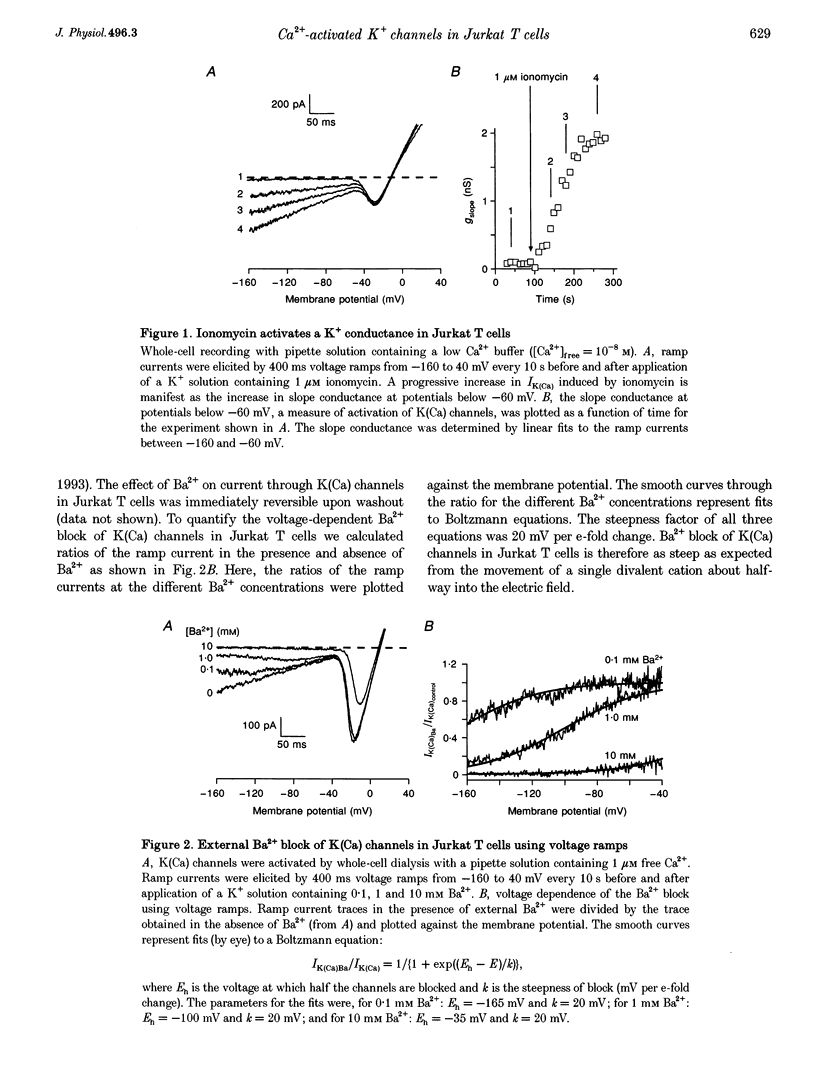
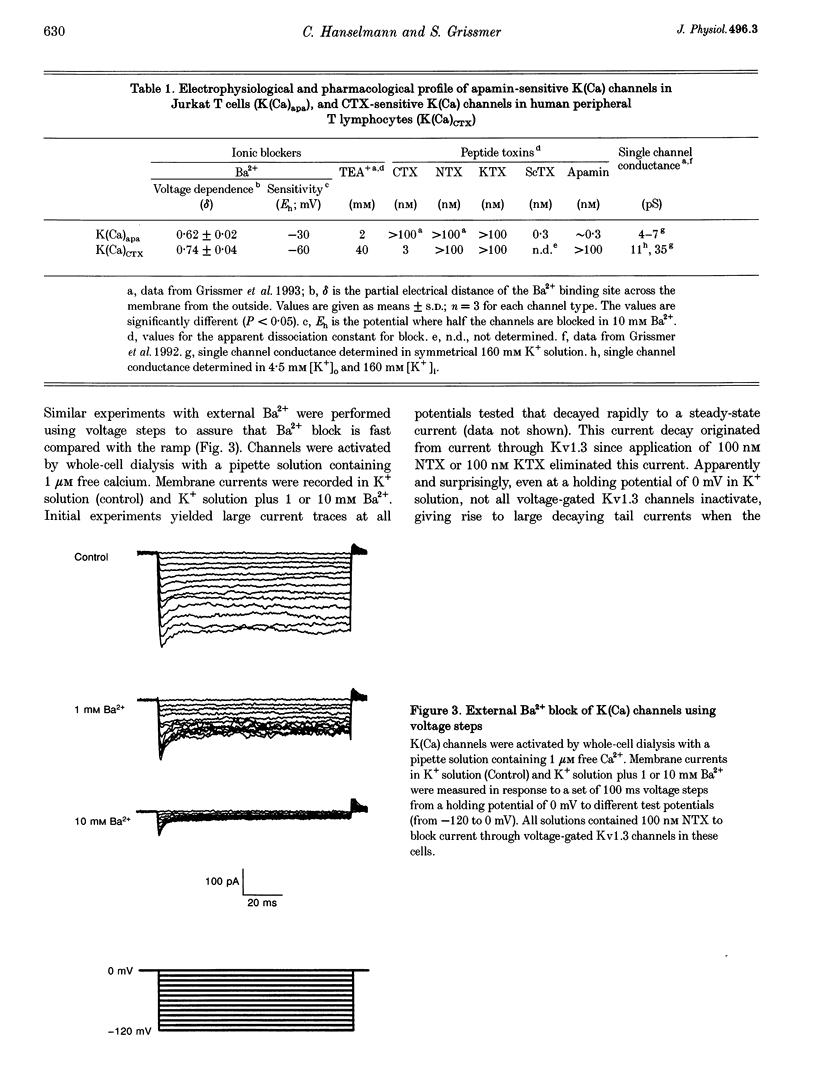
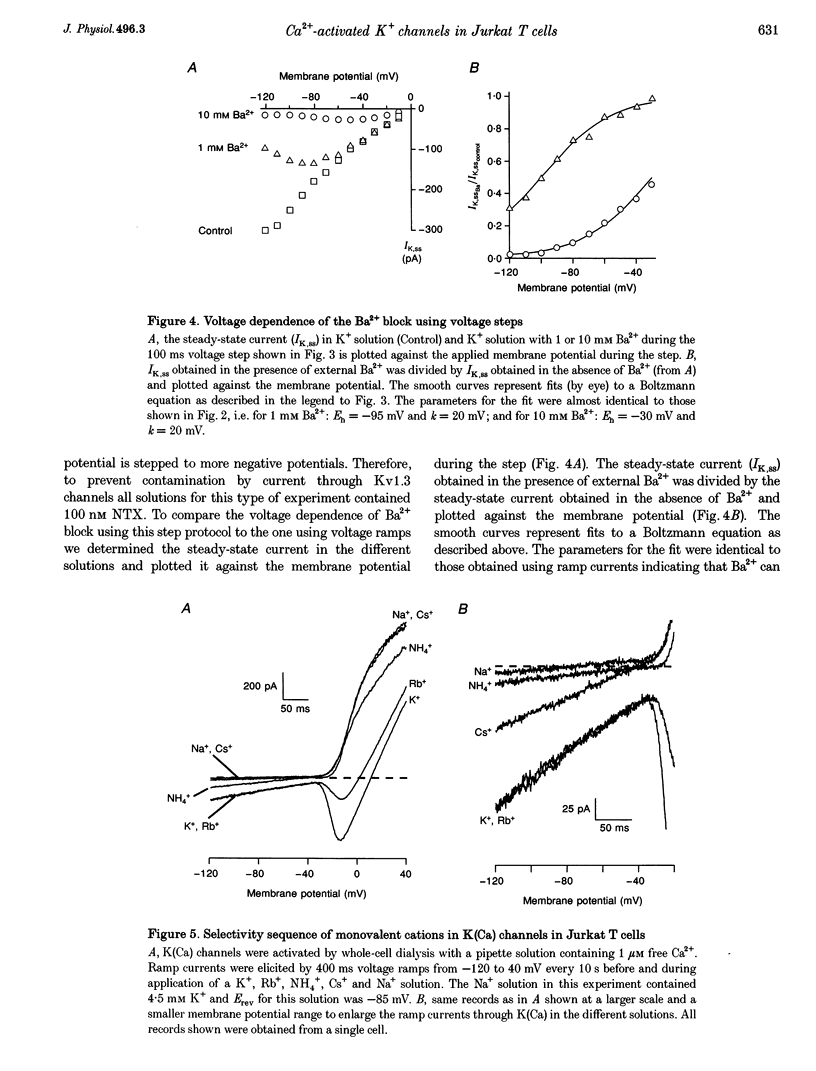
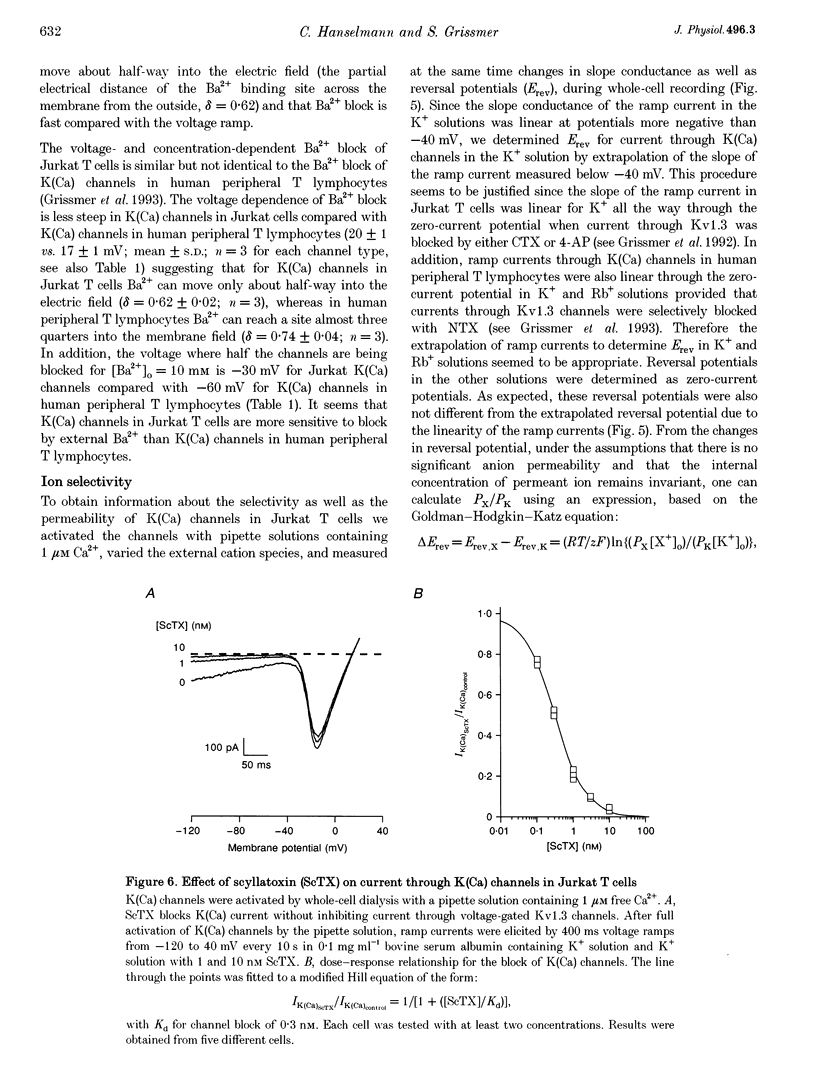
Abstract
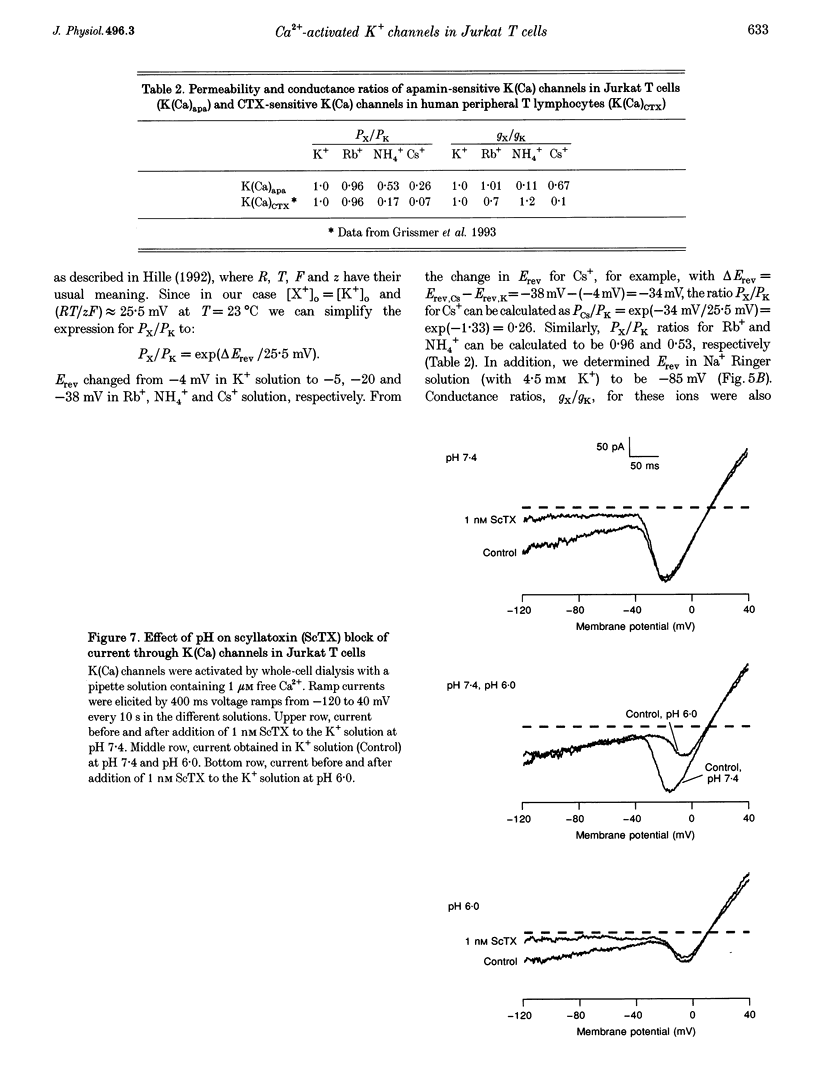
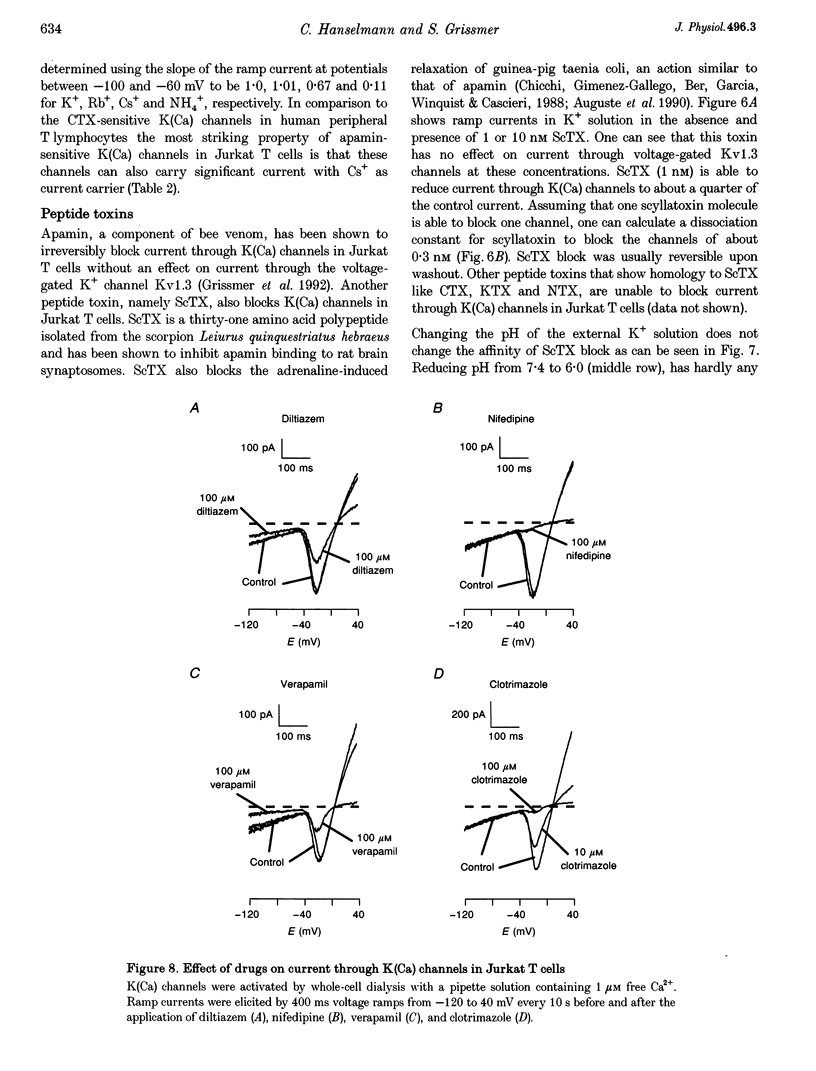
1. The whole-cell recording mode of the patch-clamp technique was used to study the effect of extracellularly applied ions, toxins and drugs on voltage-independent, apamin-sensitive Ca(2+)-activated K+ channels, K(Ca), expressed in the Jurkat human leukaemic T cell line. 2. Extracellular Ba2+ and Sr+ produced a voltage-dependent block. The equilibrium dissociation constant of the Ba2+/K(Ca) channel complex increased e-fold for a 20 mV change of potential. Ba2+ block of Jurkat K(Ca) channels is therefore as steep as expected from the movement of a single divalent cation about half-way into the electric field of the membrane from the outside. 3. We determined the ion selectivity as well as the conductance of these channels. Calculated permeability ratios, PX/PK, for these K(Ca) channels were 1.0, 0.96, 0.26 and 0.53 for K+, Rb+, Cs+ and NH4+, respectively. Conductance ratios, gX/gK, for the same ions were 1.0, 1.0, 0.67 and 0.11, respectively. Most strikingly this channel can also carry significant current with Cs+ as current carrier. 