Abstract
Background
Functional analysis (FA) for the management of challenging behaviour is a promising behavioural intervention that involves exploring the meaning or purpose of an individual’s behaviour. It extends the ‘ABC’ approach of behavioural analysis, to overcome the restriction of having to derive a single explanatory hypothesis for the person’s behaviour. It is seen as a first line alternative to traditional pharmacological management for agitation and aggression. FA typically requires the therapist to develop and evaluate hypotheses‐driven strategies that aid family and staff caregivers to reduce or resolve a person’s distress and its associated behavioural manifestations.
Objectives
To assess the effects of functional analysis‐based interventions for people with dementia (and their caregivers) living in their own home or in other settings.
Search methods
We searched ALOIS: the Cochrane Dementia and Cognitive Improvement Group’s Specialized Register on 3 March 2011 using the terms: FA, behaviour (intervention, management, modification), BPSD, psychosocial and Dementia.
Selection criteria
Randomised controlled trials (RCTs) with reported behavioural outcomes that could be associated with functional analysis for the management of challenging behaviour in dementia.
Data collection and analysis
Four reviewers selected trials for inclusion. Two reviewers worked independently to extract data and assess trial quality, including bias. Meta‐analyses for reported incidence, frequency, severity of care recipient challenging behaviour and mood (primary outcomes) and caregiver reaction, burden and mood were performed. Details of adverse effects were noted.
Main results
Eighteen trials are included in the review. The majority were in family care settings. For fourteen studies, FA was just one aspect of a broad multi‐component programme of care. Assessing the effect of FA was compromised by ill‐defined protocols for the duration of component parts of these programmes (i.e. frequency of the intervention or actual time spent). Therefore, establishing the real effect of the FA component was not possible.
Overall, positive effects were noted at post‐intervention for the frequency of reported challenging behaviour (but not for incidence or severity) and for caregiver reaction (but not burden or depression). These effects were not seen at follow‐up.
Authors' conclusions
The delivery of FA has been incorporated within wide ranging multi‐component programmes and study designs have varied according to setting ‐ i.e. family care, care homes and hospital, with surprisingly few studies located in care homes. Our findings suggest potential beneficial effects of multi‐component interventions, which utilise FA. Whilst functional analysis for challenging behaviour in dementia care shows promise, it is too early to draw conclusions about its efficacy.
Keywords: Humans; Behavior Therapy; Behavior Therapy/methods; Caregivers; Caregivers/education; Caregivers/psychology; Conditioning, Operant; Conditioning, Operant/physiology; Dementia; Dementia/psychology; Depression; Depression/therapy; Motivation; Motivation/physiology; Randomized Controlled Trials as Topic; Stress, Psychological; Stress, Psychological/therapy
Plain language summary
Inconclusive, but promising evidence for the efficacy of functional analysis interventions for challenging behaviour in dementia
The management of challenging behaviour, such as aggression and agitation in dementia has been dominated by drug therapies such as the antipsychotics, despite their modest efficacy, side effects and potential detriment to quality of life. Functional analysis (FA) is a behavioural intervention that is described by international guidelines as the first line alternative to drug therapy for challenging behaviour. FA typically requires the therapist to develop an understanding of the function or meaning behind the person’s distressed behaviour. It uses this understanding to develop individually tailored strategies aimed at both the person with dementia and the caregivers, to relieve the distress caused by the behaviour. FA can be applied in home settings where the family or informal caregiver is offered support from a therapist, or in care homes, hospitals or assisted living settings, where training in FA and specialist support to deliver interventions is provided for staff.
In this review we analysed the effectiveness of functional analysis‐based interventions for challenging behaviour in dementia. We found eighteen randomised controlled trials suitable for analysis in all four types of care settings. The majority were in family care settings and there were surprisingly few care home based studies. Most evaluated broad programmes of care, where FA was just one component of a wide range of other interventions. This made it hard to determine the real effect of FA for the management of challenging behaviour in dementia.
However, positive results were noted in the frequency of the person’s reported problem behaviours and the caregiver’s reaction to them. No significant effects were found for incidence or severity of mood and other problem behaviours. Similarly, no significant effects were found for caregiver mood or burden.
Whilst it is too early to reach a firm conclusion on the evidence for FA in the management of challenging behaviour in dementia, we note emerging beneficial effects on challenging behaviour where multi‐component psychosocial interventions have used FA as part of the programme of care.
Background
Challenging behaviours or 'behaviours that challenge us' (NICE 2006), sometimes also referred to as 'behavioural and psychological symptoms of dementia (BPSD) or neuropsychiatric symptoms' (Finkel 1997), involve disturbances in people's mood, behaviours, thoughts and perceptions. They include 'symptoms' such as delusions, hallucination anxiety, depression, apathy, agitation, aggression, wandering and disinhibition (Ballard 2001) or behaviours such as confusion, calling out, repetitive questioning, toileting difficulties, misidentifications and sexual challenge (Stokes 2000). These behaviours are described as 'challenging' because they are perceived to be 'unreasonable' and challenge the norms and rules of the contexts within which they occur. Bird 2008 propose that challenging behaviour in dementia is a manifestation of distress or suffering in the person, or distress in the caregiver. According to this definition, challenging behaviour can be seen as an active attempt by the person to meet or express a physiological or psychological need (Stokes 2000). Interventions for challenging behaviour can include those that address a family or staff caregivers' ability to cope, or their efficacy in the management of challenging behaviours. Until recently, pharmacological regimens were used to treat these problematic behaviours, but increasing concerns over their modest efficacy, significant side effects and potential detrimental impact on quality of life (Ballard 2005) have resulted in calls for non‐pharmacological approaches as the first‐line interventions (Howard 2001; NICE 2006). ‘Time for Action’ (Banerjee 2009) supports the position that pharmaceuticals have limited positive effects and they can cause significant harm to people with dementia. It is suggested that out of the 180,000 people being treated with anti‐psychotic medication each year in the UK, only 20% will derive some benefit (Banerjee 2009). As was seen in the USA following implementation of the 1987 Omnibus Budget Reconciliation Act, a 30% reduction in the use of some types of drugs, such as antipsychotics, can be achieved through legislation (Lantz 1996). In the UK, enhanced non‐pharmacological interventions that impact on the practices of prescribing doctors can also lead to reductions in the use of drugs (Fossey 2006). Despite this, psycho‐geriatricians appear to prefer pharmacological treatment (Greve 2005) and their use of drugs for managing challenging behaviour in dementia may actually be on the increase (Dempsey 2005). If professionals are required to reduce their reliance on medication, they will need to be confident in the use of non‐pharmacological alternatives.
A promising non‐pharmacological treatment has its roots in behaviour therapy, sometimes referred to as 'behavioural intervention' (Spira 2006), 'behaviour management' (DoH 2001; Livingston 2005) or 'behaviour modification'. Behavioural intervention programmes are usually based on either a behavioural analysis, or the updated approach to behavioural analysis, which is termed functional analysis (Stokes 2000). These are now collectively described as behavioural or functional analysis (NICE 2006) and can include interventions where staff and family caregivers meet the person's need (Cohen‐Mansfield 2000) by deriving an understanding of the purpose or meaning of the individual's behaviour (Moniz‐Cook 2001). In this review we refer to this non‐pharmacological approach for challenging behaviour in dementia as functional analysis‐based intervention, since, when compared with traditional behavioural analysis, this reflects a wider and conceptually stronger description of the range of such interventions.
Functional analysis‐based interventions arise out of behavioural analysis, or what is also known as the 'ABC' approach. The method requires clear specification of a problem behaviour ('B') that is understood in terms of the observed influence of events preceding it (antecedents 'A'), and the events consequent ('C') upon it (Stokes 2000). Traditional 'ABC' behavioural interventions imply that behaviour is always observable and linear in nature. However, this is not necessarily true for the development and maintenance of challenging behaviour in dementia. For example, staff anxiety may be a consequence ('C') of a challenging behaviour ('B) but staff behaviour (including anxiety) can also simultaneously act as an antecedent ('A'); see Moniz‐Cook 2000. This non‐linear relationship between antecedents, behaviour and consequences (ABCs) is also seen in a case study where a man's (unobservable) superstitious belief ('A') precipitated aggression ('B'), which led to the use of an antipsychotic ('C'), which in turn reduced his efficacy ('A'), requiring increased staff supervision ('A') and further exacerbated aggression ('B'); see Moniz‐Cook 2001. Furthermore, a given behaviour may have different functions for different individuals, or more than one function for a particular individual and it may originate for one reason and be maintained by another; or originate for more than one reason among different people (Moniz‐Cook 2003). Thus, although the principles are straightforward, in practice the relationship between features is often complex. In order to clarify such relationships, therapists therefore undertake a functional analysis of the behaviour.
Functional analysis‐based intervention builds on the empirical rigour of behaviour analysis, but is not restricted to the immediate 'observable' antecedents and/or consequences of behaviour (Moniz‐Cook 2001; Stokes 2000). It extends analysis and associated management to an understanding or a formulation (James 1999; James 2011) of the meaning or purpose of the behaviour (i.e. the function served by the behaviour). It can thus overcome the impractical search for a single explanatory hypothesis for a given behaviour that is intrinsic to the 'ABC' approach (Jones 1992; Owens 1992). For example, when examining the wider context of the challenging behaviour, one might determine that the problems may reflect coping strategies on the part of the person with dementia. Thus, aggression (which in its own right is poorly defined, see Stokes 2000) may represent a means of communicating loneliness or anxiety or avoiding shame, or a response to discomfort, pain or fear.
Functional analysis‐based interventions aim to develop hypothesis‐driven strategies to help caregivers reduce and potentially resolve a person's distress and the associated behavioural manifestation of this, in three main ways.
(1) Identifying the antecedents ('A's), consequences ('C's) or maintaining factors of challenging behaviours, and then intervening at the appropriate point in the sequence.
(2) Identifying the function of the behaviour for the individual, based on knowledge of factors such as pre‐morbid history and an understanding of the ‘unmet need’ that is being communicated by the distressed person. Based on the functional analysis and a formulation of the behaviour in its broader context, hypothesis‐driven interventions are derived.
(3) Training and supporting family and staff caregivers to apply, monitor and provide information that allows the therapist to evaluate these individually tailored hypothesis‐driven interventions.
Objectives
To evaluate the effectiveness and impact of functional analysis‐based intervention in the management and resolution of ‘behaviours that challenge’ family and staff caregivers.
Since challenging behaviour in dementia may be influenced by factors, such as training of the caregiver and the interaction between the person and the caregiver, the impact (and if possible sustainability) of interventions on the person’s behaviour and mood as well as on the caregiver’s experience, is considered.
Methods
Criteria for considering studies for this review
Types of studies
All randomised controlled trials (RCTs) which included functional analysis‐based intervention for dementia compared with control conditions of ‘care as usual’, or other types of intervention, were eligible if they had: adequate information or that which was obtained from the authors; data on the reported occurrence (incidence or frequency) of challenging behaviour, using a validated assessment measure, and were published in English as a journal article or with relevant information translated to English for evaluation by reviewers. Interrupted time series trials were excluded.
Types of participants
People with dementia, irrespective of its cause or diagnostic subtype, with reported BPSD or 'behaviours that challenge', receiving support or treatment from mental health workers, care staff or family or other informal caregivers, were included. Participants could be living at home alone or with a carer, or in care homes or cared for in psycho‐geriatric hospital wards, special care units, or other dementia facilities.
Types of interventions
Formulation‐led individualised interventions targeting reduction in the person's distress and/or resolution of the caregivers' management difficulties, by identifying the underlying 'unmet need' (Cohen‐Mansfield 2000) or 'cause' (Bird 2008) or the 'antecedents' and 'consequences' of the person's distressed behaviour (i.e. ABCs). Studies where the intervention fulfilled this criterion were identified from the published report, or written confirmation from the report's senior author.
FA intervention also needed to include all of the following criteria: (a) the intervention involved an initial elucidation by a trained professional therapist of an unmet need(s) or cause(s) of behaviour; (b) an individually tailored package of care was designed on the basis of this elucidation; and (c) the intervention was applied by a family or staff caregiver in collaboration with (or supervised by) a professional therapist.
Interventions that were limited to just one antecedent of behaviour, such as those occurring which targeted specific episodes of care, such as bathing or specific times (e.g. Sloane 2004, aggression occurring during showering or bathing) were excluded, since these protocols were weighted towards using episode‐specific strategies, rather than individualised solutions based on the function or meaning of the person’s particular behaviour.
Studies could compare the intervention to 'care as usual' that is normally provided in the study setting, including medication for behavioural problems and referral to psychiatric or community mental health services. Concurrent interventions, such as medication use (e.g. for pain relief), psychotropic drug withdrawal programmes, aromatherapy, bright‐light, other psychosocial interventions or admission to another setting to reduce challenging behaviour, were recorded.
Where more than one control condition existed (e.g. studies which compared alternative treatments), FA was evaluated against the ‘usual care’ condition. Where studies used more than two intervention conditions the most salient treatment that aligned to FA criteria was used. For example, Chenoweth 2009 used three conditions (Person Centred Care ‐ PCC, Dementia Care Mapping ‐ DCM and Usual Care) and we analysed data from the DCM condition against Usual Care, since DCM used individualised care plans based on an elucidation of the person’s needs, history and preferences. Teri 2000 used four conditions, three pharmacological (trazodone, haloperidol and placebo control) and one FA (Behaviour Management Techniques ‐ BMT). We analysed data from BMT and the placebo drug condition.
No restrictions were placed on the duration or number of treatment sessions, although these were noted in order to make comparisons between studies.
Types of outcome measures
Primary outcome
Changes in the reported incidence, frequency and severity of the range of challenging behaviours (e.g. verbal and physical aggression, restlessness) and mood (depression), using informant reports (caregiver ratings) on standardised measures, e.g. NPI, BEHAVE‐D, CMAI, RAGE, RMBPC, CBS, CDDS (Cornell Depression in Dementia Scale). Some commonly used measures such as the Neuro‐Psychiatric Inventory (NPI) provide behaviour and mood ratings for incidence, frequency and severity (mild, moderate or marked), whilst others such as the Revised Memory and Behaviour Problem Checklist (RMBPC) provide these for incidence and frequency only. Both tools provide ratings for the impact of challenging behaviours on the caregiver in terms of distress (NPI) or ‘bother’ (RMBPC). Although caregiver perception may influence their reports of challenging behaviour, for the purpose of this review the severity rating is seen as distinct from ratings of impact (i.e. distress, bother, coping or perceived management difficulty) on the caregiver.
Secondary outcomes
Changes in caregiver (i.e. family or care staff) self‐report of reaction to challenging behaviours, using ratings on standardised measures e.g. NPI, RMBPC and PC. Irrespective of how this was defined by authors, for the purposes of this review this outcome included ratings of caregiver distress, upset, ‘bother’, coping and perceived management difficulty associated with a given challenging behaviour. Other measures of family or staff carer well‐being (mood, morale, efficacy and burden) were also considered.
Short‐term (up to one month) and long‐term (one month to two years) outcomes were considered. Rates of attrition and reasons for this were noted.
See Table 1 for a Description of the Primary and Secondary outcome measures used by the Included Studies.
1. Table 1. Description of primary and secondary outcome measures.
| Table 1:Description of primary and secondary outcome measures | ||||||
|
Outcome |
Name of measure |
Source |
Description |
Eighteen trials | ||
| Family | Residential /Assisted Living/Hospital | |||||
| Primary outcomes: Care recipient | ||||||
| Patient behaviour | Revised Memory & Behaviour Problem Checklist (RMBPC) | Teri 1992 | Assessment of behavioural problems in people with dementia. A 24‐item checklist which provides one total score and 3 sub scores for the following problems: memory (7 items), depression (9 items) and disruption (8) items. Measures caregiver reports of Incidence (0‐24), Frequency and Reaction (0‐96) to each of the 24 problems. It was developed to measure reports of behavioural concerns by family caregivers in the US. |
Frequency: Farran 2000 Gitlin 2010 (2 items) Teri 2003 Teri 2005a Teri 2000 Zarit 1987(non revised version) Incidence: Gitlin 2003 (disruptive behaviour only) Burgio 2003 |
Teri 2005b | |
| Rating Scale for Aggressive Behaviour in the Elderly (RAGE) | Patel 1992 | Measures aggressive behaviours in the elderly ranging from being uncooperative to physical violence. A 21‐items scale where for 17 items ratings are made for the frequency of behaviour over the past 3 days on a Likert scale of 0 (never) to 3 (more than once every day in past 3 days). Items 18‐21 have descriptions for severity ratings of 0‐3 or yes /no. Scores range from 0‐62. Developed for staff working on psycho‐geriatric wards. | Gormley 2001 | |||
| Cohen Mansfield Agitation Inventory (CMAI) | Cohen‐Mansfield 1989 | Measures reported agitated behaviours in patients with cognitive impairment. A 29‐item scale of verbally/physically aggressive behaviour and verbal/physical non–aggressive behaviour. Each item is rated for frequency ‘since the last visit’ on a 7 point scale (1–7) ranging from ‘‘never’’ to ‘‘several times an hour.’’ A total score is obtained by summing the 29 individual frequency scores, yielding a total score that ranges from 29 to 203. Developed in care home settings. Chinese version: assess 43 behavioural problems; each item is scored according to the frequency ranging from 1 (never happened) to 7 (several times an hour). Scores can range from 42‐294. |
Huang 2003 (Chinese Version) |
Fossey 2006 Chenoweth 2009 | ||
| Problem Checklist (PC) | Agar 1997 | Assessment of problems experienced by family carers of patients with dementia. The 34‐Item Problem Checklist (Gilleard 1984) was adapted to include a further 5 items. Ratings are made for reported frequency (0‐2) ‐ scores ranging 0 ± 78 and management difficulty/coping (0‐2) ‐ score ranging 0 ± 78. Developed with family caregivers in the UK. | Moniz‐Cook 2008a | |||
| Severity of Problem Behaviours | Crichton Royal Behavioural Scale (CRBRS) | Wilkin 1989 | Assessment of psycho‐geriatric patients. The 11‐item scale requires ratings for each item on a 1‐5 point scale where each point has a severity description. Items are: mobility, memory, orientation, cooperation, restlessness, dressing, feeding, hearing, continence, sleep and subjective and objective mood. Scores range from 0‐55 | Proctor 1999 | ||
| Neuropsychiatric Inventory (NPI) | Cummings 1994 | Assessment of Behavioural and Psychological Symptoms of Dementia (BPSD) using a caregiver interview, with ratings of the frequency and severity of 10 or 12 neuropsychiatric domains (according to the version). Available versions include for Family / community settings and Nursing homes. Both the frequency (F) and severity (S) of each symptom are rated on a four ‐ (1–4) and three‐point (1–3) Likert scale, respectively. A separate score can be calculated for each symptom by multiplying the frequency and severity scores, resulting values ranging from 0 to 12 for each symptom. A total score can be obtained by summing the 12 F_S scores, yielding total scores that range from 0 to 144. A separate rating of caregiver distress can be made on a five point scale from 0 ‐ no distress, 1 ‐ minimal, 2 ‐ mild, 3 ‐ moderate, 4 ‐ moderately severe, 5 ‐ very severe or extreme; distress ranges 0‐60. | Gonyea 2006 | Chenoweth 2009 Teri 2005b | ||
| Pittsburgh Agitation Scale (PAS) | Rosen 1994 | Measures the severity of disruptive behaviours within four behavioural groups: aberrant vocalisations; motor agitation, aggressiveness & resisting care. Scored from 0‐4 with a maximum score 16. The score reflects the most disruptive of severe behaviour within each group. | Mador 2004 | |||
| Behavioural Pathology in Alzheimer’s Disease Rating Scale (Behave‐AD) | Rosen 1994 | Assessment of behavioural symptoms in Alzheimer’s disease. A 25‐item scale with Likert scale of 0‐4 covering paranoid and delusional ideation (7 items), hallucination (5 items), activity disturbances (3 items), aggression (3 items), diurnal variation (1 item), affective disturbance (2 items), and anxieties (4 items). Ratings range (0‐75) and a global rating of the trouble that the various behaviours are to the caregiver is also recorded (0‐3). | Gormley 2001 | |||
| Patient mood (depression) | Cornell Scale for Depression in Dementia (CSDD) | Alexopoulos 1988 | Assessment of depression in patients with a dementia syndrome administered by a clinician. The interview takes 20 minutes with the carer and 10 minutes with the patient. A 19‐item measure covering mood (4 items), behavioural disturbance (4 items), physical signs (3 items), cyclical functions (4 items), ideational disturbance (4 items). Items are rated on a 3 point scale: absent, mild or intermittent, and severe. Ratings are based on the week prior to the interview and range from 0‐38. | Teri 2003 | ||
| Automatic Geriatric Examination for Computer Assisted Taxonomy (AGECAT) | Copeland 1986 | Measures organic and depression symptoms. Ratings are made from 1 & 2 = subclinical to 5 = severe. It provides syndrome diagnoses of: organicity, schizophrenia, mania, depression, anxiety, obsessional disorder, phobia, and hypochondriasis. | Proctor 1999 | |||
| Revised Memory & Behaviour Problem Checklist (RMBPC) | Teri 1992 | Depression Subscale. Measures reported incidence (0‐9), frequency (0‐36) and caregiver reaction depression (0‐36). | Farran 2004 | Teri 2005b | ||
| Secondary outcomes: Caregiver | ||||||
| Mood (depression) | Centre for Epidemiological Studies — Depression scale (CES‐D) | Radloff 1977 | Detects depressive symptoms, particularly for use in research or screening. A 20‐item scale with scores ranging 0‐60. A score of 16 = mild depression and 23 and above is indicative of significant depression. Items are rated as occurring Rarely (< 1 day), Some (1‐2 days), Occasionally (3‐4 days) and Most (5‐7 days). |
Farran 2004 Teri 2005a Burgio 2003 Losada‐Baltar 2004 |
||
| Hospital and Anxiety Depression Scale (HADs) | Zigmond 1983 | Assessment of mood. A 14 item measure with two sub scales: anxiety and depression. Each item is rated on a four‐point Likert scale, giving maximum scores of 21 each for anxiety and depression. Scores of 11 or more on either sub scale are considered to be a significant 'case' of psychological morbidity, while scores of 8–10 represents 'borderline' and 0–7 'normal' | Moniz‐Cook 2008a | |||
| Reaction | Revised Memory & Behaviour Problem Checklist (RMBPC) | Teri 1992 | Assessment of behavioural problems in people with dementia. A 24 item checklist which provides one total score and 3 sub‐scores for the following problems: Memory (7 items), Depression (9 items) and Disruption (8 items). Measures caregiver reports of Incidence (0‐24), Frequency and Reaction (0‐96) to each of the 24 problems. Developed to measure reports of behavioural concerns by family caregivers in the US. |
Farran 2004 Gitlin 2003 Gitlin 2010 Teri 2003 Teri 2005a Zarit 1987 Burgio 2003 |
Teri 2005b | |
| Agitated Behaviour in Dementia Scale (ABID) | Logsdon 1999 | A measure of agitation in an outpatient sample of patients with mild to moderate Alzheimer’s disease. A 16‐item measure of frequency and caregiver reaction to common agitated behaviours in community residing dementia patients. Scored on a scale of 0‐3, rated in the past 2 weeks where: 0 = did not occur during the week, 1 = occurred once or twice, 2 = occurred 3‐6 times in the week, 3 = daily or more often. | Teri 2000 | |||
| Neuropsychiatric Inventory (NPI) Distress | Cummings 1994 | The NPI distress scale has an additional question on each of the 10 or 12 (depending on version) domains specifically addressing the level of distress caused to carers by each symptom. Available versions include for Family / community settings and Nursing homes. Ratings are on a five point scale from 0 ‐ no distress, 1‐ minimal, 2 ‐ mild, 3 ‐ moderate, 4 ‐ moderately severe, 5 ‐ very severe or extreme. Total distress ranges from 0‐60. | Gonyea 2006 | |||
| Problem Checklist (PC) | Agar 1997 | Assessment of problems experienced by family carers of patients with dementia. The 34‐item Problem Checklist (Gilleard 1984) was adapted to include a further 5 items. Ratings are made for reported frequency (0‐2) ‐ scores ranging 0 ± 78 and management difficulty /coping (0‐2) ‐ score ranging 0 ± 78. Developed for use with family caregivers in the UK. |
Moniz‐Cook 2008a | |||
| Burden | Zarit Burden Interview (ZBI) First described as the Burden Interview |
Zarit 1980 | Assessment of the feelings of burden of caregivers in caring for an older person with dementia. A 29‐item scale where scores are interpreted as follows: 0‐21 = little or no burden, 21‐20 = mild to moderate, 21‐40 = mild to moderate, 41‐60 = moderate to severe burden and 61‐88 = severe burden. |
Gitlin 2010 Gormley 2001 Zarit 1987 |
||
| The Screen for Caregiver Burden (SCB) | Vitaliano 1991 | Assessment of perceived burden of caring for a person with Alzheimer’s disease. A 25‐item scale with scores for objective and subjective burden. Objective = the number of caregiver experiences occurring independently of their distress. Subjective = overall distress. |
Teri 2005a Teri 2000 |
|||
Search methods for identification of studies
Electronic searches
We searched ALOIS (www.medicine.ox.ac.uk/alois): the Cochrane Dementia and Cognitive Improvement Group’s Specialized Register on 3 March 2011. The search terms used were: functional analysis, behaviour (intervention, management, modification), BPSD, psychosocial and Dementia.
ALOIS is maintained by the Trials Search Co‐ordinator of the Cochrane Dementia and Cognitive Improvement Group and contains studies in the areas of dementia prevention, dementia treatment and cognitive enhancement in healthy individuals. The studies are identified from:
monthly searches of a number of major healthcare databases: MEDLINE, EMBASE, CINAHL, PsycInfo and LILACS;
monthly searches of a number of trial registers: ISRCTN; UMIN (Japan's Trial Register); the WHO portal (which covers ClinicalTrials.gov; ISRCTN; the Chinese Clinical Trials Register; the German Clinical Trials Register; the Iranian Registry of Clinical Trials and the Netherlands National Trials Register, plus others);
quarterly searches of The Cochrane Library’s Central Register of Controlled Trials (CENTRAL);
six‐monthly searches of a number of grey literature sources: ISI Web of Knowledge Conference Proceedings; Index to Theses; Australasian Digital Theses.
To view a list of all sources searched for ALOIS see About ALOIS on the ALOIS web site.
Details of the search strategies used for the retrieval of reports of trials from the healthcare databases, CENTRAL and conference proceedings can be viewed in the ‘methods used in reviews’ section within the editorial information about the Dementia and Cognitive Improvement Group.
Additional searches were performed in many of the sources listed above to cover the timeframe from the last searches performed for ALOIS to ensure that the search for the review was as up‐to‐date and as comprehensive as possible. The search strategies used can be seen in Appendix 1.
Searches carried out in the previous version(s) of the review can be viewed in Appendix 2 and Appendix 3.
The latest search (March 2011) retrieved a total of 712 results. After a first‐assess and a de‐duplication of these results the authors were left with 165 references to further assess.
Selection of studies
Reviewers worked in two independent pairs (IJ and EM‐C; and MdV and FV) to assess publications for eligibility. First, the title was reviewed then the abstracts were examined, and finally for studies that remained, hard copies of the full texts were obtained. The reviewer pairs then considered relevant trials including additional information accessed from study authors. A standard form documenting the inclusion and exclusion criteria was used for each study by all four reviewers. The reviewers' selections of trials were compared and a list of studies to be included was reached by consensus across all four reviewers.
Data collection and analysis
Quality assessment
Two reviewers (EM‐C and KRS) assessed the methodological quality of each study using the Cochrane risk of bias tool and the guidance provided in the Cochrane Handbook (Higgins 2009), to identify potential sources of systematic bias. Criteria for appraisal of internal validity of studies covered bias in selection, performance, detection, attrition, reporting and any other bias identified, which were categorised into low, moderate or high risk of bias. Reviewer consensus was used to complete risk of bias summaries (Figure 1). Studies were also assessed for clinical quality and sustainability (Table 2).
1.
