Abstract
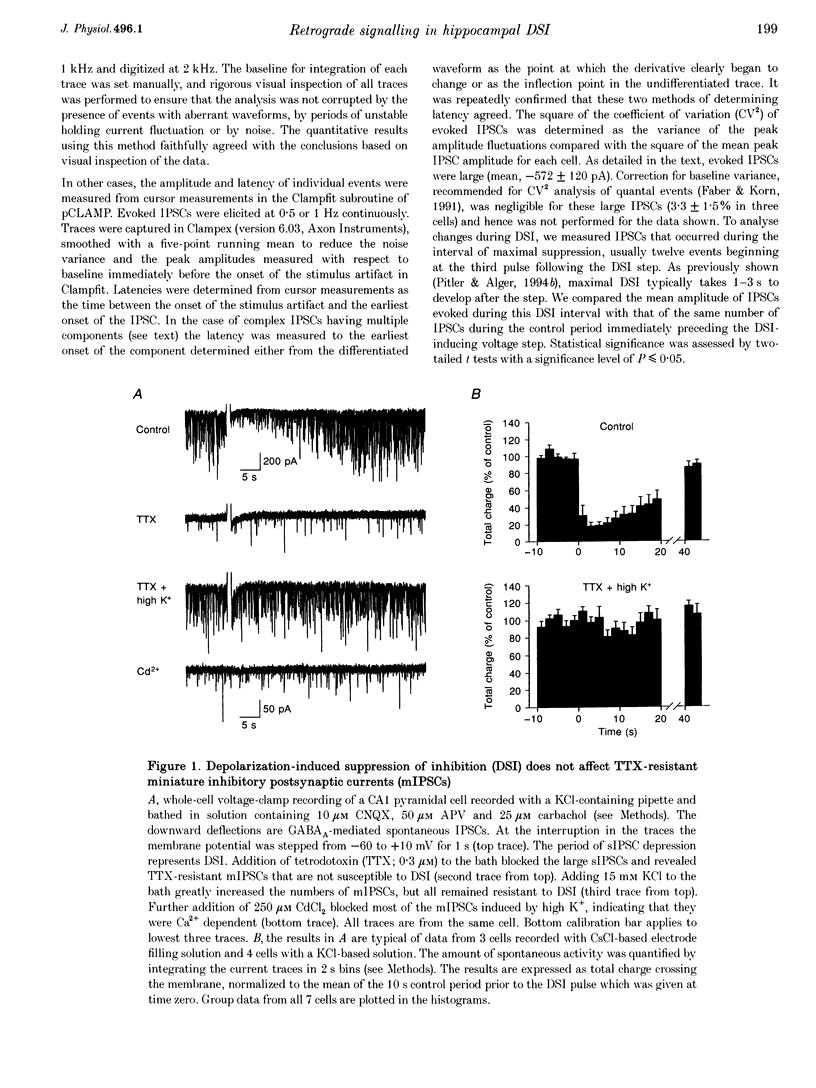
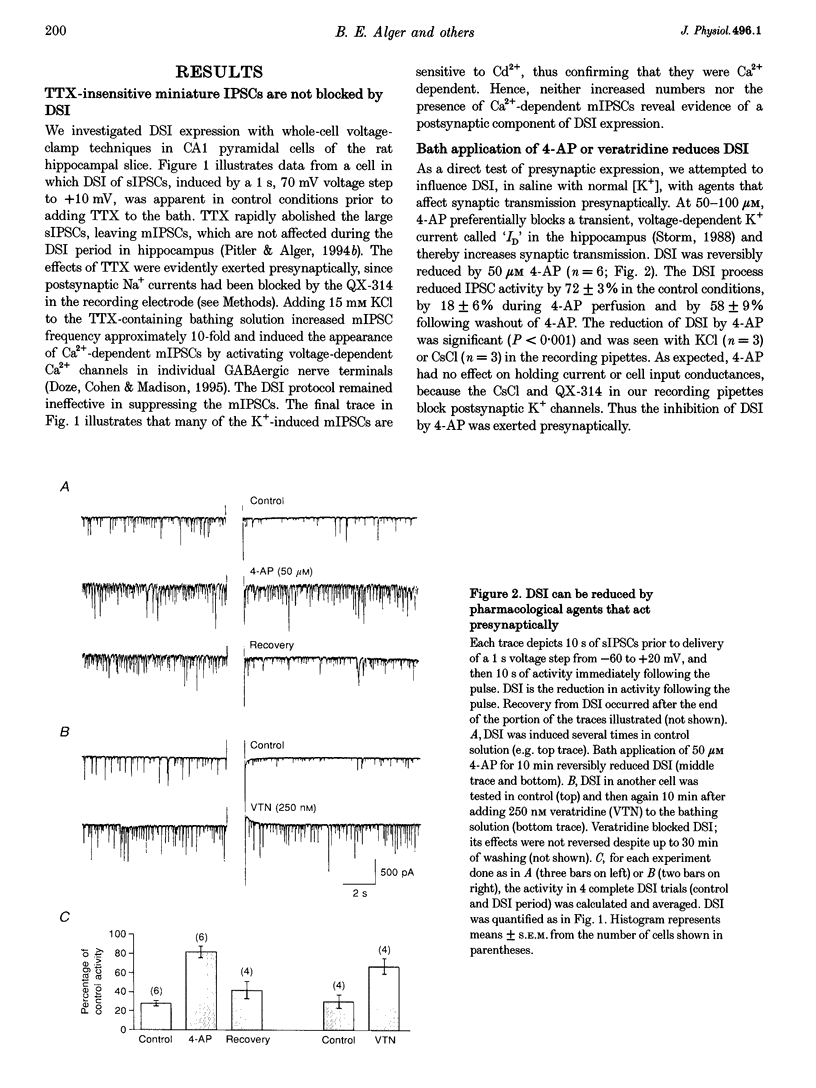
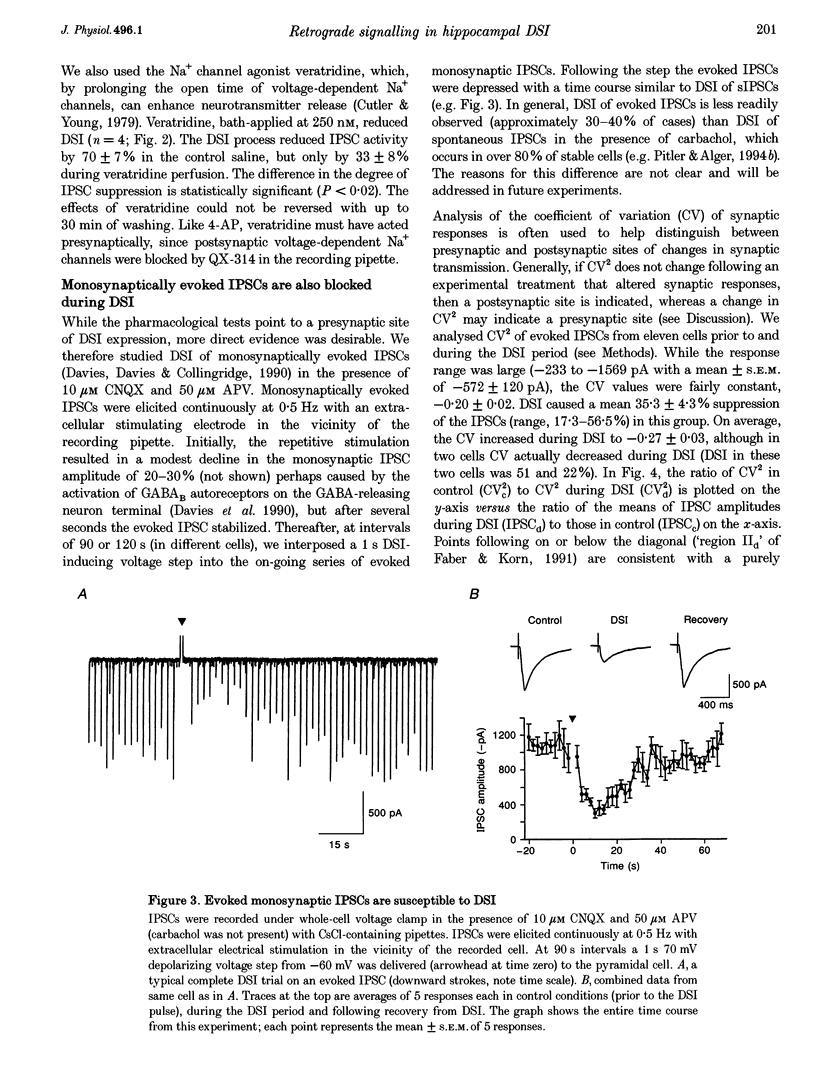
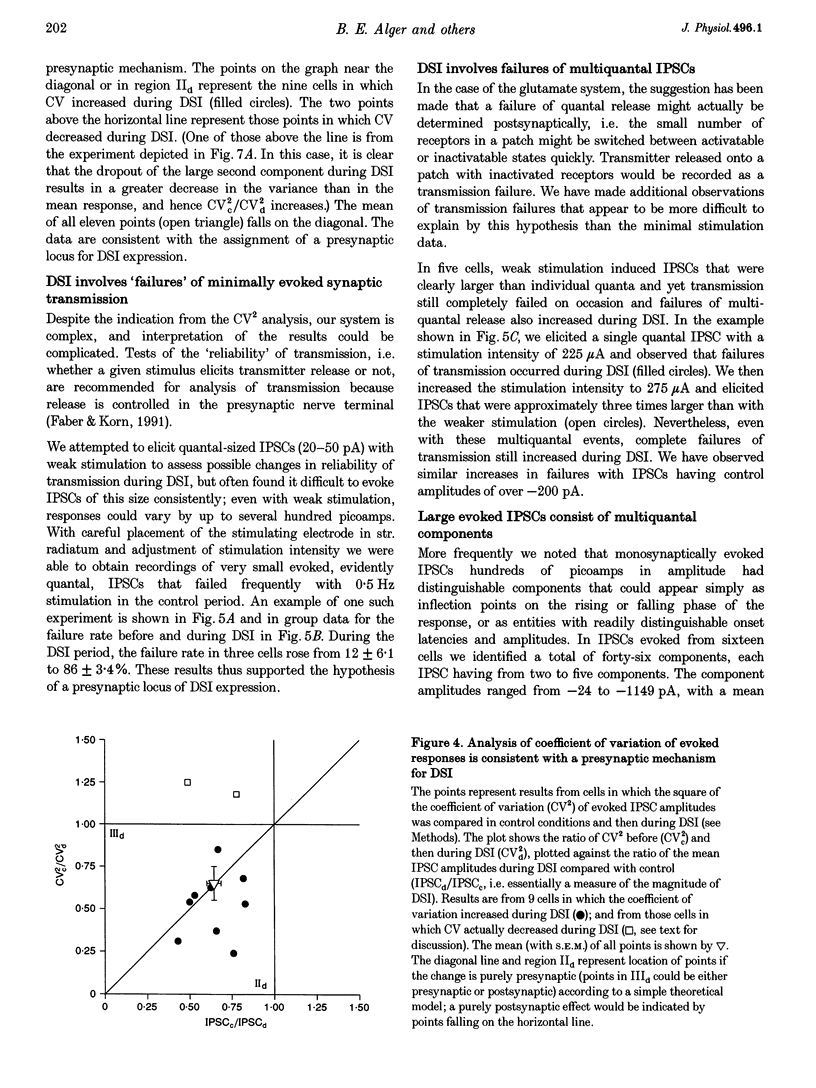
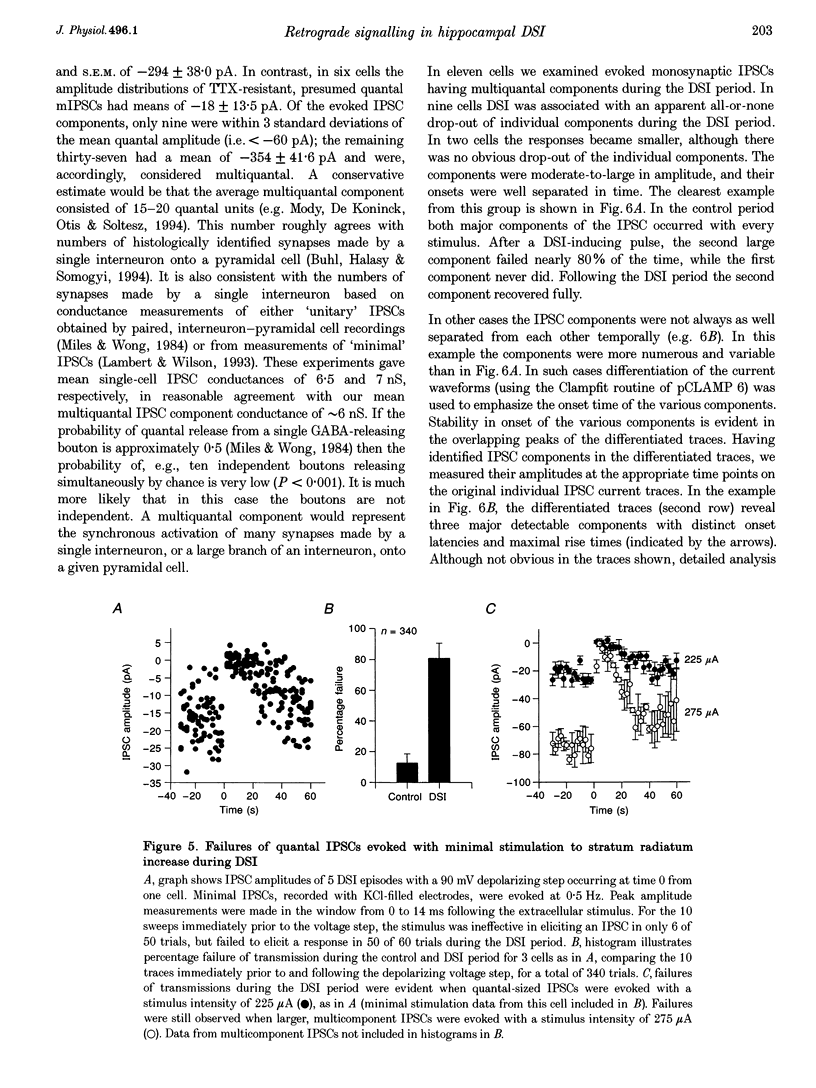
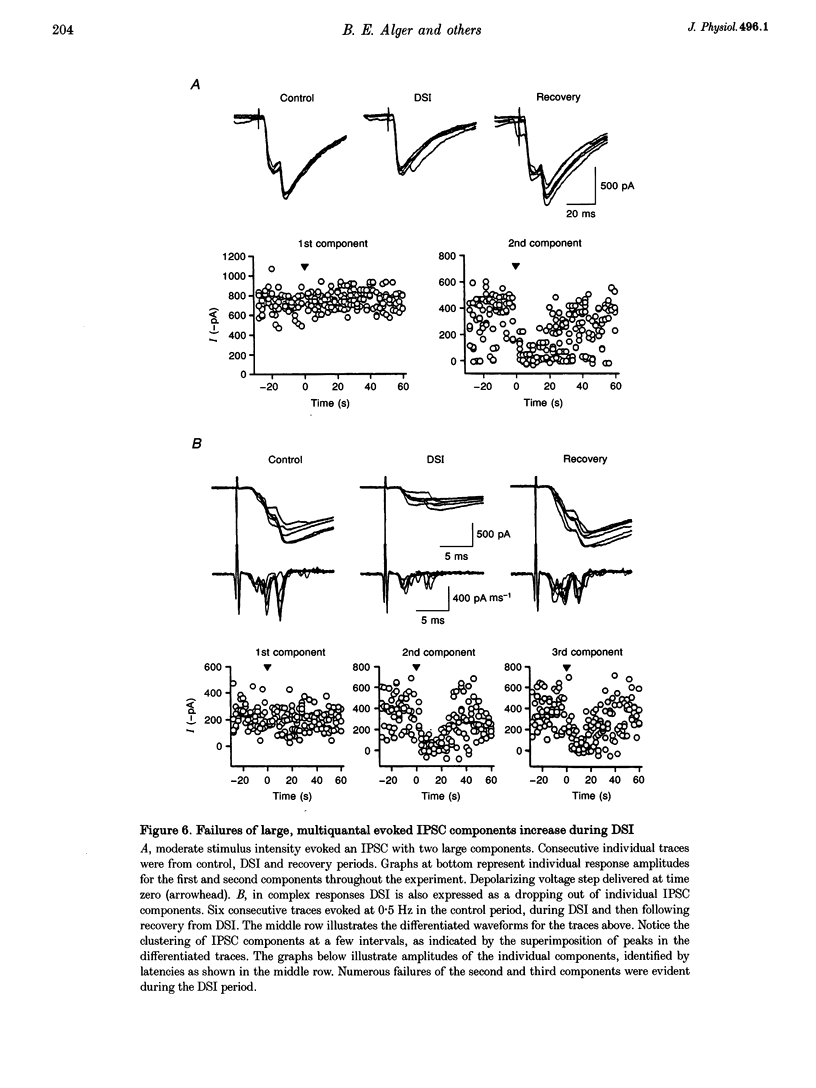
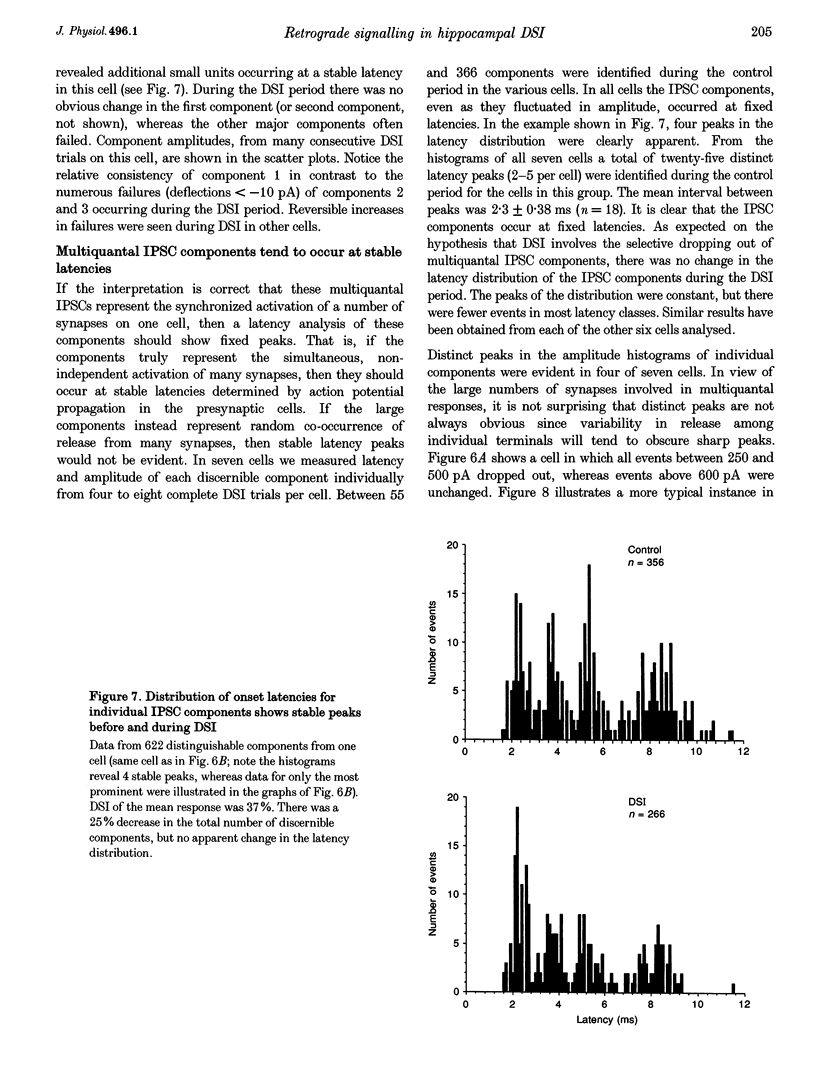
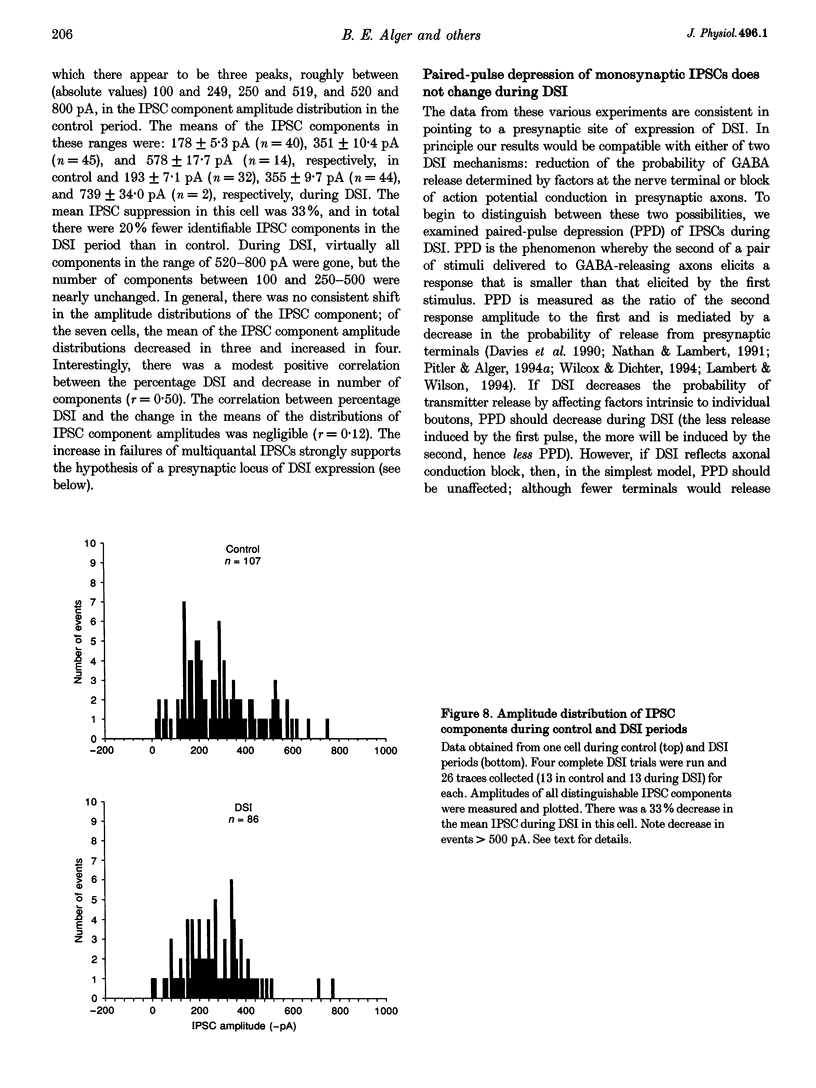
1. We have investigated the phenomenon of 'depolarization-induced suppression of inhibition' (DSI) using whole-cell voltage-clamp techniques in Ca1 pyramidal cells of rat hippocampal slices. DSI was induced by eliciting voltage-dependent calcium (Ca2+) currents with 1 s voltage steps of +60 to +90 mV from the holding potential. DSI was apparent as a reduction in synaptic GABAA responses for a period of about 1 min following the voltage step. 2. TTX-sensitive spontaneous IPSCs (sIPSCs) were susceptible to DSI, while TTX-resistant miniature inhibitory postsynaptic current (mIPSCs) were not. Miniature IPSCs are ordinarily infrequent and independent of external Ca2+ in the CA1 region. To increase the frequency of mIPSCs and to induce a population of Ca(2+)-sensitive mIPSCs, we increased the bath K+ concentration to 15 mM. The increased mIPSCs were also insensitive to DSI, however. 3. T whole-cell pipette-filling solution contained 5 mM 2(triethylamino-N-(2,6-dimethyl-phenyl)acetamide (QX-314) to block voltage-dependent Na+ currents and caesium to block K+ currents. Nevertheless, bath application of 50 microM 4-aminopyridine (4-AP) or 250 nM veratridine both clearly reduced DSI, evidently by acting at presynaptic sites. 