4. Scyllatoxin (ScTX), a thirty-one amino acid peptide toxin, reduced current through these K(Ca) channels with a half-blocking concentration of approximately 0.3 nM independent of the pH. Other drugs that were able to reduce current through these channels include the classical calcium antagonists diltiazem and verapamil. In contrast, nifedipine, clotrimazole and kaliotoxin (100 nM) were unable to block current through these channels in Jurkat T cells.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aiyar J., Withka J. M., Rizzi J. P., Singleton D. H., Andrews G. C., Lin W., Boyd J., Hanson D. C., Simon M., Dethlefs B. Topology of the pore-region of a K+ channel revealed by the NMR-derived structures of scorpion toxins. Neuron. 1995 Nov;15(5):1169–1181. doi: 10.1016/0896-6273(95)90104-3. [DOI] [PubMed] [Google Scholar]
- Alvarez J., Montero M., Garcia-Sancho J. High affinity inhibition of Ca(2+)-dependent K+ channels by cytochrome P-450 inhibitors. J Biol Chem. 1992 Jun 15;267(17):11789–11793. [PubMed] [Google Scholar]
- Auguste P., Hugues M., Gravé B., Gesquière J. C., Maes P., Tartar A., Romey G., Schweitz H., Lazdunski M. Leiurotoxin I (scyllatoxin), a peptide ligand for Ca2(+)-activated K+ channels. Chemical synthesis, radiolabeling, and receptor characterization. J Biol Chem. 1990 Mar 15;265(8):4753–4759. [PubMed] [Google Scholar]
- Blatz A. L., Magleby K. L. Single apamin-blocked Ca-activated K+ channels of small conductance in cultured rat skeletal muscle. Nature. 1986 Oct 23;323(6090):718–720. doi: 10.1038/323718a0. [DOI] [PubMed] [Google Scholar]
