Abstract
Background:
This study evaluates the differential effects of constant-load (CL-AE) and graded (G-AE) aerobic exercise training approaches on cardiopulmonary fitness and functional capacity in obese children with bronchial asthma (BA).
Methods:
Seventy-eight obese children with moderate BA (age: 14.14 ± 2.31 years; body mass index: 31.93 ± 1.26 kg/m2) were randomly assigned to 3 intervention-based groups: control, CL-AE, or G-AE group (n = 26 in a group). The cardiorespiratory fitness (peak oxygen uptake, minute ventilation [VE], ventilation-oxygen uptake ratio, stroke volume of oxygen, oxygen/carbon-dioxide exchange ratio, heart rate maximum, and heart rate recovery at one minute) and functional capacity (6-minute walk test and perceived dyspnea and fatigue) were assessed at the baseline and posttreatment.
Results:
The G-AE group exhibited more favorable changes in cardiorespiratory fitness [VO2peak (P = .03), VE (P = .021), VE/VO2 (P = .032), SVO2 (P = .025), O2/CO2 ratio (P = .004), HRmax (P = .016), HRR1 (P = .046)] and functional capacity [6-minute walk test (P = .021), dyspnea (P = .041), fatigue (P = .04)] as compared to the CL-AE group.
Conclusion:
The G-AE, compared to CL-AE, appears to be a more potent stimulus for enhancing cardiorespiratory fitness and functional capacity in obese children with BA. Further investigations are, however, required to corroborate the observed effects.
Keywords: bronchial asthma, cardiorespiratory fitness, children, conditioning exercise, functional capacity, obesity
1. Introduction
Bronchial asthma (BA) is a prevalent chronic respiratory condition characterized by airway inflammation, heightened bronchial responsiveness, and reversible airflow obstruction.[1,2] Among pediatric populations, bronchial asthma represents a significant health concern, with a rising prevalence observed globally.[3] The escalating burden of this condition necessitates a comprehensive understanding of its underlying pathophysiology, accurate diagnostic strategies, and effective management approaches to optimize outcomes and enhance the quality of life for children affected by asthma.
Children with BA manifest a spectrum of respiratory symptoms encompassing coughing, wheezing, dyspnea, and thoracic constriction, notably in the early morning hours or during the nocturnal period.[4,5] The upsurge in the incidence of muscle spasms within the bronchiolar linings in patients with BA significantly contributes to compromised respiratory function.[6] This bronchospasm results in constriction of the airways, which impedes the inflow and outflow of air from the lungs, leading to reduced oxygen uptake into the bloodstream.[7] As such, oxygen delivery is hampered, leaving those patients more vulnerable to a range of respiratory and cardiovascular issues. Prior studies have proven the association between obesity and both the incidence and severity of asthma.[8] Obesity is characterized by a chronic state of low-grade systemic inflammation.[9] This inflammatory milieu is primarily attributed to the enlarged adipocytes, which actively promote the synthesis and release of adipocytokines. These adipocytokines play a central role in activating endothelial cells and facilitating the recruitment of macrophages into the adipose tissue, thereby initiating a cascade of inflammatory responses.[10,11] Consequently, the increased production of adipocytokines, in conjunction with the underlying predisposition to asthma development, can intensify airway inflammation and contribute to the progression of asthma towards a more severe and refractory phenotype. The coexistence of obesity and BA poses unique risks and complications, necessitating tailored interventions to optimize health outcomes.
The Global Initiative for Asthma guidelines outlined explicit criteria for diagnosing and implementing various treatment modalities for individuals with asthma. The primary goals of BA management encompass symptom control, risk mitigation, and the promotion of active participation in everyday activities. Additionally, there is a focus on minimizing the adverse effects associated with medication usage.[12] It has been observed that children with asthma tend to participate in physical activities to a lesser extent than their healthy peers, potentially attributable to the apprehension of experiencing symptoms during or following exercise.[13,14] While in contrast, epidemiological studies have established significant associations between reduced levels of physical activity and the prevalence of asthma. Notably, these findings underscore that children with lower levels of physical activity are subject to an increased risk, potentially reaching up to 35%, of developing new-onset asthma.[15]
Aerobic training has emerged as a valuable adjunctive therapy in the treatment of children with BA. This approach effectively mitigates bronchial hyperresponsiveness and exercise-induced bronchospasm, leading to a reduction in corticosteroid consumption. Moreover, it has been observed to improve dyspnea scores and social development scores, while also promoting improvements in resting lung function.[16,17] However, the optimal training approach (i.e., the exercise intensity, volume, and progression rate) remains a subject of debate. Two commonly employed exercise protocols are constant-load (CL-AE) and graded aerobic exercise (G-AE). The CL-AE refers to a training paradigm that involves maintaining a consistent level of intensity and volume throughout the entire duration of the training program. In this type of training, individuals engage in activities such as walking/running or cycling at a steady pace without any significant fluctuations in intensity.[18] The G-AE is a training method that allows for an incremental transition to practice by increasing the intensity and volume of exercise over time, leading to predominant physiological adaptations.[19,20] Studies have shown that either the CL-AE or G-AE can help improve lung function, enhance cardiorespiratory fitness, and boost functional capacity in individuals with asthma. Furthermore, these exercise modalities have been associated with a reduction in asthma symptoms, increased physical activity level, improved quality of life, and decreased reliance on medication.[16–18,20,21]
Although the therapeutic benefits of aerobic exercise in the management of BA have garnered considerable attention, there remains a lack of consensus regarding the optimal training approach. Therefore, this study was designed to compare the effects of G-AE and CL-AE protocols on the cardiopulmonary fitness and functional capacity in a cohort of obese children with BA.
2. Materials and methods
2.1. Experimental design and ethics
This was a prospective, single-blind, three-arm randomized controlled trial undertaken between October 2022 and December 2023 at the Physical Therapy Outpatient Clinic and Cardiopulmonary Assessment Unit of the College of Applied Medical Sciences at Prince Sattam bin Abdulaziz University (PSAU), Al-Kharj, KSA. The trial (Protocol #: RHPT/0022/023) was approved by the Physical Therapy Research Ethics Committee at PSAU on September 29th, 2022. The study adhered to the ethical principles outlined in the 1975 Declaration of Helsinki and its subsequent revisions. Prior to enrollment, parents/legal guardians were provided with a comprehensive explanation of the study’s objectives, benefits, and potential risks, and their informed consent was obtained. Also, the participants themselves provided assent to take part in the study. The study was registered at ClinicalTrial.gov (Identifier: NCT06326632).
