Abstract
Acute pancreatitis (AP) is an inflammatory disorder associated with a significant mortality rate in its severe form. This study aimed to evaluate the association between severity of AP and ABO/Rh blood type. Retrospective chart review was conducted on hospitalized patients who met the diagnostic criteria for AP. Data collected included patient demographics, ABO/Rh blood type, etiology of pancreatitis, severity based on the Atlanta classification, and hospital length of stay. The proportion of patients who experienced severe AP was compared amongst combinations of ABO/Rh blood group. Of the 358 patients included in the study, 20.9% had non-mild AP. The proportion of patients in each blood group with non-mild AP was as follows: A: 21.1%, B: 21.4%, AB: 5.9%, O: 30.9%, Rh+: 22.0%, Rh‐: 14.8%. When comparing across A, B, AB, O and Rh groups separately and in combination, there was no statistically significant correlation found between AP severity and ABO/Rh blood type. In this retrospective cohort study, no significant association between ABO/Rh blood group and severity of AP was found, suggesting that the inflammatory cascade in AP is not directly influenced by blood groups.
Keywords: acute pancreatitis, blood group
1. Introduction
Acute pancreatitis (AP) is a common gastrointestinal condition in the United States, accounting for more than 300,000 emergency department visits per year.[1] In the last decade, there has been an increase in the number of admissions for AP, resulting in costly hospitalizations.[1] AP can have a variable course ranging from mild to moderately severe to severe. Severe cases of AP are associated with a mortality rate as high as 10% and 30%.[2] Given the high morbidity and mortality rate, early identification of patients who are at risk for developing severe cases of AP is warranted to improve clinical outcomes.
Prior studies on clinical factors associated with developing severe AP have suggested that BMI > 30 and alcohol etiology leads to higher number of severe episodes.[3–5] Predictors of pancreatitis severity using biochemical parameters have also been explored, including CRP and specific inflammatory cytokines but the results have been variable and inconclusive.[6–8]
Blood group systems are collections of proteins and oligosaccharides expressed on red blood cells and other tissues.[9] They are known to be highly immunogenic and are associated with inflammatory conditions. Pancreatic cells express blood type antigens which may affect the intensity of the inflammatory cascade during AP, potentially playing a role in the development of severe AP (SAP). Studies have shown a weak positive correlation between certain blood types and incidence of AP as well as risk of chronic pancreatitis, but to our knowledge, no study has evaluated its association with severity of AP.[10] The aim of this study was to investigate if a correlation between severity of AP and blood type exists.
2. Materials and methods
2.1. Patient population
This was a single-center retrospective study conducted at a tertiary teaching hospital. The electronic health record was queried for hospitalizations between 2014 and 2022 with admission ICD-10 codes for acute pancreatitis. Eligible cases were those that met the diagnostic criteria for AP based on the revised Atlanta classification and those in whom a blood type was obtained. Patients had to have 2 of the following 3 criteria for diagnosis: (1) typical abdominal pain for pancreatitis; (2) 3-fold increase in serum amylase or lipase; (3) imaging findings consistent with AP.[11] If a patient was hospitalized repeatedly for AP, only the first admission was included in the study. Patients were excluded if they were missing blood type data, had chronic pancreatitis, pancreatic cancer, prior solid organ transplant, or received blood transfusion of a different type within 72 hours of admission which could confound the result given the aim of the study.
2.2. Data collection
Demographic data was collected, including age at the time of admission, gender, race, ABO/Rh blood type. The etiology of AP was grouped into alcohol related, gallstone related, or other which included post procedural pancreatitis, drug induced, idiopathic, and autoimmune pancreatitis. Severity of AP was based on the revised Atlanta classification.[11,12] Hospital length of stay was also recorded.
2.3. Statistical analysis
To summarize demographic data, categorical variables were presented as frequencies and continuous variables were presented as mean and standard deviation. Multivariate logistic regression analysis was conducted using R software to assess for risk factors for SAP and results were expressed as an odds ratio with a 95% confidence interval (CI). Chi-square testing was conducted using GraphPad software to compare proportions of patients with SAP across different blood groups. P ≤ .05 was considered statistically significant.
2.4. Ethical considerations
The study was approved by the Institutional Review Board. Collected data was anonymized.
