Abstract
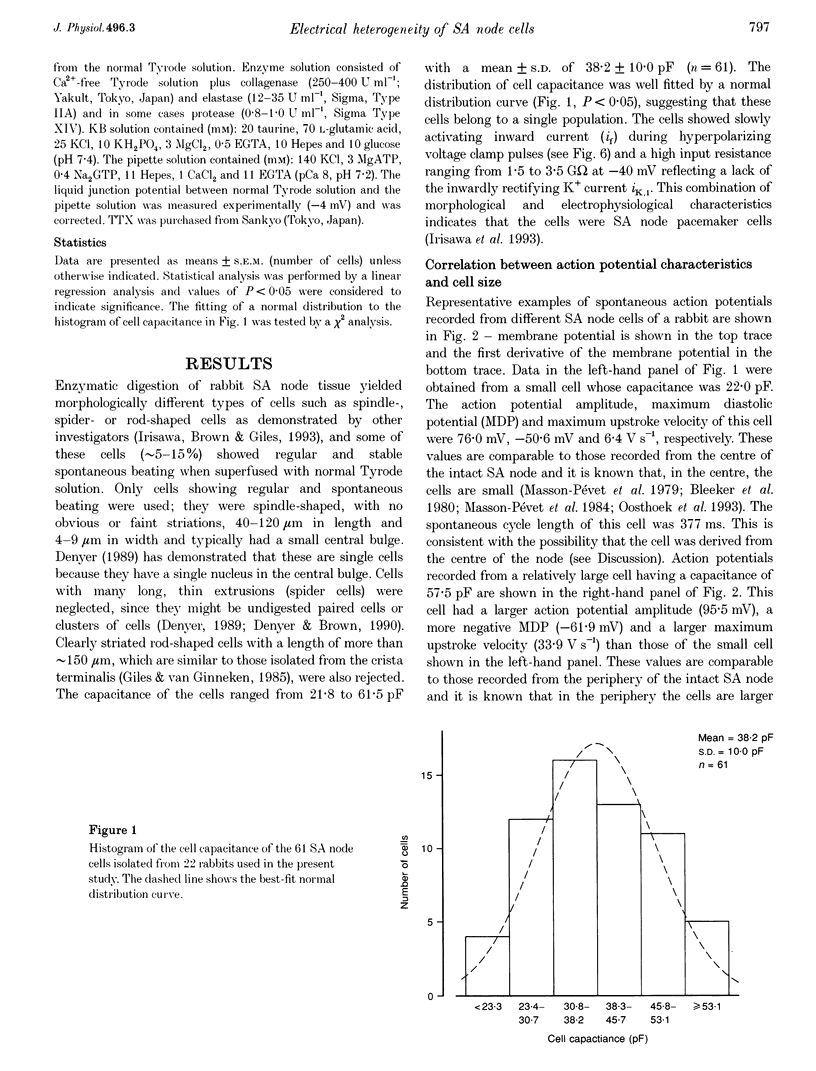
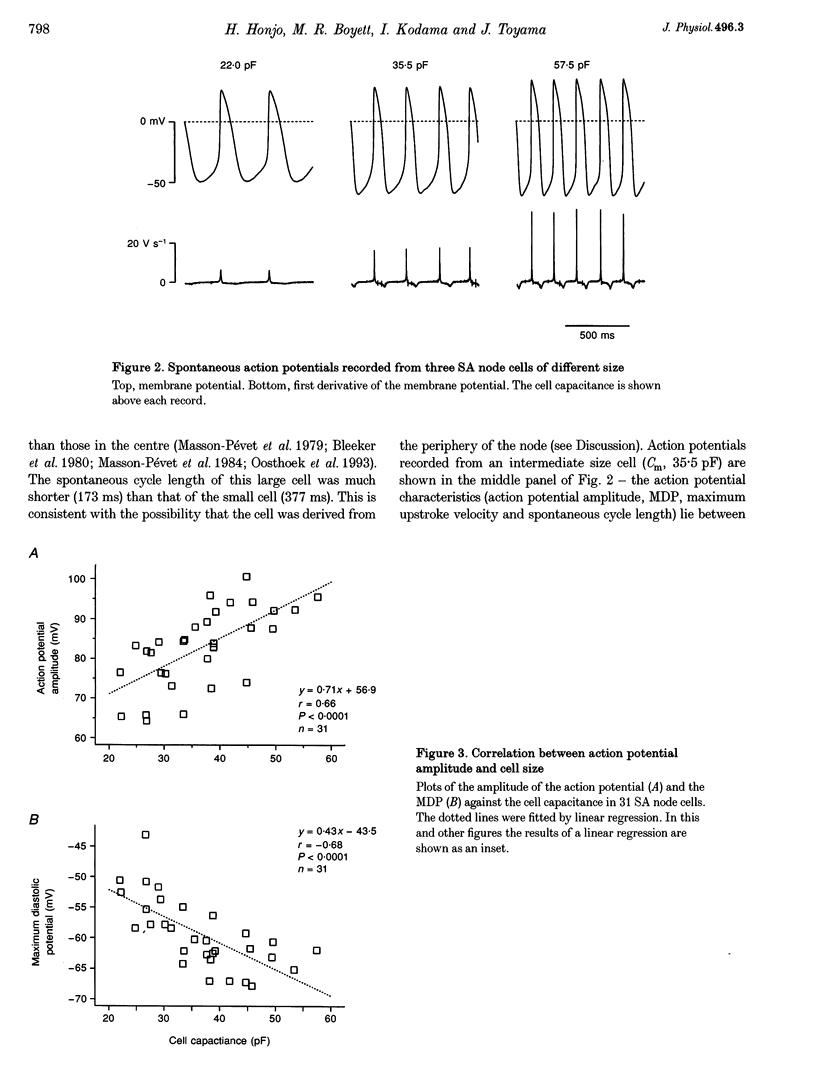
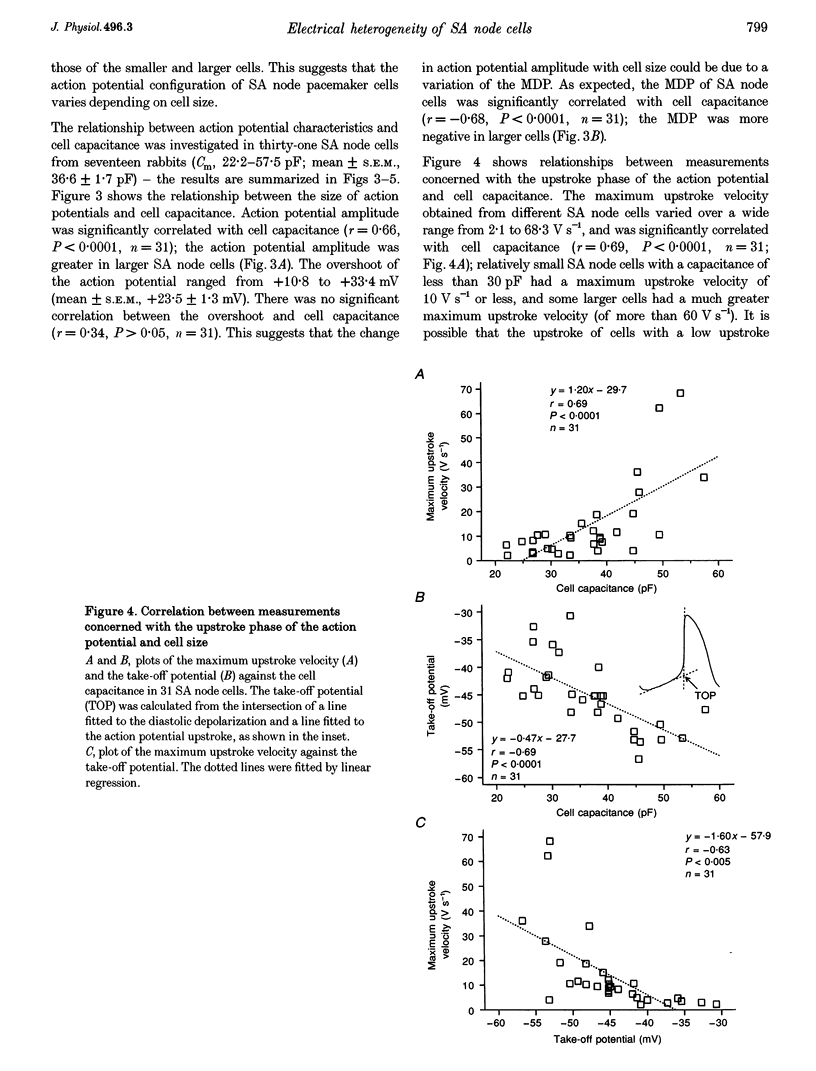
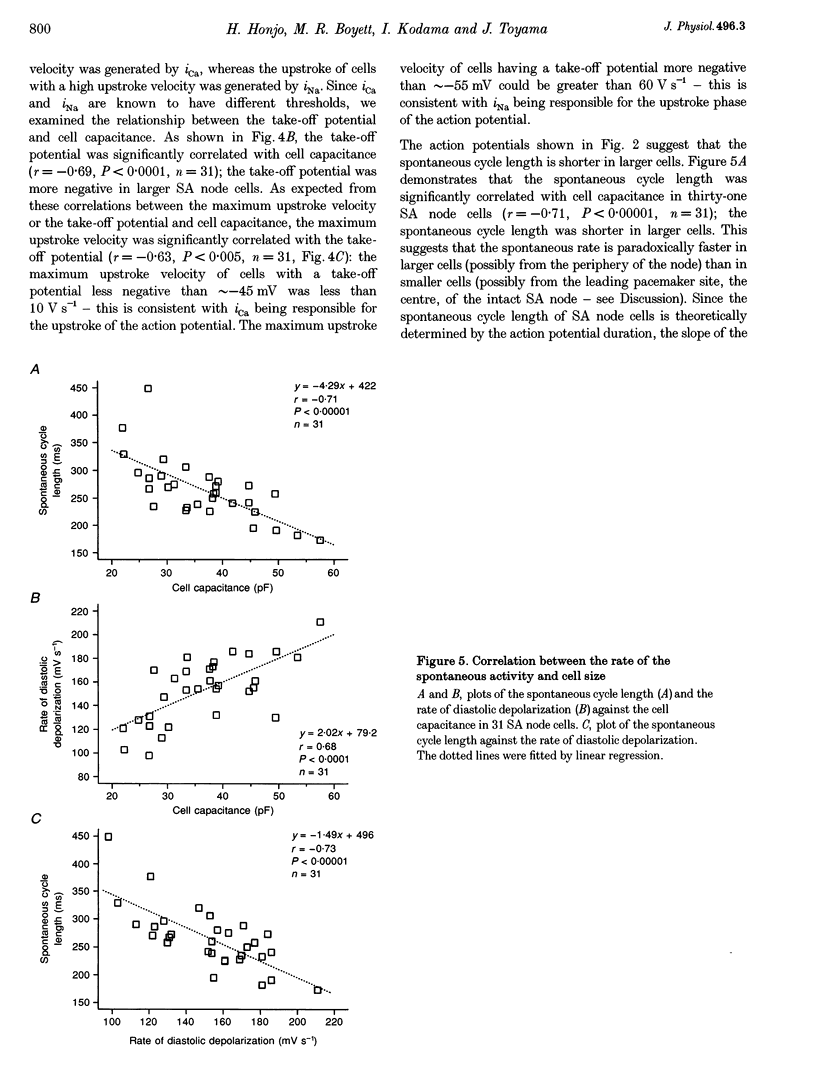
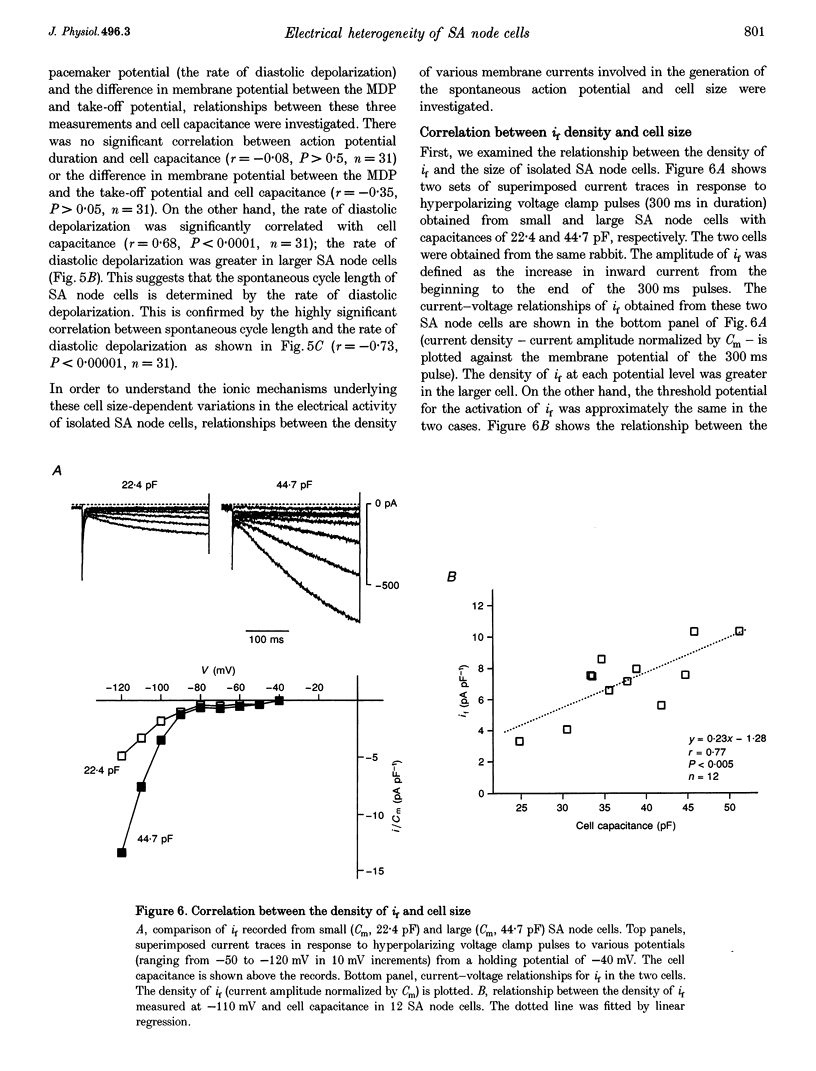
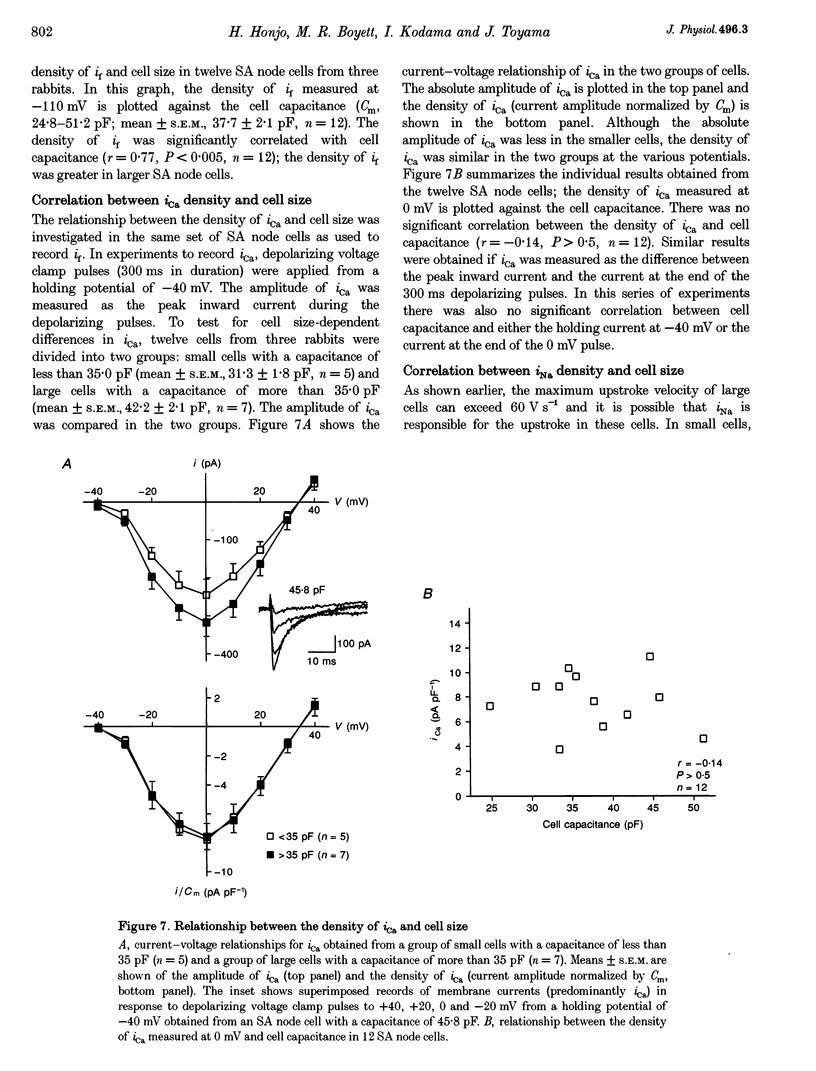
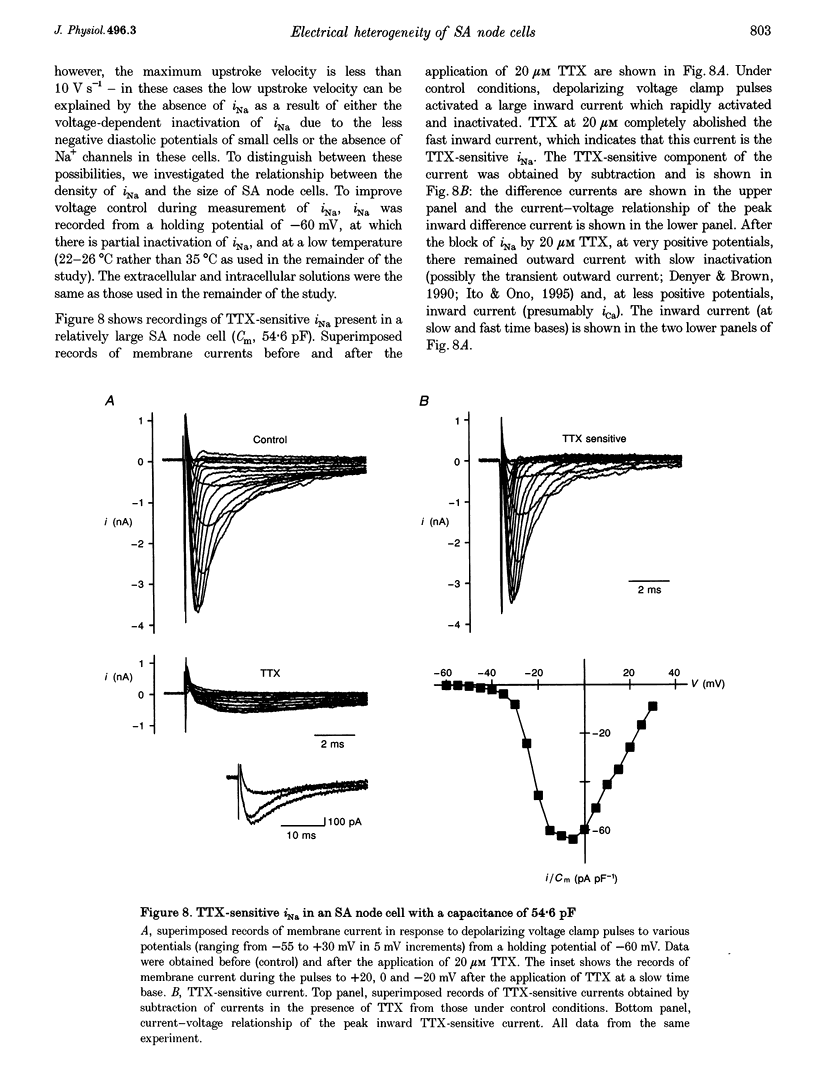
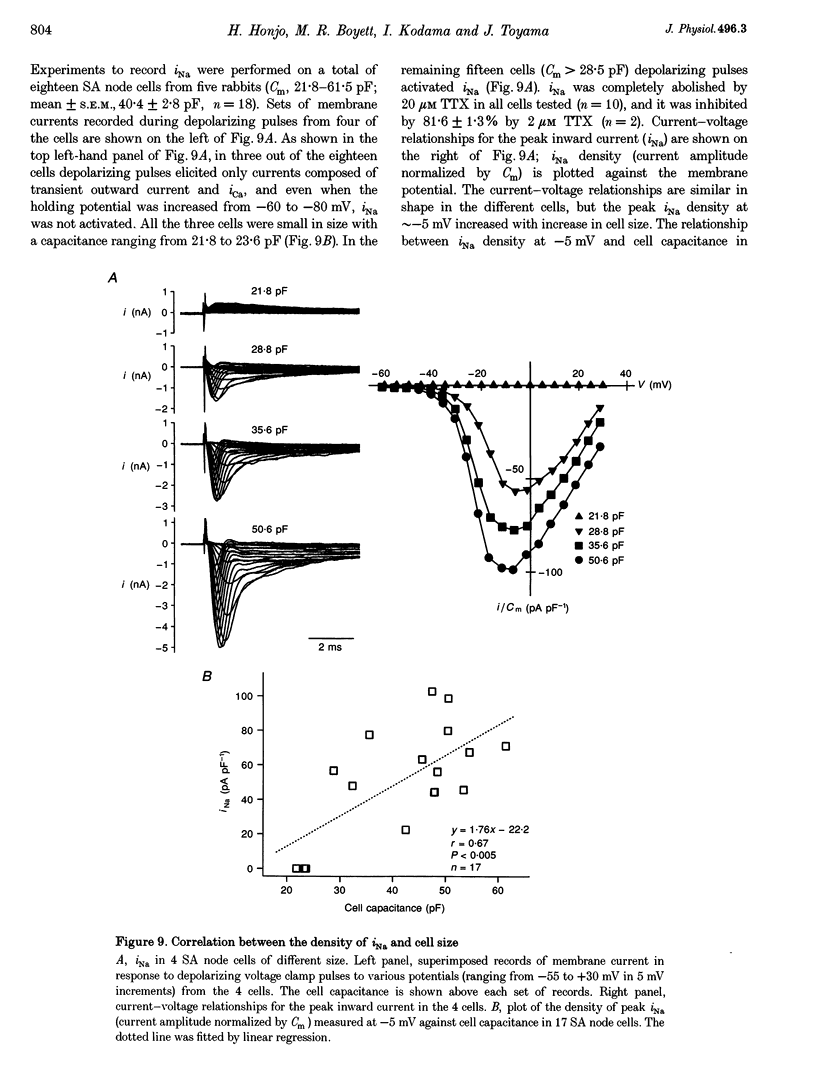
1. Single cells were isolated from rabbit sino-atrial (SA) node by enzymatic dissociation. Spontaneous action potentials and membrane currents were recorded using the whole-cell patch clamp technique to study the relationship between electrical activity and the size of the cells. 2. The size of SA node cells was estimated by measuring the cell capacitance. The cell capacitance of SA node cells ranged from 21.8 to 61.5 pF with a mean +/- S.E.M. of 38.2 +/- 1.3 pF (n = 61). 3. The action potential amplitude, maximum diastolic potential, take-off potential and action potential upstroke velocity were greater in larger cells. The rate of diastolic depolarization was greater and the intrinsic spontaneous activity was faster in larger cells. 4. The density of hyperpolarization-activated current (i(f)) was greater in larger cells, whereas the density of L-type calcium current was not correlated with the size of SA node cells. 