Abstract
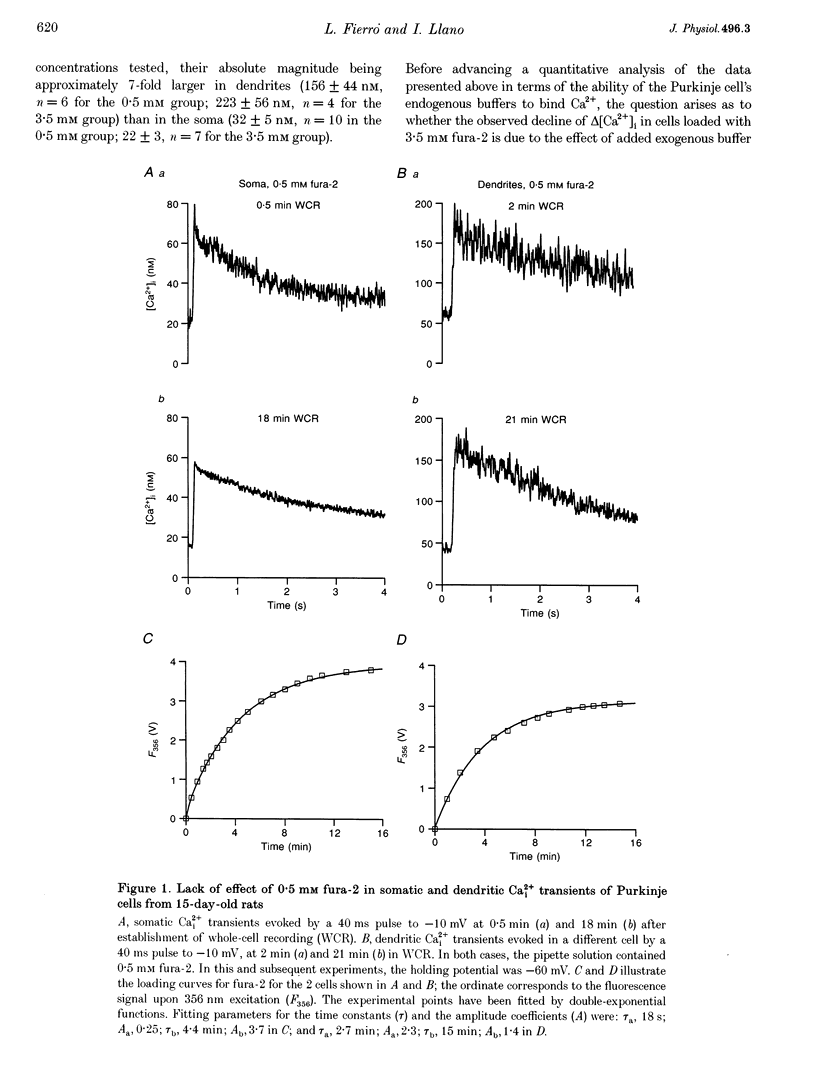
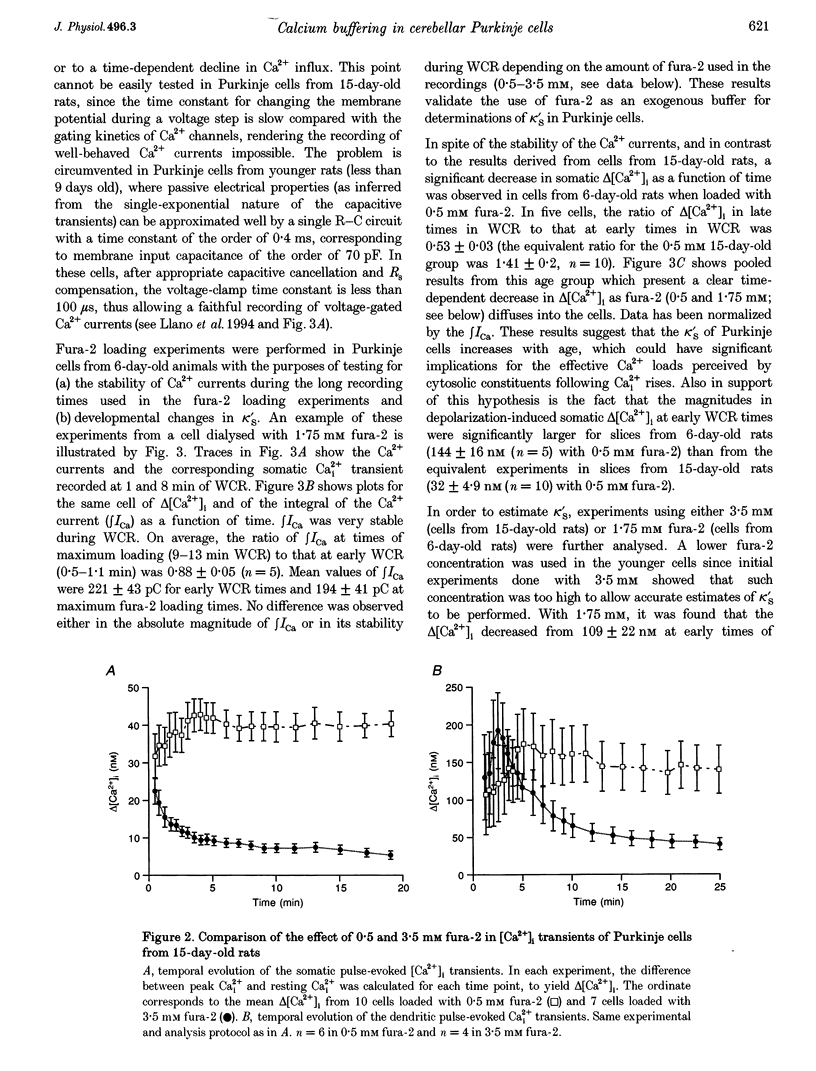
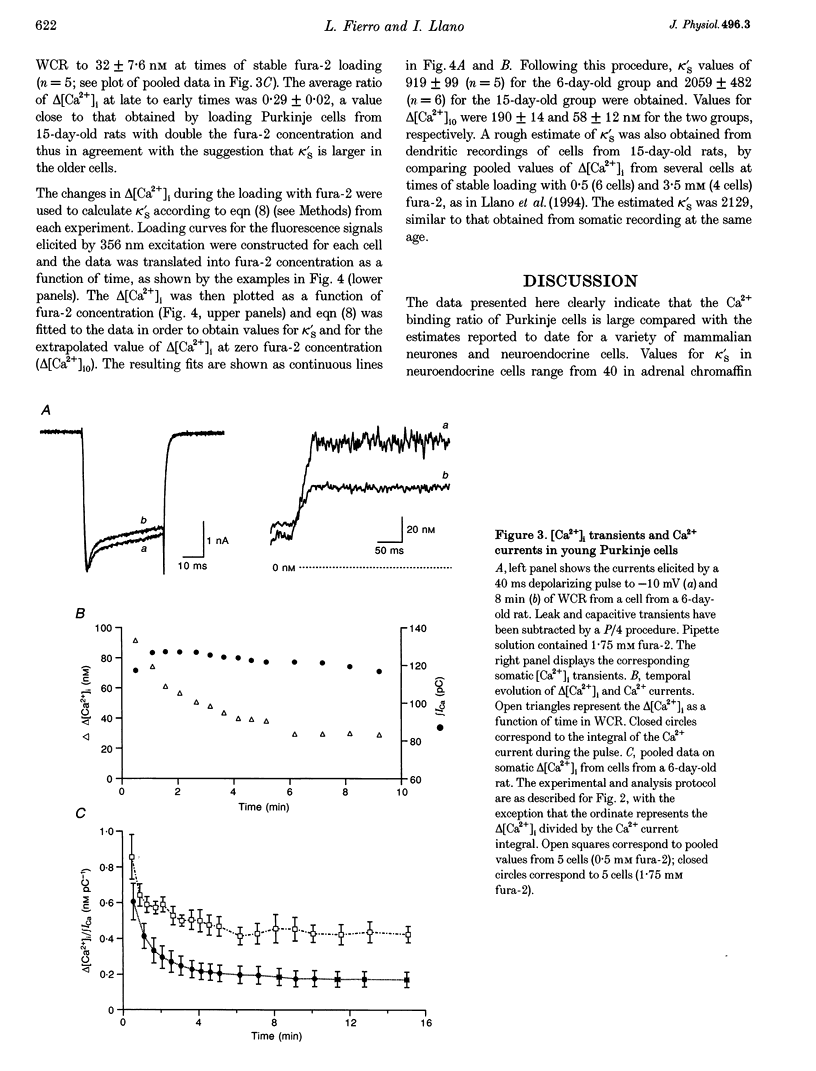
1. The ability of Purkinje cells to rapidly buffer depolarization-evoked intracellular calcium changes (delta [Ca2+]i) was estimated by titrating the endogenous buffer against incremental concentrations of the Ca(2+)-sensitive dye fura-2. 2. In cells from 15-day-old rats, pulse-evoked delta [Ca2+]i were stable during the loading with 0.5 mM fura-2 through the patch pipette. In cells from 6-day-old rats, delta [Ca2+]i decreased by approximately 50% during equivalent experiments. This decrease was not related to changes in Ca2+ influx, since the integral of the Ca2+ currents remained constant throughout the recording. 3. Experiments with high fura-2 concentrations (1.75-3.5 mM) were performed in order to obtain for each cell the curve relating delta [Ca2+]i to fura-2 concentration. From this relationship, values for the Ca2+ binding ratio (the ratio of buffer-bound Ca2+ changes over free Ca2+ changes) were calculated. 4. In Purkinje cells from 15-day-old rats, the Ca2+ binding ratio was approximately 2000, an order of magnitude larger than that of other neurones and neuroendocrine cells studied to date. This Ca2+ binding ratio was significantly smaller (approximately 900) in Purkinje cells from 6-day-old rats. 5. We propose that the large Ca2+ binding ratio of Purkinje cells is related to the presence of large concentrations of Ca2+ binding proteins and that these cells regulate their ability to handle Ca2+ loads during development through changes in the concentration of Ca2+ binding proteins.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baimbridge K. G., Celio M. R., Rogers J. H. Calcium-binding proteins in the nervous system. Trends Neurosci. 1992 Aug;15(8):303–308. doi: 10.1016/0166-2236(92)90081-i. [DOI] [PubMed] [Google Scholar]
