Abstract
This study aims to construct and validate a nomogram for the differential diagnosis of ovarian endometriosis cyst versus ovarian cystadenoma. We retrospectively studied the clinical characteristics of patients with ovarian endometriosis cysts and ovarian cystadenomas from January 1, 2021, to June 1, 2022. Independent risk factors for differential diagnosis were investigated using univariate and multivariate logistic regression analyses. Based on these factors, a differential diagnosis of ovarian endometriosis cyst versus ovarian cystadenoma was established. The performance of the nomogram model was assessed by internal validation using bootstrapping resampling. Decision curve analysis (DCA) was performed to evaluate the net clinical benefit of the model. Immunohistochemistry showed that lactate dehydrogenase (LDH) A was overexpressed in ectopic endometrial tissues compared to that in normal endometrial tissues. In multivariate analysis, LDH, CA-125, and CA19-9 were identified as independent risk factors for the differential diagnosis of ovarian endometriosis cyst versus ovarian cystadenoma. LDH levels >135.50 U/L combined with CA-125 levels >25.20 U/mL and CA19-9 levels >13.59 U/mL as single covariates had a high value in the differential diagnosis of ovarian endometriosis cysts versus ovarian cystadenoma. The area under the receiver operating characteristic curve (ROC) of the nomogram constructed using LDH, CA-125, and CA19-9 expression data was 0.873 (95% CI, 0.827–0.920), and the bootstrap-validated concordance index (C-index) was 0.871. Decision curve analysis confirmed that the nomogram model had excellent clinical utility. Based on serum lactate dehydrogenase combined with CA-125 and CA19-9, we constructed and validated a nomogram for the differential diagnosis of ovarian endometriosis cyst versus ovarian cystadenoma to help physicians formulate the optimal treatment strategy.
Keywords: lactate dehydrogenase, nomogram, ovarian cystadenoma, ovarian endometriosis cyst
1. Introduction
Ovarian endometriosis cyst is a common type of endometriosis mostly observed in women of childbearing age. The condition can lead to female infertility and severely affects the quality of life of patients.[1,2] Pathologically, endometriosis is characterized by benign morphological changes but is invasive and metastatic and exhibits recurrent biological behaviors of malignant tumors. Based on these characteristics, it is commonly considered a form of benign cancer. The pathogenesis of endometriosis is unclear thus far, and the factors that promote the implantation and growth of ectopic endometrium are yet to be studied.[3]
Ovarian cystadenoma is the most common benign tumor of the ovaries.[4] It is often necessary to distinguish endometriosis cysts of ovaries from cystadenoma of ovaries to establish a definite diagnosis to prepare an individualized treatment plan. At present, the differential diagnosis of the 2 conditions primarily relies on ultrasound examination, but the results of ultrasonic examination are affected by various factors and eventually depend on the subjective judgment of the sonographer. Thus, it is particularly important to explore objective evaluation indicators.[5,6] However, despite decades of research, clinically sensitive and specific biological indicators remain lacking.[7] Some studies have shown that serum CA-125 holds significance in the diagnosis of ovarian endometriosis cysts, but its accuracy is inadequate, and its diagnostic performance is poor.[7,8] Several factors involved in the chronic inflammatory process of endometriosis, such as angiogenesis factors and markers of oxidative stress, have been studied extensively, but their utility remains uncertain.[7]
In recent studies, the increase in the glycolysis levels in endometriosis cells has been proposed to lead to lactic acid accumulation and the M2 polarization of macrophages, which promote the formation and development of lesions.[9] These findings provide a novel idea for the diagnosis of ovarian endometriosis cysts. In this study, we evaluate serum lactate dehydrogenase (LDH) as a diagnostic marker in ovarian endometrial cysts and construct a differential diagnosis model of ovarian endometrial cysts versus ovarian cystadenomas based on serum LDH combined with CA-125 and CA19-9. We hypothesize that LDH combined with CA-125 and CA19-9 exhibit superior differential diagnostic value for ovarian endometrial cysts versus ovarian cystadenomas, which may help clinicians select the most appropriate individualized treatment strategy for each patient.
2. Materials and methods
2.1. Data sources and study population
Patients diagnosed with ovarian endometriosis cysts and ovarian cystadenomas at the First Affiliated Hospital of Wannan Medical College from January 1, 2021, to June 1, 2022, were retrospectively included in the study. The inclusion criteria were: female surgical patients aged 20 to 45 years; patients pathologically diagnosed with ovarian cystadenoma and ovarian endometriosis. The exclusion criteria were Combination of condition with hypertension or diabetes mellitus; combination of condition with rheumatic or immune diseases; Complications associated with diseases related to the heart, liver, kidney, and blood system; systemic or local infection with clinical symptoms; uterine leiomyoma or adenomyosis; intake of drugs that affect inflammatory factors and coagulation function; presence of mental health conditions and inability to cooperate actively; patients with menopause and anemia (Hb < 110 G/L). After screening according to the inclusion and exclusion criteria, 250 patients with ovarian endometriosis cysts and 213 patients with ovarian cystadenoma were found to be eligible for the study. The study protocol was approved by our Institutional Review Board, and patient data was de-identified and handled ethically. As this study was retrospective, it was not possible to obtain informed consent. All clinical data were preserved in the clinical data center of our institution. Data for our study were only accessible to researchers of this study and requests were refused without approval.
