExoHC and ExoSCAD. Combined with the fact that no significant differences in plasma low-density lipoprotein cholesterol concentration exist between the SCAD group and control group, these data eliminate the possible impact of lipoprotein and cholesterol on our overall experimental results to a certain extent.
MiRNAs have been proved to be the main biomolecules accounting for exosome-induced biological effects via negative regulation of gene expression45. Growing evidences have confirmed the regulatory effect of exosomal miRNAs on vascular diseases. Research showed that SOX17-induced overexpressed exosomal miR-224-5p and miR-361-3p maintain endothelial function in pulmonary hypertension via blocking the proliferation, apoptosis, and inflammation of ECs46. Exosomal microRNA-92a derived from cardiomyocyte was validated to be critical for post-ischemic myofibroblast activation following myocardial infarction47. Plasma exosomal miR-630 was identified as a crucial mediator of vascular function and cardiovascular disease risk in children with underlying obstructive sleep apnea and/or obesity48. Moreover, circulating exosomal miR-145 and miR-885 were indicated to play significant roles in modulating thrombosis in COVID-1949.
Increasing researches have demonstrated that exosomes from different cells or tissues modulate atherosclerosis through delivering highly expressed miRNAs into recipient cells and tissues. For instance, adipose tissue-derived exosomal miR-132/212 and nicotine-treated macrophages-derived exosomal miR-21-3p exacerbate atherosclerosis progression by promoting migration and proliferation of vascular smooth muscle cells50,51. Furthermore, sleep deprivation-induced downregulation of circulating exosomal miR-182-5p and visceral adipocytes-derived exosomal miR-27b-3p aggravate endothelial inflammation and atherogenesis14,52. In this study, HAECs treated with ExoSCAD displayed increased expression of mature miR-140-3p, decreased ZO-1 expression, and enhanced endothelial permeability. Nevertheless, an addition of exogenous antagomir-140-3p abolished the inhibitory effect of ExoSCAD on ZO-1 and attenuated its effect to increase endothelial permeability. Moreover, miR-140-3p overexpression dramatically reduced ZO-1 expression and increased endothelial permeability in HAECs, whereas silencing it had the reverse effect. Luciferase assays indicated that miR-140-3p repressed ZO-1 through directly binding the 3ʹ-UTR of ZO-1 mRNA. As predicted from our in vitro results, our in vivo studies also demonstrated that it was the miR-140-3p within the ExoSCAD that caused these above-mentioned effects of ExoSCAD, as evidenced by the fact that blockade of miR-140-3p in ExoSCAD abrogated the inhibitory effect of ExoSCAD on ZO-1 and attenuated its effect to increase endothelial permeability and exacerbate atherosclerosis in HFD-fed ApoE−/− mice. Additionally, pearson correlation analysis showed that exosomal miR-140–3p level coordinated with severity of SCAD. Taken together, these findings indicate that ExoSCAD-induced endothelial hyperpermeability via transfering of miR-140-3p into ECs to inhibit ZO-1 expression may play an crucial role in the early stage of atherosclerosis.
Interestingly, our results are supported by recent findings of similar studies about this topic. Research showed that periodontal ECs-derived exosomal miR-155-5p may deteriorate endothelial permeability and carotid atherosclerosis, accompanied by the decreased expression level of VE-Cad, ZO-1 and Claudin-153. Plasma exosomal miR-129-2-3p reportedly increases airway epithelial permeability via weakening the synthesis of ZO-1, occludin, and VE-Cad in PM2.5-aggravated asthma54. Beyond that, circulating exosomal miR-27b-3p and miR-375-3p were proved to augment endothelial permeability by decreasing the expression of endothelial junctional proteins, thereby accelerating circulating tumour cells metastasis24,55.
