ABSTRACT
Thymomas are rare, malignant, epithelial tumors of the thymus gland. Extrathoracic metastasis of thymoma is exceedingly rare, particularly when isolated to the liver. We report an 89-year-old man who presented with urinary retention. Exploratory computed tomography imaging revealed a heterogeneous mass in the aortopulmonary window and a 1.9 cm lesion in the left hepatic lobe. Results from magnetic resonance imaging, positron emission tomography-computed tomography, and histopathological analysis of biopsy samples collectively supported a diagnosis of metastatic type B2 thymoma. To the best of our knowledge, this is the oldest patient to be diagnosed with metastatic type B2 thymoma. Metastatic thymoma is difficult to identify, and patients with mediastinal mass identified after any presentation should be evaluated for malignant spread.
KEYWORDS: thymoma, metastatic thymoma, hepatic thymoma, hepatic metastasis
INTRODUCTION
Thymomas are rare, malignant, epithelial tumors of the thymus gland. The first case of thymoma was reported back in 1958 by Wessely et al1 They are mostly found in the prevascular mediastinum but can also be found in the neck, lung, pulmonary hilum, thyroid, pleura, and pericardium.2 While thymomas occur at a low frequency of 1.5 per million people, they make up about 40% of malignant tumors in the anterior mediastinum.3 Approximately 30% of patients with thymoma are asymptomatic at the time of diagnosis,4 and pathologically, thymomas predominantly affect surrounding structures through direct invasion and dissemination into the thoracic cavity.5 However, exceedingly rare occurrences of extrathoracic distant metastases can occur.6 A literature review revealed 82 reported cases of extrathoracic metastasis of thymoma, and of these, only 25 involved liver metastasis of which only 9 had isolated liver metastases. We present an exceedingly rare case of thymoma with an isolated, single liver metastasis.
CASE REPORT
An 89-year-old man with a medical history of hypertension, diabetes, and coronary artery disease presented with urinary retention. Pelvic-abdominal computed tomography (CT) with contrast was done to evaluate for the cause of retention which revealed a massively enlarged prostate with benign prostatic hyperplasia, and an indolent catheter was inserted to relieve the retention; however, the imaging also revealed a 6.8 × 5.2 cm heterogeneous mass in the aortopulmonary window and an indeterminate 1.9 cm lesion in the left hepatic lobe. Chest CT with contrast confirmed the mediastinal mass which was suspicious for malignancy, and a CT-guided biopsy of the mass was taken. Magnetic resonance imaging (MRI) of the abdomen confirmed a 1.9 cm lesion in the left hepatic lobe suggesting malignancy (Figure 1). Pathological analysis of a biopsy sample from the mediastinal mass indicated a moderate number of thymocytes admixed with polygonal epithelial cells with moderately prominent nuclei and cytoplasmic pigments. Immunostaining results were positive for a range of markers collectively consistent with thymoma type B2, including immune markers CD3, CD20, and TdT, as well as markers CK and AE1/3, used for identifying epithelial-derived tumors (Figure 2).
Figure 1.
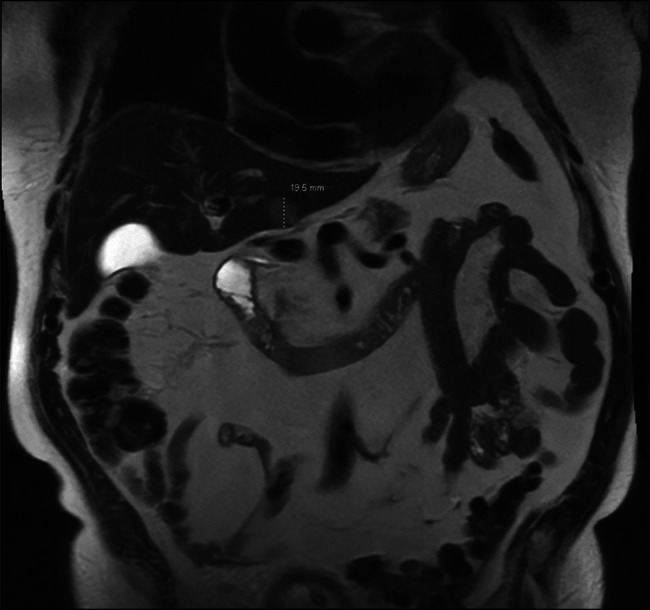
Magnetic resonance imaging picture of the liver lesion.
Figure 2.
