Abstract
The mosquito-lethal effect of commercially available standard and long-lasting ivermectin formulations were evaluated in cattle and buffalo against wild-caught Anopheles on Sumba Island, Indonesia. Cattle have substantially higher blood-level concentrations of ivermectin compared to buffalo after receiving similar doses, irrespective of formulation. In total, nine Anopheles species were captured to assess the mosquito-lethal effects of ivermectin with susceptibility ranked from lowest to highest: An. flavirostris < An. aconitus < An. annularis < An. tessellatus < An. maculatus < An. sundaicus < An. vagus < An. kochi < An. barbirostris. The duration of mosquito-lethal effect of long-lasting ivermectin was superior to standard ivermectin and in cattle it well exceeded the WHO criteria for new endectocides having a mortality hazard ratio greater than 4 through 30 days after administration. Buffalo may require higher doses of long-lasting ivermectin to achieve similar mosquito-lethal effects observed in cattle. Of the four hosts evaluated buffalo were the most attractive to Anopheles followed by cattle then horse and finally humans. This study demonstrates, for the first time, the superiority of a commercially available long-lasting ivermectin formulation for the potential deployment of mass ivermectin treatment of livestock as a vector control tool for malaria elimination in Southeast Asia.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-81743-x.
Keywords: Anopheles, Livestock, Ivermectin, Long-lasting, Survival, Sumba
Subject terms: Medical research, Translational research
Introduction
Ivermectin-treated livestock are lethal to blood-feeding Anopheles, suggesting that mass ivermectin treatment of livestock (ITL) is a potential method to reduce Plasmodium transmission1. As livestock are frequently maintained in rural areas of Southeast Asia afflicted with malaria, mass ITL could be a complementary approach to strengthen malaria control in the region. Southeast Asia has the highest Anopheles species diversity globally, and only a few Anopheles species have been evaluated for ivermectin susceptibility including Anopheles dirus s.s2–6. , An. minimus s.s2–4,6. , An. campestris2, An. sawadwongporni2, An. epiroticus5, An. farauti7–9, and An. punctulatus10. Ivermectin treatment with standard formulations and doses (200 µg/kg) in cattle has been shown to reduce the survival of blood-feeding Anophelesfrom 2 to 3 weeks post-injection5,11–14
While ITL with standard ivermectin holds promise for malaria control, it is desirable to extend the duration of mosquito-lethal effect. One study showed that a three-fold increased ivermectin dose in cattle (600 µg/kg) did not confer a substantial increase in the duration of An. gambiaes.s. mosquito-lethal effect15compared to standard dosing5,11–14. Increasing the dose of ivermectin in cattle (630 µg/kg) results in a proportional increase in the peak concentrations (Cmax)16, but this does not translate to a proportional increase in time above the critical concentration of mosquito killing effect due to the first-order process of drug elimination. Increasing the standard ivermectin dose in pigs (300 µg/kg) by two-fold (600 µg/kg) and three-fold (900 µg/kg) demonstrated significant An. coluzzii mosquito-lethal effect at day 7 for all ivermectin treatments, but only for the three-fold higher dose at day 1417. Thus, increasing the dose of standard ivermectin may not provide substantially improved cost-benefit in terms of mosquito-lethal effect.
Several commercially available, long-lasting ivermectin formulations have been developed by modifying the glycerol to propylene glycol ratio of the vehicle to alter the absorption of ivermectin from the injection site. Directly comparing standard ivermectin to two different long-lasting formulations at the same dose (630 µg/kg) in cattle demonstrated two- and three-fold longer mean residence times with the long-lasting formulations16. The extended blood ivermectin concentrations achieved with these long-lasting ivermectin formulations have superior efficacy to control tick and mite ectoparasites of cattle18–20, and thus similar outcomes are likely for mosquitoes. To date, no commercially available long-lasting ivermectin formulations for livestock have been evaluated for their effects against Anopheles survival.
Anopheles ivermectin susceptibility evaluations have never been performed in Indonesia. Indonesia represents a high level of biodiversity with three major ecozones, demarcated by the Wallace and Weber lines, with flora and fauna species changing dramatically across these three ecozones, and the same extends to the Anophelesspecies composition present in these regions21. Located in the Lesser Sundas Islands, Sumba Island is particularly of interest due to its meso-endemic malaria prevalence22 and high Anopheles species biodiversity, driven largely by the non-volcanic nature of Sumba Island, leading to different topography and water retention of the soil. Twelve different Anopheles species have been documented from the West and Southwest districts of Sumba including: An. aconitus, An. annularis, An. balabacensis, An. barbirsotris, An. flavirostris, An. indefinitus, An. kochi, An. maculatus, An. subpictus, An. sundaicus, An. tessellatus, and An. vagus23,24. None of these Anopheles species have been evaluated for their susceptibility to ivermectin. Sumba Island allows for the investigation of the mosquito-lethal effect of ivermectin on multiple Anopheles species simultaneously in a setting where the results are of relevance to the islands malaria control efforts.
Livestock on Sumba consist primarily of cattle, buffalo, and horses. Previously, pigs were common on Sumba but with the introduction of African Swine Fever in 2019, the pig population has been dramatically reduced by over 80% across the island (Southwest Sumba Livestock and Animal Health Office; personal communication). Most Anopheles mosquitoes do not exclusively bite humans, which makes mass ITL a potentially attractive vector control tool. No Anopheles host preference studies have been performed previously on Sumba Island or in the Nusa Tenggara Timor province. If ivermectin mass drug administration (MDA) to humans and/or mass ITL were to be performed on Sumba, then it would be important to understand the host preferences of the Anopheles species on the island.
This study assessed ivermectin pharmacokinetic properties in Southeast Asian cattle (Bos taurus indicus) and water buffalo (Bubalus bubalis) breeds. The ivermectin susceptibility of wild Anopheles and duration of mosquito-lethal effect of standard and long-lasting ivermectin injectable formulations in cattle and buffalo on wild Anopheles survival on Sumba Island was assessed. In addition, the host preference of wild Anopheles species on Sumba Island was evaluated.
Results
Mosquito field capture results
A total of 24 livestock, 12 cattle (7 female, 5 male) and 12 buffalo (4 female, 8 male), were used to capture mosquitoes in five different study sites.
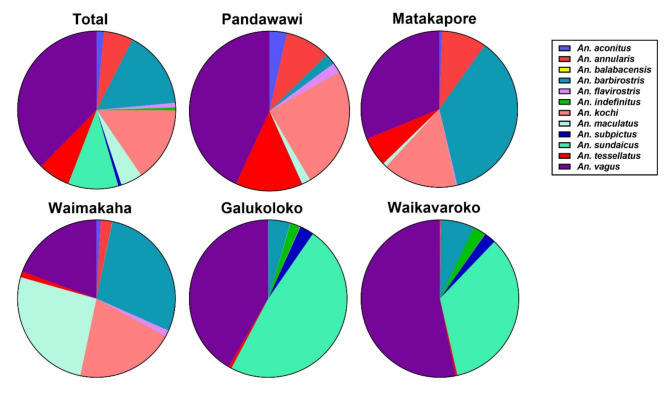
A total of 69,479 Anopheles specimens representing 12 different Anopheles species were captured from five different study sites for ivermectin susceptibility evaluation including: An. vagus (n = 26,119), An. barbirostris (n = 11,132), An. kochi (n = 10,721), An. sundaicus (n = 7,129), An. tessellatus (n = 4,556), An. annularis (n = 4,181), An. maculatus (n = 3,073), An. aconitus (n = 1,033), An. flavirostris (n = 666), An. subpictus (n = 470), An. indefinitus (n = 396), An. balabacensis (n = 3). Approximately twice the total number of Anopheles specimens were captured per site from Pandawawi (n = 23,746) and Matakapore (n = 19,047) compared to Waimakaha (n = 9,549), Galukoloko (n = 9,048), and Waikavaroko (n = 8,089) sites (Fig. 1). The number of Anopheles specimens captured from buffalo-baited traps was nearly double that from cow-baited traps, with no substantial differences between the treatment groups and includes: cow control (n = 7,506), cow standard ivermectin (n = 9,884), cow long-lasting ivermectin (n = 9,482), buffalo control (n = 14,379), buffalo standard ivermectin (n = 13,364), and buffalo long-lasting ivermectin (n = 14,864). There were no apparent differences in the proportion of Anopheles species captured by cow or buffalo across the different treatment groups (Supplemental Fig. 1).
