Abstract
Background
Diagnosis of Lyme borreliosis (LB) relies on clinical symptoms and detection of Borrelia-specific antibodies. Guidelines recommend a two-tier testing (TTT) strategy for disseminated LB: serological screening with a sensitive enzyme immunoassay (EIA) and confirmation with a specific immunoblot. Searching for the most sensitive and specific approach, this retrospective study evaluated standard (STTT) and modified (MTTT) strategies using a well-defined study population.
Methods
Cases included patients with active Lyme neuroborreliosis (LNB; n = 29) or Lyme arthritis (LA; n = 17). Controls comprised patients treated for LNB (n = 36) or LA (n = 8), healthy individuals who were either untreated (n = 75) or treated for LB (n = 15) in the past, and patients with potentially cross-reactive diseases (n = 16). Sera were subjected to three EIAs and two immunoblots. Reactive screening results were confirmed by immunoblot (STTT) or EIA (MTTT). Solitary IgM results in the screening assay and effects of antibiotic treatment on isotype-specific seropositivity rates were also assessed.
Results
Sensitivities of STTT strategies ranged from 90%–97% for LNB and were 100% for LA. MTTT strategies were 100% sensitive. Specificities ranged from 89%–95% for STTT and from 88%–93% for MTTT strategies. Differences between STTT and MTTT strategies were not statistically significant. Solitary IgM reactivity was common among controls. Antibiotic treatment significantly reduced IgM/IgG positivity for LNB patients; for LA patients, a decline was only observed for IgM.
Conclusion
In conclusion, MTTT strategies showed a slightly higher sensitivity and similar specificity compared to STTT strategies. Since EIAs are more time- and cost-efficient, MTTT strategies seem more favorable for clinical use. IgG testing enhances specificity with minimal sensitivity loss.
Keywords: Antibiotic treatment, MTTT, Sensitivity, Seroreversion, Specificity, STTT
Introduction
Lyme borreliosis (LB) is caused by infection with Ixodes-transmitted spirochetes of the Borrelia burgdorferi sensu lato (s.l.) complex, and is the most prevalent tick-borne disease in the Northern Hemisphere. In North America, B. burgdorferi sensu stricto (s.s.) is the predominant genotype, and erythema migrans (EM) and Lyme arthritis (LA) are the most frequently observed manifestations. In Europe, however, the B. burgdorferi s.l. population is more heterogenic and dissemination seems dependent on the tropism of the infecting species: B. afzelii is mainly associated with skin lesions, whereas B. garinii predominantly causes neurological syndromes and B. burgdorferi s.s. is generally involved in joint infections [1].
LB diagnosis largely relies on clinical symptoms and serology, except for EM, which is a clinical diagnosis. Guidelines for LB diagnosis and treatment recommend a standard two-tier testing (STTT) strategy that entails screening of eligible samples with a sensitive enzyme immunoassay (EIA) and confirmation of reactive screening results with a specific immunoblot [2]. The sensitivity of STTT ranges from 50% for early LB to almost 100% for late manifestations such as acrodermatitis chronica atrophicans (ACA). Specificity varies from 80% in cross-sectional settings to 95% when healthy populations are considered [3].
For correct classification of patients, STTT ideally uses a first-tier EIA with 100% sensitivity and a second-tier immunoblot with 100% specificity. Due to the suboptimal manifestation-dependent sensitivity, STTT is being debated for some time now, and modifications are being explored. Contrary to STTT, modified two-tier testing (MTTT) uses a second EIA for confirmation of reactive screening results instead of immunoblot. Besides being more cost-effective, MTTT demonstrated improved sensitivity over STTT while maintaining specificity in a North American setting [4, 5]. Therefore, the North American guidelines for serologic testing of LB now recommend MTTT as an acceptable replacement of STTT [6].
Besides optimization efforts for STTT, the role of IgM in LB diagnosis is being debated. IgM antibodies are by nature less specific than IgG antibodies and may last for years to decades after successful antibiotic treatment of LB, possibly maintained by non-borrelial antigens [7, 8]. Compared to IgG assays, IgM assays have lower sensitivity and specificity, and contribute to the screening for IgG antibodies for disseminated LB only to a limited extent [9].
The heterogenic B. burgdorferi s.l.-population in Europe demands the use of immunoblots with genotype-specific recombinant antigens, which makes immunoblot more expensive in comparison to the B. burgdorferi s.s.-specific Western blots used in North America. Moreover, immunoblot is labor-intensive and often susceptible to subjective interpretation. Therefore, the performance of MTTT was also evaluated in a European setting, and demonstrated improved sensitivity for MTTT over STTT without loss of specificity [10]. The focus of this study, however, was on GP-diagnosed EM patients, for whom serology is not recommended for diagnosis, and disseminated LB manifestations were not included. Moreover, three of the evaluated EIAs are no longer commercially available, and only one immunoblot was included in the study [10].
Despite various attempts to optimize LB serology, still no direct or indirect detection method is able to differentiate between active and past infection, or provide a measure of treatment efficacy. However, a strong association was observed between antibiotic treatment and the level of disagreement among the compared EIAs and strategies in a previous study from our group [11]. Building on these results, the present study evaluated the diagnostic performance of STTT and MTTT strategies using a well-defined study population of European disseminated LB cases and multiple control groups. To this end, two commercially available EIAs and the now-discontinued C6 Lyme ELISA, and two commercially available immunoblots were included in the study. Furthermore, the role of solitary IgM results on test performance and the effect of antibiotic treatment on isotype-specific seropositivity were evaluated.
