Abstract
Background:
Hepatosteatosis is a common condition that can lead to cirrhosis and liver cancer. Galectin-3 (GAL-3) has been implicated in liver fibrosis and inflammation.
Objectives:
The purpose of this study was to investigate the association between GAL-3 and hepatosteatosis.
Design:
This study is a retrospective secondary analysis of data from a community health screening program.
Methods:
A total of 766 participants were included in the final analysis. Hepatosteatosis was diagnosed using ultrasonography, and GAL-3 levels were measured using enzyme-linked immunosorbent assay. Logistic regression analysis was used to examine the association between GAL-3 levels and the presence of hepatosteatosis, adjusting for age, sex, and other potential confounding factors.
Results:
The prevalence of moderate-to-severe hepatosteatosis in the study population was 31.5%. The participants with hepatosteatosis had a significantly higher mean level of GAL-3 compared to those without hepatosteatosis (16.6 ± 7.3 vs 13.5 ± 7.3 ng/ml; p < 0.001). After adjusting for age, sex, body mass index, and other potential confounding factors, a higher level of GAL-3 was significantly associated with an increased risk of moderate-to-severe hepatosteatosis (adjusted odds ratio (aOR) 1.24, 95% confidence interval (CI) 1.05–1.46, p = 0.010). The coexistence of alanine transaminase/aspartate transaminase ratio >1 and GAL-3 >14.4 ng/ml was associated with a significantly increased risk (aOR 3.37, 95% CI: 1.90–5.99, p < 0.001).
Conclusion:
Our findings suggest that GAL-3 level is significantly associated with the presence of moderate-to-severe hepatosteatosis, independent of other known cardiometabolic risk factors.
Keywords: ALT/AST ratio, galectin-3, hepatosteatosis, metabolic syndrome, waist-to-height ratio
Introduction
Hepatosteatosis is one of the most common causes of chronic liver disease globally, 1 and the prevalence has dramatically increased due to the increase in obesity, type 2 diabetes mellitus, and metabolic syndrome (MetS). 2 A population-based observational study reported that the global prevalence of hepatosteatosis was 25.24%, with the highest prevalence in the Middle East and South America, followed by Asia and North America. 1 However, of note, the prevalence was shown to vary based on factors such as age, sex, and race, and further research on biomarkers is required to identify regional variations.
This study explored the feasibility of quantitative ultrasonography as a simple screening method for hepatosteatosis and its severity at the community level. Beyond several cardiometabolic indices such as MetS and waist-to-height ratio (WHR), 3 a higher alanine transaminase/aspartate transaminase (ALT/AST) ratio would increase new onset hepatosteatosis. 4 Early studies have also demonstrated that galectin-3 (GAL-3) may contribute to the progression of cancer, 5 and it is also well known to play a role in tissue repair, cardiac remodeling, fibrogenesis, and inflammation in heart failure. 6 Moreover, research on GAL-3 suggests that it may be an important protein in the pathophysiology of cardiometabolic disorders, 7 and also that it may play a role in the development of steatohepatitis8,9 and fibrosis. 10 GAL-3 has been shown to positively regulate CD36 transcription through increasing peroxisome proliferator-activated receptor activation, thereby causing fatty acid uptake and hepatosteatosis. 11 The inhibition of GAL-3 has been shown to improve and prevent the progression of fibrosis, and it has been tested in phase III clinical trials in patients with nonalcoholic steatohepatitis. 12 Furthermore, a recent study found that the inhibition of GAL-3 could abolish the hepatoprotective effects conferred by GAL-3 by promoting proptosis, but not necroptosis. 13 Nevertheless, a United Kingdom biobank investigation did not find a causal interaction between GAL-3 and hepatosteatosis. 14
In spite of multiple unknown cardiometabolic indices, we still hypothesized that GAL-3 may be strongly associated with the existence of fatty liver. Therefore, the aim of this study was to examine the influence of GAL-3 on hepatosteatosis in a community-based cohort.
Methods
Population and study design
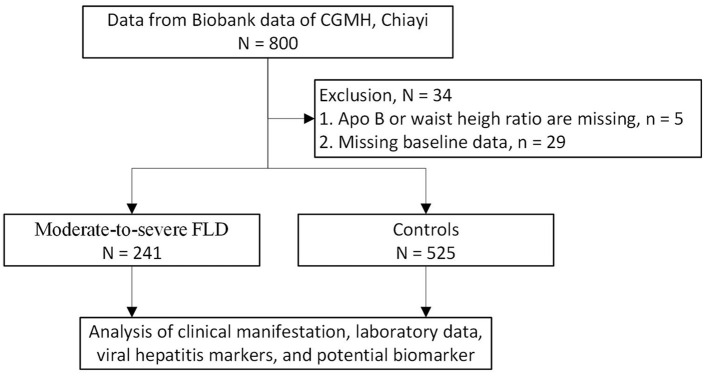
This retrospective cross-sectional study enrolled adult patients in southern Taiwan who attended annual checkups between 2017 and 2019. All of the participants provided informed consent and completed a questionnaire before enrollment. Anthropometric measurements, laboratory results, and hepatitis virus markers were then collected, and a serum sample was stored in the biobank of Chung Gung Memorial Hospital (CGMH) in Chiayi. Written informed consents for the use of stored remaining specimens were obtained from all patients prior to the participation in the study. The Ethics Committee and Institutional Review Board of Chang Gung Memorial Hospital approved this study on October 15, 2021 (IRB No. 202101596B0). A total of 800 samples were used for biomarker analysis. After excluding participants with incomplete data, 766 participants were included in the final analysis (Figure 1).
