Abstract
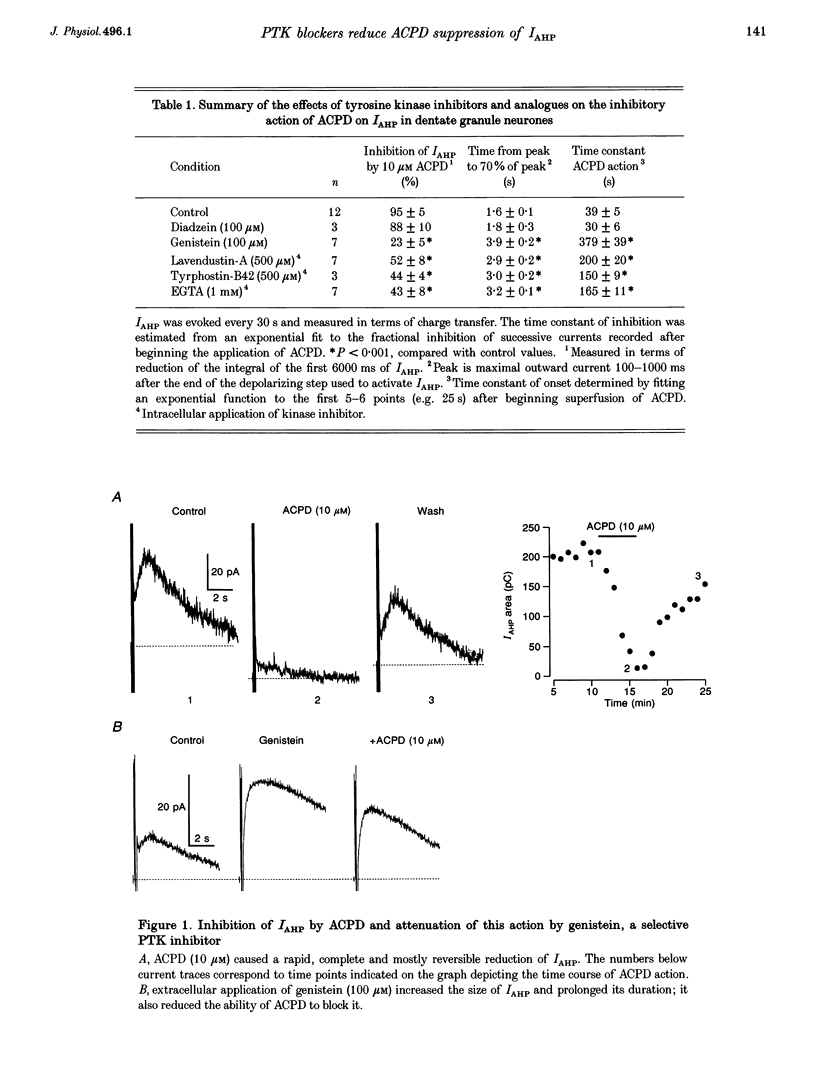
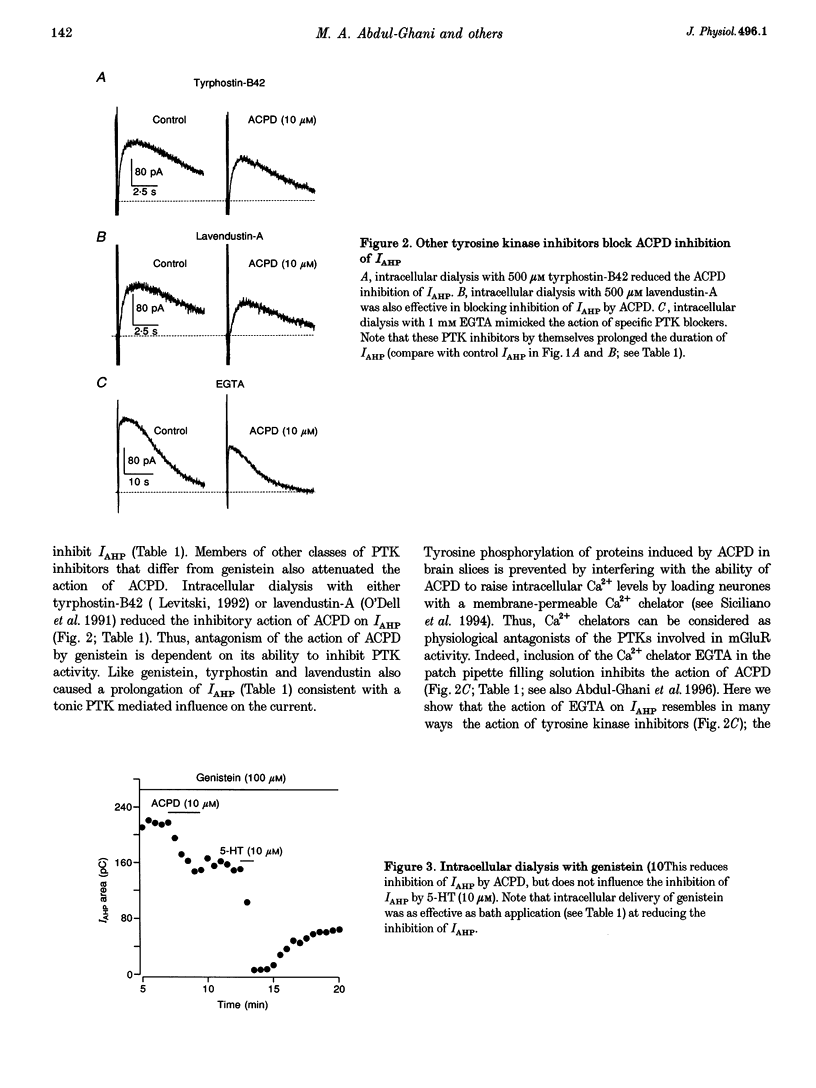
1. Activation of metabotropic glutamate receptors (mGluRs) inhibits a transient Ca(2+)-activated K+ current (IAHP) responsible for the slow after-hyperpolarization that follows depolarizations of dentate granule neurones in rat hippocampal brain slices. Here we show for the first time that this physiological consequence of mGluR stimulation is selectively attenuated by blockers of protein tyrosine kinases (PTKs). 2. Several distinct types of PTK blockers, including genistein, tyrphostin-B42 and lavendustin-A, reduced the inhibition of IAHP by the selective mGluR agonist (1S,3R)-1-aminocyclopentane-1,3-dicarboxylic acid (ACPD). Inhibition of IAHP by 5-HT was unaffected. The PTK blockers by themselves doubled the duration of IAHP suggesting that there exists a tonic inhibitory influence on IAHP that is reduced by PTK antagonists. 3. Inclusion of EGTA (1 mM) in the patch pipette also potentiated the IAHP and reduced the inhibitory action of ACPD on IAHP, consistent with the observation of others that chelation of intracellular Ca2+ prevents protein tyrosine phosphorylation induced by ACPD. 4. we propose that mGluR-initiated inositol 1,4,5-trisphosphate (InsP3) production mobilizes intracellular Ca2+ and leads to increased protein tyrosine phosphorylation which in turn leads to inhibition of IAHP.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bortolotto Z. A., Bashir Z. I., Davies C. H., Collingridge G. L. A molecular switch activated by metabotropic glutamate receptors regulates induction of long-term potentiation. Nature. 1994 Apr 21;368(6473):740–743. doi: 10.1038/368740a0. [DOI] [PubMed] [Google Scholar]
- Brown R. E., Reymann K. G. Class I metabotropic glutamate receptor agonists do not facilitate the induction of long-term potentiation in the dentate gyrus of the rat in vitro. Neurosci Lett. 1995 Dec 29;202(1-2):73–76. doi: 10.1016/0304-3940(95)12202-8. [DOI] [PubMed] [Google Scholar]
- Charpak S., Gähwiler B. H., Do K. Q., Knöpfel T. Potassium conductances in hippocampal neurons blocked by excitatory amino-acid transmitters. Nature. 1990 Oct 25;347(6295):765–767. doi: 10.1038/347765a0. [DOI] [PubMed] [Google Scholar]
- Harnick D. J., Jayaraman T., Ma Y., Mulieri P., Go L. O., Marks A. R. The human type 1 inositol 1,4,5-trisphosphate receptor from T lymphocytes. Structure, localization, and tyrosine phosphorylation. J Biol Chem. 1995 Feb 10;270(6):2833–2840. doi: 10.1074/jbc.270.6.2833. [DOI] [PubMed] [Google Scholar]