Materials and methods
Study design
This study was set up using a modified two-gate study design [12], in which cases and controls are typically selected from different study populations. The study results were reported in adherence to the Standards for Reporting Diagnostic Accuracy [13].
Study population
The sera used in this study were obtained from participants included in the prospective study “T-cell response in Lyme” that was described in detail by Van Gorkom et al. [11]. In short, the population of LB patients consisted of active LNB (aNB: n = 29) and active LA (aLA; n = 17) patients, and patients treated in the past for LNB (tNB; n = 36) or LA (tLA; n = 8). For nine of the patients included in the aNB group a follow-up serum was included in the tNB group. Similarly, follow-up sera from five aLA patients were included in the tLA group. The control group comprised healthy individuals, with (tHI; n = 15) or without (uHI; n = 75) a history of treated LB, and who were recruited from the same endemic area as the included LB patients.
Additionally, anonymized left-over sera from patients with possibly cross-reactive diseases (CR; n = 16) were selected from the Diakonessenhuis hospital serum repository.
Participants were included if sufficient serum was available to perform all selected assays. The sociodemographic and clinical characteristics of the included study participants are summarized in Table 1.
Table 1.
Sociodemographic and clinical characteristics of the study participants
| LNB patients | LA patients | Healthy individuals | Cross-reactive | ||||
|---|---|---|---|---|---|---|---|
| active | treated | active | treated | untreated | treated | patients | |
| (n = 29) | (n = 36) | (n = 17) | (n = 8) | (n = 75) | (n = 15) | (n = 16) | |
| Sex; no. of males (%) | 17 (59) | 18 (50) | 14 (82) | 7 (88) | 15 (20) | 3 (20) | 6 (38) |
| Age, years; median (range) | 57 (27—77) | 59 (21—77) | 45 (19—71) | 47 (28—59) | 46 (20—72) | 52 (23—68) | 45.5 (16—83) |
| Time of sampling after start of antibiotic treatment, years; median (range) | 0 (0—0.1) | 3.9 (1.4—9.3) | 0 (-0.1—0.3) | 2.1 (1.0—6.0) | 4.2 (2.1—12.2) | ||
| Positive PCR result; n (%)a | 4 (17) | 5 (26) | 17 (100) | 8 (100) | |||
| Definite LNBb; n (%) | 21 (72) | 30 (83) | |||||
| Possible LNBb based on clinical symptoms and | |||||||
| intrathecal Borrelia burgdorferi s.l.-specific antibody synthesis; n (%) | 1 (3) | 3 (8) | |||||
| pleocytosis; n (%) | 7 (24) | 3 (8) | |||||
LNB, Lyme neuroborreliosis; LA, Lyme arthritis
a PCR on cerebrospinal fluid (LNB patients) or synovial fluid (LA patients) at time of diagnosis. For 6 active and 17 treated LNB patients PCR was not performed
b LNB was diagnosed according to guidelines from the European Federation of Neurological Societies [14]. LNB was classified as definite based on clinical symptoms, combined with intrathecal Borrelia burgdorferi s.l.-specific antibody synthesis and pleocytosis, or as possible based on clinical symptoms, combined with either intrathecal Borrelia burgdorferi s.l.-specific antibody synthesis or pleocytosis
Laboratory analysis
All sera were subjected to the assays summarized in Table 2. The assays were performed and interpreted according to the manufacturer’s instructions. The test results were reported as negative, equivocal or positive, except for the Euroline IgG immunoblot, for which the outcome could either be negative or positive.
Table 2.
Overview of the assays included in this study and the type of antigens used
| Assay (manufacturer) | Antigens | |
|---|---|---|
| EIA | ||
| Serion IgM | Serion ELISA classic Borrelia burgdorferi IgM (Institute Virion\Serion GmbH, Würzburg, Germany) | Whole cell lysate (Ba, Bg) |
| Serion IgG | Serion ELISA classic Borrelia burgdorferi IgG (Institute Virion\Serion GmbH, Würzburg, Germany) | Whole cell lysate (Ba, Bg), VlsE (Bg) |
| Liaison IgM | Liaison® Borrelia IgM Quant (DiaSorin S.p.A, Saluggia, Italy) | OspC (Ba), VlsE (Bg) |
| Liaison IgG | Liaison® Borrelia IgG (DiaSorin S.p.A, Saluggia, Italy) | VlsE (Bg) |
| C6 IgM/IgG | C6 Lyme ELISA™ (Immunetics, Boston, USA) | Synthetic C6 peptide (derived from VlsE) |
| Immunoblot | ||
| recomLine IgM | recomLine Borrelia IgM (Mikrogen, Würzburg, Germany) | p100, VlsE (Bsl fusion protein), p58, p41, p39, OspA, OspC (Bss, Ba, Bg, Bsp), p18 (Bsl, Ba, Bbav, Bg, Bsp) |
| recomLine IgG | recomLine Borrelia IgG (Mikrogen, Würzburg, Germany) | Identical to recomLine IgM |
| Euroline IgM | Anti-Borrelia EUROLINE-RN-AT-adv IgM (Euroimmun AG, Lübeck, Germany) | VlsE (Bss), p41, p39, OspC (Ba, Bss, Bg, Bsp) |
| Euroline IgG | Anti-Borrelia EUROLINE-RN-AT IgG (Euroimmun AG, Lübeck, Germany) | VlsE (Ba, Bss, Bg), lipid (Ba, Bss), p83 (= p100), p41, p39, OspC (Bsl mixture), p58, p21, p20, p19, p18 |
Ba: B. afzelii, Bbav: B. bavariensis, Bg: B. garinii, Bsl: B. burgdorferi sensu lato, Bsp: B. spielmanii, Bss: B. burgdorferi sensu stricto, EIA: enzyme immuno assay, VlsE: variable major protein-like sequence, expressed, Osp: outer surface protein
Test strategies
The three included EIAs (Serion, Liaison and C6) were used as screening test in STTT and MTTT strategies. In STTT, reactive screening results were confirmed with either recomLine or Euroline immunoblots. In MTTT, reactive Serion results were confirmed with either Liaison or C6, and reactive Liaison results were confirmed with C6.