Figure 1.
Study enrollment flow chart.
Anthropometric measurements
Blood pressure was measured twice in each participant using an electronic sphygmomanometer (Omron HEM-1000, 6 MCN0937000; Dalian Co., Ltd, Dalian, China) in a seated position after 10 min of rest. Mean arterial pressures were measured during both the diastolic and systolic phases. Waist circumference was measured directly between the lowest rib and iliac crest with the participants standing. All measurements were performed by a trained nurse in accordance with standard procedures.
Laboratory analysis
Blood samples were collected from each participant after 12 h of fasting, and then tested in the laboratory at CGMH. The biochemical tests included serum creatinine (Cr), AST, alanine aminotransferase (ALT), fasting sugar, uric acid, triglycerides (TGs), low-density lipoprotein, high-density lipoprotein (HDL-C), total cholesterol, and apolipoprotein B (apo-B) (Roche Diagnostics, Mannheim, Germany; Cobas 6000, C501). Hemograms were performed using an XN-3000 system (Sysmex Taiwan Co., Ltd, Taipei, Taiwan). The platelet-to-lymphocyte ratio (PLR) was calculated by dividing the platelet count by the lymphocyte count, and the neutrophil-to-lymphocyte ratio (NLR) by dividing the neutrophil count by the lymphocyte count.
Biomarkers associated with hepatosteatosis
Serum levels of GAL-3, interleukin-6 (IL-6), and lipoprotein(a) were determined using enzyme-linked immunosorbent assays (GAL-3 and IL-6: Elabscience, Houston, TX, USA; lipoprotein(a): Abcam, Cambridge, UK).
Moderate-to-severe fatty liver defined by ultrasonography
The grading of hepatosteatosis is usually assessed using ultrasonography features including liver brightness, contrast between the liver and the kidneys, and appearance of the intrahepatic vessels, liver parenchyma, and diaphragm. Steatosis was graded as follows: absent (score 0) when the echotexture of the liver was normal; mild (score 1) when there was a slight and diffuse increase in liver echogenicity with normal visualization of the diaphragm and portal vein wall; moderate (score 2) when there was a moderate increase in liver echogenicity with slightly impaired appearance of the portal vein wall and diaphragm; and severe (score 3) when there was a marked increase in liver echogenicity with poor or no visualization of the portal vein wall, diaphragm, and posterior part of the right liver lobe. 15 Moderate-to-severe hepatosteatosis was defined as a score 2 + 3 compared with “controls” group as score 0 + 1.
Statistical analysis
The demographic characteristics of the participants according to the grade of hepatosteatosis were compared using independent sample t-tests for continuous variables and Chi-square tests for categorical variables. The continuous data with non-normal distribution should be presented as median (interquartile range, IQR). To identify factors potentially associated with moderate-to-severe hepatosteatosis, univariate logistic regression analysis was conducted using demographics and characteristics as explanatory variables. Based on a multivariable logistic regression model, we calculated odds ratios (ORs) with 95% confidence intervals (CIs) for moderate-to-severe hepatosteatosis after adjusting for age, sex, body mass index (BMI), WHR, and laboratory data. The analysis was conducted with two-tailed tests, and a p-value less than 0.05 was considered significant. Statistical analysis of the data was carried out using SAS version 9.4 (SAS Inc., Cary, NC, USA).
Results
Basic characteristics as determined by hepatosteatosis
A total of 766 participants were recruited and included in the final analysis (Table 1). The average age of the moderate-to-severe hepatosteatosis group was 63.4 ± 9.2 years, which was younger than that of the participants without hepatosteatosis (66.5 ± 10.3 years), and nearly half of them were male (50.1%). The moderate-to-severe hepatosteatosis group had significantly higher BMI, waist circumference, WHR, PLR, ALT/AST ratio, uric acid, TG, HDL-C, apo-B, and fasting sugar, but lower lipoprotein(a) (p < 0.05). Furthermore, we also found that the levels of GAL-3 were significantly higher among those who had moderate-to-severe hepatosteatosis compared to those who had mild hepatosteatosis. However, there were no significant differences in sex distribution, systolic blood pressure, kidney function, NLR, and IL-6 levels between the two groups.
Table 1.
