Abstract
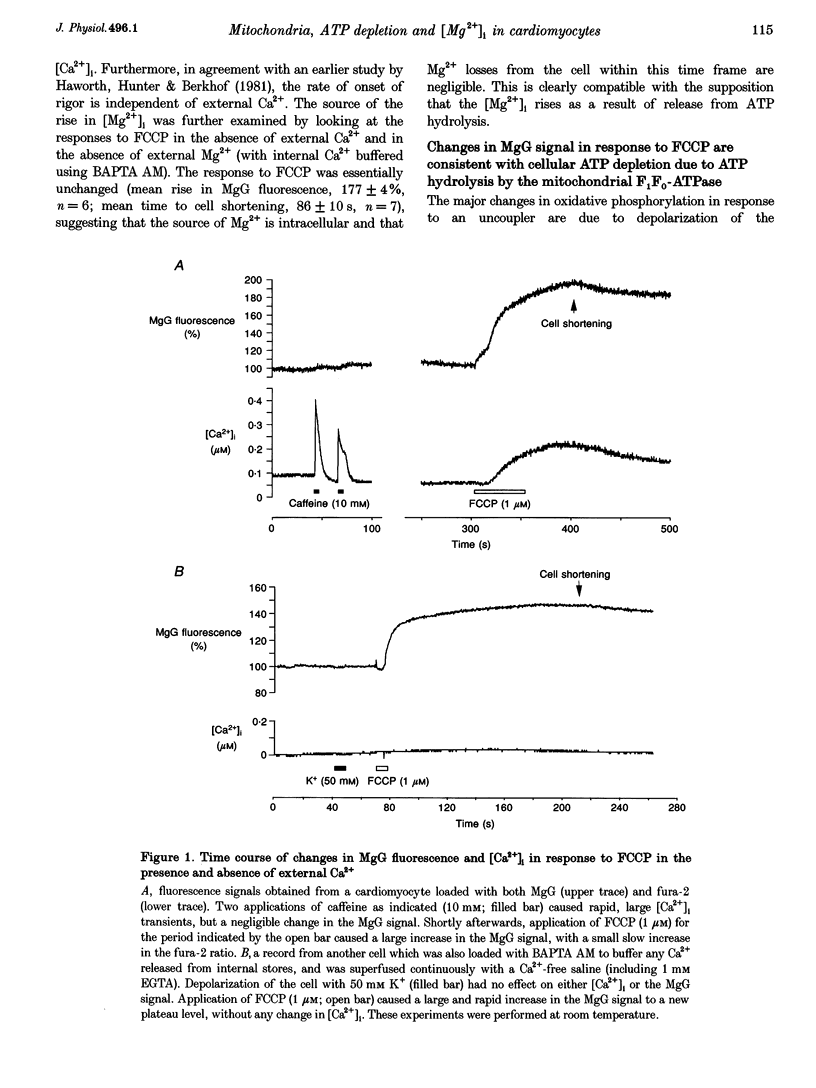
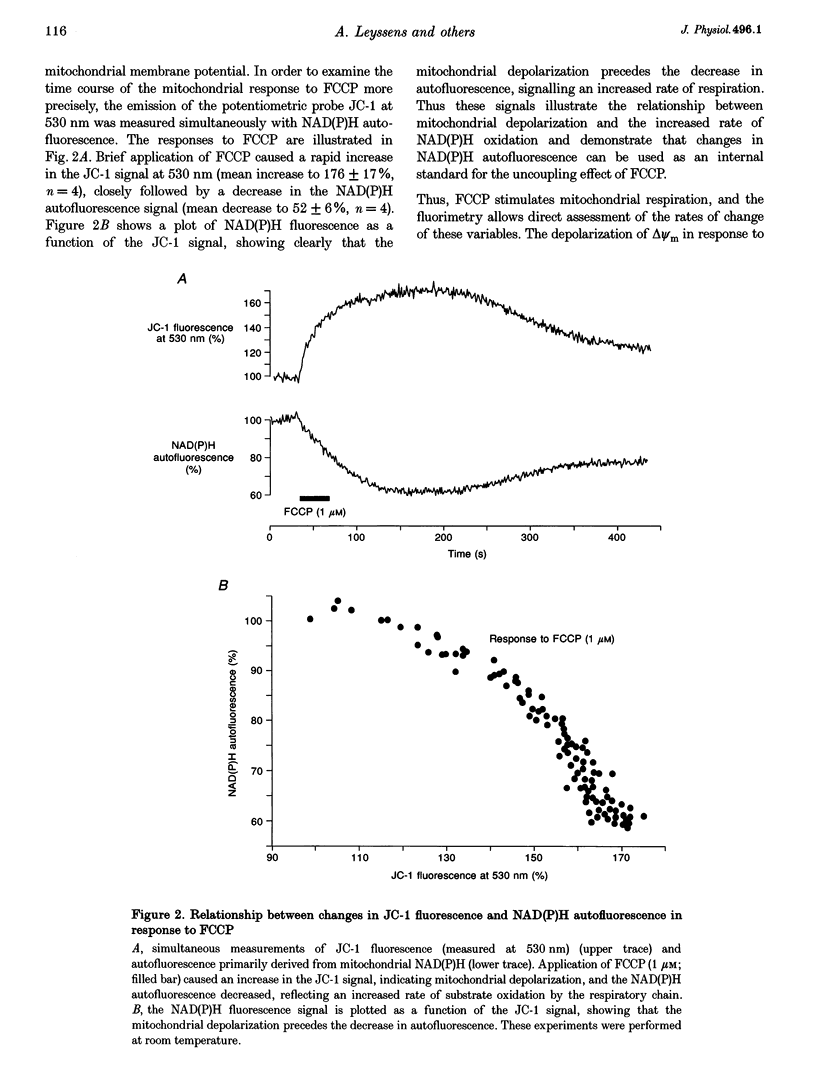
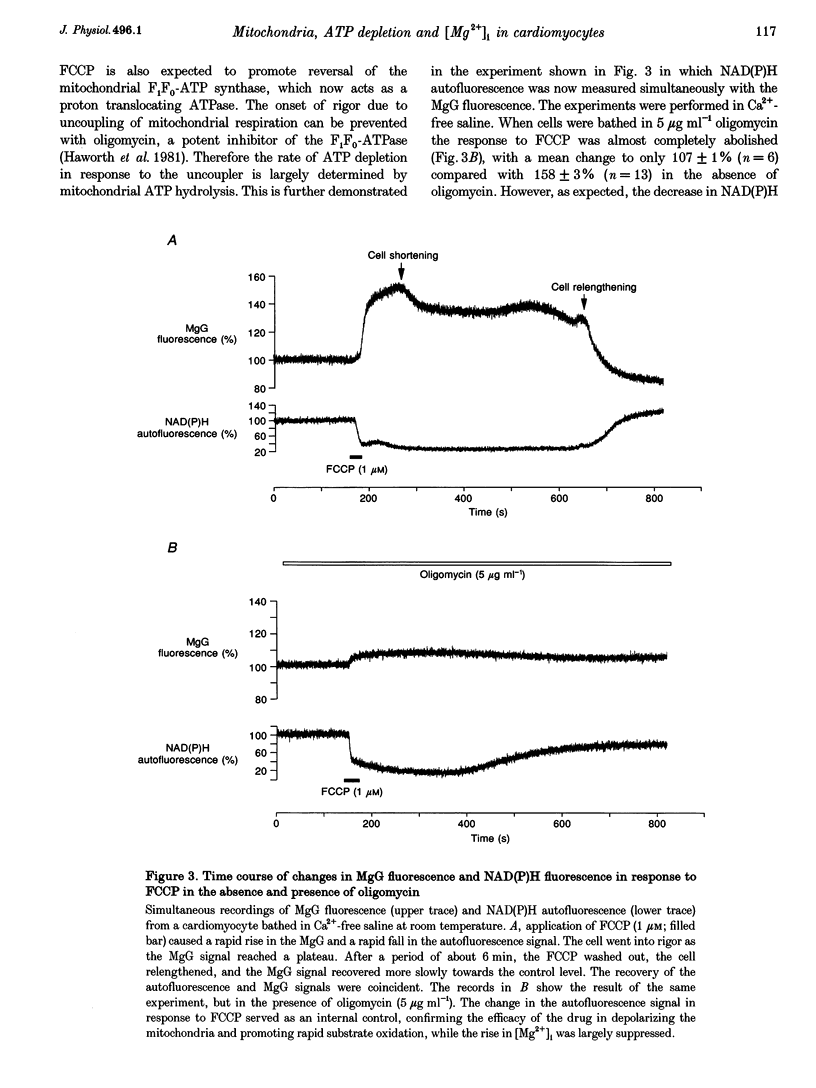
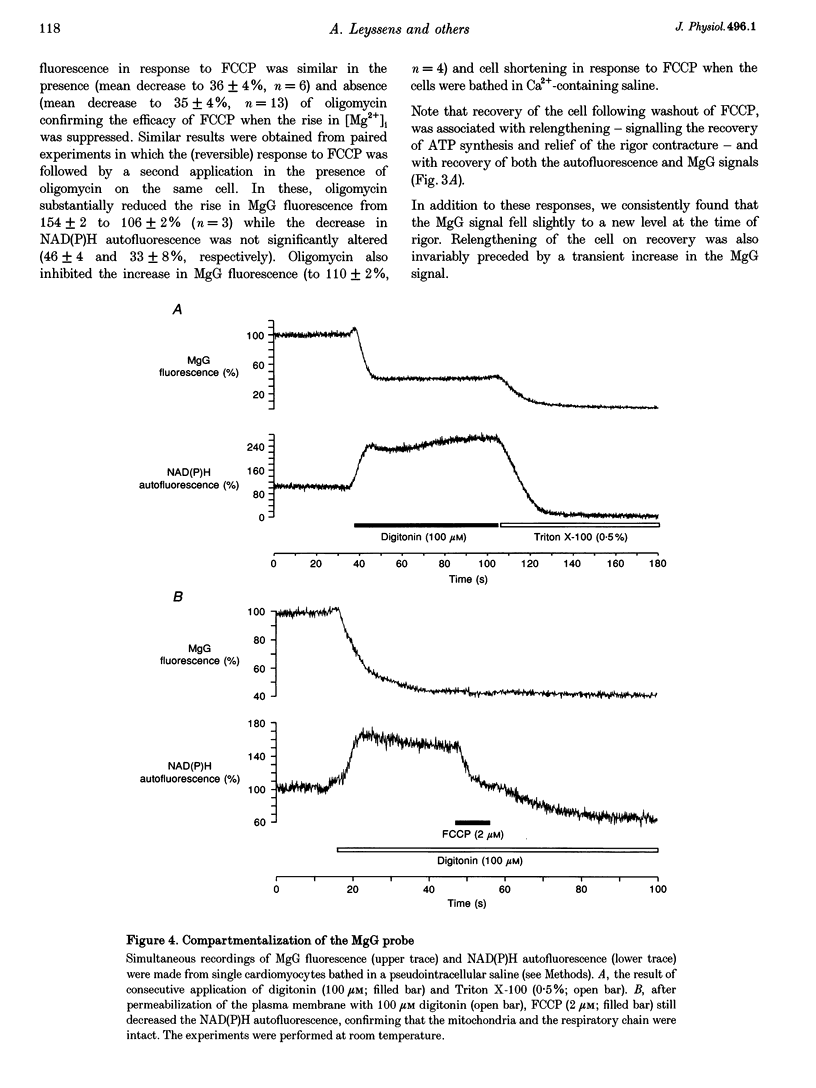
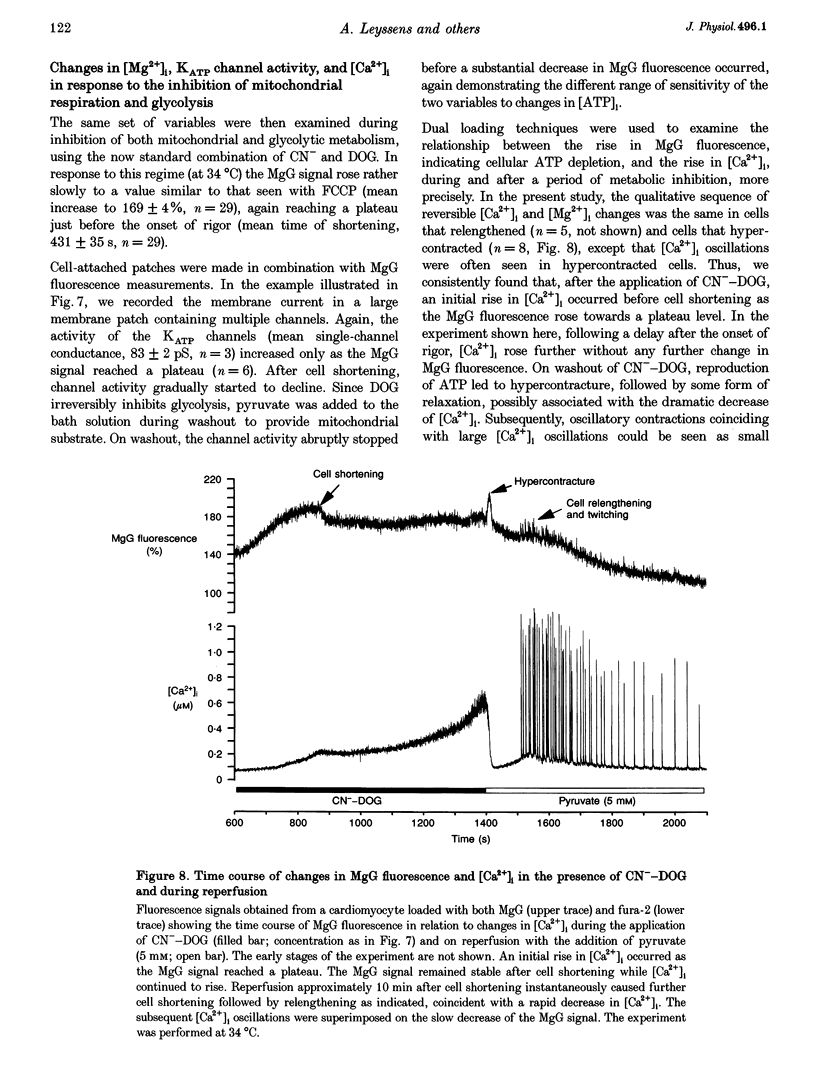
1. As ATP has a higher affinity for Mg2+ than ADP, the cytosolic magnesium concentration rises upon ATP hydrolysis. We have therefore used the Mg(2+)-sensitive fluorescent indicator Magnesium Green (MgG) to provide an index of changing ATP concentration in single rat cardiomyocytes in response to altered mitochondrial state. 2. In response to FCCP, [Mg2+]i rose towards a plateau coincident with the progression to rigor, which signals ATP depletion. Contamination of the MgG signal by changes in intracellular free Ca2+ concentration (the KD of MgG for Ca2+ is 4.7 microM) was excluded by simultaneous measurement of [Ca2+]i and [Mg2+]i in cells dual loaded with fura-2 and MgG. The response to FCCP was independent of external Mg2+, confirming an intracellular source for the rise in [Mg2+]i. 3. Simultaneous measurements of mitochondrial NAD(P)H autofluorescence and mitochondrial potential (delta psi m; .-1 fluorescence) and of autofluorescence and MgG allowed closer study of the relationship between [Mg2+]i and mitochondrial state. Oligomycin abolished the FCCP-induced rise in [Mg2+]i without altering the change in autofluorescence. Thus, the rise in [Mg2+]i in response to FCCP is consistent with the release of intracellular Mg2+ following ATP hydrolysis by the mitochondrial F1F0-ATPase. 