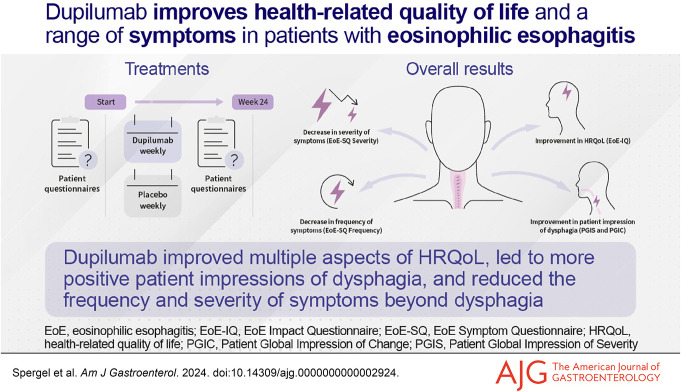
Abstract
INTRODUCTION:
Improvements in symptomatic experience and health-related quality of life (HRQoL) are among the most important treatment benefits in patients with eosinophilic esophagitis (EoE). We assessed the impact of dupilumab treatment on HRQoL, patients' impression of dysphagia, and symptoms beyond dysphagia in adults/adolescents (≥12 years) with EoE in parts A and B of the LIBERTY EoE TREET (NCT03633617) study.
METHODS:
The EoE Symptom Questionnaire (EoE-SQ; frequency and severity of nondysphagia symptoms), EoE Impact Questionnaire (impact of EoE on HRQoL), and Patient Global Impression of Severity and Patient Global Impression of Change of dysphagia were used to assess the efficacy of weekly dupilumab 300 mg vs placebo.
RESULTS:
At week 24, dupilumab reduced EoE-SQ Frequency (least squares mean difference vs placebo [95% confidence interval] part A −1.7 [–2.9, −0.5], part B −1.4 [–2.3, −0.5]; both P < 0.01) and EoE-SQ Severity (part A −2.0 [–3.9, 0.0], P < 0.05, part B −1.5 [–3.0, 0.1], P = 0.07) overall scores, and improved scores across all individual items. Improvement in the dupilumab group was clinically meaningful to patients. Dupilumab also meaningfully improved EoE Impact Questionnaire average scores and improved individual item scores at week 24, particularly emotional and sleep disturbance. More dupilumab-treated patients reported improvement in the Patient Global Impression of Change of dysphagia vs placebo or reported having no symptoms per the Patient Global Impression of Severity of dysphagia at week 24.
DISCUSSION:
Dupilumab reduced the impact of EoE on multiple aspects of HRQoL, patients' impression of dysphagia, and frequency and severity of symptoms beyond dysphagia in adults/adolescents with EoE.
KEYWORDS: dupilumab, eosinophilic esophagitis, quality of life
INTRODUCTION
Eosinophilic esophagitis (EoE) is a chronic, type 2 inflammatory disease of the esophagus with increasing incidence and prevalence (1,2). The dominant symptom in adults and adolescents with EoE is dysphagia, with other common symptoms including vomiting and abdominal pain (3). EoE has a substantial impact on health-related quality of life (HRQoL) (4–6), and patients with EoE consider improving HRQoL as one of the most important outcomes of therapy (7). Notably, EoE symptom severity is strongly correlated with a negative impact on HRQoL (8–10).
Patient-reported outcomes (PRO) are important to complement primary end points in phase 3 studies of EoE, for example, through assessing both histologic outcomes and a measure of dysphagia, such as the Dysphagia Symptom Questionnaire (DSQ) (11,12). However, PRO for EoE should also consider the measurement of disease impact on daily patient well-being, as well as the assessment of heterogeneous EoE-specific symptoms beyond dysphagia. To date, many studies of patients with EoE have measured HRQoL using instruments validated in other diseases, such as the 36-Item Short Form, which poorly correlates with EoE disease activity or improvement in symptoms (13,14).
We developed the EoE Impact Questionnaire (EoE-IQ), which assesses disease-specific impacts most relevant to patients with EoE, and the EoE Symptom Questionnaire (EoE-SQ), which measures the frequency and severity of important nondysphagia symptoms. These measures were used in the 3-part, double-blind, placebo-controlled, phase 3 LIBERTY EoE TREET study (NCT03633617) (11,15) that tested dupilumab, a fully human monoclonal antibody blocking interleukin-4 and interleukin-13 pathways, key drivers of type 2 inflammation (16–18). Dupilumab 300 mg once weekly (qw) demonstrated clinically meaningful and statistically significant improvements in DSQ score at week 24, sustained through week 52 (11). Based on the significant improvements in symptoms, histology, and other outcomes in this study, dupilumab was approved in the United States, Canada, the European Union, and the United Arab Emirates for treatment of patients with EoE aged 12 years or older and weighing ≥40 kg (19–21). This analysis further assessed the impact of dupilumab treatment on HRQoL, patients' impression of dysphagia, and symptoms beyond dysphagia in adults/adolescents with EoE.
METHODS
Study design and patients
This is an analysis of secondary and exploratory end points collected during parts A and B of the LIBERTY EoE TREET study. The study design has been previously described (11). In brief, patients aged 12 years or older with a diagnosis of EoE (peak eosinophil count, ≥15 eosinophils per high-power field), despite 8 weeks of high-dose proton-pump inhibitors (PPIs) and a DSQ biweekly total score of ≥10 were randomized 1:1 to receive subcutaneous placebo or dupilumab 300 mg qw (part A), or 1:1:1 to subcutaneous placebo, dupilumab 300 mg qw, or dupilumab 300 mg every 2 weeks (part B) for 24 weeks. Inclusion and exclusion criteria were identical in parts A and B. The current analysis focuses on the approved 300 mg qw dosage. Initiation or stopping of PPI treatment was prohibited, but maintenance of existing treatment was permitted. Background therapy with swallowed topical corticosteroids (STC) was prohibited during the study or within 8 weeks of the study baseline (study drug randomization), although STC were permitted as rescue medications.