Evidences from a number of experimental studies indicated that miR-140-3p has numerous effects including cell proliferation and migration56,57, metabolic remodeling58, inflammation59,60 etc., while most researches focused on the impact of miR-140-3p on tumors. With respect to the effects of miR-140-3p on ECs, previous studies indicated that miR-140-3p can suppress human umbilical vein ECs proliferation, migration, and tube formation through inhibiting VEGF expression61,62. Considering the above-mentioned potential effects of miR-140-3p, we speculate that the increased endothelial permeability via inhibiting ZO-1 appears to be an important, but likely not the only, mechanism for exosomal miR-140-3p-induced exacerbation of atherosclerosis. Furthermore, whether plasma exosomal miR-140-3p has similar effects as previously reported on arterial ECs during atherosclerosis will be the key direction in our future research.
Nevertheless, this study has several limitations. First, the exact component in ExoSCAD leading to downregulation of VE-Cad in HAECs remains elusive, which needs to be determined in the future. Second, with the exception of VE-Cad and ZO-1, there might be other impaired junctional proteins that are responsible for ExoSCAD-induced endothelial hyperpermeability. Additionally, apart from targeting ZO−1, miR−140 may also deteriorate endothelial permeability and atherosclerosis through other pathways, which warrants future investigation. Third, considering the genes targeted by the DEmiRNAs in ExoSCAD are enriched for other functions related to atherosclerosis, inducing endothelial hyperpermeability is probably not the only factor for ExoSCAD to promote atherosclerosis. In addition, given that exosomes contain a wide range of components, miRNAs may only be a risk factor rather than disease initiating for ExoSCAD to exacerbate endothelial permeability and atherogenesis. Finally, the source of these plasma exosomes carrying DEmiRNAs remains unknown, which is the primary focus in our future research.
Conclusion
Altogether, we find that treatment with ExoSCAD leads to decreased VE-Cad and ZO-1 expressions in ECs, endothelial hyperpermeability and aggravated atherosclerosis. Beyond that, exosomal miR-140-3p is responsible for ExoSCAD-induced inhibition of ZO-1 and may be an important causative factor in the development of endothelial hyperpermeability during atherosclerosis. Notably, exosomal miR-140–3p level was positively correlated with severity of SCAD. These findings reveal the regulatory potential of miRNA-containing plasma exosomes in atherosclerosis, which may contribute to new insights into the pathogenesis of endothelial dysfunction in early atherosclerosis and open novel options for treatment of atherosclerosis.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank the Key Lab of Medical Molecular Cell Biology of Shanxi Province, Institutes of Biomedical Sciences, Key Laboratory of Chemical Biology and Molecular Engineering of Ministry of Education, Shanxi University.
Author contributions
C.W. and Y.B. designed the study and revised the manuscript. C.W. supervised this study. J.H., X.K., and J.W. performed animal studies. J.H., Y.S. and X.C. conducted the in vitro experiments. X.K., Y.S. and J.W. helped to analyze the results and organize the figures. J.H. and X.K. wrote the manuscript. All authors reviewed the manuscript.
Funding
This work was supported by grants from the National Natural Science Foundation of China (grant no. 8207022039), and the Fundamental Research Program of Shanxi Province (grant no. 202203021222344).
Data availability
The data used to support the findings of this study are available from the corresponding author upon request.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Mensah, G. A., Fuster, V., Murray, C. J. L. & Roth, G. A. Global burden of cardiovascular diseases and risks, 1990–2022. J. Am. Coll. Cardiol.82(25), 2350–2473 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tsao, C. W. et al. Heart disease and stroke statistics-2023 update: A report from the American heart association. Circulation147(8), e93–e621 (2023). [DOI] [PubMed] [Google Scholar]
- 3.Libby, P. The changing landscape of atherosclerosis. Nature592(7855), 524–533 (2021). [DOI] [PubMed] [Google Scholar]