4. The amplitudes of monosynaptically evoked IPSCs (elicited in the presence of 10 microM 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) and 50 microM 2-amino-5-phosphonovaleric acid (APV)) were dramatically reduced during the DSI period. Weak stimulation produced small IPSCs and occasional 'failures' of transmission during the control period. The percentage of failures increased markedly during the DSI period. Moderate-intensity stimulation produced larger IPSCs that were often composed of distinguishable multiquantal components. All-or-none failures of multiquantal IPSC components also occurred during DSI. 5. The degree of paired-pulse IPSC depression did not change during DSI, whereas it was decreased, as expected, by baclofen. 6. We conclude that the data represent novel evidence that DSI is mediated by a retrograde signalling process possibly involving presynaptic axonal conduction block.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alger B. E., Pitler T. A. Retrograde signaling at GABAA-receptor synapses in the mammalian CNS. Trends Neurosci. 1995 Aug;18(8):333–340. doi: 10.1016/0166-2236(95)93923-l. [DOI] [PubMed] [Google Scholar]
- Blanton M. G., Lo Turco J. J., Kriegstein A. R. Whole cell recording from neurons in slices of reptilian and mammalian cerebral cortex. J Neurosci Methods. 1989 Dec;30(3):203–210. doi: 10.1016/0165-0270(89)90131-3. [DOI] [PubMed] [Google Scholar]