- Huang X. Y., Morielli A. D., Peralta E. G. Tyrosine kinase-dependent suppression of a potassium channel by the G protein-coupled m1 muscarinic acetylcholine receptor. Cell. 1993 Dec 17;75(6):1145–1156. doi: 10.1016/0092-8674(93)90324-j. [DOI] [PubMed] [Google Scholar]
- Huckle W. R., Dy R. C., Earp H. S. Calcium-dependent increase in tyrosine kinase activity stimulated by angiotensin II. Proc Natl Acad Sci U S A. 1992 Sep 15;89(18):8837–8841. doi: 10.1073/pnas.89.18.8837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindy M. S. Inhibition of tyrosine phosphorylation prevents delayed neuronal death following cerebral ischemia. J Cereb Blood Flow Metab. 1993 May;13(3):372–377. doi: 10.1038/jcbfm.1993.50. [DOI] [PubMed] [Google Scholar]
- Lee K. M., Toscas K., Villereal M. L. Inhibition of bradykinin- and thapsigargin-induced Ca2+ entry by tyrosine kinase inhibitors. J Biol Chem. 1993 May 15;268(14):9945–9948. [PubMed] [Google Scholar]
- Lev S., Moreno H., Martinez R., Canoll P., Peles E., Musacchio J. M., Plowman G. D., Rudy B., Schlessinger J. Protein tyrosine kinase PYK2 involved in Ca(2+)-induced regulation of ion channel and MAP kinase functions. Nature. 1995 Aug 31;376(6543):737–745. doi: 10.1038/376737a0. [DOI] [PubMed] [Google Scholar]
- Levitzki A. Tyrphostins: tyrosine kinase blockers as novel antiproliferative agents and dissectors of signal transduction. FASEB J. 1992 Nov;6(14):3275–3282. doi: 10.1096/fasebj.6.14.1426765. [DOI] [PubMed] [Google Scholar]
- McDonald J. W., Schoepp D. D. The metabotropic excitatory amino acid receptor agonist 1S,3R-ACPD selectively potentiates N-methyl-D-aspartate-induced brain injury. Eur J Pharmacol. 1992 May 14;215(2-3):353–354. doi: 10.1016/0014-2999(92)90058-c. [DOI] [PubMed] [Google Scholar]
- Meucci O., Scorziello A., Avallone A., Florio T., D'Alto V., Fattore M., Schettini G. Alpha 1 B, but not alpha 1A, adrenoreceptor activates calcium influx through the stimulation of a tyrosine kinase/phosphotyrosine phosphatase pathway, following noradrenaline-induced emptying of IP3 sensitive calcium stores, in PC Cl3 rat thyroid cell line. Biochem Biophys Res Commun. 1995 Apr 17;209(2):630–638. doi: 10.1006/bbrc.1995.1546. [DOI] [PubMed] [Google Scholar]
