Abstract
Objective:
Biochemical joint changes contribute to posttraumatic osteoarthritis (PTOA) development following anterior cruciate ligament reconstruction (ACLR). The purpose of this longitudinal cohort study was to compare tibiofemoral cartilage composition between ACLR patients with different serum biochemical profiles. We hypothesized that profiles of increased inflammation (monocyte chemoattractant protein-1 [MCP-1]), type-II collagen turnover (type-II collagen breakdown [C2C]:synthesis [CPII]), matrix degradation (matrix metalloproteinase-3 [MMP-3] and cartilage oligomeric matrix protein [COMP]) preoperatively to 6-months post-ACLR would be associated with greater tibiofemoral cartilage T1ρ relaxation times 12-months post-ACLR.
Design:
Serum was collected from 24 patients (46% female, 22.1±4.2 years old, 24.0±2.6 kg/m2 body mass index [BMI]) preoperatively (6.4±3.6 days post injury) and 6-months post-ACLR. T1ρ Magnetic Resonance Imaging (MRI) was collected for medial and lateral tibiofemoral articular cartilage at 12-months post-ACLR. A k-means cluster analysis was used to identify profiles based on biomarker changes over time and T1ρ relaxation times were compared between cluster groups controlling for sex, age, BMI, concomitant injury (either meniscal or chondral pathology), and Marx Score.
Results:
One cluster exhibited increases in MCP-1 and COMP while the other demonstrated decreases in MCP-1 and COMP preoperatively to 6-months post-ACLR. The cluster group with increases in MCP-1 and COMP demonstrated greater lateral tibial (adjusted mean difference=3.88, 95% confidence intervals [1.97–5.78]) and femoral (adjusted mean difference=12.71, 95% confidence intervals [0.41–23.81]) T1ρ relaxation times.
Conclusion:
Profiles of increased serum levels of inflammation and matrix degradation markers preoperatively to 6-months post-ACLR are associated with MRI changes consistent with lesser lateral tibiofemoral cartilage proteoglycan density 12-months post-ACLR.
Keywords: Magnetic Resonance Imaging, Biomarkers, Posttraumatic Osteoarthritis, T1rho
INTRODUCTION
Approximately 50% of individuals who sustain an anterior cruciate ligament (ACL) injury and undergo ACL reconstruction (ACLR) will develop radiographic post-traumatic osteoarthritis (PTOA) within 20 years following injury.1 Both ACL injury and surgical ACLR represent separate traumatic events capable of initiating an inflammatory response2 which, in some patients, may lead to a deleterious change in joint tissue metabolism that contributes to PTOA development.3 Previous animal models and ex vivo experiments have demonstrated the association between biochemical joint changes and articular cartilage breakdown;4–6 yet, the early biochemical changes associated with PTOA development following ACL injury in humans remain less clear. Individual synovial fluid concentrations of biomarkers related to inflammation and cartilage breakdown collected within the first 2 weeks following ACL injury were not associated with radiographic PTOA onset at a 16-year follow-up exam.7 Conversely, biochemical profiles constructed from multiple synovial fluid biomarkers collected during a single time point following ACL injury (63.9±27.1 days)8 were found to predict magnetic resonance imaging (MRI) outcomes of articular cartilage composition. Specifically, ACL injured individuals demonstrating greater synovial fluid sulfated glycosaminoglycan (sGAG) concentrations were more likely to exhibit altered articular cartilage composition within one to three years following ACLR compared to those demonstrating profiles exhibiting greater inflammation (i.e. interleukin [IL]) and enzymes related to matrix degeneration (i.e. matrix metalloproteinase [MMP]).8 Therefore, identifying biochemical profiles from the clusters of multiple biomarkers linked to various biochemical processes may be a powerful approach for early identification of patients who are most likely to develop early changes in articular cartilage composition.
Previous research has only evaluated synovial fluid biochemical profiles from a single time point collected prior to ACLR (i.e. on average nine weeks following ACL injury) for associations with altered articular cartilage composition.8 Conversely, changes in serum biomarker concentrations collected over multiple assessments has been demonstrated to be a more robust predictor of incident idiopathic osteoarthritis onset compared to biomarker concentrations collected at a single timepoint.9 Therefore, it is important to determine if serum biomarker profiles, developed from changes in concentrations before and after ACLR associate with changes in articular cartilage composition.
There is considerable evidence that increased inflammation signals degenerative processes that result in articular cartilage matrix degeneration consisting of articular cartilage proteoglycan depletion and type-II collagen turnover.2,10,11 Monocyte chemoattractant protein-1 (MCP-1) is a chemokine associated with osteoarthritis pathogenesis12 and IL-6 upregulation13 which is known to stimulate chemotaxis during the initial phases of inflammation promoting the migration of monocytes.14 Serum MCP-1 concentrations are higher in patients with knee osteoarthritis15 and patients with higher concentrations of serum MCP-1 are two times more likely to demonstrate knee osteoarthritis progression over 5 years.16 MMP-3 is a degenerative enzyme associated with the degradation of extracellular articular cartilage matrix17 which is also up-regulated by the inflammatory process18 and increases within the first 6 months post-ACLR.19 Cartilage oligomeric matrix protein (COMP) is extracellular matrix glycoprotein that binds to aggrecan, a key proteoglycan of articular cartilage, and is considered a biomarker of matrix degradation.20 Both greater serum MCP-1 and COMP have been demonstrated in the first year following ACLR compared to uninjured controls19,21 and COMP is associated with higher risk of developing knee osteoarthritis.22 Type II collagen provides tensile strength to the extracellular articular cartilage matrix and the ratio of type-II collagen breakdown (C2C) relative to type-II collagen synthesis (CPII) is estimated with a biomarker of type-II collagen turnover (C2C:CPII). Identifying biochemical profiles from using combinations of the aforementioned biomarkers would contribute to the understanding of how various processes related to joint tissue metabolism may contributed to the development of PTOA following injury.
Magnetic Resonance Imaging (MRI) has been utilized to estimate in vivo changes in cartilage composition.23,24 T1ρ relaxations times are sensitive to proteoglycan density24 and lesser proteoglycan density of the tibiofemoral articular cartilage is consistent with early compositional features related to osteoarthritis development.23 Differences in T1ρ relaxations times exist as early as 12 months post-ACLR in the articular cartilage of lateral and medial tibiofemoral condyles in the ACL injured limb compared to the contralateral limb25 and the limbs of healthy controls.26 Despite the well-supported evidence of deleterious joint tissue metabolism as a contributor to PTOA onset, the association between serum biochemical biomarker profiles over the first 6 months post-ACLR and later measures of MRI T1ρ relaxation times in tibiofemoral articular cartilage remains unclear.