2.2. Clinical data extraction
Basic preoperative clinical data, including age; body mass index; obstetrical history; blood count; the levels of CA-125, CA19-9, glutamic oxaloacetic transaminase, LDH, and D-Dimer; cyst location; and cyst diameter, were extracted from the medical records of patients. Measurements were conducted in strict accordance with the manufacturer’s requirements and kit instructions. The study was approved by the Ethics committee of the First Affiliated Hospital of Wannan Medical College, and patient data were analyzed anonymously.
2.3. Immunostaining
Immunohistochemical staining was performed on ectopic endometrial tissues and normal endometrial tissues. Paraffin-embedded sections were dewaxed in xylene and dehydrated with different concentrations of ethanol. The slices were heated in 0.01 mol/L citronic acid buffer at a high temperature for 7 minutes, maintained at a lower temperature for 7 minutes, and cooled to room temperature for antigen repair. The slices were placed in 3% hydrogen peroxide and incubated at room temperature for 15 minutes to eliminate endogenous peroxidase activity. The slices were treated with normal goat serum at 37 °C for 15 minutes to block activity. The slices were then treated overnight with diluted primary antibodies at 4 °C. This was followed by treatment with a secondary antibody at 37 °C for 20 minutes. Antibody binding was then observed using diaminobenzidine. The staining was independently scored by 2 observers with no knowledge of the clinical data. According to the intensity of cell staining, 0 indicated a negative score, 1 a weak positive score, 2 a medium positive score, and 3 a strong positive score.
2.4. Statistical analysis
The Kolmogoroff-Smirnov test was used to evaluate parameter distribution in the data. Continuous variables that are not normally distributed are expressed as medians (interquartile spacing). Categorical variables are expressed numerically and proportionally and were compared either using the Mann–Whitney U test or the Kruskal–Wallis H test. ROC curve analysis was performed to determine the prediction accuracy of ovarian endometriosis cysts. The area under the curve (AUC) is used to assess the authenticity of the diagnostic model, and the cutoff value is confirmed when the Jorden index is maximized. Univariate and multivariate logistic regression analyses were performed to identify independent risk factors for ovarian endometriosis cysts. Based on the results of the multivariate analysis, a nomogram model was constructed for the differential diagnosis of ovarian endometriosis cyst versus ovarian cystadenoma. To internally validate the nomograms, a bootstrap with 1000 resamples was performed. The performance of the nomogram model was evaluated based on the C-index and calibration plot. DCA was performed to assess the net clinical benefit of using the model. The statistical tests were bilateral. P < .05 was considered statistically significant. Statistical analysis was conducted using IBM SPSS Statistics 23.0, GraphPad Prism version 6.0, and R (v 4.2.1; https://www.R-project.org).
3. Results
3.1. Patient characteristics
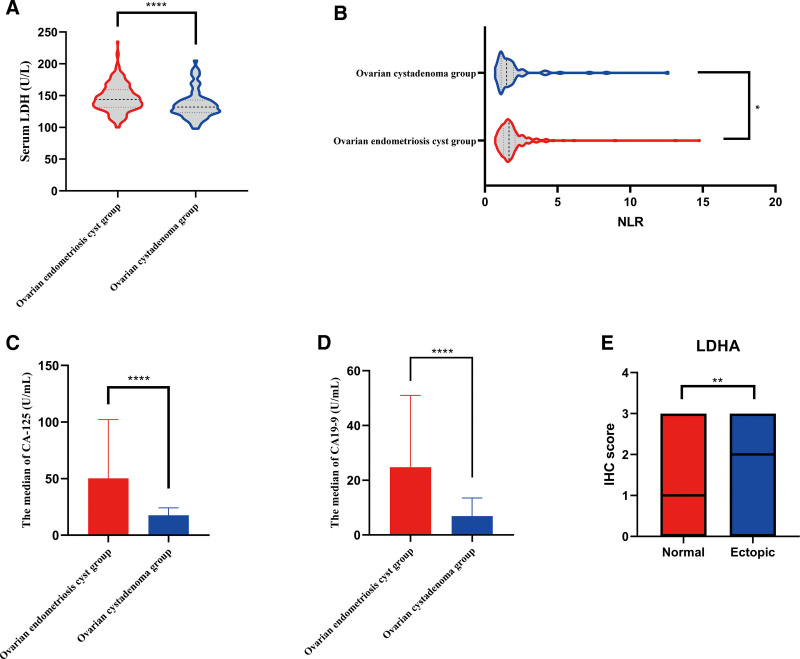
Two hundred and fifty eligible patients with ovarian endometriosis cysts and 213 patients with ovarian cystadenoma were selected for this study. The clinical characteristics of the included patients are shown in Table 1. Clinical characteristics included age; body mass index; marital history; blood count; neutrophil-to-lymphocyte ratio (NLR); the levels of CA-125, CA19-9, LDH, and D-Dimer; cyst location; and maximum cyst diameter, among others. Statistically significant differences were observed in NLR (Fig. 1B), CA-125 levels (Fig. 1C), CA19-9 levels (Fig. 1D), and LDH levels (Fig. 1A) between the ovarian endometriosis cyst group and ovarian cystadenoma group.
Table 1.
