Abstract
Background
We aimed to assess the incidence of infective endocarditis (IE) and evaluate the impact of hypertension (HTN) with underweight on the risk of IE among patients with diabetic mellitus (DM) using a nationwide population-based cohort in Korea.
Methods
We identified 2,603,012 participants (57.4 ± 12.3 years) in the national health insurance database. Of these, 374,586 were normotensive, 750,006 were at pre-HTN status, and the remainder had HTN. The risk of IE was compared between the groups, and the impact of being underweight (body mass index < 18.5) was also evaluated.
Results
During follow-up (7.14 years; interquartile range 6.01–8.08 years), 1,703 cases of IE occurred; 168 (0.0647 person per 1000 person-years [PY]), 303 (0.05836 per 1000 PY), and 1,232 (0.12235 per 1000 PY) in normotensive, pre-HTN and HTN group, respectively. Hypertensive participants presented a higher risk of IE (subdistribution hazard ratio, 1.360; 95% confidence interval, 1.152–1.607) than normotensive participants. Being underweight increased the risk of IE by 90% among subjects with HTN. In subgroup analysis, age, duration of DM, insulin use, and habitual factors were not associated with the incidence of IE.
Conclusions
Diabetic patients may require rigorous blood pressure control and simultaneous avoidance of excessive weight loss to prevent IE.
Graphical abstract
Keywords: Infective endocarditis, Diabetes mellitus, Hypertension, Underweight
Background
Despite improvement in diagnostic strategy, antimicrobial treatments, and surgical interventions, infective endocarditis (IE) remains a highly morbid medical condition with substantial mortality and morbidity [1–3]. The risk of incident IE was comparatively well understood in specific high-risk groups including patients with prosthetic valves, intracardiac devices, or heart valve disease [4–6]. Although the epidemiology of IE has been changed from young with rheumatic or congenital valve disease to old with various comorbidities over the last years, little attention has been given to people with diabetes mellitus (DM) who are at risk for various infections.
Previous studies demonstrated that people with DM present a higher susceptibility to infectious disease compared to those without DM, probably due to immune dysfunction, micro-or macro-angiopathy, neuropathy, and consequently increased medical intervention [7–10]. In addition, there is an increasing body of evidence suggesting metabolic diseases such as hypertension (HTN) and being underweight may predispose the risk of infection, and poor clinical outcomes of infectious disease in the underweight population were supported by the ‘obesity paradox’ [11–16]. It implies that HTN and underweight may play a role as a risk factor for incident IE. However, given the paucity of epidemiologic studies, the relationship between the abovementioned comorbidities and incident IE, especially in the diabetic population, remains elusive. Therefore, in the present study, we aimed to investigate the impact of HTN and underweight on incident IE in the diabetic cohort using a nationwide population-based database.
Methods
Databases
The present study analyzed the Korean National Health Insurance Service (K-NHIS) and claims database. K-NHIS, a single mandatory insurer managed by the government, covers 97.1% of the Korean population and provides a biennial national health check-up program including questionnaires regarding lifestyle behavior, anthropometric measurements, and laboratory tests. K-NHIS database contains baseline demographic data, diagnostic codes (ICD-10), clinical usage data, medical claim data, and mortality data. The institutional review board waived the need for consent from individual patients because the data used are public and anonymized under confidentiality guidelines.
Participants
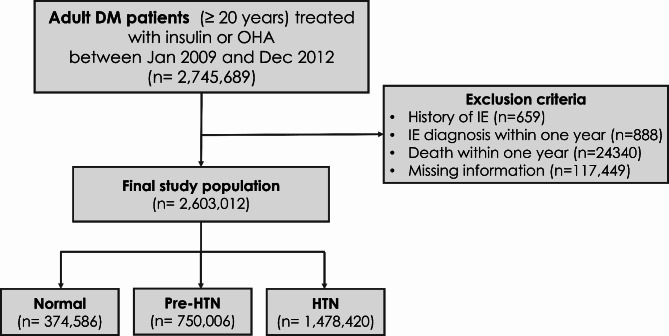
Using this database, we initially identified 2,745,689 patients (≥ 20 years old) with DM who underwent a health examination between January 2009 and December 2012. We excluded patients with a history of IE (n = 659) since the current study focused on the incidence of IE, and those with IE diagnosis (n = 229) or died (n = 24,340) within one year after the day of their health screening. Patients with missing information (n = 117,449) were excluded as well (Fig. 1). This study was approved by the institutional review board of Soongsil University (Approval no. SSU-202003-HR-201-01).