Baseline characteristics of participants.
| Variable | Moderate-to-severe hepatosteatosis | Controls | p-Value a | ||
|---|---|---|---|---|---|
| n | (%) | n | (%) | ||
| Total | 241 | 525 | |||
| Age, years | 0.007 | ||||
| <65 | 123 | (51.0) | 213 | (40.6) | |
| ⩾65 | 118 | (49.0) | 312 | (59.4) | |
| Sex | 0.938 | ||||
| Male | 121 | (50.2) | 263 | (50.1) | |
| Female | 120 | (49.8) | 262 | (49.9) | |
| Median | (Q1–Q3) | Median | (Q1–Q3) | p-Value b | |
| Age, years | 64.0 | (57–69) | 67.0 | (60–74) | <0.001 |
| BMI, kg/m2 | 27.4 | (25.6–29.6) | 24.8 | (22.6–26.9) | <0.001 |
| WHR | 0.6 | (0.5–0.6) | 0.5 | (0.5–0.6) | <0.001 |
| SBP, mmHg | 128.0 | (89–147) | 123.0 | (90–142) | 0.031 |
| Laboratory data | |||||
| NLR | 1.8 | (1.4–2.3) | 1.8 | (1.3–2.3) | 0.936 |
| PLR | 7.3 | (5.6–9.8) | 6.6 | (5.4–8.5) | 0.008 |
| Cr, mg/dl | 0.90 | (0.7–1.1) | 0.90 | (0.7–1.1) | 0.824 |
| eGFR, ml/min/1.73 m2 | 79.0 | (69.5–93.8) | 80.1 | (66.4–97.2) | 0.778 |
| ALT/AST ratio | 1.2 | (1.0–1.5) | 1.0 | (0.8–1.2) | <0.001 |
| Uric acid, mg/dl | 6.1 | (5.3–7.3) | 5.7 | (4.7–6.7) | <0.001 |
| LDL, mg/dl | 119.0 | (93.0–143.0) | 115.0 | (93.0–139.0) | 0.111 |
| TC, mg/dl | 187.0 | (161–214) | 181.0 | (156–211) | 0.086 |
| TG, mg/dl | 130 | (97–173) | 97 | (70–97) | <0.001 |
| HDL, mg/dl | 47 | (40–55) | 52 | (44–62) | <0.001 |
| Apo-B, mg/dl | 105 | (85–121) | 93 | (79–113) | <0.001 |
| Fasting sugar, mg/dl | 106 | (96–125) | 100 | (93–110) | <0.001 |
| Biomarkers | |||||
| Lipoprotein(a) (µg/ml) | 288.0 | (149.5–655.5) | 399.0 | (196.7–942.7) | 0.001 |
| GAL-3 (ng/ml) | 15.5 | (12.6–18.5) | 13.5 | (9.3–16.7) | <0.001 |
| IL-6 (pg/ml) | 1.9 | (1.0–3.7) | 1.6 | (0.9–3.1) | 0.059 |
Pearson’s Chi-squared tests.
Mann–Whitney U test.
ALT, alanine aminotransferase; apo-B, apolipoprotein B; AST, aspartate transaminase; BMI, body mass index; Cr, creatinine; eGFR, estimated glomerular filtration rate; GAL-3, galectin 3; HDL, high-density lipoprotein cholesterol; IL-6, interleukin-6; LDL, low-density lipoprotein cholesterol; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-lymphocyte ratio; SBP, systolic blood pressure; TC, total cholesterol; TG, triglyceride; WHR, waist-to-height ratio.
Univariate and multivariate analyses
The results of univariate regression analysis (Table 2) showed that WHR, uric acid, TGs, fasting sugar, ALT/AST ratio, PLR, apo-B, and GAL-3 were associated with a significantly higher risk of moderate-to-severe hepatosteatosis, while age ⩾65 years, HDL-C, and lipoprotein(a) seemed to be inversely correlated with the risk of moderate-to-severe hepatosteatosis. The results of multivariate regression analysis showed that GAL-3, female sex, WHR, uric acid, TGs, ALT/AST ratio, and PLR maintained positive correlations, but only age ⩾65 years remained inversely correlated with moderate-to-severe hepatosteatosis.
Table 2.
Logistic regression analysis of the association between moderate-to-severe hepatosteatosis and clinical variables.
| Variable | Crude | Adjusted | ||
|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | |
| Age years | ||||
| <65 | Ref. | Ref. | ||
| ⩾65 | 0.66 (0.48–0.89) | 0.007 | 0.57 (0.39–0.84) | 0.004 |
| Sex | ||||
| Male | Ref. | Ref. | ||
| Female | 1.01 (0.75–1.37) | 0.938 | 1.68 (1.10–2.58) | 0.016 |
| WHR | 3.48 (2.61–4.65) | <0.001 | 3.08 (2.17–4.38) | <0.001 |
| Laboratory data | ||||
| Uric acid | 1.56 (1.27–1.91) | <0.001 | 1.36 (1.05–1.77) | 0.018 |
| TG | 1.74 (1.48–2.05) | <0.001 | 1.27 (1.05–1.54) | 0.013 |
| HDL | 0.57 (0.45–0.72) | <0.001 | 0.80 (0.59–1.09) | 0.164 |
| Fasting sugar | 1.17 (1.08–1.28) | <0.001 | 1.01 (0.91–1.13) | 0.726 |
| ALT/AST ratio | 2.56 (2.03–3.22) | <0.001 | 1.94 (1.49–2.53) | <0.001 |
| PLR | 1.17 (1.02–1.34) | 0.029 | 1.21 (1.03–1.43) | 0.018 |
| Apo-B | 1.52 (1.24–1.86) | <0.001 | 1.19 (0.93–1.54) | 0.160 |
| Biomarkers | ||||
| Lipoprotein(a) (µg/ml) | 0.77 (0.64–0.94) | 0.008 | 0.89 (0.71–1.11) | 0.303 |
| GAL-3 (ng/ml) | 1.44 (1.25–1.65) | <0.001 | 1.24 (1.05–1.46) | 0.010 |
Adjusted: The model was adjusted for sex, age, WHR, uric acid, TG, HDL, fasting sugar, ALT/AST ratio, PLR, Apo-B, lipoprotein(a), and GAL-3.