2.2. Study population
In this trial, 78 outpatient children with verified asthma diagnosis per the Global Initiative for Asthma criteria[12] (i.e., experienced multiple asthma symptoms that tended to worsen at night or upon awakening and were often triggered by cold air, allergens, or physical activity and demonstrated positive bronchodilator reversibility: meaning that, after receiving a bronchodilator, their forced expiratory volume in 1 second increased by at least 12% and 200 mL, measured 10–15 minutes later) were recruited from the Pulmonary Medicine/Critical Care and Allergy-Immunology Departments at King Khalid Hospital and 2 referral hospitals at Al-Kharj, KSA. These children were between the ages of 8 and 18 years, had a body mass index ranging from 30 to 35 kg/m2, had a moderate onset of asthma characterized by a predicted peak expiratory flow rate value between 60% to 80%,[22] were under optimal medical treatment, were clinically stable (that is, they experienced daytime symptoms no more than twice per week, had no asthma-related activity limitations, experienced nighttime symptoms or awakenings no more than twice per month, and used rescue or reliever medication no more than twice per week),[12] maintained consistent medication dosages over the previous 3 months and were not in need to alter types/dosages throughout the study’s duration, and were not engaged in regular exercise regimens excluding the occasional physical therapy sessions and school-based physical education classes. Children who had exacerbated asthma symptoms requiring the administration of systemic corticosteroids, chronic lung conditions that posed higher health risks compared to asthma, or cardiovascular and/or musculoskeletal conditions that were expected to hinder their ability to take part in the intended exercise regimen were deemed ineligible and were excluded.
2.3. Sample size determination
A priori power analysis was conducted through the PASS software, version 14.0.15 (NCSS, Kaysville, UT) to ascertain the appropriate sample size necessary to yield clinically meaningful results. In a one-way analysis of variance (ANOVA) study, a total sample size of 63 children (i.e., 21 children for each of the 3 groups under investigation) was required to achieve 90% power to detect differences among the means versus the alternative of equal means, while utilizing an F-test with an alpha level of.05. The analysis was conducted based on peak oxygen uptake (VO2peak) data obtained from a preliminary small-scale study of 12 children with BA. These children underwent interventions that were reflective of the approaches employed in the current large-scale study. The size of the variation in the VO2peak means was represented by their standard deviation which was 2.26 mL/min/kg. The common within-group standard deviation was assumed to be 4.90 mL/min/kg. The sample size was inflated to 78 children (26 per group) in consideration of an anticipated dropout rate of 20% in each group.
2.4. Randomization and masking
The random allocation of participating children into the 3 intervention arms (control group; n = 26, CL-AE group; n = 26, or G-AE group; n = 26) was carried out by an independent researcher who had no involvement in the current study. To ensure group equivalence and unbiased participant allocation, a permuted block randomization approach was adopted, utilizing blocks of varying sizes. Within each block, a series of sealed opaque envelopes, numbered accordingly, was prepared. Upon formal enrollment of each participant, the independent researcher systematically opened the next envelope in the predetermined block sequence, thereby allocating the participant to the corresponding intervention arm.
To uphold the scientific rigor and impartiality of the research, a stringent single-blind approach was adopted in this study. The outcome assessor was completely independent of the treatment delivery process, remained blinded to the treatment allocation of participating children, and was not involved in statistical analyses.
2.5. Measurements
2.5.1. Cardiopulmonary fitness
The cardiopulmonary fitness was assessed through the McMaster incremental cycling protocol,[23] which uses an electro-magnetically braked cycle ergometer (EE; Excalibur Sport, Lode TM, Netherlands). The study measured several specific parameters during a symptom-free exercise tolerance test (ETT), including peak oxygen uptake (VO2peak, mL/kg/min), minute ventilation (VE, L/min), ventilation-oxygen uptake ratio (VE/VO2), stroke volume of oxygen (SVO2, mL/min/beat), oxygen/carbon-dioxide exchange ratio (O2/CO2 ratio), heart rate maximum (HRmax, beat/min), and heart rate recovery at one minute (HRR1, beat/min). Detailed tips and guidelines were provided to all participants both in written and oral form prior to the testing. In order to establish standardized EET procedures, participants were instructed to adhere to certain guidelines. These guidelines included avoiding the consumption of large meals within 3 hours before the test, refraining from consuming beverages containing caffeine on both the day before and the day of the test, abstaining from engaging in strenuous activities the day before the measurement, and wearing loose-fitting comfortable clothing and athletic sneakers. A pretest familiarization session was dedicated to all participants to provide them with an opportunity to familiarize themselves with the testing methodologies. The test was rigorously implemented on both the pre- and post-intervention occasions, with meticulous attention given to maintaining identical circumstances and scheduling the test at the same designated daytime.
The test protocol involved an initial load of 25 W, which was progressively increased by 12.5 W, 25 W, or 50 W at 2-minute intervals, depending on each participant’s gender and height, while maintaining a steady cycling pace of 50 to 70 rpm. Once the test was completed, a 3-minute active rest period was allocated, during which participants continued cycling at a load of 25 W, followed by a subsequent 3-minute passive rest period. Participants were urged verbally to sustain their exercise efforts until they reached their maximum capacity to maintain the required pedaling pace during the ETT. A portable gas analyzer (Med-graphics, CPX/D system, St. Paul, MN) was used to analyze expired gases continuously on a breath-by-breath basis. Throughout the test, participants were continuously monitored using a 12-lead electrocardiogram (CASE™ Exercise Testing, GE Healthcare Inc., Milwaukee, WI). The participants’ perceived exertion levels during the ETT were assessed using the 11-point children’s OMNI-cycle scale, which involved the use of pictorial descriptions and numerical categories ranging from 0 (“Extremely easy”) to 10 (“Extremely hard”).[24] To validate VO2peak values, several factors were considered. These included the leveling-off of VO2 (indicating a plateau in oxygen consumption despite increased effort), the presence of symptoms of extreme exhaustion: such as flushing face, hyperpnea, and/or unsteady posture, a perceived exertion rate on the OMNI-cycle scale that was higher than 7, an O2/CO2 exchange ratio equal to or >1.10, and a HRmax equal to or >85% of the age-predicted value.[20,25]
2.5.2. Functional capacity
The submaximal functional capacity was evaluated using the 6-minute walk test (6-MWT), in strict accordance with the established guidelines promulgated by the American Thoracic Society.[26] After a period of rest lasting 20 minutes, participants were instructed to walk back and forth along across a level corridor measuring 30 m in length, refraining from engaging in any form of running, for a duration of 6 minutes. Participants were explicitly notified of their prerogative to discontinue the test at their own discretion and request. In compliance with the standardized protocol, verbal encouragement was provided to the children at one-minute intervals, while they were directed to discontinue walking and maintain their current position as the designated time elapsed. Subsequently, the distance covered by each child was meticulously quantified in meters.
After the 6MWT, additional exploratory measures were employed to evaluate perceived dyspnea and fatigue, utilizing Borg’s category ratio scale (CR-10).[20,27] To evaluate dyspnea, participants were instructed to rate the level of difficulty they experienced in breathing after completing the 6MWT on a 12-point scale. This scale ranged from 0, representing the lowest value indicating no breathing difficulty, to 10, representing the highest value indicating the presence of maximal breathing difficulty. To assess fatigue perception, participants were similarly prompted to rate the level of exertion they felt after the 6-MWT on the 12-point scale. This scale ranged from 0, indicating the minimum score signifying no fatigue, to 10, representing the maximum score signifying the presence of significant fatigue.
