Abstract
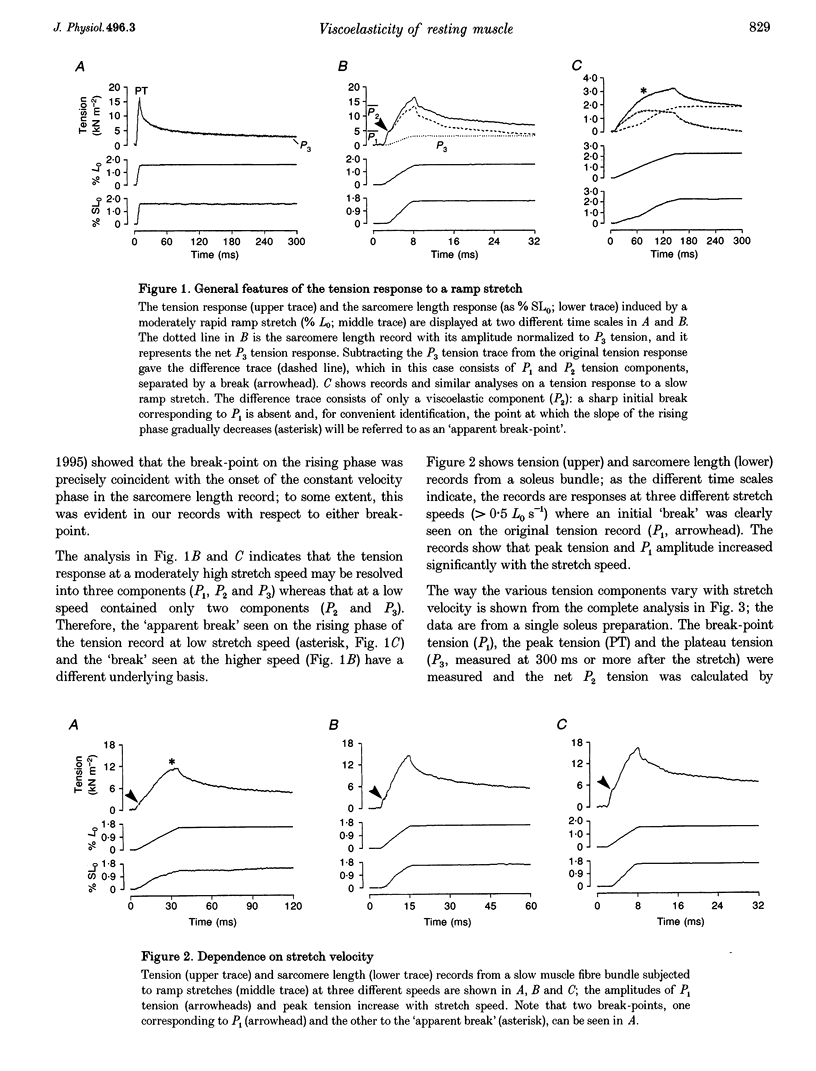
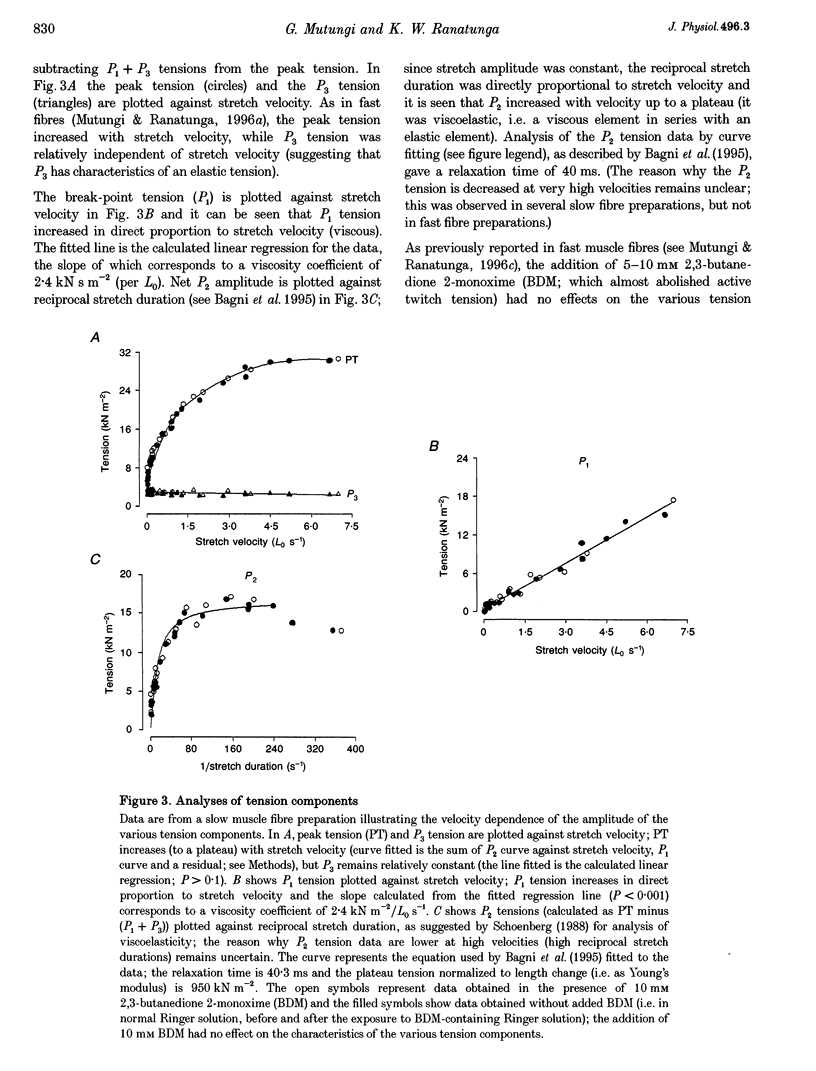
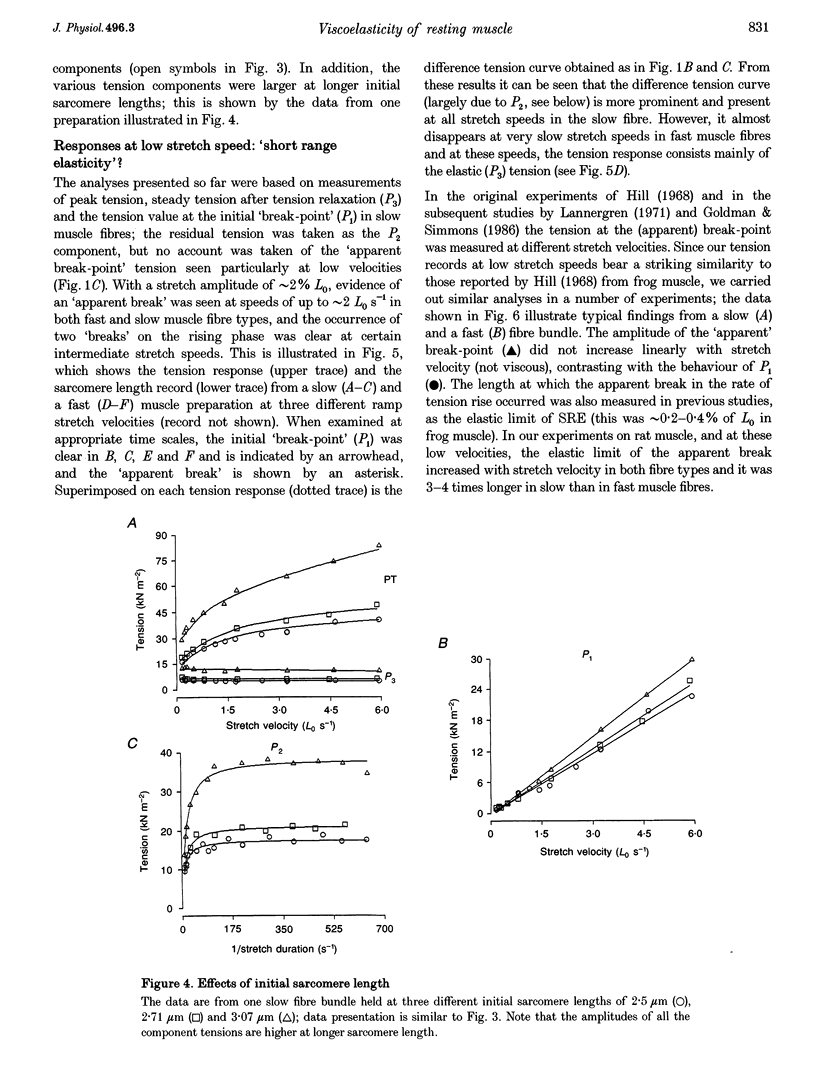
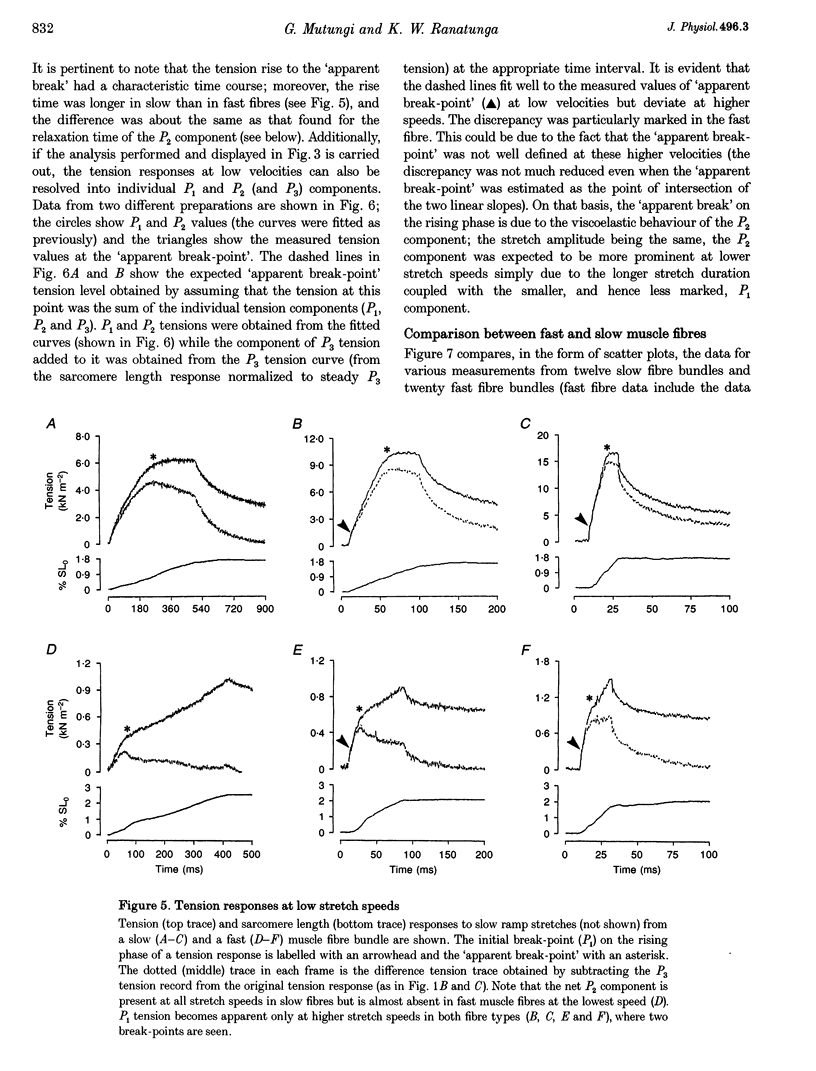
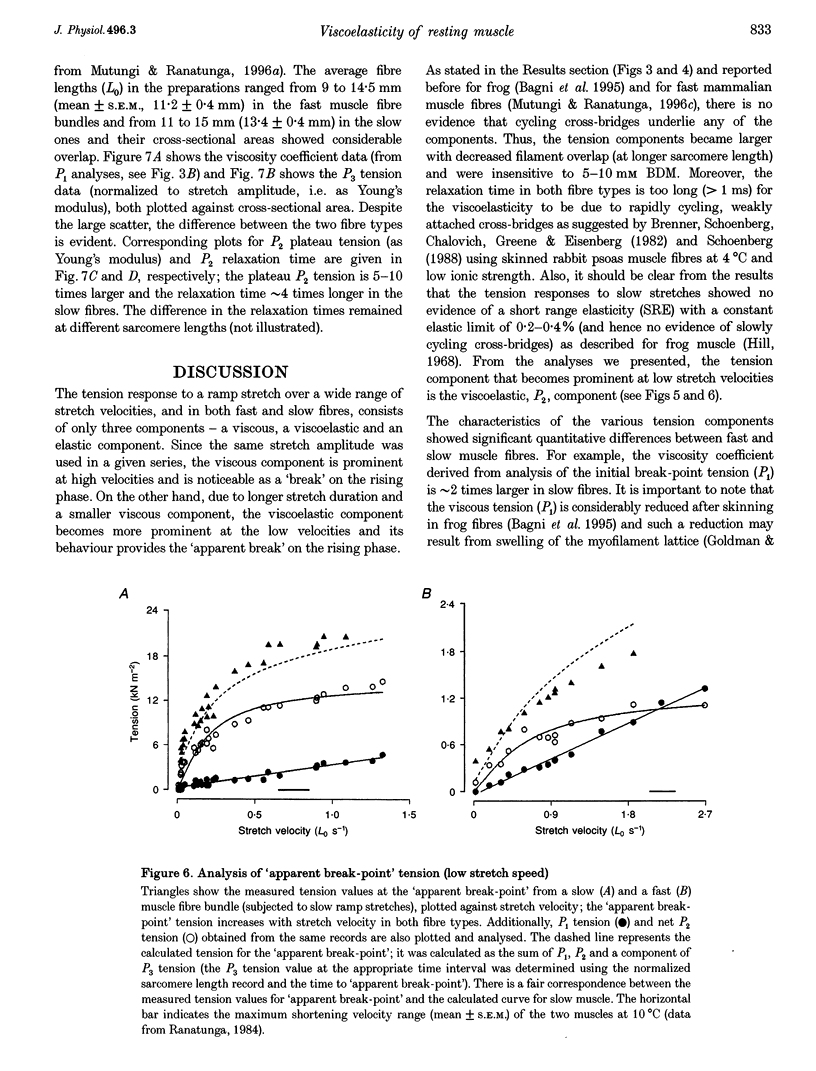
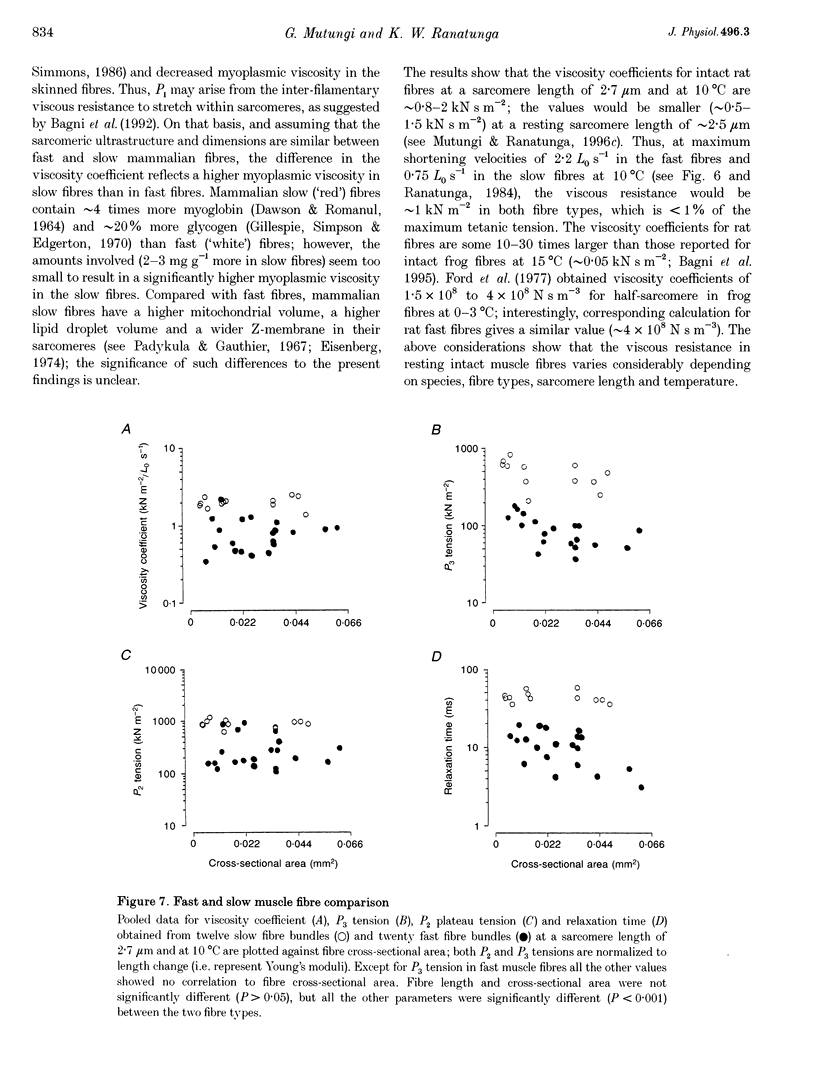
1. The tension and sarcomere length responses induced by ramp stretches (amplitude 1-3% of initial fibre length (Lzero) and speeds of 0.01-12 Lzero s-1) were examined, at 10 degrees C and sarcomere lengths of approximately 2.7 microns, in resting intact muscle fibre bundles isolated from the soleus (a slow muscle) and extensor digitorum longus (a fast muscle) of the rat. 2. In both fibre types, the tension response to moderately fast ramp stretches consists of a viscous, a viscoelastic and an elastic component. At low stretch velocities, where the viscous component is very small, the tension response consists of only the viscoelastic and elastic components. 3. The viscosity coefficient (mean +/- S.E.M., 2 +/- 0.01 kN s m-2, n = 12) and the relaxation time of the viscoelasticity (44 +/- 2 ms, n = 12) of the slow muscle fibres were significantly larger than those of the fast muscle fibres (0.8 +/- 0.1 kN s m-2 and 11 +/- 1 ms, respectively, n = 20). 