The ORs calculated for an interquartile range increase in WHR, laboratory data, and biomarkers.
ALT, alanine aminotransferase; apo-B, apolipoprotein B; AST, aspartate transaminase; CI, confidence interval; GAL-3, galectin 3; HDL, high-density lipoprotein cholesterol; OR, odds ratio; PLR, platelet-lymphocyte ratio; TG, triglyceride; WHR, waist-to-height ratio.
The impact of ALT/AST ratio and GAL-3 level on moderate-to-severe hepatosteatosis
A GAL-3 cut-off value of 14.4 is considered to indicate moderate-to-severe hepatosteatosis while ALT/AST ratio >1 reflected a higher risk of nonalcoholic fatty liver disease (NAFLD) in the previous study. 4 The coexistence of ALT/AST ratio >1 and GAL-3 >14.4 ng/ml was associated with moderate-to-severe hepatosteatosis (aOR 3.37, 95% CI: 1.90–5.99, p < 0.001) (Table 3). The participants with an ALT/AST ratio >1 had higher GAL-3 levels than those with a ratio <1 (Supplemental Figure S1), while the ALT/AST ratio has a distribution difference among the IQR of GAL-3 (Figure 2). After excluding alcohol consumption, the variable distribution and correlation between GAL-3 and NAFLD remained constant in Supplemental Tables S1–S3.
Table 3.
Logistic regression analysis of the association between moderate-to-severe hepatosteatosis and different ALT/AST ratios and GAL-3 groupings.
| Groupings | Crude | Adjusted | ||
|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | |
| ALT/AST ratio ⩽1 and GAL-3 ⩽14.4 | Ref. | Ref. | ||
| ALT/AST ratio ⩽1 and GAL-3 >14.4 | 2.29 (1.30–4.04) | 0.004 | 1.27 (0.67–2.40) | 0.457 |
| ALT/AST ratio >1 and GAL-3 ⩽14.4 | 3.94 (2.33–6.64) | <0.001 | 2.63 (1.48–4.64) | 0.001 |
| ALT/AST ratio >1 and GAL-3 >14.4 | 7.17 (4.29–11.96) | <0.001 | 3.37 (1.90–5.99) | <0.001 |
Adjusted: The model was adjusted for sex, age, WHR, uric acid, TG, HDL, fasting sugar, PLR, Apo-B, and lipoprotein(a).
ALT, alanine aminotransferase; apo-B, apolipoprotein B; AST, aspartate transaminase; CI, confidence interval; GAL-3, galectin 3; HDL, high-density lipoprotein cholesterol; OR, odds ratio; PLR, platelet-lymphocyte ratio; TG, triglyceride; WHR, waist-to-height ratio.
Figure 2.
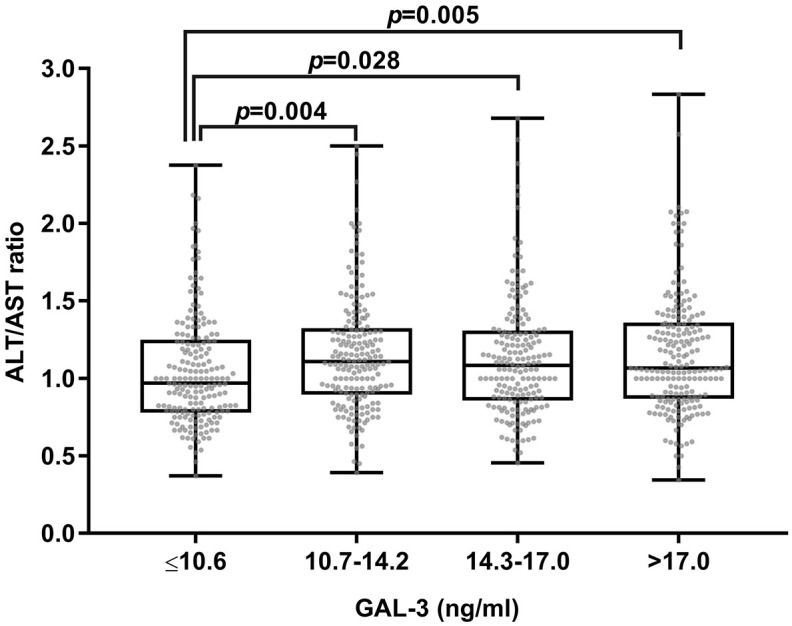
Boxplot depicting the ALT/AST ratio within GAL-3’s IQR.