Fig. 1.
CONSORT Diagram of Study Cohort
Definition of diabetes
The presence of DM was diagnosed if [1] International Statistical Classification of Disease and Related Health Problems, Tenth Revision (ICD-10) codes for diabetes (E11-E14) were claimed with at least one prescription of hypoglycemic agents or insulin before the health screening or [2] fasting blood glucose exceeded 126 mg/dL at the health screening.
Exposure: hypertension and underweight
HTN was diagnosed if ICD-10 codes for HTN (I10-I13 and I15) were claimed, and antihypertensive agents were prescribed within six months of nationwide health examination. The participants were categorized by ordinal variables as follows: normotension (systolic blood pressure [SBP] < 120 mmHg and diastolic blood pressure [DBP] < 80mmg), prehypertension (120 ≤ SBP < 140 mmHg and 80 ≤ DBP < 90 mmHg) and hypertension (SBP ≥ 140mmHg, DBP ≥ 90mmHg). Participants diagnosed with hypertension were classified into the hypertension (HTN) group, irrespective of the blood pressure measured.
Body mass index (BMI) was calculated as weight in kilograms divided by the square of height in meters (kg/m2). Following the World Health Organization recommendations for Asians, subjects with a BMI lower than 18.5 kg/m2 were defined as underweight.
Outcome: incident IE
The study population was followed up from the date of health screening to the date of incident IE diagnosis, censoring (e.g., death), or the end of the study period (December 31, 2018), whichever came first. The primary study of interest was a new diagnosis of IE, which was defined using the ICD-10 codes for IE (I33.x, I38.x, and I39.8). Of the patients who conform to ICD codes of IE, only those who died within 14 days or were hospitalized longer than 14 days were included in the present study.
Covariates and definition
Information regarding general health behaviors was obtained by a self-completed questionnaire. Smoking status was classified as a non-smoker (< 100 cigarettes in a lifetime), ex-smoker (smoked at least 100 cigarettes in a lifetime but had not smoked within one month of national health check-up), or current smoker (smoked at least 100 cigarettes in a lifetime and continued smoking within one month of the national health check-up). Alcohol assumption status was categorized into three groups as follows: non-drinker (0 g of alcohol per week), mild-drinker (0 g < alcohol < 210 g per week), or heavy-drinker (≥ 210 g of alcohol per week). Regular exercise was defined as either vigorous exercise of at least 20 min more than three times per week or moderate intensity exercise of at least 30 min five times per week. Regarding the comorbidities, the presence of dyslipidemia was determined if [1] the ICD-10 code (E78) was claimed with a prescription of an antihyperlipidemic agent or [2] total cholesterol was ≥ 240 mg/dL. Glomerular filtration rate less than 60mL/min/1·73m2, as estimated by the Modification of Diet in Renal Disease equation, was the diagnostic criteria for chronic kidney disease (CKD). Blood samples were obtained after an overnight fast to measure serum glucose and lipid parameters, including total cholesterol and low- and high-density lipoprotein cholesterol. The low income level was defined as the bottom 20% of the house income level.
