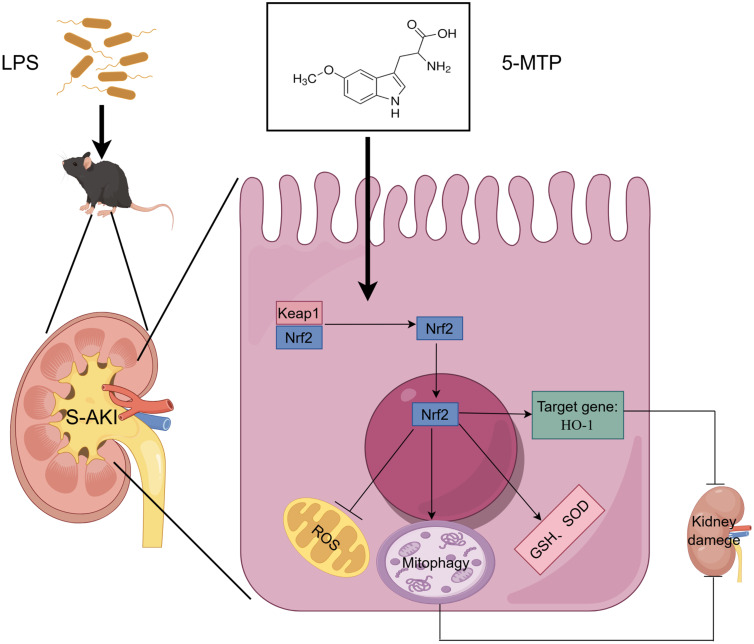
Abstract
Purpose
The effects of 5-methoxytryptophan (5-MTP) on mitophagy in sepsis-induced acute kidney injury (S-AKI) and its possible role in the Nrf2/HO-1 signaling pathway are unclear. In this study, we aimed to examine the levels of serum 5-MTP and mitophagy in patients with S-AKI and to evaluate the influence of 5-MTP on a lipopolysaccharide(LPS)-induced AKI model. Additionally, we sought to elucidate the mechanisms by which 5-MTP regulates mitophagy via Nrf2 mediation.
Patients and Methods
We initially included 52 patients with sepsis, 25 of whom were diagnosed with AKI, and used metabolomics to analyze the serum levels of 5-MTP. We investigated the effects of exogenous 5-MTP on the kidneys of a mouse model with LPS-induced AKI. We explored the underlying mechanisms by assessing oxidative stress and mitophagy in the kidneys following the administration of different doses of 5-MTP to S-AKI mice. In addition, we used ML385 to inhibit Nrf2 expression and assessed mitophagy levels in kidney damage to investigate the specific mechanism by which 5-MTP mitigates S-AKI.
Results
The plasma 5-MTP levels were significantly higher in patients with S-AKI than in those with sepsis, showing a correlation with renal function. Administration of 5-MTP led to a decrease in inflammatory and oxidative stress reactions and stimulated the Nrf2 signaling pathway to alleviate kidney injury following the induction of sepsis. However, this protective effect was reversed by ML385. In S-AKI, 5-MTP therapy enhanced mitophagy and decreased kidney injury by upregulating the Nrf2/HO-1 pathway.
Conclusion
Serum 5-MTP levels correlate with renal function and upregulate Nrf2 expression by activating the Nrf2 signaling pathway, thereby promoting renal tubular mitophagy and alleviating S-AKI.
Keywords: sepsis-induced acute kidney injury1, 5-methoxytryptophan2, Nrf23, mitophagy4, oxidative stress5
Graphical Abstract
Introduction
Approximately 13.3 million patients are diagnosed with acute kidney injury (AKI) annually, with 1.7 million resulting in mortality.1 Sepsis is a widespread inflammatory reaction to an infection, frequently resulting in multiple organ failure and impairment.2–4 The kidneys, which are targeted by inflammatory cytokines, are easily susceptible to damage. The release of numerous inflammatory mediators, such as interleukin-1 (IL-1), IL-6, and tumor necrosis factor-alpha (TNF-α), results in cytokine storms. Microvascular dysfunction originating from inflammation causes hypoxia and tissue damage. Mitochondrial injury is the primary cause of fatal damage to tubular cells.5 Studies suggest that mitochondrial dysfunction may occur before an increase in serum creatinine (a marker of AKI).5 Sepsis-induced acute kidney injury (S-AKI) significantly increases the risk of death by 6–8 times. Moreover, survivors of S-AKI face an elevated risk of progressing to chronic kidney disease (CKD).6 Therefore, discussing strategies for the prevention and treatment of S-AKI is crucial due to its immense clinical importance.
5-methoxytryptophan (5-MTP), a newly discovered tryptophan metabolite, is produced by various cell types, including kidney epithelial cells.7,8 Studies have indicated that 5-MTP acts as a protective metabolite against fibrosis and influences the mitochondria in macrophages. It enhances mitochondrial membrane rigidity and branching, thereby preventing oxidation.9 Additionally, in lipopolysaccharide (LPS)-treated human mesangial cells (HMC) and sepsis-induced lung injury models, 5-MTP significantly upregulated the antioxidant proteins, Nrf2 and HO-1.10,11 There are differences in the serological levels of 5-MTP across various diseases and disease stages. Studies have shown a positive relationship between serum 5-MTP concentration and disease activity and prognosis in patients with lupus nephritis.12 Serological 5-MTP expression decreases in patients with sepsis7 and CKD.10 However, the molecular mechanism by which 5-MTP ameliorates S-AKI remains unclear. Studies reporting on the changes in serum 5-MTP levels in patients with S-AKI or a correlation between 5-MTP and renal function are lacking.
Mitophagy is a specialized form of autophagy.13 Under stress, mitophagy is activated as a protective mechanism for maintaining healthy mitochondria and promoting cell survival. Studies have shown impaired mitophagy and cellular damage to S-AKI. Impaired selective autophagy and mitochondrial clearance can result in the accumulation of damaged mitochondria, oxidative stress, and cell death.14–16 Therefore, enhancing mitophagy, specifically during S-AKI, could be a promising therapeutic approach. Recent studies indicate that the expression of mitophagy-associated proteins in the serum of patients can be a potential diagnostic biomarker for diseases.17,18 The content of mitophagy-related proteins correlates with the severity of clinical indicators. Determining mitophagy in the serum of patients with S-AKI may be beneficial for its evaluation.
Nrf2 plays a crucial role in sepsis. Studies have shown its involvement in regulating various kidney diseases through the modulation of mitophagy. Diabetic mice showed a partial reversal of renal tubule injury, improvement in mitochondrial fragmentation, and a reduction in apoptosis via the regulation of Nrf2/ PTEN-induced putative kinase 1 (PINK1)-mediated mitophagy in renal tubule cells.19 Nrf2 activation occurs in both LPS-induced S-AKI cell models and CLP-induced animal models, where it exerts a protective effect through oxidative stress and inflammatory response reduction while promoting mitophagy.20 These results indicate that focusing on Nrf2 activation may be a promising clinical strategy for S-AKI management.
In this study, we aimed to examine the levels of serum 5-MTP and mitophagy in patients with S-AKI and evaluate 5-MTP’s influence on an LPS-induced AKI model. In addition, we sought to elucidate the mechanisms by which 5-MTP regulates mitophagy through Nrf2 mediation.
Materials and Methods
Patients’ Cohort
A prospective cohort study was conducted in the intensive care unit (ICU) of Qinghai University Affiliated Hospital located in Xining (China) between February and December 2023, during which 72 cases of sepsis were documented. The following patients were excluded: those with previously diagnosed tumors, autoimmune diseases, chronic kidney disease, pregnant women, those undergoing continuous hemofiltration therapy, children, and older adult patients. Consequently, 52 patients were included in the analysis. Patients were categorized into the S-AKI and sepsis groups based on the presence of renal function impairment. AKI was diagnosed according to the KDIGO clinical practice guidelines.21 The study were performed in compliance with the Declaration of Helsinki and approved by the Ethics Committee of Affiliated Hospital of Qinghai University (Clinical Medical School) (No:P-SL-2023-467). Informed consent was obtained from all the patients.
Sample Collection and Storage
Blood samples were promptly collected 24 h after the diagnosis of sepsis to ensure accurate experimental outcomes. Specifically, 3 mL of fasting venous blood was extracted by medical staff and placed in an EDTA-containing collection vessel. The sample was left at room temperature for 30 min before being centrifuged for 10 min at a speed of 3000 r/min at 4°C. The resulting serum was carefully transferred with a pipette into an eppendorf tube, with the corresponding number labeled on the tube. Thereafter, the sample was stored in a refrigerator at −80°C for future measurements.