Outcomes and assessments
EoE-SQ.
The EoE-SQ assesses 5 symptoms (chest pain, stomach pain, heartburn, regurgitation, throwing up) during the past 7 days. Response options for symptom frequency questions are on a 5-point scale (1 = “Never”, 2 = “1 day”, 3 = “2–6 days”, 4 = “Once a day”, 5 = “More than once a day”). The EoE-SQ Frequency score is calculated as the sum of the frequency scores from the 5 items (range: 5–25); higher scores indicate higher frequency. Responses for questions on the severity of each symptom based on the patients' worst experience in the past 7 days are on a scale of 0–10 (higher is worse). The EoE-SQ Severity score is calculated as the sum of the severity scores from questions 1 to 3 (chest pain, stomach pain, heartburn [range: 0–30], patient feedback indicated difficulty assessing severity of regurgitation, and throwing up); higher scores indicates more severe symptoms. The change from baseline in total EoE-SQ Frequency and Severity scores was calculated at weeks 12 and 24. The change from baseline in individual item scores was calculated at week 24. The EoE-SQ has been validated with data from the LIBERTY EoE TREET study, with a ≥3.7-point reduction in total Frequency score and a ≥5.3-point reduction in total Severity score considered clinically meaningful. Development of the EoE-SQ is described in the Supplementary Methods (Supplementary Digital Content 1, http://links.lww.com/AJG/D323).
Further subgroup analyses of the EoE-SQ Frequency and Severity scores were completed for patients symptomatic at baseline (baseline Frequency score ≥2; Severity score ≥1 for each individual item). The change from baseline in individual item scores for this patient population was calculated at week 24.
EoE-IQ.
The EoE-IQ comprises 11 questions, assessing the impact of EoE on emotional, social, work and school, and sleep aspects during the past 7 days. Item responses are measured on a 5-point scale (1 = “Not at all”, 2 = “A little”, 3 = “Somewhat”, 4 = “Quite a bit”, 5 = “Extremely”). The EoE-IQ average score (range: 1–5) is the sum of the nonmissing responses divided by the number of items with nonmissing response; higher scores indicate a more negative impact on QoL. The EoE-IQ has been validated with data from the LIBERTY EoE TREET study, with a ≥0.6-point reduction in average score considered clinically meaningful (22). Development of the EoE-IQ is described in the Supplementary Methods (Supplementary Digital Content 1, http://links.lww.com/AJG/D323).
The change from baseline in EoE-IQ average score was calculated at weeks 12 and 24 for all patients in parts A and B. Change from baseline for individual item scores was calculated at week 24. Subgroup analyses of patients symptomatic at baseline (baseline score ≥2 for average score or each individual item score) were performed. For these subgroups, the change from baseline in average score and individual item scores was calculated at week 24.
Patient Global Impression of Change of dysphagia.
The Patient Global Impression of Change (PGIC) is a 1-item questionnaire that assesses change in the difficulty of swallowing food compared with just before starting the study medication. Assessment is on a 7-point scale (0 = “Very much better”, 1 = “Moderately better”, 2 = “A little better”, 3 = “No change”, 4 = “A little worse”, 5 = “Moderately worse”, 6 = “Very much worse”).
The proportion of patients with responses indicating improvement (i.e., a score of 0, 1, or 2) on the PGIC of dysphagia was calculated at weeks 12, 20, and 24 for parts A and B.
Patient Global Impression of Severity of dysphagia.
The Patient Global Impression of Severity (PGIS) is a 1-item questionnaire that assesses the severity of difficulty swallowing food for the previous 7 days. Assessment is on a 4-point scale (1 = “None”, 2 = “Mild”, 3 = “Moderate”, 4 = “Severe”). The proportion of patients who responded “None”, “Mild”, “Moderate”, or “Severe” to the PGIS of dysphagia was calculated at baseline, and weeks 12, 20, and 24 for parts A and B.
PRO validation.
Psychometric analysis was performed to evaluate the degree to which the scores of a PRO are associated with other measures known to assess the same construct (convergent validity) and the degree to which the scores of a PRO are less or not associated with measures not designed to measure the same construct (divergent validity). Pearson correlation coefficients were calculated between the change scores (from baseline to week 24) for the EoE-IQ, EoE-SQ, PGIS, and PGIC and other measures to assess convergent and divergent validity. Correlations of <0.3 were considered to be weak or small, ≥0.3 to <0.7 to be moderate, ≥0.7 to <0.9 to be strong, and ≥0.9 to be very strong (23,24).
Statistical analyses
Efficacy analyses were performed using the full analysis set, which included all randomized patients, according to the treatment allocated. For binary variables, P values were derived using the Cochran-Mantel-Haenszel test stratified by age group (≥12 to <18 vs ≥18 years) and the use of PPI at randomization (yes vs no). For continuous variables, P values were based on least squares (LS) mean changes using an analysis of covariance model with baseline measurement as covariate and the treatment, age group (≥12 to <18 vs ≥18 years), and PPI use at randomization (yes vs no) strata as fixed factors. All calculated P-values are nominal.
For all end points, values after first rescue treatment used were set to missing (censored). For the EoE-IQ and EoE-SQ, missing values were imputed by multiple imputations. For the proportion of EoE-SQ responders reporting “Never” having experienced symptoms during the past 7 days, patients with missing scores at week 24 were considered as being in the worst possible category (i.e., not included in the “Never” category). For the PGIC and PGIS of dysphagia, patients with missing scores at each visit or after rescue treatment were considered nonresponders in the analysis.