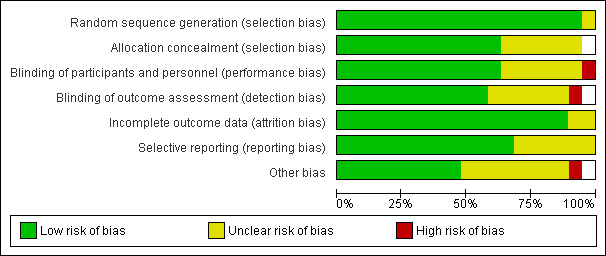
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
2. Table 2. Description of interventions and quality of included studies.
| Table 2. Description of interventions and quality of included studies | |||||||||
| Trial setting | Trial | Study duration from baseline | Intervention duration | Follow‐up assessments | Details of intervention sessions & format | Intervention type, aims and components | Delivered by |
Intervention dosage¹ Minimal 1‐2 sessions Moderate 3‐5 Medium High 6‐10 High > 10 Behaviour Management² = BM |
Intervention Information to enable replication of trial. 1. Procedural clarity 2. Manual /protocol 3. Treatment fidelity assessments 4. Follow‐up |
| Family Care | Teri 2003 | 24 months | 3 months | Post intervention = 3 months. Follow‐up data for: Problem Behaviour (PB) Frequency & Caregiver (CG) Reaction = 6 months; Patient Depression = 6, 12, 18 & 24. |
12 x 1 hour sessions, 2 per week for 3 weeks, Weekly for 4 weeks and biweekly for 4 weeks, plus 3 follow‐up sessions |
CG Skills Training Intervention Aims: CGs taught to identify and modify patient behaviours that impaired day‐to‐day function and adversely affected CR/CG interactions. Taught how to reduce the occurrences of PB, learn skills to identify and modify precipitants of patient distress. Exercise and Education |
Health care professionals delivered sessions (doesn't state how many) Trainers supervised by clinical geropsychologist (received weekly supervision). |
High | 1. Reported what components were included in the intervention; but detail on which components were addressed in each hour long session is absent. 2. Treatment protocol/manual 3. Treatment adherence was monitored by weekly supervision of each trainer by a clinical geropsychologist. Protocol sessions videotaped and reviewed by independent raters 4. Followed up to 24 months. |
|
Zarit 1987 |
24 months |
2 months |
Post‐intervention = 2 months Follow‐up = 12 months (data not available) |
8 sessions, the last used for Post‐intervention assessment |
CG Support Intervention Aims: Stress‐ Coping Model. Training teach CG to modify situations linked to stress, increase understanding of patient disease, improve management of PBs and identify useful formal and informal supports |
2 Therapists for each group. |
Medium High |
1. The paper reports what usually occurred in the second session of the intervention, but does not state each session’s agenda. 2. Conceived from a stress‐management approach treatment model, but no mention of a manual. 3. Interventions monitored using audiotapes and supervision sessions to ensure therapists implemented treatment approach. 4. 2 Year longitudinal study but only post‐intervention (2 month) data available. |
|
| Gitlin 2003 | 12 months |
6 months | Post‐intervention = 6 months Follow‐up = 12 months (data not extractable) |
Active phase: First 6 months, 5 (90 min) home contacts, 1 (30 min) telephone contact. Maintenance Phase: Subsequent 6 months |
CG Skills Training Intervention Problem solving Intervention Includes: modifying home environments and simplifying daily tasks to address CG concerns; Education, Problem solving, Use of environmental strategies |
Occupational therapist (does not state how many) | Moderate | 1. The paper reports what happens in each intervention session as run by the OT. 2. Protocol 3. Interventions monitored using case review, feedback, checklist & telephone interviews to evaluate satisfaction 4. The paper reports 6 month post‐intervention assessment, but not the results of the 12 month follow‐up. |
|
| Farran 2004 | 18 months | 3 months | Post‐intervention = 3 months Follow‐up = 6, 9, 12 & 18 months |
12 x weekly sessions (5 group, 7 individual) 2 group booster sessions at 6 & 12 months + as needed telephone contacts |
CG Skills Training Intervention Aims: Improve CG skill in dealing with PB. Content included: Potential causes/contributors to behavioural symptoms, prevention & management of BPSD, building self efficacy. |
Trained professionals (nurses, social workers) trained for 40 hours. 4 people functioned as intervention staff at any one time. | High | 1. Paper reports contents of intervention but not each session in detail. 2. Detailed manual of prescribed material for each session 3. Project director and principal investigator supervised implementation & provided corrective feedback on a weekly basis. Group sessions were taped and randomly selected for review. 4. All follow‐up data up to 18 months available. |
|
| Moniz‐Cook 2008a | 18 months | 18 months | Post‐intervention = 6 Follow‐up = 12 & 18 months |
4 consecutive weekly in home visits + clinical judgement for future contact & attend in‐service clinical supervision for the 18 month duration. (Interventions were taught prior to the study over 5 half days) |
CG Support Intervention Aims: To train community mental health nurses (CMHNs) to help family carers manage behavioural changes. Includes: Problem solving approaches, Stress‐coping interventions and Functional analysis. |
9 CMHNs (usual group 20 CMHNs) ‐ 20 hrs training initially plus supervision 2 hrs per week for 1st 6 months, 1 per fortnight for next 6 months, 1 per month for last 5 months. | High | 1. The total number of sessions or content of the sessions is not reported. 2. Protocol for CMHNs to conduct 4 in‐home visits & attend supervision. No manual. 3. Only two CMHNs with dementia specific caseloads completed the ongoing supervision and adhered to the four consecutive family treatment sessions. 4. Follow‐up data for 6, 12 & 18 months |
|
| Burgio 2003 | 18 months | 12 months | Post‐intervention = 6 months Follow‐up data not available |
16 in‐home treatment sessions (over 12 month period). Skill Training condition vs. Minimal Support Condition. 3 hour workshop, 4 weekly in home visits for 1 month & 2 in the second month. In the following 10 months home visits were alternated. |
CG Skills Training Aims: To establish a knowledge base for CGs in behaviour management, problem solving, & cognitive restructuring. Basic information in behaviour management techniques (BMT) & support on the application of behavioural and environmental treatments. Individual behaviour prescriptions. |
11 REACH interventionists. |
High |
1. Reports the intervention procedure & components covered. 2. Manual guided intervention based on common needs and cultural preferences of American family caregivers. Manual available from authors. 3. Research personnel functioned as both interventionists and assessors. Feedback on accuracy was provided in weekly clinical case review meetings. All therapeutic contacts were audio taped to check accuracy of delivery. 4. Only 6 month data reported. |
|
|
Teri 2000/ Weiner 2002 |
12 months | 4 months | Post‐intervention = 4 months Follow‐up = 12 months (Weiner 2002) |
BMT 8 weekly and 3 biweekly sessions. 16 week parallel design requiring 11 clinical visits. Randomisation to medication, BMT or placebo. |
Behaviour Management Aims: Compare Behaviour Management Techniques – BMT‐ with pharmacological treatments for agitation. BMT included: information about AD, strategies for decreasing agitated behaviours. |
Therapists with a master’s degree and 1 year clinical experience (doesn't state how many therapists) |
High BM |
1. BMT intervention sessions not reported in detail. Paper only reports number and components of sessions. 2. Protocol 3. Raters participated in ongoing training to assure standardisation. All were trained prior to starting the trial. 4. Post‐treatment data only reported; Weiner 2002 reports 12 month follow‐up. |
|
| Gitlin 2010 | 6 months | 4 months | Post‐intervention = 4 months Follow‐up = 6 months |
Up to 11 home & telephone contacts over 16 weeks. Up to 9 occupational therapy (OT) sessions, two nursing home (one home and one telephone) and a maintenance phase of 3 brief OT telephone contacts. |
CG Support Intervention Aims: To help eliminate, reduce or prevent problem behaviours within 3 interacting domains: ‐ Patient based (unmet need, discomfort, pain), Caregiver based (stress & communication style) & Environment based (clutter, hazards). |
10 OTs & 2 practice nurses received 35 hours training | High | 1. Reports what took place during the intervention but not a specific outline for each session. 2. No mention of a manual. 3. Treatment fidelity maintained through twice monthly meetings & audiotapes of 10% of home sessions. Each home session was documented in terms of time spent & content covered. 4. Four and six month follow‐up. |
|
|
Teri 2005a |
6 months |
2 months |
Post‐intervention = 2 months Follow‐up = 6 months |
8 weekly sessions followed by 4 monthly phone calls |
CG Support Intervention Aims: To teach family CGs a systematic behavioural approach for reducing mood and behaviour problems in persons with AD. Teaching ABC rationale and use Improving CG communication Increasing pleasant events, enhancing CG support. |
5 community consultants – trained by clinical gero‐psychologist. ‐ 2 hour orientation, 2nd training session & pilot case. |
High |
1. Paper reports on the contents of each treatment session 2.Treatment manual 3. Protocol, Audio taped treatment sessions and rated quality 4. Post‐test and 6 month follow‐up. |
|
|
Huang 2003 |
12 Weeks |
3 Weeks (main phase) |
Post‐intervention = 3 weeks Follow‐up = 12 weeks |
2 in home sessions over 3 weeks, plus telephone calls every 2 weeks. |
CG Skills Training Intervention Aims: Conceptually built around the Progressively Lowered Stress Threshold (PLST) model. Helping CGs identify the timing & frequency of behavioural problems & explore the causative stressors. Plan environmental and daily schedule modifications. Nurse caregiver collaboration with individualised training to develop individual plans of care. |
Investigator – Experienced Gerontological nurse |
Minimal |
1. The paper reports what was conducted by the investigator on each visit. 2. Manual developed by research team as a guide for the training program 3. It is not reported whether there were any checks to insure adherence to the manual, however the principal investigator wrote the manual and conducted the intervention. 4. Followed 12 weeks from baseline. |
|
| Gormley 2001 | 10 Weeks | 8 Weeks | Post‐intervention = 10 weeks No follow‐up |
4 sessions conducted over 8 weeks. |
Behaviour Management Training Aims: To train CGs in: Dementia education & the development of behavioural interventions by behavioural analysis. CGs taught to identify the precipitating & maintaining factors of behaviour. |
Conducted by author. |
Moderate BM |
1. The paper reports what the 1st, 2nd and subsequent sessions focused on. 2. No mention of manual, the program was developed following a review of guidelines and descriptive studies 3. The paper does not report information on treatment fidelity checks. 4. No follow‐up |
|
| Losada‐Baltar 2004 | 5 months | 2months | Post‐intervention = 2 months Follow‐up = 5 months |
8 Sessions, 2 hours per week (16 hour in total) |
CG Skills Training Intervention Aims: To train CGs in modifying behavioural problems of their relative through: Managing challenging behaviours, defining & identifying the problems, possible causes (ABC) and develop strategies and solutions. |
Two psychologists | Medium High | 1. States the components of the intervention but not which components were implemented in each session. 2. Due to difficulty translating the paper we are unsure if a manual was used. 3. Unsure regarding treatment fidelity checks 4. Followed up 5 months from Baseline. |
|
| Gonyea 2006 | 6 Weeks | 5 Weeks | Post‐intervention = 6 weeks No follow‐up |
5 weekly group sessions (90 mins) including 15 minutes of individual time. |
CG Support Intervention Aims: CG multi‐component behavioural intervention to reduce CG distress through: Behavioural management (identifying ABC), Pleasant events & Relaxation. |
Therapists (16‐20 hours training). | Moderate | 1. Session topics outlined 2. Highly structured groups with 5 main themes documented in the paper. 3. To monitor treatment fidelity the principle investigator consulted with therapists on a regular basis to review the group session experience and assess group progress. 4. No follow‐up |
|
|
Assisted Living |
Teri 2005b | 2 months | 2 months | Post‐intervention = 2 months No follow‐up |
2 half day group workshops and 4 individualised sessions |
CG Skills Training Intervention Aims: To reinforce values of dignity and respect for residents, improve staff responsiveness to resident needs, build specific staff skills to enhance resident care, improve job skill and satisfaction. |
Clinical psychologist & graduate student in nursing. |
Medium High |
1. The paper reports all the essential components and features of the intervention. 2. Manual detailing all specific aspects of training. 3. Three separate meetings were held to discuss site specific issues that might hinder implementation or sustainability. 4. No follow‐up. |
| Residential Care | Fossey 2006 | 12 months | 10 months | Post‐intervention = 12 months No follow‐up |
Trial clinician worked with homes 2 days a week over 10 months |
CG Skills Training Intervention Aims: Training in the delivery of Person‐centred care and Skills development training. Included: skills training, behavioural management techniques (ABC) and ongoing training and support |
Psychologist, occupational therapist or nurse – supervised weekly by authors. |
High |
1. Reports the components of the intervention but detail of each session. 2. No mention of a manual just reference to a specific ‘package’ of components. 3. Staff offered supervision but no report assessing treatment fidelity. Reports the intervention took a consultation approach. 4. 10 month intervention with 12 month follow‐up (for the purposes of this review classed as post‐intervention assessment). No other follow‐ups. |
| Chenoweth 2009 | 8 months | 4 months | Post‐intervention = 4 months Follow‐up = 8 months |
Training was delivered to 2 care staff selected by managers for 6 hours per day over 2 days, trained staff then helped their colleagues to implement care plans over the 4 month intervention period |
Dementia Care Mapping and Caregiver Skills Training Aims: Person centred care Need‐driven behaviour model. where staff are educated to Included: Understand behaviour as a form of communication; recognise that feelings persist despite cognitive impairment; behaviour is a way of expressing needs; understand the impact of staff actions and use of ABC |
3 authors trained by Bradford University led training. |
High |
1. Details of the interventions components are reported, but additional information was required from the author to clarify the intervention content before this trial could be included into the review. 2. Bradford University training manual 3. No detail on checking adherence to the manual or treatment fidelity. 4. Follow‐up at 8 months from baseline. |
|
| Proctor 1999 | 6 months | 6 months | Post‐intervention = 6 months No follow‐up |
7 x 1 hour seminars delivered by hospital outreach team. An experienced psychiatric nurse visited every week to give advice and support individual workers in care planning. |
Behaviour Management Aims: Staff training and psychosocial management of residents PB. Includes: Formulation of detailed and specific care plans & increasing the interval between non‐contingent interactions (not in response to need) |
Hospital outreach team & psychiatric nurse |
Medium High BM |
1. The paper reports only the components of each of the seminars 2.No report of a manual 3. No reports of checking treatment fidelity or adherence. 4. No follow‐up. |
|
| Hospital Care | Mador 2004 | 9 Days | 9 Days | Post‐intervention = 9 days No follow‐up |
Extended Practice Nurse (EPN) saw patients within 24 hours of randomisation and formulation of a non‐pharmacological management plan of strategies to manage challenging behaviour. Assumption that Control condition Geriatric assessment was also |
Behaviour Management Aims: Specialist support and education to the ward nursing staff to enable them to facilitate behaviour strategies. Included: Understanding patients needs, patient safety, minimising restraint usage, communication, nursing care & targeted behavioural strategies. |
? Geriatrician review as in Control Group + Extended practice nurse and ward staff. |
High BM |
1. The paper reports the components of the intervention only. 2. No mention of a manual 3. No reporting of assessments of treatment fidelity and adherence 4. No long‐term follow‐up |
| ¹ = Intervention dosage is based on the number of contact sessions, not the amount of functional analysis | |||||||||
| ² = Intervention focused on Behaviour Management with relatively few other components | |||||||||
Data extraction
Data were extracted from each published report or from author information, using a standard form.
For each trial, data for primary outcomes of behaviour (i.e. incidence, frequency, severity) and mood (patient depression) were extracted first, followed by the secondary outcome of caregiver reaction. Finally, we extracted data of other secondary outcomes for caregiver mood and burden. Details on intervals from post‐intervention to post‐test were not always clear from the text. Where clarification from authors was not available, this was assumed as the first data collection period from baseline.
Since the primary aim of this review was to examine the behavioural outcomes of FA, where these were absent and other (secondary) outcomes were reported, studies were excluded from the review (see for example Robinson 1994). Some rating scales, such as the RMPBC provided data for both primary and secondary outcomes, e.g. reported frequency of care recipient behaviour and caregiver reaction. Identical outcome measures, where these existed across the 18 studies, were used in the pooled analysis. The exception was where a particular assessment was specifically noted in text as the primary outcome measure. The RMPBC was used to assess either incidence or frequency or both domains of patient behaviour in nine out of 14 potential studies. For caregiver reaction eight studies used the RMBPC to measure caregiver ‘bother’ associated with care recipient challenging behaviours. The CMAI was used to assess patient behaviour in two of the three care home studies. The NPI was used as to measure the severity of patient behaviour or caregiver reaction (distress) in just three of the 18 studies (See Table 1 for an overview of measures where data were used for analysis).
The summary statistics obtained for each trial and each outcome for continuous data were the mean change from baseline, the standard error of the mean change, and the number of patients for each treatment group at each assessment. The baseline assessment was defined as the latest available assessment prior to randomisation, but no longer than two months prior to randomisation.
For each outcome measure, to allow an intention‐to‐treat analysis, the data were sought irrespective of compliance, whether or not the patient was subsequently deemed ineligible or otherwise excluded from treatment or follow‐up. If intention‐to‐treat data were not available in the publication, 'on‐treatment' data were sought (i.e. the data of those who completed the trial). For crossover trials, only data from the first treatment phase after randomisation were extracted because of the likelihood of carryover effects.
Data on adverse effects and drop‐outs were also noted.
Data analysis
Summary statistics (N, mean and standard deviation) were required for each rating scale at all assessment points, for both treatment groups in each trial for change from baseline. For continuous data (or ordinal data approximating a normal distribution), the mean change from baseline, the standard deviation, and the number of patients for each treatment group at all assessments were analysed.
The meta‐analysis required the combination of data from trials that used or did not use the same rating scale or test to assess an outcome. The measure of the treatment difference for any outcome was the weighted mean difference when the pooled trials used the same scale, and the standardised mean difference (the absolute mean difference divided by the standard deviation) when they used different scales.
We have presented overall estimates of the treatment difference from both fixed‐effect and random‐effects models and performed a test for heterogeneity using a standard Chi‐squared statistic and an I2 statistic. If there was a significant heterogeneity a random‐effects model was preferred. In using a fixed‐effect model, the true effect of intervention is assumed to be identical across studies (e.g. ‘a fixed value’). This is assuming no statistical heterogeneity between studies. In the random‐effects model, the estimated effects are not identical across trials with more weight awarded to smaller studies. If both random‐effects and fixed‐effect models give the same results this indicates that no important heterogeneity was found across studies.
Sensitivity analyses were also undertaken to assess the robustness of the results of fixed‐effect versus random‐effects models via excluding studies. If the treatment effects in the sensitivity analysis were of similar magnitude to the main analysis, a definite conclusion about the treatment effectiveness could be stated; otherwise no firm conclusion could be made on the effectiveness of the treatment.
The following comparisons were undertaken:
Primary outcomes
1) Incidence (presence) of a challenging behaviour: comparing intervention versus usual care in family care settings only, at post‐intervention and six month follow‐up. Pooled data from four trials using the Revised Memory Behaviour Problem Checklist (RMBPC) were included in the meta‐analysis.
2) Frequency of behaviour: comparing intervention versus usual care in 14 studies and three settings (i.e. family, residential and assisted living care). Frequency data at post‐intervention, six and 12 month follow‐up assessments were analysed. Of the 10 family care studies included, pooled data from eight of these using the RMBPC (or its precursor Memory Behaviour Checklist – MBCL) contributed to the meta‐analysis and for care home studies data from the Cohen Mansfield Agitation Inventory – CMAI were used. In order to examine whether studies with a strong focus on FA interventions were effective, frequency data at post‐intervention for two family care studies were pooled for analysis.
3) Severity of behaviour: comparing intervention versus usual care in four care settings (family, residential, assisted and hospital care) at post‐intervention only.
The meta‐analysis included only five out of the 18 studies, of which two used the Neuropsychiatric Inventory (NPI). In order to examine whether studies with a strong focus on FA interventions were effective, severity data at post‐intervention for two ‘institutional’ (care home and hospital) studies were pooled for analysis.
4) Patient depression: comparing intervention versus usual care in three settings (family care, residential care and assisted living) at post‐intervention only. Of the four studies included in the meta‐analysis, two studies used the Depression sub scale of the RMBPC, one study used the Cornell Depression in Dementia Scale – CDDS and one study used the Automatic Geriatric Examination for Computer Assisted Taxonomy – AGECAT.
Secondary outcomes
1) Caregiver reaction in two settings (family care and assisted living care) at post‐intervention and six months follow‐up. The meta‐analysis comprised 10 family care studies and one in an assisted living setting. Of these, nine studies used the RMBPC scale (or its precursor MBCL).
2) Caregiver depression for family care setting only at post‐intervention and six months follow‐up. The meta‐analysis comprised four family care studies, of which three used the Centre for Epidemiological Studies depression scale (CES‐D) and one used the Hospital and Anxiety depression scale (HADs).
3) Caregiver burden for family setting only at post‐intervention and six months follow‐up. The meta‐analysis included six family care studies, of which four used the Zarit Burden Interview (ZBI) and two used the Scale for Caregiver Burden (SCB). In order to examine whether studies with a strong focus on FA interventions were effective, caregiver burden at post‐intervention for two family care studies were pooled for analysis.
Sustainability of interventions was examined using follow‐up data where these existed. The most commonly occurring follow‐up time‐points were 6 and 12 months, so data from these studies were analysed.
The reviewers discussed and reached consensus on the interpretation of the statistical analysis, seeking specialist statistical advice from CDCIG as required. The review was then drafted and circulated for comment to peer reviewers and commentators with knowledge in the area, leading to production of the final version for submission to CDCIG.
Results
Description of studies
A total of 3335 references were identified by the Cochrane Dementia and Cognitive Improvement group, of which 262 abstracts were reviewed. From these abstracts, 144 papers were retrieved in full text, of which 19 were selected for inclusion into the review; one paper (Weiner 2002) reports follow‐up data of an included study (Teri 2000), therefore, the total number of studies included in the review is 18.
See: Characteristics of included studies and Characteristics of excluded studies.
Four studies (Mador 2004; Moniz‐Cook 2008a; Teri 2000; Zarit 1987) had all data required documented in the published paper. Additional data or information were provided by authors for nine studies(Chenoweth 2009; Farran 2004; Fossey 2006; Gitlin 2003; Gitlin 2010; Losada‐Baltar 2004; Teri 2003; Teri 2005a; Teri 2005b) . For two studies (Burgio 2003; Gonyea 2006) additional data were unavailable and for a further three studies the authors did not respond to requests for additional information (Gormley 2001; Huang 2003; Proctor 1999).
Included studies
See: Characteristics of included studies and Table 2.
Eighteen RCTs, with a baseline total of 2558 care recipients were included in the review. All but one study from Taiwan were carried out in Europe (England and Spain), America and Australia. The majority, 13 studies with a total of 1713 care recipients, were carried out in family care settings (Burgio 2003; Farran 2004; Gitlin 2003; Gitlin 2010; Gonyea 2006; Gormley 2001; Huang 2003; Losada‐Baltar 2004; Moniz‐Cook 2008a; Teri 2000; Teri 2003; Teri 2005a; Zarit 1987). Only three studies with a total of 743 residents were located in care homes (Chenoweth 2009; Fossey 2006; Proctor 1999). Two further studies were found, one in an assisted living setting (Teri 2005b) and the other in a hospital setting (Mador 2004), with 31 and 71 participants, respectively. Data from the latter two were pooled with that of care homes since delivery of the intervention involved staff (rather than family) caregivers.
Characteristics of the interventions
Interventions in the majority of studies were multi‐component programmes, where FA was just one part of the intervention. Fourteen interventions focused on enhancing knowledge and skills in family and staff caregivers through training support and supervision. Four trials (one residential, one hospital and two family care) utilised interventions which appeared to focus mostly on FA and are described as Behaviour Management. The time devoted to FA interventions in these trials were determined to some extent by the setting (see Table 2 ). For example, the hospital intervention had daily treatment for just nine days whilst the care home study lasted six months with weekly visits from a specialist therapist. One family care study consisted of just four sessions delivered over eight weeks (Gormley 2001).
Across the 18 trials included in the review there was considerable diversity on a number of parameters. Firstly, concepts underlying the development of the intervention were varied and included, for example, knowledge and/or training approaches, the stress‐coping model, the Progressively Lowered Stress Threshold model and Problem‐Solving approaches. Secondly, the time spent in delivering the intervention varied in length from nine days to 18 months. Similarly, variation in the degree of contact (i.e. intensity or ‘dosage’ of therapist contact) was wide ranging and in turn influenced pre‐post test intervals. Reviewers measured this as minimal (1‐2 sessions), moderate (3‐5 sessions), medium (6‐10 sessions) and high or intensive (>10 sessions). Thirdly, follow‐up data varied from no follow‐up/data unavailable/data not suitable for analysis (9 studies) to 24 month follow‐up (Zarit 1987). Table 2 provides an overview of the interventions examined.
Excluded studies
One hundred and twenty‐five studies from a total of 144 were excluded (see Characteristics of included studies and Characteristics of excluded studies).
FA was observed in a number of studies, which were not included in the review due to the following reasons:
a) Not an RCT: Four studies (Ballard 2009; Bird 2007; Cohen‐Mansfield 2007; Davison 2007).
b) Case series: Five studies (Baker 2006; Buchanan 2002;Heard 1999; Moniz‐Cook 2001; Moniz‐Cook 2003).
c) RCT but focused on one episode of care such as bathing: Two studies (Hoeffer 2006; Sloane 2004).
d) No extractable data for primary outcome: Three studies (Hinchliffe 1995; Robinson 1994; Visser 2008). Reasons for exclusion: Dichotomous data for the primary outcome (Hinchliffe 1995); only secondary caregiver outcomes reported (Robinson 1994); only sub‐scale scores reported for the primary outcome; author unable to provide total scores (Visser 2008).
Interventions for challenging behaviour that targeted specific behaviours, such as wandering (see for example Mayer 1991) were excluded as FA is based on the observation that a given function or ‘unmet need’ can manifest itself in a variety of differing behavioural repertoires. Seven trials of psychosocial interventions were excluded since descriptions of FA, according to our criteria, were absent from the text (Belle 2006;Burns 2003; Callahan 2006; Deudon 2009; Kovach 2006; Opie 2002; Tibaldi 2004). Other reasons for exclusion of studies were: review papers, no pre–post data available (e.g. Hoehn‐Anderson 1992), pharmacological studies, other psychosocial therapies (e.g. reminiscence therapy, cognitive stimulation therapy, caregiver counselling, activity programmes, occupational therapy, emotion‐orientated care) and other types of non‐pharmacological therapies (e.g. bright light therapy, snoezelen, multisensory stimulation).
Risk of bias in included studies
Quality and risk of bias in included studies
See: Risk of bias summary (Figure 1) and Characteristics of included studies .
Overall the quality of combined studies included in this review was judged as low to moderate since it was compromised in a number of ways. First, there was bias towards publishing results using test instruments, or parts of instruments that demonstrated significant post‐test gains (see Table 1), requiring additional information from authors (see Description of studies). This may explain why some researchers used two or more similar instruments to measure the same or a similar outcome (such as caregiver reaction) and were, thus, able to report significant effects on some but not all of these equivalent measures. Fifty‐three different assessment tools were used to measure outcomes across the 18 studies (see Table 3: Overview of outcome measures). Of these, 35 were relevant to our stated outcomes (see Table 3) but in order to avoid aggregating data for wide‐ranging clinical outcomes, only 15 instruments (see Table 1) were pooled for meta‐analyses. Secondly, despite our precautions in extracting data from identical measures, data from outcomes for the same domain (such as care recipient behaviour) were pooled for similar but not identical instruments. Thirdly, publication bias, where studies reporting significant results are more likely to be published, may have led to the difficulty we encountered in extracting definitive information on the extent or ‘dosage’ of FA elements of an intervention to determine the cause of the improvement. Authors did not record the time devoted to component parts of multi‐component interventions and although outcomes on behaviour and mood were taken for care recipients, not all studies had a primary aim of reducing challenging behaviour (for example, see Farran 2004 where the primary aim was to reduce emotional distress in caregivers). Fourthly, variation in concepts underlying the development of the intervention, which were not always indicated, may have resulted in reporting bias on primary and secondary outcomes. Finally, sample sizes varied across the studies. At baseline, three trials (Huang 2003; Losada‐Baltar 2004; Teri 2005b) had < 50 participants; four trials had < 100 participants (Gonyea 2006; Gormley 2001; Mador 2004; Teri 2005a). Seven trials had between 100‐200 hundred participants at baseline (Burgio 2003; Gitlin 2003; Moniz‐Cook 2008a; Teri 2000; Teri 2003; Proctor 1999; Zarit 1987). The remaining four trials had > 200 participants at baseline (Chenoweth 2009; Farran 2004; Fossey 2006; Gitlin 2010).