Comparison of general data between the observation and control groups.
| General information | Control group | Observation group | t/Z value | P-value |
|---|---|---|---|---|
| Number of cases | 213 | 250 | ||
| Age (yr) | 30 (11) | 31 (10) | −1.108 | .268 |
| Matrimony | ||||
| No | 66 (31%) | 61 (24.4%) | −1.117 | .264 |
| Yes | 147 (69%) | 189 (75.6%) | ||
| BMI (kg/m2) | 22.21 (5.79) | 21.96 (4.32) | −0.980 | .327 |
| Leukocyte (109/L) | 6.1 (2.1) | 6.2 (2.0) | −0.807 | .420 |
| NLR | 1.49 (0.84) | 1.66 (0.78) | −2.080 | .038 |
| Monocyte (109/L) | 0.37 (0.30–0.42) | 0.39 (0.33–0.46) | −1.015 | .310 |
| CA-125 (U/mL) | 17.50 (12.10) | 50.25 (72.95) | −8.691 | <.001 |
| CA19-9 (U/mL) | 6.89 (9.95) | 24.79 (39.66) | −6.853 | <.001 |
| Aspartate aminotransferase (U/L) | 13 (6) | 14 (4) | −0.360 | .719 |
| Lactate dehydrogenase (U/L) | 132 (20) | 144 (28) | −4.160 | <.001 |
| D-D (µg/mL) | 0.24 (0.20) | 0.25 (0.20) | −0.917 | .359 |
| Pregnancy | ||||
| No | 78 (36.6%) | 88 (35.2%) | −0.220 | .826 |
| Yes | 135 (63.4%) | 162 (64.8%) | ||
| Abortion | ||||
| No | 141 (66.2%) | 148 (59.2%) | −1.064 | .287 |
| Yes | 72 (33.8%) | 102 (40.8%) | ||
| Location | ||||
| Bilateral | 69 (32.4%) | 69 (27.6%) | −0.787 | .431 |
| Unilateral | 144 (67.6%) | 181 (72.4%) | ||
| Maximum cyst diameter | ||||
| ≤50 mm | 99 (46.5%) | 119 (47.6%) | −0.167 | .868 |
| >50 mm | 114 (53.5%) | 131 (52.4%) | ||
NLR = neutrophil-to-lymphocyte ratio.
Figure 1.
Analysis of relevant differences in the data. Differential analysis of serum lactate dehydrogenase. (A), CA-125 levels (C), CA19-9 levels (D), and NLR (B) in patients with ovarian endometriosis cyst and patients with ovarian cystadenoma. (E) Quantification of immunohistochemical scores in normal endometrial tissues (n = 50) and ectopic endometrial tissues (n = 50). NLR = neutrophil-to-lymphocyte ratio.
3.2. LDHA expression in ectopic endometrial tissue
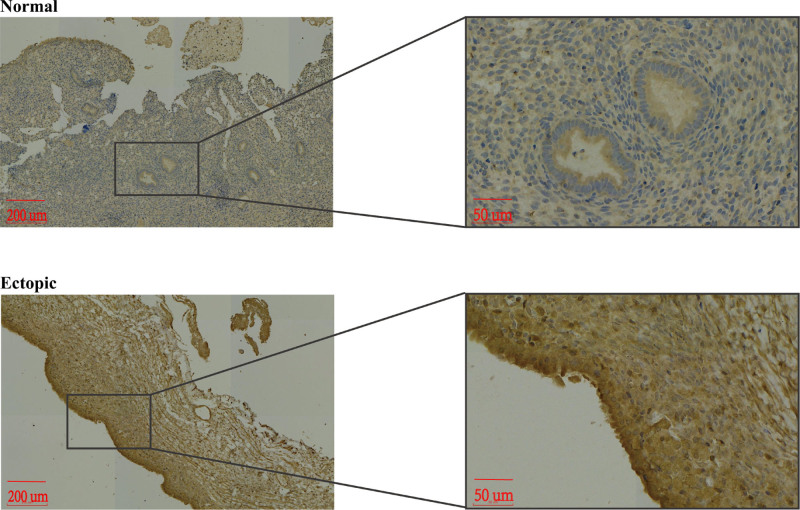
To compare the differences in LDHA expression levels between ectopic endometrial tissue and normal endometrial tissue, we used IHC and measured LDHA expression levels in the 2 tissue types. The results revealed that LDHA was expressed in the cytoplasm of the cells (Fig. 2), and the LDHA expression levels in ectopic endometrial tissues were higher than those in normal endometrial tissues (P = .008; Fig. 1E).
Figure 2.
Immunohistochemical staining of lactate dehydrogenase (LDH) A in normal endometrial tissues and ectopic endometrial tissues.