2.6. Interventions
2.6.1. Graded aerobic training
The G-AE group participants took part in a 12-week aerobic training, 3 times a week, in accordance with the protocol outlined in a previous study.[20] A qualified exercise physiologist guided the program, ensuring adherence to recommended exercise prescription guidelines.[28] The training was conducted on a motor-driven, indoor treadmill (HP Cosmos Mercury® Med, Nussdoerf-Traunstien, Germany) and was adapted to accommodate each child’s capacity. Initially (i.e., over the first 2 weeks), the training lasted 25 minutes, with a walking pace set at 50% of their HRmax. Thereafter, the training was systematically elevated by 5% HRmax and 5 minutes every 2 weeks, culminating in an intensity target of 75% HRmax for a duration of 50 minutes during the last 2 weeks. The initial training intensity and subsequent adjustments were based on the HRmax measurements derived from the pretreatment ETT. The specifics and progression pace of the G-AE training are presented in Table S1, Supplemental Digital Content, http://links.lww.com/MD/O58.
To gear up for the G-AE workout, participants engaged in a 5-minute warm-up. During this phase, they performed a range of activities, including general stretching exercises (both static and dynamic), pedaling on a stationary bicycle without resistance, brisk walking on a treadmill, and arm movements with controlled breathing. To wind down after the workout, a 5-minute cool-down was implemented, which mirrored these activities and aided in returning them to the resting state. The participants’ HR was continuously monitored at 5-second intervals using an HR sensor (S810i; Polar, Kempele, Finland). They were advised to avoid strenuous physical activities or consume food for a minimum of 2 hours before training. Importantly, treadmill training was chosen for the study due to its well-established benefits: it engages a wide range of muscles, keeps the whole body active, and provides a relatively comfortable experience during extended training as indicated by previous research.[19,20,29]
2.6.2. Constant-load aerobic training
Participants in the CL-AE group underwent a moderate-intensity aerobic training program, with an intensity set at 65% of their HRmax. They engaged in 45-minute training sessions, 3 times a week, over a consecutive 12-week period. The training sessions were conducted under the close guidance of an experienced (>5 years) exercise therapist. All training sessions took place on a treadmill, providing a controlled and standardized environment. Both the training intensity and duration were maintained at the same level throughout the entire program. A rigorous attention was given to the preparation and safety measures for the CL-AE group, which mirrored those implemented for the G-AE group. This entailed a standardized warm-up routine to prepare the participants physically and mentally, continuous monitoring of the HR during the workout, and a systematic cool-down to facilitate recovery after training.
2.6.3. Concomitant respiratory retraining
A 30-minute respiratory retraining program was administered to participants in the control, CL-AE, and G-AE groups, 3 times weekly for 12 weeks in succession. The primary focus was on alleviating hyperventilation, promoting a normal pattern of breathing, and enhancing the strength of respiratory muscles. Each participant received an individually tailored program that was carefully designed to address their unique needs. The implementation of the program was overseen by 3 proficient physical therapists who possessed specialized knowledge in the area of respiratory rehabilitation for BA. The program incorporated several components to target different facets of respiratory function, as outlined below.
i. Diaphragmatic breathing exercises: children were instructed to adopt a slow and deep breathing pattern, emphasizing the engagement of the diaphragm.
ii. Breath control and breath-hold: children engaged in breath-holding exercises at the end of the inspiratory and expiratory phases to enhance alveolar ventilation and correct hyperventilation, respectively.
iii. Pursed-lip breathing: children were guided to inhale through the nose and focus on generating expiratory pressure against pursed lips.
iv. Respiratory muscle strengthening: specifically, threshold inspiratory muscle training was employed, whereby children were trained to breathe through a device equipped with a valve that opened at predetermined pressure thresholds.
v. Manual techniques: the therapist administered diaphragmatic release and thoracic lymphatic pumping techniques in order to facilitate optimal respiratory function.
vi. Postural orientation: children were taught to lean forward, aiming to minimize respiratory effort and optimize breathing mechanics.
vii. Relaxation exercises: children learned to sequentially tighten and release various muscle groups (i.e., neck, shoulder, chest, and abdominal muscles), focusing on the sensations associated with muscle relaxation.
2.7. Data analysis
The normality assumptions of all the data sets were checked using the Anderson-Darling normality test. The group-by-time interaction effect was calculated through a 2 × 3 mixed-model ANOVA test, upon which the difference between the study groups was decided. The ANOVA model included one within-subject factor (2 time-points: pre/post) and one between-subject factor (3 intervention-based groups: control/CL-AE/G-AE). To determine if significant differences exist between the pairs of groups, pairwise comparisons were carried out using Tukey honest significance test. In instances where the ANOVA interaction effect was found to be statistically significant, the paired t test was applied to calculate the pre-to-post changes within each group. The effect size for the significant between-group differences was computed using the partial eta-squared (η2partial) formula. On the other hand, the effect size of significant within-group changes was calculated using Hedges’ g formula. Minitab statistical software, version 19.2 (Minitab Inc., State College, PA), was utilized to perform all statistical computations. A significance level of less than.05 was adopted for all statistical tests.
3. Results
3.1. Participants’ flow and retention
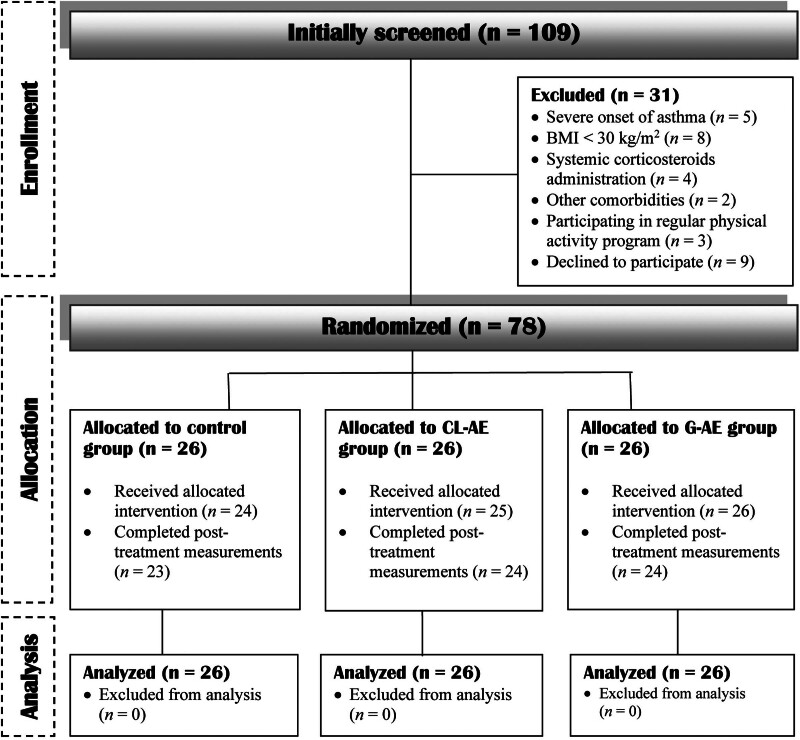
The progress of the study participants is illustrated in Figure 1. Seventy-eight, out of 109 potentially-eligible children, met the inclusion criteria and were randomized to the study groups (26 for each group). Seven participants, corresponding to ~9% of the total sample, faced challenges in either completing the assigned treatment or undergoing the follow-up measurements. Specifically, 3 participants hailed from the control group, 2 from the CL-AE group, and 2 from the G-AE group. Withdrawal or loss to follow-up stemmed from various factors, including scheduling conflicts, travel outside the working area, and unrevealed personal circumstances. Nonetheless, in line with the intention-to-treat principle, missing data were handled using a multiple regression imputation approach and were incorporated into the analysis.