Fig. 1.
Illustrates the proportion of Anopheles species captured in total and from each of the five study sites for survival analysis: Pandawawi (34%, n = 23,746), Matakapore (27%, n = 19,047), Waimakaha (14%, n = 9,549), Galukoloko (13%, n = 9,048), and Waikavaroko (12%, n = 8,089).
Ivermectin pharmacokinetic results
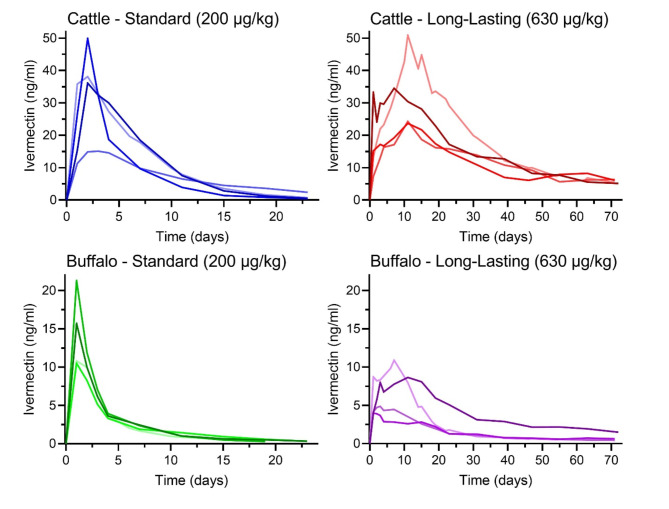
A total of 283 ivermectin post-treatment venous blood samples were collected from cattle (n = 142) and buffalo (n = 141). Blood samples from cattle (n = 41) and buffalo (n = 41) treated with standard ivermectin collected up to days post treatment (DPT) 24 were quantified for ivermectin. Blood samples from cattle (n = 71) and buffalo (n = 70) treated with long-lasting ivermectin collected up to DPT 72 were quantified for ivermectin. One blood sample from the buffalo treated with long-lasting ivermectin from Waikavaroko on DPT 4 was not collected. Three blood samples from two buffalo injected with standard ivermectin were below the lower limit of quantification (LLOQ) of 0.25 ng/ml at DPT 23 and/or 24. There was an error in quantifying the ivermectin concentrations from the cow treated with long-lasting ivermectin from Matakapore on DPT 2 and buffalo treated with long-lasting ivermectin from Waimakaha on DPT 11. Cattle injected with standard ivermectin (blue lines) had substantially higher blood ivermectin concentrations compared to buffalo injected with standard ivermectin (green lines). Similarly, cattle injected with long-lasting ivermectin (red lines) had substantially higher blood ivermectin concentrations compared to buffalo injected with long-lasting ivermectin (purple lines) (Fig. 2; Supplemental Table 1).
Fig. 2.
Depicts the ivermectin concentrations found in cattle (top panels) and buffalo (bottom panels) following injection of standard (left panels) and long-lasting (right panels) ivermectin formulations. Each line represents the ivermectin concentrations from an individual animal. The ivermectin pharmacokinetic concentrations of livestock from Pandawawi which were injected with slightly higher doses (1 ml more than manufacturer recommended dose) are represented as the darkest lines for each species and treatment group.
Ivermectin susceptibility results
One blood sample was not collected from the buffalo treated with long-lasting ivermectin and the control cow from Waikavaroko on DPT 4, therefore mosquito results were not included in the lethal concentration that kills 50% of mosquitoes (LC50) calculations. The buffalo treated with long-lasting ivermectin from Waimakaha was not available on DPT 63, therefore mosquito results from this day could not be included in the LC50 calculations. There was an error in quantifying the ivermectin concentration from the buffalo treated with long-lasting ivermectin from Waimakaha on DPT 11 post injection, therefore these mosquito results were not included in the LC50 calculations. Three blood samples from two buffalo (Matakapore, and Galukoloko/Waikavaroko) injected with standard ivermectin were below the LLOQ (0.25 ng/ml) and thus assigned values half the LLOQ (0.125 ng/ml), as is common practice in pharmacokinetic analyses.
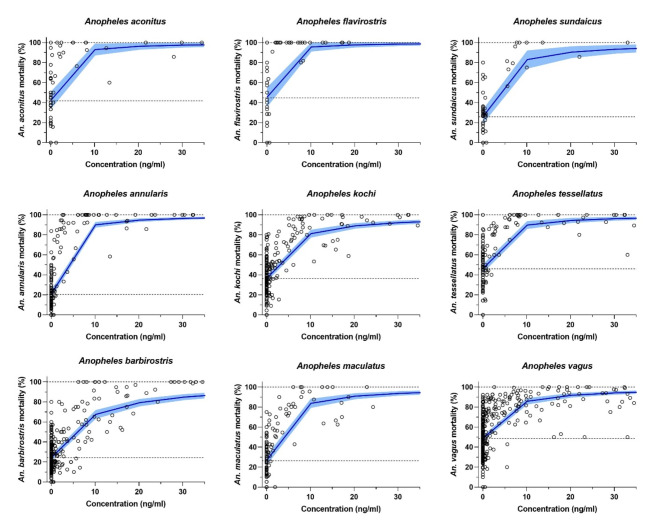
Mosquitoes collected from control animals showed > 20% mortality in the insectary between days 7 to 10 post capture, therefore survival data was analyzed at day 7 post capture instead of the full 10 days of observation. A total of 58,972 Anopheles specimens from 1,407 collection observation points (Table 1) were used to calculate ivermectin 7-day-LC50 values for 9 of the 12 species captured, including: An. aconitus, An. annularis, An. barbirostris, An. flavirostris, An. kochi, An. maculatus, An. sundaicus, An. tessellatus, and An. vagus. The LC50 results ranked the most ivermectin-susceptible to ivermectin-tolerant species as follows: An. flavirostris < An. aconitus < An. annularis < An. tessellatus < An. maculatus < An. sundaicus < An. vagus < An. kochi < An. barbirostris (Table 1; Fig. 4).
Table 1.
Presents the lethal concentration that kills 50% (LC50) of wild Anopheles spp. at seven days after a blood meal from cattle or buffalo treated with standard ivermectin, long-lasting ivermectin or untreated control. *Observations ≥ 5 represents the number of mosquito collection observation points with at least five specimens observed for mortality monitoring from a given animal on a single mosquito collection night. **Num. Mosquitoes is the total number of Anopheles specimens observed for mortality monitoring to calculate a given LC50 value for each species.
| Species | LC50 [95% CI] (ng/ml) | Observations ≥ 5* | Num. mosquitoes** |
|---|---|---|---|
| An. aconitus | 1.38 [0.56–3.41] | 55 | 608 |
| An. annularis | 1.45 [1.09–1.92] | 151 | 3,500 |
| An. barbirostris | 7.60 [6.11–9.51] | 265 | 9,951 |
| An. flavirostris | 0.89 [0.32–2.01] | 45 | 406 |
| An. kochi | 4.27 [3.26–5.58] | 223 | 9,377 |
| An. maculatus | 2.87 [2.02–4.10] | 109 | 2,604 |
| An. sundaicus | 3.00 [1.64–5.48] | 49 | 5,015 |
| An. tessellatus | 2.37 [1.57–3.58] | 138 | 3,945 |
| An. vagus | 3.86 [2.81–5.35] | 372 | 23,566 |
| Totals | 1,407 | 58,972 |
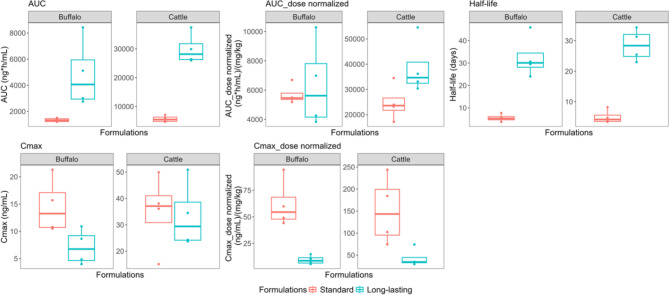
Fig. 3.