- Bliss T. V., Collingridge G. L. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993 Jan 7;361(6407):31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Buhl E. H., Halasy K., Somogyi P. Diverse sources of hippocampal unitary inhibitory postsynaptic potentials and the number of synaptic release sites. Nature. 1994 Apr 28;368(6474):823–828. doi: 10.1038/368823a0. [DOI] [PubMed] [Google Scholar]
- Chen Q. X., Stelzer A., Kay A. R., Wong R. K. GABAA receptor function is regulated by phosphorylation in acutely dissociated guinea-pig hippocampal neurones. J Physiol. 1990 Jan;420:207–221. doi: 10.1113/jphysiol.1990.sp017908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler R. W., Young J. The effect of penicillin on the release of gamma-aminobutyric acid from cerebral cortex slices. Brain Res. 1979 Jul 6;170(1):157–163. doi: 10.1016/0006-8993(79)90947-8. [DOI] [PubMed] [Google Scholar]
- Davies C. H., Davies S. N., Collingridge G. L. Paired-pulse depression of monosynaptic GABA-mediated inhibitory postsynaptic responses in rat hippocampus. J Physiol. 1990 May;424:513–531. doi: 10.1113/jphysiol.1990.sp018080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis G. W., Murphey R. K. Long-term regulation of short-term transmitter release properties: retrograde signaling and synaptic development. Trends Neurosci. 1994 Jan;17(1):9–13. doi: 10.1016/0166-2236(94)90028-0. [DOI] [PubMed] [Google Scholar]
