Abstract
PURPOSE
The aim of this randomized, placebo-controlled, two-stage, phase II/III trial was to determine the efficacy of an oral cannabis extract in adults with refractory nausea and/or vomiting during moderately or highly emetogenic, intravenous chemotherapy despite guideline-consistent antiemetic prophylaxis. Here, we report results of the prespecified combined analysis including the initial phase II and subsequent phase III components.
PATIENTS AND METHODS
Study treatment consisted of oral capsules containing either tetrahydrocannabinol 2.5 mg plus cannabidiol 2.5 mg capsules (THC:CBD) or matching placebo, taken three times a day from days –1 to 5, in addition to guideline-consistent antiemetics. The primary measure of effect was the difference in the proportions of participants with no vomiting or retching and no use of rescue medications (a complete response) during hours 0-120 after the first cycle of chemotherapy on study (cycle A).
RESULTS
We recruited 147 evaluable of a planned 250 participants from 2016 to 2022. Background antiemetic prophylaxis included a corticosteroid and 5-hydroxytryptamine antagonist in 97%, a neurokinin-1 antagonist in 80%, and olanzapine in 10%. THC:CBD compared with placebo improved the complete response rate from 8% to 24% (absolute difference 16%, 95% CI, 4 to 28, P = .01), with similar effects for absence of significant nausea, use of rescue medications, daily vomits, and the nausea scale on the Functional Living Index—Emesis quality-of-life questionnaire. More frequent bothersome adverse events of special interest included sedation (18% v 7%), dizziness (10% v 0%), and transient anxiety (4% v 1%). There were no serious adverse events attributed to THC:CBD.
CONCLUSION
THC:CBD is an effective adjunct for chemotherapy-induced nausea and vomiting despite standard antiemetic prophylaxis, but was associated with additional adverse events. Drug availability, cultural attitudes, legal status, and preferences may affect implementation. Future analyses will evaluate the cost-effectiveness of THC:CBD.
Oral cannabis versus placebo for refractory chemo-induced vomiting improves control from 8% to 24% in RCT.
INTRODUCTION
Chemotherapy-induced nausea and vomiting (CINV) remain feared complications of anticancer therapy that are associated with worse quality of life, increased use of health care resources, and reduced adherence to chemotherapy.1 Corticosteroids, 5-hydroxytryptamine (5-HT3) antagonists, and neurokinin-1 (NK-1) antagonists mitigate CINV in many patients.2 However, one third or more of patients treated with moderately or highly emetogenic chemotherapy report significant nausea or vomiting despite guideline-consistent prophylaxis.3-5
CONTEXT
Key Objective
To investigate the effectiveness of oral capsules of tetrahydrocannabinol (THC):cannabidiol (CBD) in a 1:1 ratio at improving the control of chemotherapy-induced nausea and vomiting (CINV) in patients with refractory symptoms despite modern guideline-consistent antiemetic prophylaxis including dexamethasone, 5-hydroxytryptamine antagonist, and neurokinin-1 antagonist, with or without olanzapine.
Knowledge Generated
Patients who received oral THC:CBD compared with placebo, in addition to guideline-consistent antiemetic prophylaxis, had better control of CINV, albeit with more frequent sedation, dizziness, and anxiety. The rates of complete response (no emesis nor use of rescue medications) were 24% for cannabis and 8% for placebo (P = .01).
Relevance (C. Zimmermann)
For patients with CINV despite guideline consistent antiemetic prophylaxis, oral THC:CBD is a safe option, although potential side effects should be kept in mind. Implementation may be limited due to restricted access and availability.*
*Relevance section written by JCO Associate Editor Camilla Zimmermann, MD, PhD, MPH, FRCPC.
CINV is mediated by a complex network of pathways linked to receptors for serotonin, dopamine, substance P, and the cannabinoid CB1.6-8 An understanding of the role of the cannabinoid receptor in CINV and empiric observations of the effects of cannabis led to early trials of tetrahydrocannabinol (THC) and synthetic cannabinoids (eg, nabilone), which reported improvements in CINV, at the cost of dose-dependent side effects including dizziness, sedation, and anxiety. Implementation of cannabinoids for CINV has been limited by questions resulting from trials with suboptimal methods, and the development of alternative antiemetics.9,10 Distinct from THC, cannabidiol (CBD) is a cannabinoid that has anxiolytic properties and may counteract neuropsychiatric effects of THC.11 The oral cannabinoid regimen selected for our study was based on a pilot randomized, placebo-controlled trial including 16 participants, reporting that a buccal spray of 1:1 THC:CBD (nabiximols) improved complete response rates from 22% to 71% (difference 49%, 95% CI, 1 to 75).12
The aim of this multicenter, randomized, double-blinded, placebo-controlled, two-stage, phase II/III trial was to determine the efficacy of an oral cannabis extract (THC:CBD) in adults who experienced refractory CINV during moderate and highly emetogenic intravenous chemotherapy despite guideline-consistent antiemetic prophylaxis. We previously reported that complete response rates were higher with THC:CBD than with placebo in the first 81 participants recruited to the phase II component of this trial using a crossover analysis of the first two cycles (25% v 14%, 90% CI, 1.12 to 2.79, P = .04), and that 83% of participants preferred cannabis to placebo.13 Here, we report results of the prespecified parallel-group analysis of the first cycle including all recruited participants.
PATIENTS AND METHODS
Patients
Eligible patients were age 18 years and older with a solid tumor or hematologic malignancy of any stage, being treated with intravenous chemotherapy of moderate or high emetogenic risk, and scheduled for at least two more consecutive cycles of the same chemotherapy. Patients must have experienced refractory CINV (defined as emesis and/or nausea of at least moderate severity on a five-point rating scale and/or requiring the use of rescue medications) in an earlier treatment cycle of the same chemotherapy regimen, despite eviQ14 and/or MASCC3,15 guideline-consistent antiemetic prophylaxis including a corticosteroid, 5-HT3 antagonist, and NK-1 antagonist, with or without olanzapine. Exclusion criteria were Eastern Cooperative Oncology Group performance status (ECOG) >2; a contraindication to medicinal cannabis such as unstable cardiovascular disease, substance use disorder, or significant mental health disorder; disease-related nausea or vomiting; oral chemotherapy; or radiotherapy to the brain or GI tract during the study period. Participants were to abstain from other cannabis products before or during the trial, and underwent a urinary drug screen for THC within 30 days before enrollment. Driving was not permitted for legal reasons during study treatment or for 3 days after. All participants provided signed, written, informed consent. The protocol and all amendments were approved by human research ethics committees covering all participating centers. Australian New Zealand Clinical Trials Registry Registration No. ACTRN12616001036404.