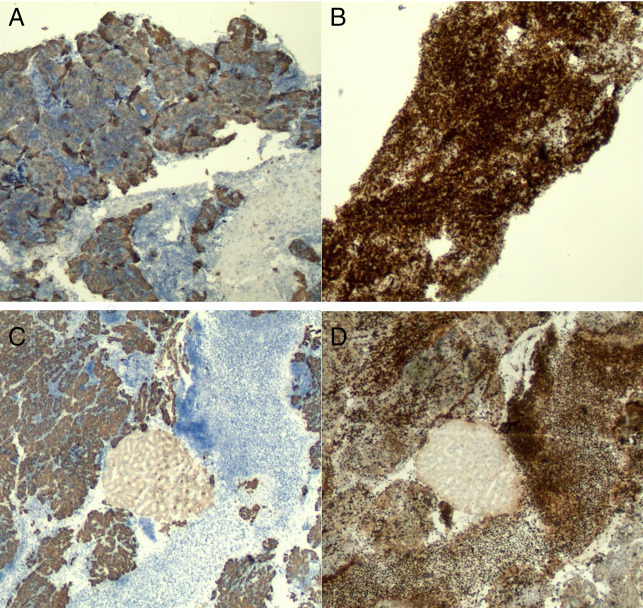
Immunostaining (40×) of the mediastinal and liver lesions. (A) AE1/AE3 immunohistochemical stain of the mediastinal lesion showing neoplastic thymic epithelial cells. (B) TdT immunohistochemical stain of the mediastinal lesion highlighting thymocytes (immature T-cells). (C) AE1/AE3 of the liver lesion highlighting the neoplastic epithelial cells. Hepatocytes showed weak-to-moderate positivity. (D) TdT of the liver lesion showing immature lymphocytes.
Hepatologists and gastroenterologists were consulted about the liver lesion, and they performed endoscopic ultrasound (EUS)-guided liver biopsy of the lesion, which appeared hypoechoic, heterogeneous, and solid with well-defined borders (Figure 3); liver biopsy results were consistent with metastatic thymoma (Figure 2).
Figure 3.
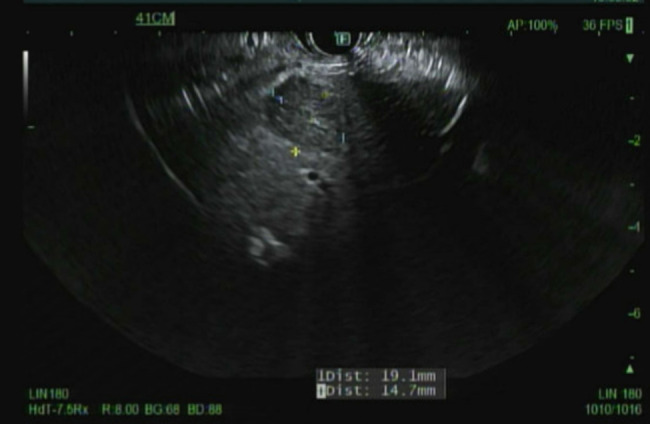
Endoscopic ultrasound picture of the liver lesion.
A tumor board containing hepatologists, gastroenterologists, and oncologists recommended a positron emission tomography (PET)-CT scan. PET-CT confirmed a segment 3 malignant liver mass and hypermetabolic anterior mediastinal mass consistent with metastatic thymoma. PET scan also showed a hypermetabolic area in the pyloric canal, upper endoscopy was done which revealed normal mucosa, and a concurrent CT scan showed no abnormality in the stomach as well.
Radiation therapy was pursued rather than surgery or chemotherapy because of the patient's advanced age and comorbidities. The patient received radiation therapy to the mediastinum over 6 weeks and is currently undergoing stereotactic body radiotherapy to the liver lesion as of the writing of this report.
DISCUSSION
Thymoma, though constituting only 0.2%–1.5% of all malignancies, is the most common tumor of the anterior mediastinum. Remarkably, extrathoracic metastasis is exceptionally rare, especially to the liver, with only a handful of documented cases. Our case presents a striking example of this rarity.
Patients with thymoma can present with a wide range of clinical symptoms, often related to the tumor's pressure on nearby structures. Common manifestations include chest pain, a neck mass, or superior vena cava syndrome. In addition, thymomas are frequently associated with myasthenia gravis and are often discovered incidentally during chest imaging.7,8 In our case, the patient initially presented with unrelated urinary retention and metastatic thymoma was identified incidentally through exploratory imaging.