3.3. ROC analysis of CA-125, and CA19-9, and LDH levels and NLR
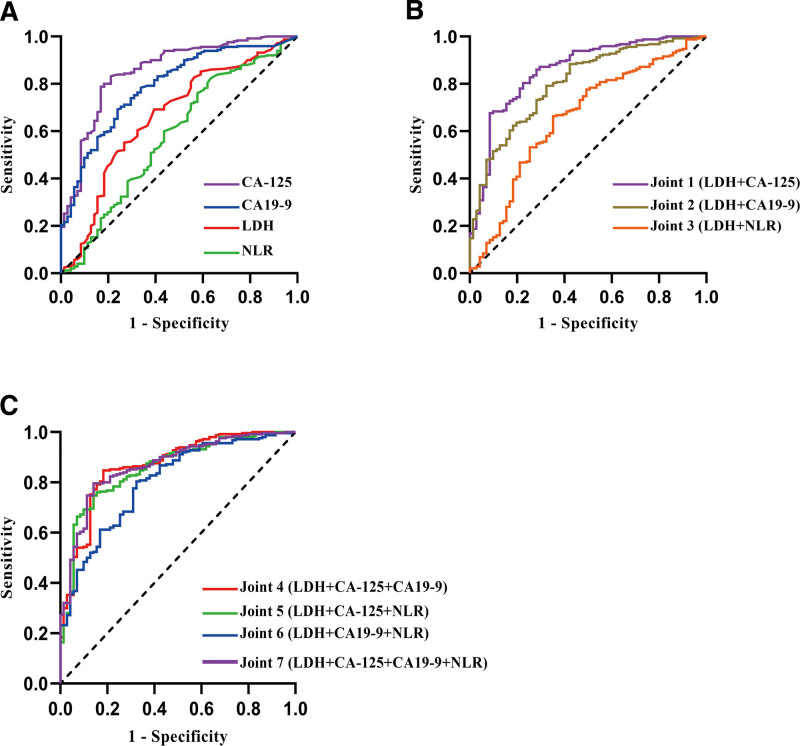
The results of ROC analysis are shown in Figure 3 and Table 2, which includes the AUC, optimal critical point, sensitivity, and specificity for the detection of ovarian endometriosis cysts and the corresponding P-values. As shown in Figure 3A and Table 2, the AUC of LDH in the differential diagnosis of ovarian endometriosis cyst was 0.662 (P < .001), and the sensitivity and specificity were 0.692 and 0.606, respectively. The AUC of CA-125 in the differential diagnosis of ovarian endometriosis cysts was 0.843 (P < .001), and the sensitivity and specificity were 0.822 and 0.779. The AUC of CA19-9 in the differential diagnosis of ovarian endometriosis cysts was 0.790 (P < .001), and the sensitivity and specificity were 0.692 and 0.761. The AUC of NLR in the differential diagnosis of ovarian endometriosis cysts was 0.580 (P = .039), and the sensitivity and specificity were 0.824 and 0.366. The AUC of combined LDH and CA-125 levels in the differential diagnosis of ovarian endometrial cysts was 0.858 (P < .001), and the sensitivity and specificity were 0.676 and 0.916, respectively. The findings indicated that the combination of CA-125 and LDH significantly improved the specificity of the differential diagnosis of ovarian endometrial cysts (Fig. 3B, Table 2). The AUC of LDH levels combined with CA-125 and CA19-9 levels in the differential diagnosis of ovarian endometrial cysts was 0.867 (P < .001), and the sensitivity and specificity were 0.848 and 0.817, respectively (Fig. 3C, Table 2). Therefore, LDH level combined with CA-125 and CA19-9 levels performs well in the differential diagnosis of ovarian endometriosis cysts versus ovarian cystadenoma. In addition, ROC curves were prepared to confirm the best cutoff levels. The best cutoff levels of NLR, CA-125, CA19-9, and LDH in the differential diagnosis of ovarian endometrial cysts versus ovarian cystadenomas were 1.185, 25.20 U/mL, 13.59 U/mL, and 135.50 U/L, respectively.
Figure 3.
ROC curves: (A) CA-125, CA19-9, and LDH levels and NLR in the differential diagnosis of ovarian endometriosis cysts with ovarian cystadenoma. (B and C) Different combinations of serum markers in the differentiation of ovarian endometriosis cysts versus ovarian cystadenoma. NLR = neutrophil-to-lymphocyte ratio, ROC = receiver operating characteristic.
Table 2.
Diagnostic performance of each index and combined index.
| Indicator | Youden index | Sensitivity | Specificity | AUC | P |
|---|---|---|---|---|---|
| LDH | 0.298 | 0.692 | 0.606 | 0.662 | <.001 |
| CA-125 (U/mL) | 0.621 | 0.822 | 0.779 | 0.843 | <.001 |
| CA19-9 (U/mL) | 0.453 | 0.692 | 0.761 | 0.790 | <.001 |
| NLR | 0.190 | 0.824 | 0.366 | 0.580 | .039 |
| Joint 1 (LDH + CA-125) | 0.592 | 0.676 | 0.916 | 0.858 | <.001 |
| Joint 2 (LDH + CA19-9) | 0.468 | 0.792 | 0.676 | 0.803 | <.001 |
| Joint 3 (LDH + NLR) | 0.312 | 0.664 | 0.648 | 0.657 | <.001 |
| Joint 4 (LDH + CA-125 + CA19-9) | 0.665 | 0.848 | 0.817 | 0.867 | <.001 |
| Joint 5 (LDH + CA-125 + NLR) | 0.607 | 0.748 | 0.859 | 0.857 | <.001 |
| Joint 6 (LDH + CA19-9 + NLR) | 0.480 | 0.804 | 0.676 | 0.801 | <.001 |
| Joint 7 (LDH + CA-125 + CA19-9 + NLR) | 0.655 | 0.796 | 0.859 | 0.868 | <.001 |
NLR = neutrophil-to-lymphocyte ratio.