Figure 1.
A flow diagram illustrating the participants’ progress throughout the various phases of the study.
3.2. Baseline characteristics
An overview of the participants’ baseline characteristics (demographic and clinical) is given in Table 1. The control, CL-AE, and G-AE groups demonstrated homogeneity in terms of age, anthropometric attributes, gender distribution, and pubertal status (all P > .05). Moreover, the 3 groups exhibited comparability in clinical aspects relevant to BA, such as age of onset (P = .30), duration of asthma symptoms (P = .25), peak expiratory flow (P = .32), medications (budesonide dose [P = .49], short β2 agonist dose [P = .41], and use of montelukast sodium [P = .56]), and number of children admitted to hospitals in the last year (P = .86).
Table 1.
The demographic and clinical baseline characteristics of participants in the study groups.
| Control group (n = 26) |
CL-AE group (n = 26) |
G-AE group (n = 26) |
P-value | |
|---|---|---|---|---|
| Age, year | 13.92 ± 2.19 | 14.38 ± 2.33 | 14.12 ± 2.45 | .77* |
| Gender (M/F), n (%) | 17 (65.4)/9 (34.6) | 14 (53.8)/12 (46.2) | 19 (73.1)/7 (26.9) | .39** |
| Weight, kg | 66.31 ± 10.43 | 68.54 ± 9.28 | 67.19 ± 11.48 | .74* |
| Height, m | 1.43 ± 0.11 | 1.47 ± 0.12 | 1.45 ± 0.13 | .59* |
| BMI, kg/m2 | 32.08 ± 1.17 | 31.75 ± 1.99 | 31.95 ± 1.26 | .64* |
| Pubertal maturation (y/n), n (%) | 14 (53.8)/12 (46.2) | 16 (61.5)/10 (38.5) | 15 (57.7)/11 (42.3) | .96** |
| Asthma duration, years | 7.19 ± 2.21 | 8.23 ± 2.44 | 7.81 ± 2.02 | .25* |
| Age at onset, year | 6.73 ± 1.56 | 6.15 ± 1.01 | 6.31 ± 1.52 | .30* |
| PEF, predicted % | 71,38 ± 3.23 | 69.88 ± 3.18 | 70.32 ± 4.54 | .32* |
| Short β2 agonist dose, μg | 466.92 ± 71.11 | 443.27 ± 62.74 | 450.58 ± 60.34 | .41* |
| Montelukast sodium (y/n), n (%) | 19 (73.1)/7 (26.9) | 15 (57.7)/11 (42.3) | 17 (65.4)/9 (34.6) | .56** |
| Budesonide, mg/day | 0.78 ± 0.10 | 0.80 ± 0.10 | 0.77 ± 0.09 | .49* |
| Hospitalized last year, (y/n), n (%) | 6 (23.1)/20 (76.9) | 3 (11.5)/23 (88.5) | 5 (19.2)/21 (80.8) | .86** |
Continuous variables are listed as mean ± StDev while categorical variables are presented as frequency (%).
BMI = body mass index, CL-AE = constant-load aerobic exercise, G-AE = graded aerobic exercise, M/F = male/female, PEF = peak expiratory flow, y/n = yes/no.
One-way ANOVA test.
Fishers’ exact test.
3.3. Cardiopulmonary fitness
The findings of the 2 × 3 mixed-model ANOVA, presented in Table 2, indicated a significant group-by-time interaction effect of large magnitude on VO2peak (F2,75 = 27.39, P < .001, η2Partial = 0.42), VE (F2,75 = 16.92, P < .001, η2Partial = 0.31), VE/VO2 (F2,75 = 32.44, P < .001, η2Partial = 0.46), SVO2 (F2,75 = 19.82, P < .001, η2Partial = 0.34), O2/CO2 (F2,75 = 8.39, P = .0005, η2Partial = 0.18), HRmax (F2,75 = 16.85, P < .001, η2Partial = 0.31), and HRR1 (F2,75 = 6.74, P = .002, η2Partial = 0.15). The post hoc Tukey pairwise analysis, as depicted in Table 3, unveiled that the G-AE group exhibited statistically significant and advantageous changes in cardiopulmonary fitness, surpassing those observed in the CL-AE group (VO2peak [P = .03], VE [P = .021], VE/VO2 [P = .032], SVO2 [P = .025], O2/CO2 [P = .004], HRmax [P = .016], and HRR1 [P = .046]) or the control group (VO2peak [P = .037], VE [P = .001], VE/VO2 [P = .02], SVO2 [P = .023], O2/CO2 [P = .0006], HRmax [P = .005], and HRR1 [P = .033]).
Table 2.
Change differences in cardiopulmonary fitness variables between the control, CL-AE, and G-AE groups.
| Control group (n = 26) |
CL-AE group (n = 26) |
G-AE group (n = 26) |
G-by-T interaction effect | ||
|---|---|---|---|---|---|
| P-value | η 2 partial | ||||
| VO2peak, mL/kg/min | |||||
| Pre | 30.73 ± 2.93 | 29.47 ± 3.72 | 28.91 ± 2.24 | <.001* | 0.42 |
| Post | 31.30 ± 3.25 | 32.45 ± 2.18 | 36.55 ± 3.44 | ||
| P-value | .19 | .0006* | <.001* | ||
| Hedges’ g (95% CI) | 0.18 (0.07–0.29) | 0.95 (0.41–1.52) | 2.55 (1.66–3.58) | ||
| VE, L/min | |||||
| Pre | 74.27 ± 3.63 | 75.53 ± 3.26 | 74.72 ± 5.10 | <.001* | 0.31 |
| Post | 75.83 ± 5.64 | 76.78 ± 4.76 | 83.61 ± 5.81 | ||
| P-value | .10 | .09 | <.001* | ||
| Hedges’ g (95% CI) | 0.32 (0.06–0.71) | 0.29 (0.05–0.65) | 1.58 (0.94–2.29) | ||
| VE/VO2 | |||||
| Pre | 41.38 ± 3.94 | 41.85 ± 4.79 | 42.69 ± 3.63 | <.001* | 0.46 |
| Post | 40.73 ± 4.56 | 39.85 ± 5.45 | 33.34 ± 4.33 | ||
| P-value | .24 | .023* | <.001* | ||
| Hedges’ g (95% CI) | 0.15 (0.17–0.48) | 0.37 (0.05–0.72) | 2.27 (1.51–3.15) | ||
| SVO2, mL/min/beat | |||||
| Pre | 7.45 ± 1.16 | 7.39 ± 1.22 | 7.52 ± 1.26 | <.001* | 0.34 |
| Post | 7.68 ± 0.84 | 7.75 ± 1.10 | 9.18 ± 1.29 | ||
| P-value | .15 | .01* | <.001* | ||
| Hedges’ g (95% CI) | 0.22 (0.07–0.53) | 0.30 (0.08–0.54) | 1.26 (0.78–1.81) | ||
| O2/CO2 ratio | |||||
| Pre | 1.11 ± 0.04 | 1.12 ± 0.06 | 1.10 ± 0.05 | .0005* | 0.18 |
| Post | 1.06 ± 0.06 | 1.03 ± 0.08 | 0.95 ± 0.09 | ||
| P-value | <.001* | <.001* | <.001* | ||
| Hedges’ g (95% CI) | 0.95 (0.51–1.44) | 1.23 (0.83–1.89) | 1.99 (1.26–2.84) | ||
| HRmax, beat/min | |||||
| Pre | 184 ± 8 | 181 ± 7 | 183 ± 8 | <.001* | 0.31 |
| Post | 187 ± 8 | 191 ± 8 | 199 ± 5 | ||
| P-value | 0.08 | <.001* | <.001* | ||
| Hedges’ g (95% CI) | 0.36 (0.01–0.73) | 1.29 (0.68–1.96) | 2.32 (1.64–3.15) | ||
| HRR1, beat/min | |||||
| Pre | 30 ± 7 | 29 ± 8 | 32 ± 7 | .002* | 0.15 |
| Post | 31 ± 6 | 33 ± 6 | 38 ± 5 | ||
| P-value | .044* | .0006* | <.001* | ||
| Hedges’ g (95% CI) | 0.15 (0.08–0.39) | 0.55 (0.23–0.89) | 0.96 (0.56–1240) | ||
The variables are displayed as (mean ± StDev).