Data analysis
Data management and analyses were conducted in R version 4.3.1. [15].
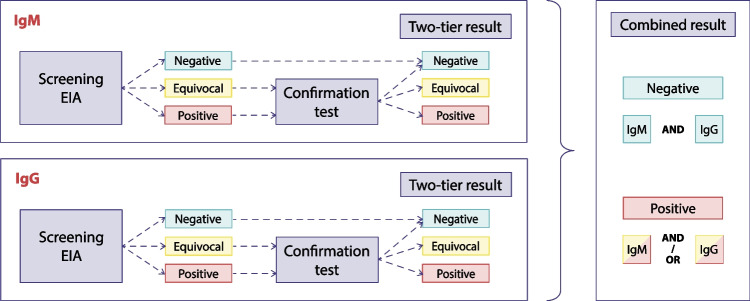
As previously mentioned, test results from all included assays were obtained for each serum (Table 2). When applying TTT strategies, the result of the confirmation assay was disregarded for sera with a negative screening result (Fig. 1). When sera were reactive in the screening assay, the result of the confirmation assay was used for further analysis (Fig. 1). For each assay with separate isotype measurements, two-tier results were first obtained for each isotype, by applying the various TTT strategies, followed by the combination of IgM and IgG results (Fig. 1). The combined IgM/IgG result from separately measured two-tier IgM and IgG results was considered positive when IgM and/or IgG was reactive, and negative when both IgM and IgG were negative (Fig. 1). The C6 does not distinguish between IgM and IgG; therefore, both isotypes of the confirmation assay were determined in case of a reactive screening result.
Fig. 1.
Flow diagrams for two-tier testing strategies for EIAs with separate isotype measurements. The confirmation test was an immunoblot in standard two-tier testing or a second EIA in modified two-tier testing strategies. Blue, yellow and red boxes represent a negative, equivocal and positive result, respectively
The sensitivity and 95% confidence intervals (CI) for proportions (Clopper-Pearson) for each of the test strategies were reported for the aNB and aLA groups. For the tNB and tLA groups, positivity rates were reported, as these groups are usually not tested in a clinical setting, nor used for test evaluations. Likewise, specificity and 95% CI were reported for the uHI group, and negativity rates were reported for the tHI and CR groups. For comparison of the different strategies, diagnostic odds ratios (DOR) were determined with R’s mada package using aNB and aLA as cases and uHI as controls [16, 17]. If no false negatives were observed, the DOR was approximated using a continuity correction of 0.5 [17].
The observed solitary equivocal and positive IgM results in the screening EIAs were described for each of the test strategies. The effect on strategy performance by exclusion of solitary equivocal or solitary equivocal and positive IgM results obtained in the screening EIAs, was evaluated using McNemar’s exact test.
The association between antibiotic treatment and seropositivity rates was evaluated at group level by comparing the aNB, aLA and uHI groups with the tNB, tLA and tHI groups, respectively, using Chi-squared test, or Fisher’s Exact test where appropriate.
Raw P-values < 0.05 were considered significant, and were interpreted after correction for multiple testing using the Benjamini–Hochberg procedure accepting a 1% false discovery rate [18].
Results
In the aNB patient group, confirmation of reactive EIA results in the STTT strategy using either the recomLine or the Euroline immunoblot resulted in sensitivities of 90% and 97%, respectively (Table 3). In the MTTT strategy, a second EIA was used for confirmation and the sensitivity was 100% for all test combinations. In the tNB group, the positivity rates of the test strategies was lower. Using STTT, the positivity rates ranged between 19% and 39%, and for MTTT strategies this was 28% to 33%. The active and treated LA patients were detected by all assays and consequently the sensitivity and positivity rates were 100% for both STTT and MTTT strategies.
Table 3.
Sensitivity (active disease patient groups) and positivity rates (post-antibiotic treatment patient groups) of the test strategies
| aNB, n = 29 | tNB, n = 36 | aLA, n = 17 | tLA, n = 8 | |||||
|---|---|---|---|---|---|---|---|---|
| npos | %pos [95% CI] | npos | %pos [95% CI] | npos | %pos [95% CI] | npos | %pos [95% CI] | |
| STTT | ||||||||
| Ser-RL | 26 | 90 [73 − 98] | 11 | 31 [16 − 48] | 17 | 100 [81 − 100] | 8 | 100 [63 − 100] |
| Lia-RL | 26 | 90 [73 − 98] | 10 | 28 [14 − 45] | 17 | 100 [81 − 100] | 8 | 100 [63 − 100] |
| C6-RL | 26 | 90 [73 − 98] | 7 | 19 [8 – 36] | 17 | 100 [81 − 100] | 8 | 100 [63 − 100] |
| Ser-EU | 28 | 97 [82 − 100] | 14 | 39 [23 − 57] | 17 | 100 [81 − 100] | 8 | 100 [63 − 100] |
| Lia-EU | 28 | 97 [82 − 100] | 14 | 39 [23 − 57] | 17 | 100 [81 − 100] | 8 | 100 [63 − 100] |
| C6-EU | 28 | 97 [82 − 100] | 11 | 31 [16 − 48] | 17 | 100 [81 − 100] | 8 | 100 [63 − 100] |
| MTTT | ||||||||
| Ser-Lia | 29 | 100 [88 − 100] | 12 | 33 [19 – 51] | 17 | 100 [81 − 100] | 8 | 100 [63 − 100] |
| Ser-C6 | 29 | 100 [88 − 100] | 10 | 28 [14 − 45] | 17 | 100 [81 − 100] | 8 | 100 [63 − 100] |
| Lia-C6 | 29 | 100 [88 − 100] | 11 | 31 [16 − 48] | 17 | 100 [81 − 100] | 8 | 100 [63 − 100] |
aNB: active Lyme neuroborreliosis, tNB: treated Lyme neuroborreliosis, aLA: active Lyme arthritis, tLA: treated Lyme arthritis, Ser: Serion, Lia: Liaison, C6: C6 Lyme ELISA, RL: recomLine, EU: Euroline, STTT: standard two-tier testing, MTTT: modified two-tier testing.