Study and Control Treatment
The study treatment was oral THC:CBD, consisting of 2.5 mg of THC and 2.5 mg of CBD in a 1:1 ratio presented in white methylcellulose capsules, derived from the Cannabis sativa L. strains, and produced by Tilray, a federally licensed producer and distributor of medicinal cannabis, under Health Canada's Marijuana for Medical Purposes Regulations. The control treatment was a placebo, presented in white capsules that were identical to that of the active treatment, and also produced by Tilray.
Trial Design and Intervention
Details of the study protocol have been previously published.16 A central, web-based randomization system was used to randomly assign participants to study treatment in cycle A with either oral THC:CBD or placebo, both given three times a day over six consecutive days, starting the morning of the day before chemotherapy (day –1) and finishing at midday on day 5. Participants commenced with an initial dose of one capsule of oral THC:CBD or matching placebo, with self-titration of the study drug dose up or down on the basis of experience of CINV or side effects, up to a maximum of four tablets three times a day. During the initial phase II component of the trial, which used a crossover design, participants were treated with the alternative treatment for cycle B, then nominated their preferred treatment for cycle C. During the subsequent phase III component of the trial, which used a parallel design, participants were to continue the same treatment for cycle B and cycle C (but following a protocol amendment to enhance recruitment, were allowed to choose the alternative treatment if they experienced significant CINV during cycle A). In addition to the study treatment, all participants received guideline-recommended CINV prophylaxis (as described above), including a corticosteroid and a 5-HT3 antagonist, and where indicated, an NK-1 inhibitor and olanzapine. Rescue medications were to be used for nausea or vomiting that occurred during the study as per standard guidelines.14,15,17
Assessments
Participants underwent clinical assessment by the study investigator on day –1 of cycles A, B, and C, and at 30 to 42 days after the last dose of study treatment. Participants recorded their experience of nausea, vomiting, use of rescue medications, and numbers of doses of study treatment in a diary for days –1 to day 5 of each cycle. There was daily contact with trial staff on the days of study treatment to ensure the appropriate use of study medication, compliance with the patient diary, recording of a structured checklist of adverse events of special interest known to be associated with cannabinoids, and provision of advice on the management of adverse events. Health-related quality of life (HRQL) outcome measures included the Functional Living Index—Emesis (FLIE)18 and the Assessment of Quality of Life—eight dimensions (AQOL-8D),19 a multiattribute utility instrument, completed at baseline, day 6 of each cycle, and at 30-42 days after the last dose of study treatment.
Objectives and End Points
The primary measure of effect was the difference between the proportions of participants allocated THC:CBD versus placebo in cycle A achieving a complete response (primary end point, defined as no emesis [vomiting or dry retching] and no use of rescue medications during hours 0-120), as recorded in the patient diary. Secondary end points included self-reported complete response, no emesis (vomiting or retching), no use of rescue medications, no clinically significant nausea (<2 on a 10-point scale), and complete control (defined as complete response and no clinically significant nausea) during the acute phase (0-24 hours), delayed phase (24-120 hours), and overall phase (0-120 hours) for cycle A; summary scales for nausea and vomiting of the FLIE with 5-day recall; and individual items, domains, and utilities of the AQOL-8D. Adverse events were recorded by clinicians using the National Cancer Institute Common Terminology Criteria for Adverse Events v4.0320 and by participants using a trial-specific structured checklist of self-rated adverse events of special interest, including dizziness, disorientation, hallucinations, anxiety, palpitation, and sedation. Health economic analyses will be reported separately in the future.
Statistical Analyses
The planned sample size of 250 participants (125 per arm), comprising 80 in the phase II component and a further 170 in the phase III component, provided 80% power with a two-sided type I-error rate of 0.05 to detect improvement in complete response from 22% to 42.5%. This sample size was calculated on a prospective basis and allowed for a dropout and ineligibility rate of 20% using a conservative inflation factor (inflated sample size = sample size/[1-rate of dropout]2). Efficacy analyses were by intention to treat among all patients with available data and included participants who received the intervention for cycle A. Participants without available data are identified in the CONSORT diagram (Fig 1). Safety analyses included all participants who received at least one dose of study drug. Binary outcomes were analyzed using a model with a log link, and continuous outcomes were analyzed with a linear model. HRQL outcome measures were analyzed with linear models adjusting for baseline values. All reported P values are two-sided and without adjustment for multiple comparisons. Analyses were completed using SAS 9.4 (SAS Institute Inc, Cary, NC).
FIG 1.
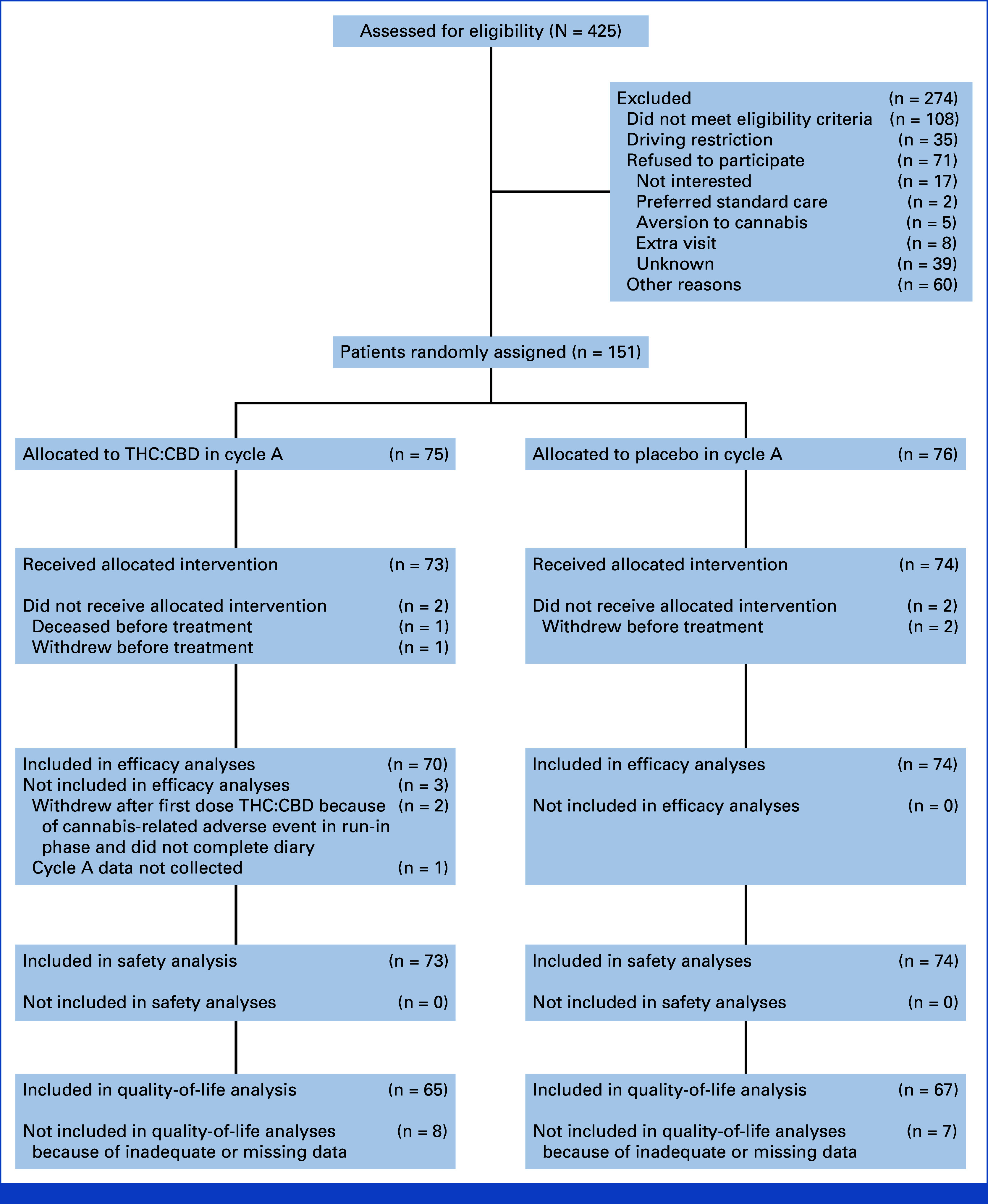
CONSORT diagram. CBD, cannabidiol; THC, tetrahydrocannabinol.
