Abstract
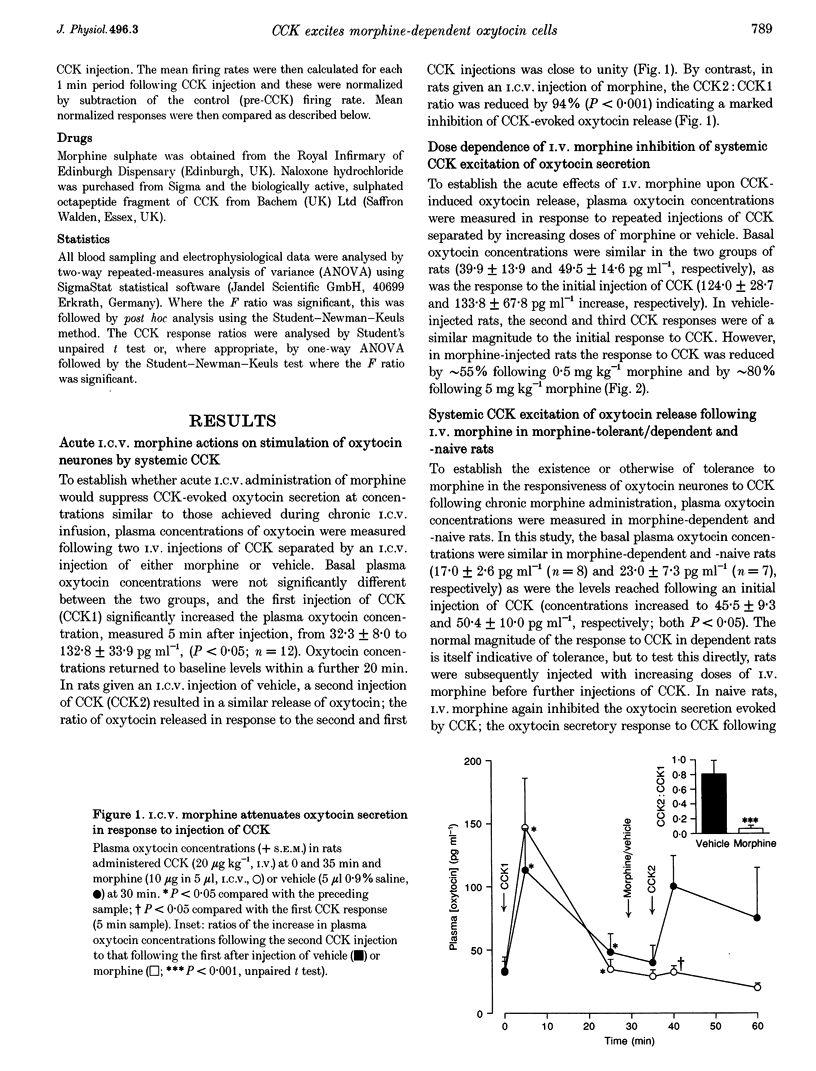
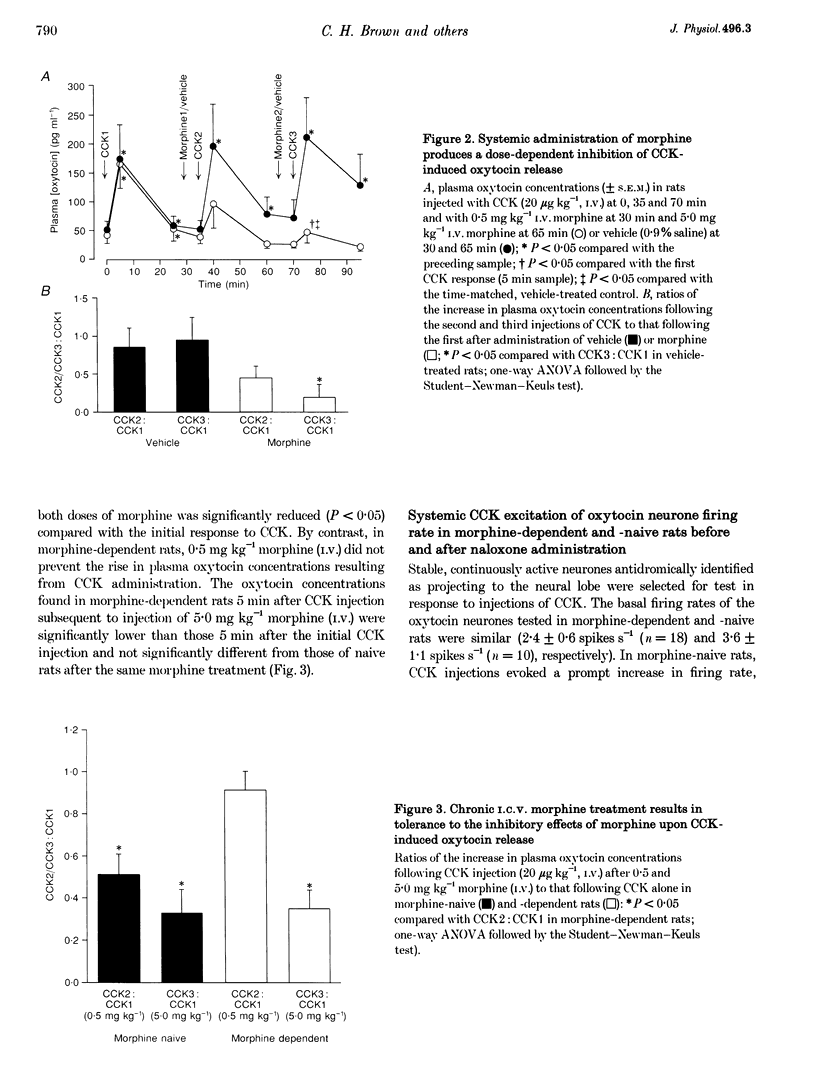
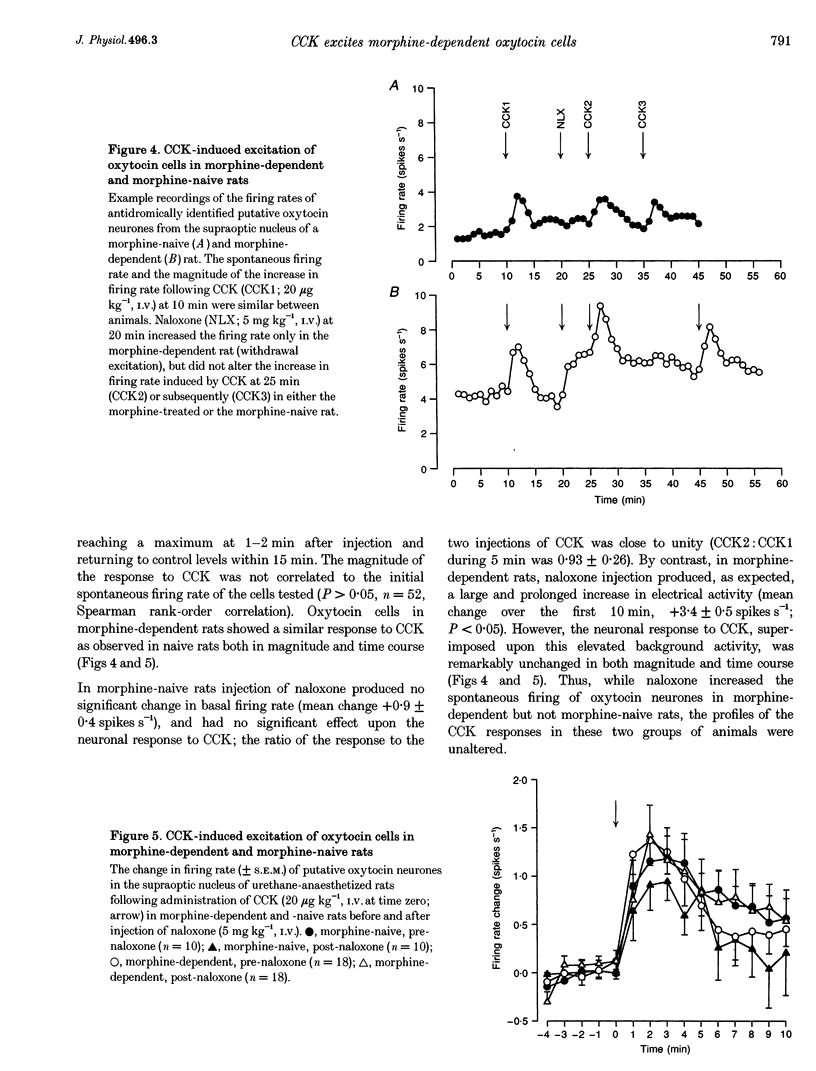
1. Morphine inhibits supraoptic nucleus oxytocin neurones directly and presynaptically via inhibition of afferent noradrenergic endings. 2. We studied whether morphine tolerance/dependence (induced by intracerebroventricular (I.C.V.) morphine infusion) alters the responsiveness of oxytocin neurones to systemic cholecystokinin (CCK), a stimulus which activates oxytocin neurones via the release of noradrenaline. 3. CCK (20 micrograms kg-1, i.v.) increased plasma oxytocin concentrations similarly in urethane-anaesthetized morphine-naive and -dependent rats. In naive rats, I.C.V. (10 micrograms) and i.v. morphine (0.5 mg kg-1) reduced CCK-induced oxytocin secretion by 95 +/- 4 and 49 +/- 10%, respectively. In dependent rats, i.v. morphine reduced CCK-induced release by only 8 +/- 9%, indicating tolerance. 4. In urethane-anaesthetized rats, i.v. CCK increased the firing rates of oxytocin neurones similarly in morphine-naive and -dependent rats (by 1.2 +/- 0.2 and 1.4 +/- 0.3 spikes s-1 maximum, respectively, over 5 min). Naloxone did not alter spontaneous or CCK-induced activity in naive rats but increased activity in dependent rats (by 3.4 +/- 0.5 spikes s-1), indicative of withdrawal excitation; however, the response to CCK remained unchanged after naloxone. 5. Systemic CCK did not trigger withdrawal, nor did it have a greater excitatory effect in dependent rats. Thus, morphine withdrawal excitation of oxytocin neurones does not involve supersensitivity to the noradrenergic input, or hypersensitivity of this input to i.v. CCK. Tolerance apparently occurs both at the cell bodies of oxytocin neurones in the supraoptic nucleus and in their noradrenergic input. However, dependence is apparent only at the cell bodies.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aghajanian G. K. Tolerance of locus coeruleus neurones to morphine and suppression of withdrawal response by clonidine. Nature. 1978 Nov 9;276(5684):186–188. doi: 10.1038/276186a0. [DOI] [PubMed] [Google Scholar]