Determination of Plasma 5-MTP in Patients with Sepsis
Metabolite Extraction
Serum 5-MTP measurement was commissioned by Shanghai Bioprofile Technology Co., Ltd. After thawing and mixing the samples in the refrigerator at 4°C, 200 μL of each sample was extracted. Subsequently, 800 μL of pre-cooled pure methanol was added to each sample, followed by vortex mixing and ultrasound treatment in an ice bath for 20 min. Afterward, the samples were stored at −20°C overnight, centrifuged at 16,000 g at 4°C for 20 min, and the supernatant was collected. The supernatant was dehydrated in a high-speed vacuum enrichment centrifuge at low temperature. Next, the samples were redissolved in 40 μL of pre-cooled 50% acetonitrile solution, centrifuged at 4°C for 15 min at 20,000 g. The resulting supernatant was used for mass spectrometry analysis. A detailed list of the primary instruments and reagents is provided in Supplementary Table 1.
LC-MS/MS Analysis
A Shimadzu Nexera X2 A8-30AD high-performance liquid chromatography system was used for the analysis. The mobile phase utilized in this experiment was formulated using two distinct liquids: Liquid A was composed of water containing 0.1% formic acid, whereas Liquid B consisted of acetonitrile containing 0.1% formic acid. The sample was redissolved in 50% acetonitrile at 4°C in the automatic injector, with a flow rate of 300 µL/min, and a sample size of 10 µL. The liquid-phase gradient protocol was as follows: liquid B was maintained at 5% from 0–3 min, liquid B linearly changed from 5 to 40% from 3–9 min, liquid B remained at 95% from 9.1–9.9 min, liquid B linearly changed from 95 to 5% from 9.9–10 min, and liquid B was maintained at 5% from 10–12 min. Mass spectrometry was performed using a QTRAP5500 mass spectrometer (SCIEX,MA,USA) in positive/negative ion mode. The chromatographic peak area and retention time were extracted using MultiQuant software (SCIEX,MA,USA), with retention time correction performed using standards for metabolite identification.
Experimental Animals
C57BL/6 male mice, aged 8–12 weeks, weighing 20–24 g, were procured from Jiangsu Huachuang Xinuo Pharmaceutical Co. Ltd. (Suzhou, China). They were housed in the animal laboratory of the Plateau Medicine Research Center and randomly assigned to one of seven groups: Control, LPS, LPS + 5-MTP low-dose (10 mg/kg), LPS + 5-MTP medium-dose (50 mg/kg), LPS + 5-MTP high-dose (100 mg/kg), LPS + 5-MTP + 3-Methyladenine (3-MA) and LPS + 5-MTP + ML385. AKI was induced by an intraperitoneal injection of LPS at a dose of 10 mg/kg.22 AKI is characterized by a 50% rise in baseline serum creatinine (Scr) within 48 hours, as outlined by the KDIGO guidelines.23 Two hours before the LPS treatment, 5-MTP (M4001, Sigma,MO, USA) was administered at different doses as follows: low (10 mg/kg), medium (50 mg/kg), and high (100 mg/kg). In the LPS + 5-MTP + ML385 and LPS + 5-MTP + 3-MA groups, ML385 (HY-100523, MedChemExpress, NJ, USA) dissolved in dimethyl sulfoxide (DMSO) at 30 mg/kg24 and 3-MA (HY-19312, MedChemExpress, NJ, USA) dissolved in phosphate-buffered saline (PBS) at 20 mg/kg25 were administered intraperitoneally 2 h before LPS administration. 5-MTP was dissolved in DMSO and then in sterile water. The control group received injections of 0.9% normal saline and an equal amount of solvent solution.
Determination of Serum Mitophagy Related Protein Expression Levels
The analysis was performed using PINK1 (JL11175, Jonln, Shanghai, China) and Parkin kits (JL11195, Jonln, Shanghai, China) for the corresponding index measurement, following the manufacturer’s instructions.
Renal Function
Renal function was assessed with a serum creatinine kit (E-BC-K188-M,Elabscience, Wuhan, China), a blood urea nitrogen kit (E-BC-K183-M,Elabscience, Wuhan, China), and a renal damage molecule-1 kit (E-EL-M3039, Elabscience, Wuhan, China), for renal injury detection.
Renal Histology
Renal histology analysis involved collecting kidney tissue samples, fixing them with 4% paraformaldehyde for 48 h, cutting them into 3 µm thick sections after dehydration and paraffin embedding, and staining with periodic acid-Schiff (PAS) stain. Tubular injury was characterized by the loss of brush boundaries, tubular dilation and destruction, cast formation, and cytolysis.
Transmission Electron Microscopy (TEM)
Cortical renal tissue was preserved with 2.5% glutaraldehyde and processed using standard methods, which involved dehydration, infiltration, embedding, ultrathin sectioning, and staining. Mitochondria and autophagosomes in renal tubular epithelial cells of mouse kidneys were visualized using JEM-1400 FLASH TEM.
Western Blot(WB)
Proteins were extracted from the kidney tissue after cracking. They were quantified, separated by electrophoresis, and transferred to a polyvinylidene fluoride membrane, lasting for 90 min. The membrane was blocked with skim milk and incubated overnight with the primary antibody. On the second day, the membranes were washed and incubated with the secondary antibody for 1 h. The bands were washed in tris-buffered saline with 0.1% Tween® 20 for 49 min and visualized using an Amersham Imager 600 chemiluminescence instrument (Cytiva,WA, USA). The band density was analyzed using Image-J software. The antibodies used in this experiment were LC3 (Abcam, ab192890), P62 (Abcam, ab109012), GAPDH (Proteintech, 60004-1-lg), PINK1 (Proteintech, 23274-1-AP), Nrf2 (Proteintech, 16396-1-AP), Parkin (Proteintech, 14060-1-AP), Keap-1 (Proteintech, 10503-2-AP), COX4 (Proteintech, 11242-1-AP), HO-1 (Proteintech, 10701-1-AP).
Immunohistochemical (IHC)
Deparaffinization and rehydration of the stained paraffin-embedded kidney sections were performed using a slide heater along with alcohol. Subsequently, the sections were embedded in 800 mL of citrate buffer at a pH of 6.0, covered with a punctured plastic wrap, and microwaved on medium heat for 15 min. Subsequently, all sections were treated with 0.3% hydrogen peroxide for 10 min to block the endogenous peroxidase activity. The slides were incubated overnight with a primary antibody (Proteintech, 16396-1-AP). After washing with PBS, the tissue sections were incubated with secondary antibodies (1:200) for 1 h and developed with diaminobenzidine. This step is crucial for the detection of specific proteins or antigens within the samples. Upon visualization of the positive signal, the reaction was quenched with ddH2O and counterstained with hematoxylin for light microscopy observation.
Immunofluorescence (IF)
For IF staining, the kidney sections were processed according to a previously published protocol,26 using LC3 (Proteintech, 14600-1-AP) and COX4 (Proteintech, 11242-1-AP).
Measurement of Inflammation Index
IL-6, MCP-1, and TNF-α levels were measured using the Cytometric Bead Array (CBA) Mouse Inflammation Kit (552364, Becton,Dickinson and Company,CA, USA). To begin 10 μL of microspheres were taken from each tube after being scrolled for 10 sec. The serum was diluted four times (15 μL stock solution + 45 μL diluent), and 50 μL of serum was transferred into an EP tube and a standard tube. All tubes were incubated with a mixture of 50 μL microspheres and 50 μL PE at room temperature and shielded from light, for 2 h. Following incubation, 1 mL of wash buffer was added to each tube, centrifuged at 200 g for 5 min, and the supernatant was discarded. Subsequently, samples were re-suspended with 500 μL of wash buffer in each tube before being transferred to the original flow tube for machine testing. IL-1β level was assessed using an IL-1β kit (JLW18442, Jonln, Shanghai, China).
Oxidative Stress Index
The oxidative stress index was determined by measuring the glutathione (GSH) level, superoxide dismutase (SOD) activity, and malondialdehyde (MDA) level in the kidney tissue. The tissues were homogenized and extracted into PBS, followed by centrifugation at 12000 r for 10 min. The analysis was performed using MDA kits (E-EL-0060-c, Elabscience, Wuhan, China), SOD kits (BC-K022-M, Elabscience, Wuhan, China), and GSH kits (E-BC-K030-M, Elabscience, Wuhan, China), for the corresponding index measurement, following the manufacturer’s instructions.