3. Table 3. Overview of outcome measures.
| Table 3. Overview of outcome measures | |||
| Trial |
Setting |
Outcomes Author’s description of care recipient (CR) & caregiver (CG) outcomes |
Assessment Tools ◊ Measure abbreviated after one full description ∞ Outcome measure not a rating scale ∆ Inadequate number of equivalent instruments for data aggregation □ Instrument not relevant or alternative measure used |
| Burgio 2003 | Family | Care Recipient (CR) Behaviour & Caregiver (CG) Reaction | Revised Memory and Behaviour Problem Checklist (RMBPC) (incidence only) & RMBPC ‘bother or upset’ |
| CG Appraisal of benefits from Caregiving | ∆ Positive Aspects of Caregiving (PAC) (developed by REACH investigators) | ||
| CG Social Support | ∆ Lubben Social Network Index (LSNI) 28 item measure (Berkman 1979 adapted scale) | ||
| CG Leisure Time satisfaction | ∆ 6‐item scale developed by interventionists | ||
| CG Mood | ∆ State‐trait personality inventory (anxiety sub scale 10 items) | ||
| The Centre for Epidemiologic studies –Depression Scale (CES‐D) | |||
| CG Desire to institutionalise | □ 7 Item scale by Morycz 1985 | ||
| Farran 2004 | Family | CR Behaviour/CG Depression | ◊ RMBPC |
| CG Mood | ◊ CES‐D | ||
| CG Skill | ∆ Behavioural Management Skill –Revised (BMS‐R) | ||
| Time to institutionalisation | ∞ Interval from Baseline to initial entry into long‐term care Facility | ||
|
Gitlin 2003 |
Family | CR Behaviour | ◊ RMBPC (incidence only) |
| CR Level of ADL assistance required | □ Functional Independence Measure (FIM) | ||
| CG Objective & Subjective Burden | ∞ Vigilance, Total hours of ADL help & Help received for ADLs. | ||
| ◊ RMBPC (upset sub scale) | |||
| CG Perceived Mastery | ∆ Care‐giving Mastery Index (CMI) | ||
| CG Skill Enhancement | ∆ Task Management Strategy Index (TMSI) | ||
| CG Wellbeing | ∆ Perceived Change Index (PCI) | ||
| CR Cognitive Ability | □ Mini Mental State Exam (MMSE) | ||
| Gitlin 2010 | Family | CR Behaviour & CG Reaction (upset) | 16‐item Agitated Behaviors in Dementia Scale and 2 items (repetitive questioning, hiding/hoarding) from RMBPC, plus 3 other items (wandering, incontinence, shadowing). |
| CG Mood | ◊ CES‐D | ||
| CG Burden | Zarit Burden Interview (ZBI) | ||
| CG Skill enhancement | ∆ ◊ TMSI | ||
| CG Perceived Benefits | ∆ 11 item survey developed by investigators. | ||
| CG change | ∆ ◊ PCI | ||
| Gonyea 2006 | Family | CR behaviour (Severity & Frequency) & CG Distress | Neuropsychiatric Inventory (NPI) |
| CG Burden | ◊ ZBI | ||
| CR Functional Impairment | □ Activities of Daily Living (ADL) | ||
| Gormley 2001 | Family | CR Behaviour (Severity & Frequency) | Behavioural Pathology in Alzheimer’s disease scale (BEHAVE‐AD) |
| Rating Scale for Aggressive Behaviour in the Elderly (RAGE) | |||
| CG Burden | ◊ ZBI | ||
| CR Cognitive Ability | □ ◊ MMSE | ||
| CR Functional Ability | □ Blessed Dementia Rating Scale | ||
| Huang 2003 | Family | CR Behaviour | Cohen Mansfield Agitation Inventory (CMAI) |
| CG self efficacy for managing agitation | ∆ Agitation Management Self Efficacy Scale (AMSS) | ||
| CR Cognitive Ability | □ ◊ MMSE | ||
| CR Dementia Severity | □ Clinical Dementia Rating (CDR) | ||
| CR Activities of Daily Living | □ Barthel Index | ||
| Losada‐Baltar 2004 | Family | CR Behaviour & CG reaction (upset) | Memory & Behaviour Checklist (MBCL‐A & MBCL‐B) |
| CG Dysfunctional thoughts about care | ∆ Beliefs about Care‐giving Questionnaire (BACS) | ||
| CG Mood | ◊ CES‐D | ||
| CG Perceived Support | ∆ Perceived Support Questionnaire (PSQ) | ||
| CG Perceived Stress | ∆ Perceived Stress Scale (PSS) | ||
| Moniz‐Cook 2008a | Family | CR Behaviour & CG Management/difficulty coping | Problem Checklist (PC) |
| CR Global Dependency | □ Global Deterioration Scale (GDS) | ||
| CG psychiatric morbidity | ∆ General Health Questionnaire (GHQ) | ||
| CG Mood | Hospital Anxiety and Depression Scale (HADS) | ||
| Teri 2000 | Family | Clinically meaningful change in CR | □ ADCS Clinical Global Impression of Change scale (ADCS‐CGIC) |
| CR function (physical and cognitive) | □ Physical Self maintenance (PSM) | ||
| □ Instrumental activities of daily living (IADL) | |||
| □ MMSE | |||
| CG Burden & Reactivity to specific disruptive behaviours | Screen for Caregiver Burden (SCB) | ||
| ◊ RMBPC reaction (not reported) | |||
| CR behaviour | □ Consortium to establish a registry for Alzheimer’s disease (CERAD) | ||
| □ Behavioural Rating scale for Dementia (BRSD) | |||
| ◊ RMBPC (Frequency) | |||
| □ ◊ CMAI (Frequency) | |||
| Agitated behaviour in dementia scale (ABID) (Frequency & Reaction) | |||
| Teri 2003 | Family | CR Behaviour & CG distress | ◊ RMBPC |
| CR Physical Health and Function | □ Short Form Health Survey (SF‐36) | ||
| □ Sickness Impact Profile Mobility (SIP) | |||
| CR Mood | Cornell Depression in Dementia Scale (CDDS) | ||
| □ Hamilton Depression Scale (HDRS) | |||
| CR Cognitive Ability | ◊ MMSE | ||
| Other outcomes: | ∞ CR walking speed, functional reach and standing balance. | ||
| Teri 2005a | Family | CR Behaviour | ◊ RMBPC |
| ◊ NPI | |||
| CR Quality of life | □ Quality of Life in Alzheimer’s disease scale (QOL‐AD) | ||
| CG Mood | ◊ CES‐D | ||
| CG Mood | □ ◊ HDRS | ||
| CG Perceived Stress | ∆ ◊ PSS | ||
| CG Burden | ◊ SCB | ||
| CG Sleep Problems | ∆ Caregiver Sleep questionnaire | ||
| CG Feelings of Competence | ∆ Short sense of Competence Questionnaire (SSCQ) | ||
| CR Cognitive status | □ ◊ MMSE | ||
| Adverse reactions | ∞ | ||
| Zarit 1987 | Family | CR Behaviour & CG distress | Memory and Behaviour Problem Checklist (MBPC) |
| CG Stress associated with care giving | Burden Interview (BI) | ||
| CR Frequency of psychiatric symptoms | □ Brief Symptom Inventory (BSI) | ||
| Social Support | ∞ Amount of interaction with informal support network, amount of assistance by others & caregiver rating of adequacy of social support. | ||
| Therapeutic dimensions of Intervention | ∆ Caregiver Change Interview (CCI) | ||
| CG Perception of intervention | ∆ Global rating of situation improvement | ||
| CR Cognitive Ability | □ ◊ MMSE | ||
| Chenoweth 2009 | Residential | CR Behaviour | ◊ CMAI |
| ◊ NPI | |||
| CR Quality of life in later stage dementia | □ Quality of Life Index (QUALID) | ||
| Amount of physical restraint | □ Quality of Interaction Schedule (QUIS) observations | ||
| CR Global Dependency | □ ◊ GDS | ||
| Other outcomes: | ∞ Antipsychotics & benzodiazepine doses, incidents and admissions to hospital. Also conducted an economic analysis. | ||
| Fossey 2006 | Residential | CR Behaviour | ◊ CMAI |
| CR Dementia Severity | □ ◊ CDR | ||
| Neuroleptic use | ∞ Daily chlorpromazine amounts to national formulary | ||
| CR Falls | ∞ Observations | ||
| CR Quality of life and well‐being | Measurement scale not reported. | ||
| Proctor 1999 | Residential | CR Behaviour | Crichton Royal Behavioural Rating Scale |
| CR Organic and Depressive symptoms | Automatic Geriatric Examination for Computer assisted taxonomy (AGECAT) | ||
| CR Activities of daily living | □ Barthel Index | ||
| Teri 2005b | Assisted Living | CR Behaviour & CG Reaction |
◊ RMBPC |
| □ ◊ ABID | |||
| ◊ NPI | |||
| CR Mood | RMBPC sub scale | ||
| □ Geriatric Depression Scale | |||
| □ Clinical Anxiety Scale (CAS) | |||
| Staff feelings on capability to provide care for a person with dementia | ∆ ◊ SSCQ | ||
| CR Cognitive ability | □ ◊ MMSE | ||
| Mador 2004 | Hospital | CR Behaviour (severity) | Pittsburgh Agitation Scale (PAS) |
| Appropriateness of psychotropic medication | □ Medication Appropriateness Index (MAI) | ||
| Other outcomes | ∞ Total daily doses of benzodiazepines and antipsychotics administered, length of stay, discharge destination, number of falls, nursing satisfaction, next of kin (NOK) satisfaction with care. | ||
This variation became important when four studies of FA‐focused interventions were analysed since it was not possible to determine whether lack of positive effects were due to insufficient intervention ‘dosage’ (see Table 2 for example Gormley 2001) or reduced power, or both.
Selection bias was judged as low risk for randomisation, although in six studies the procedure used was not stated and some studies did not report detail for concealment. Bias in performance was judged as low risk but this is often hard to evaluate in non‐pharmacological studies, since staff can have different expectations of treatment groups and control conditions varied. Six of the included studies had an attention control condition where minimal support, advice, education or placebo drug was provided. Eleven trials reported a ‘usual care’ condition and one used a wait list control. The content of ‘usual care’ was unclear. Blinding of care‐recipients and caregivers was seldom reported so it was not possible to judge whether patients in some studies were aware that they might be receiving preferential support. Authors reported the methods used to achieve and maintain blinding of interventionists and some reported where blinding had failed. Blinding of outcome assessors was not always clear and one study reported that the interventionists had a dual function as outcome assessors. Most reports outlined protocols and procedures for treatment fidelity and adherence (see Table 2) but this did not mean that variation across therapists did not occur in all studies. Only one study was judged as high risk as the authors documented ‘poor adherence’ to the protocol. Attrition reporting was achieved in the majority of trials, although reasons for dropout were often unclear, often described as ‘lost to follow‐up’ or ‘missed’. One study reported withdrawal due to adverse effects in a pharmacological comparison treatment condition.
Effects of interventions
All comparisons for analyses compared functional analysis based intervention with control group (usual care). See Data and analyses.
Primary outcomes
The primary outcomes were measurement of the reported incidence, frequency and severity of problematic behaviours. Patient depression was also included in this category.
Effects on behaviour
There were no significant reductions in the incidence of challenging behaviours as reported by four family care studies at post‐intervention (SMD 0.02, 95% CI ‐0.13 to 0.17, P = 0.80, N = 722; Figure 2). Post‐test intervals ranged from three months to six months. At six months follow‐up, no significant effect was seen for two studies (Farran 2004; Gitlin 2010) (SMD 0.08, 95%CI ‐0.11 to 0.27, P = 0.41, N = 436).
2.
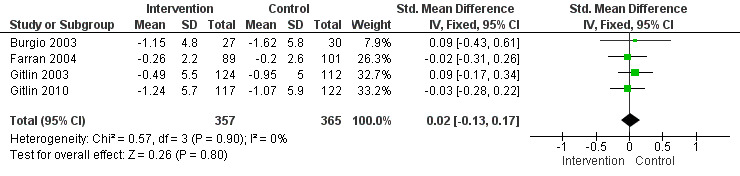
Forest plot of comparison: 1 Functional analysis versus usual care ‐ primary outcomes at post‐intervention, outcome: 1.1 Incidence of problem behaviours ‐ family care only. [Instruments used: RMPBC]
There was a significant reduction in the frequency of challenging behaviours at post‐intervention for 10 family care studies (N = 1046), two residential studies (N = 505) and one assisted living study (N = 31), (SMD ‐0.12, 95% CI ‐0.22 to ‐0.02, P = 0.02, N = 1582). Sensitivity analysis due to moderate heterogeneity (I² = 37%), resulted in removal of the assisted living study (Teri 2005b) and no heterogeneity (I² = 0%) (SMD ‐0.10, 95% CI ‐0.20 to ‐0.00, P = 0.04, N = 1551), see Figure 3. Post‐test intervals ranged from 3 weeks to 12 months. At six months follow‐up, there was no effect in four family care studies (Farran 2004; Gitlin 2010; Teri 2003; Teri 2005a) (SMD 0.00, 95% CI ‐0.16 to 0.16, P = 0.99, N = 627). Similarly, no effect was found at 12 months for three family care studies (Farran 2004; Moniz‐Cook 2008a; Teri 2000) (SMD 0.02, 95% CI ‐0.22 to 0.27, P = 0.86, N = 266).
3.
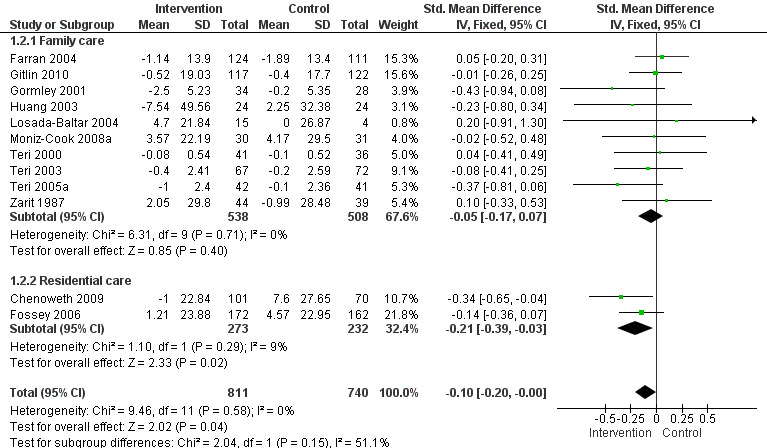
Forest plot of comparison: 1 Functional analysis versus usual care ‐ primary outcomes at post‐intervention, outcome: 1.2 Frequency of problem behaviours. [Instruments used: PC, RAGE, RMBPC, CMAI and MBCL]
Although the results of the meta‐analysis for severity of challenging behaviour were in a direction that favoured the intervention, no significant effect at post‐intervention was found Figure 4. The analysis included two family care studies, two residential care studies, one assisted living study and one hospital care study, respectively (SMD 0.02, 95% CI ‐0.15 to 0.20, P = 0.79, N = 520). Given high heterogeneity (I² = 68%), sensitivity analysis resulted in the removal of the hospital study (Mador 2004, N = 71) and 0% heterogeneity (SMD ‐0.10, 95% CI ‐0.29 to 0.08, P = 0.28, N = 449). No follow‐up data were available for analysis.
4.
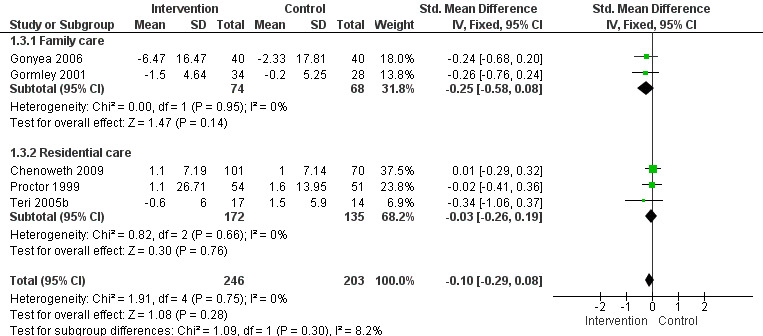
Forest plot of comparison: 1 Functional analysis versus usual care ‐ primary outcomes at post‐intervention, outcome: 1.3 Severity of problem behaviours. [Instruments used: PAS, NPI, Behave‐AD and Crichton Royal Behavioural Scale].
Effects on patient depression
A significant positive effect was found at post‐intervention on patient depression for four studies. This effect was dominated by the assisted living study for which a moderate heterogeneity occurred: I² = 44%, (SMD ‐0.19, 95% CI ‐0.36 to ‐0.01, P = 0.04). Removal of the assisted living study (Teri 2005b) from the analysis resulted in a reduction of the heterogeneity to 4%. There was no significant effect for the remaining studies ‐ two family care studies and one care home study (SMD ‐0.15, 95%CI ‐0.33 to 0.03, P = 0.10, N = 480: Figure 5).
5.
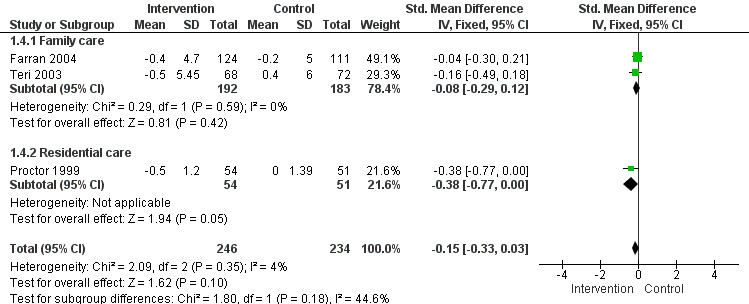
Forest plot of comparison: 1 Functional analysis versus usual care ‐ primary outcomes at post‐intervention, outcome: 1.4 Patient depression. [Instruments used: RMPBC Depression sub scale, AGECAT and CDDS]
Secondary outcomes
Self reported changes in staff or family carer management, such as reaction and burden, were included in the analysis. Data from measures of caregiver mood were also analysed. Data for aggregation of staff caregiver experience were not available.
Effects on caregiver reaction and burden
A significant positive effect on caregiver reaction to challenging behaviour for 10 family care studies and one assisted living study at post‐intervention was found (SMD ‐0.13, 95% CI ‐0.24 to ‐0.02, P = 0.02, N = 1284). Sensitivity analysis due to mild heterogeneity (21%), suggested removal of the assisted living study (Teri 2005b), which reduced the heterogeneity to 2% (SMD ‐0.11, 95% CI ‐0.22 to ‐0.00, P = 0.05, N = 1259: Figure 6). Post–test intervals ranged from two to six months. At six months follow‐up, four family care studies (Farran 2004; Gitlin 2010; Teri 2003; Teri 2005a) had available data, and analysis of the effect of the intervention was not maintained (SMD ‐0.11, 95% CI ‐0.27 to 0.04, P = 0.15, N = 653). Although the results for caregiver burden were in a direction that favoured the intervention, no significant reductions were found at post‐intervention for six studies (Gitlin 2010; Gonyea 2006; Gormley 2001; Teri 2000; Teri 2005a; Zarit 1987) (SMD ‐0.13, 95% CI ‐0.29 to 0.03, P = 0.10, N = 624 or at six months follow‐up for two studies (Gitlin 2010; Teri 2005a) (SMD ‐0.14, 95% CI ‐0.38 to 0.09, P = 0.23, N = 286).
6.
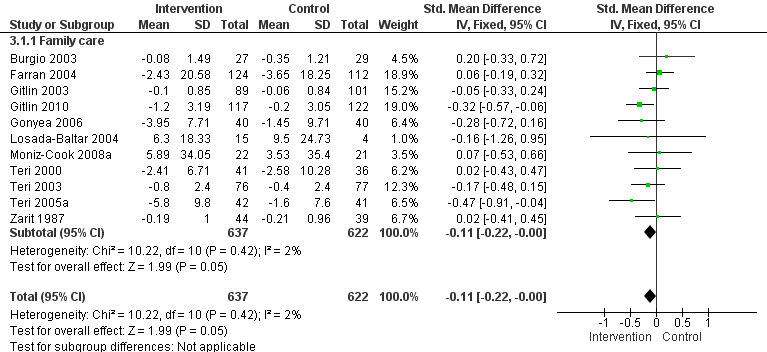
Forest plot of comparison: 3 Functional analysis versus usual care ‐ secondary outcomes at post‐intervention, outcome: 3.1 Caregiver reaction. [Instruments used: PC, RMBPC ‐reaction, NPI ‐distress and ABID ‐reaction].
Effects on caregiver depression
Although the results were in a direction that favoured the intervention, no significant reduction of caregiver depression was found at post‐intervention for five family care studies (Burgio 2003; Farran 2004; Losada‐Baltar 2004; Moniz‐Cook 2008a; Teri 2005a) (SMD ‐0.12, 95% CI ‐0.30 to 0.06, P = 0.21, N = 473). Post‐test intervals ranged from two to six months. At six months follow‐up no significant effect was seen for two studies (Farran 2004; Teri 2005a) (MD ‐0.93, 95% CI ‐2.56 to 0.70, P = 0.26, N = 290).
Behaviour management trials: effects on behaviour and caregiver burden
Three separate analyses were conducted for four trials (Gormley 2001; Mador 2004; Proctor 1999; Teri 2000) where behaviour management appeared to be the primary focus of the intervention (see Data and analyses).
Firstly, we analysed the primary outcome ‘frequency of challenging behaviours’ where two trials (Gormley 2001; Teri 2000) had data that could be pooled at post‐test, but no significant effect was found (SMD ‐0.17, 95% CI ‐0.50 to 0.17, P = 0.33, N = 139) and moderate heterogeneity (I² = 46%) was noted. Secondly, we examined the severity of challenging behaviours at post‐test where data from two trials of hospital care and a care home setting were available (Mador 2004; Proctor 1999). A significant effect was found (SMD 0.33, 95% CI 0.02 to 0.63, P = 0.03, N = 176), but this was not in the direction of the intervention, and a high heterogeneity was noted (I² = 88%). Thirdly, we examined caregiver burden at post‐test for two family care studies (Gormley 2001; Teri 2000). As was the case for the previous meta‐analysis using six studies on this variable, the findings were in a direction that favoured the intervention, but no significant effect was found (SMD ‐0.13, 95% CI ‐0.46 to 0.21, P = 0.45, N = 139).
Discussion
The aim of this review was to evaluate the evidence for the efficacy of functional analysis‐based interventions for challenging behaviour in dementia. Eighteen trials, where functional analysis was usually referred to as 'behaviour management', were included in the review. The majority, 13 studies, were in family care settings. Surprisingly, there were only three care home studies and a further two smaller studies in an assisted living and a hospital setting, respectively. For 14 studies, FA was just one aspect of a broad multi‐component programme of care; thus, only four focused on FA as the main intervention, two in family settings, one in a care home and one in hospital. Data for all studies incorporating FA showed beneficial effects on both the reported frequency of challenging behaviours (Figure 3) and caregiver reaction to these (Figure 6) at post‐intervention. No significant effects were found at any follow‐up periods. Analyses using four studies where behaviour management appeared to be the main focus of the intervention did not show significant effects in favour of FA (see Data and analyses) although as we outline later, reasons for this are likely to be associated with the quality of the studies that we were able to include in the meta‐analysis.
The strength of the conclusions that can be drawn from these results at this stage is limited by a number of important caveats.
First, as noted by other reviews of BPSD (Black 2004) and family caregiver support (Parker 2008), FA interventions tended to be conceived within a range of theoretical models. These in turn influenced the time and number of sessions devoted to treatment, the overall duration of the intervention, the way it was delivered and the instruments used to measure outcomes. The caregiver stress‐coping model was used in the earliest study (Zarit 1987) and remained the commonest approach in subsequent family care studies, although other constructs such as the progressively lowered stress threshold ‐ PSLT approach (for example Huang 2003) were also described. Most family caregiver studies used what appear to be experienced professionals or highly educated therapists, such as those with Masters level qualifications, to deliver the intervention, although the level of training and experience was not always clear. This hampered the conclusions that can be made about critical process variables, such as therapeutic qualities that may have influenced the reported outcomes. This is a well documented difficulty of studies of this type (see for example Bourgeois 1996 for a review). Insufficient ‘dosage’ in behaviour management interventions (for example Gormley 2001), or detail of FA within multi‐component interventions, limit our conclusions on the effects of FA as an intervention in its own right.
Few RCTs in care home settings were located. The absence of studies arising from the USA, where psychosocial intervention in long term care has a worthy history, was surprising. This may have been a consequence of our definition of functional analysis (and associated criteria for trial selection), which arose from the growing knowledge base over the past decade (see Bird 2008; Cohen‐Mansfield 2007; James 2011; Stokes 2000) . We were over‐inclusive in our perusal of studies that offered any aspect of behaviour management and then scrutinized the reasons for excluding studies. These were associated with both methodology as well the underlying theory and the focus of the intervention, which varied across studies. For example, a key family care study was excluded, since our inclusion criterion of utilising behavioural outcomes was not met (Burns 2003). This might have been because the intervention was conceived to address caregiver distress about the care recipient's behaviour, rather than to reduce distress and associated challenging behaviours in the care recipient per se. The study described an educational intervention of behaviour management compared with behaviour management alone and demonstrated that the combined condition had better outcomes on some caregiver variables. In contrast, a landmark care home intervention (Cohen‐Mansfield 2007) was conceived to address ‘unmet need’ in distressed residents, with appropriate measurement of behavioural outcome, but had to be excluded because the researchers were unable to achieve full randomisation.
It was hard to establish the underlying conceptual basis for two of the included care home studies (Chenoweth 2009; Fossey 2006), which contributed to the positive findings of a reduction in the frequency of challenging behaviour. Person‐Centred Care and Dementia Care Mapping originate from theories of person‐centred care in dementia (Kitwood 1997) and were underlying constructs used to develop these respective interventions ( Chenoweth 2009; Fossey 2006). The latter (Chenoweth 2009) also drew heavily on the theory of behaviour as a function of 'unmet need' (Cohen‐Mansfield 2007), a notion that is also understood within functional‐analysis models of behaviour (Bird 2008; James 2011; Moniz‐Cook 2001; Stokes 2000 and). Similarly, one of the earliest studies of behaviour management in care homes (Proctor 1999) appeared to incorporate aspects of person‐centred care. The intervention involved staff training about therapeutic activities and goal planning based on the resident’s strengths and abilities; the text documented a case example where social interaction was identified as an unmet need and non‐contingent social contact combined with offering toileting care resolved the resident’s repeated requests for the toilet. Notably, the researchers found that their ‘behaviour management’ intervention improved depression but not challenging behaviours in care recipients. Future analyses of more studies (particularly in care home settings) that have conceptually defined components of interventions – for example, those that address aggression and agitation as well as those that address depression ‐ will provide better evidence for functional analysis in the management of both behavioural as well as mood problems in care recipients.
Secondly, across the studies reviewed, primary and secondary outcomes were often not clearly defined or matched to the main focus of the intervention and there was conceptual confusion on the choice of instruments selected to measure particular outcomes. For example, the RMBPC ‘caregiver reaction’ domain was described by study authors in a variety of ways as a measure of appraisal, bother, upset, subjective burden and reaction. In some studies more than one assessment was used to measure a given outcome, such as patient behaviour or mood. When conducting meta‐analyses we decided to use measures that were identical where possible but there were 20 instruments of potential relevance that were not used in analyses, many of which were associated with caregiver experience. Our analysis of data of six studies found no positive effects of FA on family caregiver burden. This is consistent with other reviews suggesting this construct may be a good outcome predictor for interventions addressing caregiver support but not for multi‐component interventions that include behaviour management (Parker 2008). The construct of family caregiver burden may in itself be outdated as some reviewers have noted that it is poorly defined and insensitive to change (see Parker 2008) whilst others suggest that a refined and more clinically relevant conceptualisation is required (Black 2004). This review adds weight to the recommendations of similar reviews (Black 2004; Parker 2008), which have highlighted inconsistencies of instruments to evaluate outcome and inadequacy of these in the measurement of constructs within the intervention, suggesting that these are priorities for future research. Thus, we conclude that the literature on FA in dementia care, unlike other areas, such as pharmacological studies or cognitive stimulation therapy, is underdeveloped, since the knowledge base has only relatively recently emerged in terms of both concepts (Bird 2008; James 2011; Stokes 2000) and associated measurement (Moniz‐Cook 2008b).
Thirdly, the development of FA interventions was often determined by setting, i.e. family care, assisted living, care home and hospital. This dissimilarity of setting is important as an explanation of the heterogeneity noted in the assisted living and hospital‐based trials. In these studies, clinical heterogeneity such as the characteristics of people, the treatments offered and the outcomes measured (which rendered pooling of data in meta‐analysis as inappropriate) may all have been due to the context within which the intervention was delivered. For example, in the short nine day hospital‐based intervention of Mador 2004, 48% of the patients had delirium, which in a relatively small study of just 71 participants compromises the conclusions that could be reached about reported findings. Also, hospital admission criteria appeared to have influenced the findings through the route of floor effects, since the authors indicate that patients in the study were possibly not agitated enough at baseline to show significant improvement. A further difficulty in comparing FA as an intervention across care settings relates to caregiver factors since not only do professional and family carers experience strain or challenges in different ways to each other, but overall the experience of challenging behaviour is thought to be reflective of caregiver appraisal and the interpersonal context, i.e. ‘in the eye of the beholder’ (Bird 2008).
