Abstract
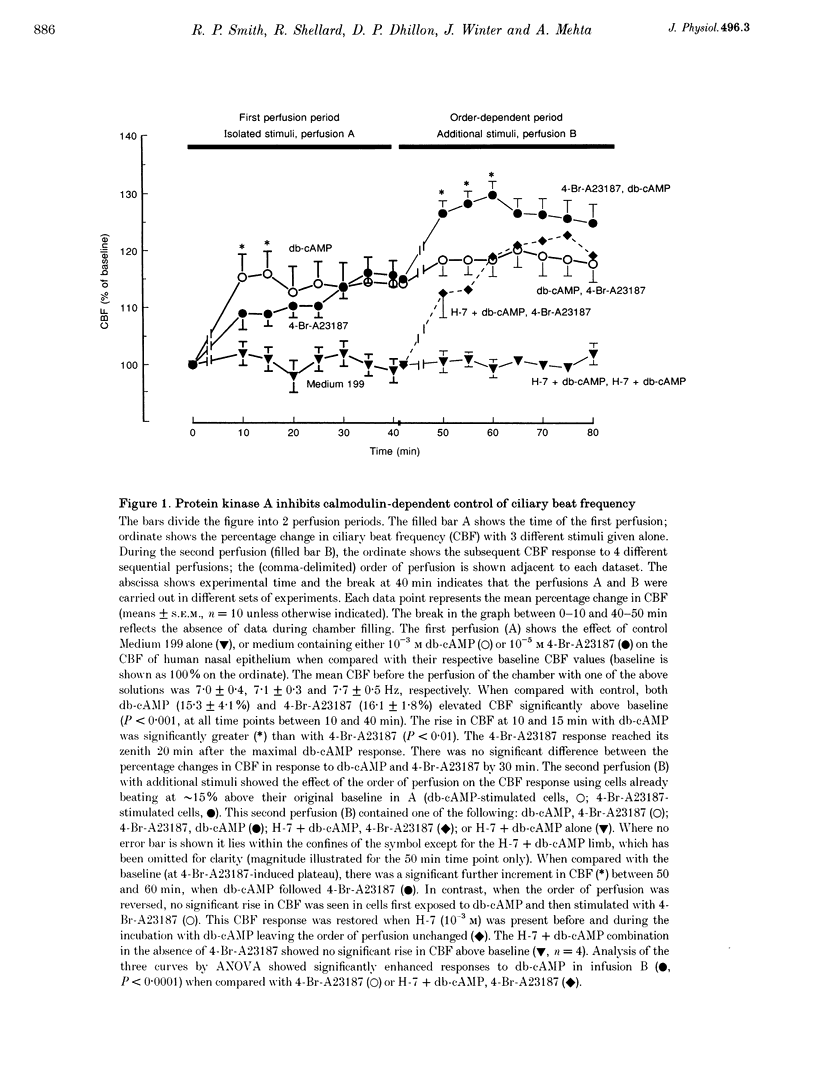
1. The effects of the sequential stimulation of ciliary beat frequency (CBF) via two different phosphorylation cascades (dependent on protein kinase A (PKA) and calmodulin, respectively) were determined using video microscopy applied to a perfused preparation of human nasal respiratory epithelium in vitro. Dibutyryl cyclic AMP (db-cAMP) (10(-3) M) was used to stimulate PKA and the calcium ionophore 4-Br-A23187 (10(-5) M) was used to stimulate calmodulin-dependent phosphorylation. 2. Perfusion with db-cAMP (10(-3) M) alone showed an early rise in CBF (15.0 +/- 4%, mean +/- S.E.M., P < 0.05) by 10 min which remained elevated for 35 min; in contrast, the highest CBF response to 4-Br-A23187 (10(-5) M) alone was not achieved until 35 min (16.1 +/- 1.8%, P < 0.05). 3. When a db-cAMP stimulus was applied to cells which had been pre-incubated with 4-Br-A23187 for 30 min, a further rise in CBF (maximal at 20 min, 14.3 +/- 2%, P < 0.05) was observed. Reversing the sequence of perfusions, cells pre-incubated with db-cAMP showed no further rise in response to stimulation with 4-Br-A23187. 4. We hypothesized that PKA inhibited the response to the 4-Br-A23187. This notion was supported by the restoration of the CBF response (22.8 +/- 4%, P < 0.05) to 4-Br-A23187 when the cells were pre-incubated with the protein kinase inhibitor 1-(5-isoquinolinyl-sulphonyl)-2-methylpiperazine (10(-3) M), before the sequential perfusions with db-cAMP and 4-Br-A23187. We conclude that the A23187-dependent pathway, which regulates intrinsic CBF, is inhibited by db-cAMP but not vice versa.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker A. P., Hillegass L. M., Holden D. A., Smith W. J. Effect of kallidin, substance P, and other basic polypeptides on the production of respiratory macromolecules. Am Rev Respir Dis. 1977 May;115(5):811–817. doi: 10.1164/arrd.1977.115.5.811. [DOI] [PubMed] [Google Scholar]
- Bylund D. B. Subtypes of alpha 1- and alpha 2-adrenergic receptors. FASEB J. 1992 Feb 1;6(3):832–839. doi: 10.1096/fasebj.6.3.1346768. [DOI] [PubMed] [Google Scholar]
- Di Benedetto G., Magnus C. J., Gray P. T., Mehta A. Calcium regulation of ciliary beat frequency in human respiratory epithelium in vitro. J Physiol. 1991 Aug;439:103–113. doi: 10.1113/jphysiol.1991.sp018659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Benedetto G., Manara-Shediac F. S., Mehta A. Effect of cyclic AMP on ciliary activity of human respiratory epithelium. Eur Respir J. 1991 Jul;4(7):789–795. [PubMed] [Google Scholar]
- Duchateau G. S., Graamans K., Zuidema J., Merkus F. W. Correlation between nasal ciliary beat frequency and mucus transport rate in volunteers. Laryngoscope. 1985 Jul;95(7 Pt 1):854–859. [PubMed] [Google Scholar]
