Abstract
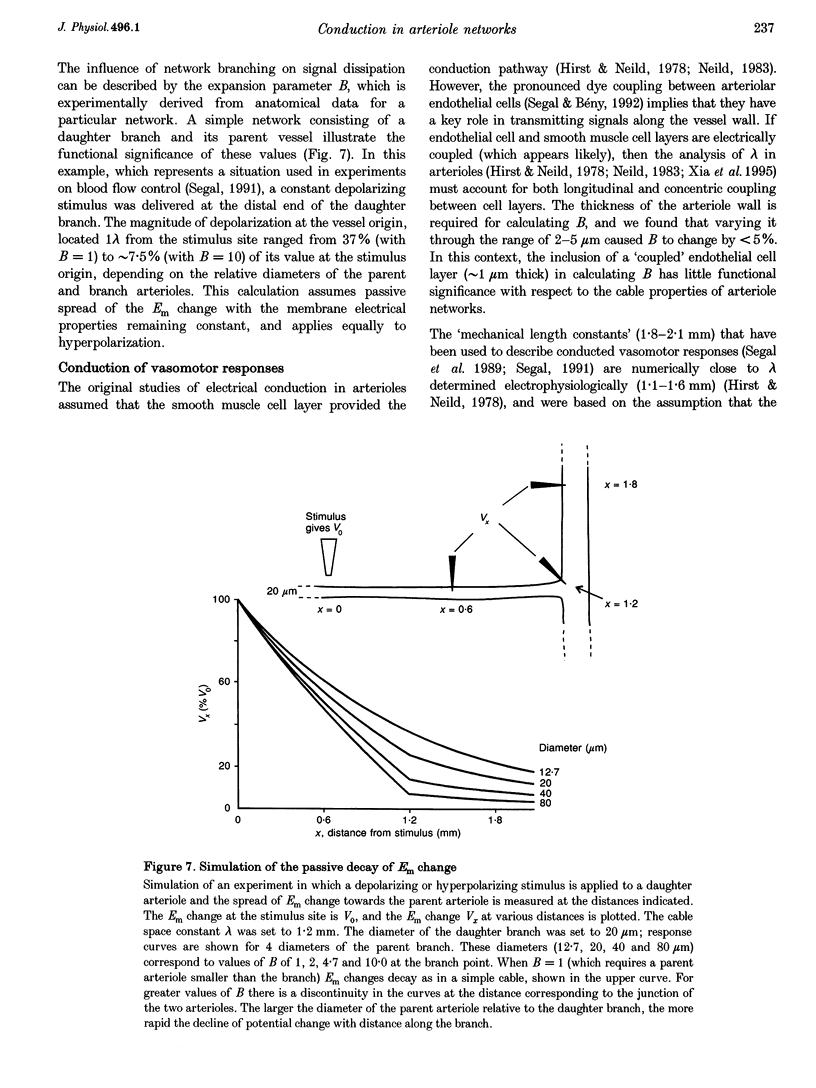
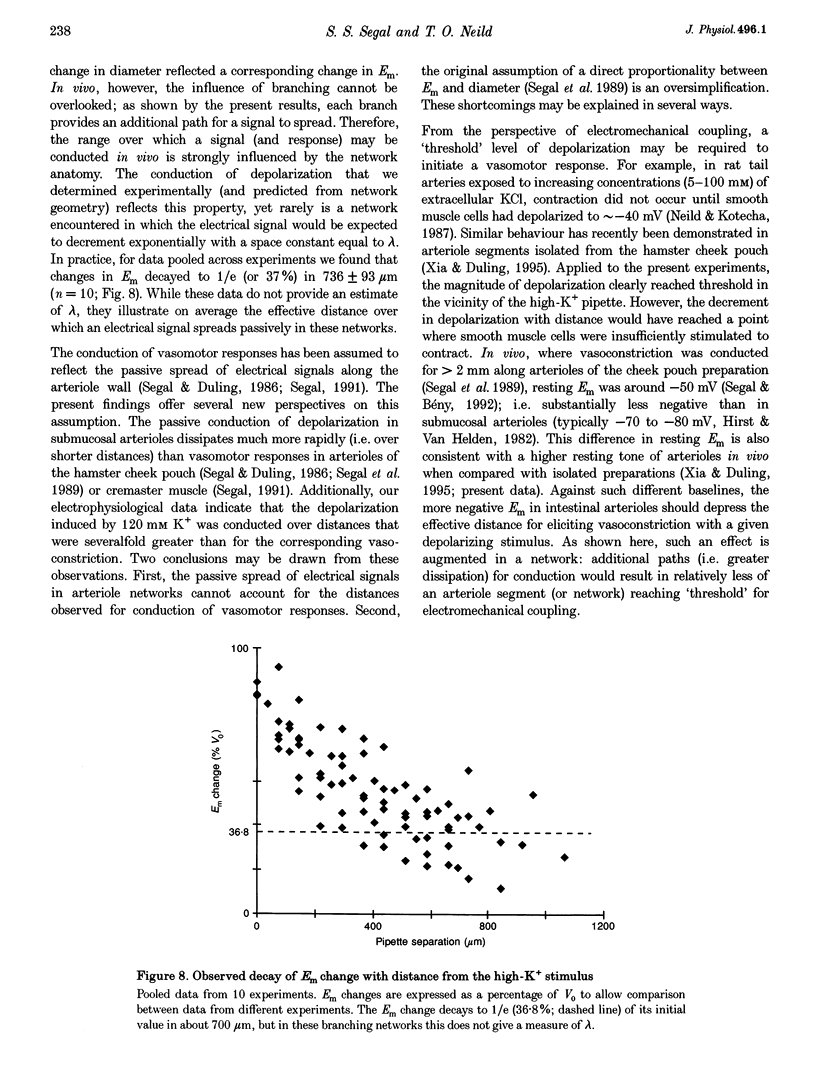
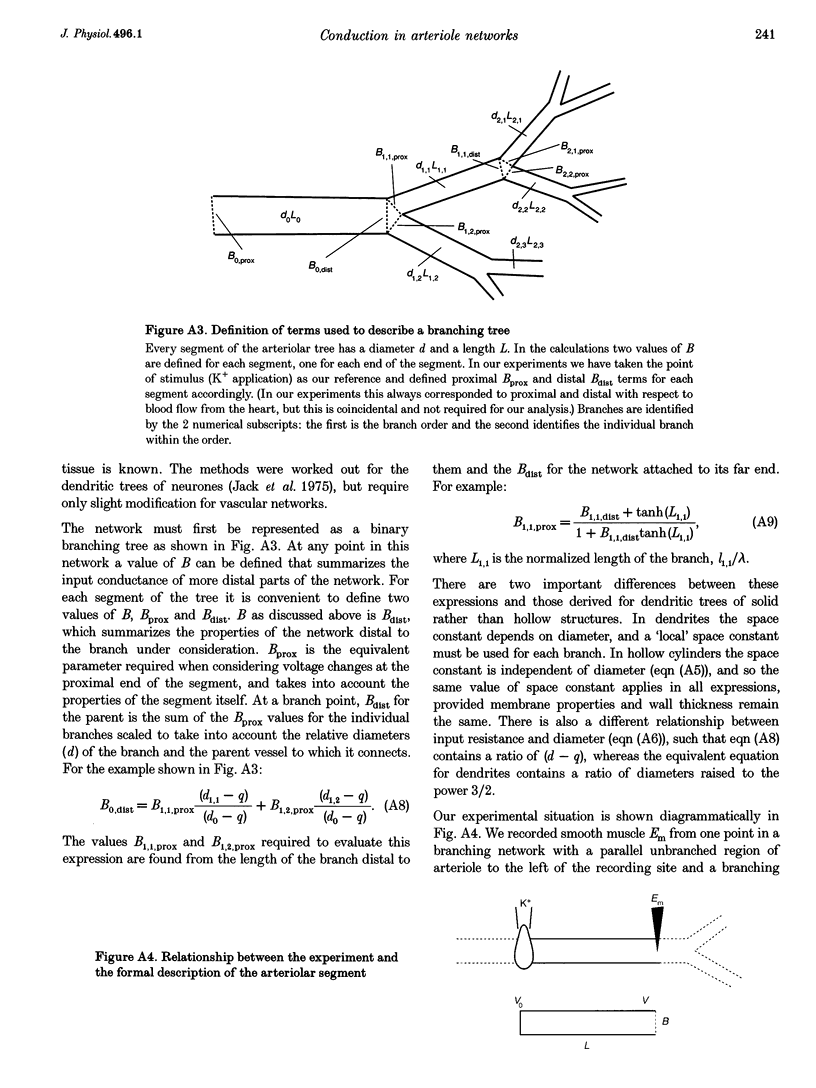
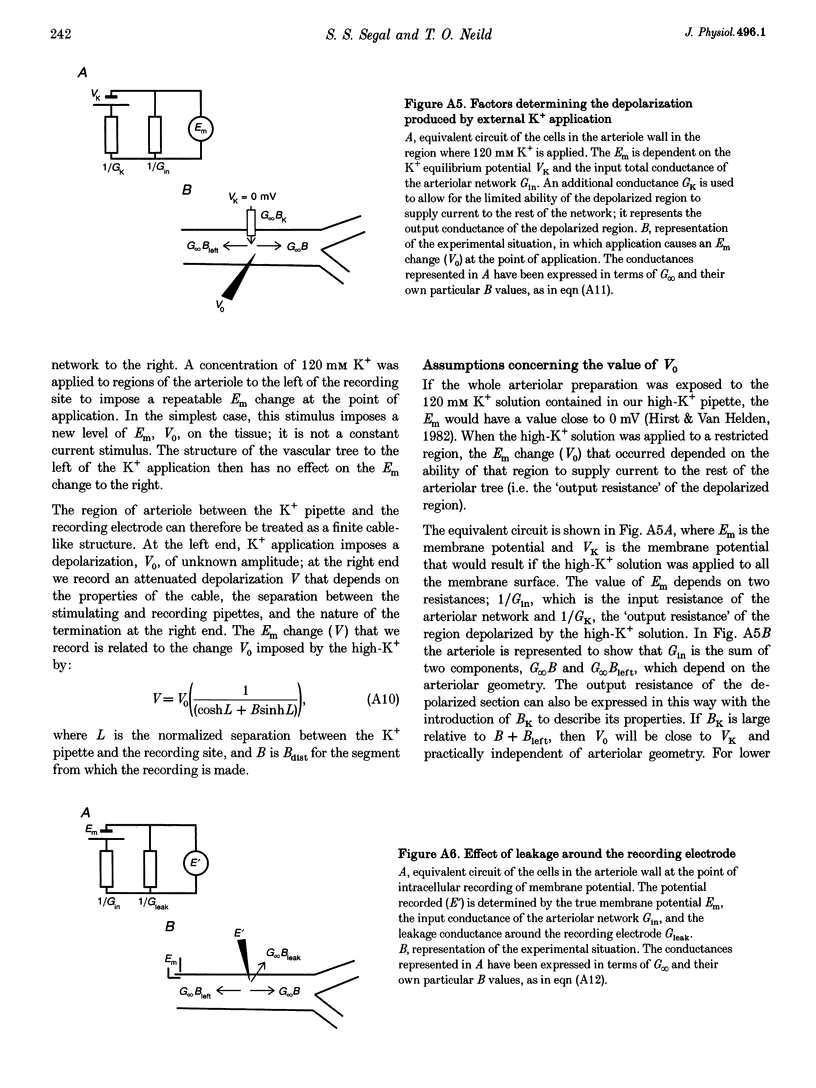
1. Blood flow control requires co-ordinated activity among many branches of arteriole networks, which may be achieved by conduction of membrane potential changes between arteriolar smooth muscle cells and endothelial cells. 2. We investigated the effect of branching upon the passive conduction of electrical signals through the syncytium of electrically coupled cells in arteriole networks (n = 12) prepared from the guinea-pig submucosa. To describe the effect of branching on cable properties, the expansion parameter B was calculated (B = 1 for an unbranched cable; B > 1 with branching) for a point in each arteriole network based on anatomy. 3. An estimate of B(B') was also obtained by measuring the spread of depolarization caused by a high-K+ stimulus applied to one region. Membrane potential (-74 +/- 4 mV (+/- S.D.) at rest) was recorded from smooth muscle cells (verified with intracellular dye labelling). A micropipette containing 120 mM KCl was positioned at 150 micron increments along an arteriole (width, 50-75 microns) up to approximately 1.2 mm from a stationary recording site, producing stable depolarization which decreased as separation distance increased. The dissipation of depolarization with separation was greater when recording near branch origins rather than continuous segments. 4. B ranged in value from 0.99 to 2.28. In any one experiment, values of B and B' were correlated (correlation coefficient, r = 0.71; P < 0.05), but B' was consistently greater than B, and we discuss methodological factors which could lead to erroneously high values for B'. 5. For pooled electrophysiological data, depolarization decayed to 37% (1/e) of initial values in approximately 700 microns, consistent with B > 1. In contrast, the conduction of vasoconstriction and vasodilatation exceeds 2 mm in arteriole networks in previous studies. To explain this discrepancy, we suggest that active electrical events in cells of the arteriole wall augment passive electrical conduction during blood flow control.
