Abstract
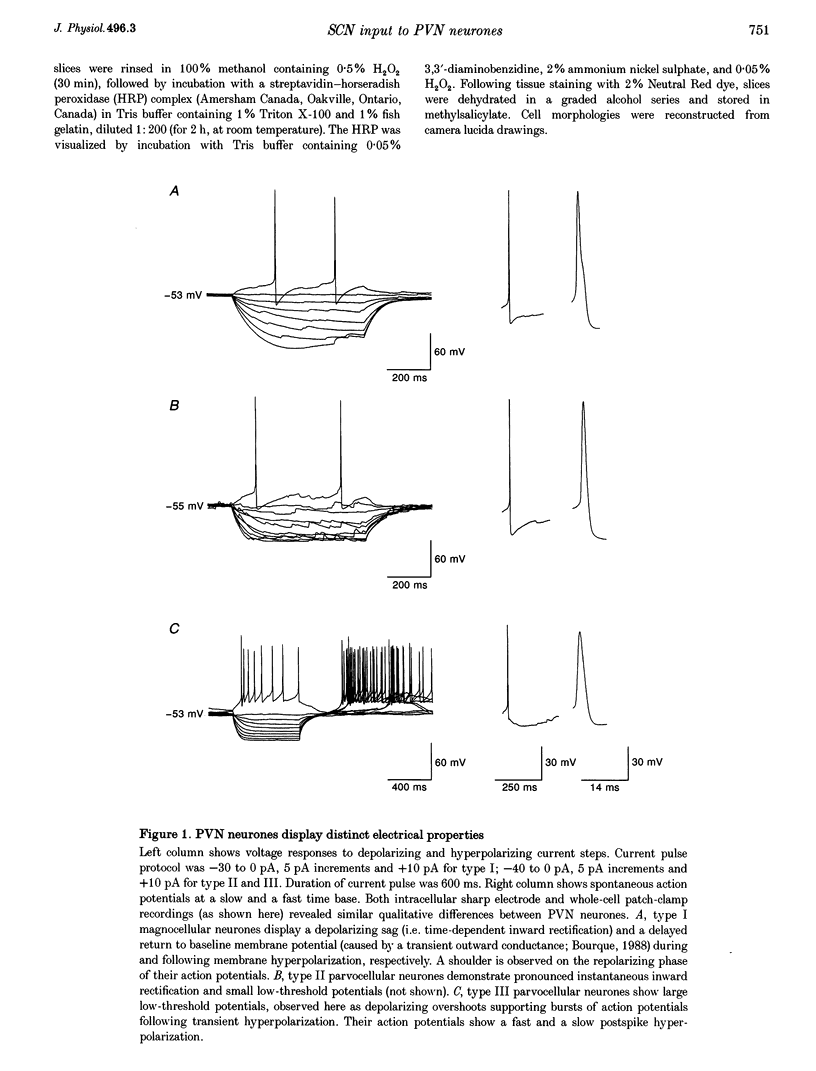
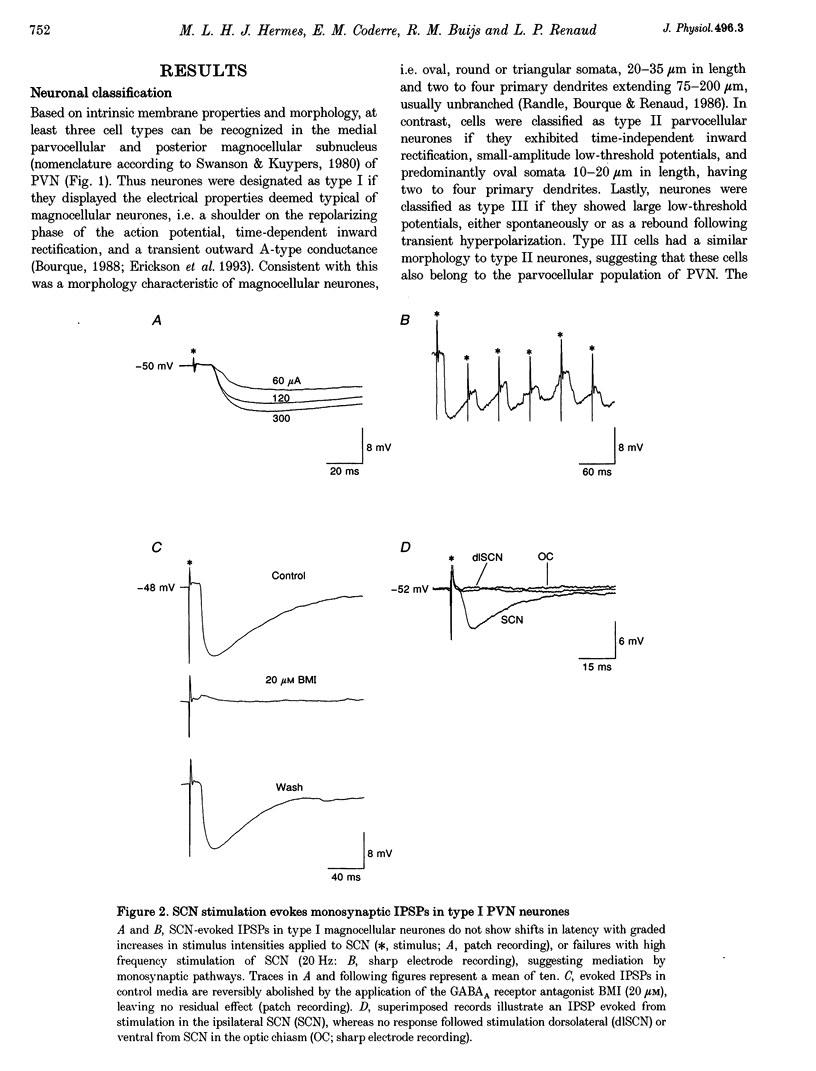
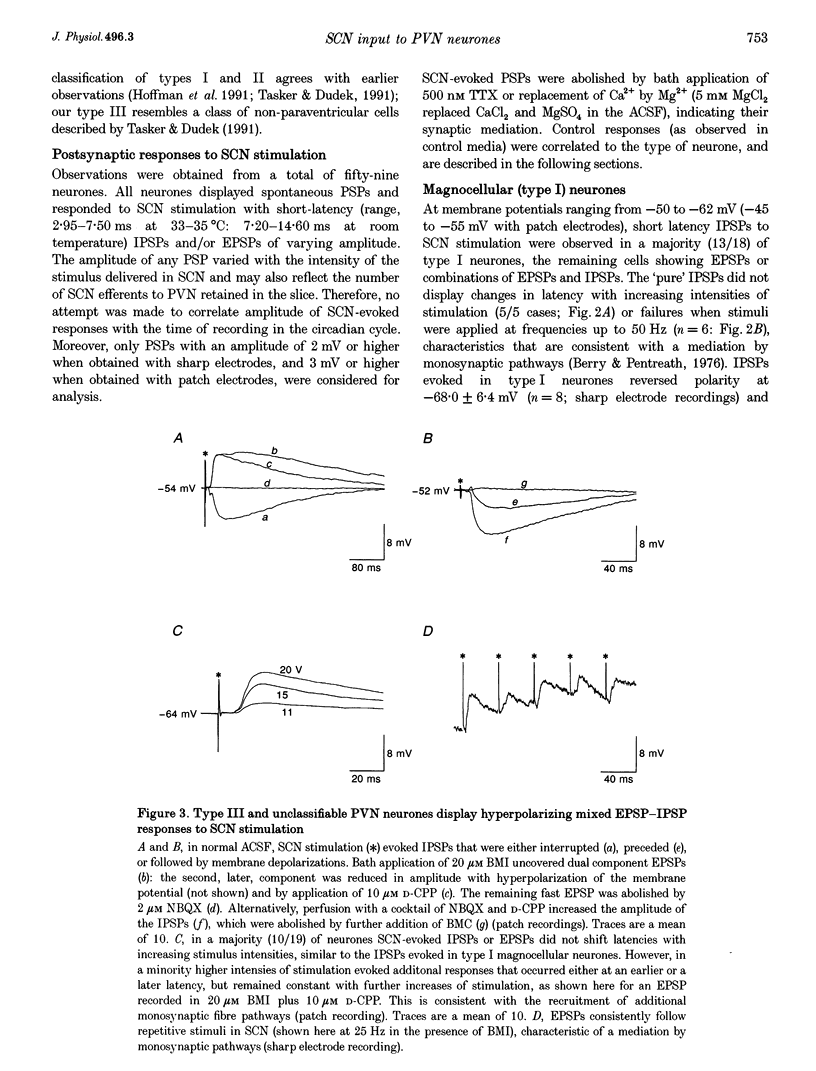
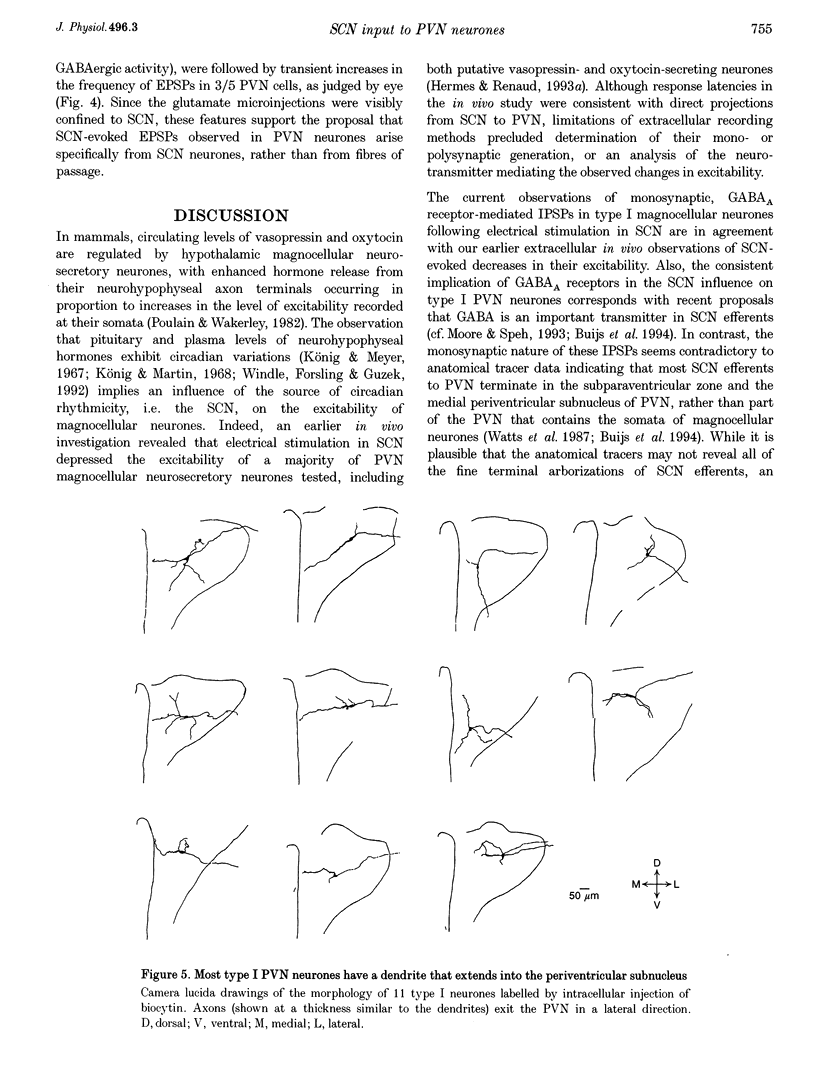
1. Intracellular sharp electrode and whole-cell patch-clamp recording from characterized paraventricular nucleus (PVN) neurones in rat hypothalamic slices were used to study the synaptic mechanism and associated neurotransmitters that mediate their response to suprachiasmatic nucleus (SCN) stimulation. 2. Electrical stimulation restricted to SCN evoked short-latency inhibitory postsynaptic potentials (IPSPs) or combinations of IPSPs and excitatory postsynaptic potentials (EPSPs) in all (n = 59) PVN neurones tested. Type I neurones (n = 18) were magnocellular and a majority (13/18) demonstrated monosynaptic IPSPs that reversed polarity at the chloride equilibrium potential and were sensitive to bicuculline. 3. Type II (n = 10) and III parvocellular (n = 13), and unclassifiable neurones (n = 18) displayed combinations of IPSPs and EPSPs following similar stimuli applied to SCN. IPSP blockade with bicuculline uncovered SCN-evoked monosynaptic dual-component EPSPs that were sensitive to N-methyl-D-aspartate (NMDA) and non-NMDA receptor antagonists. In addition, chemical microstimulation within SCN was associated with transient increases in spontaneous EPSPs recorded from these PVN neurones. 4. These data imply that the amino acids GABA and glutamate are important mediators of fast monosynaptic transmission from SCN to defined neurones in PVN, and are candidates for conveying circadian rhythmicity to PVN regulation of neuroendocrine and autonomic processes.