3.4. Univariate and multivariate logistic regression analysis for ovarian endometriosis cyst versus ovarian cystadenoma
NLR, serum LDH, serum CA-125, and serum CA19-9 levels were stratified according to the optimal cutoff values for ovarian endometriosis cysts versus ovarian cystadenoma, as determined by ROC analysis. Univariate analysis was used for determining statistical significance, which was followed by multivariate logistic regression analysis. Multifactorial logistic regression analysis revealed that serum LDH > 135.50 U/L (OR, 2.923; 95% CI, 1.468–5.818; P = .002), serum CA-125 > 25.20 U/mL (OR, 12.283; 95% CI, 5.969–25.274; P < .001), and serum CA19-9 > 13.59 U/mL (OR, 2.905; 95% CI, 1.417–5.956; P = .004) were independent factors in the differential diagnosis of ovarian endometriosis cysts versus ovarian cystadenoma (Table 3). Subsequently, LDH > 135.50 U/L was associated with CA-125 > 25.20 U/mL, CA19-9 > 13.59 U/mL, and NLR > 1.185, and each association was used as a single covariate for clustering analysis (Tables S1–S3, Supplemental Digital Content, http://links.lww.com/MD/O89). The results revealed that these combinations were independent parameters in the differential diagnosis of ovarian endometriosis cysts. Finally, LDH > 135.50 U/L combined with CA-125 > 25.20 U/mL and CA19-9 > 13.59 U/mL were used as single covariates for cluster analysis (Table 4). Multivariate regression analysis showed that LDH > 135.50 U/L combined with CA-125 > 25.20 U/mL and CA19-9 > 13.59 U/mL as single covariates had a high value in the differential diagnosis of ovarian endometriosis cysts versus ovarian cystadenoma (OR, 17.741; 95% CI, 5.419–58.075; P < .001).
Table 3.
Univariate and multivariate analyses of serum LDH, CA-125, and CA19-9 levels and NLR with consideration of ovarian endometriosis cysts versus ovarian cystadenoma.
| Variable | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| OR (95% CI) | P-value | OR (95% CI) | P-value | |
| Serum LDH | ||||
| LDH ≤ 135.50 U/L | Reference | Reference | ||
| LDH > 135.50 U/L | 3.450 (1.998–5.959) | <.001 | 2.923 (1.468–5.818) | .002 |
| Serum CA-125 | ||||
| CA-125 ≤ 25.20 U/mL | Reference | Reference | ||
| CA-125 > 25.20 U/mL | 18.489 (9.563–35.746) | <.001 | 12.283 (5.969–25.274) | <.001 |
| Serum CA19-9 | ||||
| CA19-9 ≤ 13.59 U/mL | Reference | Reference | ||
| CA19-9 > 13.59 U/mL | 7.137 (3.887–13.103) | <.001 | 2.905 (1.417–5.956) | .004 |
| NLR | ||||
| NLR ≤ 1.185 | Reference | Reference | ||
| NLR > 1.185 | 2.632 (1.473–4.704) | .001 | 1.433 (0.670–3.062) | .353 |
NLR = neutrophil-to-lymphocyte ratio.
Table 4.
Univariate and multivariate analyses of NLR and serum LDH and CA-125 + CA19-9 levels in ovarian endometriosis cysts versus ovarian cystadenoma.
| Variable | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| OR (95% CI) | P-value | OR (95% CI) | P-value | |
| Serum LDH and CA-125 and CA19-9 | ||||
| LDH > 135.50 U/L, CA-125 > 25.20 U/mL, and CA19-9 > 13.59 U/mL | 19.000 (5.822–62.004) | <.001 | 17.741 (5.419–58.075) | <.001 |
| Other | Reference | Reference | ||
| NLR | ||||
| NLR ≤ 1.185 | Reference | Reference | ||
| NLR > 1.185 | 2.632 (1.473–4.704) | .001 | 2.192 (1.176–4.086) | .014 |
NLR = neutrophil-to-lymphocyte ratio.
3.5. Construction and validation of nomogram models
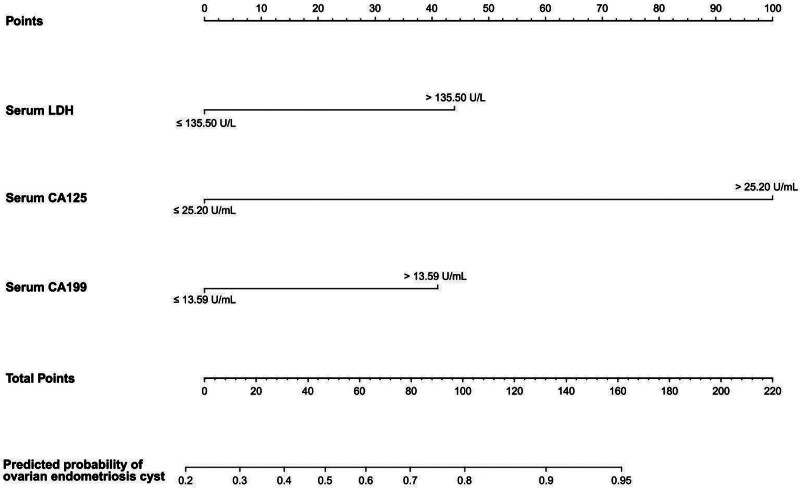
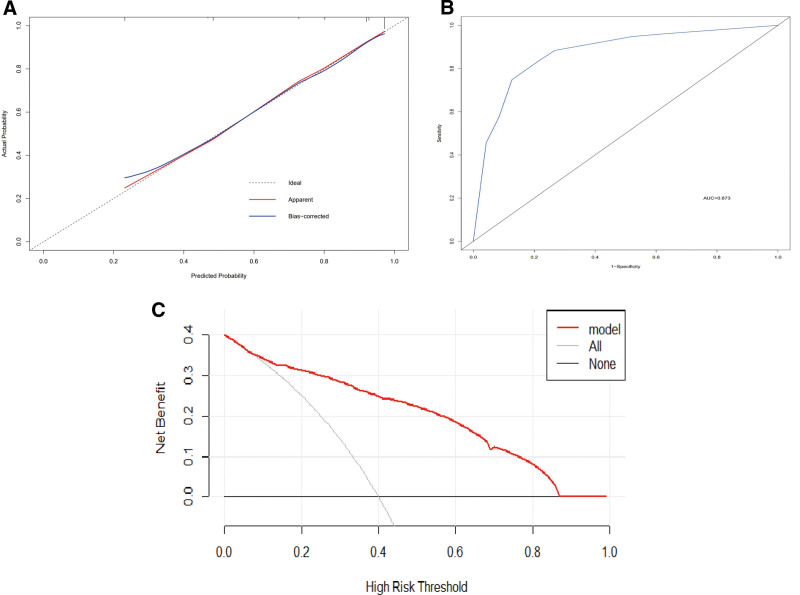
According to the ROC curve and the results of multivariate regression analysis, LDH > 135.50 U/L, CA-125 > 25.20 U/mL, and CA19-9 > 13.59 U/mL were independent parameters for the differential diagnosis of ovarian endometriosis cysts versus ovarian cystadenoma (Table 3). Therefore, we incorporated these parameters into the model (Fig. 4). The calibration curve of the forecast nomogram showed that the model was well calibrated, i.e., the predicted curve and the ideal line were roughly in agreement (Fig. 5A). The C-index value of the calibration curve was 0.871. As shown in Figure 5B, the AUC of the nomogram model was 0.873 (95% CI, 0.827–0.920), indicating that the nomogram model had better differential diagnosis performance. With respect to clinical application, the histogram was found to be a reliable tool in the differential diagnosis of ovarian endometriosis cysts versus ovarian cystadenoma (Fig. 5C), based on the DCA results.
Figure 4.
Nomogram for the differential diagnosis of ovarian endometriosis cysts versus ovarian cystadenoma.
Figure 5.