Statistical Analysis
The findings were expressed as mean ± standard deviation. For normally distributed data, t-tests or one-way analysis of variance (ANOVA) were applied. For non-normally distributed data, the Mann–Whitney U-test was used to ensure validity. Spearman correlation analysis was performed to evaluate correlations. Statistical analyses were conducted using SPSS version 22.0. All analyses were two-tailed, and statistical significance was set at p-value < 0.05.
Results
Plasma 5-MTP and Mitophagy Expression in S-AKI Patients
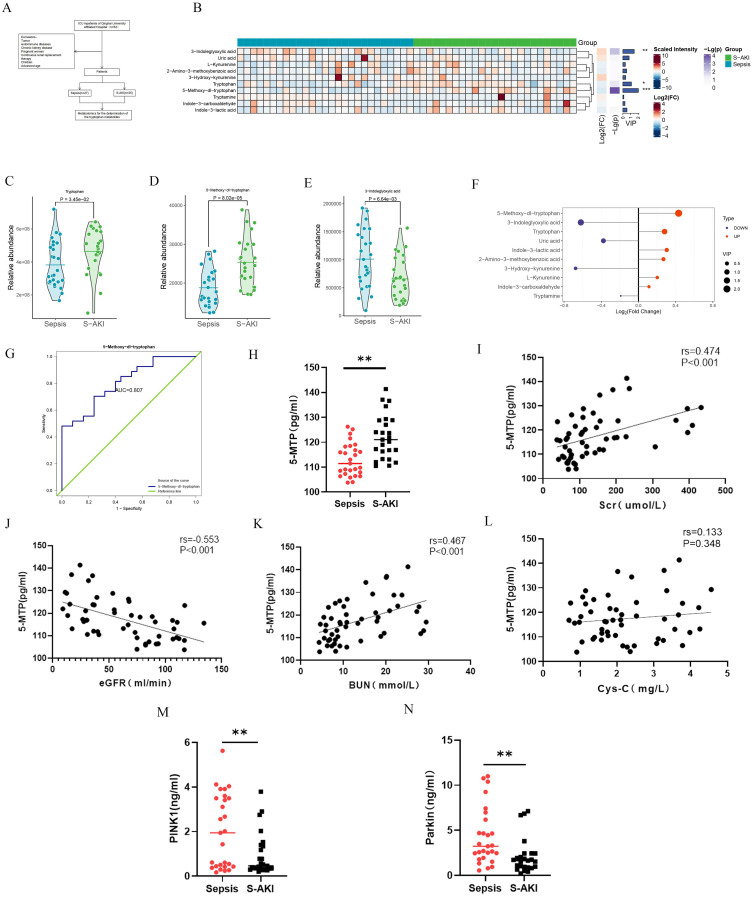
To investigate the role of 5-MTP in S-AKI, we analyzed the expression of 10 tryptophan metabolites in plasma samples from 52 patients with sepsis using targeted metabolomics (Figure 1A). The clustering results of the metabolite levels are presented in Figure 1B. Significant differences among the three metabolites were noted (Figure 1C-E). Notably, 5-MTP had the highest Variable Importance in Projection(VIP) value among the three metabolites, highlighting its importance (Figure 1F). The receiver operating characteristics (ROC) analysis produced an area under the curve (AUC) of 0.807 (95% confidence interval [CI]: 0.693–0.922, P < 0.001) (Figure 1G). Statistical analysis of the absolute quantitative results confirmed higher plasma 5-MTP levels in patients with S-AKI (Figure 1H). Spearman correlation analysis revealed a significant relationship between plasma levels of 5-MTP and renal function indicators, including Scr, eGFR, and blood urea nitrogen (BUN) (P < 0.05); however, no correlation was found with cystatin c (Cys-C) levels (Figure 1I-L). Furthermore, we determined the expression levels of mitophagy-related proteins in the serum of patients with S-AKI, and the results showed that, compared with patients with sepsis, those with S-AKI showed a decreasing trend for serum PINK1 and Parkin levels (Figure 1M and N). Detailed patient data and routine laboratory indicators are summarized in Supplementary Tables 2 and 3.
Figure 1.
Tryptophan metabolites expression and mitophagy level in S-AKI and the relationship between 5-MTP and renal function. (A) Tryptophan metabolite levels in plasma were assessed in individuals with sepsis (n = 27) and S-AKI (n = 25). (B) Hierarchical cluster analysis of tryptophan metabolites between the two groups. (C) Tryptophan peak difference between patients with sepsis and S-AKI. (D) 5-MTP peak difference between patients with sepsis and S-AKI. (E) 3-Indoleglyoxylic acid peak difference between patients with sepsis and S-AKI. (F) VIP and FC values of three metabolites. (G) ROC curve results of 5-MTP. (H) Absolute quantitative results of 5-MTP. (I) Correlation between Scr and 5-MTP. (J) Correlation between eGFR and 5-MTP. (K) Correlation between BUN and 5-MTP. (L) Correlation between Cys-C and 5-MTP. (M) Expression level of PINK1 in the patient’s serum (N) Expression level of Parkin in the patient’s serum.*P < 0.05, **P < 0.01, ***P < 0.001 denote statistically meaningful differences among the groups.
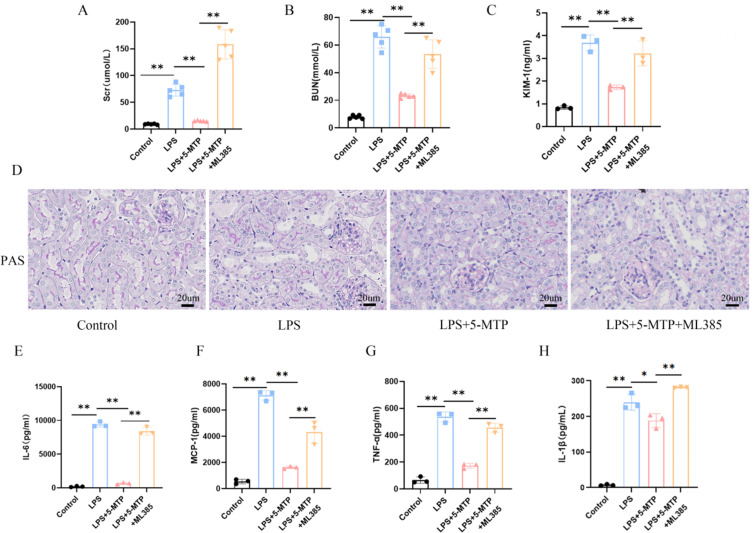
Effects of 5-MTP on Renal Dysfunction and Inflammatory Response in S-AKI Mice
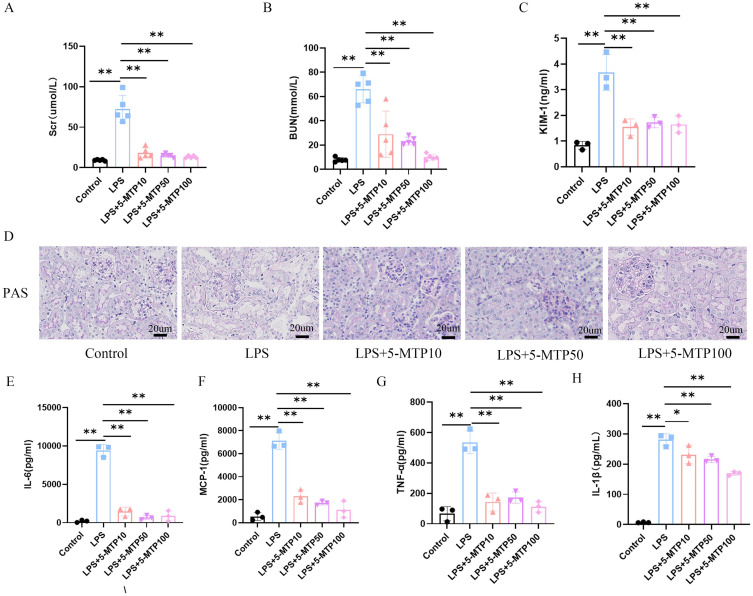
Following intraperitoneal LPS injection, the mice underwent 5-MTP measurement and renal function assessment. The level of 5-MTP are illustrated in Supplementary Table 4. The renal function experimental findings are illustrated in Figure 2. The findings indicate significant renal function alterations post-LPS injection. Compared with the control group, the model group exhibited notable increases in Scr and BUN levels after LPS injection. Administration of 5-MTP at low (10 mg/kg), medium (50 mg/kg), and high (100 mg/kg) doses resulted in significant improvements in renal function (Figure 2A and B). KIM-1, a renal tubular injury marker, was also analyzed. Compared with the control group, the LPS group showed a marked increase in KIM-1 levels, which were significantly reduced by 5-MTP treatment (Figure 2C). Additionally, significant increases in lumen dilation, vacuolation, and inflammatory infiltration were observed in the LPS group compared to the control group; although, this was reversed following treatment with varying doses of 5-MTP (Figure 2D). The experiment’s results indicated notable differences in serum IL-1β, IL-6, MCP-1, and TNF-α levels between the S-AKI and 5-MTP groups. Specifically, IL-1β, IL-6, MCP-1, and TNF-α levels were elevated in LPS-treated mice, whereas administration of 5-MTP at varying doses caused a down-regulation of these inflammatory markers (Figure 2E-H). These results suggest that 5-MTP can mitigate renal tissue inflammation and ameliorate renal function injury in S-AKI mice.