- Bicknell R. J., Leng G., Lincoln D. W., Russell J. A. Naloxone excites oxytocin neurones in the supraoptic nucleus of lactating rats after chronic morphine treatment. J Physiol. 1988 Feb;396:297–317. doi: 10.1113/jphysiol.1988.sp016963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold M. S., Redmond D. E., Jr, Kleber H. D. Clonidine blocks acute opiate-withdrawal symptoms. Lancet. 1978 Sep 16;2(8090):599–602. doi: 10.1016/s0140-6736(78)92823-4. [DOI] [PubMed] [Google Scholar]
- Gonzalvez M. L., Milanés M. V., Martinez-Piñero M. G., Marín M. T., Vargas M. L. Effects of intracerebroventricular clonidine on the hypothalamic noradrenaline and plasma corticosterone levels of opiate naive rats and after naloxone-induced withdrawal. Brain Res. 1994 Jun 6;647(2):199–203. doi: 10.1016/0006-8993(94)91318-8. [DOI] [PubMed] [Google Scholar]
- Higuchi T., Honda K., Fukuoka T., Negoro H., Wakabayashi K. Release of oxytocin during suckling and parturition in the rat. J Endocrinol. 1985 Jun;105(3):339–346. doi: 10.1677/joe.0.1050339. [DOI] [PubMed] [Google Scholar]
- Leng G., Mansfield S., Bicknell R. J., Blackburn R. E., Brown D., Chapman C., Dyer R. G., Hollingsworth S., Shibuki K., Yates J. O. Endogenous opioid actions and effects of environmental disturbance on parturition and oxytocin secretion in rats. J Reprod Fertil. 1988 Sep;84(1):345–356. doi: 10.1530/jrf.0.0840345. [DOI] [PubMed] [Google Scholar]
