Abstract
Background:
Pulse oximetry is a valuable tool for monitoring animals during anesthesia and assessing the adequacy of administered oxygen therapy.
Aims:
To compare the pulse oximeter readings obtained by the Garmin Fenix 5X plus (GF5Xp) smartwatch and transmittance pulse oximetry (TPO) in anesthetized dogs.
Methods:
Twelve clinical canine patients requiring anesthesia for castration were prospectively enrolled in this study. The animals were premedicated with intramuscular dexmedetomidine at a dose of 5 µg/kg. Anesthesia was induced through intravenous administration of propofol and maintained using sevoflurane. The arterial hemoglobin oxygen saturation (SpO2) readings obtained from the tongue using TPO (238 readings) were compared with measurements taken over the lateral side of the tibia using a GF5Xp smart wearable device (238 readings). This comparison was performed using a Bland-Altman plot, where the differences (%) between the methods were plotted against their mean SpO2 (Gold standard - Device), and the limits of agreement were represented as the mean ± 1.96 times the standard deviation.
Results:
The SpO2 levels in dogs were overestimated by the GF5Xp relative to the readings obtained by the TPO, with the bias of -0.3% (95% CI: -3.1%-2.5%).
Conclusion:
GF5Xp may be interchangeable with TPO in dogs. Further studies are required to validate the accuracy of the GF5Xp in non-anesthetized dogs or dogs outside the physiological range.
Key Words: Canine, Hemoglobin, Oxygen, Propofol, Pulse oximetry
Introduction
Hypoxemia, characterized by low oxygen levels in the blood, can have significant health implications (Ayres, 2012). Various factors contribute to its development, including respiratory diseases, cardiovascular disorders, and external factors such as high-altitude environments. Clinical signs of hypoxemia encompass dyspnea, cyanosis, exercise intolerance, weakness, and collapse (Bach, 2017; Jagodich et al., 2020). Prolonged oxygen deprivation can compromise vital organs like the heart, brain, and kidneys, leading to severe complications and potentially life-threatening consequences. Thus, early recognition and prompt intervention are crucial in managing hypoxemia, improving outcomes, and alleviating suffering (Haskins, 2014).
The partial pressure of oxygen (PaO2) and hemoglobin oxygen saturation (SaO2) are widely acknowledged as one of the standards for assessing hypoxemia. Nonetheless, obtaining arterial blood samples can be difficult in animals experiencing respiratory distress or easily becoming distressed (Hofmeister et al., 2005). Pulse oximetry provides a minimally invasive technique to estimate arterial blood hemoglobin oxygen saturation. Pulse oximeters are valuable instruments for monitoring animals during anesthesia and determining the effectiveness of administered oxygen therapy (Arulpagasam et al., 2018).
Transmittance pulse oximetry (TPO) is a widely employed method for monitoring arterial hemoglobin oxygen saturation (SpO2). This technique involves absorbing infrared light as it passes through the tissues from one side to the opposite (Nixdorff et al., 2021). Human smart wearable devices are equipped with photoplethysmographic sensors to measure SpO2 levels. Previous studies in humans have concluded that wearable devices could monitor SpO2 with high accuracy (Buekers et al., 2019; Lauterbach et al., 2021), whereas some researchers have reported inaccurate SpO2 readings (Hermand et al., 2021; Caruso and Shefflette, 2022). While the accuracy of Garmin Fēnix® 5X Plus (GF5Xp) in measuring SpO2 has been demonstrated in cats (Yanmaz et al., 2023), there is currently no research evaluating its reliability and usefulness in dogs. Therefore, this study aimed to compare a GF5Xp smartwatch with TPO in measuring SpO2 in dogs.
Materials and Methods
The study protocol (ATA-36643897-000-2100037577) was approved by the AtatürkXX University local ethical committee for animal experiments. Written informed consent from the owners was received for each participating dog. All procedures involving animals adhered to the guidelines set forth by the European Community Council Directive of 24 November 1986.
Animals
The study included twelve healthy male Shepherd dogs, owned by clients, aged between 1 and 2 years and weighing 15 and 20 kg. These dogs were referred to Atatürk University Animal Hospital for elective orchiectomy procedures between February 10 and March 10, 2022. The health status of the dogs was evaluated, including a physical examination, complete blood count, serum biochemistry profile, and radiological analysis. Only dogs with the American Society of Anesthesiologists (ASA) score of I were included in the study. Dogs were excluded from the study if they had any underlying disease, cardiac arrhythmias, hypoxemia, were less than 12 months old or more than two years old, or had an ASA score greater than 1.