The purpose of this study was to identify serum biochemical profiles by establishing cluster groups of individuals based on the changes in concentrations of serum MCP-1, MMP-3, COMP, and CPII:C2C preoperatively to 6 months post-ACLR. Furthermore, we compared tibiofemoral articular cartilage composition (i.e. T1ρ relaxation times) at 12 months post-ACLR between cluster groups with different biochemical profiles based on changes in serum biomarkers preoperatively to 6 month post-ACLR. We hypothesized that the cluster group with increased biomarker concentrations of MCP-1, MMP-3, COMP, and CPII:C2C between the preoperative and 6 months post-ACLR visits would demonstrate greater T1ρ relaxation times at 12 months post-ACLR.
METHODS
The current study was part of a prospective, longitudinal cohort study that included individuals who presented to the orthopedic clinic within 15 days of ACL injury (preoperative visit; days between injury and preoperative visit=6.4±3.6) and elected to undergo ACLR (days between injury and surgery=27.7±13.6). We only included individuals that attended the preoperative visit, as well as both the 6 and 12 month follow-up visits. Serum samples were collected at the preoperative visit and 6 months post-ACLR, and T1ρ MRIs were collected at 12 months post-ACLR. Serum samples were analyzed following the 6 month visit and participants were assigned to cluster profiles retrospectively. Patient reported outcomes were also collected at 6 months post-ACLR and reported as part of the participant demographics to help characterize the cluster groups when the final serum samples were collected. This study was approved by the Institutional Review Board at the University of North Carolina at Chapel Hill and all participants provided written, informed consent at the beginning of the study.
Participants
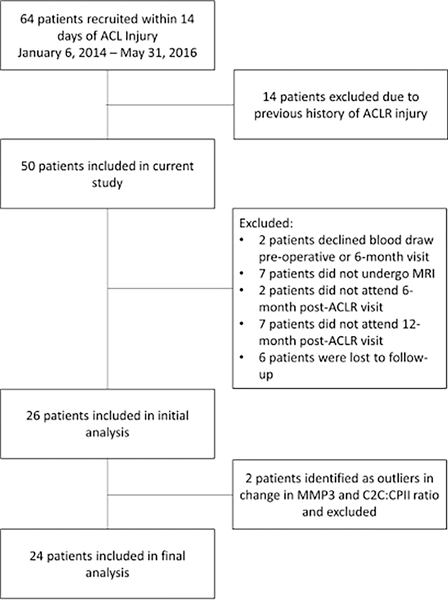
Participants were recruited from the orthopedic clinic at the University of North Carolina at Chapel Hill and treated by one of three fellowship trained orthopedic surgeons. We included individuals between the ages of 16 and 35 years old who were diagnosed at the preoperative visit with an ACL injury. As part of the prospective study, we excluded individuals who were not planning to undergo ACLR, required surgical reconstruction or repair of more than one ligament, or had previously been diagnosed with inflammatory arthritis. Participants were excluded from MRI testing if they had claustrophobia. Additionally, we excluded all individuals who were pregnant or planning on becoming pregnant over the course of 12 months. For purpose of the current analysis, we also excluded those with a previous history of ACL injury or ACLR in either limb or failed to attend both follow-up visits at 6 and 12 months post-ACLR (Figure 1). Comparisons of pre-operative characteristics between participants included and excluded from the analysis are reported in Supplementary Table 1.
Figure 1.
Strengthening the Reporting of Observational Studies in Epidemiology (STROBE)42 Flowchart of patients included in the original prospective, longitudinal cohort study and reasons for exclusion from the final analysis
Surgical Procedure and Rehabilitation
All participants underwent the same arthroscopic assisted single incision ACLR using a patellar tendon autograft procedure as previously described.25 Femoral and tibial tunnels were created by drilling into the lateral wall of the femoral intercondylar notch and drilling a pin into tibial through an infra-medial arthroscopic portal. Proximal and distal bone plugs of the patellar tendon were affixed to the femur and tibia with mental interference screws. The patellar tendon autograft was tensioned in 5° of knee flexion. Concomitant injuries were documented by the surgeon at the time of ACLR and are described in the results. After ACLR, surgeons referred participants for supervised rehabilitation by a licensed physical therapist or athletic trainer. The rehabilitation program consisted of six structured phases starting within the first week after ACLR and continuing for 6 to 9 months.27
Joint Tissue Metabolism (Collection and Processing of Serum-Biomarkers)
Blood samples were collected from the antecubital fossa of participants preoperatively and at 6 months post-ACLR. Blood samples were placed in a centrifuge 30 minutes after collection to allow for separation of serum and aliquoted into cryovials and freezer-stored at −80° C. Commercial enzyme-linked immunosorbent assays (ELISA) were used to assess for biomarker concentrations of MCP-1, MMP-3, and COMP (ng/mL; R&D Systems, Minneapolis, MN, USA) and C2C:CPII (μg/ml; IBEX Technologies, Inc. Montréal, Québec, Canada) in a batch analysis after the completion of data collection. Serum biomarkers were pre-determined for analysis inclusion due to their associations with osteoarthritis development.15,16,22 Assay detection sensitivities (mean minimal detectable dose [MDD]) were reported as: MCP-1=1.7 pg/ml, MMP-3=0.009 ng/ml, COMP=0.01 ng/ml, C2C=10 ng/ml, and CPII=35 ng/ml. Assays were performed in duplicate for standards and unknown serum samples and all assays demonstrated less than 10% inter- and intra- assay variability (Supplementary Table 2). All samples were greater than the lower limits of detection. A type-II collagen degradation to synthesis ratio type-II (C2C:CPII) was calculated from C2C and CPII concentrations in order to estimate type-II collagen turnover (collagen turnover = C2C/CPII).28 Greater concentrations of MCP-1, C2C:CPII, COMP, and MMP-3 were interpreted as greater inflammation, type-II collagen turnover, and matrix degradation, respectively.
Patient Reported Outcomes
Participants completed the Knee Injury and Osteoarthritis Outcome Score (KOOS)29 and Marx Activity Scale30 surveys preoperatively and 6 months post-ACLR to assess self-reported knee function (i.e. related to pain, symptoms, activities of daily living, sport, and quality of life) and level of physical activity, respectively. Higher scores on the KOOS and the Marx Activity Scale indicate better self-reported knee function and greater levels of activity.