Hedges’ g: effect size for the within-group difference, η2partial: effect size for the between-group difference.
CI = confidence interval, CL-AE = constant-load aerobic exercise, G-AE = graded aerobic exercise, G-by-T = group-by-time, HRmax = heart rate maximum, HRR1 = heart rate recovery at one minute, O2/CO2 = oxygen/carbon-dioxide exchange ratio, SVO2 = stroke volume of oxygen, VO2peak = peak oxygen uptake, VE = minute ventilation, VE/VO2 = ventilation-oxygen uptake ratio.
Significant at P ˂ .05.
Table 3.
Summary of pairwise comparisons (Tukey honest significance) regarding measures of cardiopulmonary fitness.
| Variable | CL-AE vs Control | G-AE vs Control | CL-AE vs G-AE | |||
|---|---|---|---|---|---|---|
| MD | P-value | MD | P-value | MD | P-value | |
| VO2peak, mL/kg/min | –0.059 | .99 | 1.714 | .037* | –1.773 | .03* |
| VE, L/min | 1.10 | .57 | 4.12 | .001* | –3.11 | .021* |
| VE/VO2 | –0.21 | .98 | –3.04 | .02* | 2.83 | .032* |
| SVO2, mL/min/beat | 0.009 | .99 | 0.79 | .023* | –0.78 | .025* |
| O2/CO2 ratio | –0.008 | .83 | –0.052 | .0006* | 0.044 | .004* |
| HRmax, beat/min | 1 | .91 | 5 | .005* | –5 | .016* |
| HRR1, beat/min | 0.25 | .98 | 5 | .033* | –4 | .046* |
CL-AE = constant-load aerobic exercise, G-AE = graded aerobic exercise, HRmax = heart rate maximum, HRR1 = heart rate recovery at one minute, MD = mean difference of pre-to-post changes, O2/CO2 = oxygen/carbon-dioxide exchange ratio, SVO2 = stroke volume of oxygen, VE/VO2 = ventilation-oxygen uptake ratio, VE = minute ventilation, VO2peak = peak oxygen uptake.
Significant at P < .05.
3.4. Functional capacity
The analysis, as demonstrated in Table 4, yielded a significant group-by-time interaction of large magnitude on the 6-MWT distance (F2,75 = 36.96, P < .001, η2Partial = 0.49), Borg’s CR-10 dyspnea score (F2,75 = 19.02, P < .001, η2Partial = 0.33), and Borg’s CR-10 fatigue score (F2,75 = 9.92, P = .0002, η2Partial = 0.21). The post hoc pairwise comparison, as shown in Table 5, indicated that the G-AE group exhibited statistically significant and more favorable changes in the functional capacity as compared to the CL-AE group (6-MWT distance [P = .021], Borg’s CR-10 dyspnea score [P = .041], Borg’s CR-10 fatigue score [P = .04]) or the control group (6-MWT [P = .044], Borg’s CR-10 dyspnea score [P = .002], Borg’s CR-10 fatigue score [P = .032]).
Table 4.
Change-differences in functional capacity (6-MWT distance) and associated dyspnea and fatigue perception.
| Control group (n = 26) |
CL-AE group (n = 26) |
G-AE group (n = 26) |
G-by-T interaction effect | ||
|---|---|---|---|---|---|
| P-value | η 2 partial | ||||
| 6-MWT, m | |||||
| Pre | 458.77 ± 60.79 | 445.27 ± 63.30 | 456.81 ± 66.88 | <.001* | 0.49 |
| Post | 460.50 ± 58.38 | 463.81 ± 60.29 | 547.23 ± 81.65 | ||
| P-value | .71 | .016* | <.001* | ||
| Hedges’ g (95% CI) | 0.03 (-0.18–0.12) | 0.29 (0.05–0.55) | 1.17 (0.77–1.64) | ||
| Borg’s CR-10, dyspnea | |||||
| Pre | 6.81 ± 0.69 | 6.65 ± 1.02 | 6.96 ± 0.92 | <.001* | 0.33 |
| Post | 5.92 ± 0.98 | 5.58 ± 1.14 | 4.12 ± 1.42 | ||
| P-value | <.001* | .0001* | <.001* | ||
| Hedges’ g (95% CI) | 1.02 (0.54–1.55) | 0.96 (0.47–1.49) | 2.30 (1.53–3.19) | ||
| Borg’s CR-10, fatigue | |||||
| Pre | 6.54 ± 1.03 | 6.65 ± 0.94 | 6.72 ± 0.92 | .0002* | 0.21 |
| Post | 5.42 ± 1.14 | 5.27 ± 1.17 | 4.10 ± 1.10 | ||
| P-value | .0001* | <.001* | |||
| Hedges’ g (95% CI) | 0.99 (0.52–1.53) | 1.26 (0.70–1.89) | 2.51 (1.66–3.49) | ||
The variables are displayed as (mean ± StDev).
Hedges’ g: effect size for the within-group difference, η2partial: effect size for the between-group difference.
6-MWT = six-minute walking test, CL-AE = constant-load aerobic exercise, CR = category ratio-scale, G-AE = graded aerobic exercise, G-by-T = group-by-time.
Significant at P ˂ .05.
Table 5.