Depicts pharmacokinetic parameters of buffalo and cattle injected with standard (red box plots) and long-lasting (blue box plots) ivermectin. From left to right, the figure comprises area under the concentration-time curve (AUC), AUC_dose normalized, maximum concentration (Cmax), Cmax_dose normalized, half-life. AUC_dose normalized is dose normalized AUC (ng*h/mL)/(mg/kg). Cmax_dose normalized is dose normalized Cmax (ng/mL)/(mg/kg). Each panel presents box and whisker plots comparing each pharmacokinetic parameter between standard (orange) and long-lasting formulations (turquoise) within species, buffalo and cattle. Dots represent observed data.
Duration of ivermectin mosquito-lethal efficacy
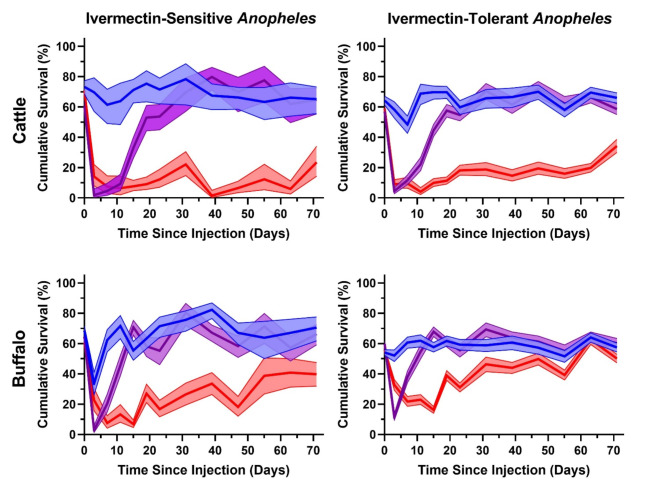
The nine Anopheles species collected in enough abundance for LC50 determination were analyzed for duration of mosquito-lethal effect of cattle and buffalo treated with standard and long-lasting ivermectin. A total of 52,452 Anopheles specimens from 359 animal collection nights were used to determine the duration of mosquito-lethal effect, including: An. aconitus (n = 843), An. annularis (n = 2,981), An. barbirostris (n = 8,153), An. flavirostris (n = 528), An. kochi (n = 8,386), An. maculatus (n = 2,015), An. sundaicus (n = 7,030), An. tessellatus (n = 3,543), and An. vagus (n = 18,973). All Anopheles specimens collected post-treatment from each animal species and treatment group were included in survival analyses including: cow control (n = 5,952), cow standard ivermectin (n = 6,949), cow long-lasting ivermectin (n = 6,848), buffalo control (n = 10,680), buffalo standard ivermectin (n = 10,550), and buffalo long-lasting ivermectin (n = 11,473). The buffalo treated with long-lasting ivermectin from Waimakaha was not available on DPT 63 for mosquito collection, therefore mosquito results from this day could not be included in the survival analyses. Due to too few Anopheles specimens captured for some species at each DPT for each individual cow or buffalo, the survival curve analyses were combined for all ivermectin-susceptible species with 7-day-LC50 values below 3 ng/ml (An. aconitus, An. annularis, An. flavirostris, An. maculatus, An. tessellatus) and ivermectin-tolerant species with 7-day-LC50 values equal to or above 3 ng/ml (An. barbirostris, An. kochi, An. sundaicus, An. vagus) (Table 1). Cattle treated with standard ivermectin were lethal to ivermectin-susceptible (7-day-LC50s < 3 ng/ml) Anopheles species through DPT 23/24 and lethal to ivermectin-tolerant (7-day-LC50s ≥ 3 ng/ml) Anopheles species through DPT 19/20. Cattle treated with long-lasting ivermectin were lethal to ivermectin-susceptible and ivermectin-tolerant Anopheles species through DPT 71/72 (Fig. 5; Supplemental Table 2). Buffalo treated with standard ivermectin were lethal to ivermectin-susceptible and ivermectin-tolerant Anopheles species through DPT 11/12. Buffalo treated with long-lasting ivermectin were lethal to ivermectin-susceptible Anopheles species through DPT 71/72 and ivermectin-tolerant Anopheles species through DPT 55/56 (Fig. 5; Supplemental Table 3).
Fig. 4.
Presents the mortality results of nine species of wild Anopheles when blood fed on cattle and buffalo treated with standard ivermectin, long-lasting ivermectin, or no ivermectin controls. Circles represent cumulative mosquito mortality at 7 days after blood meal ingestion from a single collection observation point. Solid blue lines represent the mean concentration-response relationship and the shaded area represents the 95% confidence interval associated with the nonlinear fit. Dashed black lines represent the fixed maximum effects of 100% mortality and the estimated minimum effect associated with mortality observed from control mosquitoes.
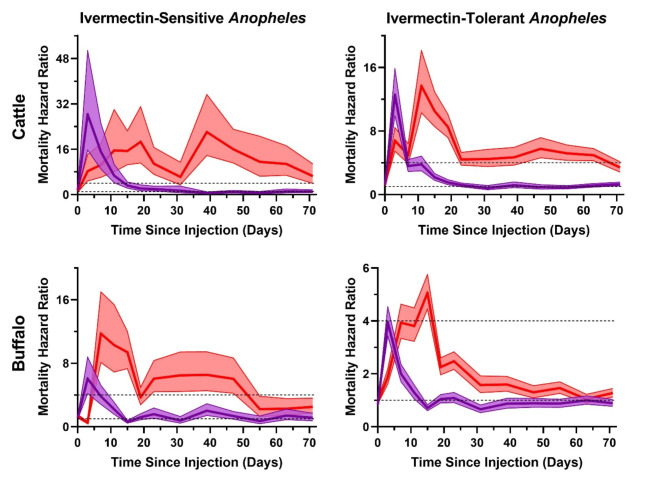
Mortality hazard ratios (Mantel-Haenszel) were greater than 4 for cattle treated with standard ivermectin for ivermectin-susceptible Anopheles through DPT 11/12, and ivermectin-tolerant Anopheles only at DPT 3/4. Mortality hazard ratios were greater than 4 for cattle treated with long-lasting ivermectin for ivermectin-susceptible Anopheles through DPT 71/72, and ivermectin-tolerant Anopheles through DPT 63/64. Mortality hazard ratios were greater than 4 for buffalo treated with standard ivermectin for ivermectin-susceptible Anopheles only at DPT 3/4, and never for ivermectin-tolerant Anopheles. Mortality hazard ratios were greater than 4 for buffalo treated with long-lasting ivermectin for ivermectin-susceptible Anopheles from DPT 7/8 to DPT 15/16 and DPT 23/24 to DPT 46/48, and ivermectin-tolerant Anopheles only at DPT 15/16 (Fig. 6).
Fig. 5.
presents the duration of mosquito-lethal mortality results of ivermectin-susceptible (An. aconitus, An. annularis, An. flavirostris, An. maculatus, An. tessellatus) (left column) and ivermectin-tolerant (An. barbirostris, An. kochi, An. sundaicus, An. vagus) (right column) Anopheles when blood fed on cattle (top row) or buffalo (bottom row) treated with standard ivermectin (purple lines), long-lasting ivermectin (red lines), or untreated controls (blue lines). All pre-dose collections are combined and depicted at day 0. Solid lines represent the mean cumulative mosquito mortality at 7 days after blood meal ingestion and the shaded area represents the 95% confidence interval.