- Doze V. A., Cohen G. A., Madison D. V. Calcium channel involvement in GABAB receptor-mediated inhibition of GABA release in area CA1 of the rat hippocampus. J Neurophysiol. 1995 Jul;74(1):43–53. doi: 10.1152/jn.1995.74.1.43. [DOI] [PubMed] [Google Scholar]
- Edwards F. A., Konnerth A., Sakmann B. Quantal analysis of inhibitory synaptic transmission in the dentate gyrus of rat hippocampal slices: a patch-clamp study. J Physiol. 1990 Nov;430:213–249. doi: 10.1113/jphysiol.1990.sp018289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards F. Neurobiology. LTP is a long term problem. Nature. 1991 Mar 28;350(6316):271–272. doi: 10.1038/350271a0. [DOI] [PubMed] [Google Scholar]
- Faber D. S., Korn H. Applicability of the coefficient of variation method for analyzing synaptic plasticity. Biophys J. 1991 Nov;60(5):1288–1294. doi: 10.1016/S0006-3495(91)82162-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geppert M., Goda Y., Hammer R. E., Li C., Rosahl T. W., Stevens C. F., Südhof T. C. Synaptotagmin I: a major Ca2+ sensor for transmitter release at a central synapse. Cell. 1994 Nov 18;79(4):717–727. doi: 10.1016/0092-8674(94)90556-8. [DOI] [PubMed] [Google Scholar]
- Gulyás A. I., Miles R., Sík A., Tóth K., Tamamaki N., Freund T. F. Hippocampal pyramidal cells excite inhibitory neurons through a single release site. Nature. 1993 Dec 16;366(6456):683–687. doi: 10.1038/366683a0. [DOI] [PubMed] [Google Scholar]