RESULTS
Participants
We randomly assigned a total of 151 participants from 17 sites between December 2016 and September 2022, of whom 147 received the allocated intervention (THC:CBD or placebo) for cycle A. One hundred and forty-four were included in efficacy analyses. One hundred and forty-seven were included in safety analyses. One hundred and thirty-two were included in quality-of-life analyses. A further 274 patients were screened but not enrolled, most commonly because of not meeting eligibility criteria due to recent use of cannabis or ineligible chemotherapy regimens, refusal to participate, or unwillingness to comply with driving restrictions. Further details are presented in the CONSORT diagram. Study recruitment was closed early because of slow accrual without knowledge of differences in study outcomes between treatment arms for cycle A.
Characteristics of the 147 participants who received the allocated intervention were median age 56 years (range, 25-80), 78% female, 39% reporting previous cannabis use, and 65% being treated with curative intent. Emetic severity of chemotherapy was high in 53% and moderate in 47%. All participants had received at least one cycle of the same chemotherapy before enrollment; 46% had received two or more cycles. Antiemetic prophylaxis included dexamethasone and a 5-HT3 antagonist in 97%, NK-1 antagonist in 80%, and olanzapine in 10%. Further baseline characteristics are summarized in Table 1.
TABLE 1.
Baseline Characteristics
| Characteristic | THC:CBD (n = 73), No. (%) | Placebo (n = 74), No. (%) | Overall (N = 147), No. (%) |
|---|---|---|---|
| Age, years | |||
| Median (min-max) | 55 (25-76) | 59 (29-80) | 56 (25-80) |
| Sex | |||
| Female | 56 (77) | 59 (80) | 115 (78) |
| Eastern Cooperative Oncology Group status | |||
| 0-1 | 70 (96) | 73 (99) | 143 (97) |
| Previous cannabis use | |||
| Yes | 28 (38) | 30 (41) | 58 (39) |
| Alcohol (standard drinks/week) | |||
| 0 | 51 (70) | 42 (57) | 93 (63) |
| 1-7 | 22 (30) | 27 (36) | 49 (33) |
| >7 | 0 | 5 (7) | 5 (3) |
| History of motion sickness | |||
| Yes | 23 (32) | 23 (31) | 46 (31) |
| History of nausea during pregnancy | |||
| Yes | 26/50 (52) | 27/51 (53) | 53/101 (52) |
| Primary cancer site | |||
| Breast | 26 (36) | 29 (39) | 55 (37) |
| GI | 25 (34) | 20 (27) | 45 (31) |
| Lung | 9 (12) | 8 (11) | 17 (12) |
| Gynecologic | 4 (5) | 7 (9) | 11 (7) |
| Genitourinary | 3 (4) | 5 (7) | 8 (5) |
| Hematologic | 2 (3) | 3 (4) | 5 (3) |
| Sarcoma | 1 (1) | 0 | 1 (1) |
| Head and neck | 1 (1) | 0 | 1 (1) |
| Unknown primary | 2 (3) | 2 (3) | 4 (3) |
| Treatment intent | |||
| Curative | 41 (56) | 54 (73) | 95 (65) |
| Palliative | 32 (44) | 20 (27) | 52 (35) |
| Chemotherapy emetic severity | |||
| High | 36 (49) | 42 (57) | 78 (53) |
| Moderate | 37 (51) | 32 (43) | 69 (47) |
| Chemotherapy cycle duration, days | |||
| 14 | 36 (49) | 40 (54) | 76 (52) |
| 21 | 37 (51) | 34 (46) | 71 (48) |
| Chemotherapy regimen | |||
| Anthracycline-based | 20 (27) | 28 (38) | 48 (33) |
| Carboplatin-based | 13 (18) | 12 (16) | 25 (17) |
| FOLFOX ± biological | 14 (19) | 12 (16) | 26 (18) |
| Cisplatin-based | 13 (18) | 12 (16) | 25 (17) |
| FOLFIRINOX | 7 (10) | 4 (5) | 11 (7) |
| Other | 6 (8) | 6 (8) | 12 (8) |
| Background antiemetic prophylaxis | |||
| Dexamethasone | 72 (99) | 71 (96) | 143 (97) |
| 5-HT3 antagonist | 71 (97) | 72 (97) | 143 (97) |
| NK-1 antagonist | 59 (81) | 58 (78) | 117 (80) |
| Olanzapine | 7 (10) | 8 (11) | 15 (10) |
Abbreviations: 5-HT3, 5-hydroxytryptamine; CBD, cannabidiol; FOLFIRINOX, infusional fluorouracil, leucovorin, irinotecan, and oxaliplatin; FOLFOX, infusional fluorouracil, leucovorin, and oxaliplatin; NK-1, neurokinin-1; THC, tetrahydrocannabinol.
Study Treatment Exposure
The median number of capsules (IQR) taken per dose in cycle A were two (1-2) for THC:CBD (equating to 5 mg THC and 5 mg CBD tds) versus 3 (2-4) for placebo.