Anopheles host choice assays
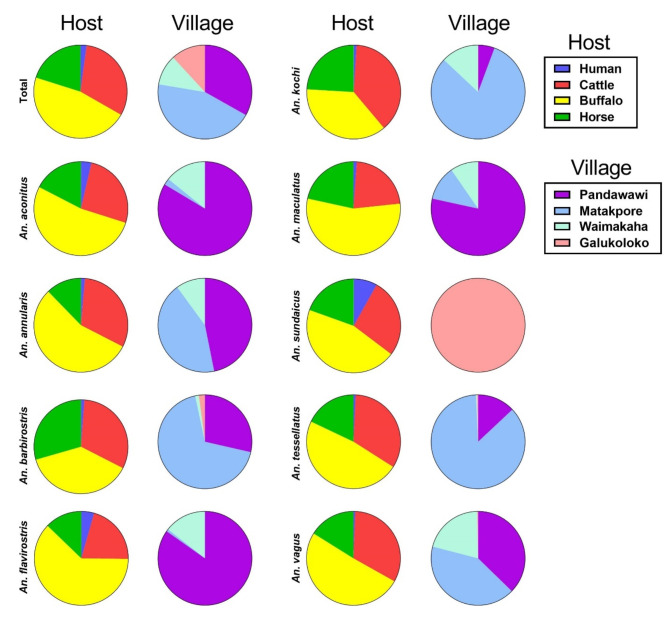
A total of 10,426 Anopheles specimens representing 12 different Anopheles species were captured from four different study sites for host choice evaluation including: An. vagus (n = 2,815), An. barbirostris (n = 1,820), An. kochi (n = 1,479), An. sundaicus (n = 1,184), An. aconitus (n = 1,137), An. tessellatus (n = 808), An. annularis (n = 587), An. maculatus (n = 343), An. flavirostris (n = 250), An. subpictus (n = 1), An. indefinitus (n = 1), and An. balabacensis (n = 1). The total number of Anopheles specimens captured per site was: Pandawawi (n = 3,458), Matakapore (n = 4,636), Waimakaha (n = 1,097), Galukoloko (n = 1,235) (Supplemental Fig. 2). The total number of Anopheles specimens captured per host was: human (n = 220), cattle (n = 3,249), buffalo (n = 4,859), horse (n = 2,098) (Supplemental Fig. 3). A two-way ANOVA revealed a significant interaction between host and study location (village) (F(9, 48) = 6.36, P < 0.0001). Simple main effects analysis showed that host (F(3, 48) = 114.70, P < 0.0001), and study location (F(3, 48) = 25.06, P < 0.0001) had a significant effect on total Anopheles captured and for each individual species (results not shown). For total Anopheles and An. annularis human caught significantly less mosquitoes compared to cattle, buffalo, and horse, while cattle and horse caught less than buffalo, and cattle caught more than horse. For An. barbirostris and An. maculatus human caught significantly less mosquitoes compared to cattle, buffalo, horse, while cattle and horse caught less than buffalo. For An. kochi, An. tessellatus and An. vagus human caught significantly less mosquitoes compared to cattle, buffalo, horse while buffalo caught more than horse. For An. sundaicus human caught significantly less mosquitoes compared to cattle, buffalo, and horse. For An. aconitus human caught significantly less mosquitoes compared to cattle, buffalo, while cattle and buffalo caught more than horse. For An. flavirostris human caught significantly less mosquitoes compared to buffalo, while buffalo caught more than horse (Fig. 7; Supplemental Table 4).
Fig. 6.
Presents the Mantel-Haenszel hazard ratios for the survival curve comparison of ivermectin-susceptible Anopheles (An. aconitus, An. annularis, An. flavirostris, An. maculatus, An. tessellatus) (left column) and ivermectin-tolerant Anopheles (An. barbirostris, An. kochi, An. sundaicus, An. vagus) (right column) when blood fed on cattle (top row) or buffalo (bottom row) treated with standard ivermectin (purple lines) or long-lasting ivermectin (red lines). Solid lines represent the mean hazard ratio for mortality at 7 days after blood meal ingestion and the shaded area represents the 95% confidence interval. The dashed lines indicate hazard ratios of 1 and 4, with 4 being the standard set by the WHO target product profile for new endectocides27.
Discussion
This study is the first evaluation of a commercially available long-lasting ivermectin against Anopheles mosquito survival and it demonstrates a clear superiority over a standard ivermectin formulation in both Southeast Asian cattle and buffalo (Fig. 5). This study is also the first to evaluate ivermectin pharmacokinetics in Southeast Asian cattle and buffalo, and the first pharmacokinetic evaluation of a long-lasting ivermectin formulation in buffalo (Figs. 2 and 3; Supplemental Table 1). This study is also the first evaluation of ivermectin susceptibility of Anopheles species in Indonesia, adding nine new Anopheles species to the list of species evaluated globally, including: An. aconitus, An. annularis, An. barbirostris, An. flavirostris, An. kochi, An. maculatus, An. sundaicus, An. tessellatus, and An. vagus (Fig. 4; Table 1). Finally, this study is the first to evaluate Anopheles host choice preference on Sumba Island, and in greater Nusa Tenggara Timor province (Fig. 7; Supplemental Table 4).
Fig. 7.
Illustrates the proportion from each host species and collection location that each Anopheles species was captured from.
The duration of mosquito-lethal effect of standard ivermectin in cattle lasted through DPT 23/24 for ivermectin-susceptible Anopheles and DPT 19/20 for ivermectin-tolerant Anopheles (Fig. 5), while mortality hazard ratios were greater than 4 for ivermectin-susceptible Anopheles through DPT 11/12, and ivermectin-tolerant Anopheles only at DPT 3/4 (Fig. 6). Buffalo ownership is common in Southeast Asia25and in some localities more likely than cattle ownership, thus it is critical to evaluate the mosquito-lethal effect of ivermectin in this regionally important livestock species. There is very limited pharmacokinetic evaluation of ivermectin in buffalo, restricted to one publication in European lactating buffalo26. It is clear from the results here that the pharmacokinetics (Figs. 2 and 3; Supplemental Table 1) and duration of mosquito-lethal effect of buffalo injected with standard ivermectin is inferior to that of cattle at the 200 µg/kg dose (Fig. 5; Supplemental Tables 2,3), and crosses the mortality hazard ratio above 4 only on DPT 3/4 for ivermectin-susceptible Anopheles (Fig. 6). Thus, buffalo may require a higher standard ivermectin dose compared to cattle to achieve a similar duration of mosquito-lethal effect. Since neither cattle nor buffalo treated with standard ivermectin met the WHO preferred products characteristics criteria for new endectocides of a > 4 mortality hazard ratio through 30 days post administration27, it may not be worthwhile to invest further in standard ivermectin formulations for malaria control purposes in Southeast Asia, and instead focus on long-lasting ivermectin formulations.
In cattle, the long-lasting ivermectin formulation was substantially superior compared to standard ivermectin, achieving mosquito-lethal effect for all Anopheles through DPT 71/72 (Fig. 5; Supplemental Table 2). It was an unfortunate limitation of this study that the duration of field evaluation was not carried out beyond DPT 72. In cattle treated with long-lasting ivermectin the mortality hazard ratio was greater than 4 through DPT 71/72 for ivermectin-susceptible Anopheles and through DPT 63/64 for ivermectin-tolerant Anopheles (Fig. 6). Thus, for cattle treated with long-lasting ivermectin, the desired efficacy target set by the WHO for new endectocides27 was well exceeded. When comparing cattle ivermectin pharmacokinetics of Ivergen®Platinum28 (Figs. 2 and 3; Supplemental Table 1), to two other commercially available long-lasting ivermectin formulations16, Ivergen® Platinum appears to be the superior option for use in malaria control.