Before the procedures, the dogs underwent a fasting period of 6 h, during which water was not withheld. For premedication, an intramuscular dose of 5 µg/kg dexmedetomidine (Precedex 0.1 mg/ml, Hospira, Inc., NC, USA) was administered. Anesthesia was induced by intravenous administration of propofol (Propofol, 20 mg/ml, Fresenius Kabi, Austria) until jaw relaxation was achieved. Following induction, the dogs were orotracheally intubated and maintained under anesthesia using 2% sevoflurane (Sevorane, 200 ml, Aesica Queenborough Ltd., UK). Subsequently, the dogs were placed in a dorsal recumbency position for the duration of the procedures.
Study design
After the induction, a TPO pulse oximeter probe (Cardell 9405, Sharn Veterinary Inc., Tampa, Florida) was placed on the animal’s tongue to measure SpO2 levels. The hair surrounding the tibia was shaved using a No. 40 hair clipper. The GF5Xp smart wearable device (Garmin Ltd., KS, USA) was attached to the lateral side of the tibia using a watch strap without any bandage material for fixation (Fig. 1). Each SpO2 reading was manually recorded by the same person. Two individuals, one for the GF5Xp and another for the TPO, simultaneously collected the recordings. The GF5Xp measurements were obtained by pressing the activation button on the wearable device. For each dog, the GF5Xp and TPO data were recorded at one-minute intervals for 20 min. Following the surgery, the connections of the GF5Xp and TPO were removed, and the animal was then moved to the recovery room.
Fig. 1.
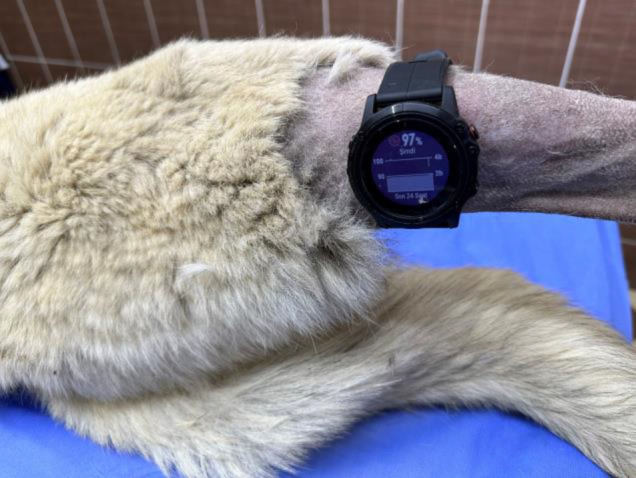
Illustration demonstrating the positioning of the Garmin Fenix 5X plus smart wearable device on the tibia to collect arterial hemoglobin oxygen saturation readings
Statistical analysis
The sample size for the study was determined using PS-Power and Sample Size calculation software (Version 3.1.2, Tennessee). It aimed to detect the minimum number of measurements required in each device to observe a 5% difference in SpO2 levels. Thus, a total of 12 animals (238 measurements) were deemed necessary. A significance level of 0.05 and a power of 90% were chosen for the analysis. The analysis was based on data from a previous study that compared SpO2 measurements in cats using the same smartwatch (Yanmaz et al., 2023). Statistical analysis was conducted using commercial software (Version 13.2.2; MedCalc, Belgium). The normality of continuous variables was assessed using the Shapiro-Wilk test. Student’s t-tests were applied to analyze the readings obtained from the GF5Xp and TPO. The Chi-square analysis was used to compare the number of unsuccessful SpO2 measurements from each device. The agreement between TPO and GF5Xp was assessed using the Bland-Altman plot (Bland and Altman, 1999). This plot displayed the percentage differences between the methods against their mean SpO2 (Gold standard - Device). The limit of agreement was represented as the mean ± 1.96 times the standard deviation. A P-value of less than 0.05 was considered statistically significant. The data were reported as mean ± standard deviation or median (range).