Overview of pairwise comparisons (Tukey honest significance) for the functional capacity variables.
| Variable | CL-AE vs Control | G-AE vs Control | CL-AE vs G-AE | |||
|---|---|---|---|---|---|---|
| MD | P-value | MD | P-value | MD | P-value | |
| 6-MWT, m | –5.10 | .95 | 42.38 | .044* | –47.48 | .021* |
| CR-10, dyspnea | –0.25 | .53 | –0.83 | .002* | 0.57 | .041* |
| CR-10, fatigue | –0.02 | .99 | –0.57 | .032* | 0.56 | .04* |
6-MWT = six-minute walking test, CL-AE = constant-load aerobic exercise, CR = category ratio-scale, G-AE = graded aerobic exercise, MD = mean difference of pre-to-post changes.
Significant at P < .05.
4. Discussion
This study set out with the aim of analyzing the effect of CL-AE versus G-AE on cardiopulmonary fitness and functional capacity of children with BA. Perhaps, the most clinically relevant findings of the present study are the remarkable improvement in all cardiopulmonary fitness indicators (i.e., VO2peak, VE, VE/VO2, SVO2, O2/CO2 ratio, HRmax, HRR1) in the G-AE group in comparison with the CL-AE group or the control group. Another finding that stands out from the results reported earlier is the significantly enhanced functional capacity (evidenced by a greater distance coverage in the 6-MWT and lesser dyspnea and fatigue perception on the Borg’s CR-10 scale) in the G-AE group relative to the CL-AE or control group.
Although extensive research has been carried out on the effectiveness of aerobic exercise for children with BA,[16–18,20,21] the present report represents a novel initiative in conducting a comparative analysis of the effects of an intensity-/duration-G-AE regimen versus a CL-AE regimen in pursuance of determining the training strategy that exhibits heightened effectiveness and engagement, yields favorable health outcomes, and aligns more appropriately with the needs of children diagnosed with BA. Specifically, the intensity-/duration-G-AE regimen entailed a 2-week training cycle, commencing with an intensity equivalent to 50% HRmax, performed for 25 minutes in the inaugural 2 weeks. The cycle culminated with a training intensity corresponding to 75% HRmax, applied for 50 minutes during the final couple of weeks. In contrast, the CL-AE regimen involved training at a constant intensity corresponding to 65% HRmax for a duration of 45 minutes, sustained over a period of 12 weeks. Therewith, the significance of the current findings cannot be disregarded, as they provide a compelling and unprecedented insight that can guide and optimize the effectiveness of aerobic training regimens, hastening the advancement of cardiopulmonary fitness and functional capacity in children and/or adolescents with moderate BA.
The current study yielded a prominent finding indicating that G-AE exhibited superior efficacy in enhancing VO2peak compared to CL-AE among children with BA. While the specific underlying mechanism remains uncertain, it is reasonable to posit that the observed increase in VO2peak can be attributed to enhanced cardiac function and/or improved oxygen extraction. This supposition gains support from the elevated levels of HRmax and SVO2, which lend credence to this claim. Alternatively, it is conceivable that the observed increase is linked to a partial alleviation of respiratory constraints experienced by these children. From our vantage point, combining the elements of both hypotheses holds the potential to yield a robust and persuasive explanation. The G-AE may have evoked adaptive changes within the respiratory system, enabling the acclimation of cardiac functions. It may have also prompted an enhanced capacity of the muscular system to extract and utilize oxygen during exercise.[19,20,29,30] The favorable increase in VE following G-AE is another intriguing finding. This phenomenon may be attributed to exercise-induced increases in respiratory rate, depth, and tidal volume, which collectively serve to uphold physiological homeostasis.[31,32] It is also feasible that the expiratory time, in relation to the respiratory cycle, has sufficiently been increased to raise the VE volume to a physiologically appropriate level during exercise.[33] What is more in this study, the VE/VO2 ratio exhibited a greater decline after G-AE. This observation suggests a harmonious interplay between perfusion and ventilation processes in response to such a training paradigm, wherein an elevation in ventilation coincides with an increase in oxygen uptake. Besides, the SVO2, HRmax, and HRR1 demonstrated a greater rise following the G-AE, indicating improved cardiovascular and hemodynamic responses. These findings offer further substantiation that the participation of these children in the training regimen was not hindered by cardiac factors, allowing them to engage in the conditioning activities for extended durations.
These findings seem to be consistent with prior research that has emphasized the advantageous effects of G-AE on various parameters of cardiopulmonary fitness. A recent randomized clinical trial has explored the effect of a G-AE regimen in obese children with BA, wherein the training regimen followed a progressive approach, beginning in the first week with an intensity equivalent to 45% HRmax for a duration of 15 minutes. Subsequently, over the course of 8 weeks, the training intensity was incrementally raised, culminating in 80% HRmax and a duration of 50 minutes. Importantly, the findings of this trial revealed significant improvements in VO2peak, VE, HRmax, and HRR1.[20] Furthermore, the findings derived from this study substantiate the prevailing research in this area, indicating a strong association between G-AE and notable improvements in multiple aspects of cardiopulmonary fitness among children presenting with various health conditions, including those who have experienced chest burn injuries,[19] or those who have survived of lymphoblastic leukemia.[29] The consistent alignment of these findings across multiple studies bolsters the scientific backing for G-AE as an intervention strategy in optimizing rehabilitation outcomes for children with BA.
On the question of functional capacity, this study found that, after 12 weeks of G-AE, children covered a significantly longer distance during the 6-MWT and reported lesser dyspnea and fatigue perception right after the test than those who underwent CL-AE. These findings corroborate earlier reports that demonstrated the physical and functional advantages of aerobic exercise for children with BA.[20,34] While still requiring further investigation, the noted improvement in functional capacity may be attributable to an increase in peripheral muscular strength or it could be directly associated with the enhancement in cardiopulmonary fitness. More likely, the interaction between these factors may have been responsible for the observed outcomes. In the present study, a significant difference of 83.42 meters in the 6-MWT distance was identified between the G-AE and CL-AE groups, exceeding the 53-meter difference reported in a previous study.[20] Nonetheless, the clinical relevance of these changes is still a subject of debate, especially considering the lack of established thresholds for minimum clinically important changes in this metric, which would be deemed meaningful to children with BA.[35]
4.1 . Merits and limitations
The rigorous and methodologically sound approach adopted herein (i.e., randomized, controlled, single-blinded design), the sufficient sample size, which was determined a priori to strike the balance between statistical power and practical feasibility, and the relatively high statistical power (β = 90%) are noteworthy merits of the present inquiry. These features synergistically potentiate the study’s capacity to establish true causal relationships, generate reliable findings, and contribute valuable insights into the research question under investigation. Additionally, participants in the 3 groups demonstrated a high level of adherence to the treatment regimens, with minimal reports of any negative training-related effects. Nevertheless, it is crucial to acknowledge that, while the present study yields valuable insights into the research question, there exist limitations that should be taken into account to ensure a nuanced interpretation and generalization of the findings. This study focused on children with clinically stable moderate BA. It is worth noting that clinical stability, being reflective of physician practice rather than the children’s actual experiences, introduces the possibility of sampling bias. As a result, the generalizability of the data to all children with BA may be limited. To address this limitation, future research should consider conducting subgroup analyses based on asthma severity (e.g., mild, moderate, and severe) to obtain more comprehensive and broadly applicable data. The sustainability of the treatment effect presents another element of uncertainty. Given that the outcome measures in this trial were assessed immediately posttreatment, it is imperative to conduct further research to explore the long-term effects at various follow-up time points.