Buffalo treated with the long-lasting ivermectin formulation provided a duration of mosquito-lethal effect through DPT 71/72 for ivermectin-susceptible Anopheles and through DPT 55/56 for ivermectin-tolerant Anopheles (Fig. 5; Supplemental Table 3), and the mortality hazard ratio with long-lasting ivermectin was greater than 4 for ivermectin-susceptible Anopheles from DPT 7/8 to 15/16 and DPT 23/24 to 46/48, and ivermectin-tolerant Anopheles only at DPT 15/16 (Fig. 6). Thus, in order to achieve the desired WHO efficacy target, it may be necessary to increase the dose of long-lasting ivermectin in buffalo. Ongoing pharmacokinetic-pharmacodynamic modeling of long-lasting ivermectin in buffalo can guide appropriate dosing strategies for further evaluation of mosquito-lethal efficacy.
Based on a non-compartmental analysis, for both buffalo and cattle, ivermectin half-lives of the long-lasting formulation were longer than those of the standard formulation, 30.1 days vs. 5.3 days in buffalo and 28.4 days vs. 4.2 days in cattle. This result corresponded to a substantially increased time above critical blood concentration for mosquito killing effect with the long-lasting ivermectin formulation. Additionally, dose-normalized peak concentrations showed substantially lower ivermectin concentrations in both buffalo and cattle (84% and 76%) associated with long-lasting formulation compared to standard formulation. Thus, the long-lasting formulation resulted in sustained mosquito-lethal effects and a reduced risk of acute adverse effects associated with high peak concentrations.
There are several advantages of using long-lasting ivermectin compared to standard ivermectin. First, the cost of the long-lasting ivermectin was substantially cheaper than standard ivermectin acquired in this study and has options to come in larger size volumes reducing bulk shipping costs. Second, the reduced number of long-lasting injections that would be administered over the malaria transmission season compared to standard ivermectin to achieve similar duration of effect means reduced amount of veterinarian and community labor time required. Third, the reduced number of visits and interruption to daily routines means community compliance should remain higher when implementing mass ITL with long-lasting ivermectin. The direct benefits of mass ITL to the livestock and their owners in terms of improved animal health and weight gains fulfills desirable targets for One Health and improves community well-being beyond malaria control alone.
Although application of veterinary standard ivermectin to livestock is widespread in Southeast Asia, there is a paucity of data of its effects on non-target organisms. These non-target effects of ivermectin could be exacerbated with the implementation of mass ITL with long-lasting formulations. Thus, if mass ITL for malaria control were performed in this region there should also be evaluation of the potential environmental impact. Potential for ivermectin resistance development in livestock helminths should be monitored as well, as resistance has been observed in many regions. However, it should be noted that mass ITL for malaria control should not be a decades long approach but used as a time limited tool to accelerate to malaria elimination during peak transmission season, thus reducing long-term exposure of the environment and livestock helminths to ivermectin for malaria control purposes. Another limitation of long-lasting ivermectin is the withdrawal time before slaughter of 120 days in cattle which was assigned by Argentinian regulators29, compared to the withdrawal time of 21–35 days for standard ivermectin in cattle which varies depending on country of registration (e.g. South Africa or United States)30,31. Further evaluation of ivermectin withdrawal times in Southeast Asian cattle and buffalo in the context of mass ITL for malaria control may be warranted. Extensive community engagement will be required to communicate this withdrawal time issue to livestock owners participating in mass ITL for malaria control. Currently, long-lasting ivermectin formulations are not registered in Southeast Asia, so international procurement, shipping, and customs processing costs should be considered as well.
The nine Anopheles species evaluated for ivermectin susceptibility here (Fig. 4; Table 1) are all new species to add to the global list1. Some species here are closely related to others in the region that have been previously evaluated, and they show similar patterns of ivermectin susceptibility. An. flavirostris and An. aconitus and are in the same Funestus Group as An. minimuss.s., which to date is the most ivermectin-susceptible species evaluated worldwide1–4,6, and similarly in these analyses An. flavirostris and An. aconitus were the most ivermectin-susceptible species. An. maculatus and An. sawadwongporni both belong to the Maculatus Group and both display moderate susceptibility to ivermectin2. An. barbirostris and An. campestris are in the same Barbirostris Group, while An. campestris displayed moderate ivermectin susceptibility in the laboratory2, the An. barbirostris evaluated here was the most ivermectin-tolerant species. An. sundaicus and An. epiroticus belong to the Sundaicus Complex, previously it was shown that An. epiroticus fed on cattle treated with standard ivermectin displayed substantial mortality through day 8 post-treatment5. Unfortunately, An. sundaicus was only caught in abundance from Galukoloko and Waikavaroko (Fig. 1) at DPT 39–40 and beyond, a point at which the cattle and buffalo provided standard ivermectin would have eliminated all blood-level ivermectin. It should be noted that these species comparisons previously reported were all assays performed with colonized mosquitoes evaluated in long-standing insectary environments, while the evaluations performed on Sumba were with wild-caught Anopheles of unknown age at time of capture then held in a makeshift field insectary environment. This explains the higher control baseline mosquito mortality observed when fed on cattle and buffalo pre-dose and control mosquito mortality when fed on untreated cattle and buffalo post-treatment (Fig. 4). Additionally, there was excessive mortality (~ 20%) observed in the insectary from days 7 to 10 post capture, even for mosquitoes fed on untreated animals, which is why the survival analyses were performed with survival observations through day 7 and not the full 10 days post capture. However, for mosquito survival curve analyses, it is important to note that only mosquitoes captured on the same night post-treatment from the three different treatment groups (control, standard ivermectin, long-lasting ivermectin) for both cattle and buffalo were compared, thus these mosquitoes were held in the same insectary conditions and were likely from similar emergence cohorts.
The Anopheles host choice experiment clearly establishes the attractiveness of buffalo being higher than the other livestock, followed by cattle, horse, and then human (Fig. 7; Supplemental Table 4). To our knowledge this is the first analysis of Anopheles host choice performed on Sumba Island and in the greater Nusa Tenggara Timor Province, adding useful information on the behavior of Anopheles in this region. There were some limitations to our host choice evaluation including: limited to four nights of sampling per location, needing to use two different collectors per location because one collector could not work for four consecutive nights, and not being able to adjust biomass across all hosts.
For security reasons to prevent theft, livestock on Sumba Island are typically kept close to the home at night time, sometimes even directly underneath the house. Thus, host-seeking Anopheles approaching a home may be diverted to feed on ivermectin-treated livestock before entering the home, making a strong case for the use of mass ITL for vector control on Sumba. However, in several villages on Sumba, livestock ownership was not observed, thus a mass ITL approach would deliver no malaria control benefit in these villages. Villages located closer to the ocean were less likely to have livestock, but they are the only villages afflicted with An. sundaicus, a species associated with brackish water32 and the most efficient malaria vector on Sumba Island. In this context, it is important to consider simultaneously performing ivermectin MDA to humans with mass ITL for malaria control to ensure effective delivery of the vector control intervention.
Horses are not treated with injectable ivermectin because of the risk of necrosis, secondary bacterial infection, and potentially death33. Since horses on Sumba have great cultural and economic significance, it would be risky to include these animals in mass ITL utilizing injectable ivermectin. Due to safety concerns, injectable ivermectin for horses was withdrawn from the market and replaced with oral paste, oral solutions, and pour-on ivermectin formulations. However, an oral paste and an oral solution did not establish sustained ivermectin blood-level concentrations compared to an injectable formulation34to merit substantial duration of mosquito-lethal effect limiting utility for mass ITL, and a pour-on formulation is even more inferior compared to oral formulation in horses35. In the host choice analysis conducted on Sumba, horses were the least efficient livestock for capturing Anopheles (Fig. 7; Supplemental Table 4). When these factors are considered together, the inclusion of horses in mass ITL for malaria control on Sumba Island or other regions where horses are prevalent may not be warranted.
Pigs are the predominant livestock on several Indonesian islands east of Sumba and the South Pacific, however, pigs may not be an ideal species for mass ITL. Pigs have a higher standard ivermectin dose (300 µg/kg) compared to cattle (200 µg/kg), and while both animals achieve comparable peak concentrations (Cmax) at this dose, pigs only reach half the total exposure of cattle36, likely limiting their duration of mosquito-lethal effect. Pigs administered two-fold the standard ivermectin dose (600 µg/kg) were only lethal to colonized An. farauti through DPT 157. Pigs administered two-fold the standard ivermectin dose (600 µg/kg) were no more lethal to colonized An. colluzzii compared to standard dose (300 µg/kg) (i.e. DPT 7), while three-fold standard dose (900 µg/kg) were mosquito-lethal through DPT 1417. Thus, in regions where pigs are the predominant livestock, it would be ideal to evaluate the duration of mosquito-lethal effect of long-lasting ivermectin in pigs.