Thymomas can be discovered at any stage, with most detected at stages I thymomas primarily exhibit local invasion within the thoracic cavity and often spread to adjacent structures, such as the pleura, lungs, and pericardium. Extrathoracic metastasis is relatively rare, occurring in less than 7% of cases, and indicating a more aggressive and advanced disease. When metastasis does occur beyond the thorax, it can involve distant organs such as the liver, bones, or brain and is usually associated with poor prognosis. The exact mechanism of thymoma metastasis to the liver is not fully understood, but it is likely due to several factors. One possibility is hematogenous spread, as tumor cells may enter the bloodstream through major vessels near the thymus and travel to distant organs, including the liver, which is highly vascular. The liver's central role in immune regulation may also make it more vulnerable to metastasis, particularly in the context of thymoma, a tumor closely associated with immune dysfunction. In addition, the proximity of the thymus to the diaphragm and thoracoabdominal venous pathways may facilitate the spread of cancer cells to the liver.9
Serologic tumor markers are frequently used to evaluate liver lesions, aiding in diagnosis and differentiation between primary liver cancers and metastatic lesions from other malignancies. Common markers include alpha-fetoprotein, which is elevated in hepatocellular carcinoma, and carcinoembryonic antigen and CA 19-9, which are typically elevated in metastatic liver lesions originating from colorectal or pancreatic cancers, respectively. CA-125 may also be used, particularly in the context of ovarian cancer metastasis to the liver. These markers help guide clinical decision making by suggesting the potential source of a liver lesion. However, in the case of thymoma metastasis to the liver, serologic tumor markers have limited utility. Thymoma and thymic carcinoma do not typically produce commonly used markers such as alpha-fetoprotein, carcinoembryonic antigen, or CA 19-9. Therefore, these markers are not helpful in diagnosing or monitoring liver metastases from thymoma. Instead, imaging studies (such as CT or MRI) and histopathological examination following a biopsy of the liver lesion are the primary methods used to confirm thymoma metastasis.
For our patient, CT was instrumental for initially identifying a heterogeneous mass in the aortopulmonary window and an indeterminate hepatic lesion, which were further evaluated with MRI. The pathological features of the mediastinal biopsy sample were consistent with thymoma type B2, involving a predominant epithelial component, and an EUS-guided biopsy of the liver lesion further supported the findings. In addition, PET-CT clearly revealed a hypermetabolic anterior mediastinal mass and segment 3 liver mass, confirming our patient's diagnosis of metastatic thymoma. PET-CT can be used to confirm thymoma diagnoses and may be beneficial for malignancy grading.10 Well-differentiated thymomas tend to be PET-negative, whereas thymic carcinomas tend to be PET-positive.11
The prognosis for patients with thymoma depends on tumor stage and resection potential.12 After treatment, the National Comprehensive Cancer Network recommends a surveillance regimen including chest CT scans every 6 months for the first 2 years, then yearly over 10 years for thymoma, and over 5 years for thymic carcinoma.13 Overall, thymomas can be challenging to diagnose and classify, and an overview of reported cases can shed some light on key characteristics, such as metastatic potential and likely sites of metastasis. In one review of 283 patients with thymoma, extrathoracic metastasis occurred in only 8 individuals, about 3%.3 In a study of 35 patients with metastatic thymoma or thymic carcinoma, liver metastasis was found in 5 patients (14%), all within the context of concurrent metastasis to other organs and none being isolated to the liver.14 Similarly, in a study that assessed the metastatic potential of initially diagnosed types A and AB thymomas, 4 of 18 patients with extrathoracic metastasis showed disease in the liver, again only within the setting of concurrent metastasis and with no occurrences of isolated liver involvement.15 In a single case report, a man with B3 thymoma had liver metastasis identified 2 years after primary surgery and metastasis to the pancreas 4 years after that.16 Overall, we identified only 8 cases of metastatic thymoma isolated to the liver,17–22 with 2 rare reports of ruptured metastatic liver tumors.23,24 (Table 1).
Table 1.