4. The rise in [Mg2+]i was correlated with cell-attached recordings of ATP-sensitive K+ channel (KATP) activity. In response to FCCP, an increase in KATP channel activity was seen only as [Mg2+]i reached a plateau. In response to blockade of mitochondrial respiration and glycolysis with cyanide (CN-) and 2-deoxyglucose (DOG), [Mg2+]i rose more slowly but again KATP channel opening increased only when [Mg2+]i reached a plateau and the cells shortened. 5. Oligomycin decreased the rate of rise of [Mg2+]i delayed the onset of rigor and increased the rate of mitochondrial depolarization in response to CN-_DOG. Thus, with blockade of mitochondrial respiration delta psi m is maintained by the mitochondrial F1F0-ATPase at the expense of ATP reserves. 6. In response to CN-_DOG, the initial rise in [Mg2+]i was accompanied by a small rise in [Ca2+]i. After [Mg2+]i reached a plateau and rigor developed, [Ca2+]i rose progressively. On reperfusion, in hypercontracted cells, [Ca2+]i recovered before [Mg2+]i and [ca2+]i oscillations were sustained while [Mg2+]i decreased. Thus on reperfusion, full recovery of [ATP]i is slow, but the activation of contractile elements and the restoration of [Ca2+]i does not require the re-establishment of millimolar concentrations of ATP.
Full text
PDF



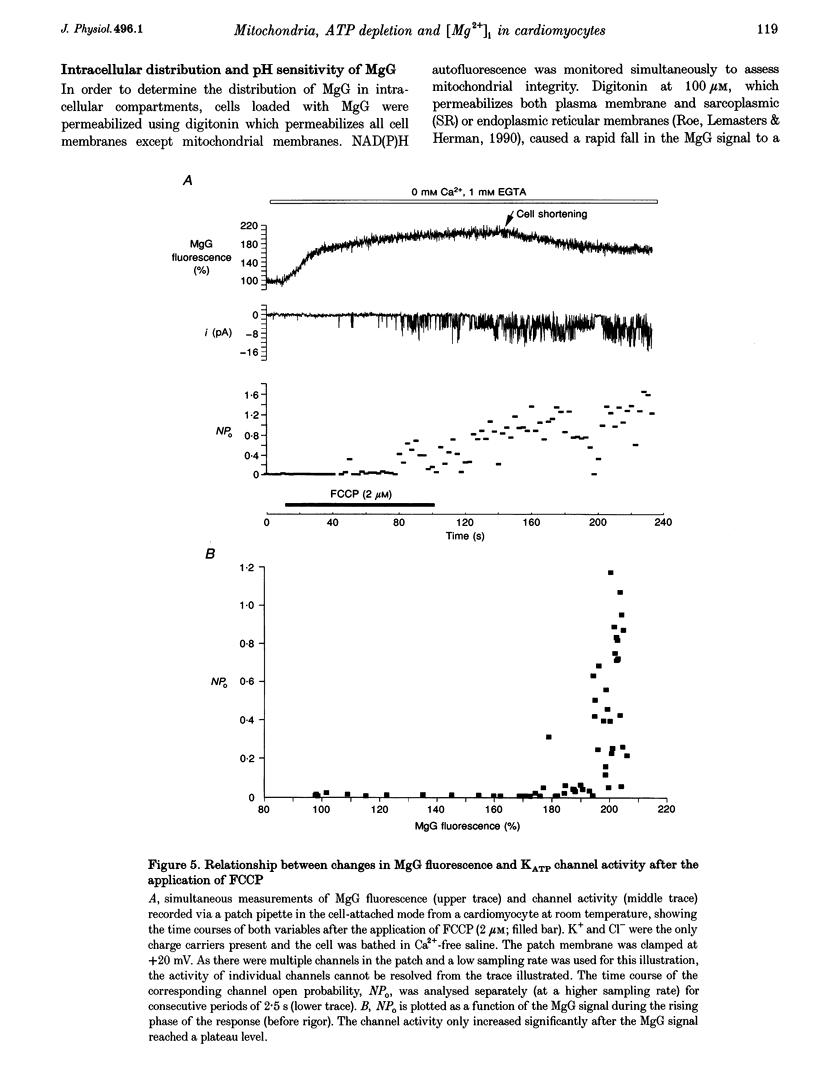
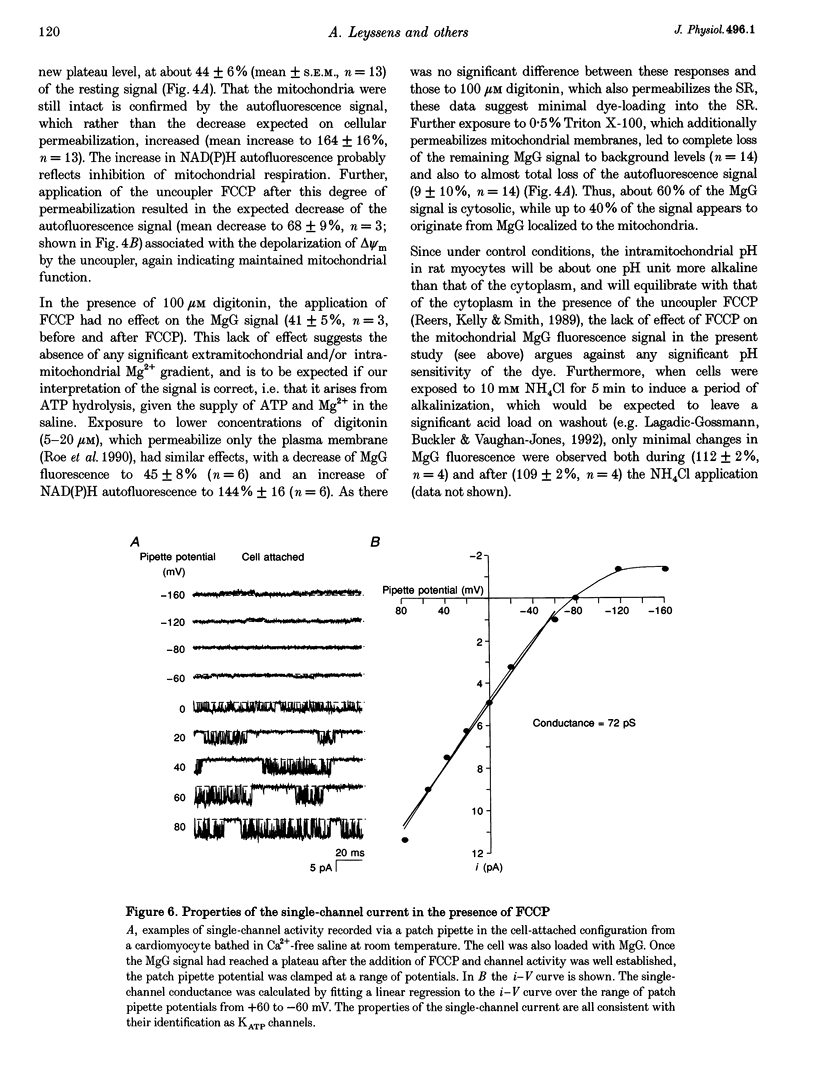
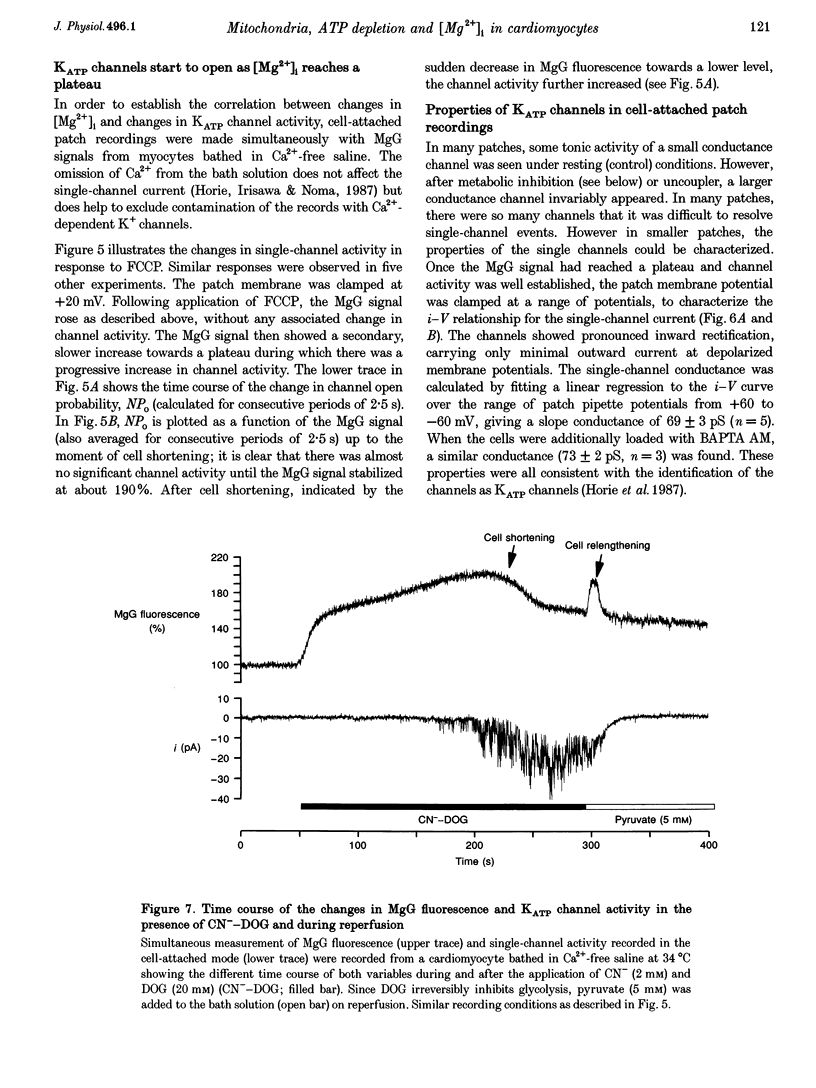




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bowers K. C., Allshire A. P., Cobbold P. H. Continuous measurements of cytoplasmic ATP in single cardiomyocytes during simulation of the "oxygen paradox". Cardiovasc Res. 1993 Oct;27(10):1836–1839. doi: 10.1093/cvr/27.10.1836. [DOI] [PubMed] [Google Scholar]