5. TTX-sensitive sodium current (iNa) was absent in small cells with a capacitance of less than approximately 25 pF, and the density of iNa was greater in larger cells. 6. The greater density of iNa in larger cells may explain the higher upstroke velocity of the action potential in large cells, and the greater density of i(f) and iNa could be responsible for the faster intrinsic spontaneous activity of large cells. These results suggest that the SA node consists of electrophysiologically heterogeneous pacemaker cells with different electrical membrane properties.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alings A. M., Bouman L. N. Electrophysiology of the ageing rabbit and cat sinoatrial node--a comparative study. Eur Heart J. 1993 Sep;14(9):1278–1288. doi: 10.1093/eurheartj/14.9.1278. [DOI] [PubMed] [Google Scholar]
- Baruscotti M., DiFrancesco D., Robinson R. B. A TTX-sensitive inward sodium current contributes to spontaneous activity in newborn rabbit sino-atrial node cells. J Physiol. 1996 Apr 1;492(Pt 1):21–30. doi: 10.1113/jphysiol.1996.sp021285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleeker W. K., Mackaay A. J., Masson-Pévet M., Bouman L. N., Becker A. E. Functional and morphological organization of the rabbit sinus node. Circ Res. 1980 Jan;46(1):11–22. doi: 10.1161/01.res.46.1.11. [DOI] [PubMed] [Google Scholar]
