Abstract
Background
Care from the family physician is generally interrupted when patients with cancer come under the care of second‐line and third‐line healthcare professionals who may also manage the patient’s comorbid conditions. This situation may lead to fragmented and uncoordinated care, and results in an increased likelihood of not receiving recommended preventive services or recommended care.
Objectives
To classify, describe and evaluate the effectiveness of interventions aiming to improve continuity of cancer care on patient, healthcare provider and process outcomes.
Search methods
We searched the Cochrane Effective Practice and Organization of Care Group (EPOC) Specialised Register, the Cochrane Central Register of Controlled Trials (CENTRAL), PubMed, EMBASE, CINAHL, and PsycINFO, using a strategy incorporating an EPOC Methodological filter. Reference lists of the included study reports and relevant reviews were also scanned, and ISI Web of Science and Google Scholar were used to identify relevant reports having cited the studies included in this review.
Selection criteria
Randomised controlled trials (including cluster trials), controlled clinical trials, controlled before and after studies and interrupted time series evaluating interventions to improve continuity of cancer care were considered for inclusion. We included studies that involved a majority (> 50%) of adults with cancer or healthcare providers of adults with cancer. Primary outcomes considered for inclusion were the processes of healthcare services, objectively measured healthcare professional, informal carer and patient outcomes, and self‐reported measures performed with scales deemed valid and reliable. Healthcare professional satisfaction was included as a secondary outcome.
Data collection and analysis
Two reviewers described the interventions, extracted data and assessed risk of bias. The authors contacted several investigators to obtain missing information. Interventions were regrouped by type of continuity targeted, model of care or interventional strategy and were compared to usual care. Given the expected clinical and methodological diversity, median changes in outcomes (and bootstrap confidence intervals) among groups of studies that shared specific features of interest were chosen to analyse the effectiveness of included interventions.
Main results
Fifty‐one studies were included. They used three different models, namely case management, shared care, and interdisciplinary teams. Six additional interventional strategies were used besides these models: (1) patient‐held record, (2) telephone follow‐up, (3) communication and case discussion between distant healthcare professionals, (4) change in medical record system, (5) care protocols, directives and guidelines, and (6) coordination of assessments and treatment.
Based on the median effect size estimates, no significant difference in patient health‐related outcomes was found between patients assigned to interventions and those assigned to usual care. A limited number of studies reported psychological health, satisfaction of providers, or process of care measures. However, they could not be regrouped to calculate median effect size estimates because of a high heterogeneity among studies.
Authors' conclusions
Results from this Cochrane review do not allow us to conclude on the effectiveness of included interventions to improve continuity of care on patient, healthcare provider or process of care outcomes. Future research should evaluate interventions that target an improvement in continuity as their primary objective and describe these interventions with the categories proposed in this review. Also of importance, continuity measures should be validated with persons with cancer who have been followed in various settings.
Keywords: Adult, Humans, Case Management, Continuity of Patient Care, Continuity of Patient Care/standards, Health Personnel, Health Personnel/psychology, Job Satisfaction, Neoplasms, Neoplasms/therapy, Patient Care Team, Quality Improvement, Quality Improvement/standards
Plain language summary
Interventions to improve the continuity of care in the follow‐up of patients with cancer
Cancer is a very complex disease characterised by varying clinical features and treatment phases. The continuum of cancer care includes risk assessment, primary prevention, screening, detection, diagnosis, treatment, survivorship, and end‐of‐life care. Continuity of care is defined as how one patient experiences care over time, as coherent and linked, and is the result of good information flow, good interpersonal skills, and good coordination of care. The objectives of this review were to classify, describe and determine the effectiveness of interventions tested in the literature to improve continuity of care in the follow‐up of patients with cancer.
Three main models of care (case management, shared care and interdisciplinary team) designed to improve continuity of care were identified in the 51 studies included in this review. We found no standard instruments that allow to specifically measure continuity of care in patients with cancer. According to our analysis, there was no clear evidence that the interventions assessed in this review either improved or worsened patient health‐related outcomes. Therefore, our analyses did not allow us to draw firm conclusions on the effectiveness of interventions designed to improve continuity of care in the follow‐up of patients with cancer.
Few studies reported provider and informal caregiver outcomes, as well as process of care outcomes, so they could not be regrouped for analysis. The main limitations of this review were the various differences between the included studies, especially in their study designs, interventions, participants, patients' phase of care, measured outcomes, healthcare settings, and length of follow‐up.
More relevant research is needed to sort out which interventions aiming to improve continuity of care in the follow‐up of patients with cancer are the most beneficial to improve patient, provider and process of care outcomes. Future research should identify which outcomes are the most sensitive to change and the most meaningful regarding continuity of care. Also, it would be valuable to develop a standardised instrument to measure continuity of care in patients with cancer.