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albers H. E., Stopa E. G., Zoeller R. T., Kauer J. S., King J. C., Fink J. S., Mobtaker H., Wolfe H. Day-night variation in prepro vasoactive intestinal peptide/peptide histidine isoleucine mRNA within the rat suprachiasmatic nucleus. Brain Res Mol Brain Res. 1990 Jan;7(1):85–89. doi: 10.1016/0169-328x(90)90077-q. [DOI] [PubMed] [Google Scholar]
- Berry M. S., Pentreath V. W. Criteria for distinguishing between monosynaptic and polysynaptic transmission. Brain Res. 1976 Mar 19;105(1):1–20. doi: 10.1016/0006-8993(76)90919-7. [DOI] [PubMed] [Google Scholar]
- Blanton M. G., Lo Turco J. J., Kriegstein A. R. Whole cell recording from neurons in slices of reptilian and mammalian cerebral cortex. J Neurosci Methods. 1989 Dec;30(3):203–210. doi: 10.1016/0165-0270(89)90131-3. [DOI] [PubMed] [Google Scholar]
- Bourque C. W. Transient calcium-dependent potassium current in magnocellular neurosecretory cells of the rat supraoptic nucleus. J Physiol. 1988 Mar;397:331–347. doi: 10.1113/jphysiol.1988.sp017004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buijs R. M., Hou Y. X., Shinn S., Renaud L. P. Ultrastructural evidence for intra- and extranuclear projections of GABAergic neurons of the suprachiasmatic nucleus. J Comp Neurol. 1994 Feb 15;340(3):381–391. doi: 10.1002/cne.903400308. [DOI] [PubMed] [Google Scholar]
- Card J. P., Brecha N., Karten H. J., Moore R. Y. Immunocytochemical localization of vasoactive intestinal polypeptide-containing cells and processes in the suprachiasmatic nucleus of the rat: light and electron microscopic analysis. J Neurosci. 1981 Nov;1(11):1289–1303. doi: 10.1523/JNEUROSCI.01-11-01289.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earnest D. J., Sladek C. D. Circadian rhythms of vasopressin release from individual rat suprachiasmatic explants in vitro. Brain Res. 1986 Sep 10;382(1):129–133. doi: 10.1016/0006-8993(86)90119-8. [DOI] [PubMed] [Google Scholar]