- Baylor S. M., Hollingworth S. Fura-2 calcium transients in frog skeletal muscle fibres. J Physiol. 1988 Sep;403:151–192. doi: 10.1113/jphysiol.1988.sp017244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatter L. A., Wier W. G. Intracellular diffusion, binding, and compartmentalization of the fluorescent calcium indicators indo-1 and fura-2. Biophys J. 1990 Dec;58(6):1491–1499. doi: 10.1016/S0006-3495(90)82494-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borst J. G., Helmchen F., Sakmann B. Pre- and postsynaptic whole-cell recordings in the medial nucleus of the trapezoid body of the rat. J Physiol. 1995 Dec 15;489(Pt 3):825–840. doi: 10.1113/jphysiol.1995.sp021095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chard P. S., Bleakman D., Christakos S., Fullmer C. S., Miller R. J. Calcium buffering properties of calbindin D28k and parvalbumin in rat sensory neurones. J Physiol. 1993 Dec;472:341–357. doi: 10.1113/jphysiol.1993.sp019950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Virgilio F., Steinberg T. H., Swanson J. A., Silverstein S. C. Fura-2 secretion and sequestration in macrophages. A blocker of organic anion transport reveals that these processes occur via a membrane transport system for organic anions. J Immunol. 1988 Feb 1;140(3):915–920. [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Heizmann C. W., Hunziker W. Intracellular calcium-binding proteins: more sites than insights. Trends Biochem Sci. 1991 Mar;16(3):98–103. doi: 10.1016/0968-0004(91)90041-s. [DOI] [PubMed] [Google Scholar]
- Helmchen F., Imoto K., Sakmann B. Ca2+ buffering and action potential-evoked Ca2+ signaling in dendrites of pyramidal neurons. Biophys J. 1996 Feb;70(2):1069–1081. doi: 10.1016/S0006-3495(96)79653-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrickson A. E., Van Brederode J. F., Mulligan K. A., Celio M. R. Development of the calcium-binding protein parvalbumin and calbindin in monkey striate cortex. J Comp Neurol. 1991 May 22;307(4):626–646. doi: 10.1002/cne.903070409. [DOI] [PubMed] [Google Scholar]