Statistical analysis
The categorical variables were expressed as frequencies and percentages and were compared with the Chi-square or Fisher’s exact tests. The continuous variables were presented as mean ± standard deviation or median with range and were compared using Student’s t-test and Mann-Whitney U test as appropriate. Incidence rates were estimated using the total number of IE events during the follow-up period divided by 1000 person-years at risk. Kaplan-Meier plots were constructed to delineate the cumulative incidence of IE, and the log-rank test was used to compare the intergroup differences in the Kaplan-Meier estimates. A multivariable-adjusted Cox proportional hazards model and a competing risk model were employed to evaluate the association between HTN/ underweight and the incidence of IE. The subdistribution hazard ratio (SHR) was estimated using the Fine and Gray method, which consider death a competing risk. Age, sex, economic status, smoking, drinking, regular exercise, dyslipidemia, chronic kidney disease, diabetes duration, insulin use, and oral hypoglycemic agent medications were adjusted in the multivariable models. Stratified analyses of the association of HTN status of incident IE by age, sex, and habitual patterns were conducted. The risk of IE according to DM duration and treatment was analyzed as well. All significant tests were two-sided, and p-values < 0.05 were considered statistically significant. SAS statistical software version 9·4 (SAS Institute, Cary, NC) was used for statistical analyses.
Results
Baseline characteristics
The final study population included 2,603,012 patients, with a mean age of 57.4 ± 12.3 years, and Normal, Pre-HTN and HTN group consisted of 374,586 (14·4%), 750,006 (28·8%) and 1,478,420 (56.8%), respectively (Fig. 1). Table 1 summarizes the baseline characteristics of the study population according to HTN status. Patients in the HTN group were older, had a higher prevalence of females, and were more likely to have higher BMI, CKD, insulin therapy, and use of three or more oral hypoglycemic agents compared to the reference group. The three groups differed significantly across various parameters listed in Table 1 (all p < 0.001).
Table 1.
Baseline profile
| Variables, n (%) | Normal | Pre-HTN | HTN | P value |
|---|---|---|---|---|
| (n=374,586) | (n=750,006) | (n=1,478,420) | ||
| Age, years | 52·8±12·1 | 53·0±12·3 | 60·9±11·3 | <0·0001 |
| Age | <0·0001 | |||
| <40 | 46,832(12·5) | 101,870(13·6) | 47,946(3·2) | |
| 40-64 | 263,953(70·5) | 509,356(67·9) | 847,446(57·3) | |
| ≥65 | 63,801(17·0) | 138,780(18·5) | 583,028(39·4) | |
| Sex | <0·001 | |||
| Male | 213,800(57·1) | 501,997(66·9) | 846,875(57·3) | |
| Female | 160,786(42·9) | 248,009(33·1) | 631,545(42·7) | |
| BMI, kg/m2 | 23·7±3·1 | 24·8±3·3 | 25·6±3·4 | <0·001 |
| BMI level, | <0·001 | |||
| <18.5 | 13,243(3·5) | 12,396(1·7) | 15,979(1·1) | |