RESULTS
Patients
In part A, 42 received dupilumab 300 mg qw and 39 placebo. In part B, 80 patients received dupilumab 300 mg qw, 81 dupilumab 300 mg every 2 weeks, and 79 placebo. The demographic and clinical characteristics of the patients at baseline were similar across all trial groups, as detailed previously (11). EoE-SQ Frequency and Severity total scores and individual item scores, along with EoE-IQ average and individual item scores, were similar between the dupilumab 300 mg qw and placebo groups at baseline (see Supplementary Figure 1, Supplementary Tables 1, 2, and 3, Supplementary Digital Content 1, http://links.lww.com/AJG/D323).
Nondysphagia EoE symptoms (EoE-SQ)
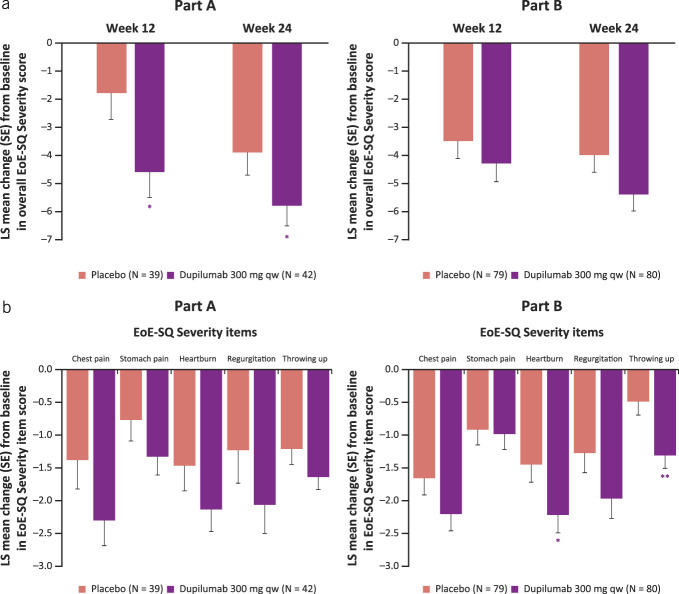
Dupilumab 300 mg qw reduced the frequency of symptoms beyond dysphagia, as measured by EoE-SQ Frequency score vs placebo at week 24 (LS mean difference [95% confidence interval (CI)] dupilumab vs placebo part A −1.7 [–2.9, −0.5], part B −1.4 [–2.3, −0.5], P < 0.01 for both parts; Figure 1a). Reductions were seen as early as week 12 (LS mean difference [95% CI] dupilumab vs placebo part A −1.7 [–2.8, −0.6], P < 0.01, part B −0.9 [–1.9, 0.0], P < 0.05; Figure 1a). The absolute change from baseline to week 24 in EoE-SQ Frequency score exceeded the minimal clinically important difference (MCID) of 3.7 points in part B and therefore represents a clinically meaningful improvement in patients treated with dupilumab 300 mg qw (LS mean change [SE] part A −3.4 [0.45], part B −3.9 [0.35], respectively). Dupilumab improved (decreased) all Frequency item scores (chest pain, stomach pain, heartburn, regurgitation, throwing up) of the EoE-SQ vs placebo (Figure 1b). This was the same when only symptomatic patients (EoE-SQ Frequency baseline score ≥2) were analyzed (see Supplementary Figure 2a, Supplementary Digital Content 1, http://links.lww.com/AJG/D323). In addition, dupilumab increased the proportion of all patients who reported “Never” having had symptoms during the past 7 days, at week 24 (Figure 1c). Similar results were seen with symptomatic patients, except with stomach pain in part B (see Supplementary Figure 2b, Supplementary Digital Content 1, http://links.lww.com/AJG/D323).
Figure 1.
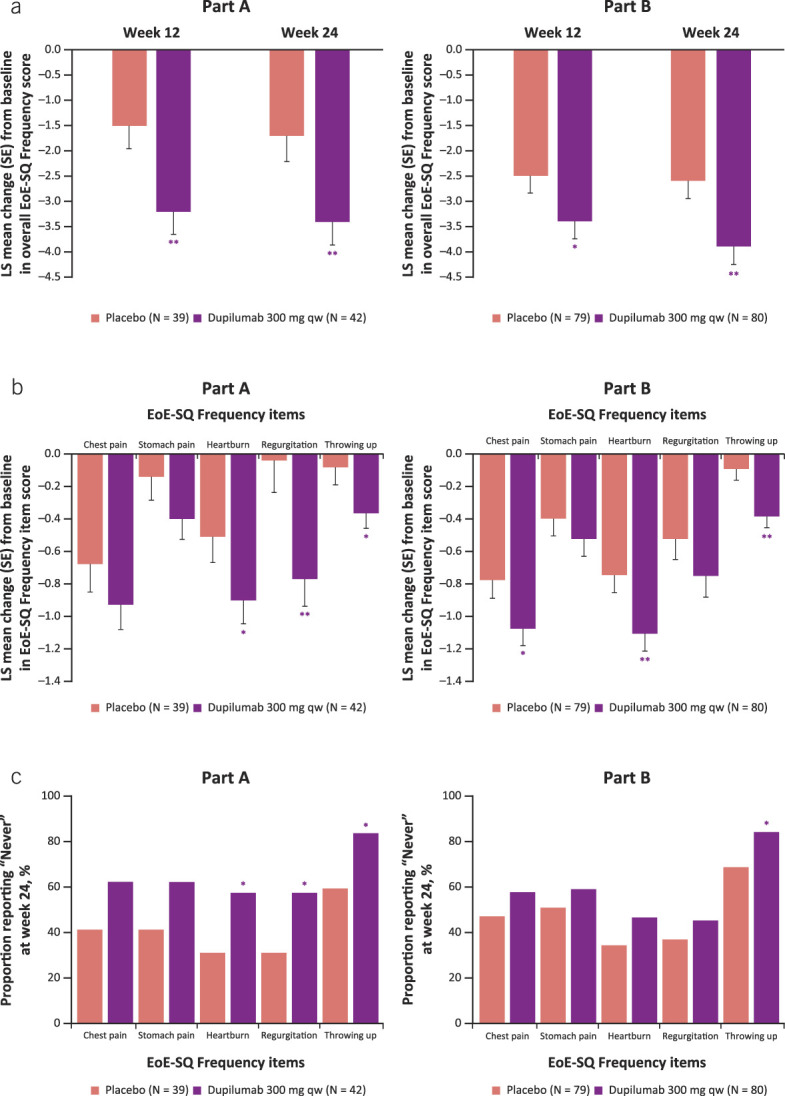
Change from baseline in overall EoE-SQ Frequency score at weeks 12 and 24 (a), individual frequency items of the EoE-SQ at week 24 (b), and proportion of patients reporting never having symptoms assessed by the EoE-SQ at week 24 (c). Note: For proportion of patients reporting never having symptoms, values after first rescue treatment used were set to missing (censoring). Patients with missing score at week 24 are considered as being in the worst possible category (i.e., not included in the “Never” category). *Nominal P ≤ 0.05, **nominal P ≤ 0.01 dupilumab vs placebo. EoE-SQ, eosinophilic esophagitis Symptom Questionnaire; LS, least squares; qw, once weekly.