Calibration curve (A), receiver operating characteristic (ROC) curve (B), and DCA (C) of the nomogram for ovarian endometriosis cyst. DCA = decision curve analysis.
4. Discussion
There are significant challenges in diagnosing endometriosis. Despite decades of research, sensitive and specific signs and symptoms of the condition are rare, and there are no blood tests to confirm endometriosis.[10] Clinically, it is difficult to distinguish ovarian endometrial cysts from ovarian cystadenoma. Imaging examination is the preferred method for the diagnosis of ovarian endometrial cyst, but it cannot reliably distinguish ovarian endometrial cysts from ovarian cystadenoma.[6,10] In this study, we observed differences in test indicators, including CA-125, CA19-9, LDH, and NLR, between patients with ovarian cystadenoma and patients with ovarian endometrial cysts, and determined that CA-125, CA19-9, and LDH levels are independent risk factors for ovarian endometrial cysts. Based on this, we established the differential diagnosis model of ovarian endometriosis cysts versus ovarian cystadenoma. Our model had high sensitivity and specificity, which make it convenient to use in clinical diagnosis.
Glucose is the primary source of energy in normal tissues. In the presence of sufficient oxygen, glucose can be completely decomposed into carbon dioxide and water through various methods of sugar metabolism, such as aerobic oxidation, anaerobic glycolysis, and the pentose phosphate pathway, and ATP can be generated to supply a large amount of energy for cells.[11] Under normal circumstances, aerobic oxidation is the primary metabolic pathway for glucose, and glycolysis only provides energy for the body under hypoxia or other stressful conditions. However, in malignant tumors, tumor cells do not decompose glucose through the highly efficient oxidative phosphorylation process in the mitochondria even in the presence of sufficient oxygen; instead, the cells metabolize glucose via the high-speed and low-yield process of glycolysis.[12,13] This special mode of energy metabolism is called the Warburg effect. The acidic microenvironment formed by lactic acid, the final product of metabolism, is conducive to the rapid growth and distant metastasis of tumor cells. Therefore, aerobic glycolysis is an important process in tumor cells and is closely related to the aggressiveness of tumors.[14,15] In recent years, ectopic endometrium was shown to have a higher glycolysis level than normal endometrium for the first time.[9] The high concentration of lactic acid in the microenvironment plays an important role in the development of endometriosis.
At present, the pathogenesis of ovarian endometriosis cyst is not clear, and immune dysfunction is a widespread concern.[2,3,16–18] The high concentration of lactic acid resulting from higher glycolysis levels in ectopic endometrial tissues promotes the M2 polarization of macrophages. M2 macrophages show immune inhibition via decreased phagocytosis function.[9,19–21] Therefore, the change in the phenotypic balance of M1/M2 macrophages is closely related to the occurrence and development of endometriosis.
LDH is a key enzyme in glycolysis.[22] Several clinical studies have confirmed that the migration and invasion of tumors, including oral squamous cell carcinoma, head and neck cancer,[23] laryngeal squamous cell carcinoma,[24] breast cancer,[25] pancreatic cancer,[26] kidney cancer,[27] prostate cancer,[28] gastric cancer,[29] and cervical cancer,[30,31] are associated with LDH levels,.[32] At present, there is no clinical study on LDH levels in patients with ovarian endometriosis cysts. In this study, we observed that the combination of CA-125, CA19-9, and LDH had excellent specificity in the differential diagnosis of ovarian endometriosis cyst versus ovarian cystadenoma. To aid the clinical application of these markers, we calculated their optimal critical value. Based on the ROC curve, the value of CA-125 was 25.20 U/mL, that of CA19-9 was 13.59 U/mL, and that of LDH was 135.50 U/L. As single covariates, CA-125 levels >25.20 U/mL, CA19-9 levels >13.59 U/mL, and LDH levels >135.50 U/L are considered to be independent factors in the diagnosis of ovarian endometrial cysts. A clinical nomogram for the differential diagnosis of ovarian endometrial cysts versus ovarian cystadenomas was established, and the diagnostic efficiency was calculated to be 0.873 (95% CI, 0.827–0.920).