- Denyer J. C., Brown H. F. Rabbit sino-atrial node cells: isolation and electrophysiological properties. J Physiol. 1990 Sep;428:405–424. doi: 10.1113/jphysiol.1990.sp018219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFrancesco D., Ferroni A., Mazzanti M., Tromba C. Properties of the hyperpolarizing-activated current (if) in cells isolated from the rabbit sino-atrial node. J Physiol. 1986 Aug;377:61–88. doi: 10.1113/jphysiol.1986.sp016177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerr T., Denger R., Trautwein W. Calcium currents in single SA nodal cells of the rabbit heart studied with action potential clamp. Pflugers Arch. 1989 Apr;413(6):599–603. doi: 10.1007/BF00581808. [DOI] [PubMed] [Google Scholar]
- Giles W. R., van Ginneken A. C. A transient outward current in isolated cells from the crista terminalis of rabbit heart. J Physiol. 1985 Nov;368:243–264. doi: 10.1113/jphysiol.1985.sp015856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J., Ono K., Noma A. A sustained inward current activated at the diastolic potential range in rabbit sino-atrial node cells. J Physiol. 1995 Feb 15;483(Pt 1):1–13. doi: 10.1113/jphysiol.1995.sp020563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara N., Irisawa H., Kameyama M. Contribution of two types of calcium currents to the pacemaker potentials of rabbit sino-atrial node cells. J Physiol. 1988 Jan;395:233–253. doi: 10.1113/jphysiol.1988.sp016916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara N., Irisawa H., Kasanuki H., Hosoda S. Background current in sino-atrial node cells of the rabbit heart. J Physiol. 1992 Mar;448:53–72. doi: 10.1113/jphysiol.1992.sp019029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irisawa H., Brown H. F., Giles W. Cardiac pacemaking in the sinoatrial node. Physiol Rev. 1993 Jan;73(1):197–227. doi: 10.1152/physrev.1993.73.1.197. [DOI] [PubMed] [Google Scholar]
- Ito H., Ono K. A rapidly activating delayed rectifier K+ channel in rabbit sinoatrial node cells. Am J Physiol. 1995 Aug;269(2 Pt 2):H443–H452. doi: 10.1152/ajpheart.1995.269.2.H443. [DOI] [PubMed] [Google Scholar]
- Ito H., Ono K., Noma A. Background conductance attributable to spontaneous opening of muscarinic K+ channels in rabbit sino-atrial node cells. J Physiol. 1994 Apr 1;476(1):55–68. [PMC free article] [PubMed] [Google Scholar]