Another limitation was that comparison conditions differed. Some studies used an active treatment ‘attention control’ condition, which may have masked the comparative effects of the intervention. Details of what the usual care condition might involve were often absent and became important in interpretation of the meta‐analysis of the four studies where behaviour management was the main focus of the intervention. For example, in the behaviour management study of Teri 2000, data from a drug placebo condition was used for comparison. In the hospital study (Mador 2004) it was not clear whether ‘usual’ pharmacological treatment, ‘usual’ nursing care or the experimental ‘enhanced care intervention’ influenced the reported outcome of the study. Other aspects of quality might also have contributed to the results of the four focused behaviour management studies (for example, allocation and performance bias in Gormley 2001). Our review concurs with others suggesting that the literature on behaviour management as an effective intervention is emerging but requires well designed and conducted RCTs (Livingston 2005). Studies should also address the need for adequate sample sizes, well defined interventions and control groups and adequate follow‐up periods (Parker 2008).
Finally, despite suggestions that psychosocial interventions may have a suppressant effect on challenging behaviours, which becomes evident over a long period in the trajectory of dementia care (Moniz‐Cook 2008b), judgment of the sustainability of FA remains in the balance. Conclusions are hampered by the variation in the length of interventions and the variation in time intervals to post‐ and follow‐up measurements.
Despite the limitations noted above, a promising finding of this review is that where FA is a component of the intervention, positive post‐intervention effects can be seen on the frequency of challenging behaviours in both family care and care home settings and on caregiver reaction. Studies that give due attention to the methodological constraints we document, including using standard primary and secondary outcomes (Moniz‐Cook 2008b), that are matched to the main focus of the intervention could in the future clarify the true effectiveness of functional analysis as an intervention for challenging behaviour in dementia care. Implementation studies of successful randomised controlled trials will need to address the translational potential of the evidence for FA given the noted expertise and qualities of trainers or therapists who deliver interventions in research studies.
Adverse effects for haloperidol were reported in a dropout analysis carried out by the authors of the four arm agitation trial of Teri 2000, but our meta‐analysis did not include data from this condition. There were no indications from the outcome measures of the eighteen studies included in the current analysis of any harm or distress to participants with dementia.
Authors' conclusions
Implications for practice.
The evidence of functional analysis‐based interventions in the management and resolution of challenging behaviour in dementia is promising but it is too early to draw robust conclusions about its efficacy.
The evidence base for the effectiveness of functional analysis‐based interventions continues to rest on randomised controlled trials that incorporate multiple components, leaving the dosage and intensity of functional analysis within the intervention variable and unclear. It is too early to provide indication of the true effectiveness of functional analysis‐based intervention in comparison to other psychosocial interventions for the management and resolution of challenging behaviour in dementia. However, as a component part of psychosocial intervention programmes, including those that focus on training and supporting caregivers, it remains a promising intervention. The finding that positive effects were seen post‐intervention on not only frequency of challenging behaviour but also caregiver reaction to it has clinical relevance, as this is an important predictor of nursing home placement (de Vugt 2005). Other reviewers have also noted that behaviour management can have lasting effects for both care recipients (Livingston 2005) and family caregivers (Selwood 2007).
Implications for research.
There is a clear need for more RCTs of functional analysis‐based interventions in family care and care homes settings. We suggest that the current knowledge base that we have outlined in this review, including the development for manuals of FA for behaviours that challenge (James 2011) has potential for developing interventions that are conceptually and theoretically sound. RCTs of functional analysis will require clear treatment protocols that separate caregiver training and support from care plan delivery to the patient, with research designs to measure the relative effects of these on behaviour outcomes. Studies need to also pay attention to clear definitions of: control groups; standardised instruments (see Moniz‐Cook 2008b) to measure outcomes on patient behaviour as well as caregiver experience, and time intervals to post‐intervention and follow‐up. To assist synthesis of future meta‐analysis in this area, we suggest that there is now scope for RCTs to develop mature methodologies that attend to the common sources of bias that have compromised the quality of studies that have evaluated FA to date.
History
Protocol first published: Issue 1, 2008 Review first published: Issue 2, 2012
| Date | Event | Description |
|---|---|---|
| 13 April 2010 | New search has been performed | A search was performed for this review on 12 April 2010. |
Acknowledgements
We thank Linda Clare (Editor), Rupert McShane (Co‐ordinating Editor) and Graham Stokes (expert clinician) for important advice and guidance, and Dymphna Hermans, Anne Eisinga and Helen Collins for assistance in the preparation of this protocol. We thank Carla Ramsay (Branch Manager, Hull & East Riding Branch, Alzheimer's Society) for assisting with and coordinating the consumer (people with personal experience of dementia) response and Tracie Jennings (family carer) for her detailed comments which have contributed to this protocol. We would like to thank the authors of both included and excluded studies for the provision of further information and data. For the provision of further data and willingness to help with this review, we thank authors Clive Ballard & Jane Fossey, Lynn Chenoweth, Louis Burgio, Andres Losada‐ Baltar, Linda Teri, Laura Gibbons, Ken Pike, Carol Farran, Maureen O'Connor, Phillip McCallion, Sandy Burgener, Ann Steffen and Marita McCabe.
Appendices
Appendix 1. Pre‐publication search: March 2011
|
Source |
Search strategy | Hits retrieved |
| 1. ALOIS (www.medicine.ox.ac.uk/alois) | Advanced search Intervention: (contains any): "functional analysis" OR mapping OR "care management" OR psychosocial OR behavioural. Date added to ALOIS between Jan 2010 and March 2011 | 36 |
| 2. MEDLINE In‐process and other non‐indexed citations and MEDLINE 1950‐present (Ovid SP) | 1. behavio*.mp. 2. agitat*.mp. 3. aggressi*.mp. 4. delusion*.mp. 5. hallucinat*.mp. 6. anxiety.mp. 7. anxious*.mp. 8. depress*.mp. 9. apath*.mp. 10. wandering.mp. 11. disinhibit*.mp. 12. confused.mp. 13. confusion.mp. 14. vocal*.mp. 15. BPSD.mp. 16. neuropsychiatr*.mp. 17. or/1‐16 18. ("functional analy*" or "dementia care map*" or "person‐centred care").mp. 19. (behavio* and (intervention* or manag* or modif* or chang* or analys*)).mp. [mp=protocol supplementary concept, rare disease supplementary concept, title, original title, abstract, name of substance word, subject heading word, unique identifier] 20. 18 or 19 21. 17 and 20 22. Dementia/ 23. Dementia, Multi‐Infarct/ 24. Dementia, Vascular/ 25. Alzheimer Disease/ 26. Lewy Body Disease/ 27. Delirium/ 28. Huntington Disease/ 29. "Pick Disease of the Brain"/ 30. Kluver‐Bucy Syndrome/ 31. Wernicke Encephalopathy/ 32. Creutzfeldt‐Jakob Syndrome/ 33. Delirium, Dementia, Amnestic, Cognitive Disorders/ 34. dement*.mp. 35. Alzheimer*.mp. 36. (lewy* adj2 bod*).mp. 37. deliri*.mp. 38. ("organic brain disease" or "organic brain syndrome").mp. 39. "supranuclear palsy".mp. 40. (pick* adj2 disease).mp. 41. (creutzfeldt or jcd or cjd).mp. 42. huntington*.mp. 43. binswanger*.mp. 44. korsako*.mp. 45. arteriosclerosis.mp. 46. "cerebrovascular disorder*".mp. 47. "cerebr* deteriorat*".mp. 48. "cerebr* insufficien*".mp. 49. or/22‐48 50. 49 and 21 51. randomized controlled trial.pt. 52. controlled clinical trial.pt. 53. random*.ab. 54. placebo.ab. 55. trial.ab. 56. groups.ab. 57. or/51‐56 58. (animals not (humans and animals)).sh. 59. 57 not 58 60. 50 and 59 61. (201004* or 201005* or 201006* or 201007* or 201008* or 201009* or 201010* or 201011* or 201012*).ed. 62. 2011*.ed. 63. 61 or 62 64. 60 and 63 |
156 |
| 3. EMBASE 1980‐2011 week 8 (Ovid SP) |
1. behavio*.mp. 2. agitat*.mp. 3. aggressi*.mp. 4. delusion*.mp. 5. hallucinat*.mp. 6. anxiety.mp. 7. anxious*.mp. 8. depress*.mp. 9. apath*.mp. 10. wandering.mp. 11. disinhibit*.mp. 12. confused.mp. 13. confusion.mp. 14. vocal*.mp. 15. BPSD.mp. 16. neuropsychiatr*.mp. 17. or/1‐16 18. ("functional analy*" or "dementia care map*" or "person centred car*" or "person centered car*").mp. 19. (behavio* adj3 (intervention* or manag* or modif* or chang* or analys*)).mp. 20. 18 or 19 21. 17 and 20 22. presenile dementia/ or "mixed depression and dementia"/ or semantic dementia/ or frontotemporal dementia/ or senile dementia/ or frontal variant frontotemporal dementia/ or multiinfarct dementia/ or exp dementia/ or Pick presenile dementia/ 23. Alzheimer disease/ 24. diffuse Lewy body disease/ 25. delirium/ 26. Huntington chorea/ 27. Kluver Bucy syndrome/ 28. Wernicke encephalopathy/ 29. Creutzfeldt Jakob disease/ 30. dement*.mp. 31. Alzheimer*.mp. 32. (lewy* adj2 bod*).mp. 33. deliri*.mp. 34. ("organic brain disease" or "organic brain syndrome").mp. 35. "supranuclear palsy".mp. 36. (pick* adj2 disease).mp. 37. (creutzfeldt or jcd or cjd).mp. 38. huntington*.mp. 39. binswanger*.mp. 40. korsako*.mp. 41. arteriosclerosis.mp. 42. "cerebrovascular disorder*".mp. 43. "cerebr* deteriorat*".mp. 44. "cerebr* insufficien*".mp. 45. or/22‐44 46. 45 and 21 47. randomized controlled trial/ 48. clinical trial/ 49. random*.ti,ab. 50. trial.ti,ab. 51. groups.ab. 52. ("treatment as usual" or "treatment as before").mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer] 53. placebo.ab. 54. or/47‐53 55. 46 and 54 56. (2010* or 2011*).em. 57. 55 and 56 |
108 |
| 4. PSYCINFO 1806‐March week 1 2011 (Ovid SP) |
1. behavio*.mp. 2. agitat*.mp. 3. aggressi*.mp. 4. delusion*.mp. 5. hallucinat*.mp. 6. anxiety.mp. 7. anxious*.mp. 8. depress*.mp. 9. apath*.mp. 10. wandering.mp. 11. disinhibit*.mp. 12. confused.mp. 13. confusion.mp. 14. vocal*.mp. 15. BPSD.mp. 16. neuropsychiatr*.mp. 17. or/1‐16 18. ("functional analy*" or "dementia care map*" or "person centred care" or "person centered care").mp. 19. (behavio* adj3 (intervention* or manag* or modif* or chang* or analys*)).mp. 20. 18 or 19 21. 17 and 20 22. exp Dementia/ or exp Senile Dementia/ or exp Presenile Dementia/ or exp Dementia with Lewy Bodies/ or exp Vascular Dementia/ 23. exp Alzheimers Disease/ 24. exp Dementia with Lewy Bodies/ 25. exp Delirium/ 26. Huntingtons Disease/ 27. Picks Disease/ 28. Kluver Bucy Syndrome/ 29. Wernickes Syndrome/ 30. Creutzfeldt Jakob Syndrome/ 31. dement*.mp. 32. Alzheimer*.mp. 33. (lewy* adj2 bod*).mp. 34. deliri*.mp. 35. ("organic brain disease" or "organic brain syndrome").mp. 36. "supranuclear palsy".mp. 37. (pick* adj2 disease).mp. 38. (creutzfeldt or jcd or cjd).mp. 39. huntington*.mp. 40. binswanger*.mp. 41. korsako*.mp. 42. arteriosclerosis.mp. 43. "cerebrovascular disorder*".mp. 44. "cerebr* deteriorat*".mp. 45. "cerebr* insufficien*".mp. 46. or/22‐45 47. 21 and 46 48. random*.ti,ab. 49. trial*.ti,ab. 50. exp Clinical Trials/ 51. groups*.ab. 52. ("treatment as before" or "treatment as usual").mp. 53. "control group".ab. 54. or/48‐53 55. 47 and 54 56. (2010* or 2011*).up. 57. 55 and 56 |
57 |
| 5. CINAHL (EBSCOhost) | S1 TX behavio* S2 TX agitat* S3 TX aggressi* S4 TX delusion* S5 TX hallucinat* S6 TX anxiety S7 TX anxious* S8 TX depress* S9 TX apath* S10 TX wandering S11 TX disinhibit* S12 TX confused S13 TX confusion S14 TX vocal* S15 TX BPSD S16 TX neuropsychiatr* S17 S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9 or S10 or S11 or S12 or S13 or S14 or S15 or S16 S18 TX "functional analy*" OR “dementia care mapping” S19 TX behavio* N3 intervention* S20 TX behavio* N3 manag* S21 TX behavio* N3 modif* S22 TX behavio* N3 chang* S23 TX behavio* N3 analys* S24 S18 or S19 or S20 or S21 or S22 or S23 S25 S17 and S24 S26 (MH "Dementia+") or (MH "Dementia, Vascular") or (MH "Delirium, Dementia, Amnestic, Cognitive Disorders") or (MH "Dementia, Multi‐Infarct") or (MH "Dementia, Presenile") or (MH "Dementia, Senile") S27 (MH "Alzheimer's Disease") S28 (MH "Huntington's Disease") S29 (MH "Pick Disease of the Brain") S30 Kluver‐Bucy S31 (MH "Wernicke's Encephalopathy") S32 (MH "Creutzfeldt‐Jakob Syndrome") S33 TX dement* S34 TX Alzheimer* S35 TX lewy* N2 bod* S36 TX "organic brain disease" or "organic brain syndrome" S37 TX "supranuclear palsy" S38 TX pick* N2 disease S39 TX creutzfeldt or jcd or cjd S40 TX huntington* S41 TX binswanger* S42 TX korsako* S43 TX arteriosclerosis S44 TX "cerebr* deteriorat*" S45 TX "cerebr* insufficien*" S46 S26 or S27 or S28 or S29 or S30 or S31 or S32 or S33 or S34 or S35 or S36 or S37 or S38 or S39 or S40 or S41 or S42 or S43 or S44 or S45 S47 S25 and S46 S48 TX random* S49 TX trial* S50 TX study S51 TX group S52 (MH "Clinical Trials+") S53 S48 or S49 or S50 or S51 or S52 S54 S47 and S53 S55 EM 2010 S56 EM 2011 S57 S55 OR S56 S58 S54 AND S57 |
48 |
| 6. Web of Science (1945‐present) with conference proceedings | Topic=(Dement* OR Alzheimer* OR "Lewy bod*" OR Huntington* OR "Kluver Bucy" OR "Pick* disease" OR delirium OR "cerebrovascular disorder*" OR "Wernicke encephalopathy" OR "Korsakoff psychosis") AND Topic=(((BPSD OR neuropsychiatr* OR behavio*) AND ("functional analy*" OR "dementia care mapping" OR (behavio* AND (intervention* OR manag* OR modif* OR chang* OR analys*))))) AND Topic=(random* OR trial* OR "double‐blind" OR "crossover" OR "cross‐over" OR "cluster rct") AND Year Published=(2010‐2011) Timespan=All Years. |
235 |
| 7. LILACS (BIREME) | Behave$ AND demen$ | 28 |
| 8. CENTRAL (The Cochrane Library) (Issue 1 of 4, Jan 2011) | #1 behavio* #2 agitat* #3 aggressi* #4 delusion* #5 hallucinat* #6 anxiety #7 anxious* #8 depress* #9 apath* #10 wandering #11 disinhibit* #12 confused #13 confusion #14 vocal* #15 BPSD #16 neuropsychiatr* #17 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16) #18 "functional analy*" #19 (behavio* and (intervention* or manag* or modif* or chang* or analys*)) #20 (#18 OR #19) #21 (#17 AND #20) #22 MeSH descriptor Dementia explode all trees #23 MeSH descriptor Alzheimer Disease explode all trees #24 MeSH descriptor Lewy Body Disease explode all trees #25 dement* #26 alzheimer* #27 lewy* adj2 bod* #28 deliri* #29 "organic brain disease" or "organic brain syndrome" #30 "supranuclear palsy" #31 pick* adj2 disease #32 creutzfeldt or jcd or cjd #33 huntington* #34 binswanger* #35 korsako* #36 arterioscerosis #37 "cerebrovascular disorder*" #38 "cerebr* deteriorat*" #39 "cerebr* insufficien*" #40 (#22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39) #41 (#21 AND #40), from 2010 to 2011 |
44 |
| TOTAL before de‐duplication | 712 | |
| TOTAL after de‐dupe and first‐assess | 165 | |
Appendix 2. Second search: April 2010
| Source | Search strategy | Hits |
| MEDLINE In‐process and other non‐indexed citations and MEDLINE 1950‐present (Ovid SP) | 1. behavio*.mp. 2. agitat*.mp. 3. aggressi*.mp. 4. delusion*.mp. 5. hallucinat*.mp. 6. anxiety.mp. 7. anxious*.mp. 8. depress*.mp. 9. apath*.mp. 10. wandering.mp. 11. disinhibit*.mp. 12. confused.mp. 13. confusion.mp. 14. vocal*.mp. 15. BPSD.mp. 16. neuropsychiatr*.mp. 17. or/1‐16 18. ("functional analy*" OR “dementia‐care map*” OR “person centred care” OR “person centered care”).mp. 19. (behavio* and (intervention* or manag* or modif* or chang* or analys*)).mp. 20. 18 or 19 21. 17 and 20 22. Dementia/ 23. Dementia, Multi‐Infarct/ 24. Dementia, Vascular/ 25. Alzheimer Disease/ 26. Lewy Body Disease/ 27. Delirium/ 28. Huntington Disease/ 29. "Pick Disease of the Brain"/ 30. Kluver‐Bucy Syndrome/ 31. Wernicke Encephalopathy/ 32. Creutzfeldt‐Jakob Syndrome/ 33. Delirium, Dementia, Amnestic, Cognitive Disorders/ 34. dement*.mp. 35. Alzheimer*.mp. 36. (lewy* adj2 bod*).mp. 37. deliri*.mp. 38. ("organic brain disease" or "organic brain syndrome").mp. 39. "supranuclear palsy".mp. 40. (pick* adj2 disease).mp. 41. (creutzfeldt or jcd or cjd).mp. 42. huntington*.mp. 43. binswanger*.mp. 44. korsako*.mp. 45. arteriosclerosis.mp. 46. "cerebrovascular disorder*".mp. 47. "cerebr* deteriorat*".mp. 48. "cerebr* insufficien*".mp. 49. or/22‐48 50. 49 and 21 51. randomized controlled trial.pt. 52. controlled clinical trial.pt. 53. random*.ab. 54. placebo.ab. 55. trial.ab. 56. groups.ab. 57. or/51‐56 58. (animals not (humans and animals)).sh. 59. 57 not 58 60. 50 and 59 61. (200709* or 200710* or 200711* or 200712*).ed. 62. 2008*.ed. 63. 2009*.ed. 64. 2010*.ed. 65. or/61‐64 66. 60 and 65 |
389 |
| EMBASE (Ovid SP) 1980‐2010 week 13 |
1. behavio*.mp. 2. agitat*.mp. 3. aggressi*.mp. 4. delusion*.mp. 5. hallucinat*.mp. 6. anxiety.mp. 7. anxious*.mp. 8. depress*.mp. 9. apath*.mp. 10. wandering.mp. 11. disinhibit*.mp. 12. confused.mp. 13. confusion.mp. 14. vocal*.mp. 15. BPSD.mp. 16. neuropsychiatr*.mp. 17. or/1‐16 18. ("functional analy*" OR “dementia care map*” OR “person centered car*” OR “person centred car*”).mp. 19. (behavio* adj3 (intervention* or manag* or modif* or chang* or analys*)).mp. 20. 18 or 19 21. 17 and 20 22. presenile dementia/ or "mixed depression and dementia"/ or semantic dementia/ or frontotemporal dementia/ or senile dementia/ or frontal variant frontotemporal dementia/ or multiinfarct dementia/ or exp dementia/ or Pick presenile dementia/ 23. Alzheimer disease/ 24. diffuse Lewy body disease/ 25. delirium/ 26. Huntington chorea/ 27. Kluver Bucy syndrome/ 28. Wernicke encephalopathy/ 29. Creutzfeldt Jakob disease/ 30. dement*.mp. 31. Alzheimer*.mp. 32. (lewy* adj2 bod*).mp. 33. deliri*.mp. 34. ("organic brain disease" or "organic brain syndrome").mp. 35. "supranuclear palsy".mp. 36. (pick* adj2 disease).mp. 37. (creutzfeldt or jcd or cjd).mp. 38. huntington*.mp. 39. binswanger*.mp. 40. korsako*.mp. 41. arteriosclerosis.mp. 42. "cerebrovascular disorder*".mp. 43. "cerebr* deteriorat*".mp. 44. "cerebr* insufficien*".mp. 45. or/22‐44 46. 45 and 21 47. randomized controlled trial/ 48. clinical trial/ 49. random*.ti,ab. 50. trial.ti,ab. 51. groups.ab. 52. ("treatment as usual" or "treatment as before").mp. 53. placebo.ab. 54. or/47‐53 55. 46 and 54 56. (2007* or 2008* or 2009* or 2010*).em. 57. 55 and 56 |
199 |
| PSYCINFO (Ovid SP) 1806‐April week 1 2010 |
1. behavio*.mp. 2. agitat*.mp. 3. aggressi*.mp. 4. delusion*.mp. 5. hallucinat*.mp. 6. anxiety.mp. 7. anxious*.mp. 8. depress*.mp. 9. apath*.mp. 10. wandering.mp. 11. disinhibit*.mp. 12. confused.mp. 13. confusion.mp. 14. vocal*.mp. 15. BPSD.mp. 16. neuropsychiatr*.mp. 17. or/1‐16 18. ("functional analy*" OR “dementia care mapping” OR “person centred care” OR “person centered care”).mp. 19. (behavio* adj3 (intervention* or manag* or modif* or chang* or analys*)).mp. 20. 18 or 19 21. 17 and 20 22. exp Dementia/ or exp Senile Dementia/ or exp Presenile Dementia/ or exp Dementia with Lewy Bodies/ or exp Vascular Dementia/ 23. exp Alzheimers Disease/ 24. exp Dementia with Lewy Bodies/ 25. exp Delirium/ 26. Huntingtons Disease/ 27. Picks Disease/ 28. Kluver Bucy Syndrome/ 29. Wernickes Syndrome/ 30. Creutzfeldt Jakob Syndrome/ 31. dement*.mp. 32. Alzheimer*.mp. 33. (lewy* adj2 bod*).mp. 34. deliri*.mp. 35. ("organic brain disease" or "organic brain syndrome").mp. 36. "supranuclear palsy".mp. 37. (pick* adj2 disease).mp. 38. (creutzfeldt or jcd or cjd).mp. 39. huntington*.mp. 40. binswanger*.mp. 41. korsako*.mp. 42. arteriosclerosis.mp. 43. "cerebrovascular disorder*".mp. 44. "cerebr* deteriorat*".mp. 45. "cerebr* insufficien*".mp. 46. or/22‐45 47. 21 and 46 48. random*.ti,ab. 49. trial*.ti,ab. 50. exp Clinical Trials/ 51. groups*.ab. 52. ("treatment as before" or "treatment as usual").mp. 53. "control group".ab. 54. or/48‐53 55. 47 and 54 56. (2007* or 2008* or 2009* or 2010*).up. 57. 55 and 56 |
104 |
| CINAHL (EBSCOhost) | S1 TX behavio* S2 TX agitat* S3 TX aggressi* S4 TX delusion* S5 TX hallucinat* S6 TX anxiety S7 TX anxious* S8 TX depress* S9 TX apath* S10 TX wandering S11 TX disinhibit* S12 TX confused S13 TX confusion S14 TX vocal* S15 TX BPSD S16 TX neuropsychiatr* S17 S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9 or S10 or S11 or S12 or S13 or S14 or S15 or S16 S18 TX "functional analy*" OR “dementia care mapping” S19 TX behavio* N3 intervention* S20 TX behavio* N3 manag* S21 TX behavio* N3 modif* S22 TX behavio* N3 chang* S23 TX behavio* N3 analys* S24 S18 or S19 or S20 or S21 or S22 or S23 S25 S17 and S24 S26 (MH "Dementia+") or (MH "Dementia, Vascular") or (MH "Delirium, Dementia, Amnestic, Cognitive Disorders") or (MH "Dementia, Multi‐Infarct") or (MH "Dementia, Presenile") or (MH "Dementia, Senile") S27 (MH "Alzheimer's Disease") S28 (MH "Huntington's Disease") S29 (MH "Pick Disease of the Brain") S30 Kluver‐Bucy S31 (MH "Wernicke's Encephalopathy") S32 (MH "Creutzfeldt‐Jakob Syndrome") S33 TX dement* S34 TX Alzheimer* S35 TX lewy* N2 bod* S36 TX "organic brain disease" or "organic brain syndrome" S37 TX "supranuclear palsy" S38 TX pick* N2 disease S39 TX creutzfeldt or jcd or cjd S40 TX huntington* S41 TX binswanger* S42 TX korsako* S43 TX arteriosclerosis S44 TX "cerebr* deteriorat*" S45 TX "cerebr* insufficien*" S46 S26 or S27 or S28 or S29 or S30 or S31 or S32 or S33 or S34 or S35 or S36 or S37 or S38 or S39 or S40 or S41 or S42 or S43 or S44 or S45 S47 S25 and S46 S48 TX random* S49 TX trial* S50 TX study S51 TX group S52 (MH "Clinical Trials+") S53 S48 or S49 or S50 or S51 or S52 S54 S47 and S53 S55 EM 2007 S56 EM 2008 S57 EM 2009 S58 EM 2010 S59 S55 or S56 or S57 or S58 S60 S54 and S59 |
251 |
| Web of Science with Conference Proceedings (1945 to present) | Topic=((Dementia OR Alzheimer* OR (Lewy body) OR arteriosclerosis OR (Huntington disease) OR (Kluver Bucy) OR (Pick disease) OR delirium OR (cerebrovascular disorder*) OR (Wernicke encephalopathy) OR (Korsakoff psychosis))) AND Topic=(((BPSD OR neuropsychiatr* OR behavio*) AND ("functional analy*" OR "dementia care mapping" OR (behavio* AND (intervention* OR manag* OR modif* OR chang* OR analys*))))) AND Topic=((random* OR trial* OR "double‐blind" OR "crossover" OR "cross‐over")) AND Year Published=((2007‐2010)) Timespan=2007‐2010. Databases=SCI‐EXPANDED, CPCI‐S |
595 |
| LILACS (BIREME) | (dement$ OR alzheimer$) AND (behavior$ OR agitate$ OR aggressi$ OR delusion$ OR hallucinate$ OR anxiety OR anxious$ OR depress$ OR apath$ OR wandering OR disinhibit$ OR confused OR confusion OR vocal$) OR (BPSD) OR neuropsychiatr$) [Words] and "functional analy$" OR "dementia care map$" OR (behavior$ AND (intervention$ OR manag$ OR modif$ OR chang$ OR analys$) [Words] and 2007 OR 2008 OR 2009 OR 2010 [Country, year publication] | 34 |
| ALOIS (www.medicine.ox.ac.uk/alois) | Advanced search: [Intervention: Contains any word: functional analysis mapping] | 1 |
| Umin (Clinical Trial register of Japan) (www.umin.ac.jp/ctr/) | Condition: dementia | 8 |
| CENTRAL (The Cochrane Library) | #1 behavio* #2 agitat* #3 aggressi* #4 delusion* #5 hallucinat* #6 anxiety #7 anxious* #8 depress* #9 apath* #10 wandering #11 disinhibit* #12 confused #13 confusion #14 vocal* #15 BPSD #16 neuropsychiatr* #17 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16) #18 "functional analy*" #19 (behavio* and (intervention* or manag* or modif* or chang* or analys*)) #20 (#18 OR #19) #21 (#17 AND #20) #22 MeSH descriptor Dementia explode all trees #23 MeSH descriptor Alzheimer Disease explode all trees #24 MeSH descriptor Lewy Body Disease explode all trees #25 dement* #26 alzheimer* #27 lewy* adj2 bod* #28 deliri* #29 "organic brain disease" or "organic brain syndrome" #30 "supranuclear palsy" #31 pick* adj2 disease #32 creutzfeldt or jcd or cjd #33 huntington* #34 binswanger* #35 korsako* #36 arterioscerosis #37 "cerebrovascular disorder*" #38 "cerebr* deteriorat*" #39 "cerebr* insufficien*" #40 (#22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39) #41 (#21 AND #40), from 2007 to 2010 |
200 |
| Total | 1781 | |
| Total after deduplication | 1376 | |
Appendix 3. First search: November 2007
| Source | Serach strategy | hits |
| CDCIG Specialized Register (now ALOIS) [searched 17 Nov 2007] | ((behavio* OR agitat* OR aggressi* OR delusion* OR hallucinat* OR anxiety OR anxious* OR depress* OR apath* OR wandering OR disinhibit* OR confused OR confusion OR vocal*) OR (BPSD) OR (neuropsychiatr*)) AND ("functional analy*" OR (behavio* AND (intervention* OR manag* OR modif* OR chang* OR analys*))) | 1091 |
| MEDLINE, EMBASE, PSYCINFO, CINAHL (Ovid SP) [searched 15 Nov 2007] | (all terms were searched as: title, abstract, keyword, controlled vocabulary). ((behavio* OR agitat* OR aggressi* OR delusion* OR hallucinat* OR anxiety OR anxious* OR depress* OR apath* OR wandering OR disinhibit* OR confused OR confusion OR vocal*) OR (BPSD) OR (neuropsychiatr*)) AND ("functional analy*" OR (behavio* AND (intervention* OR manag* OR modif* OR chang* OR analys*))).” AND (((Dementia OR Alzheimer$ OR (Lewy body) OR arteriosclerosis OR (Huntington disease) OR (Kluver Bucy) OR (Pick disease) OR delirium OR (cerebrovascular disorder$) OR (Wernicke encephalopathy) OR (Korsakoff psychosis) OR ((cognit$ or memory$ or mental$) AND (declin$ or impair$ or los$ or deteriorat$)) OR (cerebr$ deteriorat$) OR (cerebr$ insufficien$) AND Phases 1‐3 of the Highly sensitive search strategies for identifying reports of randomized controlled trials in Medline (APPENDIX 5b, Cochrane Handbook, 2006), all terms searched as Title, abstract, keyword, Publication type. |
128 |
| LILACS (BIREME) [searched 15 Nov 2007] | ((behavio* OR agitat* OR aggressi* OR delusion* OR hallucinat* OR anxiety OR anxious* OR depress* OR apath* OR wandering OR disinhibit* OR confused OR confusion OR vocal*) OR (BPSD) OR (neuropsychiatr*)) AND ("functional analy*" OR (behavio* AND (intervention* OR manag* OR modif* OR chang* OR analys*))).” AND (LILACS search strategy from “Dementia Group Search strategy for Specialized Register ie dementia terms) + trial terms |
0 |
| Science Citation index and Social Science Citation index [searched 18 Nov 2007] | TS = ((behavio* OR agitat* OR aggressi* OR delusion* OR hallucinat* OR anxiety OR anxious* OR depress* OR apath* OR wandering OR disinhibit* OR confused OR confusion OR vocal*) OR (BPSD) OR (neuropsychiatr*)) AND ("functional analy*" OR (behavio* AND (intervention* OR manag* OR modif* OR chang* OR analys*))).” AND TS = (((Dementia OR Alzheimer* OR (Lewy body) OR arteriosclerosis OR (Huntington disease) OR (Kluver Bucy) OR (Pick disease) OR delirium OR (cerebrovascular disorder*) OR (Wernicke encephalopathy) OR (Korsakoff psychosis) OR ((cognit* or memory* or mental*) AND (decline* or impair* or los* or deteriorat*)) OR (cerebr* deteriorat*) OR (cerebr* insufficien*) AND TS = (randomized controlled trial*) OR (randomised controlled trial*) OR (controlled clinical trial*) OR placebo* OR crossover OR cross‐over OR (double blind*) OR (single blind*) |
842 |
| Total | 1884 | |
| Total after de‐duplication | 1570 | |
Data and analyses
Comparison 1. Functional analysis versus usual care ‐ primary outcomes at post‐intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incidence of problem behaviours ‐ family care only | 4 | 722 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.13, 0.17] |
| 2 Frequency of problem behaviours | 12 | 1551 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.20, ‐0.00] |
| 2.1 Family care | 10 | 1046 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.05 [‐0.17, 0.07] |
| 2.2 Residential care | 2 | 505 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.21 [‐0.39, ‐0.03] |
| 3 Severity of problem behaviours | 5 | 449 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.29, 0.08] |
| 3.1 Family care | 2 | 142 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.25 [‐0.58, 0.08] |
| 3.2 Residential care | 3 | 307 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.03 [‐0.26, 0.19] |
| 4 Patient depression | 3 | 480 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.15 [‐0.33, 0.03] |
| 4.1 Family care | 2 | 375 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.08 [‐0.29, 0.12] |
| 4.2 Residential care | 1 | 105 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.38 [‐0.77, 0.00] |