| <23 | 141,932(37·9) | 204,318(27·2) | 302,093(20·4) | |
| <25 | 98,228(26·2) | 194,702(26·0) | 352,609(23·9) | |
| <30 | 109,479(29·2) | 292,413(39·0) | 664,679(45·0) | |
| ≥30 | 11,704(3·1) | 46,177(6·2) | 143,060(9·7) | |
| Smoking | <0·001 | |||
| Non-smoker | 206,036(55·0) | 368,567(49·1) | 873,831(59·1) | |
| Ex-smoker | 58,207(15·5) | 138,849(18·5) | 284,087(19·2) | |
| Current-smoker | 110,343(29·5) | 242,590(32·4) | 320,502(21·7) | |
| Drinking | <0·001 | |||
| Non-drinker | 223,487(59·7) | 378,963(50·5) | 889,328(60·2) | |
| Mild-drinker | 123,548(33·0) | 286,664(38·2) | 442,002(29·9) | |
| Heavy-drinker | 27,551(7·4) | 84,379(11·3) | 147,090(10·0) | |
| Regular exercise | 75,250(20·1) | 151,455(20·2) | 309,411(20·9) | <0·001 |
| Chronic kidney disease | 23,681(6·3) | 48,066(6·4) | 229,487(15·5) | <0·001 |
| GFR, mL/min/1.73m2 | 88·7±35·7 | 88·6±36·2 | 82·1±36·2 | <0·001 |
| DM Duration, ≥5 years | 98,278(26·2) | 168,939(22·5) | 542,560(36·7) | <0·001 |
| DM treatment | ||||
| Insulin | 29,287(7·8) | 41,622(5·6) | 157,194(10·6) | <0·001 |
| OHA, ≥3 | 50,091(13·4) | 80,709(10·8) | 246,596(16·7) | <0·001 |
| Fasting glucose, mg/dL | 147·9±49·7 | 149·6±47·6 | 141·5±45·8 | <0·001 |
| Waist circumference, cm | 81·6±8·4 | 84·5±8·3 | 86·9±8·6 | <0·001 |
| Systolic BP, mmHg | 109·7±7·0 | 127·0±6·9 | 135·0±16·5 | <0·001 |
| Diastolic BP, mmHg | 68·5±6·0 | 78·5±5·9 | 82·0±11·1 | <0·001 |
| Dyslipidemia | 123,114(32·9) | 251,699(33·6) | 716,824(48·5) | <0·001 |
| Total cholesterol, mg/dL | 195·4±41·6 | 201·7±42·1 | 194·2±43·2 | <0·001 |
| HDL -C, mg/dL | 52·7±23·5 | 52·4±23·2 | 51·7±24·2 | <0·001 |
| LDL -C, mg/dL | 113·2±39·4 | 114·9±41·1 | 108·6±41·4 | <0·001 |
| Income, low 20% | 79,140(21·1) | 152,459(20·3) | 314,996(21·3) | <0·001 |
Values are n (%), mean ± standard deviation, or median [quartile 1- quartile 3]
BP, blood pressure; BMI, body mass index; DM, diabetes mellitus; eGFR, glomerular filtration rate; HDL-C, high density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol; OHA, oral hypoglycemic agent
Impact of hypertension on IE
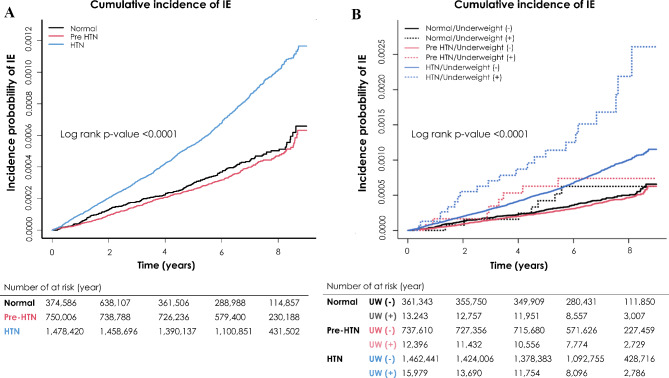
During the follow-up of 7.14 years (interquartile range 1–3, 6.01–8.08 years), a total of 1703 cases of IE were diagnosed. The incidence of IE in the Normal, Pre-HTN and HTN groups was 0.065, 0.058 and 0.122 person per 1000 person-years, respectively. The patients with HTN presented a 1.36-fold increased risk of IE compared to the normal reference group (SHR, 1.36; 95% confidence interval [CI], 1.152–1.607). However, no difference in the risk of IE was found between pre-hypertensive and normotensive participants (Table 2). The Kaplan-Meier plot demonstrated the cumulative hazard of IE according to hypertension status, showing that the HTN group had the highest incidence of IE among all groups (log-rank p < 0.001) (Fig. 2).
Table 2.