- James T. N. Anatomy of the cardiac conduction system in the rabbit. Circ Res. 1967 Jun;20(6):638–648. doi: 10.1161/01.res.20.6.638. [DOI] [PubMed] [Google Scholar]
- Kirchhof C. J., Bonke F. I., Allessie M. A., Lammers W. J. The influence of the atrial myocardium on impulse formation in the rabbit sinus node. Pflugers Arch. 1987 Sep;410(1-2):198–203. doi: 10.1007/BF00581916. [DOI] [PubMed] [Google Scholar]
- Kodama I., Boyett M. R. Regional differences in the electrical activity of the rabbit sinus node. Pflugers Arch. 1985 Jul;404(3):214–226. doi: 10.1007/BF00581242. [DOI] [PubMed] [Google Scholar]
- Kreitner D. Electrophysiological study of the two main pacemaker mechanisms in the rabbit sinus node. Cardiovasc Res. 1985 May;19(5):304–318. doi: 10.1093/cvr/19.5.304. [DOI] [PubMed] [Google Scholar]
- Mackaay A. J., Op't Hof T., Bleeker W. K., Jongsma H. J., Bouman L. N. Interaction of adrenaline and acetylcholine on cardiac pacemaker function. Functional inhomogeneity of the rabbit sinus node. J Pharmacol Exp Ther. 1980 Aug;214(2):417–422. [PubMed] [Google Scholar]
- Masson-Pévet M. A., Bleeker W. K., Besselsen E., Treytel B. W., Jongsma H. J., Bouman L. N. Pacemaker cell types in the rabbit sinus node: a correlative ultrastructural and electrophysiological study. J Mol Cell Cardiol. 1984 Jan;16(1):53–63. doi: 10.1016/s0022-2828(84)80714-2. [DOI] [PubMed] [Google Scholar]