In the uHI group, the specificity ranged from 89% to 95% for the STTT strategies, and from 88 to 93% for the MTTT strategies (Table 4). In the tHI group, the overall negativity rates ranged from 87% to 100% for both the STTT and the MTTT strategies. In the CR group, negativity rates ranged from 69% to 100% and from 75% to 94% for STTT and MTTT, respectively.
Table 4.
Specificity (untreated healthy individuals) and negativity rates (treated healthy individuals and cross-reactivity controls) of the test strategies
| uHI | tHI | CR | ||||
|---|---|---|---|---|---|---|
| n = 75 | n = 15 | n = 16 | ||||
| npos | %neg [95% CI] | npos | %neg [95% CI] | npos | %neg [95% CI] | |
| STTT | ||||||
| Ser-RL | 5 | 93 [85 − 98] | 2 | 87 [60 − 98] | 0 | 100 [80 − 100] |
| Lia-RL | 5 | 93 [85 − 98] | 2 | 87 [60 − 98] | 0 | 100 [80 − 100] |
| C6-RL | 4 | 95 [87 − 99] | 0 | 100 [78 − 100] | 0 | 100 [80 − 100] |
| Ser-EU | 8 | 89 [80 − 95] | 2 | 87 [60 − 98] | 5 | 69 [41 − 89] |
| Lia-EU | 8 | 89 [80 − 95] | 2 | 87 [60 − 98] | 4 | 75 [48 − 93] |
| C6-EU | 4 | 95 [87 − 99] | 0 | 100 [78 − 100] | 1 | 94 [70 − 100] |
| MTTT | ||||||
| Ser-Lia | 9 | 88 [78 − 94] | 2 | 87 [60 − 98] | 4 | 75 [48 − 93] |
| Ser-C6 | 5 | 93 [85 − 98] | 0 | 100 [78 − 100] | 1 | 94 [70 − 100] |
| Lia-C6 | 5 | 93 [85 − 98] | 0 | 100 [78 − 100] | 2 | 88 [62 − 98] |
uHI: untreated healthy individuals, tHI: treated healthy individuals, CR: cross-reactivity controls, Ser: Serion, Lia: Liaison, C6: C6 Lyme ELISA, RL: recomLine, EU: Euroline, STTT: standard two-tier testing, MTTT: modified two-tier testing
The DOR was used to compare the test strategies under evaluation (Fig. 2). The DORs ranged between 201 and 799 for STTT strategies, and between 651 and 1192 for MTTT strategies. Irrespective of the evaluated test strategy, highest DORs were obtained for test combinations that included the C6.
Fig. 2.
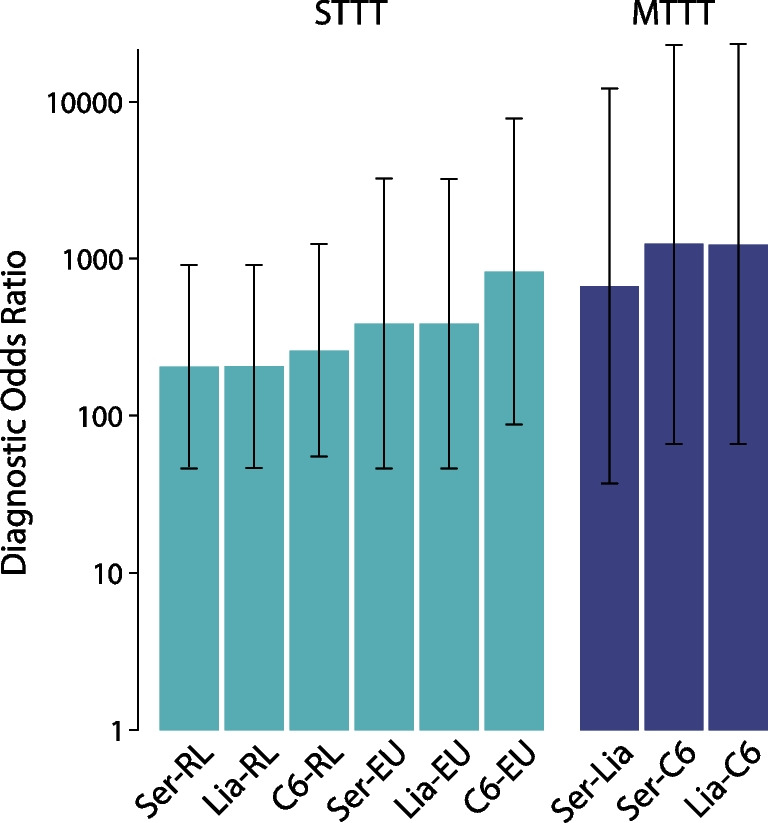
Diagnostic odds ratios (DORs) of the test strategies under evaluation, determined using active Lyme neuroborreliosis and Lyme arthritis patients as cases and untreated healthy individuals as controls. The DORs of the MTTT strategies were approximated using a continuity correction. Whiskers indicate 95% confidence intervals. Ser: Serion, Lia: Liaison, C6: C6 Lyme ELISA, RL: recomLine, EU: Euroline, STTT: standard two-tier testing, MTTT: modified two-tier testing
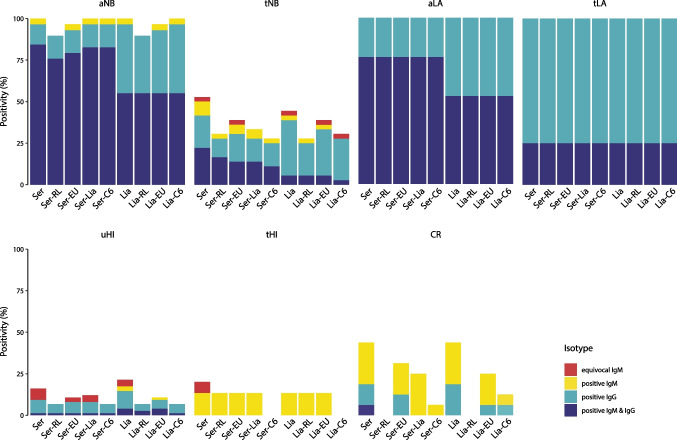
The contribution of solitary equivocal and positive IgM results in the screening assay (Serion or Liaison) to the final test result was evaluated for each of the strategies (Fig. 3). Solitary equivocal IgM results were obtained with the Serion as screening assay in the tNB (n = 1), uHI (n = 5) and tHI (n = 1) groups. In the tNB group, the solitary equivocal IgM result was confirmed by the Euroline only. In the uHI group, two and three of the five solitary equivocal IgM results were confirmed by Euroline and Liaison, respectively. The solitary equivocal IgM result observed in the tHI group was not confirmed by any second-tier assay. Using the Liaison for screening, solitary equivocal IgM results were obtained in the tNB (n = 1) and uHI (n = 3) groups. Of these, only the solitary equivocal IgM result in the tNB group was confirmed by Euroline and C6. No solitary IgM results were obtained for any of the assays and test strategies in the aLA and tLA groups.
Fig. 3.