Full text
PDF


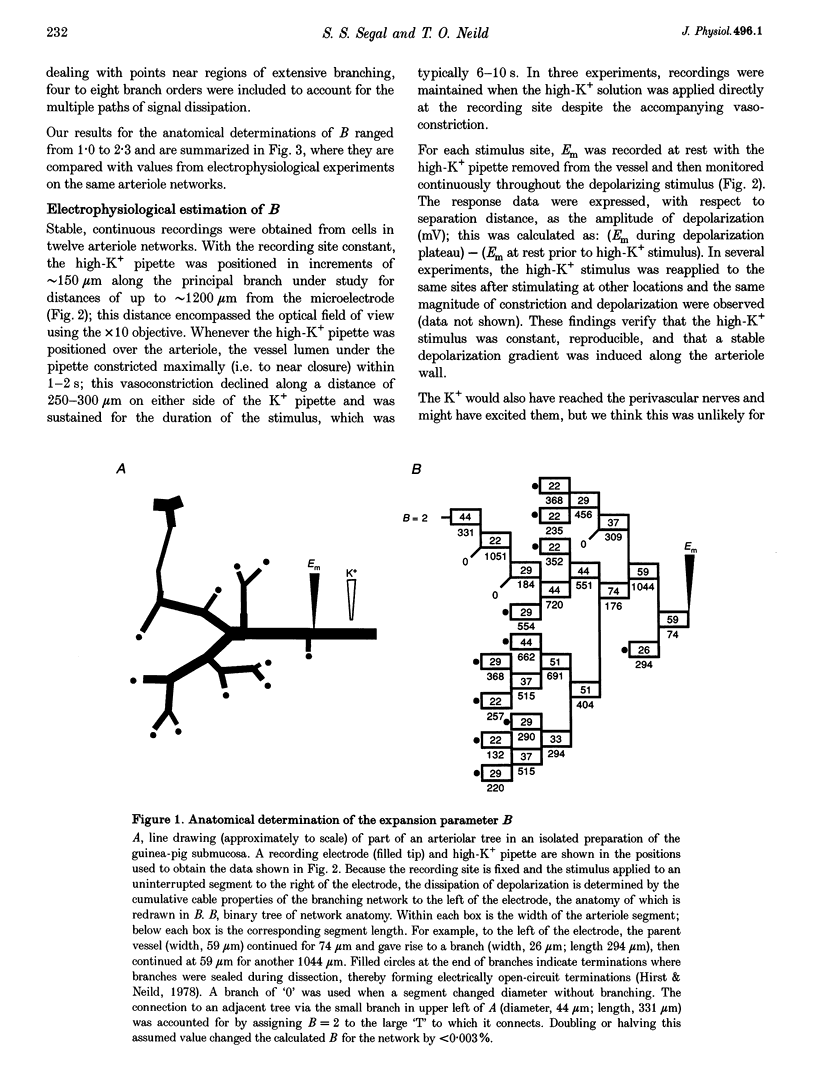
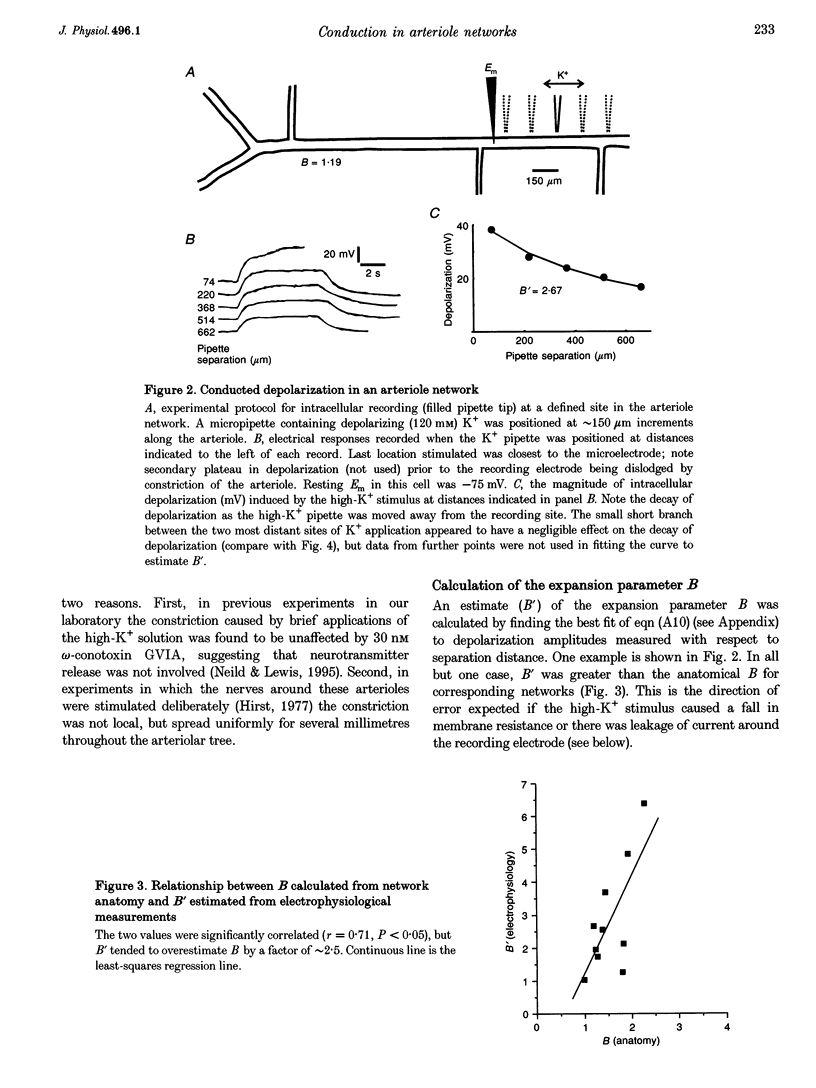
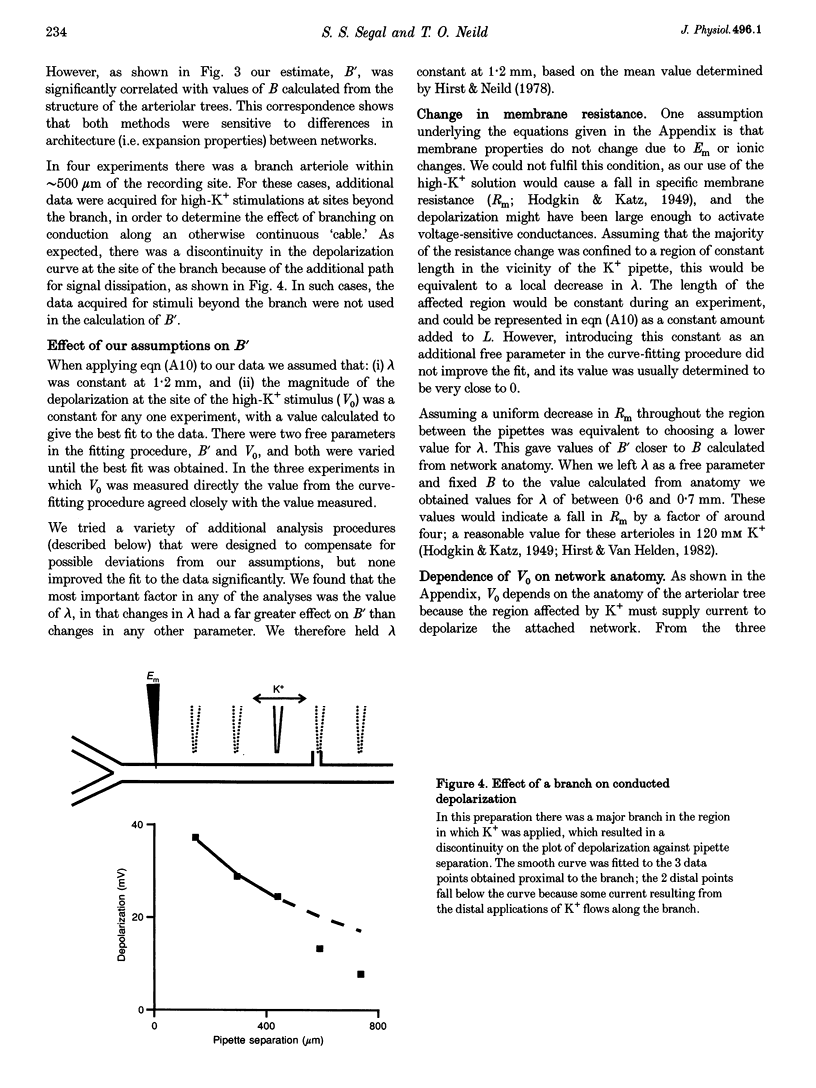

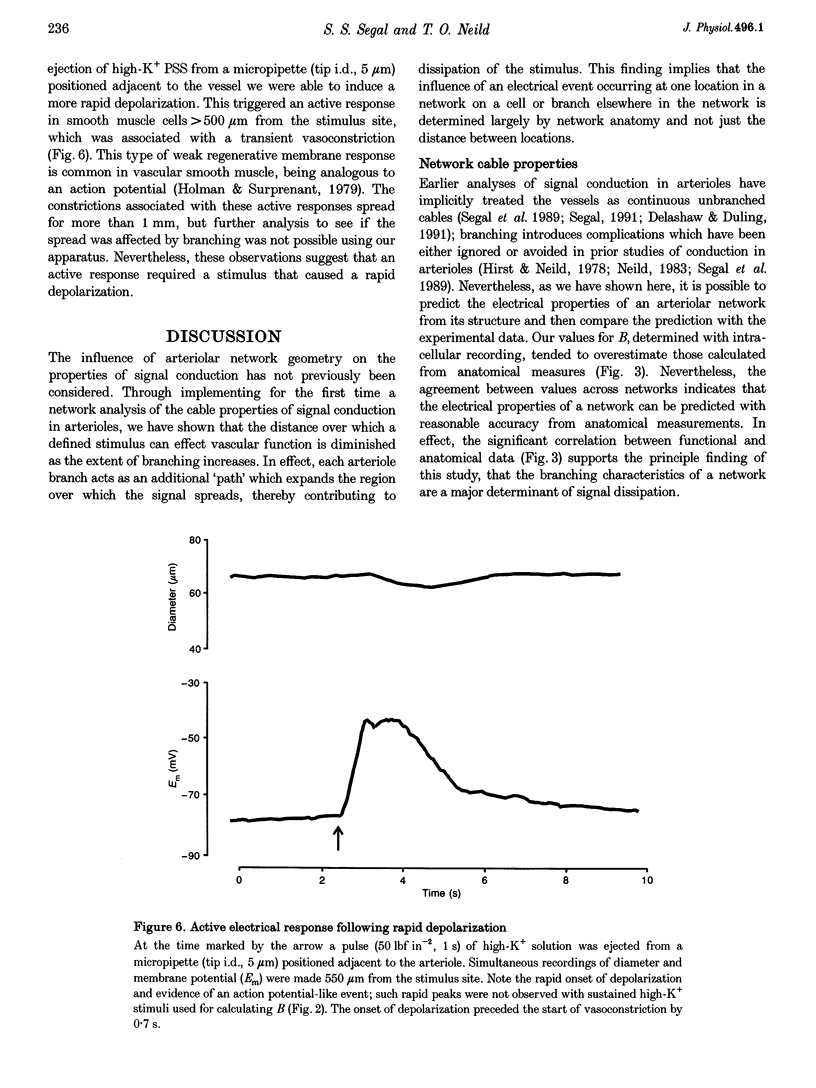




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bény J. L., Pacicca C. Bidirectional electrical communication between smooth muscle and endothelial cells in the pig coronary artery. Am J Physiol. 1994 Apr;266(4 Pt 2):H1465–H1472. doi: 10.1152/ajpheart.1994.266.4.H1465. [DOI] [PubMed] [Google Scholar]
- Chen G. F., Cheung D. W. Characterization of acetylcholine-induced membrane hyperpolarization in endothelial cells. Circ Res. 1992 Feb;70(2):257–263. doi: 10.1161/01.res.70.2.257. [DOI] [PubMed] [Google Scholar]
- Davis M. J., Donovitz J. A., Hood J. D. Stretch-activated single-channel and whole cell currents in vascular smooth muscle cells. Am J Physiol. 1992 Apr;262(4 Pt 1):C1083–C1088. doi: 10.1152/ajpcell.1992.262.4.C1083. [DOI] [PubMed] [Google Scholar]
- Delashaw J. B., Duling B. R. Heterogeneity in conducted arteriolar vasomotor response is agonist dependent. Am J Physiol. 1991 Apr;260(4 Pt 2):H1276–H1282. doi: 10.1152/ajpheart.1991.260.4.H1276. [DOI] [PubMed] [Google Scholar]
- Duling B. R., Berne R. M. Propagated vasodilation in the microcirculation of the hamster cheek pouch. Circ Res. 1970 Feb;26(2):163–170. doi: 10.1161/01.res.26.2.163. [DOI] [PubMed] [Google Scholar]