Results
During SpO2 measurements, four unsuccessful readings were observed, with two occurring in the TPO group and two in the GF5Xp group (P>0.05). In total, 476 readings (238 for TPO, 238 for GF5Xp) were obtained. The mean SpO2 readings with GF5Xp was 96.63 ± 2.39% and with the TPO was 96.33 ± 2.28% (P=0.16). The Bland-Altman scatter plot showed that the mean difference between the two devices was -0.29, with LOA in the range of -0.47 to -0.10 (Table 1). The SpO2 levels in dogs were overestimated by the GF5Xp relative to the data obtained by the TPO, wherein the bias was -0.3% (95% Confidence of Interval: -3.1%-2.5%) (P=0.0023; Fig. 2).
Table 1.
Regression equations from Bland-Altman analysis comparing bias of arterial hemoglobin oxygen saturation (%) between Garmin Fenix 5X Plus (Y) and transmittance pulse oximetry (X)
| Mean (95% CI) | Difference (%) | ||
|---|---|---|---|
| Lower limit (95% CI) | Upper limit (95% CI) | Regression equation R2=0.6595 | |
| -0.29 (-0.47 to -0.10) | -3.10 (-3.42 to -2.79) | 2.53 (2.21 to 2.84) | Y=4.2413 + -0.04696 X, P<0.01 |
CI: Confidence of interval
Fig. 2.
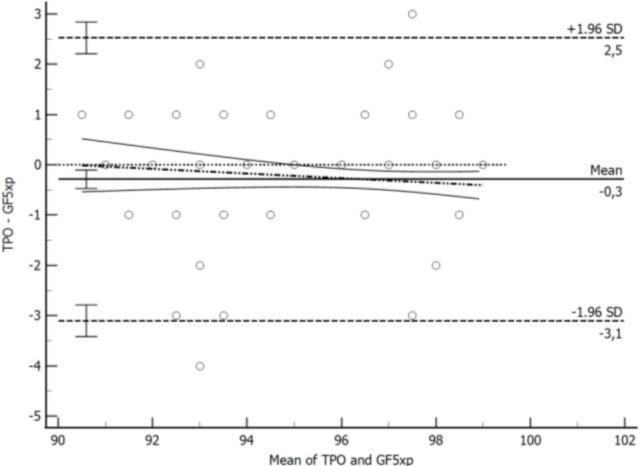
The figure illustrates the concordance between arterial hemoglobin oxygen saturation measurements obtained from the Garmin Fenix 5X Plus (GF5Xp) smartwatch and transmittance pulse oximetry (TPO) using Bland-Altman analysis. The Bland-Altman plots provide insight into the level of agreement between the GF5Xp and TPO measurements. The solid black line represents a mean bias -0.3% (95% CI: -3.1%-2.5%). The uppermost and lowermost dashed lines indicate the upper and lower agreement limits. The dotted line signifies a 0% difference, while the dotted-and-dashed lines represent the best fit. The curvilinear solid lines represent the 95% CI of the best fit. Error bars display the respective 95% CIs of the mean bias and limits of agreement
Discussion
This study aimed to compare the SpO2 readings obtained using a GF5Xp wearable smart device and TPO in dogs. The main finding was that the SpO2 levels in dogs were overestimated by GF5Xp relative to the readings obtained by TPO, wherein the bias was -0.3% (95% CI: -3.1%-2.5%). A previous study emphasized low validity at high attitudes using GF5Xp (Schiefer et al., 2021). However, another study that used GF5Xp reported a minimal overestimation of SpO2 during exposure to different simulated altitudes (Lauterbach et al., 2021). Similar findings were observed in the present study. The low limit of agreement between devices in the current study should be related to the stable conditions of animals due to anesthesia. A study has reported that even a slight change in the measurement site may affect SpO2 readings, and smart devices should be in close contact with the arteries (Lee et al., 2016). This study demonstrated that SpO2 readings are reliably obtained on the lateral side of the tibia as they contain the saphenous artery.