ALT, alanine aminotransferase; AST, aspartate transaminase; GAL-3, galectin 3; IQR, interquartile range.
The relationships between cardiometabolic risk factors, WHR, and GAL-3 level
We also examined differences in WHR among the GAL-3 quartile groups. The participants with a higher WHR had a higher GAL-3 level than those with a WHR <0.5 (Supplemental Figure S2), and there were differences in the WHR among the GAL-3 quartile groups (Figure 3). The participants with a GAL-3 level between 10.7 and 17 ng/ml seemed to have a higher cardiometabolic risk than those with a lower level; however, the difference tended to disappear if the GAL-3 level was >17.0 ng/ml (Supplemental Figure S3).
Figure 3.
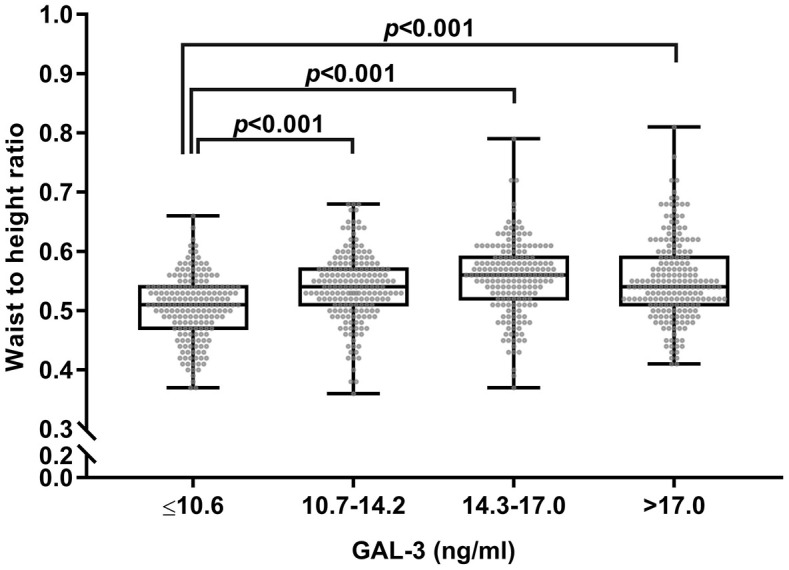
A box plot is shown below representing waist-to-height ratios across different levels of GAL-3.
GAL-3, galectin 3.
Development of a predictive nomogram
Considering the prognostic significance of GAL-3, we sought to combine it with seven common clinical factors (age, sex, ALT/AST ratio, WHR, PLR, uric acid, and TGs) to better predict the presence of moderate-to-severe hepatosteatosis (Supplemental Figure S4).
Discussion
The present retrospective study with secondary biomarker analysis demonstrated that GAL-3 level and comprehensive cardiometabolic index were associated with significant fatty liver disease, which was prevalent among those who were younger, overweight, and hypertensive. Despite potential confounders such as ALT/AST ratio, WHR, MetS, dyslipidemia, and inflammatory markers, GAL-3 remained consistently associated with moderate-to-severe hepatosteatosis regardless of alcohol consumption.
Hepatosteatosis and cardiometabolic burden
Our findings showed strong associations between cardiometabolic burden, including GAL-3, uric acid, components of MetS, WHR, and apo-B, with the severity of hepatosteatosis, compatible with previous studies.16,17 In addition, the ALT/AST ratio reflects hepatic dysfunction or inflammation, and it might be a predictive marker for hepatosteatosis in our study. 18 PLR and IL-6 are also inflammatory markers associated with anthropometric indices, metabolic factors, 19 hepatosteatosis,20,21 and hepatic fibrosis,20,22 which also found lower lipoprotein(a) in those populations. 23 Regarding alcoholic effects, the patients with NAFLD presented with similar features of cardiometabolic risks since GAL-3 still predicted high-grade hepatosteatosis in the final analysis. Therefore, we generated a nomogram including age, sex, ALT/AST ratio, WHR, PLR, uric acid, and TGs to predict the presence of fatty liver in patients attending community-based checkups (Supplemental Figure S4).
GAL-3 and hepatosteatosis
Previous studies on the association between GAL-3 and liver disease have been inconsistent. A high GAL-3 level has been reported to be a marker of hepatosteatosis, 24 hepatic fibrosis, and therapeutic response 25 ; however, Maxime et al. 14 reported that circulating GAL-3 was not correlated with NAFLD. In addition, GAL-3 has also been reported to contribute to inflammation, cell proliferation, differentiation, apoptosis, fibrosis, and host defense in chronic liver disease.6–8 Moreover, GAL-3 may play a critical role in mediating hepatoprotection and M2-like macrophages by inhibiting pyroptosis, 13 while an obesity-driven increase in steatosis was uncoupled with attenuated fibrotic nonalcoholic steatohepatitis in GAL-3-deficient mice. 9 After adjusting for multiple cardiometabolic risk factors and inflammatory biomarkers, our results showed that GAL-3 was significantly correlated with hepatosteatosis. This finding is compatible with a human study that reported that the number of GAL-3-positive cells in the liver was correlated with both the severity of biopsy-proven hepatosteatosis and liver damage. 10 Another animal study also observed that a chronic high-fat diet led to the up-regulation of GAL-3 and Toll-like receptor 4, subsequently activating NLRP3 inflammasomes. 26 In view of this, we propose that further studies are warranted to investigate a causal relationship between GAL-3 and hepatosteatosis.