- GEORGE E. P., JOHNSON E. A. Solutions of the Hodgkin-Huxley equations for squid axon treated with tetraethylammonium and in potassium-rich media. Aust J Exp Biol Med Sci. 1961 Jun;39:275–293. doi: 10.1038/icb.1961.28. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., KATZ B. The effect of sodium ions on the electrical activity of giant axon of the squid. J Physiol. 1949 Mar 1;108(1):37–77. doi: 10.1113/jphysiol.1949.sp004310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst G. D., Edwards F. R. Sympathetic neuroeffector transmission in arteries and arterioles. Physiol Rev. 1989 Apr;69(2):546–604. doi: 10.1152/physrev.1989.69.2.546. [DOI] [PubMed] [Google Scholar]
- Hirst G. D., Neild T. O. An analysis of excitatory junctional potentials recorded from arterioles. J Physiol. 1978 Jul;280:87–104. doi: 10.1113/jphysiol.1978.sp012374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst G. D. Neuromuscular transmission in arterioles of guinea-pig submucosa. J Physiol. 1977 Dec;273(1):263–275. doi: 10.1113/jphysiol.1977.sp012093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst G. D., van Helden D. F. Ionic basis of the resting potential of submucosal arterioles in the ileum of the guinea-pig. J Physiol. 1982 Dec;333:53–67. doi: 10.1113/jphysiol.1982.sp014438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holman M. E., Surprenant A. M. Some properties of the excitatory junction potentials recorded from saphenous arteries of rabbits. J Physiol. 1979 Feb;287:337–351. doi: 10.1113/jphysiol.1979.sp012663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua C., Cragg B. Measurements of smooth muscle cells in arterioles of guinea pig ileum. Acta Anat (Basel) 1980;107(2):224–230. doi: 10.1159/000145246. [DOI] [PubMed] [Google Scholar]