Articular Cartilage Composition
MRI Acquisition
Participants were seated for 30 minutes with knees extended prior to the MRI data collection session to minimize the effect of loading on knee cartilage. MRI images were collected in the ACLR limb using a Siemens Magnetom TIM Trio 3-T scanner and a 4-channel Siemens larger flex coil (516 × 224 mm; Siemens Munich Germany) or a Siemens Magnetom Prisma 3-T Powerpack scanner with a XR 80/200 gradient coil (60 × 213 cm; Siemens). Our laboratory has previously reported excellent inter-scanner reliability of the medial (ICC2,1=0.99) and lateral (ICC2,1=0.96) tibiofemoral compartments of six knees.31 An MRI T1ρ sequence was used at a three-dimensional fast low-angle shot with a spin lock power of 500 Hz at five different spin lock durations (0, 10, 20, 30, 40 ms) and a voxel size of 0.8 mm x 0.4 mm x 3 mm (field of view=288 mm, slice sequence=3.0 mm, repetition time=9.2 ms, 160 × 320 matrix, gap=0 mm, flip angle=10°, echo-train duration time=443 ms, phase encode direction of anterior/posterior).31 The five-image sequences were used to calculate a voxel by voxel T1ρ map with an in-house Matlab program (Matlab R2014 Mathworks, Natick, MA, USA).
T1ρ Relaxation Time Quantification and Articular Cartilage Segmentation
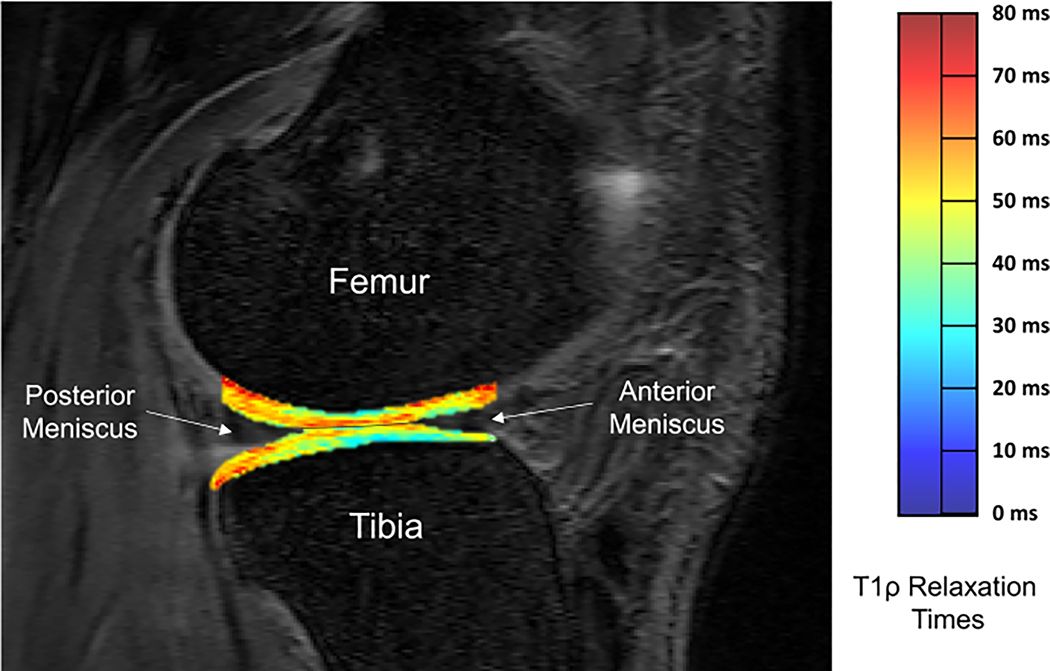
The articular cartilage from the medial and lateral condyles of the tibia and femur were manually segmented using ITK-SNAP software32 from the first image collected from the 0-ms spin-lock duration. Our laboratory has previously establish excellent intra- (ICC=0.80–0.97) and inter-segmentor reliability (ICC=0.75–0.98) for manual segmentation of medial and lateral tibial and femoral condyles.25 Anatomical accuracy of the segmentations were confirmed by a fellowship trained radiologist (DN). Weightbearing tibiofemoral regions of interest, used for analysis, were defined in the sagittal plane as articular cartilage residing between the posterior edge of the posterior horn of the meniscus and the anterior edge of the anterior horn of the meniscus, as previously described (Figure 2).25
Figure 2.
T1ρ relaxation time map of lateral tibiofemoral articular cartilage with manually segmented weight bearing regions of interest of global lateral femoral condyle and lateral tibial condyle compartments of a representative participant (Lateral Tibial Condyle T1ρ relaxation time = 48.0 ms and Lateral Femoral Condyle T1ρ relaxation time = 48.8 ms). Anterior and posterior boarders of the global regions of interest in the femur and tibia were identified based on the anterior edge of the anterior horn of the meniscus and the posterior edge of the posterior horn of the meniscus.
A customized Matlab program (MatLab R2014b (8.4.0) MathWorks, Natick, MA) was used to create voxel-by-voxel T1ρ relaxation maps (Figure 2) using the five-image sequence using the equation (TSL = duration of spin-lock time, S0= signal intensity when TSL equals 0, S = signal intensity):
The segmented image masks from the 0 ms duration spin lock were transposed over the T1ρ image to determine the T1ρ relaxation times for each region of interest. Mean T1ρ relaxation times were calculated using the ITK-SNAP software.32 Involved limb tibiofemoral articular cartilage T1ρ relaxation times were calculated for the weight bearing lateral femoral condyle (LFC), lateral tibial condyle (LTC), medial femoral condyle (MFC), and medial tibial condyle (MTC) compartments (Figure 2). Greater T1ρ relaxation times were interpreted as articular cartilage compartment consisting of lesser proteoglycan density.24
Statistical Analysis
k-Means Cluster Analysis for Determining Biochemical Profiles
A k-means cluster analysis was prespecified based on similar analyses utilized in a previous study8 and used to determine groups with similar biochemical profiles based on changes in serum-biomarker concentrations from the preoperative visit to 6 months post-ACLR. Z-scores were calculated for all serum-biomarker change scores in order to reduce the influence of differing scale magnitudes between the biomarker concentrations. The process of outlier removal was preestablished because k-means cluster analyses are sensitive to outliers.33 Therefore, participants with z-scores greater than 3 were identified as outliers and removed before the k-means cluster analysis.34 Two participants were classified as outliers based on the z-scores for change in MMP-3 or C2C:CPII ratio concentrations (Figure 1). A sensitivity analysis was performed to determine cluster groups with the inclusion of the two participants with outlier data. Using a two-means cluster analysis, a group consisting of the two participants with outlier data and another group consisting of all other 24 participants were identified. We deemed these results uninterpretable for group comparisons due to the small sample size of the cluster group that contained the outliers. Therefore, the two participants classified as outliers were removed and 24 participants were included in the final analysis. Silhouette Ranking Measure of mean silhouette coefficients were calculated and compared between a two-means cluster and three-means cluster analysis of the 24 participants with outliers removed in order to determine the optimal number of clusters used for our analysis. The silhouette coefficients were categorized as fair for both the two-means (coefficient =0.26) and three means cluster analysis (coefficient = 0.25);35 therefore, we used the two-means cluster analysis of the 24 participants to maximize the number of participants per cluster. Separate independent t-test were used to compare z-score biomarker changes between final cluster groups. Biomarker changes that were different between groups were used to characterize the profile of each cluster.