Efficacy During Cycle A
Efficacy analyses are summarized in Table 2. Regarding the primary end point, the proportion of participants with complete response during the overall phase (0-120 hours) of treatment was better among those assigned THC:CBD versus placebo (24% v 8%, absolute difference 16%, 95% CI, 4 to 28, P = .01). There were similar effects on no use of rescue medications (28% v 9%, absolute difference 19%, 95% CI, 6 to 31, P = .01) and no significant nausea (20% v 7%, absolute difference 13%, 95% CI, 2 to 24, P = .03). The number of vomits per day and scores for nausea were also better for those assigned THC:CBD rather than placebo.
TABLE 2.
Efficacy Outcomes Over the First 5 Days (hours 0 to 120) of Cycle A
| Outcome | THC:CBD (n = 73), No. (%) |
Placebo (n = 74), No. (%) |
Difference (95% CI) | Relative Risk (95% CI) | P |
|---|---|---|---|---|---|
| Complete response (primary outcome)a | 17/70 (24) | 6/74 (8) | 16 (4 to 28) | 3.0 (1 to 7) | .01 |
| No use of rescue medications | 20/71 (28) | 7/74 (9) | 19 (6 to 31) | 3.0 (1 to 7) | .01 |
| No significant nausea (score <2) | 14/70 (20) | 5/74 (7) | 13 (2 to 24) | 3.0 (1 to 8) | .03 |
| Complete response and no significant nausea | 7/70 (10) | 2/74 (3) | 7 (–1 to 15) | 3.7 (1 to 17) | .10 |
| No vomiting or retching | 49/70 (70) | 43/74 (58) | 12 (–4 to 27) | 1.2 (1 to 1.5) | .14 |
| No. of vomits per day, mean | 0.2 | 0.5 | –0.3 (–0.6, –0.1) | .01 | |
| Maximum No. of vomits per day, mean | 0.6 | 1.3 | –0.7 (–1.3, –0.10) | .02 | |
| Nausea score, mean | 2.8 | 4.3 | –1.5 (–2.2 to –0.9) | <.001 | |
| Maximum nausea score, mean | 3.8 | 5.7 | –1.9 (–2.8 to –1.1) | <.001 |
Abbreviations: CBD, cannabidiol; THC, tetrahydrocannabinol.
No vomiting or retching and no use of rescue medications during the overall phase of cycle A (0-120 hours).
There was no evidence of heterogeneity between the results for participants in the phase II component (crossover design) versus the phase III component (parallel design): the relative risk of complete response for the first stage was 3.33 (95% CI, 0.99 to 11.19) and for the second stage was 2.63 (95% CI, 0.74 to 9.32), I2 = 0%. After adjusting for known confounders individually because of low rates of complete response, only sex, with men having a higher response rate than women, was independently associated with rates of complete response. Sensitivity analyses for the primary end point tested the effect of including the three patients who received the allocated intervention but had no available data (all conservatively assigned as treatment failures), and alternatively tested the effect of including all seven patients who were randomly assigned but had no available data (all conservatively assigned as treatment failures), and provided the same conclusion as the primary analysis (P = .011, P = .012, and P = .01 respectively, CONSORT diagram).
Quality-of-life results are summarized in Table 3. Scores for the FLIE nausea summary scale were higher (better) among those assigned THC:CBD than placebo (means 67 v 48, difference 19, 95% CI, 9 to 28, P < .001) with limited evidence of effects on the FLIE vomit summary scale. For the AQOL-8D, after adjustment for baseline scores, there was a significant improvement in the mean values for the pain domain, but no significant differences in other domains nor the summary utility.
TABLE 3.
Quality-of-Life Analyses for Cycle A
| Outcome | Baseline | Day 6 (or 7, 8) | Mean Differencea | P | ||
|---|---|---|---|---|---|---|
| THC:CBD | Placebo | THC:CBD | Placebo | |||
| FLIE summary scalesb | ||||||
| Nausea domain | 63 | 62 | 67 | 48 | 19 | <.01 |
| Vomiting domain | 83 | 84 | 89 | 84 | 6 | .11 |
| AQOL-8D summary scalesc | ||||||
| Independent living | 0.77 | 0.78 | 0.70 | 0.71 | –0.00 | .91 |
| Happiness | 0.75 | 0.76 | 0.70 | 0.69 | 0.02 | .40 |
| Mental health | 0.63 | 0.62 | 0.64 | 0.62 | 0.01 | .60 |
| Coping | 0.74 | 0.73 | 0.66 | 0.67 | –0.01 | .59 |
| Relationships | 0.69 | 0.67 | 0.64 | 0.64 | –0.01 | .68 |
| Self-worth | 0.77 | 0.79 | 0.73 | 0.75 | –0.00 | .96 |
| Pain | 0.69 | 0.76 | 0.75 | 0.74 | 0.06 | .05 |
| Sensitivity | 0.85 | 0.86 | 0.86 | 0.85 | 0.01 | .44 |
| Super dimension mental | 0.36 | 0.35 | 0.30 | 0.31 | –0.01 | .76 |
| Super dimension physical | 0.61 | 0.66 | 0.60 | 0.60 | 0.03 | .24 |
| AQOL-8D utility | 0.66 | 0.67 | 0.62 | 0.61 | 0.01 | .68 |
Abbreviations: AQOL-8D, Assessment of Quality of Life—eight dimensions; CBD, cannabidiol; FLIE, Functional Living Index—Emesis; THC, tetrahydrocannabinol.
Adjusted for baseline, positive results favor THC:CBD.
Scale 0-100, higher score indicated better quality of life.
Scale 0-1, higher score indicates better quality of life.
Adverse Events During Cycle A
Self-rated adverse events of special interest (any severity, and moderate to severe) during cycle A were more frequent among those assigned THC:CBD than placebo (74% v 38% and 25% v 8%, respectively; Table 4). The most frequently reported moderate to severe adverse effects were sedation (18% v 7%) and dizziness (10% v 0%). Anxiety, disorientation, hallucinations, and palpitation were uncommon. Two participants withdrew from the study after the first dose of THC:CBD because of transient anxiety.
TABLE 4.