1.1. Analysis.
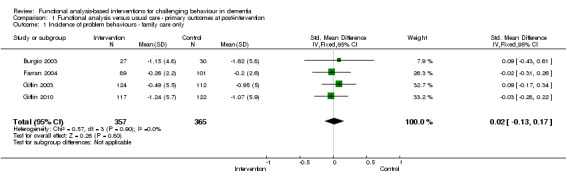
Comparison 1 Functional analysis versus usual care ‐ primary outcomes at post‐intervention, Outcome 1 Incidence of problem behaviours ‐ family care only.
1.2. Analysis.
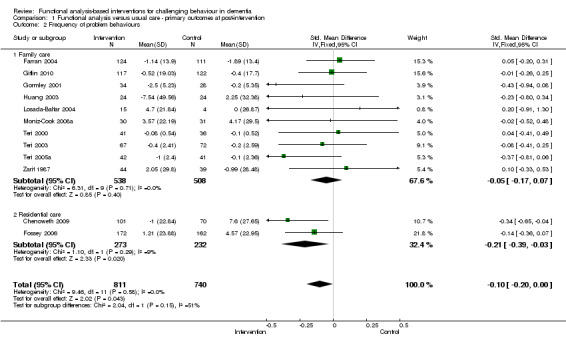
Comparison 1 Functional analysis versus usual care ‐ primary outcomes at post‐intervention, Outcome 2 Frequency of problem behaviours.
1.3. Analysis.
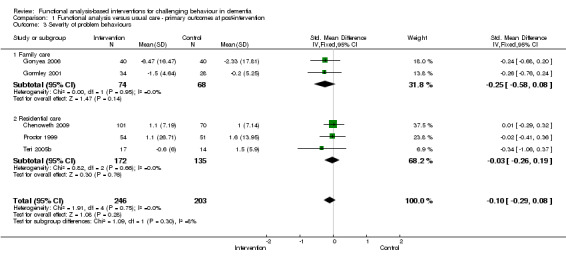
Comparison 1 Functional analysis versus usual care ‐ primary outcomes at post‐intervention, Outcome 3 Severity of problem behaviours.
1.4. Analysis.
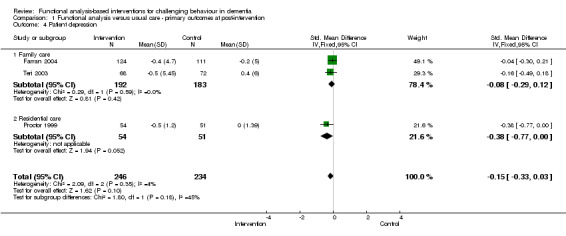
Comparison 1 Functional analysis versus usual care ‐ primary outcomes at post‐intervention, Outcome 4 Patient depression.
Comparison 2. Functional analysis versus usual care ‐ primary outcomes at follow‐up.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incidence of problem behaviours ‐ family care only at 6 month follow‐up | 2 | 436 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.08 [‐0.11, 0.27] |
| 2 Frequency of problem behaviours at 6 month follow‐up | 4 | 627 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.00 [‐0.16, 0.16] |
| 3 Frequency of problem behaviours at 12 month follow‐up | 3 | 266 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.22, 0.27] |
| 3.1 Family care | 3 | 266 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.22, 0.27] |
2.1. Analysis.
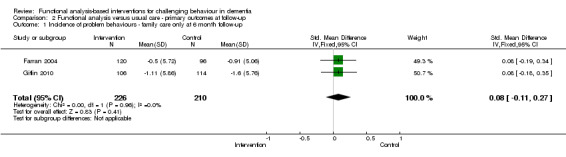
Comparison 2 Functional analysis versus usual care ‐ primary outcomes at follow‐up, Outcome 1 Incidence of problem behaviours ‐ family care only at 6 month follow‐up.
2.2. Analysis.
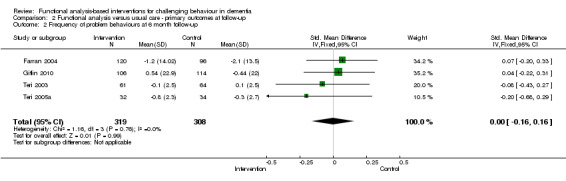
Comparison 2 Functional analysis versus usual care ‐ primary outcomes at follow‐up, Outcome 2 Frequency of problem behaviours at 6 month follow‐up.
2.3. Analysis.
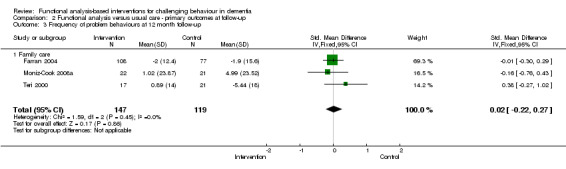
Comparison 2 Functional analysis versus usual care ‐ primary outcomes at follow‐up, Outcome 3 Frequency of problem behaviours at 12 month follow‐up.
Comparison 3. Functional analysis versus usual care ‐ secondary outcomes at post‐intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Caregiver reaction | 11 | 1259 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.11 [‐0.22, ‐0.00] |
| 1.1 Family care | 11 | 1259 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.11 [‐0.22, ‐0.00] |
| 2 Caregiver burden | 6 | 624 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.13 [‐0.29, 0.03] |
| 3 Caregiver well‐being (depression) | 5 | 473 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.12 [‐0.30, 0.06] |
3.1. Analysis.
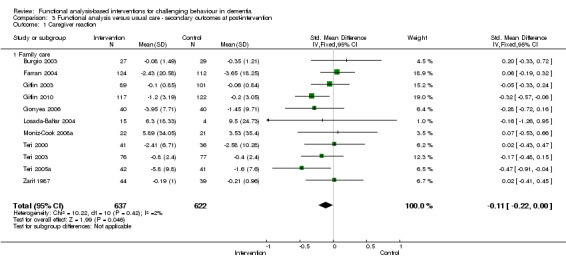
Comparison 3 Functional analysis versus usual care ‐ secondary outcomes at post‐intervention, Outcome 1 Caregiver reaction.
3.2. Analysis.
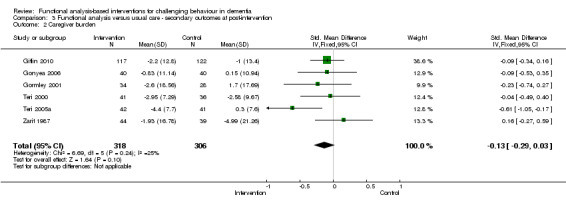
Comparison 3 Functional analysis versus usual care ‐ secondary outcomes at post‐intervention, Outcome 2 Caregiver burden.
3.3. Analysis.
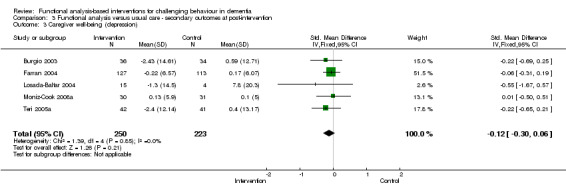
Comparison 3 Functional analysis versus usual care ‐ secondary outcomes at post‐intervention, Outcome 3 Caregiver well‐being (depression).
Comparison 4. Functional analysis versus usual care ‐ secondary outcomes at 6 month follow‐up.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Caregiver reaction | 4 | 653 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.11 [‐0.27, 0.04] |
| 2 Caregiver burden | 2 | 286 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.14 [‐0.38, 0.09] |
| 3 Caregiver well‐being (depression) | 2 | 290 | Mean Difference (IV, Fixed, 95% CI) | ‐0.93 [‐2.56, 0.70] |
4.1. Analysis.
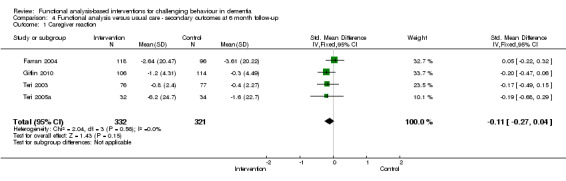
Comparison 4 Functional analysis versus usual care ‐ secondary outcomes at 6 month follow‐up, Outcome 1 Caregiver reaction.
4.2. Analysis.
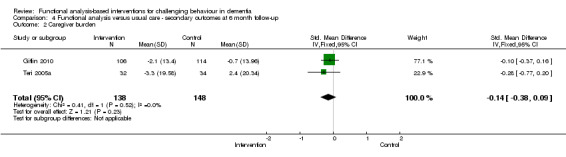
Comparison 4 Functional analysis versus usual care ‐ secondary outcomes at 6 month follow‐up, Outcome 2 Caregiver burden.
4.3. Analysis.
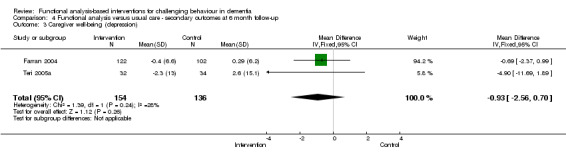
Comparison 4 Functional analysis versus usual care ‐ secondary outcomes at 6 month follow‐up, Outcome 3 Caregiver well‐being (depression).
Comparison 5. Functional analysis versus usual care ‐ outcomes for behaviour management studies only at post‐intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Frequency of problem behaviours at post‐intervention | 2 | 139 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.17 [‐0.50, 0.17] |
| 2 Severity of problem behaviours at post‐intervention | 2 | 176 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.33 [0.02, 0.63] |
| 3 Caregiver burden at post‐intervention | 2 | 139 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.13 [‐0.46, 0.21] |
5.1. Analysis.
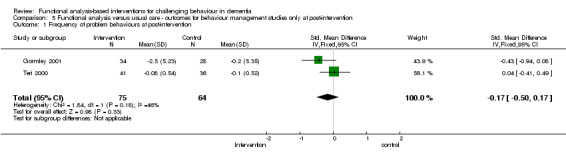
Comparison 5 Functional analysis versus usual care ‐ outcomes for behaviour management studies only at post‐intervention, Outcome 1 Frequency of problem behaviours at post‐intervention.
5.2. Analysis.
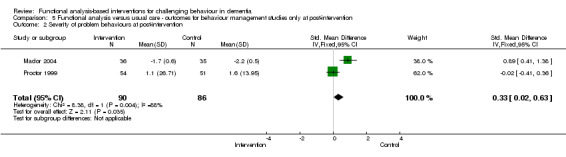
Comparison 5 Functional analysis versus usual care ‐ outcomes for behaviour management studies only at post‐intervention, Outcome 2 Severity of problem behaviours at post‐intervention.
5.3. Analysis.
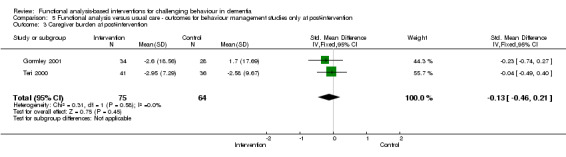
Comparison 5 Functional analysis versus usual care ‐ outcomes for behaviour management studies only at post‐intervention, Outcome 3 Caregiver burden at post‐intervention.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Burgio 2003.
| Methods | Random assignment to intervention or control condition. The intervention was delivered through a group workshop followed by 16 in‐home treatment sessions over 12 months. The paper reports only 6 months follow‐up. | |
| Participants | 70 white and 48 African American primary caregivers (PCG) of individuals with dementia. Care recipients (CR) were required to score < 24 on MMSE, exhibit one limitation in ADLs or IADLs and display 3 problem behaviours as identified by the PCG. CR mean MMSE score was 14.53 for white participants and 10.98 for African American participants, with a mean age of 78.83. | |
| Interventions | Caregiver Skill Training Intervention based on a manual Minimal Support Condition (control) Primary aim of intervention: CR problem behaviour, CG appraisal, social support, activity, well‐being (e.g. depression & anxiety) and desire to institutionalise CR. (See Table 2) |
|
| Outcomes | Revised Memory and Behaviour Problem Checklist (RMBPC) RMBPC Appraisal Leisure Time Satisfaction Measure The Center for Epidemiologic studies‐Depression Scale (CES‐D) State‐Trait Personality Inventory Desire to Institutionalise (see Table 3) |
|
| Notes | Country of origin: America | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | 'Staff were not blinded to group assignment; however. intervention and assessment were never conducted by same individual'. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Low risk | |
Chenoweth 2009.
| Methods | Cluster randomised controlled trial. Study duration 8 months. | |
| Participants | 289 residents from 15 residential homes, of similar management structure, standards and size. Residents had to show need‐driven behaviours, which made it difficult for staff to provide them with quality care. Residents mean age was 85 years. | |
| Interventions | Caregiver training and support intervention in either: Person Centered Care (PCC) or Dementia Care Mapping (DCM) Control (Usual Care) Primary aim of the intervention: To decrease need driven dementia compromised behaviours, improve resident quality of life and reduce the use of psychotropic drugs, restraints, rates of accidents and injuries. (See Table 2) |
|
| Outcomes | Cohen Mansfield Agitation Inventory (CMAI) Neuropsychiatric Inventory (NPI) Quality of life in late stage dementia (QUALID) Quality interactions schedule (QUIS) (See Table 3) |
|
| Notes | Country of origin: Sydney, Australia For the purpose of this review the DCM condition was compared with usual care. Interventionist visited sites for 6 hours per day over 2 days. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'Randomised at site level, using an SAS system'. |
| Allocation concealment (selection bias) | Low risk | Allocation performed by study statistician unaware of sites' identities, using a balanced incomplete‐block design, remaining sites used a complete block design. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Used a protocol and manual. There is no report of checking treatment fidelity or adherence to the manual. Membership to the intervention or control group was masked to outcome assessors; however, it is not reported if participants and other staff members were blind to allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | 'Research assistants were trained in measurement and remained masked to group intervention by means of a signed agreement with staff and managers not to mention the intervention information' |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 26 died and 4 transferred after randomisation. A further 21 died and 2 transferred after the intervention. |
| Selective reporting (reporting bias) | Low risk | Only reported total NPI score, not sub scale scores for frequency and severity. |
| Other bias | Unclear risk | Not other sources of bias identified. |
Farran 2004.
| Methods | Randomised clinical trial. Study duration 18 months. | |
| Participants | 295 care recipients (CR) with Alzheimer’s disease (AD) or other dementia syndrome, with MMSE < 24, and their family caregiver (CG), who provided a minimum of 6 months care, with four hours direct contact per day. CR mean MMSE score was 12.6, CR mean age was not reported. CGs had a mean age of 64.4, 225 were female and 70 male. | |
| Interventions | Caregiver skill (CSB) Intervention Information and Support Orientated Group Intervention (ISO) (Comparison Condition) Primary aim of intervention: Reducing emotional distress in CG & improving CG management of behaviour problems. (See Table 2) |
|
| Outcomes | The Center for Epidemiologic studies‐Depression Scale (CES‐D) for CG Behaviour Management Skill Revised (BMS‐R) The Revised Memory and Problem Behaviour Checklist (RMPBC) Time to Institutionalisation (see Table 3) |
|
| Notes | Country of Origin: Chicago, USA 12 weekly sessions, 5 group sessions, 7 individualised telephone contact sessions, 2 group booster sessions (6 and 12 months after enrolment) and as needed telephone contact during 12 month period. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'Participants were randomly assigned to treatment condition' |
| Allocation concealment (selection bias) | Low risk | Statistician generated randomised sequence of binary codes (1 or 2) for each block of 10 to 20 participants. Sequence position determined by an alphabetically ordered list of participant names within each block. Coin toss to determine group 1 or 2 as intervention or control. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participant assignment list and identification number exclusive to project director. Trained interviewers blind to assignment. Treatment protocol for intervention. To assure fidelity, each staff member received 40 hours training and followed a detailed manual of prescribed material for each session. Supervised implementation, corrective feedback and group sessions taped and reviewed. Intervention staff remained blind to baseline and follow‐up assessment data. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessments conducted over the telephone. Assessment of key outcomes by reviewers blind to treatment condition. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition reported (23 participants terminated early, reasons included: transportation/schedule difficulties (30%), health status (22%), nursing home placement or death (13%) and other reasons/not interested (26%)). |
| Selective reporting (reporting bias) | Low risk | Only coefficients reported, however, full data set supplied by author. |
| Other bias | Low risk | No crossover or carryover effects reported. |
Fossey 2006.
| Methods | Cluster randomised controlled trial with blinded assessment of outcome. Study duration: 12 months. | |
| Participants | 346 residents from 12 residential homes. The mean age of residents was 82 years. The majority of residents had a clinical dementia rating of severe. | |
| Interventions | Training and Support Intervention for nursing home staff. Control (treatment as usual) Primary aim of intervention: To reduce the proportion of residents with dementia who are prescribe neuroleptics. CG training in behavioural management techniques and person centred care, positive care planning, awareness of environmental design, ABC models, development of individualised interventions, active listening and communication and reminiscence techniques. (See Table 2) |
|
| Outcomes | Cohen Mansfield Agitation Inventory (CMAI) Daily drug dosage of residents (See Table 3) |
|
| Notes | Country of origin: London, Newcastle and Oxford, UK. The intervention was delivered over two days a week for 10 months by a psychologist, occupational therapist or nurse. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'Randomly assigned' |
| Allocation concealment (selection bias) | Low risk | 'Statistician randomly assigned homes to intervention or control, stratified by region and baseline neuroleptic use. Allocations were computer generated using stratified block randomisation (fixed block size of two) with strata version 8' |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Statistician blinded to identification of homes. Follow‐up assessments completed by blinded research assistants. Intervention described as 'the package', however, it is not reported whether there was a manual or assessments of adherence. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Follow‐up assessments completed by a research assistant who was not employed during the intervention period. However, the paper reports that 'because the package was designed to influence the whole care approach of staff, it is likely that the research assistant would have been able to detect which homes had received the intervention'. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All reported, some reasons reported as unknown (105 participants died, 4 moved home, 14 unknown reason). |
| Selective reporting (reporting bias) | Low risk | All data reported. |
| Other bias | Low risk | No other risks identified. |
Gitlin 2003.
| Methods | Randomised controlled trial. Study duration: 12 months. | |
| Participants | The participants were 255 persons with Alzheimer’s disease or related disorder and their family caregiver, of which 190 were available at follow‐up. CR were to have a MMSE of < 24. Care recipients had a mean age of 80.85, with an average MMSE score of 12.05. CGs had to be at least 21 years of age, providing care for 4 hours per day for 6 months. CGs were predominantly African American with a mean age of 60.45. | |
| Interventions | Home Environmental Skill‐Building program (ESP) for family CG Control (usual care) Primary aim of intervention: CG well‐being (e.g. Mastery, skill enhancement), Burden & Distress & CR functioning (behaviour & ADL/IADL) delivered by interventionists who received 25 hours of training (See Table 2) |
|
| Outcomes | Revised Memory and Behaviour Problem Checklist (RMBPC) Caregiver Burden (RMBPC) Caregiving Mastery Index Task Management Strategy Index (See Table 3) |
|
| Notes | Country of origin: Philadelphia, USA 5 in home contacts, one telephone contact, Active treatment phase for the first 6 months, maintenance phase for the subsequent 6 months which consisted of 1 home contact and 3 brief telephone sessions. 12 month follow‐up data are reported in Gitlin 2005 but data reported for CR behaviour for the primary outcome measure not equivalent to the data reported in Gitlin 2003. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | Low risk | |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Low risk | |
Gitlin 2010.
| Methods | Randomised trial with comparison group. Study duration: 6 months. | |
| Participants | 272 caregivers (CG) and people with dementia (CR) with a mean age: 82.1 years, of which 220 were available at follow‐up. CR MMSE score of < 24. Caregivers had to be at least 21 years of age, English speaking and planning to live in the area for 6 months, not actively seeking a nursing home placement, managing problem behaviours and reporting upset. | |
| Interventions | Caregiver skills training in managing problem behaviours ‐ the Advanced Caregiver Training (ACT) No treatment control group Primary aim of intervention: CG confidence in managing problem behaviours and associated upset. CG, well‐being (e.g. skill enhancement, management skills, communication, perceived change and perceived benefits), burden and mood. (See Table 2) |
|
| Outcomes | Incidence and frequency of problem behaviours, measured by: Agitated Behaviours in Dementia Scale ‐16 items, Revised Memory and Behaviour Problem Checklist (RMBPC) ‐ 3 items, and other behaviours ‐ families could specify other behaviours which were not listed. Caregiver upset was measured by averaging caregiver responses over all occurring behaviours, with higher scores indicating greater upset. Caregiver depression measured by CES‐D Caregiver burden measured by Zarit Burden Interview (ZBI) Caregiver change (managing care challenges, affect and somatic) ‐ Perceived Change Index (PCI) Task Managment Strategy Index Communication Index Perceived Benefits (See Table 3) |
|
| Notes | Country of origin: Philadelphia, USA Occupational therapists and nurse delivered intervention.16 week active phase of 9 occupational therapy sessions and two nursing sessions (one home, one telephone) and a maintenance phase (16‐24 weeks) of three brief OT telephone contacts to reinforce strategy use. Help caregivers identify antecedents and consequences or potential modifiable triggers of the target problem behaviour. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Two group randomised trial. |
| Allocation concealment (selection bias) | Low risk | Stratified according to relationship (spouse vs non spouse) and randomised within each of two strata using permuted blocks. Study statistician developed a blocking number which was unknown to others. Randomisation lists and two sets of randomisation forms were prepared using opaque envelopes. The Project director randomised each participant within 48 hours of baseline interview. Project director performed randomisation. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | 10 licensed OTs and 1 nurse had 35 hours training. Treatment fidelity monitored and maintained through twice monthly meetings involving case presentations. Audiotaped 10% of home sessions for review and feedback. Documentation of contacts was kept in order to review delivery adherence. Interviewers were masked to treatment assignment. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Interviewers masked to participants assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Incomplete outcome data addressed but specific reasons for dropout not noted, only reported as % lost to follow‐up or missed. |
| Selective reporting (reporting bias) | Low risk | All data reported. |
| Other bias | Low risk | None reported or determined. |
Gonyea 2006.
| Methods | Randomised controlled trial. Study duration: 6 weeks. | |
| Participants | 80 caregivers (CG) with a mean age of 64.4 years, providing a weekly minimum of 4 hours care to 80 care recipients (CR) with a confirmed diagnosis of Alzheimer’s disease (mean age: 77) in the mild to moderate severity range, with at least one neuropsychiatric symptom. Caregivers were mostly spouses, female and Caucasian. | |
| Interventions | Caregiver group based training intervention (Project CARE) Psychoeducational control group using similar structure to the intervention group. Primary aim: CG distress associated with CR behaviour, CG burden and CR behaviour problems. (See Table 2) |
|
| Outcomes | Neuropsychiatric inventory (NPI) ‐ Severity & Distress Zarit Burden Interview (ZBI) (See Table 3) |
|
| Notes | Country of origin: Boston, USA. Caregiver based multi‐component behavioural group intervention, delivered over 5‐weekly 90 minute sessions with 15 minutes individual time. The intervention was delivered in a group format (5 ‐10 members). The intervention was based on the principles of behaviour therapy and activation and designed to teach behavioural techniques for managing care recipients neuropsychiatric symptoms in the home environment. Caregivers were taught ABC behavioural analysis. The control group had a similar structure to the intervention, but consisted of only general information on aging and Alzheimer’s disease, home safety, support and techniques for improved communication. The total study duration was 6 weeks. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'We then assigned participants by block randomisation' |
| Allocation concealment (selection bias) | Low risk | 'We assigned participants by block randomisation to one of the two conditions'. Unclear as to who performed the randomisation. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Therapists had 16‐20 hours training in intervention protocols. To monitor treatment fidelity, PI consulted with therapists on a regular basis to review group sessions and assess group progress. Not all caregivers adhered to the intervention (did not submit homework). |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Reported in the discussion 'It was also not possible to blind all interviewers to the caregivers treatment condition at the post‐intervention assessment' |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Specific reasons for withdrawal not reported; however, the number withdrawn is recorded. (11 caregivers did not complete) |
| Selective reporting (reporting bias) | Unclear risk | NPI frequency not reported, only severity. |
| Other bias | Unclear risk | Generalisabilty to the general population difficult due to low numbers of ethnically and racially diverse individuals. |
Gormley 2001.