- Krogh A., Harrop G. A., Rehberg P. B. Studies on the physiology of capillaries: III. The innervation of the blood vessels in the hind legs of the frog. J Physiol. 1922 May 16;56(3-4):179–189. doi: 10.1113/jphysiol.1922.sp002000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurjiaka D. T., Segal S. S. Conducted vasodilation elevates flow in arteriole networks of hamster striated muscle. Am J Physiol. 1995 Nov;269(5 Pt 2):H1723–H1728. doi: 10.1152/ajpheart.1995.269.5.H1723. [DOI] [PubMed] [Google Scholar]
- Lin Y., Duling B. R. Vulnerability of conducted vasomotor response to ischemia. Am J Physiol. 1994 Dec;267(6 Pt 2):H2363–H2370. doi: 10.1152/ajpheart.1994.267.6.H2363. [DOI] [PubMed] [Google Scholar]
- Little T. L., Xia J., Duling B. R. Dye tracers define differential endothelial and smooth muscle coupling patterns within the arteriolar wall. Circ Res. 1995 Mar;76(3):498–504. doi: 10.1161/01.res.76.3.498. [DOI] [PubMed] [Google Scholar]
- Morgan K. G. Electrophysiological differentiation of alpha-receptors on arteriolar smooth muscle. Am J Physiol. 1983 Apr;244(4):H540–H545. doi: 10.1152/ajpheart.1983.244.4.H540. [DOI] [PubMed] [Google Scholar]
- Neild T. O., Kotecha N. Relation between membrane potential and contractile force in smooth muscle of the rat tail artery during stimulation by norepinephrine, 5-hydroxytryptamine, and potassium. Circ Res. 1987 May;60(5):791–795. doi: 10.1161/01.res.60.5.791. [DOI] [PubMed] [Google Scholar]