The study had some limitations. First, this study was designed retrospectively. All retrospective analyses may have selection bias. Second, the sample size is relatively small, which may have affected the discrimination ability of the model. Lastly, the clinical data of the patient was missing lipid indexes, including serum total cholesterol, low density lipoprotein, triglyceride, etc. Studies have shown that women with hypercholesterolemia have a higher risk of developing ovarian endometrial cysts,[33] and that hypercholesterolemia is often accompanied by a significant increase in LDH levels.[34] This evidence provides a reliable theoretical basis for our research results, but these findings cannot be comprehensively analyzed and discussed owing to the lack of clinical data. In future studies, we intend to collect comprehensive and high-quality clinical data for prospective trials and test model performance through external validation.
5. Conclusion
We confirmed the correlation between LDH levels and ovarian endometrial cysts and established a convenient, sensitive, and specific nomogram based on serum lactate dehydrogenase combined with CA-125 and CA19-9 for the differential diagnosis of ovarian endometriosis cyst versus ovarian cystadenoma. The application of this model had achieved good results and could help physicians formulate the optimal treatment strategy.
Acknowledgments
We thank all participants for their time and effort.
Author contributions
Conceptualization: Jin Ding.
Data curation: Chang Su, Jian Yang.
Formal analysis: Chang Su.
Methodology: Jin Ding.
Writing – original draft: Chang Su.
Writing – review & editing: Huafeng Ding.
Supplementary Material
Abbreviations:
- AUC
- area under the curve
- C-index
- concordance index
- DCA
- decision curve analysis
- LDH
- lactate dehydrogenase
- OR
- odds ratio
- ROC
- receiver operating characteristic
This work was supported by grants from the National Natural Science Foundation of China (No. 82201820).
The research involving human participants strictly adhered to the ethical guidelines set forth by the Medical Ethics Committee of the First Affiliated Hospital of Wannan Medical College. Approval from the committee was obtained to conduct this retrospective analysis (approval no. 2023-109). Patient consent was waived by the Medical Ethics Committee of The First Affiliated Hospital of Wannan Medical College due to the retrospective character of the study and participants’ anonymity.
The authors have no conflicts interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Supplemental Digital Content is available for this article.
How to cite this article: Su C, Yang J, Ding J, Ding H. Differential diagnosis of ovarian endometriosis cyst versus ovarian cystadenoma based on serum lactate dehydrogenase combined with CA-125 and CA19-9: A retrospective cohort study. Medicine 2024;103:48(e40776).
Contributor Information
Chang Su, Email: 1575393269@qq.com.
Jian Yang, Email: 274663704@qq.com.
Jin Ding, Email: sc157539@163.com.
References
- [1].Chapron C, Marcellin L, Borghese B, Santulli P. Rethinking mechanisms, diagnosis and management of endometriosis. Nat Rev Endocrinol. 2019;15:666–82. [DOI] [PubMed] [Google Scholar]
- [2].Symons LK, Miller JE, Kay VR, et al. The immunopathophysiology of endometriosis. Trends Mol Med. 2018;24:748–62. [DOI] [PubMed] [Google Scholar]
- [3].Peiris AN, Chaljub E, Medlock D. Endometriosis. JAMA. 2018;320:2608. [DOI] [PubMed] [Google Scholar]
- [4].Limaiem F, Lekkala MR, Mlika M. Ovarian cystadenoma. In: StatPearls. Treasure Island (FL) ineligible companies. Treasure Island (FL): StatPearls Publishing LLC.; 2023. [Google Scholar]
- [5].Pascoal E, Wessels JM, Aas-Eng MK, et al. Strengths and limitations of diagnostic tools for endometriosis and relevance in diagnostic test accuracy research. Ultrasound Obstet Gynecol. 2022;60:309–27. [DOI] [PubMed] [Google Scholar]
- [6].Nisenblat V, Bossuyt PM, Farquhar C, Johnson N, Hull ML. Imaging modalities for the non-invasive diagnosis of endometriosis. Cochrane Database Syst Rev. 2016;2:CD009591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Nisenblat V, Bossuyt PM, Shaikh R, et al. Blood biomarkers for the non-invasive diagnosis of endometriosis. Cochrane Database Syst Rev. 2016;2016:CD012179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Bedaiwy MA, Falcone T. Laboratory testing for endometriosis. Clin Chim Acta. 2004;340:41–56. [DOI] [PubMed] [Google Scholar]
- [9].Gou Y, Wang H, Wang T, et al. Ectopic endometriotic stromal cells-derived lactate induces M2 macrophage polarization via Mettl3/Trib1/ERK/STAT3 signalling pathway in endometriosis. Immunology. 2023;168:389–402. [DOI] [PubMed] [Google Scholar]
- [10].Coutinho LM, Ferreira MC, Rocha ALL, Carneiro MM, Reis FM. New biomarkers in endometriosis. Adv Clin Chem. 2019;89:59–77. [DOI] [PubMed] [Google Scholar]