Incidence and risk of incident IE
| HR (95% CI) | SHR(95% CI) | |||||||
|---|---|---|---|---|---|---|---|---|
| HTN level | UW | Participants, No. | Event, No. | Person-years, No. | IR per 1000 PY | Model 1a | Model 2b | Model 3c |
| Normal | 374,586 | 168 | 2,596,618.93 | 0.0647 | 1 (Reference) | 1 (Reference) | 1 (Reference) | |
| Pre-HTN | 750,006 | 303 | 5,191,772.99 | 0.05836 | 0.903 (0.748-1.091) | 0.909 (0.752-1.097) | 0.918 (0.759- 1.109) | |
| HTN | 1,478,420 | 1,232 | 10,069,290.54 | 0.12235 | 1.892 (1.610-2.223) | 1.343 (1.138-1.585) | 1.360 (1.152-1.607) | |
| HR (95% CI) | SHR(95% CI) | |||||||
| Normal | No | 361,343 | 161 | 2,513,483·48 | 0.06405 | 1 (Reference) | 1 (Reference) | 1 (Reference) |
| Yes | 13,243 | 7 | 83,135·44 | 0.0842 | 1.334 (0.626-2.843) | 1.180 (0.554-2.517) | 1.028 (0.482-2.194) | |
| Pre-HTN | No | 737,610 | 295 | 5,115,472·1 | 0.05767 | 0.902 (0.744-1.093) | 0.906 (0.747-1.098) | 0.913 (0.753-1.107) |
| Yes | 12,396 | 8 | 76,300·89 | 0.10485 | 1.663 (0.818-3.383) | 1.387 (0.681-2.825) | 1.209 (0.593-2.465) | |
| HTN | No | 1,462,441 | 1210 | 9981453.39 | 0.12122 | 1.894 (1.607-2.232) | 1.344 (1.135-1.590) | 1.357 (1.145-1.609) |
| Yes | 15,979 | 22 | 87,837·15 | 0.52046 | 4.012 (2.570-6.264) | 2.221 (1.418-3.481) | 1.748 (1.108-2.755) |
a Model 1 was unadjusted
b Model 2 was adjusted for age, sex, lower income (lowest Q1), smoking, drinking, regular exercise, diabetes duration, chronic kidney disease, dyslipidemia, insulin use and oral hypoglycemic agents (≥3 types)
c Model 3 was adjusted for age, sex, lower income (lowest Q1), smoking, drinking, regular exercise, diabetes duration, chronic kidney disease, dyslipidemia, insulin use and oral hypoglycemic agents (≥3 types), considering death as a competing event
CI, confidence interval; HR, hazard ratio; HTN, hypertension; IR, incidence rate; IE, infective endocarditis; SHR, subdistribution hazard ratio; UW, underweight
Fig. 2.
Cumulative Incidence of Infective Endocarditis. Kaplan-Meier estimates of cumulative incidence of infective endocarditis during the follow-up period were compared between the groups classified according to (A) HTN status, and (B) HTN status and presence of underweight
Composite impact of hypertension and underweight on IE
The influence of HTN on the risk of IE was maintained in both patients with (SHR, 1.748; 95% CI, 1.108–2.755) and without (SHR, 1.357; 95% CI, 1.145–1.609) underweight status (Table 2). Being underweight did not significantly increase the risk of IE in the normotensive or pre-HTN diabetic cohort (Table 2). However, participants being underweight concomitant with HTN (SHR, 1.748; 95% CI, 1.108–2.755) exhibited a higher risk of IE compared to those solely with HTN (SHR, 1.357; 95% CI, 0.145–1.609).