Summary of findings
Summary of findings for the main comparison. Interventions designed to improve any type of continuity compared to usual care.
| Interventions designed to improve any type of continuity compared to usual care | |||
| Patient or population: cancer patients Settings: multiple settings Intervention: any type of continuity Comparison: usual care | |||
| Outcomes | Median effect size* (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) |
| Functional status Multiple scales. Scale from: 0 to 100. | The median Functional status in the intervention groups was 0 higher (1.69 lower to 2.65 higher) | 3966 (16 studies) | ⊕⊝⊝⊝ very low1,2 |
| Physical status Multiple scales. Scale from: 0 to 100. | The median Physical status in the intervention groups was 0 higher (0.5 lower to 0.45 higher) | 5070 (25 studies) | ⊕⊝⊝⊝ very low1,2 |
| Psychological status Multiple scales. Scale from: 0 to 100. | The median Psychological status in the intervention groups was 0.24 lower (3.04 lower to 0.44 higher) | 4634 (20 studies) | ⊕⊝⊝⊝ very low1,2 |
| Social needs Multiple scales. Scale from: 0 to 100. | The median Social needs in the intervention groups was 0.71 lower (6.96 to 0.01 lower) | 1278 (8 studies) | ⊕⊝⊝⊝ very low1,2 |
| Satisfaction Multiple scales. Scale from: 0 to 100. | The median Satisfaction in the intervention groups was 6.7 higher (6.7 to 11.5 higher) | 378 (2 studies) | ⊕⊝⊝⊝ very low1,2,3 |
| Global quality of life Multiple scales. Scale from: 0 to 100. | The median Global quality of life in the intervention groups was 2.05 higher (0.06 lower to 2.14 higher) | 2622 (10 studies) | ⊕⊝⊝⊝ very low2,4 |
| *The basis for the median effect size (e.g. the median control group risk across studies) is provided in footnotes. Differences between the value of each outcome before and after the intervention in each experimental group were calculated for each study. Then, the difference between the effects measured in the experimental and control group served to measure the overall effect of the intervention for each outcome. We then calculated the median value of all the measured effects across all the outcomes of the same type. Lastly, to pool the results from multiple studies, the median effect size (and its 95% confidence interval) was computed for each type of outcome, by calculating the median from all the median effects in outcomes obtained from individual studies. CI: Bootstrap confidence interval; | |||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||
1 Lack of blinding or unclear blinding 2 Heterogeneity of population, interventions and outcomes 3 Unclear sequence generation 4 Lack of blinding
Background
Recent estimates have measured significant increases in survival of patients diagnosed with cancer (Jemal 2007; Sant 2003), and advances in cancer control and the application of more effective treatment approaches should lead to further reduction in cancer death rates (Byers 1999). Along with these encouraging results come new challenges for cancer care providers; adults who report a history of cancer have higher levels of disability compared to the general population (Hewitt 2003) and an increased burden of illness, as shown by long periods of sick leave, inability to work, the general perception of poor health, and the need for help with daily activities (Yabroff 2004). In addition, cancer occurs predominantly in older persons, with a median age at diagnosis of 68 years, and the proportion of older persons with cancer is expected to increase dramatically over the next 50 years (Edwards 2002). Also, older long‐term cancer survivors have been shown to have higher rates of lung disease, diabetes, heart disease, arthritis, incontinence, chronic pain, and obesity than comparable persons who have never had cancer (Keating 2005; Yancik 2001).
Care from the family physician is generally interrupted when patients with cancer come under the care of second‐line and third‐line healthcare professionals who may also manage the patient’s comorbid conditions (Oeffinger 2006). On completion of cancer treatment, patients are often discharged back to their primary care physician, who does not always have access to information about the patient prognosis, treatment plans, pain medication, possible side‐effects of treatments, complications related to the illness, and whether the transition from curative to palliative care has occurred (Barnes 2000; Dworkind 1999). These patients often require treatment from multiple providers, including surgeons, oncologists, primary care providers, nutritionists, psychologists and social workers, who are often located in multiple settings. This situation may lead to fragmented and uncoordinated care (Earle 2006; Smith 1999). As a result, cancer survivorship has been associated with an increased likelihood of not receiving recommended preventive services or recommended care across a broad range of chronic medical conditions, such as heart failure or diabetes (Earle 2004). In addition, physicians and nurses often fail to detect patients' psychosocial needs (Hewitt 2007; Hopwood 2000; Passik 1998).
Description of the intervention
The Canadian Health Services Research Foundation commissioned a report to develop a common understanding of the concept of continuity of care for patients with chronic conditions requiring management by primary care providers and to recommend continuity measures for health system monitoring. To achieve this objective, published literature on continuity of care was reviewed and researchers, content experts, and Canadian policy makers were consulted. Continuity of care was then defined as how one patient experiences care over time, as coherent and linked; continuity being the result of good information flow, good interpersonal skills, and good coordination of care (Reid 2002). The purpose of the present Cochrane review was to identify interventions aiming to improve the three types of continuity of care in cancer patients:
informational continuity, which is the availability and use of information on prior events and circumstances to make current care appropriate,
relational continuity (also called longitudinality), which refers to an ongoing relationship between a patient and a provider, and
management continuity, which is the provision of timely and complementary services within a shared management plan (Reid 2002).
Various types of barriers to continuity of cancer care have been identified in the literature. These barriers include inadequate communication between specialists and primary care providers and insufficient information provided for long‐term follow‐up care (Barnes 2000; Dworkind 1999; Johansson 2000; Oeffinger 2006); deficient communication between healthcare providers and the patient (Airey 2002; Dumont 2005; Hack 2005); insufficient coordination of health services and healthcare providers (Bickell 2001; Earle 2004; Earle 2006); difficulties to maintain a progressive transition between curative and palliative treatments (Lofmark 2005); sub‐optimal care plans (Miedema 2003); lack of clinical guidelines (Earle 2006); and lack of education and training of healthcare providers (Alvarez 2006; Dworkind 1999).
How the intervention might work
The literature describes a number of formal programs, care delivery approaches, roles, and interventional strategies that may be used to operationalize continuity of care. Continuity of care interventions are typically multifaceted, as they often combine multiple components such as: interdisciplinary approaches (accessible through most of the illness continuum) including case conference, shared written documentation tool, and interdisciplinary care standards; comprehensive assessment of patient and family needs and strengths; patient and family education and their involvement in decision making; implementation of a care plan with measurable goals; identification and coordination of supplemental resources; integration of care through each transition; and evaluation (Beddar 1994). These various components are sometimes encompassed within specific models of care delivery, such as shared care or case management.