3. Results
A total of 358 patients were included in the study with demographic data summarized in Table 1. The mean age at the time of hospitalization was 50.7 years with an average length of stay of 5.77 days. The cohort was predominantly Caucasian (48%) with even gender distribution. Among the blood types, type A (35.8%) and type O (45.0%) were most common as well as Rh+ status (84.9%). The distribution of etiology of acute pancreatitis was biliary (36.6%), alcohol (21.5%), and other (41.9%).
Table 1.
Patient demographics.
| Variable | # of patients | Frequency |
|---|---|---|
| Age (yrs) ± SD | 50.7 ± 18.7 | |
| Gender | ||
| Male | 163 | 45.5% |
| Female | 195 | 54.5% |
| Race | ||
| Caucasian | 172 | 48.0% |
| African American | 65 | 18.2% |
| Hispanic/Latino | 72 | 20.1% |
| Other | 49 | 13.7% |
| Blood group | ||
| A | 128 | 35.8% |
| B | 51 | 14.2% |
| AB | 18 | 5.0% |
| O | 161 | 45.0% |
| Rh blood type | ||
| Positive | 304 | 84.9% |
| Negative | 54 | 15.1% |
| Cause | ||
| Biliary | 131 | 36.6% |
| Alcohol | 77 | 21.5% |
| Other | 150 | 41.9% |
| Atlanta classification | ||
| Mild | 283 | 79.1% |
| Moderately severe (MS) | 65 | 18.1% |
| Severe (S) | 10 | 2.8% |
| Length of stay (days) ± SD | 5.77 ± 7.60 | |
| Total # of patients | 358 |
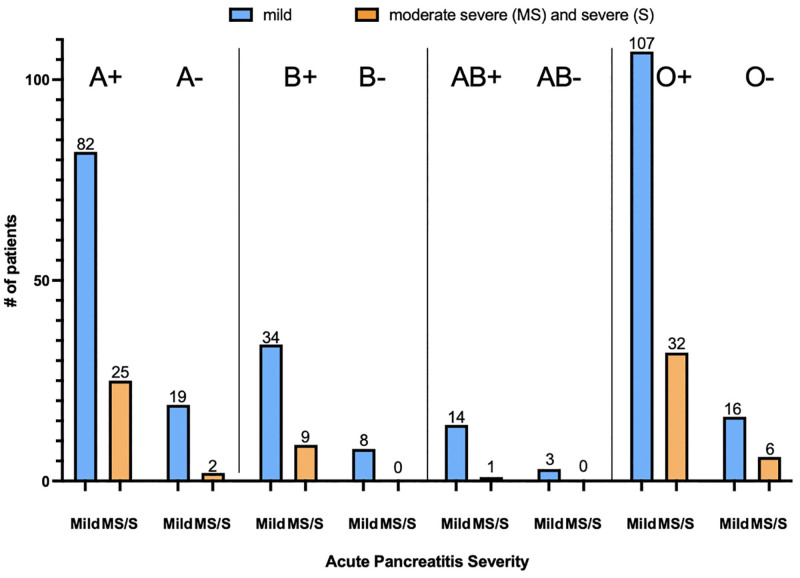
Acute pancreatitis severity was divided into 2 groups: mild vs non-mild AP which included both moderately severe and severe cases of pancreatitis based on the Atlanta classification. Of the 358 patients in this cohort, 283 (79.1%) had mild AP and 75 (20.9%) had non-mild AP. The proportion of patients in each blood group with non-mild AP was as follows: A: 21.1%, B: 21.4%, AB: 5.9%, O: 30.9%, Rh+: 22.0%, Rh‐: 14.8%. Using multivariate logistic regression analysis to exclude confounding variables from collected data, comparison between non-O blood type and O blood type, as well as between Rh+ and Rh‐ status showed no significant correlation with non-mild-AP (Table 2). The notable variable in this study that was associated with more severe cases of AP included male gender (Table 2). Combination of ABO blood type and Rh status was also evaluated using chi-square analysis, and there was no statistically significant correlation found between severe cases of pancreatitis and ABO/Rh combinations (Fig. 1; P = .36).
Table 2.