- Masson-Pévet M., Bleeker W. K., Mackaay A. J., Bouman L. N., Houtkooper J. M. Sinus node and atrium cells from the rabbit heart: a quantitative electron microscopic description after electrophysiological localization. J Mol Cell Cardiol. 1979 Jun;11(6):555–568. doi: 10.1016/0022-2828(79)90430-9. [DOI] [PubMed] [Google Scholar]
- Nathan R. D. Two electrophysiologically distinct types of cultured pacemaker cells from rabbit sinoatrial node. Am J Physiol. 1986 Feb;250(2 Pt 2):H325–H329. doi: 10.1152/ajpheart.1986.250.2.H325. [DOI] [PubMed] [Google Scholar]
- Oei H. I., Van Ginneken A. C., Jongsma H. J., Bouman L. N. Mechanisms of impulse generation in isolated cells from the rabbit sinoatrial node. J Mol Cell Cardiol. 1989 Nov;21(11):1137–1149. doi: 10.1016/0022-2828(89)90691-3. [DOI] [PubMed] [Google Scholar]
- Ono K., Ito H. Role of rapidly activating delayed rectifier K+ current in sinoatrial node pacemaker activity. Am J Physiol. 1995 Aug;269(2 Pt 2):H453–H462. doi: 10.1152/ajpheart.1995.269.2.H453. [DOI] [PubMed] [Google Scholar]