4.2. Clinical and practical implications
The findings demonstrated herein have significant implications for translating research into practice and shaping effective interventions for obese children with BA in clinical settings. Through this research endeavor, evidence-based recommendations for the development of tailored exercise interventions that can optimize health outcomes in obese children with BA could be established. The findings suggest that a graded approach to aerobic training may be the preferred method for improving cardiopulmonary fitness and functional capacity in such a patient population. Compared to CL-AE, the G-AE protocol produced superior enhancements in the key markers of aerobic fitness. This indicates that gradually ramping up the intensity and duration of the training regimen, rather than maintaining a fixed workload, allows these patients to better tolerate and adapt to the aerobic exercise stimulus. The gradual progression likely facilitates progressive physiological adaptations in the cardiopulmonary system, leading to a greater gain in exercise tolerance and functional capacity over time. For clinicians and rehabilitation specialists working with obese children with BA, these findings support the incorporation of G-AE into comprehensive rehabilitation programs, as this training modality is more effective than CL-AE for enhancing the cardiopulmonary fitness and functional capabilities of this population. Still, future research is warranted to elucidate the specific physiological mechanisms underlying the superior effect of G-AE versus CL-AE in obese children with BA.
5. Conclusions
The evidence gleaned from the present study indicates that incorporation of G-AE in the rehabilitation plans for children/adolescents diagnosed with BA yields noteworthy improvements in their cardiopulmonary fitness and functional capacity, surpassing the outcomes achieved through the CL-AE. Nonetheless, despite the intriguing nature of these training paradigms, several unanswered questions persist. Therefore, additional investigations are imperative to corroborate the effects of G-AE in obese patients with BA.
Acknowledgments
The authors of this work are immensely grateful to the children and their families for their enthusiastic participation, as their cooperation and commitment were instrumental in the successful execution of this study.
Author contributions
Conceptualization: Ragab K. Elnaggar, Walaa E. Morsy, Mahmoud S. Elfakharany.
Data curation: Ragab K. Elnaggar, Walaa E. Morsy, Mohammed A. Shendy, Mahmoud S. Elfakharany.
Formal analysis: Ragab K. Elnaggar, Walaa E. Morsy, Mohamed S. Abdrabo, Mohammed A. Shendy, Mahmoud S. Elfakharany.
Funding acquisition: Ragab K. Elnaggar.
Investigation: Ahmad M. Osailan, Mshari Alghadier, Tamer E. Elnegamy, Fahad A. Qissi.
Methodology: Ragab K. Elnaggar, Ahmad M. Osailan, Mshari Alghadier, Tamer E. Elnegamy, Fahad A. Qissi, Mohammed A. Shendy.
Project administration: Ragab K. Elnaggar.
Resources: Ragab K. Elnaggar, Ahmad M. Osailan.
Software: Mshari Alghadier, Walaa E. Morsy, Mohamed S. Abdrabo, Rania R. Mohamed.
Supervision: Ragab K. Elnaggar, Ahmad M. Osailan.
Validation: Mshari Alghadier, Mohamed S. Abdrabo, Rania R. Mohamed, Mahmoud S. Elfakharany.
Writing – original draft: Ragab K. Elnaggar, Ahmad M. Osailan, Mshari Alghadier, Walaa E. Morsy, Mohamed S. Abdrabo, Fahad A. Qissi, Mohammed A. Shendy, Rania R. Mohamed, Mahmoud S. Elfakharany.
Writing – review & editing: Ragab K. Elnaggar, Ahmad M. Osailan, Mshari Alghadier, Walaa E. Morsy, Mohamed S. Abdrabo, Mohammed A. Shendy, Rania R. Mohamed, Mahmoud S. Elfakharany.
Supplementary Material
Abbreviations:
- 6-MWT
- 6-minute walk test
- ANOVA
- analysis of variance
- BA
- bronchial asthma
- CL-AE
- constant-load aerobic exercise
- CR-10
- Borg’s category ratio scale-10
- ETT
- exercise tolerance test
- G-AE
- graded aerobic exercise
- HR
- heart rate
- HRmax
- heart rate maximum
- HRR1
- heart rate recovery at one minute
- O2/CO2
- oxygen/carbon-dioxide exchange ratio
- StDev
- standard deviation
- SVO2
- stroke volume of oxygen
- VE/VO2
- ventilation-oxygen uptake ratio
- VE
- minute ventilation
- VO2peak
- peak oxygen uptake
The authors extend their appreciation to Prince Sattam bin Abdulaziz University for funding this research through the project number (PSAU/2024/03/29517).
The trial (Protocol #: RHPT/0022/023) was approved by the Physical Therapy Research Ethics Committee at PSAU. The study adhered to the ethical principles outlined in the 1975 Declaration of Helsinki and its subsequent revisions.
The study was registered at ClinicalTrial.gov (Identifier: NCT06326632).
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request.
Supplemental Digital Content is available for this article.
How to cite this article: Elnaggar RK, Osailan AM, Alghadier M, Elnegamy TE, Morsy WE, Abdrabo MS, Qissi FA, Shendy MA, Mohamed RR, Elfakharany MS. Exercise strategies for reversing cardiopulmonary deconditioning in obese children with bronchial asthma: A randomized comparative effectiveness study of constant-load and graded aerobic training. Medicine 2024;103:48(e40667).
Contributor Information
Ahmad M. Osailan, Email: a.osailan@psau.edu.sa.
Mshari Alghadier, Email: m.alghadier@psau.edu.sa.
Tamer E. Elnegamy, Email: t.elnegamy@psau.edu.sa.
Walaa E. Morsy, Email: morsy@jazanu.edu.sa.
Mohamed S. Abdrabo, Email: mohamed.samy@pt.cu.edu.eg.
Fahad A. Qissi, Email: ffq1411@gmail.com.
Mohammed A. Shendy, Email: mshendy@taibahu.edu.sa.
Rania R. Mohamed, Email: raniareda22@cu.edu.sa.
Mahmoud S. Elfakharany, Email: mahmoud.samier@pt.cu.edu.eg.