Cardiometabolic index and GAL-3
Our results showed that WHR, MetS, ALT/AST ratio, as well as GAL-3, were predictive markers for high-grade hepatosteatosis, which is in line with previous studies performed on this topic. 27 As a result, traditionally used indices fail to account for the paradox between fatty liver and early fibrosis.28,29 The participants with higher ALT/AST ratio and WHR tended to have a higher GAL-3 level, which was significantly linearly correlated with both indices. Despite a positive correlation between GAL-3 binding protein and MetS in adults, 30 we found that the proportion of participants with >3 MetS components was not higher in those with a GAL-3 level >17 ng/ml. Nayor et al. 27 did not find a correlation between GAL-3 with cardiometabolic diseases after multivariable adjustments, and implied modulation of the GAL-3 pathway in high-grade MetS.
There was bias in this cross-sectional study as the participants were not randomly selected but rather chosen based on their personal willingness to undergo serum collection. This limitation reduces the generalizability of the findings which may not be applicable to the general population. An investigation of secondary biomarkers based on a community-dwelling cohort cannot provide long-term observations or conclude definite relationships between the level of GAL-3 and the progression of hepatosteatosis. In addition, obtaining details of a person’s medical history (co-morbidities including coronary artery disease) and over-the-counter medications using a questionnaire is insufficient. It was impossible to ignore the effects of anti-lipid treatment on measurements. Variations in ultrasound examination findings and a lack of repetitive studies were other limitations. Despite the same examination machine, samples from different years would result in variation.
Conclusion
Our study found that GAL-3 was significantly correlated with severe hepatosteatosis in addition to cardiometabolic risks and anthropometric indices. A linear relationship was observed between GAL-3 and ALT/AST ratio as well as WHR, which indicated that an inflammatory cascade and central obesity contribute to fatty liver. Further, longitudinal studies are needed to examine this relationship on a larger scale.
Supplemental Material
Supplemental material, sj-docx-1-taj-10.1177_20406223241302719 for Association between galectin-3 and hepatosteatosis in a community-based cross-sectional study by Ming-Shyan Lin, Ya-Chi Tu, Yu-Sheng Lin, Meng-Hung Lin, Chun-Liang Lin, Ming-Horng Tsai, Yung-Yu Hsieh, Tien-Hsing Chen, Mei-Yen Chen and Chung-Sheng Shi in Therapeutic Advances in Chronic Disease
Acknowledgments
This study is based on data from Biobank, CGMH, Chiayi Branch. The authors thank Health Information and Epidemiology Laboratory, Chang Gung Memorial Hospital, Chiayi Branch for providing comments and assistance in data analysis. To obtain the laboratory equipment and to confirm the data, Miss Chia-Chien Lee (Department of Laboratory Medicine, Chiayi Chang Gung Memorial Hospital) assisted us.
Appendix
Abbreviations
ALT/AST alanine transaminase/aspartate transaminase
CGMH Chung Gung Memorial Hospital
CI confidence interval
Cr creatinine
DBP diastolic blood pressure
ELISA enzyme-linked immunosorbent assay
GAL-3 galectin-3
HDL-C high-density lipoprotein
IQR interquartile range
LDL-C low-density lipoprotein
MetS metabolic syndrome
NAFLD nonalcoholic fatty liver disease
NASH nonalcoholic steatohepatitis
NLR neutrophil-to-lymphocyte ratio
OR odds ratio
PLR platelet-lymphocyte ratio
PPAR peroxisome proliferator-activated receptor
SBP systolic blood pressure
TC total cholesterol
TG triglyceride
WHR waist-to-height ratio
Footnotes
ORCID iD: Ming-Shyan Lin  https://orcid.org/0000-0001-9348-8153
https://orcid.org/0000-0001-9348-8153
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Ming-Shyan Lin, Departments of Internal Medicine and Cardiology, Chang Gung Memorial Hospital, Chiayi, Taiwan; Graduate Institute of Clinical Medical Sciences, College of Medicine, Chang Gung University, Taoyuan, Taiwan; Department of Nursing, Chang Gung University of Science and Technology, Chiayi, Taiwan.
Ya-Chi Tu, Department of Laboratory Medicine, New Taipei Municipal Tucheng Hospital, New Taipei City, Taiwan.
Yu-Sheng Lin, Departments of Internal Medicine and Cardiology, Chang Gung Memorial Hospital, Chiayi, Taiwan; Graduate Institute of Clinical Medical Sciences, College of Medicine, Chang Gung University, Taoyuan, Taiwan.