- Chicchi G. G., Gimenez-Gallego G., Ber E., Garcia M. L., Winquist R., Cascieri M. A. Purification and characterization of a unique, potent inhibitor of apamin binding from Leiurus quinquestriatus hebraeus venom. J Biol Chem. 1988 Jul 25;263(21):10192–10197. [PubMed] [Google Scholar]
- Cognard C., Traoré F., Potreau D., Raymond G. Effects of apamin on the outward potassium current of isolated frog skeletal muscle fibres. Pflugers Arch. 1984 Oct;402(2):222–224. doi: 10.1007/BF00583339. [DOI] [PubMed] [Google Scholar]
- Goldstein S. A., Miller C. A point mutation in a Shaker K+ channel changes its charybdotoxin binding site from low to high affinity. Biophys J. 1992 Apr;62(1):5–7. doi: 10.1016/S0006-3495(92)81760-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grissmer S., Cahalan M. D. Divalent ion trapping inside potassium channels of human T lymphocytes. J Gen Physiol. 1989 Apr;93(4):609–630. doi: 10.1085/jgp.93.4.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grissmer S., Dethlefs B., Wasmuth J. J., Goldin A. L., Gutman G. A., Cahalan M. D., Chandy K. G. Expression and chromosomal localization of a lymphocyte K+ channel gene. Proc Natl Acad Sci U S A. 1990 Dec;87(23):9411–9415. doi: 10.1073/pnas.87.23.9411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grissmer S., Lewis R. S., Cahalan M. D. Ca(2+)-activated K+ channels in human leukemic T cells. J Gen Physiol. 