4. The relaxation time, in either fibre type, is too long for the viscoelasticity to be due to rapidly cycling, weakly attached cross-bridges. Moreover, the tension components increased with sarcomere length and were insensitive to 5-10 mM 2,3-butanedione 2-monoxime (BDM), which inhibited active contractions. 5. The possibility that the fast-slow fibre differences may reflect differences in myoplasmic viscosity and connectin (titin) isoforms (in their gap filaments) is discussed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bagni M. A., Cecchi G., Colomo F., Garzella P. Absence of mechanical evidence for attached weakly binding cross-bridges in frog relaxed muscle fibres. J Physiol. 1995 Jan 15;482(Pt 2):391–400. doi: 10.1113/jphysiol.1995.sp020526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagni M. A., Cecchi G., Colomo F., Garzella P. Are weakly binding bridges present in resting intact muscle fibers? Biophys J. 1992 Nov;63(5):1412–1415. doi: 10.1016/S0006-3495(92)81718-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner B., Schoenberg M., Chalovich J. M., Greene L. E., Eisenberg E. Evidence for cross-bridge attachment in relaxed muscle at low ionic strength. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7288–7291. doi: 10.1073/pnas.79.23.7288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAWSON D. M., ROMANUL F. C. ENZYMES IN MUSCLES. II. HISTOCHEMICAL AND QUANTITATIVE STUDIES. Arch Neurol. 1964 Oct;11:369–378. doi: 10.1001/archneur.1964.00460220031004. [DOI] [PubMed] [Google Scholar]
- Ford L. E., Huxley A. F., Simmons R. M. Tension responses to sudden length change in stimulated frog muscle fibres near slack length. J Physiol. 1977 Jul;269(2):441–515. doi: 10.1113/jphysiol.1977.sp011911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie C. A., Simpson D. R., Edgerton V. R. High glycogen content of red as opposed to white skeletal muscle fibers of guinea pigs. J Histochem Cytochem. 1970 Aug;18(8):552–558. doi: 10.1177/18.8.552. [DOI] [PubMed] [Google Scholar]