- 4.Kalluri, R. & LeBleu, V. S. The biology function and biomedical applications of exosomes. Science367(6478), eaau6977 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garcia-Martin, R. et al. MicroRNA sequence codes for small extracellular vesicle release and cellular retention. Nature601(7893), 446–451 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Makarova, J., Turchinovich, A., Shkurnikov, M. & Tonevitsky, A. Extracellular miRNAs and cell-cell communication: problems and prospects. Trends Biochem. Sci.46(8), 640–651 (2021). [DOI] [PubMed] [Google Scholar]
- 7.Agbu, P. & Carthew, R. W. MicroRNA-mediated regulation of glucose and lipid metabolism. Nat. Rev. Mol. Cell. Biol.22(6), 425–438 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang, C. et al. Nicotine exacerbates endothelial dysfunction and drives atherosclerosis via extracellular vesicle-miRNA. Cardiovasc. Res.119(3), 729–742 (2022). [DOI] [PubMed] [Google Scholar]
- 9.Mao, S. et al. Exosomal miR-141 promotes tumor angiogenesis via KLF12 in small cell lung cancer. J. Exp. Clin. Cancer Res.39(1), 193 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang, Y. et al. miR-181a, delivered by hypoxic PTC-secreted exosomes, inhibits DACT2 by downregulating MLL3, leading to YAP-VEGF-mediated angiogenesis. Mol. Ther. Nucleic Acids. 24, 610–621 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu, Y. et al. Müller glia-derived exosomal mir-9-3p promotes angiogenesis by restricting sphingosine-1-phosphate receptor S1P(1) in diabetic retinopathy. Mol. Ther. Nucleic Acids. 27, 491–504 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu, S. et al. M1-like macrophage-derived exosomes suppress angiogenesis and exacerbate cardiac dysfunction in a myocardial infarction microenvironment. Basic. Res. Cardiol.115(2), 22 (2020). [DOI] [PubMed] [Google Scholar]
- 13.Yu, H. et al. Diabetes is accompanied by secretion of pro-atherosclerotic exosomes from vascular smooth muscle cells. Cardiovasc. Diabetol.22(1), 112 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li, X. et al. Sleep deprivation promotes endothelial inflammation and atherogenesis by reducing exosomal miR-182-5p. Arterioscler. Thromb. Vasc Biol.43(6), 995–1014 (2023). [DOI] [PubMed] [Google Scholar]
- 15.Ye, Z. et al. Serum exosomal microRNA-27-3p aggravates cerebral injury and inflammation in patients with acute cerebral infarction by targeting PPARγ. Inflammation44(3), 1035–1048 (2021). [DOI] [PubMed] [Google Scholar]
- 16.Geng, T. et al. Exosome derived from coronary serum of patients with myocardial infarction promotes angiogenesis through the miRNA-143/IGF-IR pathway. Int. J. Nanomed.15, 2647–2658 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duan, S. et al. Peripheral serum exosomes isolated from patients with acute myocardial infarction promote endothelial cell angiogenesis via the miR-126-3p/TSC1/mTORC1/HIF-1α pathway. Int. J. Nanomed.17, 1577–1592 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shih, Y. T. et al. Vinculin phosphorylation impairs vascular endothelial junctions promoting atherosclerosis. Eur. Heart J.44(4), 304–318 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou, W. et al. Targeting VEGF-A/VEGFR2 Y949 signaling-mediated vascular permeability alleviates hypoxic pulmonary hypertension. Circulation146(24), 1855–1881 (2022). [DOI] [PubMed] [Google Scholar]
- 20.Yang, X. et al. Targeting endothelial tight junctions to predict and protect thoracic aortic aneurysm and dissection. Eur. Heart J.44(14), 1248–1261 (2023). [DOI] [PubMed] [Google Scholar]
- 21.Mussbacher, M. et al. More than just a monolayer: the multifaceted role of endothelial cells in the pathophysiology of atherosclerosis. Curr. Atheroscler Rep.24(6), 483–492 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Björkegren, J. L. M. & Lusis, A. J. Atherosclerosis: recent developments. Cell185(10), 1630–1645 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun, C., Wu, M. H. & Yuan, S. Y. Nonmuscle myosin light-chain kinase deficiency attenuates atherosclerosis in apolipoprotein E-deficient mice via reduced endothelial barrier dysfunction and monocyte migration. Circulation124(1), 48–57 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mao, S. et al. Exosomal