- Jessell T. M., Kandel E. R. Synaptic transmission: a bidirectional and self-modifiable form of cell-cell communication. Cell. 1993 Jan;72 (Suppl):1–30. doi: 10.1016/s0092-8674(05)80025-x. [DOI] [PubMed] [Google Scholar]
- Lambert N. A., Wilson W. A. Heterogeneity in presynaptic regulation of GABA release from hippocampal inhibitory neurons. Neuron. 1993 Dec;11(6):1057–1067. doi: 10.1016/0896-6273(93)90219-h. [DOI] [PubMed] [Google Scholar]
- Lambert N. A., Wilson W. A. Temporally distinct mechanisms of use-dependent depression at inhibitory synapses in the rat hippocampus in vitro. J Neurophysiol. 1994 Jul;72(1):121–130. doi: 10.1152/jn.1994.72.1.121. [DOI] [PubMed] [Google Scholar]
- Llano I., Leresche N., Marty A. Calcium entry increases the sensitivity of cerebellar Purkinje cells to applied GABA and decreases inhibitory synaptic currents. Neuron. 1991 Apr;6(4):565–574. doi: 10.1016/0896-6273(91)90059-9. [DOI] [PubMed] [Google Scholar]
- Manabe T., Nicoll R. A. Long-term potentiation: evidence against an increase in transmitter release probability in the CA1 region of the hippocampus. Science. 1994 Sep 23;265(5180):1888–1892. doi: 10.1126/science.7916483. [DOI] [PubMed] [Google Scholar]