- Oosthoek P. W., Virágh S., Mayen A. E., van Kempen M. J., Lamers W. H., Moorman A. F. Immunohistochemical delineation of the conduction system. I: The sinoatrial node. Circ Res. 1993 Sep;73(3):473–481. doi: 10.1161/01.res.73.3.473. [DOI] [PubMed] [Google Scholar]
- Opthof T., VanGinneken A. C., Bouman L. N., Jongsma H. J. The intrinsic cycle length in small pieces isolated from the rabbit sinoatrial node. J Mol Cell Cardiol. 1987 Sep;19(9):923–934. doi: 10.1016/s0022-2828(87)80621-1. [DOI] [PubMed] [Google Scholar]
- Opthof T., de Jonge B., Jongsma H. J., Bouman L. N. Functional morphology of the pig sinoatrial node. J Mol Cell Cardiol. 1987 Dec;19(12):1221–1236. doi: 10.1016/s0022-2828(87)80532-1. [DOI] [PubMed] [Google Scholar]
- Opthof T., de Jonge B., Mackaay A. J., Bleeker W. K., Masson-Pevet M., Jongsma H. J., Bouman L. N. Functional and morphological organization of the guinea-pig sinoatrial node compared with the rabbit sinoatrial node. J Mol Cell Cardiol. 1985 Jun;17(6):549–564. doi: 10.1016/s0022-2828(85)80024-9. [DOI] [PubMed] [Google Scholar]
- Opthof T., de Jonge B., Masson-Pevet M., Jongsma H. J., Bouman L. N. Functional and morphological organization of the cat sinoatrial node. J Mol Cell Cardiol. 1986 Oct;18(10):1015–1031. doi: 10.1016/s0022-2828(86)80290-5. [DOI] [PubMed] [Google Scholar]