- Erickson K. R., Ronnekleiv O. K., Kelly M. J. Electrophysiology of guinea-pig supraoptic neurones: role of a hyperpolarization-activated cation current in phasic firing. J Physiol. 1993 Jan;460:407–425. doi: 10.1113/jphysiol.1993.sp019478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francois-Bellan A. M., Kachidian P., Dusticier G., Tonon M. C., Vaudry H., Bosler O. GABA neurons in the rat suprachiasmatic nucleus: involvement in chemospecific synaptic circuitry and evidence for GAD-peptide colocalization. J Neurocytol. 1990 Dec;19(6):937–947. doi: 10.1007/BF01186821. [DOI] [PubMed] [Google Scholar]
- Groos G., Hendriks J. Circadian rhythms in electrical discharge of rat suprachiasmatic neurones recorded in vitro. Neurosci Lett. 1982 Dec 31;34(3):283–288. doi: 10.1016/0304-3940(82)90189-6. [DOI] [PubMed] [Google Scholar]
- Hermes M. L., Renaud L. P. Differential responses of identified rat hypothalamic paraventricular neurons to suprachiasmatic nucleus stimulation. Neuroscience. 1993 Oct;56(4):823–832. doi: 10.1016/0306-4522(93)90130-8. [DOI] [PubMed] [Google Scholar]
- Hoffman N. W., Tasker J. G., Dudek F. E. Immunohistochemical differentiation of electrophysiologically defined neuronal populations in the region of the rat hypothalamic paraventricular nucleus. J Comp Neurol. 1991 May 15;307(3):405–416. doi: 10.1002/cne.903070306. [DOI] [PubMed] [Google Scholar]