Analysis of Covariance (ANCOVA) to Compare MRI T1ρ between Biochemical Profiles
Separate ANCOVAs were also used to compare T1ρ relaxation times of the weightbearing compartments of the medial and lateral tibial and femoral condyles (i.e. global LFC, LTC, MFC, MTC) between cluster assignments. Five covariates including age36, sex37, BMI38, level of physical activity39, and concomitant injuries40 were predefined as previous research demonstrates that tibiofemoral articular cartilage health is influenced by these factors. Participants were classified as sustaining or not sustaining either a meniscal or tibiofemoral chondral injury based on data collected by a single orthopaedic surgeon (JTS) from the surgical records. The Marx Activity Scale was collected as a measure of physical activity level because it has previously been utilized as a covariate when estimating the associations between biochemical biomarkers and articular cartilage composition.8 First, all covariates were entered into the analysis simultaneously; this was followed by adding cluster assignment. We reported the change in R2 and unstandardized β’s interpreted as adjusted mean differences of tibiofemoral T1ρ relaxation times with corresponding 95% confidence intervals to compare between cluster groups and estimate associations after accounting for all covariates. Alpha was set to 0.05 a priori, and no adjustment was made for multiple testing. Silhouette Ranking Measures were performed using RStudio package “cluster” (Version 1.2.5033, RStudio, Inc., Boston MA). All other analyses were performed using the Statistical Package for the Social Sciences software (SPSS, Version 26.0, IBM Corp., Somers, NY)
Sample Size Estimation
A previous study demonstrated a moderate association (R2=0.27) between serum COMP and idiopathic knee osteoarthritis progression.41 Therefore, we estimated a priori that a sample size of 24 participants would be needed to detect a two-tailed statistically significant moderate association (R2=0.27) between cluster groups and T1ρ relaxation times (α=0.05, β=0.80).
RESULTS
Demographics for all participants are reported in Table 1.
Table 1.
Participant Characteristic Comparisons Between Cluster Groups
| Participant Characteristics | All participants (n=24) | Increases in Inflammation and Cartilage Degradation Group (n=11) | Decreases in Inflammation and Cartilage Degradation Group (n=13) | p-value |
|---|---|---|---|---|
| Sex (% Female) | 46% | 55.0% | 38.0% | 0.68 |
| Age (years)* | 22.1±4.2 | 23.4±5.5 | 21.1±2.6 | 0.19 |
| BMI (kg/m2)* | 24.0±2.6 | 24.9±2.3 | 23.2±2.6 | 0.10 |
| Concomitant meniscal/chondral injury* | 88% | 92% | 85% | 1.00 |
| PROs at Preoperative Visit | ||||
| Marx Activity Scale | 8.7±7.2 | 10.5±7.1 | 7.4±7.3 | 0.36 |
| KOOS Pain | 64.7±19.5 | 64.8±22.2 | 64.7±17.6 | 0.99 |
| KOOS Symptoms | 51.7±16.1 | 48.3±13.0 | 54.8±18.5 | 0.35 |
| KOOS ADL | 71.0±17.7 | 68.91±16.15 | 72.92±19.5 | 0.60 |
| KOOS Sport | 27.5±30.2 | 19.1±24.8 | 35.3±33.7 | 0.21 |
| KOOS QOL | 32.8±22.3 | 32.0±20.0 | 33.5±25.2 | 0.88 |
| PROs at 6 Months Post-ACLR | ||||
| Marx Activity Scale | 9.3±4.47 | 10±3.5 | 8.6±5.4 | 0.45 |
| KOOS Pain | 84.5±13.7 | 84.2±17.1 | 84.8±9.1 | 0.91 |
| KOOS Symptoms | 78.1±16.6 | 79.3±19.6 | 78.2±13.2 | 0.87 |
| KOOS ADL | 94±12.6 | 91.5±16.6 | 97.0±3.9 | 0.29 |
| KOOS Sport | 63.1±21.3 | 65.0±26.1 | 60.9±14.5 | 0.65 |
| KOOS QOL | 56.8±17.0 | 58.2±18.4 | 55.2±15.7 | 0.67 |
| PROs at 12 Months Post-ACLR | ||||
| Marx Activity Scale | 12.9±3.4 | 13.7±2.1 | 12.4±4.0 | 0.42 |
| KOOS Pain | 92.5±6.6 | 92.6±6.0 | 92.4±7.3 | 0.94 |
| KOOS Symptoms | 86.8±10.6 | 84.5±10.1 | 88.8±10.9 | 0.33 |
| KOOS ADL | 97.8±2.8 | 98.6±2.1 | 97.2±3.2 | 0.20 |
| KOOS Sport | 86.0±14.0 | 86.4±11.0 | 85.8±16.7 | 0.92 |
| KOOS QOL | 78.2±16.0 | 76.8±16.0 | 79.5±16.5 | 0.69 |
Abbreviations: kg = kilograms, m = meters, PROs = patient reported outcomes, KOOS = Knee Injury and Osteoarthritis Outcome Score, ADL = activities of daily living, QOL = quality of life
k-Means Cluster Profile Groups
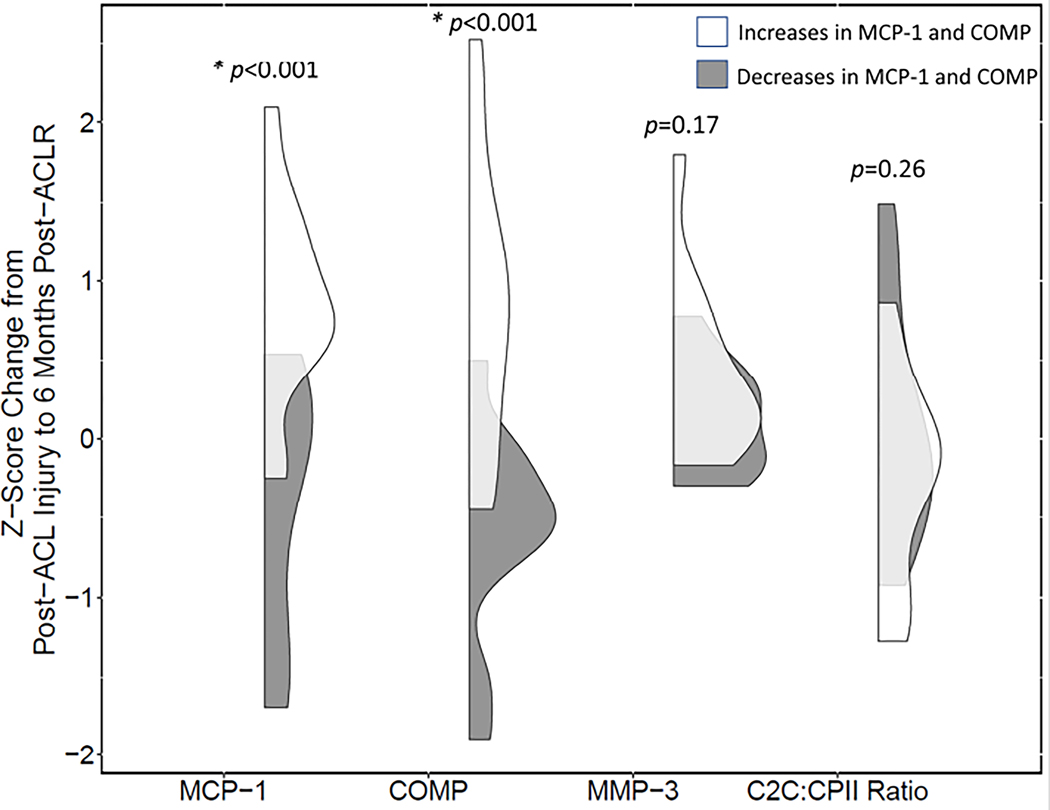
The biochemical profile of the first cluster group (n=11) included increases in MCP-1 (p<0.001) and COMP (p<0.001) compared to the second cluster group (n=13) (Figure 3). There were no statistically significant differences in MMP-3 (p=0.17) or C2C:CPII ratio (p=0.26) between clusters. There were no statistically significant differences in demographics between cluster groups (Table 1).