Multivariable logistic regression for acute pancreatitis severity.
| Variables | Odds ratio (OR) | P-value | 95% Confidence interval (CI) |
|---|---|---|---|
| Age | 0.99 | .61 | 0.98–1.01 |
| Male gender | 2.06 | .0076* | 1.22–3.53 |
| Race | 0.76 | .33 | 0.43–1.32 |
| Caucasian | |||
| Non-caucasian | |||
| Blood type | 1.18 | .54 | 0.70–1.99 |
| Non-O type (A, B, and AB) | |||
| O type | |||
| Rh blood type | 0.62 | .26 | 0.25–1.36 |
| Positive | |||
| Negative | |||
| Cause | 0.80 | .41 | 0.46–1.36 |
| Biliary | |||
| Alcohol | |||
| Other | |||
| Length of stay (LOS) ≥ 3 days | 2.24 | .033* | 1.10–4.98 |
indicates P-value <.05.
Figure 1.
Depicts acute pancreatitis severity stratified by ABO/Rh blood type combination with number of patients per category included on the graph. Chi-square analysis showed no significant association between ABO/Rh blood type combinations and acute pancreatitis severity (P = .36). MS = moderately severe, S = severe, + = Rh positive, ‐ = Rh negative.
4. Discussion
The current consensus is that identifying SAP early is critical to providing more aggressive treatment and improve patient outcomes. Predicting severity of AP was first suggested in 1974 by John Ranson, who developed the Ranson score to identify patients who might benefit from early surgical intervention.[13] Since then, many scoring systems, including the BISAP, MGS, and APACHE II scores which combine laboratory and clinical findings have been introduced but no official consensus has recommended one over the others.[14] Select criteria in these scores were adopted by a panel of experts to develop the Atlanta criteria, which was recently adopted as the best method to date to predict SAP given its consistent performance in many clinical scenarios worldwide.[11,12] The Atlanta criteria bases its prediction on the presence of persistent organ failure based on renal, respiratory, or cardiovascular parameters and localized complications of acute pancreatitis such as necrosis or pseudocyst formation.[11,12] On the other hand, relying on any single or combination of biochemical markers on admission to risk stratify AP patients has not been consistently agreed upon. Studies have found laboratory markers predicting SAP to include admission hematocrit and IL-6.[15,16] Other studies suggest measuring markers that activate trypsinogen since trypsin is one of the main drivers of the inflammatory process in AP.[17,18] These include trypsin activation peptide and carboxypeptidase B activation peptide. Other clinical factors that have been linked with severity of AP include smoking status, pancreatic necrosis, obesity, and bacteremia.[19]
Blood group has been associated with proinflammatory and neoplastic states. Patients with non-O blood groups have approximately a 2-fold increased risk of developing venous thromboembolism, with the B allele also associated with an increased risk of stroke.[20] In Caucasian patients with sepsis or trauma, those with blood type A have increased risk of developing acute respiratory distress syndrome.[21] These associations stem from the idea that ABO antigens are modifiers of glycoproteins, among which include von Willebrand factor. Differential alteration of oligosaccharide chains on von Willebrand factor can alter its metabolism leading to its persistent circulation and a proinflammatory, hypercoagulable state.[22,23] In the context of pancreatic disease, blood group has been associated with pancreatic cancer. In Wolpin et al, analysis of blood groups in 2 large cohorts of men and women revealed that non-O blood type was statistically significantly associated with a 17% increased risk of pancreatic cancer, however, the mechanistic links are unclear.[24] On other hand, with relation to chronic pancreatitis, Greer et al did not find an association between blood groups A, AB, or B and the development of chronic pancreatitis.[10]
Literature examining association between blood groups and acute pancreatitis is limited. Guler et al examined blood groups and its association with mortality secondary to AP.[25] This study suggested that blood group O was associated with a higher mortality rate (8.3%) compared to A (3.3%) and B (4.1%), however, the study did not control for other prognostic factors such as comorbidities and severity of the AP episode itself. Separately, an epidemiological study from China evaluating correlative factors in hospitalized patients with acute pancreatitis found that in the subgroup with SAP, there was an increased proportion of patients with AB and B blood type, however this study did not control for the baseline distribution of patients with each blood type.[26]
Given the above-mentioned evidence, we hypothesized that ABO antigens play a role in the severity of the inflammatory reaction in AP. At the molecular level, blood group antigens are distributed in different areas of the pancreas. One of the only tissue antigen studies examining the distribution of blood group antigens in the adult pancreas showed that A and B antigens were expressed in the cytoplasm of acinar cells, rather than on the surface.[27] During AP, intracellular activation of pancreatic zymogens results in autodigestion of the acinar cells leading to exposure of intracellular antigens that ultimately lead to the release of inflammatory mediators.[28] Therefore, a logical deduction is that the release of these intracellular antigens during AP would lead to a more intense inflammatory state and as a consequence, to a more severe disease. However, our results suggest that the activation of the intracellular inflammatory cascade in AP is not directly influenced by intracellular blood group antigens.