Subgroup analysis
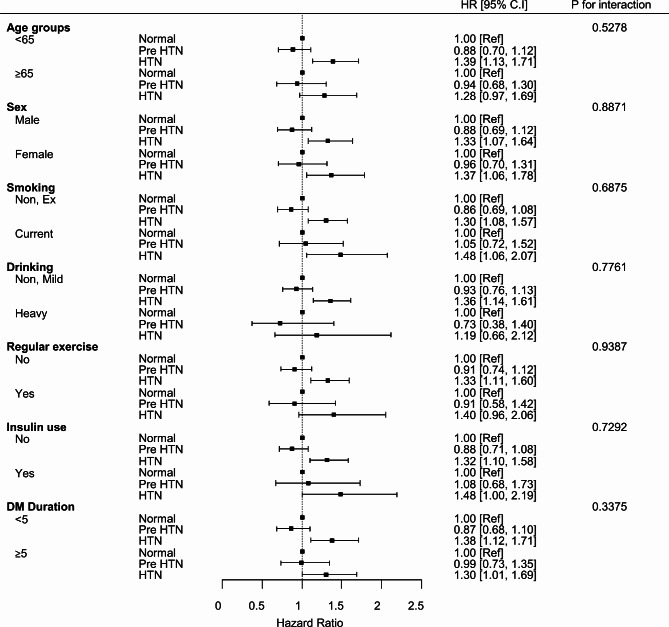
In the subgroup analysis for incidence of IE among diabetic patients, there was no interaction observed with a duration of DM (< 5 years vs. ≥ 5 years), insulin use, age (< 65 years vs. ≥ 65years, sex or habitual patterns including smoking, drinking and regular exercise (Fig. 3).
Fig. 3.
Subgroup Analysis for Incidence of Infective Endocarditis. Forest-plot summarizing the associated risk of infective endocarditis in the subgroup of age, sex, smoking, drinking, regular exercise, insulin use and duration of diabetes mellitus
Discussion
The present study demonstrates that HTN is an independent risk factor for incident IE in people with diabetes. In addition, being underweight was associated with an increased risk of incident IE in those with concomitant HTN.
A higher risk of various infectious diseases, including IE, in diabetic individuals than non-diabetic people has been shown in several series [7, 9, 17, 18]. A population-based study conducted in Spain showed that people with type 2 DM had more than twice the incidence of IE than those without DM [8]. Furthermore, a stepwise increase in the risk of IE with DM duration was indicated in the cited study using Danish nationwide registries [19]. However, there is an unmet need for risk analysis of incident IE in the diabetic population, which may provide a chance to prevent and improve the management of IE.
In a recent publication using national cohort data, Lee et al. demonstrated that increased blood pressure was associated with an increased risk for IE in a dose-dependent manner [12]. In line with this, our study showed that HTN was an independent predictor of incident IE in the diabetic cohort as well. However, unlike DM, which is known to cause immune dysfunction, angiopathy, or gastrointestinal and urinary dysmotility, the mechanism by which HTN causes incident IE is still unclear [10]. One of possible suggestion is a high shear stress applied on the valve leaflets, and another is the cardiac valvular endothelial injury aggravating secondary atherosclerosis and calcification [20–22]. These pathologic changes may further predispose diabetic patients to IE.
We also found that being underweight concomitant to HTN was a secondary risk factor for incident IE. Cited studies showed that being underweight was associated with a higher risk of infection than obesity, and diabetic people who were underweight were more likely to have a poorer prognosis in the treatment of infectious disease compared to those with obesity, so-called obesity paradox [9, 23, 24]. In our analysis, although underweight was not identified as an independent risk factor for IE, it exacerbated the risk of incident IE in diabetic patients with HTN. This finding is in line with previous diabetes studies demonstrating adverse outcomes in underweight individuals compared to those with overweight.
Limitations
The present study has several limitations to be acknowledged. First, the study contains inherent limitations of retrospective design. Second, the direct causality between HTN and incident IE was not established in the current study. Additionally, since hypertension was diagnosed using ICD-10 codes in the present study, there is a potential for misclassification in patients who have been prescribed angiotensin receptor blockers due to proteinuria. Finally, the study results have limited applicability to other ethnic groups as the study cohort was composed mostly of Korean diabetic populations.
Conclusion
In the diabetic population, HTN played a role as a predictor of incident IE. Moreover, being underweight may cause those with concomitant HTN to be more susceptible to IE. Therefore, a rigorous and timely management of HTN and simultaneous avoidance of excessive weight loss may be required to prevent the incident IE in the diabetic population.
Acknowledgements
Not applicable.