References
- [1].Noutsios GT, Floros J. Childhood asthma: causes, risks, and protective factors; a role of innate immunity. Swiss Med Wkly. 2014;144:w14036. [DOI] [PubMed] [Google Scholar]
- [2].Elnaggar RK, Osailan AM, Elbanna MF. The rationale of applying inspiratory/expiratory muscle training within the same respiratory cycle in children with bronchial asthma: a placebo-controlled randomized clinical investigation. J Asthma. 2023;60:900–11. [DOI] [PubMed] [Google Scholar]
- [3].Serebrisky D, Wiznia A. Pediatric asthma: a global epidemic. Ann Glob Health. 2019;85:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Lizzo JM, Cortes S. Pediatric asthma. StatPearls. Treasure Island (FL): StatPearls Publishing; 2021. [Google Scholar]
- [5].Hellebrandová L, Chlumský J, Vostatek P, Novák D, Rýznarová Z, Bunc V. Airflow limitation is accompanied by diaphragm dysfunction. Physiol Res. 2016;65:469–79. [DOI] [PubMed] [Google Scholar]
- [6].Elnaggar RK, Shendy MA. Efficacy of noninvasive respiratory techniques in the treatment of children with bronchial asthma: a randomized controlled trial. Bull Fac Phys Ther. 2016;21:1–10. [Google Scholar]
- [7].Elnaggar RK, Shendy MA, Mahmoud MZ. Prospective effects of manual diaphragmatic release and thoracic lymphatic pumping in childhood asthma. Respir Care. 2019;64:1422–32. [DOI] [PubMed] [Google Scholar]
- [8].Kim S-H, Sutherland ER, Gelfand EW. Is there a link between obesity and asthma? Allergy Asthma Immunol Res. 2014;6:189–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Lumeng CN, Saltiel AR. Inflammatory links between obesity and metabolic disease. J Clin Invest. 2011;121:2111–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Castro A, Macedo-de la Concha L, Pantoja-Meléndez C. Low-grade inflammation and its relation to obesity and chronic degenerative diseases. Revista Médica del Hospital General de México. 2017;80:101–5. [Google Scholar]
- [11].Elnaggar RK, Shendy MA. Aerobic interval exercises versus dietary control induced changes of adipokines levels, lipid profile and quality of life in overweight or obese children. Int J Physiother. 2016;3:587–93. [Google Scholar]
- [12].Global Initiative for Asthma: global strategy for asthma management and prevention. 2020. Available at: https://ginasthma.org/wp-content/uploads/2020/04/Main-pocket-guide_2020_04_03-final-wms.pdf. Accessed July 13, 2020. [Google Scholar]
- [13].Crosbie A. The effect of physical training in children with asthma on pulmonary function, aerobic capacity and health-related quality of life: a systematic review of randomized control trials. Pediatr Exerc Sci. 2012;24:472–89. [DOI] [PubMed] [Google Scholar]
- [14].Wanrooij VHM, Willeboordse M, Dompeling E, van de Kant KDG. Exercise training in children with asthma: a systematic review. Br J Sports Med. 2014;48:1024–31. [DOI] [PubMed] [Google Scholar]
- [15].Lochte L, Nielsen KG, Petersen PE, Platts-Mills TAE. Childhood asthma and physical activity: a systematic review with meta-analysis and graphic appraisal tool for epidemiology assessment. BMC Pediatr. 2016;16:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Hallstrand TS, Bates PW, Schoene RB. Aerobic conditioning in mild asthma decreases the hyperpnea of exercise and improves exercise and ventilatory capacity. Chest. 2000;118:1460–9. [DOI] [PubMed] [Google Scholar]
- [17].Wu X, Gao S, Lian Y. Effects of continuous aerobic exercise on lung function and quality of life with asthma: a systematic review and meta-analysis. J Thorac Dis. 2020;12:4781–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Aparecido da Silva R, Leite Rocco PG, Stelmach R, et al. Constant-load exercise versus high-intensity interval training on aerobic fitness in moderate-to-severe asthma: a randomized controlled trial. J Allergy Clin Immunol Pract. 2022;10:2596–604.e7. [DOI] [PubMed] [Google Scholar]
- [19].Elnaggar RK, Osailan AM, Alsubaie SF, Moawd SA, Abd El-Nabie WA. Graded aerobic exercise (GAEx): an effective exercise regimen to improve cardio-respiratory fitness and physical and psychosocial functioning in children with burn sequelae of the chest. Burns. 2022;48:337–44. [DOI] [PubMed] [Google Scholar]
- [20].Elnaggar RK, Shendy MA, Elfakharany MS. Effect of 8 weeks of incremental aerobic training on inflammatory mediators, cardiorespiratory indices, and functional capacity in obese children with bronchial asthma. Pediatr Exerc Sci. 2021;33:23–31. [DOI] [PubMed] [Google Scholar]
- [21].Welsh L, Kemp JG, Roberts RG. Effects of physical conditioning on children and adolescents with asthma. Sports Med. 2005;35:127–41. [DOI] [PubMed] [Google Scholar]
- [22].Lemanske RF, Jr. A review of the current guidelines for allergic rhinitis and asthma. J Allergy Clin Immunol. 1998;101(2 Pt 2):S392–6. [DOI] [PubMed] [Google Scholar]
- [23].Bar-Or O, Rowland TW. Pediatric Exercise Medicine: From Physiologic Principles to Health Care Application. Champaign: Human Kinetics; 2004. [Google Scholar]
- [24].Utter AC, Robertson RJ, Nieman DC, Kang J. Children’s OMNI Scale of Perceived Exertion: walking/running evaluation. Med Sci Sports Exerc. 2002;34:139–44. [DOI] [PubMed] [Google Scholar]
- [25].Guazzi M, Adams V, Conraads V, et al. EACPR/AHA Scientific Statement. Clinical recommendations for cardiopulmonary exercise testing data assessment in specific patient populations. Circulation. 2012;126:2261–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111–7. [DOI] [PubMed] [Google Scholar]
- [27].Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14:377–81. [PubMed] [Google Scholar]
- [28].Nyenhuis SM, Kahwash B, Cooke A, Gregory KL, Greiwe J, Nanda A. Recommendations for physical activity in asthma: a work group report of the AAAAI Sports, Exercise, and Fitness Committee. J Allergy Clin Immunol Pract. 2022;10:433–43. [DOI] [PubMed] [Google Scholar]
- [29].Elnaggar RK, Osailan AM, Elbanna MF, Abd-Elmonem AM. Effectiveness of a dose-graded aerobic exercise regimen on cardiopulmonary fitness and physical performance in pediatric survivors of acute lymphoblastic leukemia: a randomized clinical trial. J Cancer Surviv. 2024. doi: 10.1007/s11764-024-01534-1. [DOI] [PubMed] [Google Scholar]
- [30].Ahmaidi SB, Varray AL, Savy-Pacaux AM, Pnefaut CG. Cardiorespiratory fitness evaluation by the shuttle test in asthmatic subjects during aerobic training. Chest. 1993;103:1135–41. [DOI] [PubMed] [Google Scholar]
- [31].Nicolò A, Girardi M, Sacchetti M. Control of the depth and rate of breathing: metabolic vs. non-metabolic inputs. J Physiol. 2017;595:6363–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Nicolò A, Marcora SM, Bazzucchi I, Sacchetti M. Differential control of respiratory frequency and tidal volume during high-intensity interval training. Exp Physiol. 2017;102:934–49. [DOI] [PubMed] [Google Scholar]
- [33].Sim K, Keogh B. Ventilation in severe acute asthma: is there safety in numbers? Thorax. 1994;49:297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Latorre-Román PA, Navarro-Martínez AV, García-Pinillos F. The effectiveness of an indoor intermittent training program for improving lung function, physical capacity, body composition and quality of life in children with asthma. J Asthma. 2014;51:544–51. [DOI] [PubMed] [Google Scholar]
- [35].Westerterp KR. Pattern and intensity of physical activity. Nature. 2001;410:539. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.