Dupilumab reduced the severity of symptoms beyond dysphagia, as measured by the EoE-SQ Severity score vs placebo at week 24 (LS mean difference [95% CI] −2.0 [–3.9, −0.0], P < 0.05, part B −1.5 [–3.0, 0.1], P = 0.07; Figure 2a) and as early as week 12 (LS mean difference [95% CI] part A −2.8 [–5.1, −0.4], P < 0.05, part B −0.8 [–2.5, 0.8], P = 0.33). The absolute change from baseline to week 24 in EoE-SQ Severity score exceeded the MCID of 5.3 points and therefore represents a clinically meaningful improvement in patients treated with dupilumab 300 mg qw in parts A and B (LS mean change [SE] −5.8 [0.71], −5.4 [0.59], respectively). At week 24, dupilumab reduced the severity of each individual symptom vs placebo, in both analyses with all patients and with only symptomatic patients (EoE-SQ Severity score ≥1 at baseline) (see Figure 2b, Supplementary Figure 3a, Supplementary Digital Content 1, http://links.lww.com/AJG/D323).
Figure 2.
Change from baseline in overall EoE-SQ Severity score at weeks 12 and 24 (a) and individual severity items of the EoE-SQ at week 24 (b). *Nominal P ≤ 0.05, **nominal P ≤ 0.01 dupilumab vs placebo. EoE-SQ, eosinophilic esophagitis Symptom Questionnaire; LS, least squares; qw, once weekly.
Impact of EoE (EoE-IQ)
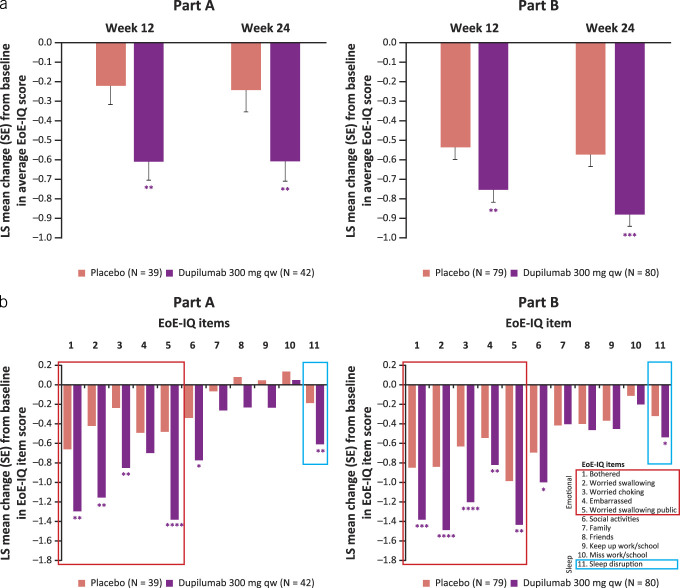
The EoE-IQ scores of patients treated with dupilumab 300 mg qw improved (decreased) vs placebo at week 24 (LS mean difference [95% CI] dupilumab vs placebo part A −0.37 [–0.64, −0.10], P < 0.01, part B −0.31 [–0.47, −0.15], P < 0.001; Figure 3a). A clinically meaningful improvement, exceeding the MCID of 0.6 points, in EoE-IQ score was observed in patients treated with dupilumab 300 mg qw at week 24 in parts A and B (LS mean change [SE] −0.61 [0.10], −0.89 [0.06], respectively). Improvements were seen as early as week 12 (LS mean difference [95% CI] part A −0.39 [–0.63, −0.15], Part B −0.22 [–0.38, −0.06], P < 0.01 for both parts; Figure 3a). Improvements to individual items of the EoE-IQ were particularly seen in items relating to emotional status, with patients feeling significantly less bothered, worried about swallowing, worried about choking, and worried about swallowing in public (Figure 3b). Patients who received dupilumab also had significantly less sleep disruption than those who received placebo.
Figure 3.
Change from baseline in EoE-IQ average score at weeks 12 and 24 (a), and individual item scores at week 24 (b). *Nominal P ≤ 0.05, **nominal P ≤ 0.01, ***nominal P ≤ 0.001, ****nominal P ≤ 0.0001 dupilumab vs placebo. EoE-IQ, eosinophilic esophagitis Impact Questionnaire; LS, least squares; qw, once weekly.