- Iacopino A. M., Rhoten W. B., Christakos S. Calcium binding protein (calbindin-D28k) gene expression in the developing and aging mouse cerebellum. Brain Res Mol Brain Res. 1990 Oct;8(4):283–290. doi: 10.1016/0169-328x(90)90041-b. [DOI] [PubMed] [Google Scholar]
- Kosaka T., Kosaka K., Nakayama T., Hunziker W., Heizmann C. W. Axons and axon terminals of cerebellar Purkinje cells and basket cells have higher levels of parvalbumin immunoreactivity than somata and dendrites: quantitative analysis by immunogold labeling. Exp Brain Res. 1993;93(3):483–491. doi: 10.1007/BF00229363. [DOI] [PubMed] [Google Scholar]
- Kurobe N., Inaguma Y., Shinohara H., Semba R., Inagaki T., Kato K. Developmental and age-dependent changes of 28-kDa calbindin-D in the central nervous tissue determined with a sensitive immunoassay method. J Neurochem. 1992 Jan;58(1):128–134. doi: 10.1111/j.1471-4159.1992.tb09287.x. [DOI] [PubMed] [Google Scholar]
- Li Z., Decavel C., Hatton G. I. Calbindin-D28k: role in determining intrinsically generated firing patterns in rat supraoptic neurones. J Physiol. 1995 Nov 1;488(Pt 3):601–608. doi: 10.1113/jphysiol.1995.sp020993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llano I., DiPolo R., Marty A. Calcium-induced calcium release in cerebellar Purkinje cells. Neuron. 1994 Mar;12(3):663–673. doi: 10.1016/0896-6273(94)90221-6. [DOI] [PubMed] [Google Scholar]
- Llano I., Marty A., Armstrong C. M., Konnerth A. Synaptic- and agonist-induced excitatory currents of Purkinje cells in rat cerebellar slices. J Physiol. 1991 Mar;434:183–213. doi: 10.1113/jphysiol.1991.sp018465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lledo P. M., Somasundaram B., Morton A. J., Emson P. C., Mason W. T. Stable transfection of calbindin-D28k into the GH3 cell line alters calcium currents and intracellular calcium homeostasis. Neuron. 1992 Nov;9(5):943–954. doi: 10.1016/0896-6273(92)90246-a. [DOI] [PubMed] [Google Scholar]