- CHANCE B., WILLIAMS G. R. The respiratory chain and oxidative phosphorylation. Adv Enzymol Relat Subj Biochem. 1956;17:65–134. doi: 10.1002/9780470122624.ch2. [DOI] [PubMed] [Google Scholar]
- Di Lisa F., Blank P. S., Colonna R., Gambassi G., Silverman H. S., Stern M. D., Hansford R. G. Mitochondrial membrane potential in single living adult rat cardiac myocytes exposed to anoxia or metabolic inhibition. J Physiol. 1995 Jul 1;486(Pt 1):1–13. doi: 10.1113/jphysiol.1995.sp020786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoso P., Mill J. G., O'Neill S. C., Eisner D. A. Fluorescence measurements of cytoplasmic and mitochondrial sodium concentration in rat ventricular myocytes. J Physiol. 1992 Mar;448:493–509. doi: 10.1113/jphysiol.1992.sp019053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchen M. R., McGuinness O., Brown L. A., Crompton M. On the involvement of a cyclosporin A sensitive mitochondrial pore in myocardial reperfusion injury. Cardiovasc Res. 1993 Oct;27(10):1790–1794. doi: 10.1093/cvr/27.10.1790. [DOI] [PubMed] [Google Scholar]
- Eisner D. A., Nichols C. G., O'Neill S. C., Smith G. L., Valdeolmillos M. The effects of metabolic inhibition on intracellular calcium and pH in isolated rat ventricular cells. J Physiol. 1989 Apr;411:393–418. doi: 10.1113/jphysiol.1989.sp017580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott A. C., Smith G. L., Allen D. G. Simultaneous measurements of action potential duration and intracellular ATP in isolated ferret hearts exposed to cyanide. Circ Res. 1989 Mar;64(3):583–591. doi: 10.1161/01.res.64.3.583. [DOI] [PubMed] [Google Scholar]