Distribution of the results per isotype for the two-tier test strategies using Serion and Liaison as screening assay, stratified by study group. aNB: active Lyme neuroborreliosis, tNB: treated Lyme neuroborreliosis, aLA: active Lyme arthritis, tLA: treated Lyme arthritis, uHI: untreated healthy individuals, tHI: treated healthy individuals, CR: cross-reactivity controls, Ser: Serion, Lia: Liaison, C6: C6 Lyme ELISA, RL: recomLine, EU: Euroline
Solitary positive IgM results were observed for the Serion and the Liaison, and especially contributed to seropositivity in the tHI and CR groups (Fig. 3). In the aNB group, one patient had a solitary positive IgM result in both screening assays that was confirmed by all second-tier assays, except the recomLine. Furthermore, solitary positive IgM results were obtained with the Serion as screening assay in the tNB (n = 3), tHI (n = 2) and CR (n = 4) groups. In the tNB group, one of these was confirmed by recomLine and C6, and two by Euroline or Liaison. In the tHI group, both solitary positive IgM results were confirmed by recomLine, Euroline and Liaison, but not by C6. In the CR group, none of the four solitary positive IgM results were confirmed by recomLine, whereas one, three and four were confirmed by C6, Euroline, and Liaison, respectively. Using the Liaison for screening, solitary positive IgM results were obtained in the tNB (n = 1), uHI (n = 2), tHI (n = 2) and CR (n = 4) groups. The solitary positive IgM result in the tNB group was confirmed by recomLine and Euroline, but not by C6. Of the two solitary positive IgM results in the uHI group, one was confirmed using Euroline. The two solitary positive IgM results in the tHI group were confirmed by recomLine and Euroline, but not by C6. None of the four solitary positive IgM results in the CR group were confirmed by recomLine, whereas one and three of these were confirmed by C6 and Euroline, respectively.
Overall, solitary equivocal IgM results were observed in the tNB patient group, and in the uHI and tHI control groups (Fig. 3). Exclusion of these solitary equivocal IgM results resulted in a minor gain in specificity (not significant) without loss in sensitivity. Solitary positive IgM results were obtained in the aNB and tNB patient groups and all three control groups. Exclusion of both solitary equivocal and positive IgM results resulted in a minor gain in specificity and a minor loss in sensitivity, both of which were not significant.
The effect of antibiotic treatment on the seropositivity rate was evaluated for LNB patients, LA patients and healthy individuals, by comparing the positivity rates of the untreated groups and the treated groups for each test strategy. For LNB patients, antibiotic treatment resulted in a significant decrease (p < 0.001, FDR < 1%) in positivity rates for all test strategies (Fig. 3, Table 3), primarily due to a significant decrease in the proportion of patients with detectable IgM and IgG antibodies (p < 0.001, FDR < 1%). Conversely, the proportion of solitary IgM results and solitary IgG results did not change significantly after antibiotic treatment. For LA patients, antibiotic treatment did not result in a decrease in positivity rates for any of the test strategies. However, post-antibiotic treatment, IgM seropositivity decreased, while IgG remained positive. These changes were only significant for strategies that used the Serion as screening assay (p = 0.028, FDR > 1%). Among healthy individuals, the positivity rates for all test strategies were comparable among untreated and treated healthy individuals (Fig. 3, Table 4). Interestingly, the response was dominated by solitary IgG and combined IgM/IgG responses in the untreated group, whereas only solitary IgM responses were observed in the treated group.