Figure 2.
5-MTP reduces LPS-induced renal injury and inflammation. Scr (A) and BUN (B) concentrations were measured across various groups of mice. (C) The expression levels of KIM-1 in the mice were analyzed. (D) Typical images showcasing PAS staining of the renal cortex were presented. Scale bar: 20 μm. (E) The levels of serum IL-6 in mice were evaluated. (F) MCP-1 levels in the serum of mice were measured. (G) The expression of TNF-α in serum from the mice was determined. (H) Analysis of serum IL-1β expression in mice was performed. The results are presented as mean ± standard deviation.*P < 0.05, **P < 0.01 denote statistically meaningful differences among the groups.
Effect of 5-MTP on Mitophagy in S-AKI Mouse Model
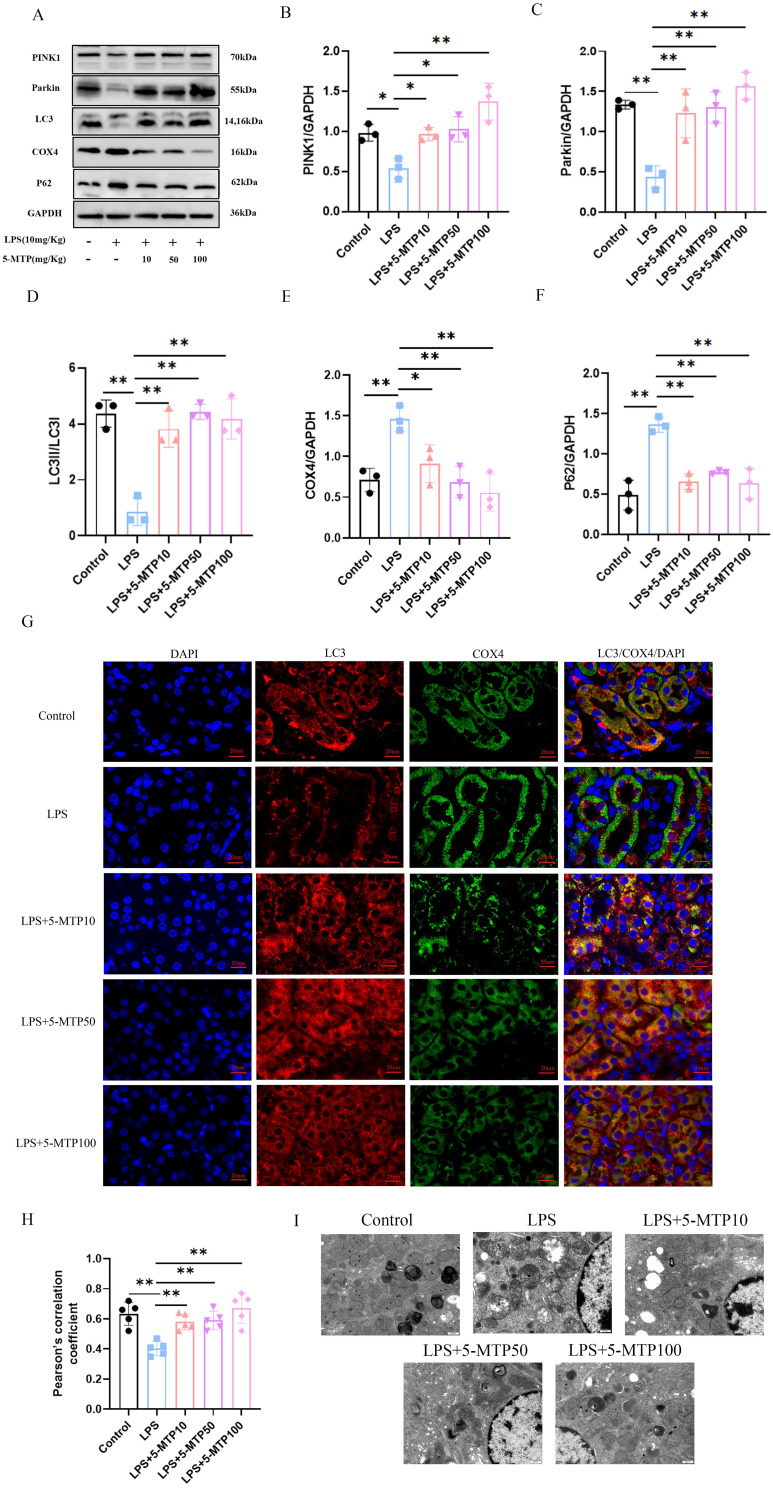
To investigate the influence of 5-MTP on mitophagy in the kidneys of S-AKI mice and renal protection, we examined the expression of mitophagy-related proteins. Following LPS intraperitoneal injection, notable variations in the expression levels of mitophagy-related proteins was among the different groups. Specifically, post-LPS treatment, an increase in P62 and COX4 protein expression levels was noted in the kidney tissues, accompanied by a significant decrease in PINK1, Parkin, and LC3-II/LC3-I ratios. 5-MTP treatment resulted in the downregulation of P62 and the mitochondrial endometrial protein, COX4, and the upregulation of Parkin and PINK1 proteins, and an increase in the LC3-II/LC3-I ratio. (Figure 3A-F). Furthermore, the results of the IF assay and WB correlated, revealing a significant upregulation of mitophagy markers following 5-MTP administration. This was demonstrated by the co-localization of LC3 and COX4 (Figure 3G and H). These results suggest the potential for 5-MTP to augment mitophagy levels. Subsequently, we observed the mitochondria and autophagosomes in the renal tubular epithelial cells using TEM. The ultrastructure of the mitochondria in renal tissue was examined (Figure 3I), and it was revealed that the mitochondria in the renal tubular epithelial cells displayed a relatively normal morphology and structure. They exhibited clear ridges, a uniform matrix, and a consistent gray appearance under TEM. Additionally, increased numbers of lysosomes and autophagosomes were observed. However, following LPS treatment, the mitochondria showed severe swelling, enlargement, and rounding, with a disrupted outer membrane, fragmented ridges, and a thin, flocculent matrix. Furthermore, they appeared vacuolated, with a decreased number of autophagosomes. Subsequent 5-MTP treatments restored the normal mitochondrial morphology and structure in renal tubular epithelial cells. The ridges were clear with a uniform matrix and a consistent gray appearance was observed under TEM. A few mitochondria were slightly swollen, with an increased number of lysosomes and autophagosomes. Subsequently, we used 3-MA as an autophagy inhibitor and explored the role of 5-MTP in protecting renal function through the regulation of mitophagy. The results showed that the expressions of LC3, Parkin, and PINK1 were downregulated, and the expressions of P62 and COX4 were upregulated after 3-MA treatment (Supplementary Figure 1A-F). The IF and WB results were consistent (Supplementary Figure 1G-H). Under an electron microscope, mitochondrial swelling and enlargement, outer membrane rupture, ridge breakage, and a thin flocculent matrix were observed after 3-MA treatment. Mitochondria were vacuolated, and the number of autophagosomes decreased (Supplementary Figure 1I). These results suggest that the effect of 5-MTP on mitophagy is inhibited by 3-MA. After 3-MA application, the effect of 5-MTP in improving renal function disappeared, Scr, BUN, and KIM-1 levels increased (Supplementary Figure 1J-L), and renal tissue damage worsened (Supplementary Figure 1M). In summary, 5-MTP alleviates kidney damage by promoting mitophagy in LPS-treated mice.