- Eng J., Lynch R. M., Balaban R. S. Nicotinamide adenine dinucleotide fluorescence spectroscopy and imaging of isolated cardiac myocytes. Biophys J. 1989 Apr;55(4):621–630. doi: 10.1016/S0006-3495(89)82859-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahn S., Koval G. J., Albers R. W. Sodium-potassium-activated adenosine triphosphatase of Electrophorus electric organ. I. An associated sodium-activated transphosphorylation. J Biol Chem. 1966 Apr 25;241(8):1882–1889. [PubMed] [Google Scholar]
- Findlay I. ATP-sensitive K+ channels in rat ventricular myocytes are blocked and inactivated by internal divalent cations. Pflugers Arch. 1987 Oct;410(3):313–320. doi: 10.1007/BF00580282. [DOI] [PubMed] [Google Scholar]
- Geisbuhler T., Altschuld R. A., Trewyn R. W., Ansel A. Z., Lamka K., Brierley G. P. Adenine nucleotide metabolism and compartmentalization in isolated adult rat heart cells. Circ Res. 1984 May;54(5):536–546. doi: 10.1161/01.res.54.5.536. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Guarnieri T. Intracellular sodium-calcium dissociation in early contractile failure in hypoxic ferret papillary muscles. J Physiol. 1987 Jul;388:449–465. doi: 10.1113/jphysiol.1987.sp016624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harman A. W., Nieminen A. L., Lemasters J. J., Herman B. Cytosolic free magnesium, ATP and blebbing during chemical hypoxia in cultured rat hepatocytes. Biochem Biophys Res Commun. 1990 Jul 31;170(2):477–483. doi: 10.1016/0006-291x(90)92116-h. [DOI] [PubMed] [Google Scholar]