Shared care refers to the joint participation of primary care physicians and specialists in the planned delivery of care for patients with a chronic condition and involves enhanced information exchange over and above routine discharge and referrals (Hickman 1994; Oeffinger 2006; Smith 2009). Recent descriptive studies suggest that the majority of older patients with breast and colorectal cancer are receiving care from both primary care physicians and oncology specialists and that preventive services (e.g. monitoring for chronic conditions such as diabetes, heart disease) are more often received when a primary care physician is involved (Earle 2004; Earle 2006; Etim 2006; Ganz 2006). However, cancer screening services are received more reliably when an oncology specialist is also caring for the patient (Etim 2006; Keating 2006). For example, a shared care intervention using a collaborative home‐care record to improve communication between caregivers has led to a significant reduction in the use of hospital services and improved patient/caregiver communication (Smeenk 1998a; Smeenk 1998b; Smeenk 2000).
Case management can be defined as a client‐level strategy for promoting the coordination of human services, opportunities or benefits. The case manager, a designated person or a team, organizes, coordinates and sustains a network of formal and informal support and activities designed to optimise the functioning and well‐being of people with multiple needs (Moxley 1989). In the context of continuity of care of patients with cancer, the case manager will often be a nurse specialist. Indeed, nurse‐led follow‐up care interventions have been developed to ensure safe monitoring of disease status, continuity of care, and close liaison with primary and secondary/tertiary care teams (Moore 2006). According to this model, patients who are stable on completion of treatment are supported by nurse specialists responsible for coordinating follow‐up care and, depending on their needs, the nurse specialists would provide information, emotional support, symptom management, and referral to oncologists, palliative care teams, social care and/or primary healthcare provider (Moore 2006). Nurses can either be based in the community or in specialised oncology clinics, and are sometimes referred to as case manager, patient navigator, advanced practice nurse, breast cancer coordinator, clinical coordinator or follow‐up nurse (Fillion 2006). There are examples of successful nurse‐led follow‐up interventions applied to patients with cancer which led to a reduction in the number and severity of symptoms, an increased survival (Addington‐Hall 1992), an improved quality of life, and an improved patient satisfaction with care (Faithfull 2001).
Interdisciplinary teams refer to healthcare professionals from different disciplines working together, usually for the same organisation and in the same setting. They discuss and analyse clinical situations in order to identify common goals. They harmonise links between disciplines into a coordinated and coherent whole (Choi 2006).
Why it is important to do this review
Many authors have recognised the lack of continuity in the services needed by patients throughout their trajectory of care as one of the main problems of cancer care (Dudgeon 2007; Dumont 2005; Grunfeld 2006; Gysels 2007; Haggerty 2003). To address this problem, the Institute of Medicine (USA) (Institute of Medicine 2006) recommends that patients completing primary cancer treatment be given a comprehensive care summary and follow‐up care plan to optimise both the continuity and the coordination of their care. However, the essential elements of follow‐up care plans need to be identified, the optimal levels of involvement of various specialists and primary care providers in the creation and application of the care plans need to be determined, and ways to optimise communication between providers should also be evaluated. The current evidence provides little guidance on whether one approach is superior to another and there is a need to identify evidence that will guide health care planning and provide a framework for the follow‐up of patients with cancer. The present review will thus summarize the various approaches tested to date and evaluate their effects in order to identify the best evidence‐based interventions within the reach of existing resources.
To our knowledge, no recent systematic review has covered all aspects of continuity (relational, informational, and management) and examined all types of interventions to assess their effectiveness on patients and their relatives, on professional and informal caregivers and on care processes.
Objectives
1. To describe and classify the various interventions studied in the literature to improve continuity of care in the follow‐up of patients with cancer.
2. To determine the effectiveness of interventions aiming to improve continuity of cancer care, on patient, healthcare provider and process outcomes.
Methods
Criteria for considering studies for this review
Types of studies
This review considered randomised controlled trials (including cluster trials); controlled clinical trials in which participants were definitely assigned prospectively to alternative forms of health care using quasi‐randomised allocation methods, e.g. alternation, date of birth, patient identifier, or possibly assigned prospectively to alternative forms of health care using a process of randomised or quasi‐randomised allocation; controlled before and after studies and interrupted time series. Studies published in all languages were included.
Types of participants
We included studies if a majority of participants (> 50%) were adults with cancer or healthcare providers of adults with cancer.
Types of interventions
We included well‐defined interventions that explicitly stated "aiming to improve the continuity of cancer care". However, since most interventions answering a continuity problem are not necessarily described as such, we also searched among interventions described as shared care, case management, inter‐ and multidisciplinary teams, discharge planning, implementation of individual follow‐up care plans, and telephone follow‐up. Additionally, we searched among strategies to improve communication between healthcare professionals such as referral guidelines, transmission of comprehensive treatment summaries, transmission of treatment plans or patient‐held records. We excluded studies which evaluated specialised teams accessible through a single phase of patient follow‐up unless they explicitly included an intervention to improve continuity of care. If improving continuity of care was not an explicit goal of the study and if the intervention was not described as one listed here above, then it could still be included provided the data collected and results reported indicated that the intervention was aimed at improving continuity of care. To limit bias from including studies that did not specify an improvement in continuity as their objective, inclusion was initially done independently by two reviewers, and following this process, all included and excluded studies were approved by the complete panel of authors (N = 7). Included studies were expected to compare an intervention with usual care or with another intervention in equivalent settings.