In addition, the EoE-IQ scores of symptomatic patients (baseline score ≥ 2) treated with dupilumab improved at week 24 (LS mean difference [95% CI] part A −0.64 [–0.97, −0.30], P ≤ 0.001, part B −0.35 [–0.57, −0.12], P ≤ 0.01; see Supplementary Figure 4a, Supplementary Digital Content 1, http://links.lww.com/AJG/D323). Similarly, individual improvements were mostly present in emotional status items in both study parts (see Supplementary Figure 4b, Supplementary Digital Content 1, http://links.lww.com/AJG/D323).
PGIC of dysphagia
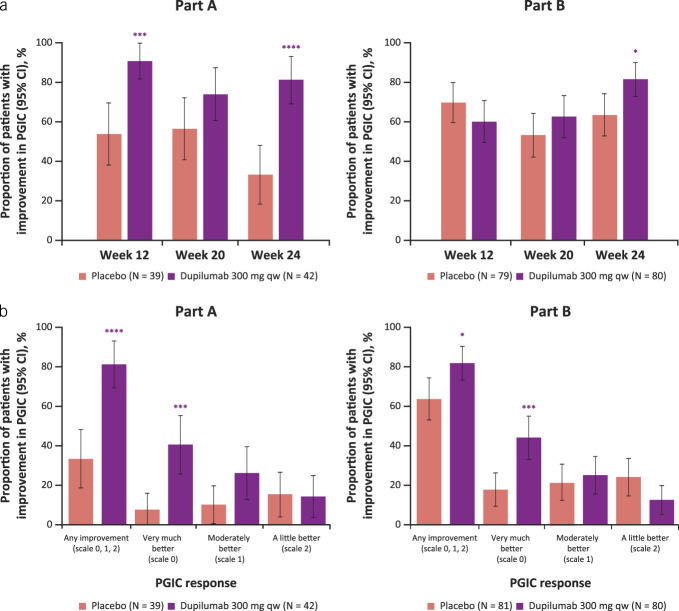
Dupilumab 300 mg qw increased the proportion of patients with any level of improvement per the PGIC of dysphagia vs placebo at week 24 (81% vs 33%, P < 0.0001, and 81% vs 63%, P < 0.05, in parts A and B, respectively; Figure 4a). Furthermore, the proportion of patients who reported feeling “Very much better” (the highest level of improvement) was significantly higher in the dupilumab group vs placebo at week 24 (41% vs 8%, P < 0.001, and 44% vs 18%, P < 0.001, in parts A and B, respectively; Figure 4b).
Figure 4.
Proportion of patients reporting any improvement on the PGIC of dysphagia (i.e., score of 0, 1, or 2) at weeks 12, 20, and 24 (a) and reporting each level of improvement at week 24 (b). *Nominal P ≤ 0.05, ***nominal P ≤ 0.001, ****nominal P ≤ 0.0001 dupilumab vs placebo. CI, confidence interval; PGIC, Patient Global Impression of Change; qw, once weekly.
PGIS of dysphagia
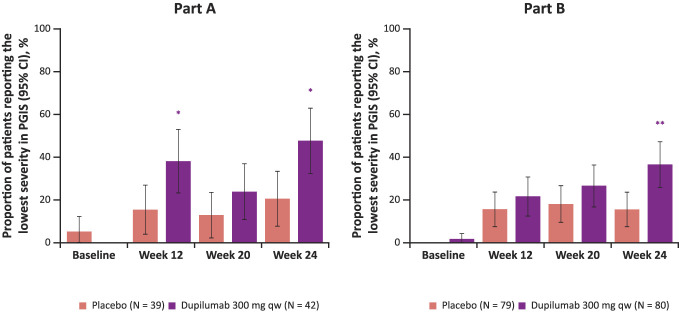
At baseline, similar proportions of patients reported no symptoms (i.e., “None”) in response to the PGIS of dysphagia in the dupilumab 300 mg qw and placebo groups (0% and 5%, P = 0.1175, and 1% and 0%, P = 0.3042, in parts A and B, respectively). By week 24, dupilumab increased the proportion of patients reporting no symptoms vs placebo (48% vs 21%, P < 0.05, and 36% vs 15%, P < 0.01, in parts A and B, respectively; Figure 5).
Figure 5.
Proportion of patients reporting the lowest severity level on the PGIS of dysphagia at baseline and weeks 12, 20, and 24. *Nominal P ≤ 0.05, **nominal P ≤ 0.01 dupilumab vs placebo. CI, confidence interval; PGIS, Patient Global Impression of Severity; qw, once weekly.
Convergent and divergent validity
Moderate-to-strong (r = 0.3–0.7) positive correlations between changes in score from baseline to week 24 (or score at week 24 for PGIC) were observed between EoE-IQ, EoE-SQ Frequency, EoE-SQ Severity, PGIC, and PGIS and with the DSQ (see Supplementary Table 4, Supplementary Digital Content 1 http://links.lww.com/AJG/D323). Correlations between PRO and endoscopic (total endoscopic reference score) and histologic measures (peak eosinophils per high-power field) were typically weak to moderate (r < 0.4) as expected due to the different constructs being measured.
DISCUSSION
Symptoms are a strong determinant for EoE-specific HRQoL (25). In this study, dupilumab 300 mg qw demonstrated improvement in PRO measures of disease-specific QoL, and symptom frequency and severity. Specifically, dupilumab reduced the frequency and severity of symptoms beyond dysphagia, as demonstrated by a clinically meaningful reduction in EoE-SQ Frequency and Severity scores. Furthermore, patient perception of dysphagia measured by PGIC and PGIS of dysphagia showed a greater proportion of patients treated with dupilumab reporting an improvement in dysphagia and its severity at week 24 compared with placebo.