- Haworth R. A., Hunter D. R., Berkoff H. A. Contracture in isolated adult rat heart cells. Role of Ca2+, ATP, and compartmentation. Circ Res. 1981 Nov;49(5):1119–1128. doi: 10.1161/01.res.49.5.1119. [DOI] [PubMed] [Google Scholar]
- Hayashi H., Satoh H., Noda N., Terada H., Hirano M., Yamashita Y., Kobayashi A., Yamazaki N. Simultaneous measurement of intracellular Na+ and Ca2+ during K(+)-free perfusion in isolated myocytes. Am J Physiol. 1994 Feb;266(2 Pt 1):C416–C422. doi: 10.1152/ajpcell.1994.266.2.C416. [DOI] [PubMed] [Google Scholar]
- Horie M., Irisawa H., Noma A. Voltage-dependent magnesium block of adenosine-triphosphate-sensitive potassium channel in guinea-pig ventricular cells. J Physiol. 1987 Jun;387:251–272. doi: 10.1113/jphysiol.1987.sp016572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung D. W., Brierley G. P. Magnesium transport by mitochondria. J Bioenerg Biomembr. 1994 Oct;26(5):527–535. doi: 10.1007/BF00762737. [DOI] [PubMed] [Google Scholar]
- Katoh H., Satoh H., Nakamura T., Terada H., Hayashi H. The role of Na+/H+ exchange and the Na+/K+ pump in the regulation of [Na+]i during metabolic inhibition in guinea pig myocytes. Biochem Biophys Res Commun. 1994 Aug 30;203(1):93–98. doi: 10.1006/bbrc.1994.2153. [DOI] [PubMed] [Google Scholar]