Abbreviations
- CKD
Chronic Kidney Disease
- CI
Confidence Interval
- DM
Diabetes Mellitus
- DBP
Diastolic Blood Pressure
- HR
Hazard Ratio
- HTN
Hypertension
- IE
Infective Endocarditis
- ICD
International Statistical Classification of Disease and Related Health Problems
- SBP
Systolic Blood Pressure
Author contributions
SHL, HJK, NSK, and KH contributed to conception and design of the present study. HJK, JNK, SJL, JHK, and WKP contributed to interpretation of the data. SHL, HJK, WKP, and NSK contributed critical revision of the manuscript. KH contributed to acquisition, analysis and interpretation of the data. WKP contributed to drafting and critical revision of the manuscript. HJK and SHL are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and accuracy of data analysis. All authors read and approved the final manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for profit sectors.
Data availability
The data that supported the findings of the present study are available from the K-NHIS. However, restrictions apply regarding the availability of the data, which were used with permission for this specific study.
Declarations
Ethics approval and consent to participate
This study was approved by the institutional review board of Soongsil University (Approval no. SSU-202003-HR-201-01). The requirement for written informed consent was waived in the present study because data in the K-NHIS database are anonymized in adherence to strict confidentiality guidelines.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Won Kyung Pyo, Hee-Jung Kim, Kyungdo Han and Seung-Hyun Lee contributed equally to this work.
Contributor Information
Hee-Jung Kim, Email: heejung440@hanmail.net.
Seung Hyun Lee, Email: henry75@yuhs.ac.
References
- 1.Talha KM, Baddour LM, Thornhill MH, Arshad V, Tariq W, Tleyjeh IM et al. Escalating incidence of infective endocarditis in Europe in the 21st century. Open Heart. 2021;8(2). [DOI] [PMC free article] [PubMed]
- 2.Murdoch DR, Corey GR, Hoen B, Miró JM, Fowler VG Jr., Bayer AS, et al. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century: the international collaboration on endocarditis-prospective cohort study. Arch Intern Med. 2009;169(5):463–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pant S, Patel NJ, Deshmukh A, Golwala H, Patel N, Badheka A, et al. Trends in infective endocarditis incidence, microbiology, and valve replacement in the United States from 2000 to 2011. J Am Coll Cardiol. 2015;65(19):2070–6. [DOI] [PubMed] [Google Scholar]
- 4.Cahill TJ, Raby J, Jewell PD, Brennan PF, Banning AP, Byrne J, et al. Risk of infective endocarditis after surgical and transcatheter aortic valve replacement. Heart. 2022;108(8):639–47. [DOI] [PubMed] [Google Scholar]
- 5.Kim JY, Park SJ, Lee SH, Seo GH, Jang SW. Risk of infective endocarditis associated with invasive dental procedures in patients with cardiac rhythm devices. Europace. 2022;24(12):1967–72. [DOI] [PubMed] [Google Scholar]
- 6.Østergaard L, Valeur N, Wang A, Bundgaard H, Aslam M, Gislason G, et al. Incidence of infective endocarditis in patients considered at moderate risk. Eur Heart J. 2019;40(17):1355–61. [DOI] [PubMed] [Google Scholar]