mir-375-3p breaks vascular barrier and promotes small cell lung cancer metastasis by targeting claudin-1. Transl Lung Cancer Res.10(7), 3155–3172 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yokota, Y. et al. Serum exosomal miR-638 is a prognostic marker of HCC via downregulation of VE-cadherin and ZO-1 of endothelial cells. Cancer Sci.112(3), 1275–1288 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gao, P. et al. Pericyte-derived exosomal miR-210 improves mitochondrial function and inhibits lipid peroxidation in vascular endothelial cells after traumatic spinal cord injury by activating JAK1/STAT3 signaling pathway. J. Nanobiotechnol.21(1), 452 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Montalescot, G. et al. ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur. Heart J. 34(38), 2949–3003 (2013). [DOI] [PubMed]
- 28.Ying, W. et al. Adipose tissue macrophage-derived exosomal miRNAs can modulate in vivo and in vitro insulin sensitivity. Cell171(2), 372–384 (2017). [DOI] [PubMed] [Google Scholar]
- 29.Chen, G. et al. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature560(7718), 382–386 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jeppesen, D. K. et al. Reassessment of exosome composition. Cell177(2), 428–445 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mone, P. et al. Exosome-mediated angiogenesis underlies LVAD-induced bleeding in patients with end-stage heart failure. JACC Basic. Transl Sci.7(3), 262–264 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yi, M. et al. Exosomes secreted from osteocalcin-overexpressing endothelial progenitor cells promote endothelial cell angiogenesis. Am. J. Physiol. Cell. Physiol.317(5), C932–C941 (2019). [DOI] [PubMed] [Google Scholar]
- 33.Lin, B., Yang, J., Song, Y., Dang, G. & Feng, J. Exosomes and atherogenesis. Front. Cardiovasc. Med.8, 738031 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kita, S., Maeda, N. & Shimomura, I. Interorgan communication by exosomes, adipose tissue, and adiponectin in metabolic syndrome. J. Clin. Invest.129(10), 4041–4049 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ginsberg, H. N. et al. Triglyceride-rich lipoproteins and their remnants: metabolic insights, role in atherosclerotic cardiovascular disease, and emerging therapeutic strategies-a consensus statement from the European Atherosclerosis Society. Eur. Heart J.42(47), 4791–4806 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dejana, E., Tournier-Lasserve, E. & Weinstein, B. M. The control of vascular integrity by endothelial cell junctions: molecular basis and pathological implications. Dev. Cell.16(2), 209–221 (2009). [DOI] [PubMed] [Google Scholar]
- 37.Liang, S. et al. The critical role of endothelial function in fine particulate matter-induced atherosclerosis. Part. Fibre Toxicol.17(1), 61 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guo, X. et al. Reducing the damage of ox-LDL/LOX-1 pathway to vascular endothelial barrier can inhibit atherosclerosis. Oxid. Med. Cell. Longev. 7541411 (2022). [DOI] [PMC free article] [PubMed]
- 39.Higashi, Y. et al. Endothelial deficiency of insulin-like growth factor-1 receptor reduces endothelial barrier function and promotes atherosclerosis in apoe-deficient mice. Am. J. Physiol. Heart Circ. Physiol.319(4), H730–H743 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang, H., Uhlmann, K., Vedula, V., Balzani, D. & Varnik, F. Fluid-structure interaction simulation of tissue degradation and its effects on intra-aneurysm hemodynamics. Biomech. Model. Mechanobiol.21(2), 671–683 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang, H., Balzani, D., Vedula, V., Uhlmann, K. & Varnik, F. On the potential self-amplification of aneurysms due to tissue degradation and blood flow revealed from FSI simulations. Front. Physiol.12, 785780 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guo, L. et al. CD163 + macrophages promote angiogenesis and vascular permeability accompanied by inflammation in atherosclerosis. J. Clin. Invest.128(3), 1106–1124 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nikdoust, F., Pazoki, M., Mohammadtaghizadeh, M., Aghaali, M. K. & Amrovani, M. Exosomes: Potential player in endothelial dysfunction in cardiovascular disease. Cardiovasc. Toxicol.22(3), 225–235 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Giró, O. et al. Extracellular vesicles in atherothrombosis and cardiovascular disease: friends and foes. Atherosclerosis330, 61–75 (2021). [DOI] [PubMed] [Google Scholar]