- Kirkels J. H., van Echteld C. J., Ruigrok T. J. Intracellular magnesium during myocardial ischemia and reperfusion: possible consequences for postischemic recovery. J Mol Cell Cardiol. 1989 Nov;21(11):1209–1218. doi: 10.1016/0022-2828(89)90697-4. [DOI] [PubMed] [Google Scholar]
- Lagadic-Gossmann D., Buckler K. J., Vaughan-Jones R. D. Role of bicarbonate in pH recovery from intracellular acidosis in the guinea-pig ventricular myocyte. J Physiol. 1992 Dec;458:361–384. doi: 10.1113/jphysiol.1992.sp019422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederer W. J., Nichols C. G. Nucleotide modulation of the activity of rat heart ATP-sensitive K+ channels in isolated membrane patches. J Physiol. 1989 Dec;419:193–211. doi: 10.1113/jphysiol.1989.sp017869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meissner G., Henderson J. S. Rapid calcium release from cardiac sarcoplasmic reticulum vesicles is dependent on Ca2+ and is modulated by Mg2+, adenine nucleotide, and calmodulin. J Biol Chem. 1987 Mar 5;262(7):3065–3073. [PubMed] [Google Scholar]
- Miyata H., Lakatta E. G., Stern M. D., Silverman H. S. Relation of mitochondrial and cytosolic free calcium to cardiac myocyte recovery after exposure to anoxia. Circ Res. 1992 Sep;71(3):605–613. doi: 10.1161/01.res.71.3.605. [DOI] [PubMed] [Google Scholar]
- Murphy E., Steenbergen C., Levy L. A., Raju B., London R. E. Cytosolic free magnesium levels in ischemic rat heart. J Biol Chem. 1989 Apr 5;264(10):5622–5627. [PubMed] [Google Scholar]
- Nazareth W., Yafei N., Crompton M. Inhibition of anoxia-induced injury in heart myocytes by cyclosporin A. J Mol Cell Cardiol. 1991 Dec;23(12):1351–1354. doi: 10.1016/0022-2828(91)90181-k. [DOI] [PubMed] [Google Scholar]