- Felder C. C. Muscarinic acetylcholine receptors: signal transduction through multiple effectors. FASEB J. 1995 May;9(8):619–625. [PubMed] [Google Scholar]
- Girard P. R., Kennedy J. R. Calcium regulation of ciliary activity in rabbit tracheal epithelial explants and outgrowth. Eur J Cell Biol. 1986 Apr;40(2):203–209. [PubMed] [Google Scholar]
- Hidaka H., Inagaki M., Kawamoto S., Sasaki Y. Isoquinolinesulfonamides, novel and potent inhibitors of cyclic nucleotide dependent protein kinase and protein kinase C. Biochemistry. 1984 Oct 9;23(21):5036–5041. doi: 10.1021/bi00316a032. [DOI] [PubMed] [Google Scholar]
- Ismailov I. I., Benos D. J. Effects of phosphorylation on ion channel function. Kidney Int. 1995 Oct;48(4):1167–1179. doi: 10.1038/ki.1995.400. [DOI] [PubMed] [Google Scholar]
- Iyengar R. Molecular and functional diversity of mammalian Gs-stimulated adenylyl cyclases. FASEB J. 1993 Jun;7(9):768–775. doi: 10.1096/fasebj.7.9.8330684. [DOI] [PubMed] [Google Scholar]
- Jain B., Rubinstein I., Robbins R. A., Leise K. L., Sisson J. H. Modulation of airway epithelial cell ciliary beat frequency by nitric oxide. Biochem Biophys Res Commun. 1993 Feb 26;191(1):83–88. doi: 10.1006/bbrc.1993.1187. [DOI] [PubMed] [Google Scholar]
- Kobayashi K., Salathé M., Pratt M. M., Cartagena N. J., Soloni F., Seybold Z. V., Wanner A. Mechanism of hydrogen peroxide-induced inhibition of sheep airway cilia. Am J Respir Cell Mol Biol. 1992 Jun;6(6):667–673. doi: 10.1165/ajrcmb/6.6.667. [DOI] [PubMed] [Google Scholar]
- Kobayashi K., Tamaoki J., Sakai N., Chiyotani A., Takizawa T. Inhibition of ciliary activity by phorbol esters in rabbit tracheal epithelial cells. Lung. 1989;167(5):277–284. doi: 10.1007/BF02714957. [DOI] [PubMed] [Google Scholar]
- Lansley A. B., Sanderson M. J., Dirksen E. R. Control of the beat cycle of respiratory tract cilia by Ca2+ and cAMP. Am J Physiol. 1992 Aug;263(2 Pt 1):L232–L242. doi: 10.1152/ajplung.1992.263.2.L232. [DOI] [PubMed] [Google Scholar]
- Liedtke C. M. Electrolyte transport in the epithelium of pulmonary segments of normal and cystic fibrosis lung. FASEB J. 1992 Sep;6(12):3076–3084. doi: 10.1096/fasebj.6.12.1521739. [DOI] [PubMed] [Google Scholar]
- Luk C. K., Dulfano M. J. Effect of pH, viscosity and ionic-strength changes on ciliary beating frequency of human bronchial explants. Clin Sci (Lond) 1983 Apr;64(4):449–451. doi: 10.1042/cs0640449. [DOI] [PubMed] [Google Scholar]