- O'Dell T. J., Kandel E. R., Grant S. G. Long-term potentiation in the hippocampus is blocked by tyrosine kinase inhibitors. Nature. 1991 Oct 10;353(6344):558–560. doi: 10.1038/353558a0. [DOI] [PubMed] [Google Scholar]
- Piiper A., Stryjek-Kaminska D., Stein J., Caspary W. F., Zeuzem S. Tyrphostins inhibit secretagogue-induced 1,4,5-IP3 production and amylase release in pancreatic acini. Am J Physiol. 1994 Mar;266(3 Pt 1):G363–G371. doi: 10.1152/ajpgi.1994.266.3.G363. [DOI] [PubMed] [Google Scholar]
- Pin J. P., Duvoisin R. The metabotropic glutamate receptors: structure and functions. Neuropharmacology. 1995 Jan;34(1):1–26. doi: 10.1016/0028-3908(94)00129-g. [DOI] [PubMed] [Google Scholar]
- Sargeant P., Farndale R. W., Sage S. O. Calcium store depletion in dimethyl BAPTA-loaded human platelets increases protein tyrosine phosphorylation in the absence of a rise in cytosolic calcium. Exp Physiol. 1994 Mar;79(2):269–272. doi: 10.1113/expphysiol.1994.sp003762. [DOI] [PubMed] [Google Scholar]
- Schlessinger J., Ullrich A. Growth factor signaling by receptor tyrosine kinases. Neuron. 1992 Sep;9(3):383–391. doi: 10.1016/0896-6273(92)90177-f. [DOI] [PubMed] [Google Scholar]
- Siciliano J. C., Gelman M., Girault J. A. Depolarization and neurotransmitters increase neuronal protein tyrosine phosphorylation. J Neurochem. 1994 Mar;62(3):950–959. doi: 10.1046/j.1471-4159.1994.62030950.x. [DOI] [PubMed] [Google Scholar]
- Stratton K. R., Worley P. F., Huganir R. L., Baraban J. M. Muscarinic agonists and phorbol esters increase tyrosine phosphorylation of a 40-kilodalton protein in hippocampal slices. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2498–2501. doi: 10.1073/pnas.86.7.2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez M. P., Tapley P., Saini S. S., He B., Pulido D., Barbacid M. Multiple tyrosine protein kinases in rat hippocampal neurons: isolation of Ptk-3, a receptor expressed in proliferative zones of the developing brain. Proc Natl Acad Sci U S A. 1994 Mar 1;91(5):1819–1823. doi: 10.1073/pnas.91.5.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y. T., Salter M. W. Regulation of NMDA receptors by tyrosine kinases and phosphatases. Nature. 1994 May 19;369(6477):233–235. doi: 10.1038/369233a0. [DOI] [PubMed] [Google Scholar]
- Wilson G. F., Kaczmarek L. K. Mode-switching of a voltage-gated cation channel is mediated by a protein kinase A-regulated tyrosine phosphatase. Nature. 1993 Dec 2;366(6454):433–438. doi: 10.1038/366433a0. [DOI] [PubMed] [Google Scholar]