Figure 3.
Data distributions between two cluster profile groups for all serum biomarker outcomes. Shaded gray = cluster group profile with decreased inflammation and cartilage degradation; White = cluster group with increased inflammation and cartilage degradation; * = differences in z-scores between cluster profile groups (p<0.001)
Articular Cartilage Composition Associations With k-Means Cluster Profile Groups
Individuals assigned to the first cluster with increased MCP-1 and COMP demonstrated greater lateral femoral (adjusted mean difference=12.71, ΔR2=0.18, p=0.04) and lateral tibial (adjusted mean difference=3.88, ΔR2=0.40, p=0.001) condyle T1ρ relaxation times (Table 2) after accounting for age, sex, BMI, concomitant injury or procedure, and Marx Activity Scale (Table 3). There were no statistically significant differences between k-means cluster profile groups and involved limb T1ρ relaxation times in the medial tibial (adjusted mean difference=1.11, ΔR2=0.04, p=0.33) and medial femoral (adjusted mean difference=1.85, ΔR2=0.02, p=0.58) condyle T1ρ relaxation times after accounting for all covariates (Table 2). Adjusted and unadjusted β coefficients with 95% confidence intervals of T1ρ relaxation times for covariates and cluster group profiles are reported in Table 3 and Supplementary Table 3, respectively.
Table 2.
Associations between Tibiofemoral T1ρ Outcomes with Cluster Group, after controlling for age, sex, and BMI
| Tibiofemoral T1ρ Outcomes (ms) | Predictor Variables | Total R2 | ΔR2 | ΔR2 p-value |
|---|---|---|---|---|
| Lateral Femoral Condyle (ms) | Covariates | 0.24 | ||
| Cluster Group | 0.41 | 0.18 | 0.04* | |
| Lateral Tibial Condyle (ms) | Covariates | 0.25 | ||
| Cluster Group | 0.65 | 0.40 | 0.001* | |
| Lateral Tibial Condyle (ms) | Covariates | 0.25 | ||
| Cluster Group | 0.65 | 0.40 | 0.001* | |
| Medial Femoral Condyle (ms) | Covariates | 0.33 | ||
| Cluster Group | 0.37 | 0.04 | 0.33 | |
| Medial Tibia Condyle (ms) | Covariates | 0.24 | ||
| Cluster Group | 0.25 | 0.02 |
p<.05 from F-statistic testing Change in R2 at p<0.05
Table 3.
Adjusted β coefficients and 95% confidence intervals of T1ρ relaxation times for covariates and cluster group profiles
| Lateral Tibia | Lateral Femur | |||
|---|---|---|---|---|
| Predictor | β (95% CI) | p-value | β (95% CI) | p-value |
|
| ||||
| Age (years) | 0.15 (−0.06 to 0.37) | 0.15 | −0.11 (−1.42 to 1.21) | 0.86 |
| Sex† | 0.59 (−1.52 to 2.70) | 0.56 | 4.99 (−7.93 to 17.89) | 0.43 |
| BMI (kg/m2) | 0.07 (−0.34 to 0.48) | 0.73 | 2.48 (−0.03 to 5.00) | 0.05 |
| Concomitant Injury‡ | 1.57 (−1.52 to 4.66) | 0.30 | 5.02 (−13.93 to 23.97) | 0.58 |
| Marx Scale | 0.19 (−0.01 to 0.38) | 0.07 | 0.73 (−0.49 to 1.95) | 0.22 |
| Cluster Group § | 3.88 (1.97 to 5.78) | 0.001* | 12.71 (0.41 to 23.81) | 0.04* |
|
| ||||
| Medial Tibia | Medial Femur | |||
| Predictor | β (95% CI) | p-value | β (95% CI) | p-value |
|
| ||||
| Age (years) | 0.21 (−0.27 to 0.68) | 0.37 | 0.13 (−0.30 to 0.57) | 0.52 |
| Sex† | 1.31 (−3.32 to 5.94) | 0.56 | 0.99 (−3.27 to 5.25) | 0.63 |
| BMI (kg/m2) | -0.21 (−1.11 to 0.70) | 0.64 | -0.33 (−1.16 to 0.50) | 0.41 |
| Concomitant Injury‡ | 4.71 (−2.09 to 11.50 | 0.16 | 4.78 (−1.47 to 11.04) | 0.12 |
| Marx Scale | 0.24 (−0.20 to 0.68) | 0.26 | 0.30 (−0.11 to 0.70) | 0.14 |
| Cluster Group§ | 1.11 (−3.10 to 5.26) | 0.58 | 1.85 (−2.02 to 5.71) | 0.33 |
indicates associations where p<0.05
positive β indicates higher mean relaxation times for females
positive β indicates higher mean relaxation times for individuals with concomitant meniscal or chondral injury or pathology
positive β indicates higher mean relaxation times for individuals in the cluster group with increases in inflammation and cartilage degradation
DISCUSSION
In this prospective, longitudinal cohort study, we identified two biochemical profiles based on changes in four different serum biomarkers between a preoperative timepoint and 6 months post-ACLR. Specifically, the first cluster group exhibited a biochemical profile characterized by increases in MCP-1 and COMP between the preoperative timepoint and 6 months post-ACLR while the second cluster demonstrated decreases in MCP-1 and COMP. No differences were found for changes in MMP-3 and C2C:CPII ratio between biochemical cluster groups. Changes in C2C:CPII ratio may not occur until 12 months post-ACLR.19 Greater concentrations of serum MCP-1 and COMP have been independently associated with worsening idiopathic OA16,22 but have not been well explored in patient populations at high-risk for PTOA (i.e. ACL injury and ACLR). In support of our hypotheses, the first cluster group exhibiting increases in MCP-1 and COMP between the preoperative and 6 month post-ACLR timepoints demonstrated greater T1ρ relaxation times (i.e. lesser proteoglycan density) in the lateral tibiofemoral compartment at 12 months post-ACLR after controlling for age, sex, BMI, and the Marx Activity Scale as well as presence of any concomitant meniscal or chondral injury. The identification of biochemical profiles associated with early worsening tibiofemoral articular cartilage composition may aid clinical detection of individuals at elevated risk for PTOA onset and improve therapeutic targets for pharmacological and non-pharmacological interventions for PTOA prevention preoperatively and post-ACLR.