Figure 3.
5-MTP promotes mitophagy in mice subjected to AKI caused by LPS. (A) Representative blot bands of PINK1, Parkin, LC3, COX4 and P62 in the renal cortex. (B-F) The relative band density of PINK1, Parkin, LC3, COX4 and P62 in renal cortex. (G) Representative IF images of LC3 (red) and COX4 (green) in renal tissue of each group. Nuclei stained with DAPI (blue), scale bar: 20 μm. (H) The Pearson correlation coefficient was utilized to assess the co-localization of LC3 and COX4. (I) The proximal tubule mitochondria were detected by transmission electron microscopy. Scale bar: 500 nm. The results are presented as mean ± standard deviation. *P < 0.05, **P < 0.01 denote statistically meaningful differences among the groups.
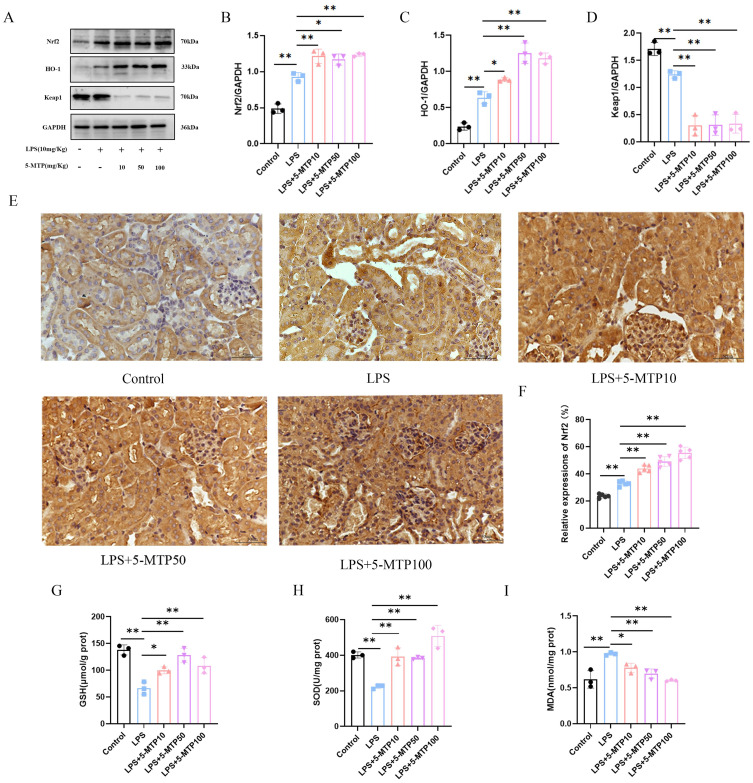
Effects of 5-MTP on Renal Nrf2 Pathway in S-AKI Mouse Model
To further verify the effect of 5-MTP on S-AKI, we measured the expression of Nrf2 pathway-associated proteins in S-AKI mouse models. WB showed notable changes in Nrf2, HO-1, and E3 ubiquitin-associated protein 1 (Keap1) in the kidney tissues of S-AKI mice that received LPS treatment, in contrast to the control group. Specifically, Nrf2 and HO-1 expression levels were elevated in LPS-treated mice. Moreover, they were augmented following 5-MTP administration (Figure 4A-C). Compared with the control mice, those subjected to intraperitoneally injected LPS showed decreased Keap1 expression in the renal tissues, and treatment with different doses of 5-MTP significantly reduced Keap1 expression (Figure 4D). The results of IHC staining demonstrated a rise in the expression and nuclear translocation of Nrf2 in the mesorenal cortex of S-AKI mice, induced by LPS. Additionally, varying doses of 5-MTP further augmented these effects (Figure 4E and F). This result suggested that 5-MTP can activate Nrf2 pathway.
Figure 4.
5-MTP stimulates Nrf2 and decreases oxidative stress in mice subjected to AKI caused by LPS. (A) Representative blot bands for Nrf2, Keap-1 and HO-1 in the renal cortex. (B-D) Comparative band density measurements of Nrf2, Keap-1 and HO-1 within the renal cortex. (E) Examples of IHC staining images displaying Nrf2 in renal tissue. Scale bar: 50 μm. (F) The percentage of Nrf2 expression in the renal cortex. (G) GSH content was quantified in the kidney tissue of the mice. (H) The activity of SOD was assessed in the kidney tissue of mice. (I) MDA levels in the kidney tissue of the mice were evaluated. The results are presented as mean ± standard deviation. *P < 0.05, **P < 0.01 denote statistically meaningful differences among the groups.
Furthermore, compared with the control mice, LPS-treated S-AKI mice exhibited significantly different SOD activity, GSH level, and MDA level in the kidney tissues. The SOD activity and GSH content were reduced in LPS-treated mice; however, supplementation with varying doses of 5-MTP resulted in their upregulation (Figure 4G and H). MDA levels were elevated in the renal tissues of LPS-treated mice, and this increase was reversed by 5-MTP administration at varying doses (Figure 4I). Overall, these findings suggest that 5-MTP can effectively ameliorate oxidative stress-induced damage caused by sepsis in the renal tissue by activating the Nrf2 pathway.
5-MTP Activates Mitophagy by Activating the Nrf2 Pathway, Thereby Alleviating LPS-Stimulated Kidney Injury
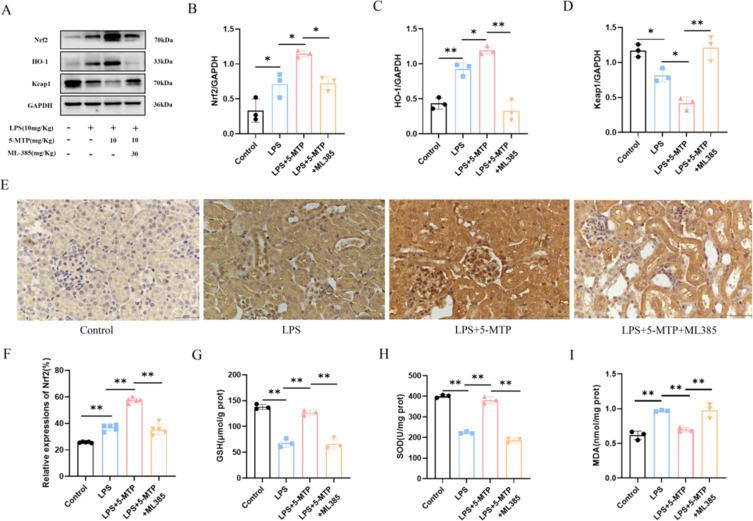
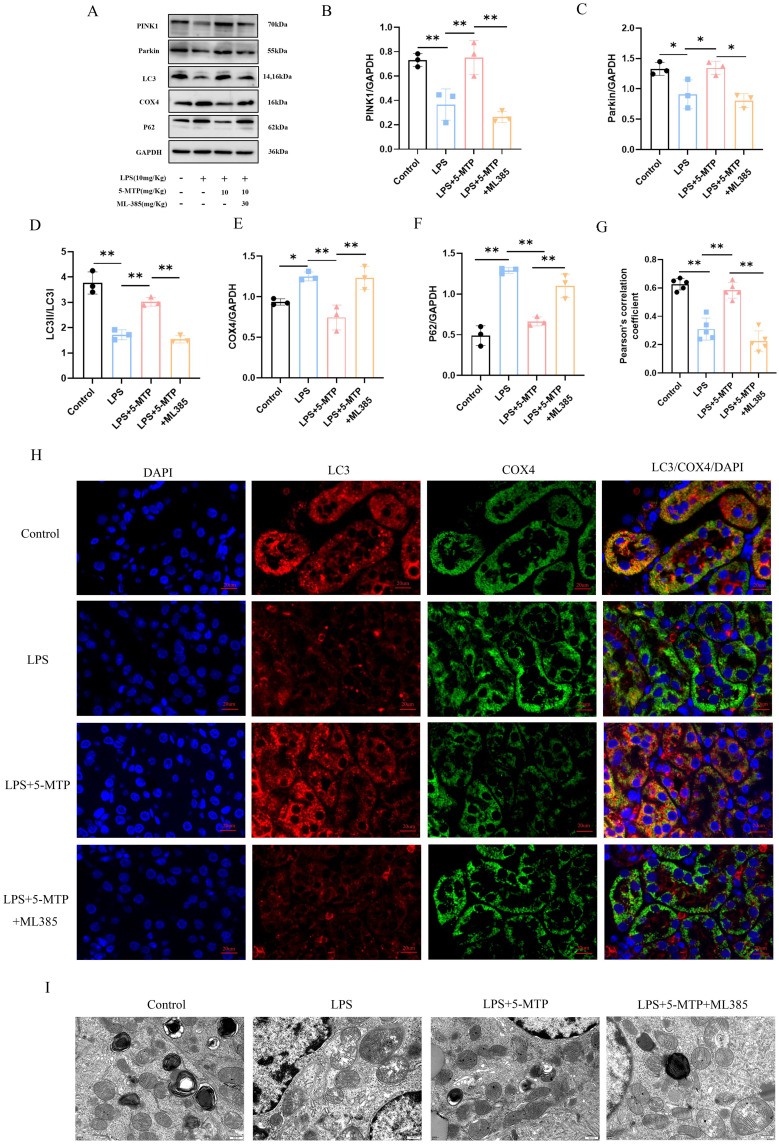
To further elucidate the mechanism of 5-MTP against S-AKI, we used ML-385 to inhibit Nrf2 activation to verify whether the protective effect of 5-MTP is related to Nrf2 activation. Compared with the group treated with 5-MTP alone, that treated with ML385, a significant increase in Scr, BUN, and KIM-1 levels (Figure 5A-C) and exacerbated kidney tissue damage (Figure 5D) were observed. Additionally, IL-6 (Figure 5E), MCP-1 (Figure 5F), TNF-α (Figure 5G), and IL-1β (Figure 5H) levels were elevated. Protein imprinting revealed significant changes in the expression levels of Nrf2 and mitophagy-related proteins. Following ML385 administration, a significant decrease in Nrf2 and HO-1 expressions were observed, accompanied by an increase in Keap1 expression (Figure 6A-D). IHC results further indicated that the ML385 group showed decreased Nrf2 expression and nuclear translocation in the mesorenal cortex compared to the 5-MTP group (Figure 6E and F). The ML385 group exhibited decreased GSH level (Figure 6G), reduced SOD activity (Figure 6H), and elevated MDA level (Figure 6I). Analysis of mitophagy-related proteins showed that in the ML385 group, P62 expression increased; the ratio of PINK1, Parkin, and LC3-II/LC3-I significantly decreased; and the expression of the mitochondrial endometrial protein, COX4, was upregulated (Figure 7A-F). IF assay results were consistent with the WB findings, demonstrating a decrease in LC3 and COX4 co-localization after ML385 administration (Figure 7G and H). TEM revealed severe mitochondrial swelling, enlarged and rounded mitochondria, outer membrane rupture, a large amount of ridge fracture dissolution, a thin matrix containing flocculent material, mitochondrial vacuolation, and a decreased number of autophagosomes (Figure 7I). These findings indicate the significant role of 5-MTP in safeguarding kidney function. This protective effect is achieved through the activation of the Nrf2 signaling pathway, which is crucial for promoting mitophagy; 5-MTP provides kidney protection by enhancing mitophagy.