- Nichols C. G., Ripoll C., Lederer W. J. ATP-sensitive potassium channel modulation of the guinea pig ventricular action potential and contraction. Circ Res. 1991 Jan;68(1):280–287. doi: 10.1161/01.res.68.1.280. [DOI] [PubMed] [Google Scholar]
- Noll T., Koop A., Piper H. M. Mitochondrial ATP-synthase activity in cardiomyocytes after aerobic-anaerobic metabolic transition. Am J Physiol. 1992 May;262(5 Pt 1):C1297–C1303. doi: 10.1152/ajpcell.1992.262.5.C1297. [DOI] [PubMed] [Google Scholar]
- Noma A. ATP-regulated K+ channels in cardiac muscle. Nature. 1983 Sep 8;305(5930):147–148. doi: 10.1038/305147a0. [DOI] [PubMed] [Google Scholar]
- Reers M., Kelly R. A., Smith T. W. Calcium and proton activities in rat cardiac mitochondria. Effect of matrix environment on behaviour of fluorescent probes. Biochem J. 1989 Jan 1;257(1):131–142. doi: 10.1042/bj2570131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reers M., Smith T. W., Chen L. B. J-aggregate formation of a carbocyanine as a quantitative fluorescent indicator of membrane potential. Biochemistry. 1991 May 7;30(18):4480–4486. doi: 10.1021/bi00232a015. [DOI] [PubMed] [Google Scholar]
- Reimer K. A., Hill M. L., Jennings R. B. Prolonged depletion of ATP and of the adenine nucleotide pool due to delayed resynthesis of adenine nucleotides following reversible myocardial ischemic injury in dogs. J Mol Cell Cardiol. 1981 Feb;13(2):229–239. doi: 10.1016/0022-2828(81)90219-4. [DOI] [PubMed] [Google Scholar]