Types of outcome measures
Multiple measures are needed to capture all aspects of continuity of care (Reid 2002). For the purpose of this review, the included primary outcomes were the process of healthcare services, objectively measured healthcare professional, informal carer and patient outcomes, and self‐reported measures performed with scales having known validity and reliability. Healthcare professional satisfaction was included as a secondary outcome.
Search methods for identification of studies
Electronic searches
Studies were identified using the following bibliographic databases, sources, and approaches.
Databases
The Cochrane Central Register of Controlled Trials (CENTRAL), Issue 1, 2009, part of the The Cochrane Library.www.thecochranelibrary.com
PubMed [1948 to 2009]
EMBASE, embase.com [1947 to 2009]
Cumulative Index to Nursing and Allied Health Literature (CINAHL), EbscoHost [1980 to 2009]
PsycINFO, APA PsycNET [1806 to 2009]
The EPOC Specialised Register, Reference Manager
Strategy
Search strategies were developed by AG (Appendix 1; Appendix 2; Appendix 3; Appendix 4; Appendix 5; Appendix 6). The strategies were initially based on published research on continuity of care (Beddar 1994; Cox 2003; Dumont 2005; Freeman 2001; Gysels 2007; O'Hare 1993; Reid 2002; Sussman 2004) and then refined through an iterative development process whereby results of test strategies were screened for relevance. Based on this feedback, terms were added to or deleted from the final search strategies used for the review.
Search strategies are comprised of keywords and controlled vocabulary terms. Language limits were not applied. All databases were searched from database start date to February 2nd, 2009.
EPOC methodological search filter for MEDLINE, CINAHL and EMBASE was used to limit retrieval to appropriate study design and interventions of interest. See Appendices 1 to 6 for details of individual strategies and filters for each database searched.
Searching other resources
Additional studies were identified as follows:
we reviewed reference lists of relevant systematic reviews or other publications;
we contacted authors of relevant studies or reviews to clarify reported published information or seek unpublished results/data;
we contacted researchers with expertise relevant to the review topic;
we conducted cited reference searches on studies selected for inclusion in this review, studies cited in related reviews, and other relevant citations in ISI Web of Science/Web of Knowledge and in Google Scholar.
Data collection and analysis
Selection of studies
Two reviewers (AG and Marie Fortier) independently selected studies to be included in the review. Disagreements regarding study inclusion were resolved by discussion between the two reviewers.
Data extraction and management
A single reviewer (AG, MM, Marie Fortier or Nadine Tremblay) initially extracted data regarding study design, sample size at randomisation, follow‐up duration, description of the interventions in all experimental groups, setting, participants' inclusion and exclusion criteria, types of cancer, patient's phase of care (pre‐treatment, treatment, discharge, surveillance, recurrence or palliative) and type of outcome reported (patient, care provider or process) using a specially designed data extraction form based on the Cochrane EPOC data collection template. A second reviewer verified all data extracted. Disagreements were generally resolved by consensus or when needed, by consulting a third reviewer.
Several investigators (N = 40) were contacted to obtain missing information to complete data extraction. If information on the outcome results could not be found (generally because the investigators did not respond to the email or sometimes because they could not locate the information), then the outcomes were excluded from the analyses.
Assessment of risk of bias in included studies
Two reviewers (AG and Marie Fortier) independently assessed risks of bias of the selected trials, except for one trial (Vallieres 2006) which was assessed by RV and Marie Fortier because AG was a coauthor in this trial. Two reviewers (AG, MF) assessed the quality of all eligible studies using the eight criteria described in the EPOC module: sequence generation; allocation concealment; blinding; incomplete outcome data; selective reporting; baseline outcomes; baseline characteristics; protection against contamination; and other bias. Each criteria was answered by "Yes", "No" or "Unclear". We resolved any discrepancies in quality ratings by discussion and involvement of an arbitrator as necessary (MA or RV).
Data synthesis
Each intervention was described independently by two authors (MA and AG) with categorical variables relative to the type of continuity targeted (informational, relational and/or management), the model of care tested (case management, shared care, interdisciplinary team), and the type of interventional strategy used, as proposed in the EPOC data collection checklist for professional and organisational interventions (EPOC) (Table 2; Table 3; Table 4; Table 5). In addition, the type of targeted behaviour, the format or medium used, the deliverer of the intervention and its recipient were described using the categories proposed by EPOC in its data collection checklist (data not shown). Disagreements regarding intervention classification were resolved by discussion between the reviewers.