Previous work has demonstrated that profiles exhibiting greater synovial biomarker concentrations of sulfated glycosaminoglycan (i.e. sGAG) on average nine weeks following ACL injury but prior to ACLR predicted worse medial tibial cartilage composition sensitive to decreases in proteoglycan density (i.e. greater T1ρ relaxation times) and type-II collagen disorientation (i.e. greater T2 relaxation times) one to three years post-ACLR.8 Therefore, greater synovial biomarker concentrations of sGAG, which is released into synovial fluid with cartilage degeneration8 associates with later in vivo MRI measures of decreased proteoglycan density (i.e. greater T1ρ relaxation times) and diminished type-II collagen orientation (i.e. greater T2 relaxation times).24,42 Our current study builds upon the results of Amano et al. 8 by demonstrating associations between increases in serum-biomarkers of inflammation and matrix degradation within the first 6 months post-ACLR and in vivo MRI measures of decreased proteoglycan density 12 months post-ACLR. Amano et al.8 reported that patients with lower preoperative inflammatory synovial fluid biomarker profiles demonstrated worse medial cartilage composition. In comparison, participants in our study had similar MCP-1 biomarker concentrations preoperatively (Supplementary Figure 1), but participants with profiles of increasing inflammation demonstrated worse lateral cartilage composition. Our current study suggests that early prolonged or increased inflammation and matrix degradation between the preoperative and 6 month post-ACLR timepoints may be potential biological mechanisms contributing to deleterious changes in lateral tibiofemoral articular cartilage composition following ACL injury, specifically. We also identified associations between biochemical profiles and cartilage composition at an earlier and more defined time point of 12 months post-ACLR (370.0±10.0 days) compared to cartilage composition across a 3-year time period post-ACLR8 suggesting that biochemical changes may be associated with early changes in cartilage composition occurring within the first year after surgery. Our study only utilized serum biomarkers limiting our ability to make conclusions regarding tibiofemoral joint site-specific responses that can be made with synovial fluid biomarkers. Serum biomarkers may reflect systemic metabolic processes occurring outside of the involved knee. Regardless, synovial fluid aspirations may be less accessible for serial assessment following ACLR43 than venous blood draws used to collect serum.
Within 12 months post-ACLR, compositional changes occur in the medial and lateral tibiofemoral compartments.26 We demonstrated that biochemical profiles with increased MCP-1 and COMP had higher T1ρ relaxation times in the lateral tibiofemoral cartilage, which differs from previous work that demonstrated biochemical biomarker associations in the medial tibial compartment.8 There were notable associations between greater Marx Activity Scores and greater BMI with greater femoral cartilage T1ρ relaxation times. Relationships between activity level and BMI with cartilage composition were expected44,45 and controlled for in analyses. Previous work has also demonstrated that higher T1ρ relaxation times in the lateral femoral articular cartilage associated with worse patient reported outcomes 12 months post-ACLR.25 Additionally, traumatic bone marrow edema-like lesions that occur at the time of ACL injury are known to be most common in the lateral tibiofemoral compartment.46 We can speculate that lateral tibiofemoral traumatic bone marrow edema-like lesions may increase the susceptibility of or reflect early catabolic changes to the articular cartilage in the lateral tibiofemoral compartment.47 Furthermore, patients with large bone marrow edema-like lesions post-ACL injury demonstrated a 74% chance of developing worsening chondral damage five years post-ACLR.46 Nearly two-thirds of the patients in this study presented with concomitant meniscal and/or articular cartilage injuries in the lateral tibiofemoral compartment at the time of ACLR. The percentage of participants with meniscal injury, tibiofemoral chondral lesions, and bone bruises are reported in Supplementary Table 4. Most notably, 82–100% of the participants with profiles demonstrating increases in inflammation and cartilage degradation sustained lateral tibiofemoral bone bruises identified based on criteria from the Whole-Organ Magnetic Resonance Imaging Score48 by a radiologist (DN) compared to 69–85% of the participants in the other cluster group. Lateral concomitant injury in our cohort may contribute to the association found between biochemical profiles and T1ρ relaxation times lateral tibiofemoral compartment within the first 12 months post-ACLR and should be explored in future studies.
The current study demonstrates novel findings regarding biochemical changes and tibiofemoral articular cartilage composition after ACL injury and ACLR, but some limitations should be acknowledged to inform future research. Based on our relatively modest sample size we chose to evaluate two clusters which demonstrated fair cohesion. Additionally, biochemical profiles developed in the current study did not incorporate as many individual biomarkers as previous studies assessing the relationships between joint tissue metabolism and articular cartilage composition.8 Future studies with a larger group of participants and a broader assessment of joint tissue metabolism from serum biomarkers will be important for determining more robust biochemical profiles for predicting cartilage health. Baseline serum biomarkers were collected from participants excluded from the analysis. Excluded participants demonstrated higher serum MMP-3 concentrations compared to participants included in the analysis (Supplementary Table 1). While it is not clear how this may affect MMP-3 changes, future studies may consider including serum MMP-3 as a biomarker of interest. The current study only assessed tibiofemoral T1ρ relaxations at a single time point (12 months post-ACLR). Without pre-operative imaging, the associations between serum biomarkers and changes in tibiofemoral cartilage composition cannot be determined. It is possible that lateral tibiofemoral T1ρ relaxation times were elevated preoperatively or even prior to ACL injury. Future studies should include multiple MRI assessments at preoperative and post-ACLR time points to identify the effects of serum biomarker changes on changes in tibiofemoral cartilage composition.
In conclusion, our results suggest that profiles exhibiting increased concentrations of serum biomarkers of inflammation and matrix degradation in the first 6 months following ACL injury and ACLR are likely to exhibit poorer lateral tibiofemoral articular cartilage health at 12 months following ACLR. The current study serves as a hypothesis generating analysis to identify serum biochemical profiles that associate with potentially early deleterious changes in articular cartilage matrix composition.