1992 Jan;99(1):63–84. doi: 10.1085/jgp.99.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grissmer S., Nguyen A. N., Cahalan M. D. Calcium-activated potassium channels in resting and activated human T lymphocytes. Expression levels, calcium dependence, ion selectivity, and pharmacology. J Gen Physiol. 1993 Oct;102(4):601–630. doi: 10.1085/jgp.102.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hidalgo P., MacKinnon R. Revealing the architecture of a K+ channel pore through mutant cycles with a peptide inhibitor. Science. 1995 Apr 14;268(5208):307–310. doi: 10.1126/science.7716527. [DOI] [PubMed] [Google Scholar]
- Hugues M., Schmid H., Romey G., Duval D., Frelin C., Lazdunski M. The Ca2+-dependent slow K+ conductance in cultured rat muscle cells: characterization with apamin. EMBO J. 1982;1(9):1039–1042. doi: 10.1002/j.1460-2075.1982.tb01293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latorre R., Oberhauser A., Labarca P., Alvarez O. Varieties of calcium-activated potassium channels. Annu Rev Physiol. 1989;51:385–399. doi: 10.1146/annurev.ph.51.030189.002125. [DOI] [PubMed] [Google Scholar]
- Leonard R. J., Garcia M. L., Slaughter R. S., Reuben J. P. Selective blockers of voltage-gated K+ channels depolarize human T lymphocytes: mechanism of the antiproliferative effect of charybdotoxin. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):10094–10098. doi: 10.1073/pnas.89.21.10094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C. S., Boltz R. C., Blake J. T., Nguyen M., Talento A., Fischer P. A., Springer M. S., Sigal N. H., Slaughter R. S., Garcia M. L. Voltage-gated potassium channels regulate calcium-dependent pathways involved in human T lymphocyte activation. J Exp Med. 1993 Mar 1;177(3):637–645. doi: 10.1084/jem.177.3.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKinnon R., Heginbotham L., Abramson T. Mapping the receptor site for charybdotoxin, a pore-blocking potassium channel inhibitor. Neuron. 1990 Dec;5(6):767–771. doi: 10.1016/0896-6273(90)90335-d. [DOI] [PubMed] [Google Scholar]
- Mahaut-Smith M. P., Schlichter L. C. Ca2(+)-activated K+ channels in human B lymphocytes and rat thymocytes. J Physiol. 1989 Aug;415:69–83. doi: 10.1113/jphysiol.1989.sp017712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PORTZEHL H., CALDWELL P. C., RUEEGG J. C. THE DEPENDENCE OF CONTRACTION AND RELAXATION OF MUSCLE FIBRES FROM THE CRAB MAIA SQUINADO ON THE INTERNAL CONCENTRATION OF FREE CALCIUM IONS. Biochim Biophys Acta. 1964 May 25;79:581–591. doi: 10.1016/0926-6577(64)90224-4. [DOI] [PubMed] [Google Scholar]
- Partiseti M., Choquet D., Diu A., Korn H. Differential regulation of voltage- and calcium-activated potassium channels in human B lymphocytes. J Immunol. 1992 Jun 1;148(11):3361–3368. [PubMed] [Google Scholar]