- Llinás R., Sugimori M. Calcium conductances in Purkinje cell dendrites: their role in development and integration. Prog Brain Res. 1979;51:323–334. doi: 10.1016/S0079-6123(08)61312-6. [DOI] [PubMed] [Google Scholar]
- Malgaroli A., Milani D., Meldolesi J., Pozzan T. Fura-2 measurement of cytosolic free Ca2+ in monolayers and suspensions of various types of animal cells. J Cell Biol. 1987 Nov;105(5):2145–2155. doi: 10.1083/jcb.105.5.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marty A., Llano I. Modulation of inhibitory synapses in the mammalian brain. Curr Opin Neurobiol. 1995 Jun;5(3):335–341. doi: 10.1016/0959-4388(95)80046-8. [DOI] [PubMed] [Google Scholar]
- Neher E., Augustine G. J. Calcium gradients and buffers in bovine chromaffin cells. J Physiol. 1992 May;450:273–301. doi: 10.1113/jphysiol.1992.sp019127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E. The use of fura-2 for estimating Ca buffers and Ca fluxes. Neuropharmacology. 1995 Nov;34(11):1423–1442. doi: 10.1016/0028-3908(95)00144-u. [DOI] [PubMed] [Google Scholar]
- Plogmann D., Celio M. R. Intracellular concentration of parvalbumin in nerve cells. Brain Res. 1993 Jan 15;600(2):273–279. doi: 10.1016/0006-8993(93)91383-4. [DOI] [PubMed] [Google Scholar]
- Regehr W. G., Atluri P. P. Calcium transients in cerebellar granule cell presynaptic terminals. Biophys J. 1995 May;68(5):2156–2170. doi: 10.1016/S0006-3495(95)80398-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regehr W. G., Tank D. W. Dendritic calcium dynamics. Curr Opin Neurobiol. 1994 Jun;4(3):373–382. doi: 10.1016/0959-4388(94)90099-x. [DOI] [PubMed] [Google Scholar]