- Neild T. O., Lewis C. J. Reduction of vasoconstriction mediated by neuropeptide Y Y2 receptors in arterioles of the guinea-pig small intestine. Br J Pharmacol. 1995 May;115(2):220–221. doi: 10.1111/j.1476-5381.1995.tb15865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neild T. O. The relation between the structure and innervation of small arteries and arterioles and the smooth muscle membrane potential changes expected at different levels of sympathetic nerve activity. Proc R Soc Lond B Biol Sci. 1983 Dec 22;220(1219):237–249. doi: 10.1098/rspb.1983.0097. [DOI] [PubMed] [Google Scholar]
- Olesen S. P., Clapham D. E., Davies P. F. Haemodynamic shear stress activates a K+ current in vascular endothelial cells. Nature. 1988 Jan 14;331(6152):168–170. doi: 10.1038/331168a0. [DOI] [PubMed] [Google Scholar]
- Olesen S. P., Davies P. F., Clapham D. E. Muscarinic-activated K+ current in bovine aortic endothelial cells. Circ Res. 1988 Jun;62(6):1059–1064. doi: 10.1161/01.res.62.6.1059. [DOI] [PubMed] [Google Scholar]
- Ransom B. R., Kettenmann H. Electrical coupling, without dye coupling, between mammalian astrocytes and oligodendrocytes in cell culture. Glia. 1990;3(4):258–266. doi: 10.1002/glia.440030405. [DOI] [PubMed] [Google Scholar]
- Rhodin J. A. The ultrastructure of mammalian arterioles and precapillary sphincters. J Ultrastruct Res. 1967 Apr;18(1):181–223. doi: 10.1016/s0022-5320(67)80239-9. [DOI] [PubMed] [Google Scholar]
- Segal S. S. Cell-to-cell communication coordinates blood flow control. Hypertension. 1994 Jun;23(6 Pt 2):1113–1120. doi: 10.1161/01.hyp.23.6.1113. [DOI] [PubMed] [Google Scholar]