1. Characteristics of interventions involving case management.
| ID | Type of continuity targeted | Secondary model of cancer care | Type of targeted behaviour↕ | Structural organisational strategies § | Provider‐oriented organisational strategies * | Professional strategies ¥ | Format △ |
| Addington‐Hall 1992 | Relational Management |
‐ | 1 | 4 | 5, 6 | 1 | |
| Giesler 2005 | Relational | ‐ | 2, 4, 5, 6, 10, 11, 12 | 2, 3, 4 | 5, 6, 9, 10 | 7, 8 | 1, 3, 4, 5 |
| Given 2002 | Relational | ‐ | 2, 4, 5, 6, 10, 11, 12 | 2, 3, 4 | 5, 6, 9, 10 | 7 | 1, 2, 5 |
| Goodwin 2003 | Management Relational Informational |
‐ | 1, 2, 5, 11, 12 | 4 | 3‐12 | 2 | 1, 2, 3 |
| Koinberg 2004 | Relational Management |
‐ | 1, 2, 5, 6 12 | 4 | 3, 5, 6, 11 | 1, 2, 3 | |
| Liu 2006 | Relational | ‐ | 2, 5, 6, 10 | 4 | 5, 6, 9‐11 | 1, 2 | |
| McArdle 1996 | Relational | ‐ | 5, 9 | 1 | 5 | 1, 2 | |
| McCorkle 1989 | Relational | Home care | 1 | 4 | 1, 6, 11 | 1 | |
| McCorkle 2000 | Relational Management Informational |
Home care | 1, 2, 5, 11, 12 | 1, 2, 4 | 3, 5, 6, 10‐12 | 1, 2 | |
| McCorkle 2009 | Relational Management Informational |
‐ | 1, 2, 5, 11 | 4 | 3‐6, 9‐11 | 1, 2, 3 | |
| McKegney 1981 | Relational Management |
Home care | 2‐4, 6, 10 | 4 | 3, 10 | 1 | |
| McLachlan 2001 | Management Relational Informational |
‐ | 1, 2, 6, 11 | 2, 4 | 3, 5, 6, 9‐11 | 6 | 1, 5 |
| Moore 2002 | Relational Informational Management |
‐ | 1, 2, 6, 11 | 1, 2, 4 | 1, 3, 5‐7, 10, 11 | 2 | 1, 2, 3 |
| Mor 1995 | Relational | ‐ | 5, 6, 11, 10 | 1, 2, 4 | 5, 6, 9, 11, | 1, 2, 3 | |
| Oleske 1988 | Management Informational Relational |
Shared care | 2 | 2, 3, 4 | 1, 3‐6, 8, 11 | 2, 4 | 1, 3 |
| Rawl 2002 | Relational Management |
‐ | 2, 4, 10, 11 | 1, 2, 4 | 4‐6, 9, 10 | 1, 4 | 1, 2, 5 |
| Ritz 2000 | Relational Management |
‐ | 1, 5, 9, 11 | 1, 4 | 4‐6, 9, 10 | 1, 2 | |
| Schumacher 2002 | Relational | Home care | 3, 4, 5, 10, 12 | 1 | 5, 6 | 6 | 1, 2 |
| Skrutkowski 2008 | Relational Management |
‐ | 1, 5, 6, 11, 12 | 1, 2, 4 | 3‐6, 9, 11 | 2 | 1, 2 |
| Wells 2003 | Management | ‐ | 4, 10, 11, 12 | 1, 4 | 5 | 2 |
* 1 = Revision of professional roles; 2 = Clinical multidisciplinary teams; 3 = Formal integration of services; 4 = Skill mix change; 5 = Arrangement for follow‐up; 6 = Coordination of assessment and treatment; 7 = Transmission of comprehensive treatment summaries between providers; 8 = Transmission of treatment plans between providers; 9 = Implementation of follow‐up care plans; 10 = Care protocols, directives, guidelines; 11= Referral guidelines; 12 = Communication and case discussion between distant health professionals
§1 = Implementation of communication technologies (telephone, facsimile, telehealth); 2 = Change in medical records systems; 3 = Presence and organisation of quality monitoring mechanisms; 4 = Staff organisation
¥1 = Distribution of educational materials; 2 = Educational meetings; 3 = Local consensus processes; 4 = Educational outreach visits; 5 = Local opinion leader; 6 = Patient mediated interventions; 7 = Audit and feedback; 8 = Reminders; 9 = Marketing; 10 = Mass media
↕ 1 = Referrals; 2 = Procedures; 3 = Prescribing; 4 = General management of a problem; 5 =Patient education/advice; 6 = Professional‐patient communication; 7 = Record keeping; 8 = Financial; 9 = Discharge planning; 10 = Patient outcome; 11 = Assessment; 12 = Patient empowerment
△1 = Interpersonal; 2 = Telephone; 3 = Paper; 4 = Audio/visual; 5 = Computer / Interactive; 6 = Tele‐nursing; 7 = Diary; 8 = Group meetings; 9 = Algorithm
2. Characteristics of interventions involving shared care.
| ID | Type of continuity targeted | Secondary model of cancer care | Type of targeted behaviour↕ | Structural organisational strategies § | Provider‐oriented organisational strategies * | Professional strategies ¥ | Format △ |
| Bonnema 1998 | Management Informational |
‐ | 1, 2, 5, 9 | 4 | 3, 5, 7, 11 | 1, 3 | |
| de Wit 2001 | Informational Management |
Telephone follow‐up Patient‐held record |
2, 4, 5, 11, 12 | 1, 2 | 7, 12 | 6 | 1, 2, 3, 4, 7 |
| Grunfeld 1996 | Informational Management |
‐ | 1, 2, 9 | 1, 5, 7, 10, 11 | 1 | 3 | |
| Grunfeld 2006 | Informational Management |
‐ | 1 | 4 | 1, 3, 5, 8, 10, 11 | 1 | 3 |
| Jefford 2008 | Informational Management |
‐ | 2, 4, 7 | 2 | 7, 10, 11 | 1 | 2, 3, 5, |
| Johansson 1999 | Informational Management Relational |
Home care | 1 | 4 | 3‐7, 10‐12 | 2 | 1, 2, 3 |
| Jordhoy 2001 | Management Informational Relational |
Multidisciplinary team | 1, 2, 12 | 4 | 2, 3, 5‐12 | 2, 4 | 1 |
| Kousgaard 2003 | Management Informational |
‐ | 1, 5, 9, 12 | 7, 10, 11, 12 | 1 | 2, 3 | |
| Luker 2000 | Informational | ‐ | 1, 2, 9 | 7, 10, 11 | 1, 6 | 3 | |
| McWhinney 1994 | Informational Management |
Interdisciplinary team Home care |
1, 11 | 2, 4 | 2, 3, 5, 6, 8, 9, 11, 12 Palliative care team physician as backup resource |
3 | 1, 2, 3 |
| Mitchell 2008 | Management | Interdisciplinary team | 1 | 2, 5, 8, 12 | 3 | 2, 3, 4 | |