- McNamara J. O. Cellular and molecular basis of epilepsy. J Neurosci. 1994 Jun;14(6):3413–3425. doi: 10.1523/JNEUROSCI.14-06-03413.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles R., Wong R. K. Unitary inhibitory synaptic potentials in the guinea-pig hippocampus in vitro. J Physiol. 1984 Nov;356:97–113. doi: 10.1113/jphysiol.1984.sp015455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mody I., De Koninck Y., Otis T. S., Soltesz I. Bridging the cleft at GABA synapses in the brain. Trends Neurosci. 1994 Dec;17(12):517–525. doi: 10.1016/0166-2236(94)90155-4. [DOI] [PubMed] [Google Scholar]
- Nathan T., Lambert J. D. Depression of the fast IPSP underlies paired-pulse facilitation in area CA1 of the rat hippocampus. J Neurophysiol. 1991 Nov;66(5):1704–1715. doi: 10.1152/jn.1991.66.5.1704. [DOI] [PubMed] [Google Scholar]
- Nicoll R. A., Alger B. E. A simple chamber for recording from submerged brain slices. J Neurosci Methods. 1981 Aug;4(2):153–156. doi: 10.1016/0165-0270(81)90049-2. [DOI] [PubMed] [Google Scholar]
- Pitler T. A., Alger B. E. Cholinergic excitation of GABAergic interneurons in the rat hippocampal slice. J Physiol. 1992 May;450:127–142. doi: 10.1113/jphysiol.1992.sp019119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitler T. A., Alger B. E. Depolarization-induced suppression of GABAergic inhibition in rat hippocampal pyramidal cells: G protein involvement in a presynaptic mechanism. Neuron. 1994 Dec;13(6):1447–1455. doi: 10.1016/0896-6273(94)90430-8. [DOI] [PubMed] [Google Scholar]