Discussion
In this study, the performance of multiple STTT and MTTT strategies was evaluated using a well-defined study population of patients with early and late disseminated LB and multiple control groups. Furthermore, the contribution of solitary IgM results in the screening EIA to the final strategy result, and the impact of antibiotic treatment on seroreversion were evaluated.
The sensitivity of STTT with recomLine immunoblot as a second-tier assay was slightly, though not significantly, lower compared to the Euroline immunoblot, while the opposite was observed for specificity. One previous study that compared recomLine and Euroline immunoblots, reported equal sensitivity and specificity for IgM and IgG separately [19]. Unfortunately, the combined IgM/IgG results were not reported, which complicated comparison to the present study’s observations. In our study, MTTT strategies showed slightly higher sensitivities, with specificities comparable to those of STTT strategies. However, in contrast to previous MTTT evaluations in both early localized and disseminated LB patients in various epidemiological settings, the observed differences among STTT and MTTT strategies were not statistically significant in our study [4, 10, 20]. This may be due to the relatively small study population.
The specificities observed here were comparable to those reported previously [3], and reflect the background seroprevalence of 5.3% in the endemic region where the hospital is located, which is comparable to the 4.4% seroprevalence observed in the general population of the Netherlands [21]. Participants who reported treatment for LB in the past were grouped separately and, therefore, the seropositivity observed in the uHI group is likely the result of a previous (asymptomatic) Borrelia infection. Cross-reactivity seems less probable, as IgG was the dominant isotype in this group, whereas IgM positivity dominated the CR group, consistent with previous findings [9, 10].
The role of IgM testing for LB diagnosis has been debated for some time [9, 22]. In the present study, solitary IgM results were predominantly observed in the control groups, and disregarding them could possibly improve specificity for all strategies with minor loss of sensitivity. Due to the small sample size in the present study, this observation was not statistically significant, and should be confirmed in a larger cohort. The observed trend, however, underscores the recommendation that IgM testing should only be considered when clinical symptoms are suggestive for active LB with symptom duration until six to ten weeks [23, 24].
In our study, isotype-specific seropositivity in post-treatment sera seemed dependent on the disease stage in which antibiotics were administered. The solitary IgM responses observed among healthy individuals that were treated for early localized manifestations up to 6.2 years prior to inclusion suggest that seroconversion was abrogated before the isotype switch from IgM to IgG had taken place. For the disseminated manifestations LNB and LA, combined IgM/IgG seropositivity was predominantly observed among active patients, indicating that the isotype switch preceded initiation of antibiotic treatment. After antibiotic treatment, a significant decrease in seropositivity was observed among LNB patients, which was attributable to decreased IgM and IgG levels, consistent with previous findings [25]. Among treated LA patients, no seroreversion was observed; however, decreased IgM antibody levels resulted in increased solitary IgG seropositivity. As pre- and post-treatment sera were only available for some of the LB patients, associations with antibiotic treatment were made on group level. In future studies, pairwise comparisons should determine whether the observations in this study also apply to patient level.
This study was conducted on a well-defined population of patients diagnosed with early and late disseminated LB patients and various control groups. The LNB patients in our study were diagnosed based on clinical symptoms with pleocytosis and/or intrathecal B. burgdorferi s.l.-specific antibody production. For the diagnosis of LNB patients, the detection of intrathecal B. burgdorferi s.l.-specific antibody production is preferred over serology, because up to 20% of the LNB patients with intrathecal antibody synthesis had not seroconverted (yet) at the time of diagnosis [26, 27]. The LA patients in this study were diagnosed based on clinical symptoms and positive Borrelia PCR on synovial fluid, confirmed by serology. This selection bias could be considered a limitation that is difficult to resolve as serology is part of the diagnosis, and as shown here antibodies persist at least a year post-treatment. Furthermore, exclusion of EM patients in this study likely has resulted in an overestimation of the sensitivity. EM is, however, a clinical diagnosis, for which laboratory testing should not delay treatment – even with improved sensitivity of MTTT [10].
Most MTTT evaluations tested the off-market C6 Lyme ELISA as second-tier assay [4, 10, 20]. As reflected by the high DORs in this study, all test strategies that included this assay demonstrated the best performance in terms of sensitivity and specificity. As the C6 Lyme ELISA assay was discontinued, we also evaluated the Liaison as a second-tier EIA, and this MTTT strategy also outperformed commercially available STTT strategies. One could argue, however, that the antigens used in the Liaison might also be part of the screening assay, which could introduce bias. This could partially be overcome using a peptide-based and/or multiplex second-tier assay, such as the recently introduced Zeus VlsE1/pepC10 IgM/IgG ELISA (Zeus ELISA) or the BioPlex 2200 Lyme IgG and Lyme IgM assays.
The Zeus ELISA has previously been evaluated as a single-tier or first-tier assay, and performed comparably to the C6 Lyme ELISA in both single-tier and STTT strategies [9, 28]. In North America, the FDA-approved MTTT strategy uses Zeus ELISA as first-tier assay with Zeus’ whole cell sonicate (WCS)-based IgM/IgG ELISA as second-tier assay. This strategy outperformed STTT and MTTT strategies with second-tier C6 Lyme ELISA in terms of sensitivity, especially in early LB, without loss of specificity [29, 30]. However, these WCS ELISAs use inactivated B. burgdorferi B31 antigen, and might therefore be suboptimal for detection of the heterogenic B. burgdorferi s.l. population in Europe. The Bioplex, on the other hand, was also evaluated on European patient and control populations, and demonstrated comparable sensitivity, but inferior specificity to the C6 Lyme ELISA in a STTT strategy [31]. Also, the Bioplex requires specific equipment and expertise and is therefore less suitable for use in routine clinical settings. Hence, future assay developments should focus on accessible, low-cost assays for improved performance of TTT or to replace TTT altogether.