Self-Rated Adverse Events of Special Interest During Cycle A
| Adverse Event | THC:CBD, No. (%) | Placebo, No. (%) | THC:CBD, No. (%) | Placebo, No. (%) | Mean Difference (95% CI) |
P Fisher's Exact Test |
|---|---|---|---|---|---|---|
| Any severity | Moderate or severe | |||||
| Sedation | 40 (56) | 18 (24) | 13 (18) | 5 (7) | 11 (0.6 to 22) | .05 |
| Dizziness | 30 (42) | 10 (14) | 7 (10) | 0 | 10 (3 to 17) | .006 |
| Anxiety | 5 (7) | 6 (8) | 3 (4) | 1 (1) | 3 (–3 to 8) | .4 |
| Disorientation | 13 (18) | 4 (5) | 3 (4) | 0 | 4 (–0.5 to 9) | .12 |
| Hallucinations | 1 (1) | 1 (1) | 0 | 0 | ||
| Palpitations | 5 (7) | 5 (7) | 0 | 0 | ||
| Any AESIa | 53 (74) | 28 (38) | 18 (25) | 6 (8) | 17 (5 to 29) | .007 |
NOTE. Self-rated AESI were collected during daily assessment between D-1 and D6, and included the following known cannabis-related adverse events: sedation, anxiety, disorientation, dizziness, hallucinations, and palpitations.
Abbreviations: AESI, adverse events of special interest; CBD, cannabidiol; THC, tetrahydrocannabinol.
Number of participants experiencing ≥1 event during cycle A.
Clinician-rated adverse events of grade 3 or 4 during cycle A were reported with similar frequency among those assigned THC:CBD and placebo (19% v 12%; Appendix Table A1, online only). Serious adverse events were reported with similar frequency among those assigned THC:CBD than placebo (5% v 8%; Appendix Table A2). The only death was from febrile neutropenia in the placebo group. Site investigators attributed no serious adverse events to study treatment with THC:CBD.
Subsequent Cycles
Because of the high rates of crossover in the phase III component of the trial, efficacy results for cycle B and cycle C have not been analyzed. Adverse events for cycle B (in part after unblinding and crossover) were similar and are included in Appendix Table A3.
DISCUSSION
Oral THC:CBD improved CINV that was refractory despite guideline-consistent antiemetic prophylaxis. Oral THC:CBD compared with placebo was associated with a statistically significant and clinically meaningful benefit as assessed by complete response (defined as no vomiting or retching, and no use of rescue medications), absence of significant nausea, number of vomits per day, and the nausea scale of the FLIE quality-of-life questionnaire.
Moderate to severe adverse events of special interest that were more frequent among those assigned THC:CBD than placebo included sedation (18% v 7%), dizziness (10% v 0%), and transient anxiety (4% v 1%). No unexpected or serious adverse events were attributed to THC:CBD; however, two participants discontinued THC:CBD because of transient neuropsychiatric side effects after the first dose of THC:CBD. The most frequently titrated dose was 5 mg of THC with 5 mg of CBD tds. A small number of patients did not tolerate the starting dose of 2.5 mg of THC with 2.5 mg of CBD, so dose titration remains recommended.
Putting the results of our study in context, Table 5 outlines the comparative efficacy of antiemetics versus placebo in positive randomized placebo-controlled trials. The absolute improvement (16%) and relative improvement (3.0) in complete response rates in our study exceeded the absolute improvement of 10% considered sufficient to warrant changing recommendations in antiemetic guidelines,15 and—within the limits of cross-trial comparisons with heterogeneous trial designs—was at least comparable with modern studies of aprepitant and olanzapine (13%-15%, 1.2-1.7), and older studies of cannabinoids (23%-33% and 1.2-10, respectively). It should be noted that our rates of complete response (24% for THC:CBD and 8% for placebo) are lower than typically reported in modern trials of primary antiemetic prophylaxis, because our study population was patients with refractory CINV despite guideline-recommended prophylaxis, not chemotherapy-naïve patients commencing chemotherapy. As outlined in Table 5, the rate of complete response for placebo is typically <20% in trials for refractory CINV (including our study) and trials that do not include modern background antiemetic regimens.
TABLE 5.
Comparative Efficacy of Antiemetics Versus Placebo in Randomized Controlled Trials
| Antiemetic (class) | Background Antiemetic Regimen | Chemotherapy Emetogenicity | Setting | Sample Size | Duration of Follow-Up | Rate of CR (experimental arm), % | Rate of CR (placebo arm), % | Absolute Improvement in CR, % | Relative Improvement in CR | P | References |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cannabinoid | |||||||||||
| Dronabinol | Nil | Moderate/high | Refractory (91%) | 22 | 0-24 h | 33 | 0 | 33 | (10) | <.001 | Sallan, 197521 |
| Dronabinol | Nil | Moderate | Naïve | 116 | 0-24 h | 42 | 19 | 23 | 1.2 | .05 | Frytak, 197922 |
| Nabilone | UK | Moderate/high | Various | 228 | 0-24 h | 35 | 11 | 24 | 2.2 | <.001 | Wada, 198223 |
| THC:CBD | D, 5HT3 |
Moderate | Refractory | 16 | 0-120 h | 71 | 22 | 49 | 3.2 | NS | Duran, 201012 |
| THC:CBD | D, 5HT3, NK1, +/– O |
Moderate/high | Refractory | 147 | 0-120 h | 24 | 8 | 16 | 3.0 | .01 | This paper |
| 5-HT3 antagonist | Nil relevant trials identified | ||||||||||
| NK-1 inhibitor | |||||||||||
| Aprepitant | D, 5HT3 |
Moderate/high | Naïve | 848 | 0-120 h | 69 | 56 | 13 | 1.2 | <.001 | Rapoport, 200924 |
| Olanzapine | |||||||||||
| Olanzapine | D, 5HT3, NK1 |
High | Naïve | 380 | 0-120 h | 37 | 22 | 15 | 1.7 | .02 | Navari, 201625 |
| Olanzapine | D, 5HT3, NK1 |
High | Naïve | 710 | 0-120 h | 78 | 64 | 14 | 1.2 | <.001 | Hashimoto, 201926 |
Abbreviations: 5-HT3, 5-hydroxytryptamine; CBD, cannabidiol; CR, complete response; D, dexamethasone; h, hours; NK-1, neurokinin-1; NS, not significant; O, olanzapine; THC, tetrahydrocannabinol.
The main strengths of our study were its randomized, placebo-controlled design to minimize bias; use of modern, guideline-consistent background antiemetic prophylaxis to improve relevance compared with older studies of cannabinoids for CINV; and collection of utility-based quality-of-life measures and data regarding resource use for planned health economic evaluations. To improve tolerance, we used a combination of THC with CBD, and allowed dose titration starting the day before each chemotherapy cycle. We used a pharmaceutical-grade oral capsule formulation to improve accuracy and convenience of dosing, in comparison with cannabis oil or inhaled cannabis products.