Figure 5.
The effect of 5-MTP on renal injury and inflammatory response is eliminated after administration of Nrf2 inhibitor. Scr(A) and BUN (B) concentrations were measured across various groups of mice. (C) The expression levels of KIM-1 in the mice were analyzed. (D) Typical images showcasing PAS staining of the renal cortex are presented. Scale bar: 20 μm. (E) The levels of serum IL-6 in mice were evaluated. (F) MCP-1 levels in the serum of mice were measured. (G) The expression of TNF-α in serum from the mice was determined. (H) Analysis of serum IL-1β expression in mice was performed.The results are presented as mean ± standard deviation. *P < 0.05, **P < 0.01 denote statistically meaningful differences among the groups.
Figure 6.
The effect of 5-MTP on oxidative stress is eliminated after administration of Nrf2 inhibitor.(A) Representative blot bands for Nrf2, Keap-1 and HO-1 in the renal cortex. (B-D) Comparative band density measurements of Nrf2, Keap-1 and HO-1 within the renal cortex. (E) Examples of IHC staining images displaying Nrf2 in renal tissue. Scale bar: 50 μm. (F) The percentage of Nrf2 expression in the renal cortex. (G) GSH content was quantified in the kidney tissue of the mice. (H) The activity of SOD was assessed in the kidney tissue of mice. (I) MDA levels in the kidney tissue of the mice were evaluated. The results are presented as mean ± standard deviation. *P < 0.05, **P < 0.01 denote statistically meaningful differences among the groups.
Figure 7.
The effect of 5-MTP on mitophagy is eliminated after administration of Nrf2 inhibitor. (A) Representative blot bands of PINK1, Parkin, LC3, COX4 and P62 in the renal cortex. (B-F) The relative band density of PINK1, Parkin, LC3, COX4 and P62 in renal cortex. (G)The Pearson correlation coefficient was utilized to assess the co-localization of LC3 and COX4. (H) Representative IF images of LC3 (red) and COX4 (green) in renal tissue of each group. Nuclei stained with DAPI (blue), scale bar: 20 μm.(I) The proximal tubule mitochondria were detected by transmission electron microscopy. Scale bar: 500 nm. The results are presented as mean ± standard deviation. *P < 0.05, **P < 0.01 denote statistically meaningful differences among the groups.
Discussion
This study revealed a novel finding of 5-MTP expression in S-AKI and highlighted its potential as a biomarker for this condition. Concurrently, in clinical patient findings, our study highlighted the importance of mitophagy in the pathogenesis and disease progression of S-AKI. Furthermore, the study demonstrated that 5-MTP can improve kidney function and lessen tubular cell injury in S-AKI through the activation of the Nrf2 pathway and the facilitation of mitophagy. These findings suggest that 5-MTP could be a promising treatment in the future for S-AKI. The elucidation of the Nrf2 pathway and mitophagy roles in S-AKI provided a strong foundation for further investigation into the potential therapeutic effects of 5-MTP.
5-MTP, a newly discovered tryptophan metabolite, is synthesized by fibroblasts, kidney epithelial and smooth muscle cells, and bronchial and vascular endothelial cells.7,8 Previous studies have reported differences in the serological levels of 5-MTP across various diseases and disease stages. In our study, we employed a targeted metabolomics approach to analyze tryptophan and its metabolites in the serum. The analysis revealed distinct trends in tryptophan metabolite levels between the two groups, indicating alterations in tryptophan metabolism in patients with sepsis and S-AKI and significantly enhanced serum 5-MTP levels in patients with S-AKI than in those with sepsis. Through the analysis of VIP values, we identified 5-MTP as an important metabolite among the 10 metabolites assessed. The VIP value and ROC curve analysis highlighted 5-MTP as a key metabolite, distinguishing between the two groups. 5-MTP exhibited high sensitivity and a certain predictive capacity for the onset of S-AKI. A significant relationship between the levels of 5-MTP and various indicators of renal function, specifically Scr and BUN, was observed. These findings suggested a significant relationship between 5-MTP levels and the underlying mechanisms in the pathophysiology of S-AKI. This association indicated the possible role of 5-MTP in the development and progression of S-AKI. The production of 5-MTP from tryptophan via specific enzymatic reactions is triggered by an excessive inflammatory septic response, which prompts renal tubular epithelial cells to release 5-MTP to combat infection. In cases of severe inflammation, reduced effective blood volume and kidney microcirculation damage can cause AKI, thereby prompting the release of 5-MTP to protect blood vessels, regulate inflammation, and control kidney fibrosis. Discrepancies with previous studies may stem from variations in blood collection timing, study population inclusion criteria, and detection methods. Our results indicated that 5-MTP may serve as a biomarker with the potential to mitigate inflammation and preserve vascular integrity in S-AKI.