- Pitler T. A., Alger B. E. Differences between presynaptic and postsynaptic GABAB mechanisms in rat hippocampal pyramidal cells. J Neurophysiol. 1994 Nov;72(5):2317–2327. doi: 10.1152/jn.1994.72.5.2317. [DOI] [PubMed] [Google Scholar]
- Pitler T. A., Alger B. E. Postsynaptic spike firing reduces synaptic GABAA responses in hippocampal pyramidal cells. J Neurosci. 1992 Oct;12(10):4122–4132. doi: 10.1523/JNEUROSCI.12-10-04122.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sik A., Ylinen A., Penttonen M., Buzsáki G. Inhibitory CA1-CA3-hilar region feedback in the hippocampus. Science. 1994 Sep 16;265(5179):1722–1724. doi: 10.1126/science.8085161. [DOI] [PubMed] [Google Scholar]
- Soltesz I., Smetters D. K., Mody I. Tonic inhibition originates from synapses close to the soma. Neuron. 1995 Jun;14(6):1273–1283. doi: 10.1016/0896-6273(95)90274-0. [DOI] [PubMed] [Google Scholar]
- Stelzer A. GABAA receptors control the excitability of neuronal populations. Int Rev Neurobiol. 1992;33:195–287. doi: 10.1016/s0074-7742(08)60693-5. [DOI] [PubMed] [Google Scholar]
- Stelzer A., Simon G., Kovacs G., Rai R. Synaptic disinhibition during maintenance of long-term potentiation in the CA1 hippocampal subfield. Proc Natl Acad Sci U S A. 1994 Apr 12;91(8):3058–3062. doi: 10.1073/pnas.91.8.3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storm J. F. Temporal integration by a slowly inactivating K+ current in hippocampal neurons. Nature. 1988 Nov 24;336(6197):379–381. doi: 10.1038/336379a0. [DOI] [PubMed] [Google Scholar]