- Goldman Y. E., Simmons R. M. The stiffness of frog skinned muscle fibres at altered lateral filament spacing. J Physiol. 1986 Sep;378:175–194. doi: 10.1113/jphysiol.1986.sp016213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granzier H., Helmes M., Trombitás K. Nonuniform elasticity of titin in cardiac myocytes: a study using immunoelectron microscopy and cellular mechanics. Biophys J. 1996 Jan;70(1):430–442. doi: 10.1016/S0006-3495(96)79586-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill C., Weber K. Monoclonal antibodies distinguish titins from heart and skeletal muscle. J Cell Biol. 1986 Mar;102(3):1099–1108. doi: 10.1083/jcb.102.3.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill D. K. Tension due to interaction between the sliding filaments in resting striated muscle. The effect of stimulation. J Physiol. 1968 Dec;199(3):637–684. doi: 10.1113/jphysiol.1968.sp008672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowits R. Passive force generation and titin isoforms in mammalian skeletal muscle. Biophys J. 1992 Feb;61(2):392–398. doi: 10.1016/S0006-3495(92)81845-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labeit S., Kolmerer B. Titins: giant proteins in charge of muscle ultrastructure and elasticity. Science. 1995 Oct 13;270(5234):293–296. doi: 10.1126/science.270.5234.293. [DOI] [PubMed] [Google Scholar]
- Lännergren J. The effect of low-level activation on the mechanical properties of isolated frog muscle fibers. J Gen Physiol. 1971 Aug;58(2):145–162. doi: 10.1085/jgp.58.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutungi G., Ranatunga K. W. Tension relaxation after stretch in resting mammalian muscle fibers: stretch activation at physiological temperatures. Biophys J. 1996 Mar;70(3):1432–1438. doi: 10.1016/S0006-3495(96)79702-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutungi G., Ranatunga K. W. The visco-elasticity of resting intact mammalian (rat) fast muscle fibres. J Muscle Res Cell Motil. 1996 Jun;17(3):357–364. doi: 10.1007/BF00240933. [DOI] [PubMed] [Google Scholar]
- Politou A. S., Thomas D. J., Pastore A. The folding and stability of titin immunoglobulin-like modules, with implications for the mechanism of elasticity. Biophys J. 1995 Dec;69(6):2601–2610. doi: 10.1016/S0006-3495(95)80131-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranatunga K. W. The force-velocity relation of rat fast- and slow-twitch muscles examined at different temperatures. J Physiol. 1984 Jun;351:517–529. doi: 10.1113/jphysiol.1984.sp015260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranatunga K. W. Thermal stress and Ca-independent contractile activation in mammalian skeletal muscle fibers at high temperatures. Biophys J. 1994 May;66(5):1531–1541. doi: 10.1016/S0006-3495(94)80944-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenberg M. Characterization of the myosin adenosine triphosphate (M.ATP) crossbridge in rabbit and frog skeletal muscle fibers. Biophys J. 1988 Jul;54(1):135–148. doi: 10.1016/S0006-3495(88)82938-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Tombe P. P., ter Keurs H. E. An internal viscous element limits unloaded velocity of sarcomere shortening in rat myocardium. J Physiol. 1992 Aug;454:619–642. doi: 10.1113/jphysiol.1992.sp019283. [DOI] [PMC free article] [PubMed] [Google Scholar]