Numerous studies have highlighted the diverse pharmacological effects of 5-MTP, including anti-inflammatory, antioxidant, antifibrotic, and antitumor properties. It has shown promise in the treatment of various animal models of acute lung injury11 and chronic kidney disease.10 In our study, we demonstrated its ability to mitigate LPS-induced renal dysfunction by preventing renal tubular damage through anti-inflammatory mechanisms and improving oxidative stress management. Systemic and local inflammatory responses are pivotal in the development of S-AKI. The findings revealed elevated IL-1β, IL-6, MCP-1, and TNF-α levels in S-AKI mice; however, 5-MTP treatment at varying doses led to a reduction in these inflammatory markers. This indicates the potential of 5-MTP in mitigating the inflammatory response in S-AKI, aligning with previous research.7 Simultaneously, PAS staining results showed an improvement in the kidney injury after 5-MTP application. Therefore, these Results demonstrated that 5-MTP alleviates LPS-induced kidney damage in mice.
In the liver, 5-MTP regulates autophagy by inhibiting the proliferation and fibrosis of human hepatic stellate cells. Recent studies have highlighted the protective role of 5-MTP in fibrosis, demonstrating its effect on mitochondria in macrophages. 5-MTP enhanced mitochondrial structural integrity by increasing membrane rigidity and branching, leading to reduced oxidation.9 Mitochondrial damage is the primary cause of fatal injury in tubule cells.5 Mitophagy, a selective form of autophagy, is crucial in eliminating excess or damaged mitochondria to maintain a healthy mitochondrial population.13 Under stress, mitophagy is induced as an adaptive or defense mechanism for maintaining a healthy mitochondrial population that keeps cells alive. Mitophagy defects have been linked to various human diseases, such as cardiovascular diseases, cancer and sepsis.27–29 Chemicals, such as 4-octyl itaconic acid,24 and cell therapies, such as human umbilical cord blood mononuclear cells30 enhance mitophagy and attenuate experimental S-AKI. The results of our clinical part of the study show that PINK1 and Parkin are decreased in patients with S-AKI. The in vivo section of the study revealed that 5-MTP up-regulated LC3 protein, PINK1, and Parkin, and down-regulated COX4 and P62 in LPS-induced AKI. Following the inhibition of autophagy by 3-MA, mitophagy was inhibited, and 5-MTP’s role in improving renal function disappeared. Our research demonstrated the association between 5-MTP and mitophagy in patients with S-AKI. 5-MTP effectively ameliorated cristae disruption and vacuolation damage in kidney mitochondria post-LPS stimulation, thereby promoting autophagosome formation, upregulating mitophagy, and safeguarding renal tubule cells following LPS-induced injury.
Nrf2 belongs to the alkaline leucine zipper family of transcription factors and binds to its inhibitor Keap1 in the cytoplasm, where it undergoes ubiquitination by Keap1, and is continually degraded by the proteasome system.31 However, when stimulated by ROS-induced tissue damage, Nrf2 is released from Keap1 and translocated to the nucleus, where it forms heterodimers with small Maf proteins to activate the expression of various antioxidant and detoxification enzymes, such as, HO-1, NAD(P)H quinone oxidoreductase 1 (NQO1), SOD, and GSH.32,33 Previous studies have indicated that micheliolides can reduce the production of NOD-, LRR- and pyrin domain-containing 3(NLRP3) inflammasome and enhance mitophagy through Nrf2, activation thereby reducing S-AKI.34 Sulforaphane enhances mitophagy by activating the NRF2/Pink1 signaling pathway, which alleviates kidney podocyte injury in diabetic kidney disease.35 While some studies have explored the connection between 5-MTP and Nrf2/HO-1, evidence regarding 5-MTP regulation of oxidative stress and mitophagy in S-AKI remains limited. In this study, our focus was primarily on investigating the impact of 5-MTP on Nrf2/HO-1 regulation in S-AKI. We discovered increased Nrf2 levels in the renal tissue, upregulation of the downstream target HO-1, and decreased levels of GSH and SOD, alongside an increase in MDA following LPS stimulation. This alteration in the Nrf2 signaling pathway, accompanied by a decrease in Keap1 expression, indicated a cellular self-defense strategy triggered by ROS production and induced by LPS, which is aimed at sustaining homeostasis. Furthermore, WB demonstrated that 5-MTP administration upregulated Nrf2 signaling, resulting in an increased expression of the downstream target gene HO-1. Notably, KEAP1 protein levels varied significantly across the experimental groups. These findings are consistent with prior research.10 In addition, 5-MTP elevated GSH and SOD levels in the kidney tissue of S-AKI mice, and reduced MDA levels to restore homeostasis in LPS-treated tubule cells. To investigate the role of Nrf2 on 5-MTP’s effect on S-AKI, we introduced the Nrf2 inhibitor, ML385. Inhibition of Nrf2 reversed the changes in protein expression induced by 5-MTP, indicating a possible role for Nrf2 activation. Nrf2 intranuclear transfer led to the overexpression of autophagy-related genes, which enhanced mitophagy. Overall, 5-MTP may improve the inflammatory response and enhance mitophagy by up-regulating Nrf2 expression in S-AKI. Our results did not show a dose-dependent effect of 5-MTP on S-AKI.
This study had some limitations. First, the sample size was relatively small and obtained from one medical center, potentially restricting the generalizability of our findings. Second, we did not include healthy controls in our clinical case collection, and the baseline levels of 5-MTP were not clearly defined. Furthermore, we did not analyze patient prognosis, including ICU transfer, and patient survival time, respectively. Finally, the animal experiments in our study partially confirmed the involvement of kidney tissue and failed to provide sufficient evidence to establish renal tubular epithelial cells or other cells as the main contributors to mitophagy. Worthy of note, the mechanism of 5-MTP-mediated mitophagy in S-AKI may extend beyond the Nrf2 pathway, necessitating further comprehensive investigations to elucidate the impact of 5-MTP on mitochondrial function in S-AKI.
Conclusion
In this study, we demonstrated a significant increase in the plasma expression of 5-MTP in patients with S-AKI, indicating a potential protective effect of 5-MTP against septic stress in renal tissue. 5-MTP expression is closely related to renal function and can serve as a biomarker for predicting S-AKI. Mitophagy is directly associated with S-AKI pathogenesis and progression. 5-MTP inhibits LPS-induced inflammation and oxidative stress in renal tubular cells and enhances mitophagy. Mechanistically, 5-MTP upregulated Nrf2 expression, activated the Nrf2 signaling pathway, and elicited antioxidative stress and anti-inflammatory responses. Our findings provide novel insights into 5-MTP’s protective role in kidney function through mitophagy regulation and lay a strong foundation for further investigation into the potential therapeutic effects of 5-MTP, shedding light on promising treatment in the future for S-AKI.
Acknowledgments
The graphical abstract was supported by Figdraw.
Funding Statement
This research was supported by “2023 Young Scientific Foundation of Qinghai University” (No: 2023-QYY-2); Qinghai Province “Kunlun Talents · High-end Innovative and Entrepreneurial Talents” cultivate leading talents (2022).