The current study identified four instances of unsuccessful SpO2 readings during measurements, with two occurrences observed in the TPO and two in the GF5Xp devices. The presence of two unsuccessful readings in anesthetized dogs could be attributed to various factors, including challenges in tongue positioning, interference from saliva and moisture, and issues with sensor attachment (Matthews et al., 2003). Traditional pulse oximeters typically function by emitting light wavelengths through tissues to detect changes in oxygenated and deoxygenated hemoglobin absorption, while smart wearable devices such as GF5Xp utilize similar principles but in a compact, portable form. However, unsuccessful SpO2 readings in GF5Xp may also be influenced by motion or excessive movement during surgery, a phenomenon observed in human studies (Williamson et al., 2018). These findings highlight the importance of considering and addressing these factors to improve the reliability and accuracy of SpO2 measurements using both traditional pulse oximeters and smart wearable devices. Future research can explore strategies to minimize the occurrence of unsuccessful readings and enhance the overall performance of SpO2 monitoring in veterinary medicine, potentially even using human smartwatches for such purposes.
Most pulse oximeters update SpO2 data every 0.5-1.0 seconds (Hanning and Alexander-Williams, 1995). However, the GF5Xp pulse oximeter differs in its approach, as it displays SpO2 readings only when the SpO2 function is activated. This particular design characteristic poses challenges in obtaining concurrent data from the device. By requiring the activation button to be pressed, the ability to continuously evaluate oxygen status in patients may be compromised. This limitation can hinder real-time monitoring and potentially delay the detection of sudden changes in oxygen saturation levels. Consequently, healthcare professionals must consider the potential drawbacks associated with the design of the GF5Xp pulse oximeter when selecting monitoring devices for patients requiring continuous oxygen assessment. Additionally, devices designed for use in animals without the need for shaving may also be developed.
The main limitation of the present study was the lack of a validated monitor. In the authors’ university animal hospital setting, the pulse oximetry device used in this study is routinely used for monitoring pulse oximetry readings. Therefore, we assumed that the SpO2 readings were accurate. Another limitation of this study was the lack of a gold standard. The use of SpO2 instead of PaO2 to detect arterial oxygenation in a dog breathing room air is not clinically acceptable (Farrell et al., 2019). The lack of heart rate and arterial blood pressure measurements can be considered a limitation. Besides, a recent study showed accurate heart rate variables in dogs using GF5Xp (Yanmaz et al., 2022). Further studies are needed to confirm the accuracy of GF5Xp in non-anesthetized dogs or dogs outside the physiological range.
This study examined the utility of the GF5Xp smart wearable device for assessing SpO2 levels in dogs. The findings suggest that the GF5Xp device can effectively be used as an alternative to TPO in anesthetized dogs. However, it is crucial to recognize that extensive shaving of a dog’s limbs may not be tolerated by all owners. Further research is warranted to validate the accuracy and reliability of SpO2 readings obtained from other popular smart wearable devices in canine. By investigating the performance of various devices, clinicians and researchers can gain a better understanding of the potential applications and limitations of smart wearable technologies in veterinary medicine. This knowledge will ultimately contribute to enhancing the monitoring and management of oxygenation status in dogs, leading to improved patient care and outcomes.
Acknowledgements
This study did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interest
The authors declare that there is no conflict of interest.
References
- 1.Arulpagasam S, Lux C, Odunayo A, Biskup J, Sun X. Evaluation of pulse oximetry in healthy brachycephalic dogs. J. Am. Anim. Hosp. Assoc. 2018;54:344–350. doi: 10.5326/JAAHA-MS-6654. [DOI] [PubMed] [Google Scholar]