| Rutherford 2001 | Management Informational Relational |
‐ | 6, 9 | 1, 3, 7, 8 | 1, 6 | 1, 2, 3 | |
| Wattchow 2006 | Management Informational |
‐ | 2, 7 | 2 | 4, 5, 10 | 1, 8 | 3 |
| Wells 2004 | Informational Management |
Case management Patient‐held record |
1, 2, 4, 5, 7, 9, 11 |
1, 2, 4 | 3, 5‐7, 10‐12 | 6 | 1, 2, 3, 7 |
see footnotesTable 2
3. Characteristics of interventions involving Interdisciplinary teams.
| ID | Type of continuity targeted | Secondary model of cancer care | Type of targeted behaviour↕ | Structural organisational strategies § | Provider‐oriented organisational strategies * | Professional strategies ¥ | Format △ |
| Boyes 2006 | Informational Management |
Cancer centre care | 2, 7, 10, 11 | 2 | 10 | 3, 6, 7 | 5 |
| Hanks 2002 | Management Informational |
Shared care | 2, 6, 7, 9, 11 | 2, 4 | 2, 3, 5, 6, 8, 9, 12 | 3 | 1, 2, 3 |
| Hughes 1992 | Management Informational |
‐ | 2, 7 | 4 | 2, 5, 6, 8, 9 | 3 | 1, 7 |
| Kane 1984 | Management | ‐ | 4 | 2, 3 | 1 | ||
| Rao 2005 | Management Informational |
‐ | 1, 2, 10, 11 | 4 | 2, 3, 5, 6, 9, 10 | 1 |
see footnotesTable 2
4. Main interventional strategies used by interventions that could not be encompassed within identified models of care.
| ID | Type of continuity targeted | Main interventional strategies | Setting | Type of targeted behaviour↕ | Structural organisational strategies § | Provider‐oriented organisational strategies * | Professional strategies ¥ | Format △ |
| Beney 2002 | Management | Telephone follow‐up | Cancer centre care | 1, 2, 6 | 1, 4 | 5, 10 | 2, 3 | |
| Bohnenkamp 2004 | Relational | Communication technology | Home care | 5, 9 | 1 | 1, 4, 6 | ||
| Drury 2000 | Informational | Patient‐held records | Any setting | 7 | 2 | 6 | 3, 7 | |
| Du Pen 1999 | Management | Care protocol | Cancer centre care | 2, 3, 4, 10, 11, 12 | 2, 3 | 5, 10 | 1, 3, 9 | |
| King 2009 | Informational Management |
Assessments and feedback | Cancer centre care | 4, 6, 11 | 2, 3 | 6 | 6 | 1, 3 |
| Kravitz 1996 | Informational | Change in medical record system | Cancer centre care | 4, 7, 10, 11 | 2 | 3 | ||
| McDonald 2005 | Informational Management |
Communication technology | Home care | 1, 4, 10 | 1 | 10 | 1, 8 | 5 |
| Mills 2009 | Informational | Patient‐held records | Any setting | 4, 7, 10, 11 | 2 | 1, 6 | 3, 7 | |
| Trowbridge 1997 | Informational | Change in medical record system | Cancer centre care | 4, 6, 7, 10, 11 | 2 | 6 | 3 | |
| Vallières 2006 | Informational | Patient‐held records | 1, 3‐5, 7, 10‐12 | 2 | 6, 10, 11 | 6 | 1, 3, 7 | |
| Velikova 2004 | Informational | Assessments and feedback | Cancer centre care | 2, 4, 7, 10, 11 | 2 | 1, 2, 6, 10 | 3, 5 | |
| Williams 2001 | Informational | Patient‐held records | 7, 12 | 2 | 6 | 7 |
see footnotes Table 2
Given the clinical and methodological diversity with various models of interventions, disparate outcomes, many different care settings, and various study designs, a formal meta‐analysis could not be done. Instead, we have reported a modified form of meta‐analysis based on the median change in outcomes among studies. This approach was first suggested by Grimshaw and colleagues (Grimshaw 2004) and later used by a number of authors (Shojania 2009; Steinman 2006; Walsh 2006). We slightly adapted the methodology to give some inferences, using 200 bootstrap resamples to compute 95% confidence level bootstrap intervals.
Patient outcomes were initially combined into eight classes chosen by consensus by all the review authors: functional status, physical status, psychological status, social status, satisfaction with care, support, care needs, and global quality of life (Table 6). We decided to consider independently each sub‐scale of the quality of life instruments (i.e. Functional Assessment of Cancer Therapy Scale‐General, European Organisation for Research and Treatment of Cancer, and Medical Outcomes Study 36 Short form) to assess patient's functioning, physical, psychological and social status as well as global quality of life, which is a single item present in each of these scales.