- Inouye S. T., Kawamura H. Persistence of circadian rhythmicity in a mammalian hypothalamic "island" containing the suprachiasmatic nucleus. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5962–5966. doi: 10.1073/pnas.76.11.5962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalsbeek A., Buijs R. M., van Heerikhuize J. J., Arts M., van der Woude T. P. Vasopressin-containing neurons of the suprachiasmatic nuclei inhibit corticosterone release. Brain Res. 1992 May 15;580(1-2):62–67. doi: 10.1016/0006-8993(92)90927-2. [DOI] [PubMed] [Google Scholar]
- König A., Martin B. Tagesperiodische Schwankungen des neurohypophysären Oxytocingehaltes bei männlichen Wistar-Ratten. Acta Endocrinol (Copenh) 1968 May;58(1):98–100. [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L., Guthrie P. B. Voltage-dependent block by Mg2+ of NMDA responses in spinal cord neurones. Nature. 1984 May 17;309(5965):261–263. doi: 10.1038/309261a0. [DOI] [PubMed] [Google Scholar]
- Moore R. Y., Eichler V. B. Loss of a circadian adrenal corticosterone rhythm following suprachiasmatic lesions in the rat. Brain Res. 1972 Jul 13;42(1):201–206. doi: 10.1016/0006-8993(72)90054-6. [DOI] [PubMed] [Google Scholar]
- Moore R. Y., Speh J. C. GABA is the principal neurotransmitter of the circadian system. Neurosci Lett. 1993 Feb 5;150(1):112–116. doi: 10.1016/0304-3940(93)90120-a. [DOI] [PubMed] [Google Scholar]
- Nowak L., Bregestovski P., Ascher P., Herbet A., Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984 Feb 2;307(5950):462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- O'Connor J. J., Rowan M. J., Anwyl R. Tetanically induced LTP involves a similar increase in the AMPA and NMDA receptor components of the excitatory postsynaptic current: investigations of the involvement of mGlu receptors. J Neurosci. 1995 Mar;15(3 Pt 1):2013–2020. doi: 10.1523/JNEUROSCI.15-03-02013.