Our study has limitations. Accrual was stopped early because of slow recruitment before analyses of the study outcomes. Early stopping reduced power to detect differences between the study groups but would not bias the results. The preplanned primary analysis involved combining data from the randomized phase II component and the randomized phase III component, with separate randomizations. Only 10% of participants were treated with olanzapine, which has been added to clinical practice guidelines as antiemetic prophylaxis for highly emetogenic chemotherapy risk on the basis of a large phase III randomized trial,25,27,28 but was not widely used during accrual to our study. Our trial did not provide information about longer-term efficacy over multiple cycles of chemotherapy because many participants completed chemotherapy within one or two cycles of study treatment. Notably, we previously reported that participants in the phase II component (crossover design) of this trial reported a strong preference for THC:CBD.13
There are important barriers to using oral THC:CBD for CINV in routine clinical practice. These include adverse effects, cultural attitudes, and legal restrictions to the use of cannabinoids. The high rate of screen failures and early stopping for slow recruitment reflect these challenges. The commonest reasons for screen failure were current use of prescribed or nonprescribed cannabis (the former is now more easily available in Australia), aversion to cannabis, and the need to restrict driving. Most participants were recruited in the state of New South Wales, Australia, where driving after recent use of cannabis is prohibited, regardless of source, indication, or degree of impairment. Studies have reported that THC can impair driving ability, and no safe level of THC has been identified.29-31 Driving restrictions are a challenge, particularly in regional and rural areas that lack public transport. Cultural barriers to implementation, including the unwillingness of oncologists to endorse or prescribe cannabis, is another challenge. The cost of medicinal cannabis may also be prohibitive to patients. Health economic evaluations of cost-effectiveness are planned and may influence attitudes and reimbursement by public and private payers. Better control of CINV needs to be balanced by potential adverse effects from THC:CBD.
Future research should evaluate alternative cannabinoids formulations, sequencing, and/or combinations with other antiemetics; comparison of cannabinoids versus olanzapine; and the use of cannabinoids as primary prevention of CINV (rather than secondary prevention for refractory CINV). Research is also warranted regarding the use of THC:CBD for nausea, vomiting, and/or anorexia associated with oral anticancer treatments (eg, capecitabine, temozolomide, and tyrosine kinase inhibitors); and the use of THC:CBD for pain, given our finding of improvement in pain by THC:CBD on the pain scale of the AQOL-8D quality-of-life questionnaire.
In conclusion, an oral formulation of THC:CBD was an effective adjunct to standard antiemetics for prevention and treatment of refractory CINV, with adverse effects including sedation and dizziness, but no increase in serious adverse events. Our data support the claim that oral THC:CBD is an effective and safe option for the prevention of refractory CINV. Availability, access, affordability, cultural attitudes, societal barriers, and legal barriers may limit implementation.
ACKNOWLEDGMENT
The authors thank the participants; the principal investigators, coinvestigators, and study coordinators at the participating centers; staff at the National Health and Medical Research Council Clinical Trials Centre in Sydney, Australia, who were responsible for central coordination and data management of the trial; Drs Kerry Chant and Jan Fizzell from the NSW Department of Health; and consumer support from the Chris O'Brien Lifehouse Partnership Council.
APPENDIX
TABLE A1.
Number of Participants With Grade 3 to 4 Adverse Events During Cycle A
| Adverse Event | THC:CBD (n = 73) | Placebo (n = 74) |
|---|---|---|
| Neutrophil count decreased | 2 | 1 |
| Febrile neutropenia | 1 | |
| Platelet count decreased | 1 | |
| Anemia | 2 | |
| Nausea | 2 | 1 |
| Vomiting | 1 | 2 |
| Urinary tract infection | 1 | |
| Lymph gland infection | 1 | |
| Diarrhea | 1 | |
| Hypokalemia | 1 | |
| Headache | 1 | |
| Cystitis, noninfective | 1 | |
| Somnolence | 1 | |
| Agitation | 1 | |
| Anxiety | 1 | |
| Confusion | 1 | |
| No. of participants experiencing any G3-4 AE | 14 (19%) | 9 (12%) |
Abbreviations: AE, adverse event; CBD, cannabidiol; THC, tetrahydrocannabinol.
TABLE A2.
Serious Adverse Events During Cycle A
| Serious Adverse Event | THC:CBD (n = 73), No. (%) | Placebo (n = 74), No. (%) |
|---|---|---|
| Neutrophil count decreased | ||
| Vomiting | 1 | 2 |
| Febrile neutropenia | 1 | |
| Fever | ||
| Anemia | 1 | |
| Atrial flutter | ||
| Urinary tract infection | 1 | |
| Cystitis, noninfective | 1 | |
| Cerebral edema | ||
| Dehydration | ||
| Postchemotherapy reaction | ||
| Lymph gland infection | 1 | |
| Anal pain | ||
| Hypokalemia | 1 | |
| Diarrhea | 1 | |
| Abdominal pain | ||
| No. of patients experiencing any SAE | 4 (5%) | 6 (8%) |
Abbreviations: CBD, cannabidiol; SAE, serious adverse event; THC, tetrahydrocannabinol.
TABLE A3.
Self-Rated AESI During Cycle B
| Adverse Event | THC:CBD, No. (%) | Placebo, No. (%) | THC:CBD, No. (%) | Placebo, No. (%) |
|---|---|---|---|---|
| Any severity | Moderate or severe | |||
| Sedation | 51 (59) | 13 (29) | 14 (16) | 2 (4) |
| Dizziness | 6 (7) | 3 (7) | 0 | 0 |
| Anxiety | 15 (17) | 2 (4) | 3 (3) | 0 |
| Disorientation | 42 (49) | 7 (16) | 11 (13) | 2 (4) |
| Hallucinations | 9 (10) | 0 | 0 | 0 |
| Palpitations | 2 (2) | 0 | 0 | 0 |
| Any AESIa | 64 (74) | 17 (38) | 21 (24) | 3 (7) |
NOTE. Self-rated AESI were collected during daily assessment between D-1 and D6, and included the following known cannabis-related adverse events: sedation, anxiety, disorientation, dizziness, hallucinations, and palpitations.
Abbreviations: AESI, adverse events of special interest; CBD, cannabidiol; THC, tetrahydrocannabinol.
Number of participants experiencing ≥1 event during cycle B.