Limitations of our study include its retrospective nature and lack of information on patients who did not get their blood typed. Given the fewer number of patients in the severe pancreatitis group, moderately severe AP was grouped with severe AP cases in this study. These limitations may have led to underpowering of the study to detect an association between bloody group and severity of AP. Nonetheless, to our knowledge, this is the first study to investigate if a correlation between blood groups and severity of acute pancreatitis exists. Our findings could be used as background information for future studies that should include larger cohorts of patients.
In conclusion, our study found no significant association between blood group and severity of AP. Although blood group antigens have been associated with other inflammatory conditions, it does not appear directly involved in the inflammatory process of acute pancreatitis.
Author contributions
Conceptualization: Antonio H. Mendoza Ladd.
Data curation: Christine Shieh, Richard J. Dean, Spring A. Silva, Lizette Rodriguez, Jose Martinez Perez.
Formal analysis: Christine Shieh.
Investigation: Christine Shieh, Antonio H. Mendoza Ladd.
Methodology: Antonio H. Mendoza Ladd.
Project administration: Antonio H. Mendoza Ladd.
Supervision: Antonio H. Mendoza Ladd.
Visualization: Antonio H. Mendoza Ladd.
Writing – original draft: Christine Shieh.
Writing – review & editing: Christine Shieh, Richard J. Dean, Antonio H. Mendoza Ladd.
Abbreviations:
- AP
- acute pancreatitis
- SAP
- severe AP
The author have no funding and conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
How to cite this article: Shieh C, Dean RJ, Silva SA, Rodriguez L, Perez JM, Mendoza Ladd A. Association between acute pancreatitis severity and ABO/Rh blood group. Medicine 2024;103:48(e40789).
Contributor Information
Richard J. Dean, Email: rjdean@ucdavis.edu.
Spring A. Silva, Email: sasilva@ucdavis.edu.
Lizette Rodriguez, Email: lizrodriguez@ucdavis.edu.
Jose Martinez Perez, Email: jlmartinezperez@ucdavis.edu.
Antonio Mendoza Ladd, Email: ahmendozaladd@ucdavis.edu.
References
- [1].Sellers ZM, MacIsaac D, Yu H, et al. Nationwide trends in acute and chronic pancreatitis among privately insured children and non-elderly adults in the United States, 2007–2014. Gastroenterology. 2018;155:469–78.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Forsmark CE, Vege SS, Wilcox CM. Acute pancreatitis. N Engl J Med. 2016;375:1972–81. [DOI] [PubMed] [Google Scholar]
- [3].Mederos M, Reber HA, Girgis MD. Acute pancreatitis: a review. JAMA. 2021;325:382–90. [DOI] [PubMed] [Google Scholar]
- [4].Frey CF, Zhou H, Harvey DJ, White RH. The incidence and case-fatality rates of acute biliary, alcoholic, and idiopathic pancreatitis in California, 1994–2001. Pancreas. 2006;33:336–44. [DOI] [PubMed] [Google Scholar]
- [5].Papachristou GI, Papachristou DJ, Morinville VD, Slivka A, Whitcomb DC. Chronic alcohol consumption is a major risk factor for pancreatic necrosis in acute pancreatitis. Am J Gastroenterol. 2006;101:2605–10. [DOI] [PubMed] [Google Scholar]
- [6].Dobszai D, Mátrai P, Gyöngyi Z, et al. Body-mass index correlates with severity and mortality in acute pancreatitis: a meta-analysis. World J Gastroenterol. 2019;25:729–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Komolafe O, Pereira SP, Davidson BR, Gurusamy KS. Serum C-reactive protein, procalcitonin, and lactate dehydrogenase for the diagnosis of pancreatic necrosis. Cochrane Database Syst Rev. 2017;4:CD012645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Mayer J, Rau B, Gansauge F, Beger HG. Inflammatory mediators in human acute pancreatitis: clinical and pathophysiological implications. Gut. 2000;47:546–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Alsten SCV, Aversa JG, Santao L, et al. Association between ABO and Duffy blood types and circulating chemokines and cytokines. Genes Immunity. 2021;22:161–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Greer JB, LaRusch J, Brand RE, et al. ABO blood group and chronic pancreatitis risk in the NAPS2 cohort. Pancreas. 2011;40:1188–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Sarr MG. 2021 revision of the Atlanta classification of acute pancreatitis. Pol Arch Med Wewn. 2013;123:118–24. [DOI] [PubMed] [Google Scholar]