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamura H., Bérod A., Julien J. F., Geffard M., Kitahama K., Mallet J., Bobillier P. Demonstration of GABAergic cell bodies in the suprachiasmatic nucleus: in situ hybridization of glutamic acid decarboxylase (GAD) mRNA and immunocytochemistry of GAD and GABA. Neurosci Lett. 1989 Jul 31;102(2-3):131–136. doi: 10.1016/0304-3940(89)90067-0. [DOI] [PubMed] [Google Scholar]
- Poulain D. A., Wakerley J. B. Electrophysiology of hypothalamic magnocellular neurones secreting oxytocin and vasopressin. Neuroscience. 1982 Apr;7(4):773–808. doi: 10.1016/0306-4522(82)90044-6. [DOI] [PubMed] [Google Scholar]
- Ralph M. R., Foster R. G., Davis F. C., Menaker M. Transplanted suprachiasmatic nucleus determines circadian period. Science. 1990 Feb 23;247(4945):975–978. doi: 10.1126/science.2305266. [DOI] [PubMed] [Google Scholar]
- Randle J. C., Bourque C. W., Renaud L. P. Serial reconstruction of Lucifer yellow-labeled supraoptic nucleus neurons in perfused rat hypothalamic explants. Neuroscience. 1986 Feb;17(2):453–467. doi: 10.1016/0306-4522(86)90259-9. [DOI] [PubMed] [Google Scholar]
- Schwartz W. J., Gross R. A., Morton M. T. The suprachiasmatic nuclei contain a tetrodotoxin-resistant circadian pacemaker. Proc Natl Acad Sci U S A. 1987 Mar;84(6):1694–1698. doi: 10.1073/pnas.84.6.1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata S., Liou S., Ueki S., Oomura Y. Influence of environmental light-dark cycle and enucleation on activity of suprachiasmatic neurons in slice preparations. Brain Res. 1984 Jun 4;302(1):75–81. doi: 10.1016/0006-8993(84)91286-1. [DOI] [PubMed] [Google Scholar]