- Ramnarine S. I., Hirayama Y., Barnes P. J., Rogers D. F. 'Sensory-efferent' neural control of mucus secretion: characterization using tachykinin receptor antagonists in ferret trachea in vitro. Br J Pharmacol. 1994 Dec;113(4):1183–1190. doi: 10.1111/j.1476-5381.1994.tb17122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusznak C., Devalia J. L., Lozewicz S., Davies R. J. The assessment of nasal mucociliary clearance and the effect of drugs. Respir Med. 1994 Feb;88(2):89–101. doi: 10.1016/0954-6111(94)90020-5. [DOI] [PubMed] [Google Scholar]
- Rutland J., Cole P. J. Non-invasive sampling of nasal cilia for measurement of beat frequency and study of ultrastructure. Lancet. 1980 Sep 13;2(8194):564–565. doi: 10.1016/s0140-6736(80)91995-9. [DOI] [PubMed] [Google Scholar]
- Salathe M., Pratt M. M., Wanner A. Protein kinase C-dependent phosphorylation of a ciliary membrane protein and inhibition of ciliary beating. J Cell Sci. 1993 Dec;106(Pt 4):1211–1220. doi: 10.1242/jcs.106.4.1211. [DOI] [PubMed] [Google Scholar]
- Sanderson M. J., Dirksen E. R. Mechanosensitive and beta-adrenergic control of the ciliary beat frequency of mammalian respiratory tract cells in culture. Am Rev Respir Dis. 1989 Feb;139(2):432–440. doi: 10.1164/ajrccm/139.2.432. [DOI] [PubMed] [Google Scholar]
- Satir P., Barkalow K., Hamasaki T. The control of ciliary beat frequency. Trends Cell Biol. 1993 Nov;3(11):409–412. doi: 10.1016/0962-8924(93)90092-f. [DOI] [PubMed] [Google Scholar]
- Sleigh M. A., Blake J. R., Liron N. The propulsion of mucus by cilia. Am Rev Respir Dis. 1988 Mar;137(3):726–741. doi: 10.1164/ajrccm/137.3.726. [DOI] [PubMed] [Google Scholar]
- Smith R. P., Shellard R., Di Benedetto G., Magnus C. J., Mehta A. Interaction between calcium, neutral endopeptidase and the substance P mediated ciliary response in human respiratory epithelium. Eur Respir J. 1996 Jan;9(1):86–92. doi: 10.1183/09031936.96.09010086. [DOI] [PubMed] [Google Scholar]
- Stephens R. E., Prior G. Dynein from serotonin-activated cilia and flagella: extraction characteristics and distinct sites for cAMP-dependent protein phosphorylation. J Cell Sci. 1992 Dec;103(Pt 4):999–1012. doi: 10.1242/jcs.103.4.999. [DOI] [PubMed] [Google Scholar]
- Tamaoki J., Kobayashi K., Sakai N., Kanemura T., Horii S., Isono K., Takeuchi S., Chiyotani A., Yamawaki I., Takizawa T. Atrial natriuretic factor inhibits ciliary motility in cultured rabbit tracheal epithelium. Am J Physiol. 1991 Feb;260(2 Pt 1):C201–C205. doi: 10.1152/ajpcell.1991.260.2.C201. [DOI] [PubMed] [Google Scholar]
- Taylor C. W. The role of G proteins in transmembrane signalling. Biochem J. 1990 Nov 15;272(1):1–13. doi: 10.1042/bj2720001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treharne K. J., Marshall L. J., Mehta A. A novel chloride-dependent GTP-utilizing protein kinase in plasma membranes from human respiratory epithelium. Am J Physiol. 1994 Nov;267(5 Pt 1):L592–L601. doi: 10.1152/ajplung.1994.267.5.L592. [DOI] [PubMed] [Google Scholar]