- [12].Banks PA, Bollen TL, Dervenis C, et al. Classification of acute pancreatitis—2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102–11. [DOI] [PubMed] [Google Scholar]
- [13].Ranson JH, Rifkind KM, Roses DF, Fink SD, Eng K, Spencer FC. Prognostic signs and the role of operative management in acute pancreatitis. Surg Gynecol Obstet. 1974;139:69–81. [PubMed] [Google Scholar]
- [14].Papachristou G, Muddana V, Yadav D, et al. Comparison of BISAP, Ranson’s, APACHE-II, and CTSI scores in predicting organ failure, complications and mortality in acute pancreatitis. Am J Gastroenterol. 2010;105:435–41. [DOI] [PubMed] [Google Scholar]
- [15].Gan SI, Ramognuolo J. Admission hematocrit: a simple, useful and early predictor of severe pancreaitits. Dig Dis Sci. 2004;49:1946–52. [DOI] [PubMed] [Google Scholar]
- [16].Van den Berg FF, De Bruijn AC, van Santvoort HC, Issa Y, Boermeester MA. Early laboratory biomarkers for severity in acute pancreatitis; a systematic review and metanalysis. Pancreatology. 2020;20:1302–11. [DOI] [PubMed] [Google Scholar]
- [17].Hedström J, Sainio V, Kemppainen E, et al. Serum complex of trypsin 2 and alpha 1 antitrypsin as diagnostic and prognostic marker of acute pancreatitis: clinical study in consecutive patients. BMJ. 1996;313:333–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Gudgeon AM, Heath DI, Hurley P, et al. Trypsinogen activation peptides assay in the early prediction of severity of acute pancreatitis. Lancet. 1990;335:4–8. [DOI] [PubMed] [Google Scholar]
- [19].Martinez J, Sanchez-Paya J, Palazon JM, Suazo-Barahona J, Robles-Díaz G, Pérez-Mateo M. Is obesity a risk factor in acute pancreatitis? A meta-analysis. Pancreatology. 2004;4:42–8. [DOI] [PubMed] [Google Scholar]
- [20].Wiggins KL, Smith NL, Glazer NL, et al. ABO genotype and risk of thrombotic events and hemorrhagic stroke. J Thromb Haemost. 2009;7:263–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Reilly JP, Meyer NJ, Shashaty MG, et al. ABO blood type A is associated with increased risk of ARDS in whites following both major trauma and severe sepsis. Chest. 2014;145:753–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Bowen DJ. An influence of ABO blood group on the rate of proteolysis of von Willebrand factor by ADAMTS13. J Thromb Haemost. 2003;1:33–40. [DOI] [PubMed] [Google Scholar]
- [23].Wu O, Bayoumi N, Vickers MA, Clark P. ABO(H) blood groups and vascular disease: a systematic review and meta-analysis. J Thromb Haemost. 2008;6:62–9. [DOI] [PubMed] [Google Scholar]
- [24].Wolpin BM, Chan AT, Hartge P, et al. ABO blood group and the risk of pancreatic cancer. J Natl Cancer Inst. 2009;101:424–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Guler I, Ustaalioglu I. Relationship between blood groups and mortality in patients with acute pancreatitis. South. Clin. Ist Euras. 2023;34:200–4. [Google Scholar]
- [26].Fei Y, Liu X, Gao K, et al. Analysis of influencing factors of severity in acute pancreatitis using big data mining. Rev Assoc Med Bras (1992). 2018;64:454–61. [DOI] [PubMed] [Google Scholar]
- [27].Rouger P, Goossens D, Gane P, Salmon C. Distribution of blood group antigens in adult pancreas. Tissue Antigens. 1981;18:51–5. [DOI] [PubMed] [Google Scholar]
- [28].Bhatia M, Brady M, Shokuhi S, Christmas S, Neoptolemos JP, Slavin J. Inflammatory mediators in acute pancreatitis. J Pathol. 2000;190:117–25. [DOI] [PubMed] [Google Scholar]