- Luckman S. M., Hamamura M., Antonijevic I., Dye S., Leng G. Involvement of cholecystokinin receptor types in pathways controlling oxytocin secretion. Br J Pharmacol. 1993 Sep;110(1):378–384. doi: 10.1111/j.1476-5381.1993.tb13820.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Piñero M. G., Milanes M. V., Vargas M. L. Modulation by catecholamine of hypothalamus-pituitary-adrenocortical (HPA) axis activity in morphine-tolerance and withdrawal. Gen Pharmacol. 1994 Jan;25(1):187–192. doi: 10.1016/0306-3623(94)90031-0. [DOI] [PubMed] [Google Scholar]
- Onaka T., Luckman S. M., Guevara-Guzman R., Ueta Y., Kendrick K., Leng G. Presynaptic actions of morphine: blockade of cholecystokinin-induced noradrenaline release in the rat supraoptic nucleus. J Physiol. 1995 Jan 1;482(Pt 1):69–79. doi: 10.1113/jphysiol.1995.sp020500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pumford K. M., Leng G., Russell J. A. Morphine actions on supraoptic oxytocin neurones in anaesthetized rats: tolerance after i.c.v. morphine infusion. J Physiol. 1991;440:437–454. doi: 10.1113/jphysiol.1991.sp018717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pumford K. M., Russell J. A., Leng G. Effects of the selective kappa-opioid agonist U50,488 upon the electrical activity of supraoptic neurones in morphine-tolerant and morphine-naive rats. Exp Brain Res. 1993;94(2):237–246. doi: 10.1007/BF00230291. [DOI] [PubMed] [Google Scholar]
- Rasmussen K., Beitner-Johnson D. B., Krystal J. H., Aghajanian G. K., Nestler E. J. Opiate withdrawal and the rat locus coeruleus: behavioral, electrophysiological, and biochemical correlates. J Neurosci. 1990 Jul;10(7):2308–2317. doi: 10.1523/JNEUROSCI.10-07-02308.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renaud L. P., Tang M., McCann M. J., Stricker E. M., Verbalis J. G. Cholecystokinin and gastric distension activate oxytocinergic cells in rat hypothalamus. Am J Physiol. 1987 Oct;253(4 Pt 2):R661–R665. doi: 10.1152/ajpregu.1987.253.4.R661. [DOI] [PubMed] [Google Scholar]