5. Scales regrouped under each class of patient‐related outcomes.
| Classes of outcome measures | Instruments used to evaluate the endpoint |
| Functional status | Enforced Social Dependency Scale; Barthels Self‐Care Index; Functional Assessment of Cancer Therapy Scale‐General (FACT‐G); Memorial Symptom Assessment Scale (MSAS); European Organisation for Research and Treatment of Cancer (EORTC). |
| Physical status | McGill‐Melzack Pain Questionnaire; Brief Pain Inventory (BPI); Symptom Distress Scale (SDS); Supportive Care Needs Survey (SCNS); Brief Fatigue Inventory; Karnofsky Performance Status; Rotterdam Symptoms Checklist; Amsterdam Pain Management Index; Ferrell's Patient Pain Questionnaire; Symptom Experience Scale; WONCA Scale; General Health Rating Index; Functional Assessment of Cancer Therapy Scale‐General (FACT‐G); Memorial Symptom Assessment Scale (MSAS); European Organisation for Research and Treatment of Cancer (EORTC); Present, Average and Worst Pain Intensity Scale; Symptom experience scale. |
| Psychological status | Hospital Anxiety and Depression Scale (HADS); Profile of Mood States (POMS); Supportive Care Needs Survey (SCNS); Short Portable Mental Status Questionnaire (SPMSQ); Uncertainty Scale (US); General Health Questionnaire; Inventory of Current Concerns (ICC); Beck Depression Inventory; State‐Trait Anxiety Inventory (STAI); Impact of Event Scale (IES); Center for Epidemiological Studies Depression Scale (CES‐D); Functional Assessment of Cancer Therapy Scale‐General (FACT‐G); Memorial Symptom Assessment Scale (MSAS); Supportive Care Needs Survey (SCNS); European Organisation for Research and Treatment of Cancer (EORTC). |
| Social status | Functional Assessment of Cancer Therapy Scale‐General (FACT‐G); European Organisation for Research and Treatment of Cancer (EORTC); Supportive Care Needs Survey (SCNS). |
| Satisfaction with care | Satisfaction with Care Scale; Family Apgar Scale; Pain Treatement Acceptibility Scale; FAMCARE Scale; MacAdam's Assessment of suffering Questionnaire. |
| Support | Social Support Questionnaire (SSQ); Supportive Care Needs Survey (SCNS). |
| Global quality of life | Functional Assessment of Cancer Therapy Scale‐General (FACT‐G); European Organisation for Research and Treatment of Cancer (EORTC); Medical Outcomes Study 36 Short form (SF‐36); Palliative Care Quality of Life Index (PQLI); Prostate Cancer Quality of Life Instrument. |
| Care needs | Social Support Questionnaire (SSQ). |
The standard median effect size estimates across studies were calculated for patient health measures when a minimum of 4 studies were included in the analyses.
All measured outcome scales were pre‐processed to assure they were in an interval of [0‐100] and that the direction of the scales were uniform, 100 indicating a better outcome for the patient. Differences between the value of each outcome before and after the intervention in each experimental and control group were calculated for each study. Then, the difference between the effects measured in the intervention minus those in the control groups served to measure the overall effect of the intervention for each outcome. To handle the diverse set of outcomes within each individual study, we computed the median value of all the measured effects across all the outcomes of the same class. Lastly, to pool the results from multiple studies, the median effect size was calculated for each class of outcome, by computing the median from all the median effects in outcomes obtained from individual studies. Variability of this weighted median effect size was estimated using a 95% non‐parametric bootstrap confidence interval (BCI) (Efron 1993). Studies were weighted according to their sample size, so larger studies had more weight in the computation of the median effect than smaller studies. Similarly, bootstrap intervals were computed using this same weighting. All analyses were performed using SAS 9.2 (SAS Software) and figures were created using R 2.12.1 and the rmeta package (rmeta Package; R Software). This pooling strategy, based on a median instead of a mean, was chosen to be consistent with the median approach used in other reviews. Also, considering the disparate outcomes and their different scales, it seemed more appropriate to use a median instead of a mean, because it is less influenced by extreme values than would have been a mean.
As mentioned earlier, when some information on an outcome was missing to perform the calculations, then this outcome was not included in the analyses. The median effect size estimates were only calculated for patient outcome measures including four studies or more. The result significance was analysed based on the 95% BCI around the median effect size estimates.
Lastly, a descriptive analysis of single interventions on the improvement of patient health‐related outcomes was performed.
Subgroup analysis and investigation of heterogeneity
For analyses, studies were grouped either according to the type of targeted continuity of care (informational, management, and relational) or to the type of model of care or interventional strategy being evaluated. The following comparisons were studied:
Effectiveness of interventions designed to improve continuity of care on patient outcomes:
any type of continuity of care compared to usual care;
the 3 types of continuity of care simultaneously compared to usual care;
informational continuity of care compared to usual care;
management continuity of care compared to usual care;
relational continuity of care compared to usual care.
Effectiveness of different models of care or interventional strategies:
case management model of care compared to usual care;
shared care model compared to usual care;
interdisciplinary team model of care compared to usual care;
patient held‐records compared to usual care;
telephone follow‐up compared to usual care;
communication technologies compared to usual care;
changes in medical record system compared to usual care;
care protocols compared to usual care;
assessments and feedbacks compared to usual care.
Heterogeneity of the studies pooled within each analysis was explored visually using Forest plots. Then, for each comparison, studies were stratified according to the cancer phase: 1) treatment phase, 2) after discharge from the cancer centre, 3) palliative phase, and 4) any phase (many studies included patients at different phases of cancer and presented undifferentiated results).
Sensitivity analysis
For quality of life instruments, we compared effect size estimates and bootstrap confidence intervals when each subscale was considered separately (functioning, physical, psychological and social status, global quality of life) or when they were combined within a single measure. Furthermore, physical symptoms assessed within validated instruments were also considered either independently or as a whole in the physical status class of patient health‐related outcomes.