Ethics Statement
For patient samples, our study complies with the Declaration of Helsinki. All animal experiments were conducted in compliance with National Institutes of Health guidelines and approved by the Ethics Committee of the Affiliated Hospital of Qinghai University (Clinical Medical School) (No:P-SL-2023-467).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the study design, execution, acquisition of data, analysis and interpretation; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Mehta RL, Cerdá J, Burdmann EA, et al. International Society of Nephrology’s 0by25 initiative for acute kidney injury (zero preventable deaths by 2025): a human rights case for nephrology. Lancet. 2015;385(9987):2616–2643. doi: 10.1016/S0140-6736(15)60126-X [DOI] [PubMed] [Google Scholar]
- 2.Vincent JL, Opal SM, Marshall JC, Tracey KJ. Sepsis definitions: time for change. Lancet. 2013;381(9868):774–775. doi: 10.1016/S0140-6736(12)61815-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dellepiane S, Marengo M, Cantaluppi V. Detrimental cross-talk between sepsis and acute kidney injury: new pathogenic mechanisms, early biomarkers and targeted therapies. Crit Care. 2016;20(1):61. doi: 10.1186/s13054-016-1219-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang K, Xie S, Xiao K, Yan P, He W, Xie L. Biomarkers of sepsis-induced acute kidney injury. Biomed Res Int. 2018;2018:6937947. doi: 10.1155/2018/6937947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ja F, Rg S. Persistent disruption of mitochondrial homeostasis after acute kidney injury. Am J Physiol Renal Physiol. 2012;302(7):F853–F864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gómez H, Kellum JA. Sepsis-induced acute kidney injury. Curr Opin Crit Care. 2016;22(6):546–553. doi: 10.1097/MCC.0000000000000356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang YF, Hsu YJ, Wu HF, et al. Endothelium-derived 5-methoxytryptophan is a circulating anti-inflammatory molecule that blocks systemic inflammation. Circ Res. 2016;119(2):222–236. doi: 10.1161/CIRCRESAHA.116.308559 [DOI] [PubMed] [Google Scholar]
- 8.Chang TC, Hsu MF, Shih CY, Wu KK. 5-methoxytryptophan protects MSCs from stress induced premature senescence by upregulating FoxO3a and mTOR. Sci Rep. 2017;7(1):11133. doi: 10.1038/s41598-017-11077-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maassen S, Warner H, Ioannidis M, et al. Mitochondrial interaction of fibrosis-protective 5-methoxy tryptophan enhances collagen uptake by macrophages. Free Radic Biol Med. 2022;188:287–297. doi: 10.1016/j.freeradbiomed.2022.06.235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen DQ, Cao G, Chen H, et al. Identification of serum metabolites associating with chronic kidney disease progression and anti-fibrotic effect of 5-methoxytryptophan. Nat Commun. 2019;10(1):1476. doi: 10.1038/s41467-019-09329-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ma Y, Wang Z, Wu X, et al. 5-Methoxytryptophan ameliorates endotoxin-induced acute lung injury in vivo and in vitro by inhibiting NLRP3 inflammasome-mediated pyroptosis through the Nrf2/HO-1 signaling pathway. Inflamm Res. 2023;72(8):1633–1647. doi: 10.1007/s00011-023-01769-1 [DOI] [PubMed] [Google Scholar]
- 12.Lin TC, Kuo CC, Wu CY, Yeh KW, Kuo ML, Huang JL. The role of 5-methoxytryptophan in pediatric-onset lupus nephritis: a retrospective cohort study. J Microbiol Immunol Infect. 2020;53(5):797–802. doi: 10.1016/j.jmii.2018.12.003 [DOI] [PubMed] [Google Scholar]
- 13.Youle RJ, van der Bliek AM. Mitochondrial fission, fusion, and stress. Science. 2012;337(6098):1062–1065. doi: 10.1126/science.1219855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu JX, Yang C, Zhang WH, et al. Disturbance of mitochondrial dynamics and mitophagy in sepsis-induced acute kidney injury. Life Sci. 2019;235:116828. doi: 10.1016/j.lfs.2019.116828 [DOI] [PubMed] [Google Scholar]
- 15.Hsiao HW, Tsai KL, Wang LF, et al. The decline of autophagy contributes to proximal tubular dysfunction during sepsis. Shock. 2012;37(3):289–296. doi: 10.1097/SHK.0b013e318240b52a [DOI] [PubMed] [Google Scholar]
- 16.Lin Q, Li S, Jiang N, et al. PINK1-parkin pathway of mitophagy protects against contrast-induced acute kidney injury via decreasing mitochondrial ROS and NLRP3 inflammasome activation. Redox Biol. 2019;26:101254. doi: 10.1016/j.redox.2019.101254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Veverová K, Laczó J, Katonová A, et al. Alterations of human CSF and serum-based mitophagy biomarkers in the continuum of Alzheimer disease. Autophagy. 2024;20(8):1868–1878. doi: 10.1080/15548627.2024.2340408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qian S, He H, Xiong X, et al. Identification of mitophagy-associated proteins profile as potential plasma biomarkers of idiopathic Parkinson’s disease. CNS Neurosci Ther. 2024;30(4):e14532. doi: 10.1111/cns.14532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xiao L, Xu X, Zhang F, et al. The mitochondria-targeted antioxidant MitoQ ameliorated tubular injury mediated by mitophagy in diabetic kidney disease via Nrf2/PINK1. Redox Biol. 2017;11:297–311. doi: 10.1016/j.redox.2016.12.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen Z, Wang H, Hu B, et al. Transcription factor nuclear factor erythroid 2 p45-related factor 2 (NRF2) ameliorates sepsis-associated acute kidney injury by maintaining mitochondrial homeostasis and improving the mitochondrial function. Eur J Histochem. 2022;66(3):3412. doi: 10.4081/ejh.2022.3412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ostermann M, Bellomo R, Burdmann EA, et al. Controversies in acute kidney injury: conclusions from a Kidney Disease: improving Global Outcomes (KDIGO) conference. Kidney Int. 2020;98(2):294–309. doi: 10.1016/j.kint.2020.04.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu Z, Yang D, Gao J, et al. Discovery and validation of miR-452 as an effective biomarker for acute kidney injury in sepsis. Theranostics. 2020;10(26):11963–11975. doi: 10.7150/thno.50093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kellum JA, Lameire N, KDIGO AKI Guideline Work Group. Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (Part 1). Crit Care. 2013;17(1):204. doi: 10.1186/cc11454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu L, Cai J, Li C, et al. 4-Octyl itaconate attenuates LPS-induced acute kidney injury by activating Nrf2 and inhibiting STAT3 signaling. Mol Med. 2023;29(1):58. doi: 10.1186/s10020-023-00631-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deng Z, Sun M, Wu J, et al. SIRT1 attenuates sepsis-induced acute kidney injury via Beclin1 deacetylation-mediated autophagy activation. Cell Death Dis. 2021;12(2):217. doi: 10.1038/s41419-021-03508-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiang N, Zhao H, Han Y, et al. HIF-1α ameliorates tubular injury in diabetic nephropathy via HO-1-mediated control of mitochondrial dynamics. Cell Prolif. 2020;53(11):e12909. doi: 10.1111/cpr.12909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lu H, Li G, Liu L, Feng L, Wang X, Jin H. Regulation and function of mitophagy in development and cancer. Autophagy. 2013;9(11):1720–1736. doi: 10.4161/auto.26550 [DOI] [PubMed] [Google Scholar]
- 28.Lira Chavez FM, Gartzke LP, van Beuningen FE, et al. Restoring the infected powerhouse: mitochondrial quality control in sepsis. Redox Biol. 2023;68:102968. doi: 10.1016/j.redox.2023.102968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ajoolabady A, Chiong M, Lavandero S, Klionsky DJ, Ren J. Mitophagy in cardiovascular diseases: molecular mechanisms, pathogenesis, and treatment. Trends Mol Med. 2022;28(10):836–849. doi: 10.1016/j.molmed.2022.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Feng LX, Zhao F, Liu Q, et al. Role of Nrf2 in lipopolysaccharide-induced acute kidney injury: protection by human umbilical cord blood mononuclear cells. Oxid Med Cell Longev. 2020;2020:6123459. doi: 10.1155/2020/6123459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang DD, Hannink M. Distinct cysteine residues in Keap1 are required for Keap1-dependent ubiquitination of Nrf2 and for stabilization of Nrf2 by chemopreventive agents and oxidative stress. Mol Cell Biol. 2003;23(22):8137–8151. doi: 10.1128/MCB.23.22.8137-8151.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Qin T, Yin S, Yang J, et al. Sinomenine attenuates renal fibrosis through Nrf2-mediated inhibition of oxidative stress and TGFβ signaling. Toxicol Appl Pharmacol. 2016;304:1–8. doi: 10.1016/j.taap.2016.05.009 [DOI] [PubMed] [Google Scholar]
- 33.Wei W, Ma N, Fan X, Yu Q, Ci X. The role of Nrf2 in acute kidney injury: novel molecular mechanisms and therapeutic approaches. Free Radic Biol Med. 2020;158:1–12. doi: 10.1016/j.freeradbiomed.2020.06.025 [DOI] [PubMed] [Google Scholar]
- 34.Lei X, Wang J, Zhang F, et al. Micheliolide ameliorates lipopolysaccharide-induced acute kidney injury through suppression of NLRP3 activation by promoting mitophagy via Nrf2/PINK1/Parkin axis. Int Immunopharmacol. 2024;138:112527. doi: 10.1016/j.intimp.2024.112527 [DOI] [PubMed] [Google Scholar]
- 35.Wang Y, Xu Y, Wang Q, et al. Sulforaphane ameliorated podocyte injury according to regulation of the Nrf2/PINK1 pathway for mitophagy in diabetic kidney disease. Eur J Pharmacol. 2023;958:176042. doi: 10.1016/j.ejphar.2023.176042 [DOI] [PubMed] [Google Scholar]