Peter Grimison
Research Funding: Tilray (Inst), Pfizer (Inst), MSD (Inst), Genentech (Inst), Eisai (Inst), QED Therapeutics (Inst), Janssen-Cilag (Inst), Daiichi Sankyo Europe GmbH (Inst), PTC Therapeutics (Inst), InhibRx (Inst)
Antony Mersiades
Honoraria: The Limbic
Ian Olver
Consulting or Advisory Role: VieCure, Aucentra Therapeutics, BOD Australia
Paul Haber
Speakers' Bureau: Camurus
Research Funding: Indivior (Inst), Woke Pharmaceuticals (Inst)
Karen Briscoe
Consulting or Advisory Role: Bristol Myers Squibb Foundation
Speakers' Bureau: Pierre Fabre
Jasotha Sanmugarajah
Speakers' Bureau: Lilly
Craig Gedye
Consulting or Advisory Role: Novotech, Bristol Myers Squibb (Inst), Merck Sharp & Dohme (Inst), CADEX Genomics, BCAL Diagnostics
Speakers' Bureau: Limbic Oncology (Inst)
Stephen Begbie
Consulting or Advisory Role: Astellas Pharma, MSD Oncology, Merck Serono, Pfizer, Bayer
Research Funding: Astellas Medivation (Inst), Merck Serono (Inst), Janssen Oncology (Inst), Roche (Inst), Pfizer/EMD Serono (Inst), Bristol Myers Squibb (Inst), MSD (Inst), Exelixis (Inst)
R. John Simes
Consulting or Advisory Role: FivePhusion (Inst)
Research Funding: Bayer (Inst), AbbVie (Inst), Roche (Inst), Bristol Myers Squibb (Inst), AstraZeneca (Inst), Pfizer (Inst), Amgen (Inst), MSD (Inst), BeiGene (Inst), Astellas Pharma (Inst)
Martin R. Stockler
Research Funding: Astellas Pharma (Inst), Bayer (Inst), Medivation (Inst), Pfizer (Inst), AstraZeneca (Inst), Bristol Myers Squibb (Inst), Roche (Inst), Amgen (Inst), Merck Sharp & Dohme (Inst), Tilray (Inst), BeiGene (Inst), Novartis (Inst)
No other potential conflicts of interest were reported.
See accompanying Editorial, p. 4008
SUPPORT
Supported by the Department of Health, New South Wales Government, Australia. Tilray supplied and covered the cost of study treatments but had no role in data analysis.
CLINICAL TRIAL INFORMATION
ACTRN12616001036404 (CannabisCINV)
AUTHOR CONTRIBUTIONS
Conception and design: Peter Grimison, Adrienne Kirby, Ian Olver, Paul Haber, Anna Walsh, Peter Fox, R. John Simes, Martin R Stockler
Financial support: Martin R Stockler
Administrative support: Peter Grimison, Anna Walsh, R. John Simes, Martin R Stockler
Provision of study materials or patients: Peter Grimison, Ehtesham Abdi, Stephen Della-Fiorentina, Morteza Aghmesheh, Peter Fox, Karen Briscoe, Jasotha Sanmugarajah, Gavin Marx, Ganessan Kichenadasse, Matthew Chan, Jenny Shannon, Craig Gedye, Stephen Begbie, Martin R Stockler
Collection and assembly of data: Peter Grimison, Antony Mersiades, Adrienne Kirby, Annette Tognela, Ian Olver, Anna Walsh, Yvonne Lee, Ehtesham Abdi, Morteza Aghmesheh, Peter Fox, Karen Briscoe, Jasotha Sanmugarajah, Ganessan Kichenadasse, Helen Wheeler, Matthew Chan, Craig Gedye, Stephen Begbie, Martin R. Stockler
Data analysis and interpretation: Peter Grimison, Antony Mersiades, Adrienne Kirby, Ian Olver, Rachael L. Morton, Paul Haber, Stephen Della-Fiorentina, Morteza Aghmesheh, Peter Fox, Jasotha Sanmugarajah, Gavin Marx, Ganessan Kichenadasse, Matthew Chan, Jenny Shannon, Stephen Begbie, R. John Simes, Martin R. Stockler
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Oral Cannabis Extract for Secondary Prevention of Chemotherapy-Induced Nausea and Vomiting: Final Results of a Randomized, Placebo-Controlled, Phase II/III Trial
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Peter Grimison
Research Funding: Tilray (Inst), Pfizer (Inst), MSD (Inst), Genentech (Inst), Eisai (Inst), QED Therapeutics (Inst), Janssen-Cilag (Inst), Daiichi Sankyo Europe GmbH (Inst), PTC Therapeutics (Inst), InhibRx (Inst)
Antony Mersiades
Honoraria: The Limbic
Ian Olver
Consulting or Advisory Role: VieCure, Aucentra Therapeutics, BOD Australia
Paul Haber
Speakers' Bureau: Camurus
Research Funding: Indivior (Inst), Woke Pharmaceuticals (Inst)
Karen Briscoe
Consulting or Advisory Role: Bristol Myers Squibb Foundation
Speakers' Bureau: Pierre Fabre
Jasotha Sanmugarajah
Speakers' Bureau: Lilly
Craig Gedye
Consulting or Advisory Role: Novotech, Bristol Myers Squibb (Inst), Merck Sharp & Dohme (Inst), CADEX Genomics, BCAL Diagnostics
Speakers' Bureau: Limbic Oncology (Inst)
Stephen Begbie
Consulting or Advisory Role: Astellas Pharma, MSD Oncology, Merck Serono, Pfizer, Bayer
Research Funding: Astellas Medivation (Inst), Merck Serono (Inst), Janssen Oncology (Inst), Roche (Inst), Pfizer/EMD Serono (Inst), Bristol Myers Squibb (Inst), MSD (Inst), Exelixis (Inst)
R. John Simes
Consulting or Advisory Role: FivePhusion (Inst)
Research Funding: Bayer (Inst), AbbVie (Inst), Roche (Inst), Bristol Myers Squibb (Inst), AstraZeneca (Inst), Pfizer (Inst), Amgen (Inst), MSD (Inst), BeiGene (Inst), Astellas Pharma (Inst)
Martin R. Stockler
Research Funding: Astellas Pharma (Inst), Bayer (Inst), Medivation (Inst), Pfizer (Inst), AstraZeneca (Inst), Bristol Myers Squibb (Inst), Roche (Inst), Amgen (Inst), Merck Sharp & Dohme (Inst), Tilray (Inst), BeiGene (Inst), Novartis (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Sommariva S, Pongiglione B, Tarricone R: Impact of chemotherapy-induced nausea and vomiting on health-related quality of life and resource utilization: A systematic review. Crit Rev Oncol Hematol 99:13-36, 2016 [DOI] [PubMed] [Google Scholar]
- 2.Patel P, Robinson PD, Wahib N, et al. : Interventions for the prevention of acute phase chemotherapy-induced nausea and vomiting in adult and pediatric patients: A systematic review and meta-analysis. Support Care Cancer 30:8855-8869, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aapro M, Molassiotis A, Dicato M, et al. : The effect of guideline-consistent antiemetic therapy on chemotherapy-induced nausea and vomiting (CINV): The Pan European emesis Registry (PEER). Ann Oncol 23:1986-1992, 2012 [DOI] [PubMed] [Google Scholar]
- 4.Gilmore JW, Peacock NW, Gu A, et al. : Antiemetic guideline consistency and incidence of chemotherapy-induced nausea and vomiting in US Community Oncology Practice: INSPIRE study. JCO Oncol Pract 10:68-74, 2014 [DOI] [PubMed] [Google Scholar]