Reported cases of extrathoracic metastasis of thymoma to the liver and other organs
| Reference (yr) | No of patients | Mean age (range) | No. tumors w/benign cytomorphology | Thymoma subtype | Stage | Organs involved | Single vs multiple liver metastases | Treatment |
| Vladislav et al (2012)14 | 13 | 52 (31–93) | 13 | A-1 B1-3 B2-3 B3-6 |
IV IV IV I (1), II(1), III (1), IV(2), N/A (1) |
Liver (5/13), kidney, vertebra, and soft tissue | Multiple | NR |
| Jain et al15 (2010) | 18 | 53.6 (37–79) | 18 | A (9), AB (9) | I (4), II (8), N/A (6) | Pleura, lung, liver (4/18), bone, brain, and peritoneum | NR | Chemotherapy + radiotherapy + surgery |
| Passuello et al16 (2017) | 1 | 71 | 1 | B3 | III | Liver, pancreas | Single | Surgery |
| Marasco et al18 (1991) | 1 | 46 | 1 | NR | NR | Liver | Single | Surgery |
| Moretti et al19 (2000) | 1 | 58 | 1 | NR | NR | Liver | Single | Surgery |
| Hoshino et al17 (2008) | 1 | 54 | 1 | B2 | NR | Liver | Multiple | Radiofrequency ablation |
| Wang et al22 (2014) | 1 | 49 | 1 | AB | NR | Liver | Single | Surgery |
| Speisky et al (2016)21 | 1 | 55 | 1 | B1 | NR | Liver | Single | Surgery |
| Mallick et al20 (2022) | 1 | 49 | 1 | B1 | II | Liver | Single | Surgery |
| Utsunomiya et al23 (2021) | 1 | 56 | 1 | AB | IV | Liver | Single | Surgery |
| Kim et al24 (2016) | 1 | 62 | 1 | A | II | Liver | Single | Surgery |
| Dhahir Ali et al25, 2021 | 1 | 59 | 1 | B2 | NR | Liver | Single | Surgery |
| Cheng et al26, 2016 | 31 | 51 (28–78) | 31 | A-1 AB-1 B1-2 B2-11 B3-16 |
NR | Plural/pericardial, bone, brain, lymph nodes, liver (6/31) | NR | Chemotherapy + radiotherapy + surgery |
NR, not reported.
Metastasis of thymoma to the liver is relatively rare, highlighting the need for investigation into the biological mechanisms underlying the metastatic potential of this malignancy. In addition, a better understanding of the long-term prognoses for patients with liver metastasis from thymoma would help guide treatment strategies. Furthermore, treatment modalities for patients with liver metastasis from thymoma require extensive exploration to determine when surgical options vs medical and radiological approaches would be most appropriate.
In conclusion, the occurrence of liver metastasis from thymoma is extremely rare, yet it underscores the importance of thorough evaluation in patients presenting with mediastinal masses. This case highlights the need for vigilance in identifying potential metastatic disease, particularly in elderly patients with incidental liver lesions. Clinicians should be aware that even seemingly benign tumors can have metastatic potential, and a high index of suspicion is crucial for timely diagnosis and effective management. Recognizing the link between mediastinal masses and potential liver involvement can significantly influence treatment outcomes.
DISCLOSURES
Author contributions: M. Abusuliman contributed to manuscript writing, drafting, and critical revision of the manuscript and is the article guarantor. M. Aboeldahb contributed to manuscript writing, drafting, and critical revision of the manuscript. A. Olimy contributed to the drafting and critical revision of the manuscript. O. Abbas contributed to the drafting and critical revision of the manuscript. A. Abusulimanm contributed to the drafting and critical revision of the manuscript. T. Jamali contributed to manuscript writing, drafting, and critical revision of the manuscript. AG Rosario contributed to the drafting and critical revision of the manuscript. L. Yuan contributed to the drafting and critical revision of the manuscript. R. Pompa contributed to the conception and design of the manuscript.
Financial disclosure: None to report.
Informed consent was obtained for this case report.
Contributor Information
Moataz Aboeldahb, Email: aboeldahb.moataz@mayo.edu.
Abdullah Olimy, Email: Abdullahmo.olimy@gmail.com.
Omar Abbas, Email: oabbas2@hfhs.org.
Taher Jamali, Email: tjamali1@hfhs.org.
Agustin Gavidia Rosario, Email: agavidi1@hfhs.org.
Lisi Yuan, Email: lyuan2@hfhs.org.
Robert Pompa, Email: rpompa1@hfhs.org.