Both STTT and MTTT strategies do not discriminate active from past disease, and offer no measure for therapeutic success or reinfection. Here, dynamics in isotype-specific immune responses among different LB manifestations were demonstrated that should be explored further to ameliorate these shortcomings of TTT. Moreover, the output of TTT is currently categorical (i.e., positive or negative) and provides no detailed information about the type and magnitude of the antigen-specific immune responses. Preliminary data from our research group have shown promising results regarding antigen-specific immune responses. We are in the process of confirming these findings in a larger cohort of LB patients, including paired sera from active and treated patients diagnosed with EM, LA and LNB. Also, the relationship between antibody levels and predictive values has already been demonstrated for some assays [32, 33], and could be explored further to improve LB diagnostics.
In conclusion, this study showed that for disseminated LB, MTTT strategies demonstrated a slightly higher sensitivity, although not statistically significant, while maintaining similar specificity compared to STTT strategies. Since solitary IgM reactions were predominantly observed among healthy individuals, the use of IgM assays for the diagnosis of LB should be carefully considered based on disease duration and manifestation. Lastly, although antibiotic treatment does not always result in seroreversion, decline of isotype-specific antibody levels was observed, and could possibly be used to assess disease status for different Lyme manifestations.
Acknowledgements
The authors thank Robert van der Kieft, Vanessa van Schaik and Maruska Nijrolder for technical assistance during the study.
Author contributions
Conceptualization of the study and methodology: B.J.A. Hoeve-Bakker (BHB), K. Kerkhof (KK), M. Heron (MH), S.F.T. Thijsen (ST) and T. van Gorkom (TG); data collection: BHB and TG; laboratory testing and interpretation: BHB and TG; formal analysis and visualization: BHB; supervision: BHB, KK, ST and TG. BHB wrote the original draft and BHB, KK, MH, ST and TG reviewed and edited the manuscript. All authors gave their approval of the submitted version of the manuscript.
Funding
This study was financially supported by the Ministry of Health, Welfare and Sport of the Netherlands. The funder was not involved in the study setup, data collection and analysis, nor in the decision to publish the results.
Data availability
The anonymized data is available from the corresponding author upon reasonable request.
Declarations
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study “T-cell response in Lyme” (TRIL) was approved by the regional Medical Research Ethics Committees United (MEC-U), Nieuwegein, The Netherlands and registered with the Central Committee on Research Involving Human Subjects (CCMO) of the Netherlands under number NL36407.100.11 at 09–07-2020.
Consent to participate
Informed consent was obtained from all individual participants included in the TRIL study.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Stanek G, Strle F (2018) Lyme borreliosis-from tick bite to diagnosis and treatment. FEMS Microbiol Rev 42(3):233–258. 10.1093/femsre/fux047 [DOI] [PubMed] [Google Scholar]
- 2.Eldin C et al (2019) Review of European and American guidelines for the diagnosis of Lyme borreliosis. Med Mal Infect 49(2):121–132. 10.1016/j.medmal.2018.11.011 [DOI] [PubMed] [Google Scholar]
- 3.Leeflang MM et al (2016) The diagnostic accuracy of serological tests for Lyme borreliosis in Europe: a systematic review and meta-analysis. BMC Infect Dis 16:140. 10.1186/s12879-016-1468-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pegalajar-Jurado A et al (2018) Evaluation of modified two-tiered testing algorithms for lyme disease laboratory diagnosis using well-characterized serum samples. J Clin Microbiol 56 (8). 10.1128/jcm.01943-17 [DOI] [PMC free article] [PubMed]
- 5.Hatchette TF (2020) Modified two-tiered testing algorithm for Lyme disease serology: the Canadian context. Can Commun Dis Rep 46(5):125–131. 10.14745/ccdr.v46i05a05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mead P et al (2019) Updated CDC recommendation for serologic diagnosis of lyme disease. MMWR Morb Mortal Wkly Rep 68(32):703. 10.15585/mmwr.mm6832a4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Markowicz M et al (2021) Persistent anti-borrelia igm antibodies without lyme borreliosis in the clinical and immunological context. Microbiol Spectr 9(3):e0102021. 10.1128/Spectrum.01020-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kalish RA et al (2001) Persistence of immunoglobulin M or immunoglobulin G antibody responses to Borreliaburgdorferi 10–20 years after active Lyme disease. Clin Infect Dis 33(6):780–785. 10.1086/322669 [DOI] [PubMed] [Google Scholar]
- 9.Hoeve-Bakker BJA et al (2022) The performance of nine commercial serological screening assays for the diagnosis of lyme borreliosis: a multicenter modified two-gate design study. Microbiol Spectr 10(2):e0051022. 10.1128/spectrum.00510-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baarsma ME et al (2020) Diagnostic parameters of modified two-tier testing in European patients with early Lyme disease. Eur J Clin Microbiol Infect Dis 39(11):2143–2152. 10.1007/s10096-020-03946-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Gorkom T et al (2017) Disagreement between the results from three commercial tests for the detection of Borrelia-specific serum antibodies in the Netherlands associated with antibiotic treatment for Lyme borreliosis: a retrospective study. Eur J Clin Microbiol Infect Dis 36(11):2137–2146. 10.1007/s10096-017-3037-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rutjes AW et al (2005) Case-control and two-gate designs in diagnostic accuracy studies. Clin Chem 51(8):1335–1341. 10.1373/clinchem.2005.048595 [DOI] [PubMed] [Google Scholar]