Previous mathematical modeling indicates that coverage is a critical component for efficacy of human ivermectin MDA for malaria control37. The results presented here and summarized above clearly indicate that mosquito-lethal efficacy is driven by livestock species, ivermectin formulation applied, and individual Anopheles species ivermectin susceptibility. Additional efficacy components to consider are the availability of treatable hosts (e.g. humans and livestock) which varies on a village level scale, ivermectin formulations that are available for use in these hosts, and Anopheles species host choice which may vary between localities. This report illustrates the superior mosquito-lethal effect of long-lasting ivermectin compared to standard ivermectin in both cattle and buffalo, which warrants further evaluation of the long-lasting formulation in additional livestock species against important Anopheles species from other regions, and its potential use to reduce transmission for malaria control.
Methods
Field site
Southwest district of Sumba Island was selected based on Anopheles species biodiversity. Five sub-village study locations (i.e. Pandawawi, Matakapore, Waimakaha, Galukoloko, Waikavaroko) were selected based on prior knowledge of the Anopheles species composition23,24, livestock ownership, and ease of access during the rainy season.
Ivermectin susceptibility assays
In each study location, three adult Southeast Asian cattle and three adult buffalo were identified for inclusion in the study. In some cases, animals had to be imported for the duration of the trial (two cattle) or transported daily from a nearby sub-villages (five cattle) due to a lack of livestock in the immediate study area. A preliminary health check was performed by trained field veterinarians to assess animals with helminth infection by the modified McMaster fecal egg counting procedure or trypanosomiasis infection Giemsa stain microscopy. Animals with trypanosomiasis were treated with Tryponil® (Interchemie weken B.V. Metaalweg, Venray, Holand) and excluded from the study. One buffalo in Waimakaha was excluded due to trypanosome infection, while no animals were excluded due to high helminth burden. Three buffalo and three cattle were used simultaneously in two locations, Galukoloko and Waikavaroko, with mosquito collections utilizing the same animals on consecutive nights of exposure. This approach was done to maximize potential to capture An. sundaicus, a critical malaria vector in Indonesia, which is mainly present in the dry season on Sumba Island.
One cattle and one buffalo from each study location were assigned to serve as untreated controls, treated with standard ivermectin, or treated with long-lasting ivermectin (Supplemental Fig. 4). Standard ivermectin, Ivomec Classic® 1% (Boehringer Ingelheim Animal Health, Midrand, South Africa), was injected subcutaneously at 200 µg/kg (1 ml per 50 kg). Long-lasting ivermectin, Ivergen® Platinum 3.15% (Biogénesis Bagó, Buenos Aires, Argentina), was injected subcutaneously at 630 µg/kg (1 ml per 50 kg). An 18 g needle was required for injections due to the viscosity of the long-lasting ivermectin formulation. Initially the Cahaya Adil BFS-Alexa-1T livestock weight scale (PT. Alexindo Putra Mandiri, Jakarta, Indonesia) was not available at the time of ivermectin treatment for the first study site, Pandawawi, thus animal weight was estimated using the hearth girth circumference method. One week post-treatment all study animals in Pandawawi were weighed once the livestock weight scale was available in the field, and it was determined that animal weight was overestimated with the hearth girth circumference, with each animal receiving an extra one ml than was recommended for their body weight for both standard ivermectin and long-lasting ivermectin formulations. The remaining animals in the study were weighed prior to treatment and dosed according to manufacturer instruction.
The study animals were used to capture wild blood-fed Anopheles by placing the animals underneath net traps (approx. 3 m x 3 m x 2 m) (WxLxH). Six fixed locations were established for the net traps at each study site and net traps were spaced approximately 10 m apart. The location of the study animals were rotated amongst the six fixed positions on each mosquito collection night so as to not cause a location bias for mosquito trapping. The net traps were supported by bamboo poles and had two zippered entrances allowing for animals and mosquito collectors to enter and exit the net trap. Inside some of the net traps a steel cage was placed to keep particularly active livestock from moving around and disturbing the mosquitoes resting on the net trap walls or aggressive livestock from kicking the mosquito collectors. The cage was large enough (approx. 0.75 m x 1.5 m x 1.8 m) (WxLxH) that animals could still lay down, stand, and graze as desired. The net traps were tethered approximately 30 cm from the ground which allowed host-seeking mosquitoes to enter the trap (Supplemental Fig. 4). Mosquitoes would then fly into the trap and blood-feed on the livestock. Once blood-fed, the mosquitoes tended to rest on the sides of the net trap. Animals were placed inside the net traps from 18:00 until 06:00.
Three pre-dose mosquito collections up to nine days before treatment were performed in Pandawawi, Waimakaha, Galukoloko and Waikavaroko. In Matakapore four pre-dose mosquito collections were performed on days − 13 and − 11 and again at −4 and − 1 before treatment because the PI and field veterinarian contracted Dengue, delaying field collections for one week. Mosquito collections occurred at the following days post treatment (DPT) 3/4, 7/8, 11/12, 15/16, 19/20, 23/24, 31/32, 39/40, 46–48(46/48), 55/56, 63/64, 71/72. The reason for the DPT ranges were due to collection schedule shifts driven by overlapping study site collection schedules, holidays, and the Galukoloko/Waikavaroko host overlap requiring staggered nights of collection.
Blood-fed mosquitoes were collected from the six net traps every hour by two mosquito collectors. Blood-fed mosquitoes were lightly aspirated from the walls of the net trap (Supplemental Fig. 4) and placed into temporary field containers (0.2 L) cardboard drinking cups, sealed with mesh netting. In the field, mosquitoes were placed inside of Igloo coolers, which contained freezer packs, separated by a Styrofoam divider, and each mosquito container had a cotton ball lightly soaked with water to serve as a water source for the mosquitoes. Mosquitoes were transported back to the Field Insectary. Blood-fed Anopheles were gently transferred by mouth aspiration to clean cardboard containers (0.5 L), with a clean Whatman filter paper fixed to the bottom of the container. Containers were then sealed with mesh and fresh cotton balls were soaked in 10% sucrose solution which was changed daily. Mosquito mortality was observed daily for 10 days, dead mosquitoes were removed from the containers, identified morphologically, and recorded. Any mosquitoes alive at day 10 were frozen and counted as alive, identified morphologically, and recorded. Due to limited funds Anopheles could not be identified molecularly to species.
A 2 ml jugular venous blood sample was collected in EDTA tubes from each study cattle and buffalo after each mosquito collection night. Additional blood collections occurred at approximately 24, 48, 96 h post-treatment to characterize the peak blood ivermectin concentrations. The blood samples were transferred by pipette to 2 ml cryovials. The whole blood samples were transported in small coolers with ice packs back to a field station where they were maintained frozen at −20 °C until completion of the study. Cryovials were sorted by study site, animal, and date of collection and transferred into freezer boxes. The freezer boxes were then transported on freezer packs from Sumba Island to Yogyakarta via plane where they were stored frozen at −20 °C. Once shipment clearance from Indonesia occurred, then the blood samples were shipped on dry ice to Bangkok where they were stored at −80°C until they were processed. Ivermectin was quantified by liquid chromatography-mass spectrometry/mass spectrometry (LC-MS/MS) as described previously38. The ivermectin LLOQ was 0.25 ng/ml.