Supplementary Material
Supplemental Figure 1. Serum biomarker concentrations between groups from the pre-operative visit to 6 months post-ACLR. Gray Circle = cluster group profile with decreased inflammation and cartilage degradation; Black Triangle = cluster group with increased inflammation and cartilage degradation; Units MCP-1 = pg/ml, MMP-3 = ng/ml, COMP = ng/ml, C2C:CPII = ng/ml ratio.
Acknowledgments
Role of the funding sources: Research reported in this manuscript was supported by funding from the National Institute of Arthritis and Musculoskeletal and Skin Disease of the National Institutes of Health (1R03 AR066840-01A1 and P30 AR072580), North Carolina Translational and Clinical Sciences (TraCS) Institute, and National Athletic Trainers Association Research and Education Foundation (14NewINV001). The study sponsors had no role in the study design, collection, analysis and interpretation of data, in the writing of the manuscript, or in the decision to submit the manuscript for publication.
Footnotes
Competing Interest Statement: The authors declare that they have no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Luc B, Gribble PA, Pietrosimone BG. Osteoarthritis prevalence following anterior cruciate ligament reconstruction: a systematic review and numbers-needed-to-treat analysis. J Athl Train. 2014;49(6):806–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hunt ER, Jacobs CA, Conley CE, Ireland ML, Johnson DL, Lattermann C. Anterior cruciate ligament reconstruction reinitiates an inflammatory and chondrodegenerative process in the knee joint. J Orthop Res. 2021;39(6):1281–1288. [DOI] [PubMed] [Google Scholar]
- 3.Struglics A, Larsson S, Pramhed A, Frobell R, Swärd P. Changes in synovial fluid and serum concentrations of cartilage oligomeric matrix protein over 5 years after anterior cruciate ligament rupture: an exploratory analysis in the KANON trial. Osteoarthritis Cartilage. 2018;26(10):1351–1358. [DOI] [PubMed] [Google Scholar]
- 4.Carlson CS, Guilak F, Vail TP, Gardin JF, Kraus VB. Synovial fluid biomarker levels predict articular cartilage damage following complete medial meniscectomy in the canine knee. J Orthop Res. 2002;20(1):92–100. [DOI] [PubMed] [Google Scholar]
- 5.Tao H, Hu Y, Qiao Y, Xie Y, Chen T, Chen S. Alternations of metabolic profiles in synovial fluids and the correlation with T2 relaxation times of cartilage and meniscus-A study on anterior cruciate ligament- (ACL-) injured rabbit knees at early stage. Biomed Res Int. 2019;2019:8491301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lindhorst E, Wachsmuth L, Kimmig N, et al. Increase in degraded collagen type II in synovial fluid early in the rabbit meniscectomy model of osteoarthritis. Osteoarthritis Cartilage. 2005;13(2):139–145. [DOI] [PubMed] [Google Scholar]
- 7.Neuman P, Dahlberg LE, Englund M, Struglics A. Concentrations of synovial fluid biomarkers and the prediction of knee osteoarthritis 16 years after anterior cruciate ligament injury. Osteoarthritis Cartilage. 2017;25(4):492–498. [DOI] [PubMed] [Google Scholar]
- 8.Amano K, Huebner JL, Stabler TV, et al. Synovial fluid profile at the time of anterior cruciate ligament reconstruction and its association with cartilage matrix composition 3 years after surgery. Am J Sports Med. 2018;46(4):890–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kraus VB, Collins JE, Hargrove D, et al. Predictive validity of biochemical biomarkers in knee osteoarthritis: data from the FNIH OA Biomarkers Consortium. Ann Rheum Dis. 2017;76(1):186–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Catterall JB, Stabler TV, Flannery CR, Kraus VB. Changes in serum and synovial fluid biomarkers after acute injury (NCT00332254). Arthritis Res Ther. 2010;12(6):R229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.King JD, Rowland G, Villasante Tezanos AG, et al. Joint fluid proteome after anterior cruciate ligament rupture reflects an acute posttraumatic inflammatory and chondrodegenerative state. Cartilage. 2020;11(3):329–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Loeser RF, Goldring SR, Scanzello CR, Goldring MB. Osteoarthritis: a disease of the joint as an organ. Arthritis Rheum. 2012;64(6):1697–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Viedt C, Vogel J, Athanasiou T, et al. Monocyte chemoattractant protein-1 induces proliferation and interleukin-6 production in human smooth muscle cells by differential activation of nuclear factor-kappaB and activator protein-1. Arterioscler Thromb Vasc Biol. 2002;22(6):914–920. [DOI] [PubMed] [Google Scholar]
- 14.Deshmane SL, Kremlev S, Amini S, Sawaya BE. Monocyte chemoattractant protein-1 (MCP-1): an overview. J Interferon Cytokine Res. 2009;29(6):313–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ni F, Zhang Y, Peng X, Li J. Correlation between osteoarthritis and monocyte chemotactic protein-1 expression: a meta-analysis. J Orthop Surg Res. 2020;15(1):516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Longobardi L, Jordan JM, Shi XA, et al. Associations between the chemokine biomarker CCL2 and knee osteoarthritis outcomes: the Johnston County Osteoarthritis Project. Osteoarthritis Cartilage. 2018;26(9):1257–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chu XQ, Wang JJ, Dou LD, Zhao G. Cartilage oligomeric matrix protein and matrix metalloproteinase-3 expression in the serum and joint fluid of a reversible osteoarthritis rabbit model. Genet Mol Res. 2015;14(4):14207–14215. [DOI] [PubMed] [Google Scholar]
- 18.Wojdasiewicz P, Poniatowski ŁA, Szukiewicz D. The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Mediators Inflamm. 2014;2014:561459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Longobardi L, Davis-Wilson H, Pietrosimone B, et al. Longitudinal analysis of serum biochemical changes following anterior cruciate ligament injury and reconstruction: A matched comparison-control analysis. Orthop J Sports Med. 2020;8(7 suppl6):2325967120S2325900354. [Google Scholar]
- 20.Tseng S, Reddi AH, Di Cesare PE. Cartilage Oligomeric Matrix Protein (COMP): A Biomarker of Arthritis. Biomark Insights. 2009;4:33–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Palmieri-Smith RM, Wojtys EM, Potter HG. Early cartilage changes after anterior cruciate ligament injury: Evaluation with imaging and serum biomarkers-A pilot study. Arthroscopy. 2016;32(7):1309–1318. [DOI] [PubMed] [Google Scholar]