The EoE-IQ measure was used to assess psychosocial aspects of disease that affect patients with EoE and the impact of dupilumab treatment. Baseline measurements revealed that patients in the LIBERTY EoE TREET trial were most impacted by the emotional and social aspects of the disease, such as feeling bothered, worried about swallowing or choking, and difficulty taking part in social activities that involve eating. This is consistent with results using the EoE-IQ to assess QoL in a real-world study of patients with EoE (26). Dupilumab resulted in a clinically meaningful reduction in EoE-IQ score and had the greatest impact in the emotional and sleep disturbance domains. Coupled with demonstrated efficacy in the LIBERTY EoE TREET study (11), these findings show that dupilumab offers a range of benefits beyond the observed improvement of dysphagia. While previous studies have demonstrated improvements in general and EoE-specific HRQoL measures with STC (27–29), this is the first time an approved EoE treatment has demonstrated improvements in QoL aspects most important to patients. Improvements in QoL outcomes (EoE-IQ) and symptoms (DSQ, EoE-SQ Frequency and Severity, PGIC, and PGIS) were well correlated with each other but showed low correlation with histologic or endoscopic outcomes. This finding has been recognized in studies using other validated EoE-specific measures of HRQoL, such as the Adult EoE QoL Questionnaire, which correlated well with experience of dysphagia and food impaction, but not endoscopic or histologic outcomes (30,31). As such, it is important to measure multiple aspects of the disease to ensure a full picture of the patient's disease burden and to monitor the effectiveness of treatments for QoL and symptoms. In this study, dupilumab also demonstrated significant improvements in dysphagia (as measured by the DSQ) (11), and clinically meaningful improvements in other symptoms besides dysphagia (as measured by EoE-SQ) and QoL (as measured by EoE-IQ). These results highlight the need for physicians to consult with patients regarding their experience of symptoms when making decisions about treatment options.
Interpretation of results is limited because the LIBERTY EoE TREET study was not powered to measure the statistical significance of changes in the global assessments of dysphagia or the total scores or individual items of the EoE-SQ and EoE-IQ. As the EoE-SQ and EoE-IQ instruments are newly validated and used in this clinical trial for the first time, qualitative patient input on the meaningful changes in these scores would complement the clinically meaningful improvements derived through anchor-based analyses described in this study. However, this is the first study to demonstrate improvements in important aspects of QoL with an approved treatment for EoE, and it provides an in-depth assessment of the effect of dupilumab treatment on HRQoL in these patients. Treatment with dupilumab also resulted in improvements in symptoms beyond dysphagia that have been identified as important by patients and healthcare professionals. Symptom improvements following treatment with dupilumab have been demonstrated in a real-world study of patients with refractory fibrostenotic EoE. However, the study was unable to use any validated PRO measures (32). There is a need for further research to investigate the efficacy of dupilumab on symptoms and QoL in a real-world setting using validated PRO, such as the EoE-SQ and EoE-IQ.
A final limitation of this study is that the link between symptom severity and impact on mental health burden was not investigated in the patients enrolled in the LIBERTY EoE TREET study. This is an important topic for future research as EoE clinical disease severity has been associated with a high burden of mental distress, including anxiety and/or depression symptoms, with young adults (18–35 years), particularly vulnerable to anxiety (33).
Overall, dupilumab 300 mg qw improved HRQoL, most clearly in the areas of emotional health and sleep disturbance. Dupilumab also improved the patients' impressions of dysphagia symptoms and reduced the frequency and severity of important EoE symptoms beyond dysphagia.
CONFLICTS OF INTEREST
Guarantor of article: Eilish McCann, PhD.
Specific author contributions: All authors contributed to data interpretation. All authors had full access to trial data and vouch for the integrity of the data and adherence to the study protocol. All authors critically reviewed the manuscript and had final responsibility for the decision to submit the manuscript for publication. J.M.S., M.C., E.S.D., A.J.B., L.G., A.S., S.T.T., and E.M.: conceived and designed the trial; J.M.S., M.C., E.S.D., and A.J.B.: contributed to data collection; X.S.: contributed to data analysis.
Financial support: Research sponsored by Sanofi and Regeneron Pharmaceuticals Inc.
Potential competing interests: J.M.S. is a consultant for Allakos, DBV Technologies, Novartis, Regeneron Pharmaceuticals Inc., and Shire/Takeda; and receives grant support from DBV Technologies and Regeneron Pharmaceuticals Inc. M.C. serves/served as a consultant for Adare/Ellodi, Allakos, AstraZeneca, BMS, Phathom, Recludix, Regeneron Pharmaceuticals Inc., Sanofi, Shire/Takeda, and Nexstone Immunology; and receives/has received research funding from Adare/Ellodi, Allakos, AstraZeneca, Danone, Regeneron Pharmaceuticals Inc., Shire/Takeda, and BMS. E.S.D. is a consultant for Abbott, AbbVie, Adare/Ellodi, Aimmune, Akesobio, Alfasigma, ALK, Allakos, Amgen, Aqilion, Arena/Pfizer, Aslan, AstraZeneca, Avir, Biorasi, Calypso, Celgene/Receptos/BMS, Celldex, Eli Lilly, EsoCap, Eupraxia, Ferring, Gossamer Bio, GSK, Holoclara, Invea, Knightpoint, Landos, LucidDx, Morphic, Nexstone Immunology, Nutricia, Parexel/Calyx, Phathom, Regeneron Pharmaceuticals Inc., Revolo, Robarts/Alimentiv, Salix, Sanofi, Shire/Takeda, Target RWE, and Upstream Bio; receives research funding from Adare/Ellodi, Allakos, Arena, AstraZeneca, Celgene/Receptos/BMS, Eupraxia, GSK, Meritage, Miraca, Nutricia, Regeneron Pharmaceuticals Inc., Revolo, and Shire/Takeda; and receives educational grants from Allakos, Aqilion, Holoclara, and Invea. A.J.B. is a consultant for Alimentiv, Aqilion, AstraZeneca, Dr. Falk Pharma, Eupraxia, Laborie, Medtronic, Reckitt, and Regeneron Pharmaceuticals Inc.; receives/has received financial support for research/grants from Dr. Falk Pharma, Nutricia, Regeneron Pharmaceuticals Inc., Sanofi, SST, and Thelial; and receives/has received lecture fees from/is a member of the speakers bureaux for Alimentiv, Aquilon, AstraZeneca, Dr. Falk Pharma, Laborie, Medtronic, Reckitt, Regeneron Pharmaceuticals Inc., and Sanofi. X.S., A.S., and E.M. are employees and shareholders in Regeneron Pharmaceuticals Inc. L.G. and S.T.T. are employees and may hold stock and/or stock options in Sanofi.