Ivermectin survival analyses
Pre-dose mosquito collections from all animals regardless of treatment and post-dose mosquito collections from all untreated control animals were included in the LC50 analyses. Anopheles collected from cattle or buffalo treated with standard ivermectin through DPT 24 were included in the LC50 calculations. Anopheles collected from cattle or buffalo treated with long-lasting ivermectin collected through DPT 72 were included in the LC50 calculations. A minimum cutoff of five Anopheles specimens per mosquito species per animal per treatment per collection timepoint needed to be captured in order for the data to be included in the ivermectin susceptibility analyses to calculate the ivermectin lethal concentration that kills 50% of mosquitoes (LC50). The LC50 was estimated using a normalized, unweighted, four-variable concentration-response analysis (IC50, Hill, EMIN, and EMAX). The Hill slope was set to 1 and maximum mosquito mortality (EMAX) that was assumed to reach 100% at infinite concentrations. The 95% confidence intervals (95% CI) around point estimates were derived using asymmetrical (asymptotic) approximation. An initial data analyses determined that minimum of 40 observation points were necessary for the dose-response model to converge.
To estimate the duration of mosquito-lethal effect, the Log-Rank survival curve analysis (Mantel-Cox method) was used to compare mosquito mortality within animal (cow or buffalo) for each treatment (standard ivermectin or long-lasting ivermectin) to the control group mosquito mortality for each timepoint post-treatment across all study sites. Mosquito mortality hazard ratios and 95% CI were calculated using the Mantel-Haenszel method. All mosquito survival analyses were performed with GraphPad Prism v.10.2 (GraphPad Software Inc, San Diego, CA, USA).
Host choice assay
Four locations were utilized for Anopheles mosquito host choice including: Pandawawi, Matakapore, Waimakaha, and Galukoloko. At each study site four hosts (human, cattle, buffalo, horse) were placed inside the same net traps as described above. All livestock were secured with the steel inner cages (Supplemental Fig. 4). Four fixed locations were established for the net traps at each study site and net traps were spaced approximately 10 m apart in a square pattern. The location of the study animals were rotated amongst the four fixed positions on each mosquito collection night so as to not cause a location bias for mosquito trapping. The position of the vertebrates were rotated every night over four consecutive nights in a Latin square design. Hosts were exposed to mosquitoes from 18:00 to 06:00. For mosquito human landing collections, one volunteer worked throughout the night, collecting mosquitoes for 50 min with a 10 min break each hour. All mosquitoes landing on the volunteer were collected via mouth aspirator and transferred to holding containers per hourly collection. Two mosquito human landing collectors were recruited per location based on their past experience with other projects24, and a brief refresher practice training was provided. The mosquito human landing collectors were rotated each night of collection so that no collector worked for two consecutive nights. For the animal-baited mosquito collections, mosquito collectors entered the livestock-baited net traps for 10 min and mouth aspirate all mosquitoes (blood-fed and un-fed, Anopheles and non-Anopheles) and placed the mosquitoes into one 0.2L container per hourly collection. Mosquitoes were transferred back to the field lab, frozen, and identified morphologically to the lowest taxonomical unit.
Mosquito abundance for each mosquito species for each vertebrate (i.e. cattle, buffalo, horse, human) were evaluated by two-way ANOVA with variables: host and study location. Mosquito density collection data were transformed to log10 (x + 1) before analysis. Log-transformed mean comparisons were made using the Tukey’s Post-hoc test.
Ethics declaration
The human study protocol was approved by the ethics committees of the Medical and Health Research Ethics Committee (MHREC), Faculty of Medicine, Public Health and Nursing, University of Gadjah Mada (KE/FK/0773/EC) and the Oxford University Tropical Research Ethics Committee (556 − 21). All experiments involving humans were performed in accordance with relevant guidelines and regulations. Each volunteer was provided with an explanation of the study and informed consent was obtained from all participants before study entry. The animal study protocol was approved by the Animal Care and Use Committee of the MHREC, Faculty of Medicine, Public Health and Nursing, University of Gadjah Mada (KE/FK/0773/EC) and the Indonesia National Research and Innovation Agency (BRIN) (023/KE.02/SK/8/2022). All authors confirm compliance with the ARRIVE guidelines. All experiments were performed in accordance with the relevant guidelines and regulations of the above listed institutions. Permission to work in the villages and site selection for the traps was given by community leaders.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We are grateful to the volunteers for mosquito human landing collections, livestock owners, the residents of Sumba for letting us perform the study. Ferdi Mori, Karola Ina Kue, and Felisia Delsi Bebon for assistance with community engagement. The Head and Support Staff of the Southwest Sumba Livestock and Animal Health Office. Entomologists from Balai Besar Penelitian dan Pengembangan Vektor dan Reservoir Penyakit (B2P2VRP) Salatiga. The resident entomologists from Sumba Island. Dr. Adrian Lifschitz for preliminary guidance in study design and veterinary ivermectin pharmacokinetics. Dr. Alejandro Kroweleicki for assistance obtaining the long-lasting ivermectin, Jorge Casalis and Juan Manuel Rodriguez for assistance with importation to Indonesia, and Biogénesis Bagó for donating the Ivergen® Platinum. Dr. Sirikachorn Tangawattana for assistance acquiring the standard ivermectin. The Joint Global Health Trial (MR/V004670/1) UK funders including the Department of Health and Social Care, the Foreign, Commonwealth & Development Office, the Medical Research Council, and Wellcome. The funders had no role in the design of the study and collection, analysis, and interpretation of data or writing the manuscript. This research was funded in part, by the Wellcome Trust [220211/Z/20/Z]. For the purpose of Open Access, the author has applied a CC BY public copyright license to any Author Accepted Manuscript version arising from this submission.
Author contributions
Designed study KCK, TBTS, WN, CB; Executed field study KCK, PW, YP, DT; Performed animal health checks YRN, PW, YP; Community Engagement DT, MC; Study logistics VAT, CB; Sample export VAT; Ivermectin concentration measurements JT; Pharmacokinetic assessment PA, JT; Data analyses KCK, JT; Prepared figures PA, KCK; Administrative oversight TBTS, WN, MC, KB, LvS, CB. Wrote first draft of manuscript KCK, JT. All authors read and approved the final manuscript.
Data availability
Data is available upon reasonable request to the authors. Requests can be sent to the corresponding author.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
The original online version of this Article was revised: The Acknowledgements section in the original version of this Article was incomplete. It now reads: "We are grateful to the volunteers for mosquito human landing collections, livestock owners, the residents of Sumba for letting us perform the study. Ferdi Mori, Karola Ina Kue, and Felisia Delsi Bebon for assistance with community engagement. The Head and Support Staff of the Southwest Sumba Livestock and Animal Health Office. Entomologists from Balai Besar Penelitian dan Pengembangan Vektor dan Reservoir Penyakit (B2P2VRP) Salatiga. The resident entomologists from Sumba Island. Dr. Adrian Lifschitz for preliminary guidance in study design and veterinary ivermectin pharmacokinetics. Dr. Alejandro Kroweleicki for assistance obtaining the long-lasting ivermectin, Jorge Casalis and Juan Manuel Rodriguez for assistance with importation to Indonesia, and Biogénesis Bagó for donating the Ivergen® Platinum. Dr. Sirikachorn Tangawattana for assistance acquiring the standard ivermectin. The Joint Global Health Trial (MR/V004670/1) UK funders including the Department of Health and Social Care, the Foreign, Commonwealth & Development Office, the Medical Research Council, and Wellcome. The funders had no role in the design of the study and collection, analysis, and interpretation of data or writing the manuscript. This research was funded in part, by the Wellcome Trust [220211/Z/20/Z]. For the purpose of Open Access, the author has applied a CC BY public copyright license to any Author Accepted Manuscript version arising from this submission.”