- 22.Kluzek S, Bay-Jensen A-C, Judge A, et al. Serum cartilage oligomeric matrix protein and development of radiographic and painful knee osteoarthritis. A community-based cohort of middle-aged women. Biomarkers. 2015;20(8):557–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li X, Pai A, Blumenkrantz G, et al. Spatial distribution and relationship of T1rho and T2 relaxation times in knee cartilage with osteoarthritis. Magn Res Med. 2009;61(6):1310–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wong CS, Yan CH, Gong NJ, Li T, Chan Q, Chu YC. Imaging biomarker with T1ρ and T2 mappings in osteoarthritis - in vivo human articular cartilage study. Eur J Radiol. 2013;82(4):647–650. [DOI] [PubMed] [Google Scholar]
- 25.Pietrosimone B, Nissman D, Padua DA, et al. Associations between cartilage proteoglycan density and patient outcomes 12months following anterior cruciate ligament reconstruction. Knee. 2018;25(1):118–129. [DOI] [PubMed] [Google Scholar]
- 26.Li X, Kuo D, Theologis A, et al. Cartilage in anterior cruciate ligament-reconstructed knees: MR imaging T1{rho} and T2--initial experience with 1-year follow-up. Radiology. 2011;258(2):505–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Adams D, Logerstedt D, Hunter-Giordano A, Axe MJ, Snyder-Mackler L. Current concepts for anterior cruciate ligament reconstruction: A criterion-based rehabilitation progression. J Orthop Sports Phy Ther. 2012;42(7):601–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pietrosimone B, Blackburn JT, Harkey MS, et al. Greater mechanical loading during walking is associated with less collagen turnover in individuals with anterior cruciate ligament reconstruction. Am J Sports Med. 2016;44(2):425–432. [DOI] [PubMed] [Google Scholar]
- 29.Wang D, Jones MH, Khair MM, Miniaci A. Patient-reported outcome measures for the knee. J Knee Surg. 2010;23(3):137–151. [DOI] [PubMed] [Google Scholar]
- 30.Marx RG, Stump TJ, Jones EC, Wickiewicz TL, Warren RF. Development and evaluation of an activity rating scale for disorders of the knee. Am J Sports Med. 2001;29(2):213–218. [DOI] [PubMed] [Google Scholar]
- 31.Pfeiffer SJ, Spang J, Nissman D, et al. Gait mechanics and T1rho MRI of tibiofemoral cartilage 6 months post ACL reconstruction. Med Sci Sports Exerc. 2019;51(4):630–639. [DOI] [PubMed] [Google Scholar]
- 32.Yushkevich PA, Piven J, Hazlett HC, et al. User-guided 3D active contour segmentation of anatomical structures: Significantly improved efficiency and reliability. NeuroImage. 2006;31(3):1116–1128. [DOI] [PubMed] [Google Scholar]
- 33.Kaufman L, Rousseeuw PJ. Finding groups in data: an introduction to cluster analysis. Vol 344: John Wiley & Sons; 2009. [Google Scholar]
- 34.Iglewicz B, Hoaglin DC. How to detect and handle outliers. Vol 16: Asq Press; 1993. [Google Scholar]
- 35.Wendler T, Gröttrup S. Cluster Analysis. In: Wendler T, Gröttrup S, eds. Data Mining with SPSS Modeler: Theory, Exercises and Solutions. Cham: Springer International Publishing; 2016:587–712. [Google Scholar]
- 36.Goto H, Iwama Y, Fujii M, et al. The natural degeneration course in the T1rho values of normal knee cartilage. Kobe J Med Sci. 2012;57(4):E155–170. [PubMed] [Google Scholar]
- 37.Kumar D, Souza RB, Subburaj K, et al. Are there sex differences in knee cartilage composition and walking mechanics in healthy and osteoarthritis populations? Clin Orthop Relat Res. 2015;473(8):2548–2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Patterson BE, Culvenor AG, Barton CJ, et al. Worsening knee osteoarthritis features on magnetic resonance imaging 1 to 5 years after anterior cruciate ligament reconstruction. Am J Sports Med. 2018:363546518789685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kumar D, Souza RB, Singh J, et al. Physical activity and spatial differences in medial knee T1rho and t2 relaxation times in knee osteoarthritis. J Orthop Sports Phys Ther. 2014;44(12):964–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Claes S, Hermie L, Verdonk R, Bellemans J, Verdonk P. Is osteoarthritis an inevitable consequence of anterior cruciate ligament reconstruction? A meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2013;21(9):1967–1976. [DOI] [PubMed] [Google Scholar]
- 41.Vilím V, Olejárová M, Machácek S, Gatterová J, Kraus VB, Pavelka K. Serum levels of cartilage oligomeric matrix protein (COMP) correlate with radiographic progression of knee osteoarthritis. Osteoarthritis Cartilage. 2002;10(9):707–713. [DOI] [PubMed] [Google Scholar]
- 42.Li X, Benjamin Ma C, Link TM, et al. In vivo T(1rho) and T(2) mapping of articular cartilage in osteoarthritis of the knee using 3 T MRI. Osteoarthritis Cartilage. 2007;15(7):789–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hyldahl RD, Evans A, Kwon S, et al. Running decreases knee intra-articular cytokine and cartilage oligomeric matrix concentrations: a pilot study. Eur J Appl Physiol. 2016;116(11–12):2305–2314. [DOI] [PubMed] [Google Scholar]
- 44.Su F, Pedoia V, Teng HL, et al. The association between MR T1ρ and T2 of cartilage and patient-reported outcomes after ACL injury and reconstruction. Osteoarthritis Cartilage. 2016;24(7):1180–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Collins AT, Kulvaranon ML, Cutcliffe HC, et al. Obesity alters the in vivo mechanical response and biochemical properties of cartilage as measured by MRI. Arthritis Res Ther. 2018;20(1):232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Filardo G, Andriolo L, di Laura Frattura G, Napoli F, Zaffagnini S, Candrian C. Bone bruise in anterior cruciate ligament rupture entails a more severe joint damage affecting joint degenerative progression. Knee Surg Sports Traumatol Arthrosc. 2019;27(1):44–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tiderius CJ, Olsson LE, Nyquist F, Dahlberg L. Cartilage glycosaminoglycan loss in the acute phase after an anterior cruciate ligament injury: delayed gadolinium-enhanced magnetic resonance imaging of cartilage and synovial fluid analysis. Arthritis Rheum. 2005;52(1):120–127. [DOI] [PubMed] [Google Scholar]
- 48.Peterfy CG, Guermazi A, Zaim S, et al. Whole-Organ Magnetic Resonance Imaging Score (WORMS) of the knee in osteoarthritis. Osteoarthritis Cartilage. 2004;12(3):177–190. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Serum biomarker concentrations between groups from the pre-operative visit to 6 months post-ACLR. Gray Circle = cluster group profile with decreased inflammation and cartilage degradation; Black Triangle = cluster group with increased inflammation and cartilage degradation; Units MCP-1 = pg/ml, MMP-3 = ng/ml, COMP = ng/ml, C2C:CPII = ng/ml ratio.