- 45.Robson, A. Exosome-derived microRNAs improve cardiac function. Nat. Rev. Cardiol.18(3), 150–151 (2021). [DOI] [PubMed] [Google Scholar]
- 46.Zou, X. et al. SOX17 is a critical factor in maintaining endothelial function in pulmonary hypertension by an exosome-mediated autocrine manner. Adv. Sci. (Weinh)10(14), e2206139 (2023). [DOI] [PMC free article] [PubMed]
- 47.Wang, X., Morelli, M. B., Matarese, A., Sardu, C. & Santulli, G. Cardiomyocyte-derived exosomal microRNA-92a mediates post-ischemic myofibroblast activation both in vitro and ex vivo. ESC Heart Fail.7(1), 284–288 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Khalyfa, A. et al. Circulating plasma extracellular microvesicle microRNA cargo and endothelial dysfunction in children with obstructive sleep apnea. Am. J. Respir Crit. Care Med.194(9), 1116–1126 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gambardella, J. et al. Exosomal miR-145 and miR-885 regulate thrombosis in COVID-19. J. Pharmacol. Exp. Ther.384(1), 109–115 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guo, B. et al. MiRNA-132/212 encapsulated by adipose tissue-derived exosomes worsen atherosclerosis progression. Cardiovasc. Diabetol.23(1), 331 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhu, J. et al. Exosomes from nicotine-stimulated macrophages accelerate atherosclerosis through miR-21-3p/PTEN-mediated VSMC migration and proliferation. Theranostics9(23), 6901–6919 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tang, Y. et al. Exosomal miR-27b-3p secreted by visceral adipocytes contributes to endothelial inflammation and atherogenesis. Cell. Rep.42(1), 111948 (2023). [DOI] [PubMed] [Google Scholar]
- 53.Yang, W. et al. Exosomal mir-155-5p promote the occurrence of carotid atherosclerosis. J. Cell. Mol. Med.28(21), e70187 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang, C. et al. Exosomal mir-129-2-3p promotes airway epithelial barrier disruption in PM(2.5)-aggravated asthma. J. Environ. Manage.370, 123053 (2024). [DOI] [PubMed] [Google Scholar]
- 55.Dou, R. et al. EMT-cancer cells-derived exosomal miR-27b-3p promotes circulating tumour cells-mediated metastasis by modulating vascular permeability in colorectal cancer. Clin. Transl Med.11(12), e595 (2021). [DOI] [PMC free article] [PubMed]
- 56.Wang, Y. et al. Circ-AASDH functions as the progression of early stage lung adenocarcinoma by targeting mir-140-3p to activate E2F7 expression. Transl Lung Cancer Res.10(1), 57–70 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang, Z. et al. Mir-140-3p is involved in the occurrence and metastasis of gastric cancer by regulating the stability of FAM83B. Cancer Cell. Int.21(1), 537 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dou, D. et al. Circ_0008039 supports breast cancer cell proliferation, migration, invasion, and glycolysis by regulating the miR-140-3p/SKA2 axis. Mol. Oncol.15(2), 697–709 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang, D. et al. Extracorporeal cardiac shock wave-induced exosome derived from endothelial colony-forming cells carrying mir-140-3p alleviate cardiomyocyte hypoxia/reoxygenation injury via the PTEN/PI3K/AKT pathway. Front. Cell. Dev. Biol.9, 779936 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jude, J. et al. Mir-140-3p regulation of TNF-α-induced CD38 expression in human airway smooth muscle cells. Am. J. Physiol. Lung Cell. Mol. Physiol.303(5), L460–L468 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Payervand, N. et al. Exosomal circ_0084043 derived from colorectal cancer-associated fibroblasts promotes in vitro endothelial cell angiogenesis by regulating the miR-140-3p/HIF-1α/VEGF signaling axis. Heliyon10(11), e31584 (2024). [DOI] [PMC free article] [PubMed]
- 62.Liang, S. et al. LncRNA SNHG1 alleviates hypoxia-reoxygenation-induced vascular endothelial cell injury as a competing endogenous RNA through the HIF-1α/VEGF signal pathway. Mol. Cell. Biochem.465, 1–11 (2020). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.