| Methods | Randomised controlled trial. Study duration 10 weeks. | |
| Participants | 62 care recipients (CR) with a diagnosis of dementia and their co‐resident carer. Care recipients with dementia were required to be rated by their carer as mildly aggressive. Care recipient mean age was 75.95 years, with an average MMSE score of 13.3. Caregivers (CG) mean age was 68.45 and were predominantly female. | |
| Interventions | Caregiver Behaviour Management Training Programme Control group Primary aim of intervention: CR behaviour & severity and CG burden. (See Table 2) |
|
| Outcomes | Rating Scale for Aggressive Behaviour in the Elderly (RAGE) Behavioural Pathology in Alzheimer's Disease Rating Scale (BEHAVE‐AD) Zarit Burden Interview (ZBI) (See Table 3) |
|
| Notes | Country of origin: Kent, UK. 4 sessions over 8 weeks, providing education, ABC analysis and behavioural interventions. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'Randomly allocated patients and their carers to intervention or control group' |
| Allocation concealment (selection bias) | Low risk | Randomisation was concealed from the second author. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The paper does not report the use of a manual or checking adherence to the manual. The intervention and control were conducted by the first author, only the second author was blinded to allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Second author blind to treatment allocation conducted assessments. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All reasons and number of withdrawals noted. Three patients dropped out of the trial shortly after their initial assessment: two were admitted to hospital and the third was admitted to residential care. |
| Selective reporting (reporting bias) | Low risk | All results reported. |
| Other bias | Unclear risk | Author conducted the intervention. |
Huang 2003.
| Methods | Randomised controlled trial, Pilot study. Study duration: 12 weeks. | |
| Participants | 48 patients with dementia and their family caregiver (CG). Care recipients (CR) had to be aged 65 or over and score 50 or above on the CMAI. CRs were predominantly female with a mean age of 75.8 years. Twenty had a CDR rating of mild, 17 moderate, 10 severe and 1 very severe, with an average MMSE score of 13.1. CGs were predominantly female, with a mean age of 55.6. | |
| Interventions | A home‐based Caregiver Training Programme Control (written materials only) Primary aim of intervention: To improve CG self efficacy and decrease CR problem behaviours. (See Table 2) |
|
| Outcomes | Chinese version of Cohen Mansfield Agitation Inventory (CMAI) (CR Frequency of problem behaviours & CG Self efficacy). | |
| Notes | Country of origin: Northern Taiwan. 2 week in home training programme, plus telephone consultations every two weeks.The control group received educational materials and social telephone follow‐ups every two weeks. At the third week and third month assessments were conducted. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'Randomly assigned to intervention or control group' |
| Allocation concealment (selection bias) | Unclear risk | Randomised by patient registration number, odd registration numbers to intervention, even to control. The paper does not report who performed randomisation, |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | 'Although caregivers knew they were in a study, they did not know whether they were in the experimental or control group'. A manual was developed by the research team as a guide for the training program. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Investigator ran the intervention, unclear as to level of blinding and who conducted assessments. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition reported (11 participants were lost to follow‐up because either the caregiver was unwilling to continue, the patient was hospitalised or the address was changed). |
| Selective reporting (reporting bias) | Low risk | All results reported. |
| Other bias | Unclear risk | None determined. |
Losada‐Baltar 2004.
| Methods | Randomised trial with 2 treatment and 1 control arm (for the purpose of this review the PSP condition was compared with the control condition). Study duration: 5 months. | |
| Participants | 31 family caregivers (CG) of a relative with dementia. CG had a mean age of 61.1 years and were predominantly female. Care recipients (CR) had a mean age of 80.4. | |
| Interventions | Caregiver Cognitive Behavioural Intervention (PCC) Caregiver Problem‐Solving Skills Training Intervention (PSP) Control group For the purpose of this review PSP was compared to the Control group. Primary aim of intervention: Modifying CR behavioural problems, CG stress associated with problem behaviours, CG depression and CG dysfunctional thoughts. (See Table 2) |
|
| Outcomes | Memory and Behaviour Check List (MBCL) ‐ Frequency & Reaction Perception of Social Support (PSQ) Caregiver depression measured by CES‐D Perceived Stress Scale (PSS) CG dysfunctional thoughts on care (CPD) (See Table 3) |
|
| Notes | Country of origin: Madrid, Spain. The paper is reported in Spanish. Our translation of this study led us to believe it was suitable for inclusion in the review as causes of behaviour were identified and hypothesis and strategies formed to alleviate the targeted behaviour. The intervention was delivered by two psychologists in one 2‐hour session a week for 8 weeks, totalling 16 hours. A post‐intervention assessment was taken after the 8 weeks, and 3 months following the end of the intervention. The total study duration was 5 months. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'Randomly assigned' |
| Allocation concealment (selection bias) | Unclear risk | Unsure who performed the randomisation procedure and how it was conducted. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | 'Interventionists carried out assessments, however, were unaware of membership at the time'. Due to difficulty translating the paper we were unable to establish whether the intervention has a manual or whether adherence checks were executed to ensure full delivery of the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unable to determine due to difficulty translating the paper |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data reported. |
| Selective reporting (reporting bias) | Unclear risk | No risks identified, however, due to difficulty in translation of the paper, we have graded this as unclear. |
| Other bias | Low risk | None idenitified. |
Mador 2004.
| Methods | Randomised trial ‐ Behaviour Advisory Service compared with Usual Care. Study duration: 9 days. | |
| Participants | 71 patients with dementia and behavioural disturbance judged to be problematic with a mean age: 82.5. | |
| Interventions | Staff Training Hospital Behaviour Advisory Service Usual Care Primary aim of intervention: Modify level of patient agitation over time, appropriateness of psychotropics, length of stay, discharge destination, falls, restraint use and CG satisfaction with care provided. (See Table 2) |
|
| Outcomes | Pittsburgh Agitation Scale (PAS) Medication Appropriateness Index (MAI) Discharge destination Falls Restraint use CG satisfaction with care Length of stay (See Table 3) |
|
| Notes | Country or origin: South Australia Patients assessed within 24 hours of randomisation. Nurse formulated management plan with respect to non‐pharmacological strategies to help manage patients problematic behaviours, discussed the plan with ward nursing staff and provided ongoing support and education for nursing staff. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Pragmatic randomised controlled trial. 'Patients were randomised' (not by ward or hospital only by patient). |
| Allocation concealment (selection bias) | Low risk | Randomisation by hospital pharmacy department using sequential sealed opaque envelopes by external person using stratified blocks. Computer generated random numbers, allocation via external person. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | It is not reported if a manual was used or whether checks were completed to ensure accurate delivery of the intervention. The level of blinding of participants and personnel is not reported. Adherence was not formally measured 'it is possible that, although the EPN was offering advice and providing frequent follow‐up visits to reinforce their suggestions, the ward nursing staff were not carrying out the strategies suggested'. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unsure as to the level of blinding of the EPN. Ward nurses conducted assessments unsure as to level of blinding. 'Treatment and control patients were both nursed on the same wards so it is possible that nursing staff may have picked up on useful strategies and applied them to the control group'. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All reported (4 died, 67 discharged). |
| Selective reporting (reporting bias) | Unclear risk | Only follow‐up data for the PAS is reported (in the abstract). |
| Other bias | Unclear risk | No other potential sources of bias identified. |
Moniz‐Cook 2008a.
| Methods | Randomised controlled trial. Study duration:18 months | |
| Participants | 113 care recipients (CR) and their family caregiver (CG). CR had a mean age of 77.2 years; CG had a mean age of 63.2 years and were predominantly female. | |
| Interventions | Community Mental Health Nurses Training Intervention (CMHN) Control (usual practice) Primay aim of intervention: Training CMHNs in systematic psychosocial interventions (PSI) to help family caregivers manage behavioural changes in their relative with dementia. (See Table 2) |
|
| Outcomes | The General Health Questionnaire (GHQ) The adapted‐Gilleard Problem Checklist (PC) The Hospital Anxiety and Depression Scale (HADS) The Global Deterioration Scale (GDS) (See Table 3) |
|
| Notes | Country of origin: Hull, UK. 4 consecutive weekly in home visits following which CMHN exercised clinical judgment about future contact and attended in service clinical supervision with a Clinical Psychologist (Esme Moniz‐Cook) and senior nurse for the duration of the 18 month study, 2 hours, once a week for the first 6 months and once a fortnight for the following 6 months. Individual sessions were held once a month for the final 5 months. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'Dyads (i.e. CR and CG) were randomly allocated to either condition' |
| Allocation concealment (selection bias) | Unclear risk | Randomisation procedure not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Level of blinding of participants and personnel not reported. A protocol was in place. Only two CMHNs adhered to the 4 consecutive family treatment sessions. Despite protocol‐led recommendations no relaxation or anxiety management occurred. Only two CMHNs sustained clinical supervision, noted as 'poor adherence' in the text. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Researchers conducted baseline measures. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reasons for withdrawal were reported (1 neighbour disengaged, 18 caregivers disengaged, 3 carers relocated, 3 spouse deceased care provided by a child). |
| Selective reporting (reporting bias) | Low risk | All outcome results at each time period reported. |
| Other bias | Low risk | Authors supervised CMHNs. |
Proctor 1999.
| Methods | Randomised controlled trial. Study duration: 6 months. | |
| Participants | 105 subjects, 12 nursing and residential homes. Residents had a mean age of 83.1. Staff selected 10 residents in each home whose behavioural problems made them difficult to care for. | |
| Interventions | Staff training and Education Intervention including psychosocial management of resident's behavioural problems. Control Primary aim of intervention: To assess quality of care, resident depression and organic symptoms and resident behavioural characteristics. (See Table 2) |
|
| Outcomes | Crichton Royal Behavioural Rating Scale (CRBRS) Automatic Geriatric Examination for Assisted Taxonomy (AGECAT) (depression & organic symptoms) (See Table 3) |
|
| Notes | Country of origin: Manchester, UK Seven 1 hour seminars plus individual visits from a member of the hospital outreach team. An experienced psychiatric nurse then visited each residential home every week to provide support to individual staff in development of care planning skills. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'Residential homes were randomised to the control or intervention group' |
| Allocation concealment (selection bias) | Low risk | 10 residential homes and 2 nursing homes were paired according to size and accreditation status. Computer generated random numbers used independently of the researchers to assign one of each pair of homes to intervention or control. Ten residents in each home were selected by staff independently of the researchers. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Residents were unaware of carer allocation. However, 'Staff that received the training were aware of the intervention'. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The paper does not report who conducted the outcome assessments and whether they were blind to allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All reported (11 died, 2 transferred and 3 withdrew consent). |
| Selective reporting (reporting bias) | Low risk | All results reported. |
| Other bias | Unclear risk | Staff who received training were aware of intervention and may have had expectations about the effects of the programme. |
Teri 2000.
| Methods | Randomised placebo controlled clinical trial. Study duration: 12 months. | |
| Participants | 149 care recipients (CR) with Alzheimer’s disease and their caregiver (CG). CRs had a mean age of 74.8 years, whilst CGs had a mean age of 65.6 and were predominantly the CR's spouses. | |
| Interventions | Caregiver Behaviour Management Techniques Intervention Haloperidol Trazodone Placebo Primary aim of intervention: To decrease CR agitated behaviours (See Table 2) |
|
| Outcomes | Clinical Impression of Change (ADCS) The Consortium to Establish a Registry for Alzheimers Disease (CERAD) Behavioural Rating Scale for Dementia (BRSD) Revised Memory and Behaviour Problem Checklist (RMBPC) Cohen Mansfield Agitation Inventory (CMAI) Physical Self Maintenance (PSM) Agitated Behaviour Inventory for Dementia (ABID) Cognitive Function (MMSE) (See Table 3) |
|
| Notes | Country of origin: America BMT intervention delivered over 8 weekly and 3 biweekly sessions providing information about AD, strategies for decreasing agitated behaviours, assignments and videotape training program, conducted by a therapist with masters degree. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'Subjects were randomly allocated' |
| Allocation concealment (selection bias) | Low risk | 'Subjects were allocated to four study arms. Ten sites had patients randomised to medications or placebo. Eleven sites had patients randomised to medications, placebo or BMT. Treatments were assigned in randomised blocks of nine or 12'. Randomisation was concealed. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The intervention had a protocol. Ongoing training, inter‐rater reliability checks and quality control were performed to ensure standardisation. 'To insure interviewers remained blind to treatment assignment, caregivers did not discuss any aspects of treatment with the interviewer '. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Paper reports that in no instance was blinding compromised. Assessments conducted by blind interviewers. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The paper reports number of patients who withdrew and reasons and adverse effects (57 discontinued, major reasons for dropout included increased agitation in the trazodone arm (59%), unacceptable adverse effects in the haloperidol arm (43%), and caregiver difficulties or increased agitation in the BMT arm (35%)). |
| Selective reporting (reporting bias) | Low risk | The paper reports only total frequency score for the RMBPC but not reaction. The paper reports post‐treatment data only. |
| Other bias | Low risk | Clinicians had a treatment protocol but allowed discretion in strategies to employ and when; therefore, intervention not wholly standardised. |
Teri 2003.
| Methods | Randomised controlled trial. Study duration: 24 months. | |
| Participants | 153 community dwelling care recipients (CR) meeting criteria for Alzheimer’s disease and their caregiver (CG). CRs had a mean age of 78 years, with an average MMSE score of 16.8 and were predominantly male. CGs had a mean age of 70, and were predominantly female. | |
| Interventions | Caregiver training in behavioural management techniques with home‐based exercise program ‐ Reducing Disability in Alzheimers disease (RDAD) Control (routine medical care) Primary aim of intervention: CG management of CR problem behaviours and decreasing CR frailty and behavioural impairment. (See Table 2) |
|
| Outcomes | Physical Health and Function (SF36) Affective Status ‐ Hamilton Depression Rating Scale (HDRS) & Cornell Scale for Depression in Dementia CR Physical Health & Function Revised Memory and Behaviour Problem Checklist (RMBPC) (See Table 3) |
|
| Notes | Country of origin: Washington, USA. RDAD: In own home, 12 x 1 hour sessions, 2 per week for the first 3 weeks, followed by 1 for the next 4 weeks and biweekly sessions over the following 4 weeks. Followed by 3 sessions over the next 3 months conducted by health professionals experienced in dementia care. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'Patient caregiver dyads were randomly assigned to exercise plus behavioural management techniques or routine medical care' |
| Allocation concealment (selection bias) | Low risk | The random allocation sequence was obtained from a computer program that blocked groups of 8 patients. Dyads were randomised after baseline assessment by research coordinators. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | A manual was used. Treatment adherence maintained and monitored through weekly supervision. Treatment sessions were videotaped and reviewed by independent reviewers. Unsure as to the level of blinding of other personnel and participants other than outcome assessment interviewers. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessments conducted by blind interviewers. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All reported (43 institutionalised, 2 declined to continue, 2 caregivers were ill, 2 moved, 5 caregivers declined to continue, 9 patients died and 1 caregiver died). |
| Selective reporting (reporting bias) | Unclear risk | Behavioural data not reported. |
| Other bias | Unclear risk | Training by authors |
Teri 2005a.
| Methods | Randomised controlled trial. Study duration: 6 months. | |
| Participants | 95 care recipients (CR) with Alzheimer’s disease and family caregivers (CG). CR mean age: 79.95 with an average MMSE score of 14.0. CR were required to have three or more agitated or depressed behaviour problems. CG ages ranged from 22 to 91 years. CR were predominantly female. | |
| Interventions | Community Consultants Training program (STAR‐ Caregivers) Control (routine medical care) Primary aim of intervention: To train community consultants to teach CGs a systematic behavioural approach for reducing mood and behaviour problems of their CR. (See Table 2) |
|
| Outcomes | Center for Epidemiologic Studies Depression Scale (CES‐D) for CG Hamilton Depression Rating Scale (HDRS) for CG Perceived Stress Scale (PSS) Caregiver Sleep Questionnaire Screen for Caregiver Burden (SCB) Short Sense of Competence Questionnaire (SSCQ) Neuropsychiatric Inventory (NPI) Revised Memory and Behaviour Problem Checklist (RMBPC) The quality of Life in Alzheimers disease (QOL‐AD) (See Table 3) |
|
| Notes | Country of origin: Washington, USA. Counsultant training consisted of an initial 2 hour orientation with supervising gero‐psychologist. A standardised treatment manual that included instructions to consultants was disseminated and discussed. Consultants met the CGs in their home over 8 weekly sessions followed by four monthly phone calls. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'Randomly assigned caregivers and care recipients to the intervention or control' |
| Allocation concealment (selection bias) | Unclear risk | The randomisation procedure is not reported; therefore, whether adequate allocation concealment was achieved is unclear. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Follow‐up was conducted by interviewers blind to treatment assignment. Unsure as to the level of blinding of participants. A manual was used and adherence to the manual was monitored through audio taping treatment sessions and weekly supervision. (Consultants also had to successfully complete a pilot case). |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Interviewers blind to treatment assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reported withdrawal, however, some specific reasons are not recorded (3 care recipients hospitalised, 9 caregivers declined due to non‐specific reasons) |
| Selective reporting (reporting bias) | Low risk | The paper does not report RMBPC data for frequency at 6 months. |
| Other bias | Unclear risk | Ratings of consultant adherence not done by independent raters. |
Teri 2005b.
| Methods | Randomised controlled trial. Study duration: 2 months. | |
| Participants | 31 residents and 25 staff from four assisted living residences. Residents were predominantly female, had a mean age of 85.8 years and a MMSE mean score of 16.0. The mean age of staff was 37.4 years. | |
| Interventions | Staff Training in Assisted Living Residences (STAR) based on a manual. Control ‐ usual onsite training Primary aim of intervention: Dementia specific training program to teach direct care staff to improve care and reduce problems in residents with dementia. (See Table 2) |
|
| Outcomes | Geriatric Depression Scale (GDP) Clinical Anxiety Scale (CAS) Revised Memory and Behaviour Problem Checklist (RMBPC) Agitated Behaviours in Dementia (ABID) Neuropsychiatric Inventory (NPI) Short Sence of Competence Questionnaire (SSCQ) (See Table 3) |
|
| Notes | Country of origin: Seattle, Washington, USA. STAR is conducted over 2 months, through 2 half day group workshops and four individualised sessions. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'Four assisted living residences were randomly assigned to intervention or control' |
| Allocation concealment (selection bias) | Unclear risk | Randomisation procedure not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | A training manual and protocol were used. Opportunities to discuss site specific issues that might hinder implementation or sustainability were provided. Unclear as to the level of blinding of participants and staff other than outcome assessors. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Interviewers blind to treatment condition conducted pre‐training and post‐training assessments |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No attrition to report. |
| Selective reporting (reporting bias) | Unclear risk | No RMBPC frequency data reported. Doesent state the number of participants in each group, this information had to be sought by authors. |
| Other bias | Low risk | Training by authors. |
Weiner 2002.
| Methods | Randomised controlled trial | |
| Participants | 76 Care recipients with data available at 12 month follow‐up. | |
| Interventions | See Teri 2000 and Table 2 | |
| Outcomes | Agitated Behaviours in Dementia (ABID) (SeeTable 3) |
|
| Notes | Reports the maintenance effects of Teri 2000 paper. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised placebo controlled clinical trial. |
| Allocation concealment (selection bias) | Low risk | 'Subjects were allocated to four study arms. Ten sites had patients randomised to medications or placebo. Eleven sites had patients randomised to medications, placebo or BMT. Treatments were assigned in randomised blocks of nine or 12'. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | 'To insure Interviewers remained blind to treatment assignment, caregivers did not discuss any aspects of treatment with the interviewer'. Clinicians had a treatment protocol. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Paper reports that in no instance was blinding compromised. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Does not state dropout, however, this is reported in the previous paper. |
| Selective reporting (reporting bias) | Unclear risk | The paper only reports ABID data, however, other outcome measures were used. |
| Other bias | Low risk | No other forms of bias noted. |
Zarit 1987.
| Methods | Randomised controlled trial ‐ wait list control. Study duration: 24 months. | |
| Participants | 184 dementia care recipients (CR) living in the community and their primary caregivers (CG). CR mean age was 75.72 with an average MMSE score of 14.42. Mean age of CG was 62.02. 119 completed treatment. | |
| Interventions | Caregiver Support Group Intervention (SG) Caregiver Individual Family Counselling Intervention (IFC) Wait list Control Group Primary aim of intervention: To test the effectiveness of a stress‐management approach in reducing CG stress and burden. CG changes in reports of stress, improvement in management of the CR's problem behaviours, CG increased use of social support and CG perception of treatment benefits. (See Table 2) |
|
| Outcomes | Brief Symptom Inventory (BSI) Burden Interview (BI) Memory and Behaviour Problems Checklist (MBPC) Caregiver Change Interview Social Support Caregiver adequacy of support (See Table 3) |
|
| Notes | Country of origin: USA Only one experimental condition offered at a time at each site (2 sites). Subjects at one site randomly assigned to either IFC or wait list, other site randomly assigned to SG or wait list. For the purposes of this review SG was compared with wait list control. The interventions were delivered over 8 sessions (8 weeks). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomly assigned to either IFC, SG or wait list control" |
| Allocation concealment (selection bias) | Unclear risk | Crossover. 1st year of study one site received intervention and then assigned to a wait list. In the 2nd year, this was reversed. Does not state actual procedure of how sites assigned, e.g. blocks |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding of participants and staff not reported. The first author monitored sessions using audiotapes and supervision sessions to ensure that therapists implemented the treatment approach. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The paper does not report who conducted the assessments. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropout numbers reported, but specific reasons not reported |
| Selective reporting (reporting bias) | Low risk | One year outcome data not reported, only post‐intervention |
| Other bias | High risk | Crossover trial |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Alessi 1999 | Physical activity and environmental intervention to improve sleep and agitation. |
| Ashaye 2003 | Assessment of CANE measurement scale. No behavioural outcomes. |
| Assal 2004 | Pharmacological intervention only. |
| Ayalon 2006 | This paper is a systematic review. |
| Ayalon 2009 | Not an RCT, the paper reports a case series using problem solving for the management of depression and agitation in long term care. |
| Baillon 2002 | Review paper of multi‐sensory therapy in psychiatric care. |
| Baillon 2004 | Snoezelen or Reminiscence therapy intervention. |
| Baillon 2005 | Snoezelen or Reminiscence intervention |
| Baker 2001 | Snoezelen & Activity intervention |
| Baker 2003 | Snoezelen intervention |
| Baker 2006 | Case series. |
| Baldelli 2007 | Occupational Therapy intervention |
| Ballard 2009 | Period of BPST not randomised, only pharmacotherapy part of trial randomised (information supplied by Author) |
| Beauchamp 2005 | Worksite based Internet multi‐media program. No behavioural component, predominantly focused on carer stress and coping |
| Belle 2006 | Enhancing Quality of life through proving education and skills |
| Bellelli 2004 | This paper reports on the maintenance effects of the CRONOS project. |
| Bird 2007 | Not an RCT, naturalistic controlled trial with repeated measures |
| Buchanan 2002 | Case Series |
| Burgener 1998 | Instructional intervention for Caregivers on bathing and specific activities. |
| Burgio 2001 | Communication improvement intervention using memory books, no behavioural analysis |
| Burns 2003 | This paper reports the effects of the Reach study 2 year outcomes. No behavioural outcomes. |
| Callahan 2006 | Behavioural intervention, however from intervention description there is no evidence of functional analysis. |
| Cohen‐Mansfield 2006 | Identity specific intervention regarding retention of self identity and the impact of role based treatment |
| Cohen‐Mansfield 2007 | This was not a fully randomised controlled trial, due to only some of the care homes being randomly assigned |
| Conti 2008 | Recreational activities intervention |
| Coyne 1997 | Therapies were standardised not individually tailored, intervention involved the use of verbal prompts for eating behaviour |
| Davison 2007 | Not an RCT, participants were referred into the study |
| Deudon 2009 | Education and coaching intervention to provide ideas of interventions to reduce and avoid BPSD but did not involve analysis of behaviour |
| Dias 2008 | Support & education Intervention to predominantly reduce caregiver burden |
| Dwyer‐Moore 2007 | Case Series |
| Elliot 2010 | Psycho‐education and caregiver health intervention |
| Farran 2007 | Reports on a subgroup only from previous randomised controlled trial, full RUSH trial is included in the review |
| Feeney 2003 | Interactive voice response intervention.Behavioural management advice provided over the phone. |
| Finnema 2005 | Emotion oriented care intervention training staff to use an emotion oriented approach |
| Gallagher‐Thompson 2008 | Cognitive behavioural intervention to reduce depression in family caregivers |
| Garilova 2009 | Education only intervention involving two day training on problem behaviours. |
| Gerritsen 2005 | Cross sectional study in care homes to investigate the relationship between apathy and quality of life. |
| Gitlin 2001 | Home Environmental intervention proving occupational therapy to improve the environment. |
| Gitlin 2005 | Paper reports the maintenance effects of included study Gitlin 2003, however the results have not been reported in the same format and therefore this data could not be included in the review. |
| Gitlin 2007 | This paper reports on the design and method of projectACT3, however the results for this study are not yet published. |
| Gitlin 2008 | Physical activity intervention, not functional analysis |
| Graff 2006 | Occupational therapy based intervention |
| Graff 2007 | Occupational therapy based intervention. |
| Graff 2008 | Occupational therapy based intervention. |
| Grant 2007 | Initial elucidation of unmet need or cause not by trained professional as this study is distance based. All contact with a trained professional is via the telephone |
| Heard 1999 | Case Series |
| Hepburn 2001 | Psychodeuctional and coaching group intervention, providing role training to help caregivers assume a more clinical belief set about care giving |
| Hepburn 2003 | Reports only on the development and testing of the Savvy Caregiver Program. |
| Hepburn 2005 | Psychoeducational intervention to deal with caregiver distress using activity, OT and music |
| Herbert 2003 | Psycho‐educational group program for caregivers to look at caregiver appraisal of stress and problem solving |
| Hinchliffe 1995 | Primary outcome data not reported in continuous format (reported as dichotomous) therefore it could not be included in the meta‐analysis. |
| Hochhalter 2007 | Observational study. |
| Hoeffer 2006 | No behavioural outcome e.g. NPI or CMAI. Behaviour rated through 'hassle' scale only. Specific bathing intervention. |
| Hoehn‐Anderson 1992 | Psychosocial intervention to involve families in care to evoke positive responses from residents when provided with items of interest |
| Javadpour 2009 | Psychoeducational intervention. Randomisation unclear |
| Kolanowski 2001 | Therapeutic recreational activities intervention. The paper is a review with a report of a small pilot crossover experimental design |
| Kolanowski 2005 | Recreational Activities intervention, not functional analysis. |
| Kolanowski 2006 | Specific agitation study, not an RCT, cross sectional design with repeated measures |
| Koltai 2001 | Memory and coping program specifically for improving cognition not behaviour. |
| Konnert 2009 | Cognitive behavioural therapy intervention |
| Kovach 1996 | Therapeutic activities intervention, to promote comfort, QOL and dignity. |
| Kovach 2006 | Serial Trial Intervention, needs assessed but not in terms of what functions behaviours served or what where the antecedents and causes. |
| Kuiper 2009 | Dementia care mapping intervention |
| Kurz 2003 | Pharmacological intervention. |
| Lam 2010 | Activities based intervention. |
| Lavertsky 2006 | Review paper. |
| Lawton 1998 | Stimulation intervention |
| Litchenburg 2005 | Pleasant events intervention, brainstorming and activity programming |
| Lovheim 2006 | Cross sectional study to discover factors associated with the use of anti‐psychotics |
| Low 2004 | Cross sectional study to investigate the relationship between self destructive behaviours and nursing home environments |
| Lucero 2002 | Review/discussion paper of exit seeking wandering behaviour intervention strategies. |
| Magai 2002 | Increasing sensitivity to non verbal signals to improve psychological well‐being of caregiver. |
| Marriot 2000 | Cognitive behavioural therapy intervention, involving role play and problem solving. |
| Martin 2007 | Activity based intervention to improve sleep/wake patterns. |
| Mayer 1991 | Specific observational wandering intervention to assess the use of mirror. |
| McCallion 1999 | Intervention to improve communication between carer and resident by observing interactions. |
| McCurry 1998 | Specific Sleep intervention, did analyse behaviour however excluded due to targeting only night time behaviour. |
| McCurry 2005 | Sleep Education intervention to deal with nighttime insomnia only. |
| McGilton 2003 | Way‐finding intervention |
| Melis 2008 | No behavioural outcomes. |
| Mittleman 2004 | Support and education intervention where caregivers dictated sessions. |
| Mittleman 2006 | Counselling and support intervention with management of behaviours however from the description of the intervention it was not apparent functional analysis was utilised. |
| Moniz‐Cook 2001 | Case Series |
| Moniz‐Cook 2003 | Case Series, not a randomised controlled trial |
| Montgomery 2004 | Systematic review of pharmacological therapies for sleep problems in later life |
| Narayan 2000 | Reports 6 month data from an NIH‐funded study, decision making educational intervention. |
| Onder 2005 | Reality orientation intervention |
| Opie 1999 | Systematic Literature Review paper looking at the efficacy of psychosocial approaches |
| Opie 2002 | Randomised controlled trial lasted only 3 days where subjects acted as own controls (early group controls for late group). |
| Ostwald 1999 | Psychoeducational intervention only. |
| Ouslander 2006 | Sleep improvement intervention involving increasing daytime physical activity, bright light exposure and social interactions |
| Palese 2009 | Observational study. |
| Politis 2004 | Kit based activity intervention to reduce apathy and improve quality of life. |
| Poon 2005 | Cognitive intervention to test the efficacy of telemedicine vs face to face treatment |
| Qazi 2003 | Case series regarding managing anxiety in people with dementia. |
| Rasin 2007 | Qualitative study. |
| Reeve 1985 | Reality orientation. Not a randomised controlled trial. |
| Reuben 2003 | Discussion paper. |
| Richards 2005 | Social Activity Intervention. |
| Robinson 1994 | Only secondary outcomes reported. No extractable data for Primary outcomes. |
| Robinson 2007 | Psychoeducational and communication facilitation intervention. |
| Rolland 2007 | Physical Activity intervention to improve ADL's & physical performance |
| Rosendahl 2006 | High intensity Functional exercise program to improve gait. |
| Scholzel‐Dorenbos 2010 | Review paper. |
| Schrijnemaekers 2002 | Emotion‐oriented care intervention providing education on dementia |
| Schulz 2003 | Overview of REACH project, site specific outcomes and future directions. |
| Sink 2006 | Cross sectional study on caregiver characteristics and which are associated with neuropsychiatric symptoms |
| Sival 1997 | Activities intervention, case study of three participants with dementia. |
| Sloane 2004 | Intervention specifically tailored to behaviours experienced during showering/bathing |
| Sung 2006 | Group music with movement intervention |
| Teri 1994 | Review paper |
| Teri 1998 | Qualitative study reporting cases from a previous randomised controlled trial. |
| Thal 2000 | Pharmacological intervention. |
| Thal 2003 | Pharmacological intervention. |
| Tibaldi 2004 | Home hospital intervention. Reviewers could not determine a sufficient dosage of Functional Analysis to include this paper |
| Torta 2004 | Review paper |
| Tung 2005 | Physical activity intervention |
| Van de Winckel 2004 | Music based exercise intervention. |
| Van Weert 2005a | Snoezelen Intervention |
| Van Weert 2005b | Snoezelen Intervention |
| Vespa 2002 | Role of social relationships in psychosocial and psycho‐cognitive behaviour |
| Visser 2008 | No extractable data as only sub scale means reported. Author contacted‐ data unavailable |
| Williams 1987 | Reality orientation and environmental intervention |
| Zanetti 1998 | Psychoeducational intervention |
Differences between protocol and review
Addition of authors Katie Roberts‐Stride and Reem Malouf to the review.