- Stephan F. K., Zucker I. Circadian rhythms in drinking behavior and locomotor activity of rats are eliminated by hypothalamic lesions. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1583–1586. doi: 10.1073/pnas.69.6.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson L. W., Kuypers H. G. The paraventricular nucleus of the hypothalamus: cytoarchitectonic subdivisions and organization of projections to the pituitary, dorsal vagal complex, and spinal cord as demonstrated by retrograde fluorescence double-labeling methods. J Comp Neurol. 1980 Dec 1;194(3):555–570. doi: 10.1002/cne.901940306. [DOI] [PubMed] [Google Scholar]
- Swanson L. W., Sawchenko P. E. Hypothalamic integration: organization of the paraventricular and supraoptic nuclei. Annu Rev Neurosci. 1983;6:269–324. doi: 10.1146/annurev.ne.06.030183.001413. [DOI] [PubMed] [Google Scholar]
- Tasker J. G., Dudek F. E. Electrophysiological properties of neurones in the region of the paraventricular nucleus in slices of rat hypothalamus. J Physiol. 1991 Mar;434:271–293. doi: 10.1113/jphysiol.1991.sp018469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts A. G., Swanson L. W., Sanchez-Watts G. Efferent projections of the suprachiasmatic nucleus: I. Studies using anterograde transport of Phaseolus vulgaris leucoagglutinin in the rat. J Comp Neurol. 1987 Apr 8;258(2):204–229. doi: 10.1002/cne.902580204. [DOI] [PubMed] [Google Scholar]
- Welsh D. K., Logothetis D. E., Meister M., Reppert S. M. Individual neurons dissociated from rat suprachiasmatic nucleus express independently phased circadian firing rhythms. Neuron. 1995 Apr;14(4):697–706. doi: 10.1016/0896-6273(95)90214-7. [DOI] [PubMed] [Google Scholar]
- Windle R. J., Forsling M. L., Guzek J. W. Daily rhythms in the hormone content of the neurohypophysial system and release of oxytocin and vasopressin in the male rat: effect of constant light. J Endocrinol. 1992 May;133(2):283–290. doi: 10.1677/joe.0.1330283. [DOI] [PubMed] [Google Scholar]