Meng-Hung Lin, Health Information and Epidemiology Laboratory, Chang Gung Memorial Hospital Chiayi Branch, Chiayi, Taiwan.
Chun-Liang Lin, Department of Nephrology, Chang Gung Memorial Hospital, Chiayi, Taiwan.
Ming-Horng Tsai, Department of Pediatrics, Chang Gung Memorial Hospital, Yunlin, Taiwan.
Yung-Yu Hsieh, Department of Gastroenterology and Hepatology, Chang Gung Memorial Hospital, Chiayi, Taiwan.
Tien-Hsing Chen, Departments of Internal Medicine and Cardiology, Chang Gung Memorial Hospital Chiayi Branch, Keelung, Taiwan.
Mei-Yen Chen, Department of Nursing, Chang Gung University of Science and Technology, Chiayi, Taiwan; Department of Nursing, Chang Gung University, Taoyuan, Taiwan.
Chung-Sheng Shi, Graduate Institute of Clinical Medical Sciences, College of Medicine, Chang Gung University, No. 6, Sec. West, Chai-Pu Road, Pu-TZ City, Chiayi 61363, Taiwan.
Declarations
Ethics approval and consent to participate: Despite the fact that the patient’s identity (i.e., the chart number or national identification number) was encrypted, every patient was assigned a personal identification number (PIN) with de-identified information. A protocol for this study has been approved by the Chang Gung Memorial Hospital Institutional Review Board (IRB No. 202101596B0). The written informed consent to participate in the study was obtained from all participants. Further, all methods in our study adhered to the principles of the Declaration of Helsinki as well as relevant guidelines and regulations. The statement was mentioned in the section titled “Methods.”
Consent for publication: Not applicable.
Author contributions: Ming-Shyan Lin: Conceptualization; Data curation; Formal analysis; Methodology; Writing – original draft; Writing – review & editing.
Ya-Chi Tu: Formal analysis; Investigation; Methodology; Writing – original draft.
Yu-Sheng Lin: Investigation; Methodology; Validation; Writing – original draft.
Meng-Hung Lin: Data curation; Formal analysis; Methodology; Writing – original draft.
Chun-Liang Lin: Investigation; Project administration; Writing – original draft.
Ming-Horng Tsai: Methodology; Resources; Supervision; Writing – original draft.
Yung-Yu Hsieh: Investigation; Methodology; Validation; Writing – original draft.
Tien-Hsing Chen: Investigation; Methodology; Writing – original draft.
Mei-Yen Chen: Investigation; Supervision; Validation; Writing – original draft.
Chung-Sheng Shi: Conceptualization; Data curation; Methodology; Supervision; Writing – original draft; Writing – review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by research funding from Chang Gung Memorial Hospital (Grant Numbers: CORPG6L0141, CORPG6L0142).
Competing interests: The authors declare that there is no conflict of interest.
Availability of data and materials: The data used to support the findings of this study are available from the corresponding author upon request.
Role of the funder/sponsor: Chang Gung Memorial Hospital had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
References
- 1. Younossi ZM, Golabi P, Paik JM, et al. The global epidemiology of nonalcoholic fatty liver disease (HEPATOSTEATOSIS) and nonalcoholic steatohepatitis (NASH): a systematic review. Hepatology 2023; 77: 1335–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Targher G, Corey KE, Byrne CD, et al. The complex link between HEPATOSTEATOSIS and type 2 diabetes mellitus—mechanisms and treatments. Nat Rev Gastroenterol Hepatol 2021; 18: 599–612. [DOI] [PubMed] [Google Scholar]
- 3. Tuglo LS. Comparison of adiposity anthropometric indices and their associations with visceral fat levels determined by bioelectrical impedance analysis among diabetic patients. Sci Rep 2022; 12: 17824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zou Y, Zhong L, Hu C, et al. Association between the alanine aminotransferase/aspartate aminotransferase ratio and new-onset non-alcoholic fatty liver disease in a nonobese Chinese population: a population-based longitudinal study. Lipids Health Dis 2020; 19(1): 245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Farhad M, Rolig AS, Redmond WL. The role of galectin-3 in modulating tumor growth and immunosuppression within the tumor microenvironment. Oncoimmunology 2018; 7: e1434467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hara A, Niwa M, Noguchi K, et al. Galectin-3 as a next-generation biomarker for detecting early stage of various diseases. Biomolecules 2020; 10: 389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cyr B, Keane RW, de Rivero Vaccari JP. ASC, IL-18 and galectin-3 as biomarkers of non-alcoholic steatohepatitis: a proof of concept study. Int J Mol Sci 2020; 21: 8580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pejnovic N, Jeftic I, Jovicic N, et al. Galectin-3 and IL-33/ST2 axis roles and interplay in diet-induced steatohepatitis. World J Gastroenterol 2016; 22: 9706–9717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jeftic I, Jovicic N, Pantic J, et al. Galectin-3 ablation enhances liver steatosis, but attenuates inflammation and IL-33-dependent fibrosis in obesogenic mouse model of nonalcoholic steatohepatitis. Mol Med 2015; 21: 453–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. de Oliveira FL, Panera N, De Stefanis C, et al. The number of liver galectin-3 positive cells is dually correlated with HEPATOSTEATOSIS severity in children. Int J Mol Sci 2019; 20: 3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yu H, Yang F, Zhong W, et al. Secretory galectin-3 promotes hepatic steatosis via regulation of the PPARγ/CD36 signaling pathway. Cell Signal 2021; 84: 110043. [DOI] [PubMed] [Google Scholar]