- Romey G., Rieger F., Renaud J. F., Pinçon-Raymond M., Lazdunski M. The electrophysiological expression of Ca2+ channels and of apamin sensitive Ca2+ activated K+ channels is abolished in skeletal muscle cells from mice with muscular dysgenesis. Biochem Biophys Res Commun. 1986 May 14;136(3):935–940. doi: 10.1016/0006-291x(86)90422-5. [DOI] [PubMed] [Google Scholar]
- Sands S. B., Lewis R. S., Cahalan M. D. Charybdotoxin blocks voltage-gated K+ channels in human and murine T lymphocytes. J Gen Physiol. 1989 Jun;93(6):1061–1074. doi: 10.1085/jgp.93.6.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stampe P., Kolmakova-Partensky L., Miller C. Intimations of K+ channel structure from a complete functional map of the molecular surface of charybdotoxin. Biochemistry. 1994 Jan 18;33(2):443–450. doi: 10.1021/bi00168a008. [DOI] [PubMed] [Google Scholar]
- Verheugen J. A., Vijverberg H. P., Oortgiesen M., Cahalan M. D. Voltage-gated and Ca(2+)-activated K+ channels in intact human T lymphocytes. Noninvasive measurements of membrane currents, membrane potential, and intracellular calcium. J Gen Physiol. 1995 Jun;105(6):765–794. doi: 10.1085/jgp.105.6.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweifach A., Lewis R. S. Mitogen-regulated Ca2+ current of T lymphocytes is activated by depletion of intracellular Ca2+ stores. Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):6295–6299. doi: 10.1073/pnas.90.13.6295. [DOI] [PMC free article] [PubMed] [Google Scholar]