REFERENCES
- 1.Wessely Z. Malignant thymus tumor with liver metastasis in patient with myasthenia gravis. N Y State J Med. 1958;58(14):2422–4. [PubMed] [Google Scholar]
- 2.Robinson SP, Akhondi H. Thymoma. Treasure Island, Florida: StatPearls Publishing LLC; 2023. [PubMed] [Google Scholar]
- 3.Lewis JE, Wick MR, Scheithauer BW, Bernatz PE, Taylor WF. Thymoma. A clinicopathologic review. Cancer. 1987;60(11):2727–43. [DOI] [PubMed] [Google Scholar]
- 4.Walid MS, Troup EC, Robinson JS. Brain metastasis from thymic carcinoma in association with SIADH and pituitary enlargement: A case report. South Med J. 2008;101(7):764–6. [DOI] [PubMed] [Google Scholar]
- 5.Youk JH, Kim EK, Kim MJ, Oh KK, Park YN. Metastatic breast lesion from thymic carcinoma. J Ultrasound Med. 2006;25(10):1339–42. [DOI] [PubMed] [Google Scholar]
- 6.Aoki Y, Miki A, Nakano T, et al. Thymoma with an isolated splenic metastasis eight years after extended thymectomy: A case report. BMC Cancer. 2018;18(1):1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lavini C, Moran CA, Morandi U, Schoenhuber R. Thymus Gland Pathology: Clinical, Diagnostic and Therapeutic Features. Springer: Berlin/Heidelberg, Germany; 2009. [Google Scholar]
- 8.Detterbeck FC, Zeeshan A. Thymoma: Current diagnosis and treatment. Chin Med J. 2013;126(11):2186–91. [PubMed] [Google Scholar]
- 9.Remien K, Jan A. Anatomy, head and neck, thymus. Treasure Island, Florida: StatPearls Publishing LLC; 2023. [PubMed] [Google Scholar]
- 10.Tomaszek S, Wigle DA, Keshavjee S, Fischer S. Thymomas: Review of current clinical practice. Ann Thorac Surg. 2009;87(6):1973–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosenow EC, 3rd, Hurley BT. Disorders of the thymus. A review. Arch Intern Med. 1984;144(4):763–70. [PubMed] [Google Scholar]
- 12.Shamji F, Pearson FG, Todd TR, Ginsberg RJ, Ilves R, Cooper JD. Results of surgical treatment for thymoma. J Thorac Cardiovasc Surg. 1984;87(1):43–7. [PubMed] [Google Scholar]
- 13.Strange CD, Ahuja J, Shroff GS, Truong MT, Marom EM. Imaging evaluation of thymoma and thymic carcinoma. Front Oncol. 2021;11:810419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vladislav T, Jain RK, Alvarez R, et al. Extrathoracic metastases of thymic origin: A review of 35 cases. Mod Pathol. 2012;25(3):370–7. [DOI] [PubMed] [Google Scholar]
- 15.Jain RK, Mehta RJ, Henley JD, Kesler KA, Loehrer PJ, Badve S. WHO types A and AB thymomas: Not always benign. Mod Pathol. 2010;23(12):1641–9. [DOI] [PubMed] [Google Scholar]
- 16.Passuello N, Pozza G, Blandamura S, Valmasoni M, Sperti C. Thymoma metastatic to liver and pancreas: Case report and review of the literature. J Int Med Res. 2017;45(2):868–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoshino S, Furukawa M, Aragane K, et al. Successful multimodal treatment in a patient with thymoma accompanied by hepatic metastasis. J Thorac Oncol. 2008;3(1):98–100. [DOI] [PubMed] [Google Scholar]
- 18.Marasco WJ, Hergreuter CA, Pritchard E, O'Hara CJ, Steele GD. Surgical resection of a solitary liver metastasis in a 46-year-old patient with a malignant thymoma. J Surg Oncol. 1991;46(2):139–40. [DOI] [PubMed] [Google Scholar]
- 19.Moretti RD, Nasuelli D, Torre P, Antonello RM, Cazzato G, Stanta G. Hepatic metastasis of thymoma. Eur J Neurol. 2000;7(1):127–8. [DOI] [PubMed] [Google Scholar]
- 20.Mallick J, Peterson JM, Pina-Oviedo S, et al. Liver metastasis of thymoma: Case report and review of the literature. Int J Surg Pathol. 2023;31(5):755–60. [DOI] [PubMed] [Google Scholar]
- 21.Psoma E, Karkos PD, Dova S, et al. Sinonasal glomangiopericytoma treated with preoperative embolisation and endoscopic sinus surgery. Ecancermedicalscience. 2016;10:692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Z, Li H, Cao H, Zheng J. Clinicopathological features of type AB thymoma with liver metastases. Int J Clin Exp. Pathol 2014;7(12):8700–5. [PMC free article] [PubMed] [Google Scholar]
- 23.Utsunomiya T, Sakamoto K, Tsukamoto D, et al. Ruptured metastatic liver tumor secondary to a thymoma: A case report. J Surg Case Rep. 2021;2021(8):rjab341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim HJ, Park YE, Ki MS, et al. Spontaneous rupture of hepatic metastasis from a thymoma: A case report. World J Gastroenterol. 2016;22(44):9860–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dhahir Ali F, Kuebler S, Lakenberg N, Hermann L, Mall J, Fangmann J. A rare case of hepatic metastasis 20 years after surgical resection of a thymoma: A case report. Int J Surg Case Rep. 2021;87:106406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheng G, Gu C, Song Z. Impact of metastasis site for survival of patients with advanced thymic epithelial tumors. Translational Cancer Res. 2016;5(5):546–51. [Google Scholar]