ClinicalTrials.gov identifier: NCT03633617.
Data availability statement: Qualified researchers may request access to study documents (including the clinical study report, study protocol with any amendments, blank case report form, statistical analysis plan) that support the methods and findings reported in this manuscript. Individual anonymized participant data will be considered for sharing once the indication has been approved by a regulatory body, if there is legal authority to share the data and there is not a reasonable likelihood of participant reidentification. Submit requests to https://vivli.org/.
Study Highlights.
WHAT IS KNOWN
✓ Improvement in health-related quality of life (HRQoL) is viewed as one of the most important outcomes of therapy by adult patients with eosinophilic esophagitis.
✓ Dupilumab has demonstrated significant improvements in symptoms, HRQoL, histology, and endoscopic features.
WHAT IS NEW HERE
✓ Patients treated with dupilumab 300 mg once weekly had improvements in frequency and severity of symptoms beyond dysphagia.
✓ Key aspects of HRQoL and patient impression of dysphagia were also improved.
✓ Improvements in patient-reported outcome measures of HRQoL and symptoms were well correlated with each other.
✓ Improvements in patient-reported outcome measures of HRQoL and symptoms were poorly correlated with improvement in histologic and endoscopic outcomes.
Supplementary Material
ACKNOWLEDGMENTS
Medical writing support provided by Christopher Bulman, PhD, of Adelphi Group, Macclesfield, UK, funded by Sanofi and Regeneron Pharmaceuticals Inc. in accordance with Good Publication Practice.
Footnotes
SUPPLEMENTARY MATERIAL accompanies this paper at http://links.lww.com/AJG/D323
Contributor Information
Mirna Chehade, Email: mirna.chehade@mssm.edu.
Evan S. Dellon, Email: edellon@med.unc.edu.
Albert J. Bredenoord, Email: a.j.bredenoord@amsterdamumc.nl.
Xian Sun, Email: xian.sun@regeneron.com.
Lila Glotfelty, Email: lilagollogly@gmail.com.
Arsalan Shabbir, Email: ashabbir@buffalo.edu.
Sarette T. Tilton, Email: sarette.tilton@sanofi.com.
Eilish McCann, Email: Eilish.mccann@regeneron.com.
REFERENCES
- 1.Dellon ES, Hirano I. Epidemiology and natural history of eosinophilic esophagitis. Gastroenterology 2018;154(2):319–32.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Navarro P, Arias A, Arias-Gonzalez L, et al. Systematic review with meta-analysis: The growing incidence and prevalence of eosinophilic oesophagitis in children and adults in population-based studies. Aliment Pharmacol Ther 2019;49(9):1116–25. [DOI] [PubMed] [Google Scholar]
- 3.Lucendo AJ, Molina-Infante J, Arias A, et al. Guidelines on eosinophilic esophagitis: Evidence-based statements and recommendations for diagnosis and management in children and adults. United Eur Gastroenterol J 2017;5(3):335–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gonsalves NP, Aceves SS. Diagnosis and treatment of eosinophilic esophagitis. J Allergy Clin Immunol 2020;145(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harris RF, Menard-Katcher C, Atkins D, et al. Psychosocial dysfunction in children and adolescents with eosinophilic esophagitis. J Pediatr Gastroenterol Nutr 2013;57(4):500–5. [DOI] [PubMed] [Google Scholar]
- 6.Taft TH, Kern E, Keefer L, et al. Qualitative assessment of patient-reported outcomes in adults with eosinophilic esophagitis. J Clin Gastroenterol 2011;45(9):769–74. [DOI] [PubMed] [Google Scholar]
- 7.Safroneeva E, Balsiger L, Hafner D, et al. Adults with eosinophilic oesophagitis identify symptoms and quality of life as the most important outcomes. Aliment Pharmacol Ther 2018;48(10):1082–90. [DOI] [PubMed] [Google Scholar]
- 8.Mukkada V, Falk GW, Eichinger CS, et al. Health-related quality of life and costs associated with eosinophilic esophagitis: A systematic review. Clin Gastroenterol Hepatol 2018;16(4):495–503.e8. [DOI] [PubMed] [Google Scholar]
- 9.Klinnert MD, Silveira L, Harris R, et al. Health-related quality of life over time in children with eosinophilic esophagitis and their families. J Pediatr Gastroenterol Nutr 2014;59(3):308–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Safroneeva E, Coslovsky M, Kuehni CE, et al. Eosinophilic oesophagitis: Relationship of quality of life with clinical, endoscopic and histological activity. Aliment Pharmacol Ther 2015;42(8):1000–10. [DOI] [PubMed] [Google Scholar]
- 11.Dellon ES, Rothenberg ME, Collins MH, et al. Dupilumab in adults and adolescents with eosinophilic esophagitis. N Engl J Med 2022;387(25):2317–30. [DOI] [PubMed] [Google Scholar]
- 12.Hirano I, Collins MH, Katzka DA, et al. Budesonide oral suspension improves outcomes in patients with eosinophilic esophagitis: Results from a phase 3 trial. Clin Gastroenterol Hepatol 2022;20(3):525–34.e10. [DOI] [PubMed] [Google Scholar]