- Roe M. W., Lemasters J. J., Herman B. Assessment of Fura-2 for measurements of cytosolic free calcium. Cell Calcium. 1990 Feb-Mar;11(2-3):63–73. doi: 10.1016/0143-4160(90)90060-8. [DOI] [PubMed] [Google Scholar]
- Siegmund B., Schlüter K. D., Piper H. M. Calcium and the oxygen paradox. Cardiovasc Res. 1993 Oct;27(10):1778–1783. doi: 10.1093/cvr/27.10.1778. [DOI] [PubMed] [Google Scholar]
- Silverman H. S., Di Lisa F., Hui R. C., Miyata H., Sollott S. J., Hanford R. G., Lakatta E. G., Stern M. D. Regulation of intracellular free Mg2+ and contraction in single adult mammalian cardiac myocytes. Am J Physiol. 1994 Jan;266(1 Pt 1):C222–C233. doi: 10.1152/ajpcell.1994.266.1.C222. [DOI] [PubMed] [Google Scholar]
- Stern M. D., Silverman H. S., Houser S. R., Josephson R. A., Capogrossi M. C., Nichols C. G., Lederer W. J., Lakatta E. G. Anoxic contractile failure in rat heart myocytes is caused by failure of intracellular calcium release due to alteration of the action potential. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6954–6958. doi: 10.1073/pnas.85.18.6954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss J. N., Lamp S. T. Cardiac ATP-sensitive K+ channels. Evidence for preferential regulation by glycolysis. J Gen Physiol. 1989 Nov;94(5):911–935. doi: 10.1085/jgp.94.5.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R. L., Wittenberg B. A. Effects of calcium on mitochondrial NAD(P)H in paced rat ventricular myocytes. Biophys J. 1995 Dec;69(6):2790–2799. doi: 10.1016/S0006-3495(95)80152-9. [DOI] [PMC free article] [PubMed] [Google Scholar]