- 7.Kim EJ, Ha KH, Kim DJ, Choi YH. Diabetes and the risk of infection: a National Cohort Study. Diabetes Metab J. 2019;43(6):804–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Miguel-Yanes JM, Jiménez-García R, Hernández-Barrera V, de Miguel-Díez J, Méndez-Bailón M, Muñoz-Rivas N, et al. Infective endocarditis according to type 2 diabetes mellitus status: an observational study in Spain, 2001–2015. Cardiovasc Diabetol. 2019;18(1):161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tomic D, Shaw JE, Magliano DJ. The burden and risks of emerging complications of diabetes mellitus. Nat Rev Endocrinol. 2022;18(9):525–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Casqueiro J, Casqueiro J, Alves C. Infections in patients with diabetes mellitus: a review of pathogenesis. Indian J Endocrinol Metab. 2012;16(Suppl 1Suppl1):S27–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dobner J, Kaser S. Body mass index and the risk of infection-from underweight to obesity. Clin Microbiol Infect. 2018;24(1):24–8. [DOI] [PubMed] [Google Scholar]
- 12.Lee GB, Shin KE, Han K, Son HS, Jung JS, Kim YH, et al. Association between Hypertension and Incident Infective Endocarditis. Hypertension. 2022;79(7):1466–74. [DOI] [PubMed] [Google Scholar]
- 13.Higgins H, Mason AM, Larsson SC, Gill D, Langenberg C, Burgess S. Estimating the Population benefits of blood pressure lowering: a wide-angled mendelian randomization study in UK Biobank. J Am Heart Association. 2021;10(17):e021098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gribsholt SB, Pedersen L, Richelsen B, Sørensen HT, Thomsen RW. Body Mass Index and 90-Day Mortality Among 35,406 Danish Patients Hospitalized for Infection. Mayo Clin Proc. 2021;96(3):550 – 62. [DOI] [PubMed]
- 15.Rios-Diaz AJ, Lin E, Williams K, Jiang W, Patel V, Shimizu N, et al. The obesity paradox in patients with severe soft tissue infections. Am J Surg. 2017;214(3):385–9. [DOI] [PubMed] [Google Scholar]
- 16.Nie W, Zhang Y, Jee SH, Jung KJ, Li B, Xiu Q. Obesity survival paradox in pneumonia: a meta-analysis. BMC Med. 2014;12:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carey IM, Critchley JA, DeWilde S, Harris T, Hosking FJ, Cook DG. Risk of infection in type 1 and type 2 diabetes compared with the General Population: a matched cohort study. Diabetes Care. 2018;41(3):513–21. [DOI] [PubMed] [Google Scholar]
- 18.Lu P, Zhang Y, Liu Q, Ding X, Kong W, Zhu L, et al. Association of BMI, diabetes, and risk of tuberculosis: a population-based prospective cohort. Int J Infect Dis. 2021;109:168–73. [DOI] [PubMed] [Google Scholar]
- 19.Østergaard L, Mogensen UM, Bundgaard JS, Dahl A, Wang A, Torp-Pedersen C, et al. Duration and complications of diabetes mellitus and the associated risk of infective endocarditis. Int J Cardiol. 2019;278:280–4. [DOI] [PubMed] [Google Scholar]
- 20.Nazarzadeh M, Pinho-Gomes AC, Smith Byrne K, Canoy D, Raimondi F, Ayala Solares JR, et al. Systolic blood pressure and risk of Valvular Heart Disease: a mendelian randomization study. JAMA Cardiol. 2019;4(8):788–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kalra SS, Shanahan CM. Vascular calcification and hypertension: cause and effect. Ann Med. 2012;44(Suppl 1):S85–92. [DOI] [PubMed] [Google Scholar]
- 22.Sitia S, Tomasoni L, Atzeni F, Ambrosio G, Cordiano C, Catapano A, et al. From endothelial dysfunction to atherosclerosis. Autoimmun Rev. 2010;9(12):830–4. [DOI] [PubMed] [Google Scholar]
- 23.Doehner W, Erdmann E, Cairns R, Clark AL, Dormandy JA, Ferrannini E, et al. Inverse relation of body weight and weight change with mortality and morbidity in patients with type 2 diabetes and cardiovascular co-morbidity: an analysis of the PROactive study population. Int J Cardiol. 2012;162(1):20–6. [DOI] [PubMed] [Google Scholar]
- 24.Wiebe N, Muntner P, Tonelli M. Associations of body mass index, fasting insulin, and inflammation with mortality: a prospective cohort study. Int J Obes (Lond). 2022;46(12):2107–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that supported the findings of the present study are available from the K-NHIS. However, restrictions apply regarding the availability of the data, which were used with permission for this specific study.