- [11].Luengo A, Li Z, Gui DY, et al. Increased demand for NAD(+) relative to ATP drives aerobic glycolysis. Mol Cell. 2021;81:691–707.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Liberti MV, Locasale JW. The Warburg effect: how does it benefit cancer cells? Trends Biochem Sci. 2016;41:211–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Stacpoole PW. Therapeutic targeting of the pyruvate dehydrogenase complex/pyruvate dehydrogenase kinase (PDC/PDK) axis in cancer. J Natl Cancer Inst. 2017;109:1–14. [DOI] [PubMed] [Google Scholar]
- [14].Yang J, Ren B, Yang G, et al. The enhancement of glycolysis regulates pancreatic cancer metastasis. Cell Mol Life Sci. 2020;77:305–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Wu Z, Wu J, Zhao Q, Fu S, Jin J. Emerging roles of aerobic glycolysis in breast cancer. Clin Transl Oncol. 2020;22:631–46. [DOI] [PubMed] [Google Scholar]
- [16].Wang Y, Nicholes K, Shih IM. The origin and pathogenesis of endometriosis. Annu Rev Pathol. 2020;15:71–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Zondervan KT, Becker CM, Koga K, Missmer SA, Taylor RN, Viganò P. Endometriosis. Nat Rev Dis Primers. 2018;4:9. [DOI] [PubMed] [Google Scholar]
- [18].Crispim PCA, Jammal MP, Murta EFC, Nomelini RS. Endometriosis: what is the influence of immune cells? Immunol Invest. 2021;50:372–88. [DOI] [PubMed] [Google Scholar]
- [19].Funes SC, Rios M, Escobar-Vera J, Kalergis AM. Implications of macrophage polarization in autoimmunity. Immunology. 2018;154:186–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Yunna C, Mengru H, Lei W, Weidong C. Macrophage M1/M2 polarization. Eur J Pharmacol. 2020;877:173090. [DOI] [PubMed] [Google Scholar]
- [21].Liu YC, Zou XB, Chai YF, Yao YM. Macrophage polarization in inflammatory diseases. Int J Biol Sci. 2014;10:520–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Sharma D, Singh M, Rani R. Role of LDH in tumor glycolysis: regulation of LDHA by small molecules for cancer therapeutics. Semin Cancer Biol. 2022;87:184–95. [DOI] [PubMed] [Google Scholar]
- [23].Mohajertehran F, Ayatollahi H, Jafarian AH, et al. Overexpression of lactate dehydrogenase in the saliva and tissues of patients with head and neck squamous cell carcinoma. Rep Biochem Mol Biol. 2019;7:142–9. [PMC free article] [PubMed] [Google Scholar]
- [24].Guo E, Guo L, An C, et al. Prognostic significance of lactate dehydrogenase in patients undergoing surgical resection for laryngeal squamous cell carcinoma. Cancer Control. 2020;27:1073274820978795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Mack N, Mazzio EA, Bauer D, Flores-Rozas H, Soliman KF. Stable shRNA silencing of lactate dehydrogenase A (LDHA) in human MDA-MB-231 breast cancer cells fails to alter lactic acid production, glycolytic activity, ATP or survival. Anticancer Res. 2017;37:1205–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Maftouh M, Avan A, Sciarrillo R, et al. Synergistic interaction of novel lactate dehydrogenase inhibitors with gemcitabine against pancreatic cancer cells in hypoxia. Br J Cancer. 2014;110:172–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Zhao J, Huang X, Xu Z, et al. LDHA promotes tumor metastasis by facilitating epithelial-mesenchymal transition in renal cell carcinoma. Mol Med Rep. 2017;16:8335–44. [DOI] [PubMed] [Google Scholar]
- [28].Vieira FQ, Cardoso AR, Gigliano D, et al. LDHA and CPT2 association with therapy resistance in prostate cancer. Eur J Public Health. 2021;31(Supplement_2):ii22. [Google Scholar]
- [29].Michelotti A, de Scordilli M, Sperti E, et al. LDH as prognostic factor in second line treatment for advanced gastric cancer: the LINE study. J Clin Oncol. 2021;39(15_suppl):e16102–e16102. [Google Scholar]
- [30].Wang H, Wang MS, Zhou YH, Shi JP, Wang WJ. Prognostic values of LDH and CRP in cervical cancer. Onco Targets Ther. 2020;13:1255–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Liu Y, Guo JZ, Liu Y, et al. Nuclear lactate dehydrogenase A senses ROS to produce α-hydroxybutyrate for HPV-induced cervical tumor growth. Nat Commun. 2018;9:4429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Cai H, Li J, Zhang Y, et al. LDHA promotes oral squamous cell carcinoma progression through facilitating glycolysis and epithelial-mesenchymal transition. Front Oncol. 2019;9:1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Mu F, Rich-Edwards J, Rimm EB, Spiegelman D, Forman JP, Missmer SA. Association between endometriosis and hypercholesterolemia or hypertension. Hypertension. 2017;70:59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Moon CM, Oh CH, Ahn KY, et al. Metabolic biomarkers for non-alcoholic fatty liver disease induced by high-fat diet: in vivo magnetic resonance spectroscopy of hyperpolarized [1-(13)C] pyruvate. Biochem Biophys Res Commun. 2017;482:112–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.