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
1/29/2025
A Correction to this paper has been published: 10.1038/s41598-025-87906-8
References
- 1.Billingsley, P. et al. A Roadmap for the development of Ivermectin as a complementary Malaria Vector Control Tool. Am. J. Trop. Med. Hyg.102 (2s), 3–24 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kobylinski, K. et al. Ivermectin susceptibility and sporontocidal effect in Greater Mekong Subregion Anopheles. Mal. J.16, e280 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kobylinski, K. et al. Safety, pharmacokinetics, and mosquito-lethal effects of ivermectin in combination with dihydroartemisinin-piperaquine and primaquine in healthy adult Thai subjects. Clin. Pharmacol. Ther.107 (5), 1221–1230 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kobylinski, K. et al. Ivermectin metabolites reduce Anopheles survival. Sci. Rep.13(1), e8131 (2023). [DOI] [PMC free article] [PubMed]
- 5.Cramer, E. et al. Ivermectin Treatment for Cattle Reduced the Survival of Two Malaria Vectors, Anopheles dirus and Anopheles Epiroticus, under Laboratory conditions in Central Vietnam. Am. J. Trop. Med. Hyg.104 (6), 2165–2168 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khemrattrakool, P. et al. Impact of ivermectin components on Anopheles dirus and Anopheles minimus mosquito survival. Parasit. Vectors. 17, e224 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pasay, C. et al. Treatment of pigs with endectocides as a complementary tool for combating malaria transmission by Anopheles farauti (s.s.) in Papua New Guinea. Parasit. Vectors. 12 (1), e124 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Foley, D., Bryan, J. & Lawrence, G. The potential of ivermectin to control the malaria vector Anopheles farauti. Trans. R. Soc. Trop. Med. Hyg.94 (6), 625–628 (2000). [DOI] [PubMed] [Google Scholar]
- 9.Kositz, C. et al. Incidental mosquitocidal effect of an ivermectin mass drug administration on Anopheles farauti conducted for scabies control in the Solomon Islands. Trans. R Soc. Trop. Med. Hyg.111, 97–101 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bockarie, M. et al. Mass treatment with ivermectin for filariasis control in Papua New Guinea: impact on mosquito survival. Med. Vet. Entomol.13, 120–123 (1999). [DOI] [PubMed] [Google Scholar]
- 11.Makhanthisa, T., Braack, L. & Lutermann, H. The effect of cattle-administered ivermectin and fipronil on the mortality and fecundity of Anopheles arabiensis Patton. Parasit. Vectors14(1), e349 (2021). [DOI] [PMC free article] [PubMed]
- 12.Naz, S., Maqbool, A., Ahmad, M., Ahmad, A. & Zaman, S. Efficacy of ivermectin for control of zoophilic malaria vectors in Pakistan. Pakistan J. Zool.45, 1585–1591 (2013). [Google Scholar]
- 13.Pooda, H. et al. Administration of ivermectin to peridomestic cattle: a promising approach to target the residual transmission of human malaria. Mal J.14, e496 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dreyer, S. et al. Fipronil and ivermectin treatment of cattle reduced the survival and ovarian development of field-collected Anopheles albimanus in a pilot trial conducted in northern Belize. Mal J.18(1), e296 (2019). [DOI] [PMC free article] [PubMed]
- 15.Fritz, M. et al. Toxicity of bloodmeals from ivermectin-treated cattle to Anopheles gambiae s.l. Ann. Trop. Med. Parasitol.103 (6), 539–547 (2009). [DOI] [PubMed] [Google Scholar]
- 16.Lifschitz, A. et al. Ivermectin (3.15%) long-acting formulations in cattle: absorption pattern and pharmacokinetic considerations. Vet. Parasitol.147 (3–4), 303–310 (2007). [DOI] [PubMed] [Google Scholar]
- 17.Pooda, S. et al. Impact of blood meals taken on ivermectin-treated livestock on survival and egg production of the malaria vector Anopheles coluzzii under laboratory conditions. PLoS One. 19 (8), e0308293 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davey, R., Pound, J., Miller, J. & Klavons, J. Therapeutic and persistent efficacy of a long-acting (LA) formulation of ivermectin against Rhipicephalus (Boophilus) microplus (Acari: Ixodidae) and sera concentration through time in treated cattle. Vet. Parasitol.169 (1–2), 149–156 (2010). [DOI] [PubMed] [Google Scholar]
- 19.Bridi, A., Carvalho, L., Cramer, L. & Barrick, R. Efficacy of a long-acting formulation of ivermectin against Psoroptes ovis (Hering, 1838) on cattle. Vet. Parasitol.97 (4), 277–283 (2001). [DOI] [PubMed] [Google Scholar]
- 20.Nava, S. et al. Relationship between pharmacokinetics of ivermectin (3.15%) and its efficacy to control the infestation with the tick Rhipicephalus (Boophilus) microplus in cattle. Vet. Parasitol.268, 81–86 (2019). [DOI] [PubMed] [Google Scholar]
- 21.Elyazar, I. et al. The distribution and bionomics of Anopheles malaria vector mosquitoes in Indonesia. Adv. Parasitol.83, 173–266 (2013). [DOI] [PubMed] [Google Scholar]
- 22.Syafruddin, D. et al. Seasonal prevalence of malaria in West Sumba district, Indonesia. Mal J.8, e8 (2009). [DOI] [PMC free article] [PubMed]
- 23.Barbara, K. et al. Survey of Anopheles mosquitoes (Diptera:Culicidae) in West Sumba District, Indonesia. Southeast Asian J. Trop. Med. Public Health. 42 (1), 71–82 (2011). [PubMed] [Google Scholar]
- 24.Syahrani, L. et al. An inventory of human night-biting mosquitoes and their bionomics in Sumba, Indonesia. PLoS Negl. Trop. Dis.16 (3), e0010316 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nanda, A. & Nakao, T. Role of buffalo in the socioeconomic development of rural Asia: current status and future prospectus. Anim. Sci. J.74, 443–455 (2003). [Google Scholar]
- 26.Anastasio, A. et al. Residue study of ivermectin in plasma, milk, and mozzarella cheese following subcutaneous administration to buffalo (Bubalus bubalis). J. Ag Food Chem.50 (18), 5241–5245 (2002). [DOI] [PubMed] [Google Scholar]
- 27.World Health Organization. Endectocides and ectocide products for malaria transmission control: Preferred product characteristics. Geneva, Switzerland. p. 6. (2022).
- 28.Errecalde, J. Pharmacokinetic of a Formulation Based on Ivermectin 3.15% Developed for Bago S (Universidad Nacional de La Plata, 2003). [Google Scholar]
- 29.Biogénesis Bagó, S. A. Ivergen®Platinum 3.15% Endectocide for Cattle Injectable Solution (Buenos Aires, 2005). [Google Scholar]
- 30.Boehringer Ingelheim Animal Health South Africa. Ivomec® Injection. South Africa. (2019).
- 31.Merial, L. T. D. Ivomec®1% Injection for Cattle and Swine. Duluth, GA, USA. (2011).
- 32.Nixon, C. P. et al. Distance to Anopheles sundaicus larval habitats dominant among risk factors for parasitemia in meso-endemic Southwest Sumba, Indonesia. Pathog Glob Health. 108 (8), 369–380 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anderson, R. The use of ivermectin in horses: Research and clinical observations. Kans. Ag Exp. Stn. J.6 (9), S516–S521 (1984). [Google Scholar]
- 34.Saumell, C. et al. The route of administration drastically affects ivermectin activity against small strongyles in horses. Vet. Parasitol.236, 62–67 (2017). [DOI] [PubMed] [Google Scholar]
- 35.Gokbulut, C. et al. Comparative plasma disposition, bioavailability and efficacy of ivermectin following oral and pour-on administrations in horses. Vet. Parasitol.170 (1–2), 120–126 (2010). [DOI] [PubMed] [Google Scholar]
- 36.Lifschitz, A. et al. Bioequivalence of ivermectin formulations in pigs and cattle. J. Vet. Pharmacol. Ther.22 (1), 27–34 (1999). [DOI] [PubMed] [Google Scholar]
- 37.Slater, H. et al. Ivermectin as a novel complementary malaria control tool to reduce incidence and prevalence: a modelling study. Lancet Infect. Dis.20 (4), 498–508 (2020). [DOI] [PubMed] [Google Scholar]
- 38.Kaewkhao, N., Hanpithakpong, W., Tarning, J. & Blessborn, D. Determination of ivermectin in plasma and whole blood using LC-MS/MS. Wellcome Open. Res.9, e231 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is available upon reasonable request to the authors. Requests can be sent to the corresponding author.