- 2.Ayres, DA. Pulse oximetry and CO-oximetry. In: Burkitt Creedon, JM, Davis, H., editors. Advanced monitoring and procedures for small animal emergency and critical care. 1st Edn. Oxford, United Kingdom: Wiley-Blackwell ; 2012. pp. 274–285. [Google Scholar]
- 3.Bach J. A quick reference on hypoxemia. Vet. Clin. North Am. Small. Anim. Pract. 2017;47:175–179. doi: 10.1016/j.cvsm.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 4.Bland JM, Altman DG. Measuring agreement in method comparison studies. Stat. Methods Med. Res. 1999;8:135–160. doi: 10.1177/096228029900800204. [DOI] [PubMed] [Google Scholar]
- 5.Buekers J, Theunis J, De Boever P, Vaes AW, Koopman M, Janssen EV, Wouters EF, Spruit MA, Aerts J. Wearable finger pulse oximetry for continuous oxygen saturation measurements during daily home routines of patients with chronic obstructive pulmonary disease (COPD) over one week: observational study. JMIR mHealth and uHealth. 2019;7:e12866. doi: 10.2196/12866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caruso JF, Shefflette ACS. Intrarater reliability and repeatability of pulse oximetry values obtained before, between sets, and after resistive exercise. Isokinet. Exerc. Sci. 2022;30:89–97. [Google Scholar]
- 7.Farrell KS, Hopper K, Cagle LA, Epstein S. Evaluation of pulse oximetry as a surrogate for PaO2 in awake dogs breathing room air and anesthetized dogs on mechanical ventilation. J. Vet. Emerg. Crit. Care. 2019;29:622–629. doi: 10.1111/vec.12898. [DOI] [PubMed] [Google Scholar]
- 8.Hanning CD, Alexander-Williams JM. Pulse oximetry: a practical review. Br. Med. J. 1995;311:367–370. doi: 10.1136/bmj.311.7001.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haskins, SC. In: Hypoxemia. Silverstein, D, Hopper, K, editors. Elsevier; 2014. pp. 81–86. [Google Scholar]
- 10.Hermand E, Coll C, Richalet JP, Lhuissier FJ. Accuracy and reliability of pulse O2 saturation measured by a wrist-worn oximeter. Int. J. Sports M. 2021;42:1268–1273. doi: 10.1055/a-1337-2790. [DOI] [PubMed] [Google Scholar]
- 11.Hofmeister EH, Read MR, Brainard BM. Evaluating veterinarians’ and veterinary students’ knowledge and clinical use of pulse oximetry. J. Vet. Med. Educ. 2005;32:272–277. doi: 10.3138/jvme.32.2.272. [DOI] [PubMed] [Google Scholar]
- 12.Jagodich TA, Bersenas AM, Bateman SW, Kerr CL. High-flow nasal cannula oxygen therapy in acute hypoxemic respiratory failure in 22 dogs requiring oxygen support escalation. J. Vet. Emerg. Crit. Care. 2020;30:364–375. doi: 10.1111/vec.12970. [DOI] [PubMed] [Google Scholar]
- 13.Lauterbach CJ, Romano PA, Greisler LA, Brindle RA, Ford KR, Kuennen MR. Accuracy and reliability of commercial wrist-worn pulse oximeter during normobaric hypoxia exposure under resting conditions. Res. Q. Exerc. Sport. 2021;92:549–558. doi: 10.1080/02701367.2020.1759768. [DOI] [PubMed] [Google Scholar]
- 14.Lee H, Ko H, Lee J. Reflectance pulse oximetry: Practical issues and limitations. ICT. Express. 2016;2:195–198. [Google Scholar]
- 15.Matthews NS, Hartke S, Allen JC. An evaluation of pulse oximeters in dogs, cats and horses. Vet. Anaesth. Analg. 2003;30:3–14. doi: 10.1046/j.1467-2995.2003.00121.x. [DOI] [PubMed] [Google Scholar]
- 16.Nixdorff J, Zablotski Y, Hartmann K, Dorfelt R. Comparison of transmittance and reflectance pulse oximetry in anesthetized dogs. Front. Vet. Sci. 2021;8:643966. doi: 10.3389/fvets.2021.643966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schiefer LM, Treff G, Treff F, Schmidt P, Schäfer L, Niebauer J, Swenson KE, Swenson ER, Berger MM, Sareban M. Validity of peripheral oxygen saturation measurements with the Garmin Fēnix ® 5X plus wearable device at 4559 m. Sensors (Basel) 2021;21:6363. doi: 10.3390/s21196363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yanmaz LE, Okur S, Ersoz U, Senocak MG, Turgut F. Accuracy of heart rate measurements of three smartwatch models in dogs. Top. Companion Anim. Med. 2022;49:100654. doi: 10.1016/j.tcam.2022.100654. [DOI] [PubMed] [Google Scholar]
- 20.Yanmaz LE, Okur S, Ersoz U, Senocak MG, Turgut F. Two different smartwatches exhibit high accuracy in evaluating heart rate and peripheral oxygen saturation in cats when compared with the electrocardiography and transmittance pulse oximetry. J. Am. Vet. Med. Assoc. 2023;261:205–209. doi: 10.2460/javma.22.08.0357. [DOI] [PubMed] [Google Scholar]