- 13.Bossuyt PM et al (2015) STARD 2015: an updated list of essential items for reporting diagnostic accuracy studies. Clin Chem 61(12):1446–1452. 10.1373/clinchem.2015.246280 [DOI] [PubMed] [Google Scholar]
- 14.Mygland A et al (2010) EFNS guidelines on the diagnosis and management of European Lyme neuroborreliosis. Eur J Neurol 17(1):8-16.e11-14. 10.1111/j.1468-1331.2009.02862.x [DOI] [PubMed] [Google Scholar]
- 15.Team RC (2023) R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. Vienna, Austria. https://www.R-project.org/. Accessed 27 Oct 2023
- 16.Doebler P (2015) Meta-analysis of diagnostic accuracy with mada. R Packages. https://api.semanticscholar.org/CorpusID:30459830. Accessed 27 Oct 2023
- 17.Glas AS et al (2003) The diagnostic odds ratio: a single indicator of test performance. J Clin Epidemiol 56(11):1129–1135. 10.1016/S0895-4356(03)00177-X [DOI] [PubMed] [Google Scholar]
- 18.Benjamini Y, Hochberg Y (2018) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Roy Stat Soc: Ser B (Methodol) 57(1):289–300. 10.1111/j.2517-6161.1995.tb02031.x [Google Scholar]
- 19.Busson L et al (2012) Evaluation of commercial screening tests and blot assays for the diagnosis of Lyme borreliosis. Diagn Microbiol Infect Dis 73(3):246–251. 10.1016/j.diagmicrobio.2012.04.001 [DOI] [PubMed] [Google Scholar]
- 20.Davis IRC et al (2020) Performance of a modified two-tiered testing enzyme immunoassay algorithm for serologic diagnosis of lyme disease in Nova Scotia. J Clin Microbiol 58(7):10–1128. 10.1128/jcm.01841-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoeve-Bakker BJA et al (2023) Seroprevalence and risk factors of lyme borreliosis in The Netherlands: a population-based cross-sectional study. Microorganisms 11(4):1081. 10.3390/microorganisms11041081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hillerdal H, Henningsson AJ (2021) Serodiagnosis of Lyme borreliosis-is IgM in serum more harmful than helpful? Eur J Clin Microbiol Infect Dis 40(6):1161–1168. 10.1007/s10096-020-04093-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.CBO (2013) [Dutch Guideline Lyme Disease], https://www.rivm.nl/nieuws/cbo-richtlijn-lymeziekte-definitief. Accessed: 15–09–2021
- 24.Joyner G et al (2022) Introduction of IgM testing for the diagnosis of acute Lyme borreliosis: a study of the benefits, limitations and costs. Eur J Clin Microbiol Infect Dis 41(4):671–675. 10.1007/s10096-021-04366-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pietikainen A et al (2022) Borreliaburgdorferi specific serum and cerebrospinal fluid antibodies in Lyme neuroborreliosis. Diagn Microbiol Infect Dis 104(3):115782. 10.1016/j.diagmicrobio.2022.115782 [DOI] [PubMed] [Google Scholar]
- 26.Tetens MM et al (2022) The diagnostic value of serum Borreliaburgdorferi antibodies and seroconversion after Lyme neuroborreliosis, a nationwide observational study. Clin Microbiol Infect 28(11):1500.e1501-1500.e1506. 10.1016/j.cmi.2022.06.001 [DOI] [PubMed] [Google Scholar]
- 27.van Gorkom T et al (2022) Retrospective evaluation of various serological assays and multiple parameters for optimal diagnosis of lyme neuroborreliosis in a routine clinical setting. Microbiol Spectr 10(3):e0006122. 10.1128/spectrum.00061-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baarsma ME et al (2022) Diagnostic performance of the ZEUS Borrelia VlsE1/pepC10 assay in European LB patients: a case-control study. Eur J Clin Microbiol Infect Dis 41(3):387–393. 10.1007/s10096-021-04372-6 [DOI] [PubMed] [Google Scholar]
- 29.Khan F et al (2022) Modified two-tiered testing enzyme immunoassay algorithm for serologic diagnosis of lyme disease. Open Forum Infect Dis 9(7):ofac272. 10.1093/ofid/ofac272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sfeir MM et al (2022) Multicenter clinical evaluation of modified two-tiered testing algorithms for lyme disease using zeus scientific commercial assays. J Clin Microbiol 60(5):e0252821. 10.1128/jcm.02528-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baarsma ME et al (2021) Diagnostic performance of the novel BioPlex lyme serological assays in European patients with lyme disease. J Clin Microbiol 59(7):e0320520. 10.1128/jcm.03205-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dessau RB et al (2015) Multiplex assay (Mikrogen recomBead) for detection of serum IgG and IgM antibodies to 13 recombinant antigens of Borreliaburgdorferi sensu lato in patients with neuroborreliosis: the more the better? J Med Microbiol 64(Pt 3):224–231. 10.1099/jmm.0.000009 [DOI] [PubMed] [Google Scholar]
- 33.Nigrovic LE et al (2019) Higher C6 enzyme immunoassay index values correlate with a diagnosis of noncutaneous Lyme disease. Diagn Microbiol Infect Dis 94(2):160–164. 10.1016/j.diagmicrobio.2018.12.001 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The anonymized data is available from the corresponding author upon reasonable request.