- 12. Al Attar A, Antaramian A, Noureddin M. Review of galectin-3 inhibitors in the treatment of nonalcoholic steatohepatitis. Expert Rev Clin Pharmacol 2021; 14: 457–464. [DOI] [PubMed] [Google Scholar]
- 13. Bai L, Lu W, Tang S, et al. Galectin-3 critically mediates the hepatoprotection conferred by M2-like macrophages in ACLF by inhibiting pyroptosis but not necroptosis signalling. Cell Death Dis 2022; 13: 775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tremblay M, Perrot N, Ghodsian N, et al. Circulating galectin-3 levels are not associated with nonalcoholic fatty liver disease: a Mendelian Randomization Study. J Clin Endocrinol Metab 2021; 106: e3178–e3184. [DOI] [PubMed] [Google Scholar]
- 15. Dasarathy S, Dasarathy J, Khiyami A, et al. Validity of real time ultrasound in the diagnosis of hepatic steatosis: a prospective study. J Hepatol 2009; 51: 1061–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kong L, Yang Y, Li H, et al. Prevalence of nonalcoholic fatty liver disease and the related risk factors among healthy adults: a cross-sectional study in Chongqing, China. Front Public Health 2023; 11: 1127489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yang KC, Hung HF, Lu CW, et al. Association of non-alcoholic fatty liver disease with metabolic syndrome independently of central obesity and insulin resistance. Sci Rep 2016; 6: 27034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lin MS, Lin HS, Chung CM, et al. Serum aminotransferase ratio is independently correlated with hepatosteatosis in patients with HCV: a cross-sectional observational study. BMJ Open 2015; 5(9): e008797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Arefhosseini S, Aghajani T, Tutunchi H, et al. Association of systemic inflammatory indices with anthropometric measures, metabolic factors, and liver function in non-alcoholic fatty liver disease. Sci Rep 2024; 14(1): 12829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhou Y, Tian N, Li P, et al. The correlation between neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio with nonalcoholic fatty liver disease: a cross-sectional study. Eur J Gastroenterol Hepatol 2022; 34(11): 1158–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Simon TG, Trejo MEP, McClelland R, et al. Circulating interleukin-6 is a biomarker for coronary atherosclerosis in nonalcoholic fatty liver disease: results from the Multi-Ethnic Study of Atherosclerosis. Int J Cardiol 2018; 259: 198–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ding R, Zhou X, Huang D, et al. Predictive performances of blood parameter ratios for liver inflammation and advanced liver fibrosis in chronic hepatitis B infection. Biomed Res Int 2021; 2021: 6644855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jung I, Kwon H, Park SE, et al. Serum lipoprotein(a) levels and insulin resistance have opposite effects on fatty liver disease. Atherosclerosis 2020; 308: 1–5. [DOI] [PubMed] [Google Scholar]
- 24. Yilmaz Y, Eren F, Kurt R, et al. Serum galectin-3 levels in patients with nonalcoholic fatty liver disease. Clin Biochem 2011; 44: 955–958. [DOI] [PubMed] [Google Scholar]
- 25. Kram M. Galectin-3 inhibition as a potential therapeutic target in non-alcoholic steatohepatitis liver fibrosis. World J Hepatol 2023; 15: 201–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Qin ZY, Gu X, Chen YL, et al. Toll-like receptor 4 activates the NLRP3 inflammasome pathway and periodontal inflammaging by inhibiting Bmi-1 expression. Int J Mol Med 2021; 47: 137–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nayor M, Wang N, Larson MG, et al. Circulating galectin-3 is associated with cardiometabolic disease in the community. J Am Heart Assoc 2015; 5: e002347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Neuman MG, Cohen LB, Nanau RM. Biomarkers in nonalcoholic fatty liver disease. Can J Gastroenterol Hepatol 2014; 28: 607–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Alexopoulos AS, Crowley MJ, Wang Y, et al. Glycemic control predicts severity of hepatocyte ballooning and hepatic fibrosis in nonalcoholic fatty liver disease. Hepatology 2021; 74: 1220–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhen S, Cai R, Yang X, et al. Association of serum galectin-3-binding protein and metabolic syndrome in a Chinese adult population. Front Endocrinol (Lausanne) 2021; 12: 726154. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-taj-10.1177_20406223241302719 for Association between galectin-3 and hepatosteatosis in a community-based cross-sectional study by Ming-Shyan Lin, Ya-Chi Tu, Yu-Sheng Lin, Meng-Hung Lin, Chun-Liang Lin, Ming-Horng Tsai, Yung-Yu Hsieh, Tien-Hsing Chen, Mei-Yen Chen and Chung-Sheng Shi in Therapeutic Advances in Chronic Disease