- Segal S. S., Damon D. N., Duling B. R. Propagation of vasomotor responses coordinates arteriolar resistances. Am J Physiol. 1989 Mar;256(3 Pt 2):H832–H837. doi: 10.1152/ajpheart.1989.256.3.H832. [DOI] [PubMed] [Google Scholar]
- Segal S. S., Duling B. R. Conduction of vasomotor responses in arterioles: a role for cell-to-cell coupling? Am J Physiol. 1989 Mar;256(3 Pt 2):H838–H845. doi: 10.1152/ajpheart.1989.256.3.H838. [DOI] [PubMed] [Google Scholar]
- Segal S. S., Duling B. R. Flow control among microvessels coordinated by intercellular conduction. Science. 1986 Nov 14;234(4778):868–870. doi: 10.1126/science.3775368. [DOI] [PubMed] [Google Scholar]
- Somlyo A. P., Walker J. W., Goldman Y. E., Trentham D. R., Kobayashi S., Kitazawa T., Somlyo A. V. Inositol trisphosphate, calcium and muscle contraction. Philos Trans R Soc Lond B Biol Sci. 1988 Jul 26;320(1199):399–414. doi: 10.1098/rstb.1988.0084. [DOI] [PubMed] [Google Scholar]
- Veenstra R. D., Wang H. Z., Beblo D. A., Chilton M. G., Harris A. L., Beyer E. C., Brink P. R. Selectivity of connexin-specific gap junctions does not correlate with channel conductance. Circ Res. 1995 Dec;77(6):1156–1165. doi: 10.1161/01.res.77.6.1156. [DOI] [PubMed] [Google Scholar]
- Xia J., Duling B. R. Electromechanical coupling and the conducted vasomotor response. Am J Physiol. 1995 Dec;269(6 Pt 2):H2022–H2030. doi: 10.1152/ajpheart.1995.269.6.H2022. [DOI] [PubMed] [Google Scholar]
- Xia J., Little T. L., Duling B. R. Cellular pathways of the conducted electrical response in arterioles of hamster cheek pouch in vitro. Am J Physiol. 1995 Dec;269(6 Pt 2):H2031–H2038. doi: 10.1152/ajpheart.1995.269.6.H2031. [DOI] [PubMed] [Google Scholar]