Addition of Banerjee report 2009 and James 2011 to background text.
Contributions of authors
E M‐C ‐ All correspondence; drafting initial protocol and review versions; selection of trials; interpretation of data analyses; updating review. KS ‐ Extraction, entry and analysis of data; interpretation of data analyses; drafting review versions; liaising with trial and review authors; updating review.
RM ‐ Statistical advice, guidance and extraction of data.
M dV ‐ Drafting initial protocol and review versions; selection of trials; interpretation of data analyses; updating review. FV ‐ Drafting initial protocol and review versions; selection of trials; interpretation of data analyses; updating review. IJ ‐ Drafting initial protocol review versions; selection of trials; interpretation of data analyses; updating review.
Contact Editor: Linda Clare Consumer Editors: Dr Graham Stokes (expert clinician) South Staffordshire and Shropshire NHS Foundation Trust, David Parry Suite, St Michaels Court Trent Valley Road, Lichfield and Tracie Jennings (family carer), Alzheimer's Society, Hull & East Riding Branch.
Sources of support
Internal sources
E M‐C: Humber Mental Health Teaching NHS Trust & University of Hull, UK.
M deV : University of Maastricht, Netherlands.
F V : University of Maastricht, Netherlands.
I J: Centre for the Health of the Elderly, Newcastle General Hospital, Northumberland, Tyne and Wear NHS Trust, UK.
External sources
NIHR Programme Grant: 'Management of Challenging Behaviour in dementia at home and in care homes' awarded to authors E M‐C and IJ (2007), UK.
Declarations of interest
Esme Moniz‐Cook
Prof. Moniz‐Cook is an author of a study included in the review (Moniz‐Cook 2008a).
Esme Moniz‐Cook is chief investigator for two ongoing studies‐ Challenge DemCare Projects ‐ that relate to this review.
Ian James
Ian James is an author of a study included in the review (Fossey 2006). Ian James is co‐investigator for two ongoing studies ‐ Challenge DemCare Projects ‐ that relate to this review.
New
References
References to studies included in this review
Burgio 2003 {published data only}
- Burgio L, Stevens A, Guy D, Roth DL, Haley WE. Impact of two psychosocial interventions on White and African American family caregivers of individuals with dementia. The Gerontologist 2003;43(4):568‐79. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chenoweth 2009 {unpublished data only}
- Chenoweth L, King MT, Jeon YH, Brodaty H, Stein‐Parbury J, Norman Ret al. Caring for aged dementia care resident study (CADRES) of person‐centred care, dementia‐care mapping, and usual care in dementia: a cluster‐randomised trial. The Lancet Neurology 2009;8(4):317‐25. [DOI] [PubMed] [Google Scholar]
Farran 2004 {unpublished data only}
- Farran C J, Gilley DW, McCann JJ, Bienias JL, Lindeman DA, Evans DA. Psychosocial interventions to reduce depressive symptoms of dementia caregivers: A randomized clinical trial comparing two approaches. Journal of Mental Health and Aging 2004;10(4):337‐50. [Google Scholar]
Fossey 2006 {unpublished data only}
- Fossey J, Ballard C, Juszczak E, James I, Alder N, Jacoby R, et al. Effect of enhanced psychosocial care on antipsychotic use in nursing home residents with severe dementia: cluster randomised trial. BMJ 2006;332:756‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gitlin 2003 {published data only}
- Gitlin LN, Winter L, Corcoran M, Dennis MP, Schinfeld S, Hauck WW. Effects of the home environmental skill‐building program on the caregiver ‐ care recipient dyad: 6‐month outcomes from the Philadelphia REACH initiative. The Gerontologist 2003;43(4):532‐46. [DOI] [PubMed] [Google Scholar]
Gitlin 2010 {published and unpublished data}
- Gitlin LN, Winter L, Dennis MP, Hodgson N, Hauck W. Targeting and managing behavioural symptoms in individuals with dementia: A randomised trial of a nonpharmacolgical intervention. Journal of the American Geriatrics Society 2010;58:1465‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gonyea 2006 {published data only}
- Gonyea JG, O'Connor MK, Boyle PA. Project CARE: A randomized controlled trial of a behavioral intervention group for Alzheimer's disease caregivers. The Gerontologist 2006;46(6):827‐32. [DOI] [PubMed] [Google Scholar]
Gormley 2001 {published data only}
- Gormley N, Declan L, Howard R. Behavioural management of aggression in dementia: a randomised controlled trial. Age and Ageing 2001;30:141‐5. [DOI] [PubMed] [Google Scholar]
Huang 2003 {published data only}
- Huang HL, Lotus Shyu YI, Chen MC, Chen ST, Lin LC. A pilot study on a home‐based caregiver training program for improving care‐giver self efficacy and decreasing the behavioural problems of elders with dementia in Taiwan. International Journal of Geriatric Psychiatry 2003;18:337‐45. [DOI] [PubMed] [Google Scholar]
Losada‐Baltar 2004 {published data only}
- Losada‐Baltar A, Izal‐Fernández de Trocóniz M, Montorio‐Cerrato I, Màrquez‐González M, Pérez‐Rojo G. Differential efficacy of two psychoeducational interventions for dementia family caregivers [Eficacia diferential de dos intervenciones psicoeducativas para cuidadores de familiares con demencia]. Revista de Neurología 2004;38(8):701‐8. [PubMed] [Google Scholar]
Mador 2004 {published data only}
- Mador JE, Giles L, Whitehead C, Crotty M. A randomized controlled trial of a behavior advisory service for hospitalised older patients with confusion. International Journal of Geriatric Psychiatry 2004;19:858‐63. [DOI] [PubMed] [Google Scholar]
Moniz‐Cook 2008a {published data only}
- Moniz‐Cook E, Elston C, Gardiner E, Agar S, Silver M, Win T. Can training community mental health nurses to support family carers reduce behavioural problems in dementia? An exploratory pragmatic randomised controlled trial. International Journal of Geriatric Psychiatry 2008;23:185‐91. [DOI] [PubMed] [Google Scholar]
Proctor 1999 {published data only}
- Proctor R, Burns A, Powell HS, Tarrier N, Faragher B, Richardson G, et al. Behavioural management in nursing and residential homes : a randomised controlled trial. The Lancet 1999;354(9172):26‐9. [DOI] [PubMed] [Google Scholar]
Teri 2000 {published data only}
- Teri L, Logsdon RG, Peskind E, Raskind M, Weiner MF, Tractenberg RE, et al. Treatment of agitation in AD : A randomized, placebo‐controlled clinical trial. Neurology 2000;55:1271‐8. [DOI] [PubMed] [Google Scholar]
Teri 2003 {published and unpublished data}
- Teri L, Gibbons LE, McCurry SM, Logsdon RG, Buchner DM, Barlow WE, et al. Exercise plus behavioral management in patients with Alzheimer's disease: A randomized controlled trial. JAMA 2003;290(15):2015‐22. [DOI] [PubMed] [Google Scholar]
Teri 2005a {published and unpublished data}
- Teri L, McCurry SM, Logsdon R, Gibbons LE. Training community consultants to help family members improve dementia care: A randomized controlled trial. The Gerontologist 2005;45(6):802‐11. [DOI] [PubMed] [Google Scholar]
Teri 2005b {published and unpublished data}
- Teri L, Huda P, Gibbons L, Young H, Leynseele J. STAR: A dementia‐specific training program for staff in assisted living residences. The Gerontologist 2005;45(5):686‐93. [DOI] [PubMed] [Google Scholar]
Weiner 2002 {published data only}
- Weiner MF, Tractenberg RE, Sano M, Logsdon R, Teri L, Galasko D, et al. No long‐term effect of behavioural treatment on psychotropic drug use for agitation in Alzheimer's disease patients. Journal of Geriatric Psychiatry and Neurology 2002;15:95‐8. [DOI] [PubMed] [Google Scholar]
Zarit 1987 {published data only}
- Zarit SH, Anthony CR, Boutselis M. Interventions with caregivers of dementia patients: Comparison of two approaches. Psychology and Aging 1987;2(3):225‐32. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Alessi 1999 {published data only}
- Alessi CA, Yoon EJ, Schnelle JF, Al‐Samarrai NR, Cruise PAA. Randomized trial of a combined physical activity and environmental intervention in nursing home residents: Do sleep and agitation improve?. Journal of the American Geriatrics Society 1999;47:784‐91. [DOI] [PubMed] [Google Scholar]
Ashaye 2003 {published data only}
- Ashaye OA, Livingston G, Orrell MW. Does standardised needs assessment improve the outcome of psychiatric day hospital care for older people? A randomised controlled trial. Aging and Mental Health 2003;7(3):195‐9. [DOI] [PubMed] [Google Scholar]
Assal 2004 {published data only}
- Assal F, Alarcon M, Solomon EC, Masterman D, Geshwind DH, Cummings JL. Association of the serotonin transported and receptor gene polymorphisms in neuropsychiatric symptoms in Alzheimer's disease. Archives of Neurology 2004;61:1249‐53. [DOI] [PubMed] [Google Scholar]
Ayalon 2006 {published data only}
- Ayalon L, Gum AM, Feliciano L, Arean PA. Effectiveness of nonpharmalogical interventions for the management of neuropsychiatric symptoms in patients with dementia: A systematic review. JAMA 2006;166(20):2182‐8. [DOI] [PubMed] [Google Scholar]
Ayalon 2009 {published data only}
- Ayalon L, Bornfeld H, Gum AM, Arean PA. The use of problem solving therapy and restraint‐free environment for the management of depression and agitation in long‐term care. Clinical Gerontologist 2009;32:77‐90. [Google Scholar]
Baillon 2002 {published data only}
- Baillon S, Diepen E, Prettyman R. Multi‐sensory therapy in psychiatric care. Advances in Psychiatric Treatment 2002;8:444‐52. [Google Scholar]
Baillon 2004 {published data only}
- Baillon S, Diepan E, Prettyman R, Redman J, Rooke N, Campbell R. A comparison of the effects of Snoezelen and reminiscence therapy on the agitated behaviour of patients with dementia. International Journal of Geriatric Psychiatry 2004;19:1047‐52. [DOI] [PubMed] [Google Scholar]
Baillon 2005 {published data only}
- Baillon S, Diepen E, Prettyman R, Rooke N, Redman J, Campbell R. Variability in response of older people with dementia to both snoezelen and reminiscence. British Journal of Occupational Therapy 2005;68(8):367‐74. [Google Scholar]
Baker 2001 {published data only}
- Baker R, Bell S, Baker E, Gibson S, Holloway J, Pearce R, et al. A randomised controlled trial of the effects of multi‐sensory stimulation (MSS) for people with dementia. British Journal of Clinical Psychology 2001;40:81‐96. [DOI] [PubMed] [Google Scholar]
Baker 2003 {published data only}
- Baker R, Holloway J, Holtkamp CCM, Larsson A, Hartman LC, Pearce R, et al. Effects of multi‐sensory stimulation for people with dementia. Journal of Advanced Nursing 2003;43(5):465‐77. [DOI] [PubMed] [Google Scholar]
Baker 2006 {published data only}
- Baker JC, Hanley GP, Mathews RM. Staff administered functional analysis and treatment of aggression by an elder with dementia. Journal of Applied Behaviour Analysis 2006;39(4):469‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Baldelli 2007 {published data only}
- Baldelli MV, Pradelli JM, Zucchi P, Martini B, Orsi F, Fabbo A. Occupational therapy and dementia: The experience of an Alzheimer special care unit. Archives of Gerontology and Geriatrics 2007;44:49‐54. [DOI] [PubMed] [Google Scholar]
Ballard 2009 {published data only}
- Ballard C, Brown R, Fossey J, Dougals S, Bradley P, Hancock J, et al. Brief psychosocial therapy for the treatment of agitation in Alzheimer's disease (the Calm‐Ad Trial). American Journal of Geriatric Psychiatry 2009;17(9):726‐33. [DOI] [PubMed] [Google Scholar]
Beauchamp 2005 {published data only}
- Beauchamp N, Irvine BA, Seeley J, Johnson B. Worksite based Internet multimedia programme for family caregivers of participants with dementia. The Gerontological Society of America 2005;45(6):793‐801. [DOI] [PubMed] [Google Scholar]
Belle 2006 {published data only}
- Belle SH, Burgio L, Burns R, Coon D, Czaja SJ, Gallagher‐Thompson D, et al. Enhancing the quality of life of dementia caregivers from different ethnic or racial groups: A randomized, controlled trial. Annals of Internal Medicine 2006;145(10):727‐38. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bellelli 2004 {published data only}
- Bellelli G, Lucchi E, Minicuci N, Rozzini L, Bianchetti A, Padovani A, et al. Results of a multi‐level therapeutic approach for Alzheimer's disease subjects in the "real world" (CRONOS project): a 36‐ week follow‐up study. Aging Clinical and Experimental Research 2004;17(1):54‐61. [DOI] [PubMed] [Google Scholar]
Bird 2007 {published data only}
- Bird M, Llewellyn‐Jones R, Korten A, Smithers HA. Controlled trial of a predominantly psychosocial approach to BPSD: treating causality. International Psychogeriatrics 2007;19(5):874‐91. [DOI] [PubMed] [Google Scholar]
Buchanan 2002 {published data only}
- Buchanan JA, Fisher JE. Functional assessment and noncontingent reinforcement in the treatment of disruptive vocalisation in elderly dementia patients. Journal of Applied Behaviour Analysis 2002;35(1):99‐103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Burgener 1998 {published data only}
- Burgener SC, Bakas T, Murray C, Dunahee J, Tossey S. Effective caregiving approaches for patients with Alzheimer's disease. Geriatric Nursing 1998;19(3):121‐6. [DOI] [PubMed] [Google Scholar]
Burgio 2001 {published data only}
- Burgio LD, Allen‐Burge R, Roth DL, Bourgeois MS, Dijkstra K, Gerstle J, et al. Come talk with me: Improving communication between nursing assistants and nursing home residents during care routines. The Gerontologist 2001;41(4):449‐60. [DOI] [PubMed] [Google Scholar]
Burns 2003 {published data only}
- Burns R, Nichols LO, Martindale‐Adams J, Graney MJ, Lummus A. Primary care interventions for dementia caregivers: 2‐year outcomes from the REACH study. The Gerontologist 2003;43(4):547‐55. [DOI] [PubMed] [Google Scholar]
Callahan 2006 {published data only}
- Callahan C, Boustani M, Unversagt F, Austrom M, Damush T, Perkins A, et al. Effectiveness of collaborative care for older adults with Alzheimer's disease in primary care: A randomized controlled trial. JAMA 2006;295(18):2148‐57. [DOI] [PubMed] [Google Scholar]
Cohen‐Mansfield 2006 {published data only}
- Cohen‐Mansfield J, Parpura‐Gill A, Golander H. Utilization of self identity roles for designing interventions for persons with dementia. Journals of Gerontology Series B: Psychosocial Sciences & Social Sciences 2006;61B(4):P202‐12. [DOI] [PubMed] [Google Scholar]
Cohen‐Mansfield 2007 {published data only}
- Cohen‐Mansfield J, Libin A, Marx MS. Nonpharmacological treatment of agitation: A controlled trial of systematic individualised intervention. Journal of Gerontology 2007;62A(8):908‐16. [DOI] [PubMed] [Google Scholar]
Conti 2008 {published data only}
- Conti A, Voelkl JE, McGuire FA. Efficacy of meaningful activities in recreation therapy on passive behaviours of older adults with dementia. Annual in Therapeutic Recreation 2008;16:91‐104. [Google Scholar]
Coyne 1997 {published data only}
- Coyne ML, Hoskins L. Improving eating behaviors in dementia using behavioral strategies. Clinical Nursing Research 1997;6(3):275‐90. [DOI] [PubMed] [Google Scholar]
Davison 2007 {published data only}
- Davison TE, Hudgson C, McCabe MP, George K, Buchanan G. An individualized psychosocial approach for "treatment resistant" behavioural symptoms of dementia among aged care residents. International Psychogeriatrics 2007;19(5):859‐73. [DOI] [PubMed] [Google Scholar]
Deudon 2009 {published data only}
- Deudon A, Maubourguet N, Gervais X, Leone E, Brocker P, Carcaillon L, et al. Nonpharmacological management of behavioural symptoms in nursing homes. Internation Journal of Geriatric Psychiatry 2009;24(12):1386‐95. [DOI] [PubMed] [Google Scholar]
Dias 2008 {published data only}
- Dias A, Dewey ME, D'Souza J, Dhume R, Motghare DD, Shaji KS, et al. The effectiveness of a home care program for supporting caregivers of persons with dementia in developing countries: A randomised controlled trial from Goa, India. PLoS ONE 2008;3(6):e2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dwyer‐Moore 2007 {published data only}
- Dwyer‐Moore KJ, Dixon MR. Functional analysis and treatment of problem behaviour of elderly adults in long‐term care. Journal of Applied Behaviour Analysis 2007;40(4):679‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Elliot 2010 {published data only}
- Elliott AF, Burgio LD, Decoster J. Enhancing caregiver health: Findings from the resources for enhancing Alzheimer's caregiver health II intervention. The American Geriatrics Society 2010;58:30‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Farran 2007 {published data only}
- Farran C, Gilley DW, McCann JJ, Bienias JL, Lindeman DA Evans DA. Efficacy of behavioural interventions for dementia caregivers. Western Journal of Nursing Research 2007;29(8):944‐60. [DOI] [PubMed] [Google Scholar]
Feeney 2003 {published data only}
- Feeney D, Tarlow BJ, Jones RN. Effects of an automated telephone support system on caregiver burden and anxiety: findings from Reach TLC intervention study. The Gerontologist 2003;43(4):556. [DOI] [PubMed] [Google Scholar]
Finnema 2005 {published data only}
- Finnema E, Dröes R‐M, Ettema T, Ooms M, Adèr H, Ribbe M, et al. The effect of integrated emotion‐oriented care versus usual care on elderly persons with dementia in the nursing home and on nursing assistants: a randomised clinical trial. International Journal of Geriatric Psychiatry 2005;20(4):330‐43. [DOI] [PubMed] [Google Scholar]
Gallagher‐Thompson 2008 {published data only}
- Gallagher‐Thompson G, Gray HL, Dupart T, Jimenez D, Thompson LW. Effectiveness of cognitive behavioural small group intervention for reduction of depression and stress in non‐Hispanic White and Hispanic Latino women dementia family caregivers: Outcomes and mediators of change. Journal of Rational‐Emotive & Cognitive Behaviour Therapy December 2008;J26(4):286‐303. [DOI] [PMC free article] [PubMed] [Google Scholar]
Garilova 2009 {published data only}
- Garilova SI, Ferri CP, Mikhaylova N, Sokolova O, Banerjee S, Prince M. Helping carers to care ‐ the 10/66 Dementia Research Group's randomised control trial of a caregiver intervention in Russia. International Journal of Geriatric Psychiatry 2009;24(4):347‐54. [DOI] [PubMed] [Google Scholar]
Gerritsen 2005 {published data only}
- Gerritsen DL, Jongenelis K, Steverink N, Ooms ME, Ribbe MW. Down and drowsy? Do apathetic nursing home residents experience low quality of life?. Aging and Mental Health 2005;9(2):135‐41. [DOI] [PubMed] [Google Scholar]
Gitlin 2001 {published data only}
- Gitlin L, Corcoran M, Winter L, Boyce A, Hauck WW. Controlled trial of a home environmental intervention: Effect on efficacy and upset in caregivers and on a daily function of persons with dementia. The Gerontologist 2001;41(1):4‐14. [DOI] [PubMed] [Google Scholar]
Gitlin 2005 {published data only}
- Gitlin LN, Hauck WW, Dennis MP, Winter L. Maintenance of effects of the home environmental skill‐building program for family caregivers and individuals with Alzheimer's disease and related disorders. Journal of Gerontology: Medical Sciences 2005;60A(3):368‐74. [DOI] [PubMed] [Google Scholar]
Gitlin 2007 {published data only}
- Gitlin LN, Winter L, Dennis MP, Huack WW. A non‐pharmacological intervention to manage behavioural and psychological symptoms of dementia and reduce caregiver distress:design and methods of project ACT3. Clinical Interventions in Aging 2007;2(4):695‐703. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gitlin 2008 {published data only}
- Gitlin L, Winter L, Burke J, Chernett N, Dennis MP, Hauck WW. Tailored activities to manage neuropsychiatric behaviors in persons with dementia and reduce caregiver burden: A randomized pilot study. American Journal of Geriatric Psychiatry 2008;16(3):229‐39. [DOI] [PMC free article] [PubMed] [Google Scholar]
Graff 2006 {published data only}
- Graff MJL, Vernooij‐Dassen MJM, Zajec J, Olde‐Rikkert MGM, Hoefnagels WHL, Dekker J. How can occupational therapy improve the daily performance and communication of an older patient with dementia and his primary caregiver. Dementia 2006;5(4):503‐32. [Google Scholar]
Graff 2007 {published data only}
- Graff MJL, Vernooij‐Dassem MJM, Thijssen M, Dekker J, Hoefnagels WHL, Olderikkert MGM. Effects of community occupational therapy on quality of life, mood and health status in dementia patients and their caregivers: a randomised controlled trial. Journals of Gerontology Series A: Biological Sciences and Medical Sciences 2007;62(9):1002‐9. [DOI] [PubMed] [Google Scholar]
Graff 2008 {published data only}
- Graff MJL, Adang EMM, Venooij‐Dassen MJM, Dekker J, Jonsson L, Thijssen M, et al. Community occupational therapy for older patients with dementia and their caregivers: Cost effectiveness study. BMJ 2008;336(7636):134‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Grant 2007 {published data only}
- Grant JR, Steffen AM, Lauderdale SA. Comparative outcomes of two distance‐based interventions for male caregivers of family members with dementia. American Journal of Alzheimer's Disease & Other Dementias 2007;22(2):120‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Heard 1999 {published data only}
- Heard K, Watson TS. Reducing wandering by person with dementia using differential reinforcement. Journal of Applied Behaviour Analysis 1999;32(3):381‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hepburn 2001 {published data only}
- Hepburn KW, Tornatore J, Center B, Ostwald SW. Dementia family caregiver training: Affecting beliefs about caregiving and caregiver outcomes. Journal of the American Geriatrics Society 2001;49(4):450‐7. [DOI] [PubMed] [Google Scholar]
Hepburn 2003 {published data only}
- Hepburn KW, Lewis M, Sherman CW, Tornatore J. The savvy caregiver program: Developing and testing a transportable dementia family caregiver training program. The Gerontologist 2003;43(6):908‐15. [DOI] [PubMed] [Google Scholar]
Hepburn 2005 {published data only}
- Hepburn KW, Lewis M, Narayan S, Center B, Tornatore J, Bremer KL, et al. Partners in caregiving: A psychoeducation program affecting dementia family caregivers' distress and caregiving outlook. Clinical Gerontologist 2005;29(1):53‐69. [Google Scholar]
Herbert 2003 {published data only}
- Hébert R, Lévesque L, Vézina J, Lavoie JP, Ducharme F, Gendron C, et al. Efficacy of a psychoeducative group program for caregivers of demented persons living at home: A randomized controlled trial. Journal of Gerontology 2003;58B(1):S58‐S67. [DOI] [PubMed] [Google Scholar]
Hinchliffe 1995 {published data only}
- Hinchliffe AC, Hyman LL, Blizard B, Livingston G. Behavioral complications of dementia ‐ can they be treated?. International Journal of Geriatric Psychiatry 1995;10:839‐47. [Google Scholar]
Hochhalter 2007 {published data only}
- Hochhalter AK, Stevens AB, Burgio L. Rates of resident need‐driven behaviours and nursing assistant skill use in nursing homes. Long‐Term Care Interface 2007;8(2):36‐40. [Google Scholar]
Hoeffer 2006 {published data only}
- Hoeffer B, Talerico KA, Rasin J, Mitchell M, Stewart BJ, McKenzie D, et al. Assisting cognitively impaired nursing home residents with bathing: Effects of two bathing interventions on caregiving. The Gerontologist 2006;46(4):524‐32. [DOI] [PubMed] [Google Scholar]
Hoehn‐Anderson 1992 {published data only}
- Hoehn‐Anderson K, Hobson A, Steiner P, Rodel B. Patients with dementia ‐ involving families to maximise nursing care. Journal or Gerontological Nursing 1992;18:19‐25. [DOI] [PubMed] [Google Scholar]
Javadpour 2009 {published data only}
- Javadpour A, Ahmadzadeh L, Bahredar MJ. An educative support group for female family caregivers: Impact on caregivers psychological distress and patients neuropsychiatry symptoms. International Journal of Geriatric Psychiatry 2009;25(5):469‐71. [DOI] [PubMed] [Google Scholar]
Kolanowski 2001 {published data only}
- Kolanowski AM, Buettner L, Costa PT Jr, Litaker MS. Capturing interests: Therapeutic recreation activities for persons with dementia. Therapeutic Recreation Journal 2001;35(3):220‐35. [Google Scholar]
Kolanowski 2005 {published data only}
- Kolanowski AM, Litaker M, Buettner L. Efficacy of theory‐based activities for behavioral symptoms of dementia. Nursing Research 2005;54(4):219‐28. [DOI] [PubMed] [Google Scholar]
Kolanowski 2006 {published data only}
- Kolanowski A, Litaker M. Social interaction, premorbid personality, and agitation in nursing home residents with dementia. Archives of Psychiatric Nursing 2006;20(1):12‐20. [DOI] [PubMed] [Google Scholar]
Koltai 2001 {published data only}
- Koltai DC, Wlsh‐Bohmer KA, Schmechel DE. Influence of anosognosia on treatment outcome among dementia patients. Neuropsychological Rehabilitation 2001;11(34):455‐75. [Google Scholar]
Konnert 2009 {published data only}
- Konnert C, Dobson K, Stelmach L. The prevention of depression in nursing home residents: a randomised clinical trial of cognitive behavioural therapy. Aging and Mental Health 2009;13(2):288‐99. [DOI] [PubMed] [Google Scholar]
Kovach 1996 {published data only}
- Kovach C, Wilson SA, Noonan PE. The effects of hospice interventions on behaviours, discomfort, and physical complications of end stage dementia nursing home residents. American Journal of Alzheimer's Disease 1996;11(4):7‐15. [Google Scholar]
Kovach 2006 {published data only}
- Kovach CR, Logan BR, Noonan PE, Schlidt AM, Smerz J, Simpson Met al. Effects of the serial trial intervention on discomfort and behavior of nursing home residents with dementia. American Journal of Alzheimer's Disease & Other Dementias 2006;21(3):147‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kuiper 2009 {published data only}
- Kuiper D, Dijkstra GJ, Tuinstra J, Groothoff JW. The influence of Dementia Care Mapping (DCM) on behavioural problems of persons with dementia and the job satisfaction of caregivers: A pilot study. Tijdschrift voor Gerontologie en geriatrie 2009;40(3):102‐12. [DOI] [PubMed] [Google Scholar]
Kurz 2003 {published data only}