- Roberts W. M. Localization of calcium signals by a mobile calcium buffer in frog saccular hair cells. J Neurosci. 1994 May;14(5 Pt 2):3246–3262. doi: 10.1523/JNEUROSCI.14-05-03246.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai M. Calcium is an intracellular mediator of the climbing fiber in induction of cerebellar long-term depression. Proc Natl Acad Sci U S A. 1990 May;87(9):3383–3385. doi: 10.1073/pnas.87.9.3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäfer B. W., Heizmann C. W. The S100 family of EF-hand calcium-binding proteins: functions and pathology. Trends Biochem Sci. 1996 Apr;21(4):134–140. doi: 10.1016/s0968-0004(96)80167-8. [DOI] [PubMed] [Google Scholar]
- Stuenkel E. L. Regulation of intracellular calcium and calcium buffering properties of rat isolated neurohypophysial nerve endings. J Physiol. 1994 Dec 1;481(Pt 2):251–271. doi: 10.1113/jphysiol.1994.sp020436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatsumi H., Katayama Y. Regulation of the intracellular free calcium concentration in acutely dissociated neurones from rat nucleus basalis. J Physiol. 1993 May;464:165–181. doi: 10.1113/jphysiol.1993.sp019628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P., Surprenant A., Almers W. Cytosolic Ca2+, exocytosis, and endocytosis in single melanotrophs of the rat pituitary. Neuron. 1990 Nov;5(5):723–733. doi: 10.1016/0896-6273(90)90226-6. [DOI] [PubMed] [Google Scholar]
- Tse A., Tse F. W., Hille B. Calcium homeostasis in identified rat gonadotrophs. J Physiol. 1994 Jun 15;477(Pt 3):511–525. doi: 10.1113/jphysiol.1994.sp020212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villa A., Podini P., Panzeri M. C., Racchetti G., Meldolesi J. Cytosolic Ca2+ binding proteins during rat brain ageing: loss of calbindin and calretinin in the hippocampus, with no change in the cerebellum. Eur J Neurosci. 1994 Sep 1;6(9):1491–1499. doi: 10.1111/j.1460-9568.1994.tb01010.x. [DOI] [PubMed] [Google Scholar]
- Zhou Z., Neher E. Mobile and immobile calcium buffers in bovine adrenal chromaffin cells. J Physiol. 1993 Sep;469:245–273. doi: 10.1113/jphysiol.1993.sp019813. [DOI] [PMC free article] [PubMed] [Google Scholar]