- 13.Chang N, Raja S, Betancourt R, et al. Generic measures of quality of life are not correlated with disease activity in eosinophilic esophagitis. Dig Dis Sci 2021;66(10):3312–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lucendo AJ, Arias-Gonzalez L, Molina-Infante J, et al. Systematic review: Health-related quality of life in children and adults with eosinophilic oesophagitis-instruments for measurement and determinant factors. Aliment Pharmacol Ther 2017;46(4):401–9. [DOI] [PubMed] [Google Scholar]
- 15.Rothenberg ME, Dellon ES, Collins MH, et al. Efficacy and safety of dupilumab up to 52 weeks in adults and adolescents with eosinophilic oesophagitis (LIBERTY EoE TREET study): A multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Gastroenterol Hepatol 2023;8(11):990–1004. [DOI] [PubMed] [Google Scholar]
- 16.O'Shea KM, Aceves SS, Dellon ES, et al. Pathophysiology of eosinophilic esophagitis. Gastroenterology 2018;154(2):333–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Le Floc'h A, Allinne J, Nagashima K, et al. Dual blockade of IL-4 and IL-13 with dupilumab, an IL-4Rα antibody, is required to broadly inhibit type 2 inflammation. Allergy 2020;75(5):1188–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gandhi NA, Pirozzi G, Graham NMH. Commonality of the IL-4/IL-13 pathway in atopic diseases. Expert Rev Clin Immunol 2017;13(5):425–37. [DOI] [PubMed] [Google Scholar]
- 19.DUPIXENT® (dupilumab). Highlights of Prescribing Information. US Food and Drug Administration, 2024 (https://www.accessdata.fda.gov/drugsatfda_docs/label/2024/761055s059lbl.pdf). Accessed July 15, 2024. [Google Scholar]
- 20.DUPIXENT® (dupilumab). DUPIXENT® (dupilumab injection) Is Now Approved in Canada for the Treatment of Adult and Adolescent Patients (12+) With Eosinophilic Esophagitis. 2023 (https://sanoficanada.mediaroom.com/2023-05-04-DUPIXENT-R-dupilumab-injection-is-now-approved-in-Canada-for-the-treatment-of-adult-and-adolescent-patients-12-with-Eosinophilic-Esophagitis#:∼:text=DUPIXENT%C2%AE%20(dupilumab%20injection)%20is,Eosinophilic%20Esophagitis%20%2D%20May%204%2C%202023). Accessed July 15, 2024. [Google Scholar]
- 21.DUPIXENT® (dupilumab). Summary of Product Characteristics. Regeneron Pharmaceuticals, 2020. (https://www.ema.europa.eu/en/documents/product-information/dupixent-epar-product-information_en.pdf). Accessed July 15, 2024. [Google Scholar]
- 22.McCann E, Chehade M, Spergel JM, et al. Validation of the novel eosinophilic esophagitis impact questionnaire. J Patient Rep Outcomes 2023;7(1):120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cohen J. Set correlation and contingency tables. Appl Psychol Meas 1988;12(4):425–34. [Google Scholar]
- 24.Hinkle DE, Wiersma W, Jurs SG. Applied Statistics for the Behavioral Sciences. Houghton Mifflin Company: Boston, MA, 2003. [Google Scholar]
- 25.Lucendo AJ, Arias-Gonzalez L, Molina-Infante J, et al. Determinant factors of quality of life in adult patients with eosinophilic esophagitis. United Eur Gastroenterol J 2018;6(1):38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schoepfer A, Olsen S, Kamat S, et al. Symptom and quality of life burden among patients with eosinophilic oesophagitis in a real-world setting. United Eur Gastroenterol J 2021;9:290–1. [Google Scholar]
- 27.Bergquist H, Larsson H, Johansson L, et al. Dysphagia and quality of life may improve with mometasone treatment in patients with eosinophilic esophagitis: A pilot study. Otolaryngol Head Neck Surg 2011;145(4):551–6. [DOI] [PubMed] [Google Scholar]
- 28.Larsson H, Bergman K, Finizia C, et al. Dysphagia and health-related quality of life in patients with eosinophilic esophagitis: A long-term follow-up. Eur Arch Otorhinolaryngol 2015;272(12):3833–9. [DOI] [PubMed] [Google Scholar]
- 29.Kruszewski PG, Russo JM, Franciosi JP, et al. Prospective, comparative effectiveness trial of cow's milk elimination and swallowed fluticasone for pediatric eosinophilic esophagitis. Dis Esophagus 2016;29(4):377–84. [DOI] [PubMed] [Google Scholar]
- 30.Taft TH, Kern E, Kwiatek MA, et al. The adult eosinophilic oesophagitis quality of life questionnaire: A new measure of health-related quality of life. Aliment Pharmacol Ther 2011;34(7):790–8. [DOI] [PubMed] [Google Scholar]
- 31.Stern E, Taft T, Zalewski A, et al. Prospective assessment of disease-specific quality of life in adults with eosinophilic esophagitis. Dis Esophagus 2018;31(4):1–7. [DOI] [PubMed] [Google Scholar]
- 32.Lee CJ, Dellon ES. Real-world efficacy of dupilumab in severe, treatment-refractory, and fibrostenotic patients with eosinophilic esophagitis. Clin Gastroenterol Hepatol 2024;22(2):252–8. [DOI] [PubMed] [Google Scholar]
- 33.de Rooij WE, Bennebroek Evertsz F, Lei A, et al. Mental distress among adult patients with eosinophilic esophagitis. Neurogastroenterol Motil 2021;33(7):e14069. [DOI] [PMC free article] [PubMed] [Google Scholar]