- 5.Molassiotis A, Saunders MP, Valle J, et al. : A prospective observational study of chemotherapy-related nausea and vomiting in routine practice in a UK cancer centre. Support Care Cancer 16:201-208, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Bhattacharyya S, Morrison PD, Fusar-Poli P, et al. : Opposite effects of delta-9-tetrahydrocannabinol and cannabidiol on human brain function and psychopathology. Neuropsychopharmacology 35:764-774, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Navari RM, Aapro M: Antiemetic prophylaxis for chemotherapy-induced nausea and vomiting. N Engl J Med 374:1356-1367, 2016 [DOI] [PubMed] [Google Scholar]
- 8.Kramer JL: Medical marijuana for cancer. CA Cancer J Clin 65:109-122, 2015 [DOI] [PubMed] [Google Scholar]
- 9.Smith LA, Azariah F, Lavender VT, et al. : Cannabinoids for nausea and vomiting in adults with cancer receiving chemotherapy. Cochrane Database Syst Rev 2015:CD009464, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Warr D, Hesketh P: Cannabinoids as antiemetics: Everything that's old is new again. Ann Oncol 31:1425-1426, 2020 [DOI] [PubMed] [Google Scholar]
- 11.Bonaccorso S, Ricciardi A, Zangani C, et al. : Cannabidiol (CBD) use in psychiatric disorders: A systematic review. Neurotoxicology 74:282-298, 2019 [DOI] [PubMed] [Google Scholar]
- 12.Duran M, Perez E, Abanades S, et al. : Preliminary efficacy and safety of an oromucosal standardized cannabis extract in chemotherapy-induced nausea and vomiting. Br J Clin Pharmacol 70:656-663, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grimison P, Mersiades A, Kirby A, et al. : Oral THC:CBD cannabis extract for refractory chemotherapy-induced nausea and vomiting: A randomised, placebo-controlled, phase II crossover trial. Ann Oncol 31:1553-1560, 2020 [DOI] [PubMed] [Google Scholar]
- 14.Cancer Institute NSW : Prevention of anti-cancer therapy induced nausea and vomiting (AINV). https://www.eviq.org.au/clinical-resources/side-effect-and-toxicity-management/gastrointestinal/7-prevention-of-anti-cancer-therapy-induced-nausea
- 15.Roila F, Molassiotis A, Herrstedt J, et al. : 2016 MASCC and ESMO guideline update for the prevention of chemotherapy- and radiotherapy-induced nausea and vomiting and of nausea and vomiting in advanced cancer patients. Ann Oncol 27:v119-v133, 2016. (suppl 5) [DOI] [PubMed] [Google Scholar]
- 16.Mersiades AJ, Tognela A, Haber PS, et al. : Oral cannabinoid-rich THC/CBD cannabis extract for secondary prevention of chemotherapy-induced nausea and vomiting: A study protocol for a pilot and definitive randomised double-blind placebo-controlled trial (CannabisCINV). BMJ Open 8:e020745, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roila F, Ballatori E, Patoia L, et al. : Adjuvant systemic therapies in women with breast cancer: An audit of clinical practice in Italy. Ann Oncol 14:843-848, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Cai B, Elmer M, Lindley C, et al. : Assessing the impact of chemotherapy-induced nausea and vomiting on patients' daily lives: A modified version of the Functional Living Index-emesis (FLIE) with 5-day recall. Support Care Cancer 11:522-527, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Richardson J, Iezzi A, Khan MA, et al. : Validity and reliability of the Assessment of Quality of Life (AQoL)-8D multi-attribute utility instrument. Patient 7:85-96, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Health NIo : National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) V4. 03. National Institutes of Health, Bethesda, MD, Cancer Therapy Evalution Program, 2010 [Google Scholar]
- 21.Sallan SE, Zinberg NE, Frei E III: Antiemetic effect of delta-9-tetrahydrocannabinol in patients receiving cancer chemotherapy. N Engl J Med 293:795-797, 1975 [DOI] [PubMed] [Google Scholar]
- 22.Frytak S, Moertel CG, O'Fallon JR, et al. : Delta-9-tetrahydrocannabinol as an antiemetic for patients receiving cancer chemotherapy. A comparison with prochlorperazine and a placebo. Ann Intern Med 91:825-830, 1979 [DOI] [PubMed] [Google Scholar]
- 23.Wada JK, Bogdon DL, Gunnell JC, et al. : Double-blind, randomized, crossover trial of nabilone vs. placebo in cancer chemotherapy. Cancer Treat Rev 9:39-44, 1982. (suppl B) [DOI] [PubMed] [Google Scholar]
- 24.Rapoport BL, Jordan K, Boice JA, et al. : Aprepitant for the prevention of chemotherapy-induced nausea and vomiting associated with a broad range of moderately emetogenic chemotherapies and tumor types: A randomized, double-blind study. Support Care Cancer 18:423-431, 2010 [DOI] [PubMed] [Google Scholar]
- 25.Navari RM, Qin R, Ruddy KJ, et al. : Olanzapine for the prevention of chemotherapy-induced nausea and vomiting. N Engl J Med 375:134-142, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hashimoto H, Abe M, Tokuyama O, et al. : Olanzapine 5 mg plus standard antiemetic therapy for the prevention of chemotherapy-induced nausea and vomiting (J-FORCE): A multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 21:242-249, 2020 [DOI] [PubMed] [Google Scholar]
- 27.Hesketh PJ, Kris MG, Basch E, et al. : Antiemetics: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol 35:3240-3261, 2017 [DOI] [PubMed] [Google Scholar]
- 28.Chow R, Herrstedt J, Aapro M, et al. : Olanzapine for the prophylaxis and rescue of chemotherapy-induced nausea and vomiting: A systematic review, meta-analysis, cumulative meta-analysis and fragility assessment of the literature. Support Care Cancer 29:3439-3459, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arkell TR, McCartney D, McGregor IS: Medical cannabis and driving. Aust J Gen Pract 50:357-362, 2021 [DOI] [PubMed] [Google Scholar]
- 30.McCartney D, Arkell TR, Irwin C, et al. : Determining the magnitude and duration of acute Δ9-tetrahydrocannabinol (Δ9-THC)-induced driving and cognitive impairment: A systematic and meta-analytic review. Neurosci Biobehavioral Rev 126:175-193, 2021 [DOI] [PubMed] [Google Scholar]
- 31.Rudisill TM, Innes KK, Wen S, et al. : The effects of cannabidiol on the driving performance of healthy adults: A pilot RCT. AJPM Focus 2:100053, 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]