- Rossetti Z. L., Longu G., Mercuro G., Gessa G. L. Extraneuronal noradrenaline in the prefrontal cortex of morphine-dependent rats: tolerance and withdrawal mechanisms. Brain Res. 1993 Apr 23;609(1-2):316–320. doi: 10.1016/0006-8993(93)90889-u. [DOI] [PubMed] [Google Scholar]
- Russell J. A., Coombes J. E., Leng G., Bicknell R. J. Morphine tolerance and inhibition of oxytocin secretion by kappa-opioids acting on the rat neurohypophysis. J Physiol. 1993 Sep;469:365–386. doi: 10.1113/jphysiol.1993.sp019818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell J. A., Leng G., Bicknell R. J. Opioid tolerance and dependence in the magnocellular oxytocin system: a physiological mechanism? Exp Physiol. 1995 May;80(3):307–340. doi: 10.1113/expphysiol.1995.sp003850. [DOI] [PubMed] [Google Scholar]
- Silverstone P. H., Done C., Sharp T. In vivo monoamine release during naloxone-precipitated morphine withdrawal. Neuroreport. 1993 Aug;4(8):1043–1045. doi: 10.1097/00001756-199308000-00012. [DOI] [PubMed] [Google Scholar]
- Stornetta R. L., Norton F. E., Guyenet P. G. Autonomic areas of rat brain exhibit increased Fos-like immunoreactivity during opiate withdrawal in rats. Brain Res. 1993 Oct 8;624(1-2):19–28. doi: 10.1016/0006-8993(93)90055-r. [DOI] [PubMed] [Google Scholar]
- Sumner B. E., Coombes J. E., Pumford K. M., Russell J. A. Opioid receptor subtypes in the supraoptic nucleus and posterior pituitary gland of morphine-tolerant rats. Neuroscience. 1990;37(3):635–645. doi: 10.1016/0306-4522(90)90095-l. [DOI] [PubMed] [Google Scholar]
- Ueta Y., Kannan H., Higuchi T., Negoro H., Yamashita H. CCK-8 excites oxytocin-secreting neurons in the paraventricular nucleus in rats--possible involvement of noradrenergic pathway. Brain Res Bull. 1993;32(5):453–459. doi: 10.1016/0361-9230(93)90290-r. [DOI] [PubMed] [Google Scholar]