- Sakai R., Hagiwara N., Matsuda N., Kassanuki H., Hosoda S. Sodium--potassium pump current in rabbit sino-atrial node cells. J Physiol. 1996 Jan 1;490(Pt 1):51–62. doi: 10.1113/jphysiol.1996.sp021126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verheijck E. E., van Ginneken A. C., Bourier J., Bouman L. N. Effects of delayed rectifier current blockade by E-4031 on impulse generation in single sinoatrial nodal myocytes of the rabbit. Circ Res. 1995 Apr;76(4):607–615. doi: 10.1161/01.res.76.4.607. [DOI] [PubMed] [Google Scholar]
- Watanabe E. I., Honjo H., Anno T., Boyett M. R., Kodama I., Toyama J. Modulation of pacemaker activity of sinoatrial node cells by electrical load imposed by an atrial cell model. Am J Physiol. 1995 Nov;269(5 Pt 2):H1735–H1742. doi: 10.1152/ajpheart.1995.269.5.H1735. [DOI] [PubMed] [Google Scholar]
- ten Velde I., de Jonge B., Verheijck E. E., van Kempen M. J., Analbers L., Gros D., Jongsma H. J. Spatial distribution of connexin43, the major cardiac gap junction protein, visualizes the cellular network for impulse propagation from sinoatrial node to atrium. Circ Res. 1995 May;76(5):802–811. doi: 10.1161/01.res.76.5.802. [DOI] [PubMed] [Google Scholar]