- Kurz AF, Erkinjuntti T, Small GW, Lilienfeld S, Venkata Damaraju CR. Long term safety and cognitive effects of galantamine in the treatment of probable vascular dementia or alzheimer's disease with cerebrovascular disease. European Journal of Neurology 2003;10:633‐40. [DOI] [PubMed] [Google Scholar]
Lam 2010 {published data only}
- Lam LCW, Lui VWC, Luk DNY, Chau R, So C, Poon V, et al. Effectiveness of an individual function training program on affective disturbances and functional skills in mild and moderate dementia ‐ a randomised control trial. International Journal od Geriatric Psychiatry 2010;25(2):133‐41. [DOI] [PubMed] [Google Scholar]
Lavertsky 2006 {published data only}
- Lavertsky H, Nguyen LH. Diagnosis and treatment of neuropsychiatric symptoms in Alzheimer's disease. Psychiatric Services 2006;57(5):617‐9. [DOI] [PubMed] [Google Scholar]
Lawton 1998 {published data only}
- Lawton MP, Haitsma K, Klapper J, Kleban MH, Katz IR, Corn J. A stimulation ‐ retreat special care unit for elders with dementing illness. International Psychogeriatrics 1998;10(4):379‐95. [DOI] [PubMed] [Google Scholar]
Litchenburg 2005 {published data only}
- Lichtenberg PA, Kemp‐Havican J, MacNeill SE, Johnson AS. Pilot study of behavioral treatment in dementia care units. The Gerontologist 2005;45(3):406‐10. [DOI] [PubMed] [Google Scholar]
Lovheim 2006 {published data only}
- Lovheim H, Sandman PO, Kallin K, Karlsson S, Gustafson Y. Relationship between antipsychotic drug use and behavioural and psychological symptoms of dementia in old people with cognitive impairment living in geriatric care. International Psychogeriatrics 2006;18(4):713‐26. [DOI] [PubMed] [Google Scholar]
Low 2004 {published data only}
- Low LF, Draper B, Brodaty H. The relationship between self destructive behaviour and nursing home environment. Aging and Mental Health 2004;8(1):29‐33. [DOI] [PubMed] [Google Scholar]
Lucero 2002 {published data only}
- Lucero MI. Intervention strategies for exit seeking wandering behaviour in dementia residents. American Journal of Alzheimer's disease 2002;17(5):277‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Magai 2002 {published data only}
- Magai C, Cohen CI, Gomberg D. Impact of training dementia caregivers in sensitivity to nonverbal emotion signals. International Psychogeriatrics 2002;14(1):25‐38. [DOI] [PubMed] [Google Scholar]
Marriot 2000 {published data only}
- Marriott A, Donaldson C, Tarrier N, Burns A. Effectiveness of cognitive‐behavioural family intervention in reducing the burden of care in carer's of patients with Alzheimer's disease. British Journal of Psychiatry 2000;176:557‐62. [DOI] [PubMed] [Google Scholar]
Martin 2007 {published data only}
- Martin JL, Marler MR, Harker JO, Josephson KR, Alessi CA. A multicomponent nonpharmacological intervention improves activity rhythms among nursing home residents with disrupted sleep/wake patterns. Journals of Gerontology Series A: Biological Sciences and Medical Sciences 2007;62(1):67‐72. [DOI] [PubMed] [Google Scholar]
Mayer 1991 {published data only}
- Mayer R, Darby SJ. Does a mirror deter wandering in demented older people?. International Journal of Geriatric Psychiatry 1991;6:607‐9. [Google Scholar]
McCallion 1999 {published data only}
- McCallion P, Toseland RW, Freeman K. An evaluation of a family visit education program. Journal of the American Geriatrics Society 1999;47(2):203‐14. [DOI] [PubMed] [Google Scholar]
McCurry 1998 {published data only}
- McCurry S, Logsdon RG, Vitiello MV, Teri L. Sucessful behavioural treatment for reported sleep problems in elderly caregivers of dementia patients: A controlled study. The Gerontological Society of America 1998;53b(2):122‐9. [DOI] [PubMed] [Google Scholar]
McCurry 2005 {published data only}
- McCurry SM, Gibbons LE, Logsdon RG, Vitiello MV, Teri L. Nighttime insomnia treatment and education for Alzheimer's Disease: A randomized, controlled trial. Journal of the American Geriatrics Society 2005;53:793‐802. [DOI] [PubMed] [Google Scholar]
McGilton 2003 {published data only}
- McGilton KS, Rivera TM, Dawson P. Can we help persons with dementia find their way in a new environment?. Aging and Mental Health 2003;7(5):363‐71. [DOI] [PubMed] [Google Scholar]
Melis 2008 {published data only}
- Melis RJ, Eijken MI, Teerenstra S, Achterberg T, Parker SG, Borm GF, et al. A randomised study of a multi‐disciplinary program to intervene on geriatric syndromes in vulnerable older people who live at home (Dutch EASY care study). The Journals of Gerontology Series A: Biological Sciences and Medical Sciences 2008;63(3):283‐90. [DOI] [PubMed] [Google Scholar]
Mittleman 2004 {published data only}
- Mittelman MS, Roth DL, Haley WE, Zarit SH. Effects of a caregiver intervention on negative caregiver appraisals of behavior problems in patients with Alzheimer's Disease: Results of a randomized trial. Journal of Gerontology: Psychological Sciences 2004;59B(1):27‐34. [DOI] [PubMed] [Google Scholar]
Mittleman 2006 {published data only}
- Mittelman MS, Haley WE, Clay OJ, Roth DL. Improving caregiver well‐being delays nursing home placement of patients with Alzheimer disease. Neurology 2006;67:1592‐9. [DOI] [PubMed] [Google Scholar]
Moniz‐Cook 2001 {published data only}
- Moniz‐Cook E, Woods RT, Richards K. Functional analysis of challenging behaviour in dementia: the role of superstition. International Journal of Geriatric Psychiatry 2001;16(1):45‐56. [DOI] [PubMed] [Google Scholar]
Moniz‐Cook 2003 {published data only}
- Moniz‐Cook E, Stokes G, Agar S. Difficult behaviour and dementia in nursing homes: Five cases of psychosocial intervention. Clinical Psychology & Psychotherapy 2003;10(3):197‐208. [Google Scholar]
Montgomery 2004 {published data only}
- Montgomery P, Dennis J. A systematic review of non‐pharmacological therapies for sleep problems in later life. Sleep Medicine Reviews 2004;8:47‐62. [DOI] [PubMed] [Google Scholar]
Narayan 2000 {published data only}
- Narayan SM, Hepburn KW, Lewis ML, Corcoran‐Perry S. Decision‐making focused education as a mediator of family caregiving stress. Neurobiology of Aging 2000;21(1S):S269. [Google Scholar]
Onder 2005 {published data only}
- Onder G, Zanetti O, Giacobini E, Frisoni G, Bartorelli L, Cabone G, et al. Reality orientation therapy combined with cholinesterase inhibitors in alzheimer's disease: a randomised controlled trial. British Journal of Psychiatry 2005;187:450‐5. [DOI] [PubMed] [Google Scholar]
Opie 1999 {published data only}
- Opie J, Rosewarne R, O'Connor DW. The efficacy of psychosocial approaches to behaviour disorders in dementia: a systematic literature review. Australian and New Zealand Journal of Psychiatry 1999;33:789‐99. [DOI] [PubMed] [Google Scholar]
Opie 2002 {published data only}
- Opie J, Doyle C, O'Connor DW. Challenging behaviours in nursing home residents with dementia: A randomised controlled trial of multidisciplinary interventions. International Journal of Geriatric Psychiatry 2002;17:6‐13. [DOI] [PubMed] [Google Scholar]
Ostwald 1999 {published data only}
- Ostwald SK, Hepburn KW, Caron W, Burns T, Mantell R. Reducing caregiver burden: A randomized psychoeducational intervention for caregivers of persons with dementia. The Gerontologist 1999;39(3):299‐309. [DOI] [PubMed] [Google Scholar]
Ouslander 2006 {published data only}
- Ouslander JG, Connell BR, Bliwise DL, Endeshaw Y, Griffiths P, Schnelle JF. A non pharmacological intervention to improve sleep in nursing home patients: Results of a controlled clinical trial. The American Geriatrics Society 2006;54:38‐47. [DOI] [PubMed] [Google Scholar]
Palese 2009 {published data only}
- Palese A, Menegazzo E, Baulino F, Pistrino R, Papparotto C. The effectiveness of multistrategies on disruptive vocalization of people with dementia in institutions: a multi‐centred observational study. Journal of Neuroscience Nursing 2009;41(4):191‐200. [DOI] [PubMed] [Google Scholar]
Politis 2004 {published data only}
- Polotis AM, Vozzella S, Mayer LS, Onyike CU, Baker AS, Lyketos CG. A randomised controlled, clinical trial of activity therapy for apathy in patients with dementia residing in long‐term care. International Journal of Geriatric Psychiatry 2004;19:1087‐94. [DOI] [PubMed] [Google Scholar]
Poon 2005 {published data only}
- Poon P, Hui E, Dai D, Kwok T, Woo J. Cognitive intervention for community dwelling older persons with memory problems: telemedicine versus face to face treatment. International Journal of Geriatric Psychiatry 2005;20:285‐6. [DOI] [PubMed] [Google Scholar]
Qazi 2003 {published data only}
- Qazi A, Shankar K, Orrell M. Managing anxiety in people with dementia ‐ A case series. Journal of Affective Disorders 2003;76:261‐5. [DOI] [PubMed] [Google Scholar]
Rasin 2007 {published data only}
- Rasin J, Kautz DD. Knowing the resident with dementia. Journal of Gerontological Nursing 2007;33(9):30‐6. [DOI] [PubMed] [Google Scholar]
Reeve 1985 {published data only}
- Reeve W, Ivison D. Use of environmental manipulation and classroom and modified informal reality orientation with institutionalised, confused elderly patients. Age and Ageing 1985;14:119‐21. [DOI] [PubMed] [Google Scholar]
Reuben 2003 {published data only}
- Reuben DB, Roth C, Kamberg C, Wenger NS. Restructuring primary care practices to manage geriatric syndromes: The ACOVE‐2 intervention. American Geriatrics Society 2003;51:1787‐93. [DOI] [PubMed] [Google Scholar]
Richards 2005 {published data only}
- Richards KC, Beck C, O'Sullivan PS, Shue VM. Effect of individualized social activity on sleep in nursing home residents with dementia. Journal of the American Geriatrics Society 2005;53(9):1510‐7. [DOI] [PubMed] [Google Scholar]
Robinson 1994 {published data only}
- Robinson K, Yates K. Effects of two caregiver‐training programs on burden and attitude toward help. Archives of Psychiatric Nursing 1994;8(5):312‐9. [DOI] [PubMed] [Google Scholar]
Robinson 2007 {published data only}
- Robinson J, Curry L, Gruman C, Porter M, Henderson CR, Pillemer K. Partners in caregiving in a special care environment: Cooperative communication between staff and families on dementia units. The Gerontologist 2007;47(4):504‐15. [DOI] [PubMed] [Google Scholar]
Rolland 2007 {published data only}
- Rolland Y, Pillard F, Klapouszczak A, Reynish E, Thomas D, Andrieu S, et al. Exercise program for nursing home residents with Alzheimer's Disease : A 1‐year randomized, controlled trial. Journal of The American Geriatrics Society 2007;55:158‐65. [DOI] [PubMed] [Google Scholar]
Rosendahl 2006 {published data only}
- Rosendahl E, Lindelof N, Littbrand H, Yifter‐Lindgren E, Lundin‐Olsson L, Haglin L, et al. High intensity functional exercise program and protein enriched energy supplement for older persons dependent in activities of daily living: a randomised controlled trial. Australian Journal of Physiotherapy 2006;52:105‐13. [DOI] [PubMed] [Google Scholar]
Scholzel‐Dorenbos 2010 {published data only}
- Scholzel‐Dorenbos CJM, Meeuwsen EJ, Rikkert MGMO. Intergrating unmet needs into dementia health‐related quality of life research and care: Introduction of the hierarchy model of needs in dementia. Aging and Mental Health 2010;14(1):113‐9. [DOI] [PubMed] [Google Scholar]
Schrijnemaekers 2002 {published data only}
- Schrijnemaekers V, Rossum E, Candel M, Fredericks C, Derix M, Sielhorst H, et al. Effects of emotion oriented care on elderly people with cognitive impairment and behavioural problems. International Journal of Geriatric Psychiatry 2002;17:926‐37. [DOI] [PubMed] [Google Scholar]
Schulz 2003 {published data only}
- Schulz R, Burgio L, Burns R, Eisdorfer C, Gallagher‐Thompson D, Gitlin LN, et al. Resources for enhancing Alzheimer's caregiver health (REACH): Overview, site specific outcomes and future directions. The Gerontologist 2003;43(4):514‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sink 2006 {published data only}
- Sink KM, Covinsky KE, Barnes DE, Newcomer RJ, Yaffe K. Caregiver characteristics are associated with Neuropsychiatric symptoms of dementia. The American Geriatrics Society 2006;54:796‐803. [DOI] [PubMed] [Google Scholar]
Sival 1997 {published data only}
- Sival RC, Vingerhoets RW, Haffmans PMJ, Jansen PAF, Hazelhoff JNT. Effect of a program of diverse activities on disturbed behaviour in three severely demented patients. International Psychogeriatrics 1997;9(4):423‐30. [DOI] [PubMed] [Google Scholar]
Sloane 2004 {published data only}
- Sloane PD, Hoeffer B, Mitchell CM, McKenzie DA, Barrick AL, Rader J, et al. Effect of person‐centered showering and the towel bath on bathing‐associated aggression, agitation, and discomfort in nursing home residents with dementia: A randomized, controlled trial. Journal of the American Geriatrics Society 2004;52:1795‐804. [DOI] [PubMed] [Google Scholar]
Sung 2006 {published data only}
- Sung HC, Chang SM, Lee Wl, Lee MS. The effects of group music with movement intervention on agitated behaviours of institutionalised elders with dementia in Taiwan. Complementary Therapies in Medicine 2006;14:113‐9. [DOI] [PubMed] [Google Scholar]
Teri 1994 {published data only}
- Teri L. Behavioural treatment of depression in patients with dementia. Alzheimer's Disease and Associated Disorders 1994;8(supplement 3):66‐74. [PubMed] [Google Scholar]
Teri 1998 {published data only}
- Teri L, Logsdon RG, Weiner MF, Trimmer C, Thal L, Whall AL, et al. Treatment for agitation in dementia patients: A behavior management approach. Psychotherapy 1998;35(4):436‐43. [Google Scholar]
Thal 2000 {published data only}
- Thal LJ, Forrest M, Loft H, Mengel H. Lu25‐109 a muscarinic agonist fails to improve cognition in alzheimer's disease. Neurology 2000;54(2):421. [DOI] [PubMed] [Google Scholar]
Thal 2003 {published data only}
- Thal LJ, Grindman M, Berg J, Ernstrom K, Margolin R, Pfeiffer E, et al. Idebenone treatment fails to slow cognitive decline in Alzheimer's disease. Neurology 2003;61:1498‐502. [DOI] [PubMed] [Google Scholar]
Tibaldi 2004 {published data only}
- Tibaldi V, Aimonino N, Ponzetto M, Stasi MF, Amati D, Raspo S, et al. A randomised controlled trial of a home hospital intervention for frail demented patients: behavioural disturbances and caregiver stress. Archive of Gerontology and Geriatrics 2004;9:431‐6. [DOI] [PubMed] [Google Scholar]
Torta 2004 {published data only}
- Torta R, Badino E, Scalabrino A. Therapeutic strategies for behavioural and psychological symptoms (BPSD) in demented patients. Archives of Gerontology and Geriatrics 2004;supplement 9:443‐54. [DOI] [PubMed] [Google Scholar]
Tung 2005 {published data only}
- Tung WC, Gillett PA, Pattillo RE. Applying the transtheoretical model to physical activity in family caregivers in Taiwan. Public Health Nursing 2005;22(4):299‐310. [DOI] [PubMed] [Google Scholar]
Van de Winckel 2004 {published data only}
- Winckel A, Feys H, Weerdt De W. Cognitive and behavioural effects of music‐based exercises in patients with dementia. Clinical Rehabilitation 2004;18:253‐60. [DOI] [PubMed] [Google Scholar]
Van Weert 2005a {published data only}
- Weert JCM, Dulmen AM, Spreeuwenberg PMM, Ribbe ME, Bensing JM. Effects of snoezelen, integrated in 24h dementia care, on nurse‐patient communication during morning care. Patient Education and Counselling 2005;58:312‐26. [DOI] [PubMed] [Google Scholar]
Van Weert 2005b {published data only}
- Weert JCM, Dulmen AM, Spreeuwenberg PMM, Ribbe MW, Bensing JM. Behavioural and mood effects on snoezelen integrated into 24‐hour dementia care. Journal of American Geriatric Society 2005;54:24‐33. [DOI] [PubMed] [Google Scholar]
Vespa 2002 {published data only}
- Vespa A, Gori G, Spazzafumo L. Evaluation of a non pharmacological intervention on anti psychotic behaviour in patients suffering from Alzheimer's disease in a day care centre. Archives of Gerontology and Geriatrics 2002;34:1‐8. [DOI] [PubMed] [Google Scholar]
Visser 2008 {published data only}
- Visser SM, McCabe MP, Hudgson C, Buchanan G, Davison TE, George K. Managing behavioural symptoms of dementia: Effectiveness of staff education and peer support. Aging & Mental Health 2008;1:47‐55. [DOI] [PubMed] [Google Scholar]
Williams 1987 {published data only}
- Williams R, Reeve W, Ivison D, Kavanagh D. Use of manipulation and modified reality orientation with institutionalised confused elderly subjects: a replication. Age and Aging 1987;16:315‐8. [DOI] [PubMed] [Google Scholar]
Zanetti 1998 {published data only}
- Zanetti O, Metieri T, Bianchetti A, Trabucchi M. Effectiveness of an educational program for demented persons relatives. Archives of Gerontology and Geriatrics 1998;6:531‐8. [Google Scholar]
Additional references
Agar 1997
- Agar S, Moniz‐Cook ED, Orbell S, Elston C, Wang M. Measuring the outcome of psychosocial intervention for family caregivers of dementia sufferers: a factor analytic study. Aging and Mental Health 1997;1:166‐175. [Google Scholar]
Alexopoulos 1988
- Alexopoulos GS, Abrams RC, Young RC, Shamoian CA. Cornell scale for depression in dementia. Biological Psychiatry 1988;23:271‐84. [DOI] [PubMed] [Google Scholar]
Ballard 2001
- Ballard C, O'Brien J, James I, Swann A. Dementia: management of behavioural and psychological symptoms. Oxford: Oxford University Press, 2001. [Google Scholar]
Ballard 2005
- Ballard C, Cream J. Drugs used to relieve behavioural symptoms in people with dementia or an unacceptable chemical cosh?. International Psychogeriatrics 2005;17:4‐12. [DOI] [PubMed] [Google Scholar]
Banerjee 2009
- Banerjee S. The use of antipsychotic medication for people with dementia: Time for action. http://www.dh.gov.uk/prod_consum_dh/groups/dh_digitalassets/documents/digitalasset/dh_108302.pdf 2009 (Accessed 08‐11‐2010).
Berkman 1979
- Berkman LF, Syme SL. Social networks, host resistance and mortality: A nine year follow‐up study of Alameda Country residents. American Journal of Epidemiology 1979;109:186‐204. [DOI] [PubMed] [Google Scholar]
Bird 2008
- Bird M, Moniz‐Cook ED. Challenging behaviour in dementia: a psychosocial approach to intervention. In: Woods B, Clare L editor(s). Handbook of the Clinical Psychology of Ageing. 1st Edition. Chichester, UK: John Wiley and Sons, 2008. [Google Scholar]
Black 2004
- Black W, Almeida OP. A systematic review of the association between behavioural and psychological symptoms of dementia and burden of care. International Psychogeriatrics 2004;16(3):295‐315. [DOI] [PubMed] [Google Scholar]
Bourgeois 1996
- Bourgeois MS, Schulz R, Burgio L. Intervention for caregivers of patients with Alzheimer's disease: A review and analysis of content, process and outcomes. International Journal of Human Development 1996;43:35‐92. [DOI] [PubMed] [Google Scholar]
Cohen‐Mansfield 1989
- Cohen‐Mansfield J, Marx M, Rosenthal A. A description of agitation in a nursing home. Journal of Gerontology 1989;44:M77‐M84. [DOI] [PubMed] [Google Scholar]
Cohen‐Mansfield 2000
- Cohen‐Mansfield J. Use of patient characteristics to determine nonpharmacologic interventions for behavioral and psychological symptoms of dementia. International Psychogeriatrics 2000;12(1):373‐80. [Google Scholar]
Copeland 1986
- Copeland JRM, Dewey M, Griffiths‐Jones H. Computerised psychiatric diagnosis system and case nomenclature for elderly subjects: GMS and AGECAT. Psychological Medicine 1986;6:439‐49. [DOI] [PubMed] [Google Scholar]
Cummings 1994
- Cummings JL, Mega M, Gray K, Rosenberg‐Thompson S, Carusi DA, et al. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology 1994;44:2308‐14. [DOI] [PubMed] [Google Scholar]
de Vugt 2005
- Vugt ME, Stevens F, Aalten P, Lousberg R, Jaspers N, Verhey FR. A prospective study of the effects of behavioral symptoms on the institutionalisation of patients with dementia. International Psychogeriatrics 2005;17(4):577‐89. [DOI] [PubMed] [Google Scholar]
Dempsey 2005
- Dempsey OP, Moore H. Psychotropic prescribing for older people in residential care in the UK; are guidelines being followed?. Primary Care and Community Psychiatry 2005;10:13‐8. [Google Scholar]
DoH 2001
- Department of Health (DoH). National service framework for older people. London: The Stationary Office, 2001:100. [Google Scholar]
Finkel 1997
- Finkel S, Silva JC, Cohen G, Miller S, Sartorius N. Behavioral and psychological signs and symptoms of dementia: a consensus statement on current knowledge and implications for research and treatment. International Journal of Geriatric Psychiatry 1997;12:1060‐1. [DOI] [PubMed] [Google Scholar]
Greve 2005
- Greve M, O'Connor D. A survey of Australian and New Zealand old age psychiatrists' preferred medications to treat behavioural and psychological symptoms of dementia (BPSD). International Psychogeriatrics 2005;17:195‐205. [DOI] [PubMed] [Google Scholar]
Higgins 2009
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2 [updated March 2009]. The Cochrane Collaboration, 2009. Available from www.cochrane‐handbook.org 2009.
Howard 2001
- Howard R, Ballard C, O'Brien J, Burns A. Guidelines for the management of dementia in care homes. International Journal of Geriatric Psychiatry 2001;16:714‐7. [DOI] [PubMed] [Google Scholar]
James 1999
- James IA. Using a cognitive rationale to conceptualise anxiety in people in people with dementia. Behavioural and Cognitive Psychotherapy 1999;27(4):345‐51. [Google Scholar]
James 2011
- James IA. Understanding behaviour in dementia that challenges: A guide to assessment and treatment. London: Jessica Kingsley Publishers, 2011. [Google Scholar]
Jones 1992
- Jones RSP, Owens RG. Applying functional analysis: A reply to Samson and McDonnell. Behavioural Psychotherapy 1992;20:37‐40. [Google Scholar]
Kitwood 1997
- Kitwood T. Dementia reconsidered: the person comes first. Buckingham: Open University Press, 1997. [Google Scholar]
Lantz 1996
- Lantz M, Giambianco V, Buchalter EA. 10 year review of the effects of OBRA on psychotropic prescribing practices in an academic nursing home. Psychiatric Services 1996;47:951‐7. [DOI] [PubMed] [Google Scholar]
Livingston 2005
- Livingston G, Johnston K, Paton J, Lyketsos C. Systematic review of psychological approaches to the management of neuropsychiatric symptoms of dementia. American Journal of Psychiatry 2005;162:1996‐2021. [DOI] [PubMed] [Google Scholar]
Logsdon 1999
- Logsdon RG, Teri L, Weiner MF, Gibbons LE, Raskind M, Peskind E, et al. Assessment of agitation in Alzheimer's disease: The agitated behaviour in dementia scale. Journal of American Geriatrics Society 1999;47:1354‐8. [DOI] [PubMed] [Google Scholar]
Moniz‐Cook 2000
- Moniz‐Cook ED, Woods R, Gardiner E. Staff factors associated with perception of behaviour as 'challenging' in residential and nursing homes. Aging and Mental Health 2000;4:48‐55. [Google Scholar]
Moniz‐Cook 2008b
- Moniz‐Cook ED, Vernooij‐Dassen M, Woods R, Verhey F, et al. A European consensus on outcome measures for psychosocial intervention research in dementia care. Aging and Mental Health 2008;12(1):14‐29. [DOI] [PubMed] [Google Scholar]
Morycz 1985
- Morycz RK. Caregiving strain and the desire to institutionalise family members with Alzheimer's disease. Possible predictors and model development. Research on Aging 1985;7:329‐61. [DOI] [PubMed] [Google Scholar]
NICE 2006
- National Institute of Clinical Excellence. Dementia: supporting people with dementia and their carers. NICE Clinical Guideline 42 2006; Vol. November 33.
Owens 1992
- Owens RG, Jones RSP. Extending the role of functional analysis in challenging behaviour. Behavioural Psychotherapy 1992;20:45‐6. [Google Scholar]
Parker 2008
- Parker D, Mills S, Abbey J. Effectiveness of interventions that assist caregivers to support people with dementia living in the community: a systematic review. International Journal of Evidence Based Healthcare 2008;6(2):137‐72. [DOI] [PubMed] [Google Scholar]
Patel 1992
- Patel V, Hope RA. A rating scale for aggressive behaviour in the elderly‐ the RAGE. Psychological Medicine 1992;22:211‐21. [DOI] [PubMed] [Google Scholar]
Radloff 1977
- Radloff L. The Center for Epidemiological Studies Depression scale. A self report depression scale for research in the general population. Applied Psychological Measurements 1977;1:385‐401. [Google Scholar]
Rosen 1994
- Rosen J, Burgio L, Kollar M, Cain M, Allison M, Fogleman M, et al. The Pittsburgh Agitation Scale: a user friendly instrument for rating agitation in dementia patients. American Journal of Geriatric Psychiatry 1994;2:52‐9. [DOI] [PubMed] [Google Scholar]
Selwood 2007
- Selwood A, Johnston K, Katona C, Lyketsos C, Livingston G. Systematic review of the effect of psychological interventions on family caregivers of people with dementia. Journal of Affective Disorders 2007;101:75‐89. [DOI] [PubMed] [Google Scholar]
Spira 2006
- Spira A, Edelstein B. Behavioral interventions for agitation in older adults with dementia: an evaluative review. International Psychogeriatrics 2006;18:195‐225. [DOI] [PubMed] [Google Scholar]
Stokes 2000
- Stokes G. Challenging behaviour in dementia: A person centred approach. Bicester: Winslow Press, 2000. [Google Scholar]
Teri 1992
- Teri L, Traux P, Logsdon R, Uomoto J, Zarit S, Vitaliano PP. Assessment of behavioural problems in dementia: The Revised Memory and Behaviour Problems Checklist. Psychology and Aging 1992;7:622‐31. [DOI] [PubMed] [Google Scholar]
Vitaliano 1991
- Vitaliano PP, Young HM, Becker J, Maiuro RD. The screen for caregiver burden. Gerontologist 1991;31:76‐83. [DOI] [PubMed] [Google Scholar]
Wilkin 1989
- Wilkin VM, Blessed G. Crichton Royal Behavioural Rating Scale. Users guide to dependency measures in elderly people. Social services monographs. Sheffield: University of Sheffield, 1989. [Google Scholar]
Zarit 1980
- Zarit SH, Reever KE, Bach‐Peterson J. Relatives of the impaired elderly: correlates of feeling of burden. The Gerontologist 1980;20:649‐55. [DOI] [PubMed] [Google Scholar]
Zigmond 1983
- Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Psychiatrica Scandinavica 1983;67:361‐70. [DOI] [PubMed] [Google Scholar]