- Thompson S. M. Modulation of inhibitory synaptic transmission in the hippocampus. Prog Neurobiol. 1994 Apr;42(5):575–609. doi: 10.1016/0301-0082(94)90044-2. [DOI] [PubMed] [Google Scholar]
- Vincent P., Armstrong C. M., Marty A. Inhibitory synaptic currents in rat cerebellar Purkinje cells: modulation by postsynaptic depolarization. J Physiol. 1992 Oct;456:453–471. doi: 10.1113/jphysiol.1992.sp019346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent P., Marty A. Fluctuations of inhibitory postsynaptic currents in Purkinje cells from rat cerebellar slices. J Physiol. 1996 Jul 1;494(Pt 1):183–199. doi: 10.1113/jphysiol.1996.sp021484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent P., Marty A. Neighboring cerebellar Purkinje cells communicate via retrograde inhibition of common presynaptic interneurons. Neuron. 1993 Nov;11(5):885–893. doi: 10.1016/0896-6273(93)90118-b. [DOI] [PubMed] [Google Scholar]
- Wigström H., Gustafsson B. Facilitated induction of hippocampal long-lasting potentiation during blockade of inhibition. Nature. 1983 Feb 17;301(5901):603–604. doi: 10.1038/301603a0. [DOI] [PubMed] [Google Scholar]
- Wilcox K. S., Dichter M. A. Paired pulse depression in cultured hippocampal neurons is due to a presynaptic mechanism independent of GABAB autoreceptor activation. J Neurosci. 1994 Mar;14(3 Pt 2):1775–1788. doi: 10.1523/JNEUROSCI.14-03-01775.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]