Results
Description of studies
Results of the search
Electronic searching yielded a total of 6968 citations. The PubMed search generated 3502 records, CINAHL generated 1695, EMBASE generated 2472, PsycINFO generated 29, CENTRAL generated 281, EPOC Specialised Register generated 287. From these abstracts, 653 studies appeared to meet the entry criteria and were retrieved for further assessment. Fifty‐one trials published in 115 documents met all the review criteria and the remaining 541 documents were excluded. Among the excluded documents, 410 did not meet the criteria relative to the types of studies, 21 failed to meet the type of participant inclusion criteria, 104 did not evaluate an intervention judged to improve continuity of care, and 6 did not meet the criteria relative to the types of studied outcomes. All included trials were published in English.
Included studies
Description of included studies
Fifty‐one studies met all the inclusion criteria of this review (see Table: Characteristics of included studies). Nine studies included more than two treatment groups (Johansson 1999; King 2009; McArdle 1996; McCorkle 1989; McDonald 2005; Oleske 1988; Rao 2005; Rutherford 2001; Wells 2003), giving a total of 63 different interventions being tested within the 51 studies. The majority of included studies were randomised controlled trials (N = 49) and among these, eight studies allocated participants by clusters (Addington‐Hall 1992; Du Pen 1999; Goodwin 2003; Jefford 2008; Jordhoy 2001; Kousgaard 2003; McKegney 1981; Oleske 1988). Only two studies used a controlled clinical trial design (Liu 2006; Luker 2000). None of the included studies were designed as controlled before and after studies or interrupted time series.
Characteristics of participants
For included studies, sample size at randomisation ranged from 28 (Bohnenkamp 2004) to 1388 patients (Rao 2005). Twenty‐five studies included patients having any type of cancer (Addington‐Hall 1992; Beney 2002; Boyes 2006; de Wit 2001; Drury 2000; Du Pen 1999; Given 2002; Hanks 2002; Jordhoy 2001; Kane 1984; Kousgaard 2003; Kravitz 1996; McCorkle 2000; McDonald 2005; McKegney 1981; McLachlan 2001; McWhinney 1994; Mitchell 2008; Mor 1995; Oleske 1988; Rao 2005; Trowbridge 1997; Vallieres 2006; Velikova 2004; Wells 2003). Six studies included patients with various mixes of cancer types (breast, lung or colorectal cancer: King 2009; Rawl 2002; bladder, colorectal or cervical/ovarian cancer: Bohnenkamp 2004; gastric, breast, prostate or colorectal cancer: Johansson 1999; endometrial or cervical/ovarian: Rutherford 2001; breast or lung cancer: Skrutkowski 2008). Ten studies included patients having exclusively breast cancer (Bonnema 1998; Goodwin 2003; Grunfeld 1996; Grunfeld 2006; Koinberg 2004; Liu 2006; Luker 2000; McArdle 1996; Ritz 2000; Wells 2004), three only included patients with lung cancer (McCorkle 1989; Mills 2009; Moore 2002) and one of each only included patients with prostate cancer (Giesler 2005), cervical/ovarian cancer (McCorkle 2009), and colorectal cancer (Wattchow 2006). One study included participants with any type of cancer except for basal cell carcinoma of the skin (Williams 2001). Three studies did not mention which type of cancer their participants had (Hughes 1992; Jefford 2008; Schumacher 2002).
Generally, participants in all phases of cancer care were recruited and many studies followed patients going through multiple cancer phases. A few studies selected patients exclusively in the treatment phase (Boyes 2006; Drury 2000; Given 2002; Kravitz 1996; Rawl 2002; Vallieres 2006; Velikova 2004) or in the palliative phase (Addington‐Hall 1992; Du Pen 1999; Hanks 2002; Hughes 1992; Jordhoy 2001; Kane 1984; McWhinney 1994; Mills 2009; Mitchell 2008). The follow‐up duration of included studies varied between five days (Kravitz 1996) to five years (Koinberg 2004), and a few interventions targeting patients in palliative care specified that the intervention would run until participants' death (Addington‐Hall 1992; Hanks 2002; Jordhoy 2001). Twenty‐two studies were performed in the United States of America, 13 in the United Kingdom, six in Australia, three in Canada, two in the Netherlands and in Sweden, and one of each in Norway, Denmark and Taiwan (please see Characteristics of included studies).
Description of the interventions
Among the 63 tested interventions (51 studies), 20 evaluated case management models (Table 2), 14 evaluated shared care models (Table 3), and five evaluated interdisciplinary team models (Table 4), among which three tested palliative care teams (Hanks 2002; Hughes 1992; Kane 1984). Some of the reviewed interventions could not be encompassed to any main model of care but the main interventional strategy used was identified: four studies used patient‐held records (Drury 2000; Mills 2009; Vallieres 2006; Williams 2001), one used telephone follow‐up (Beney 2002), two used communication technologies (Bohnenkamp 2004; McDonald 2005), two used changes in medical record system (Kravitz 1996; Trowbridge 1997), one tested a care protocol (Du Pen 1999) and two used strategies of regular assessments and feedbacks (King 2009; Velikova 2004) (Table 5).
Nine studies targeted simultaneously informational, relational and management continuity, including six using a case management model of care (Goodwin 2003; McCorkle 2000; McCorkle 2009; McLachlan 2001; Moore 2002; Oleske 1988) and three using a shared care model (Johansson 1999; Jordhoy 2001; Rutherford 2001).
Six studies targeted relational and management continuity, and all of these used a case management model of care (Addington‐Hall 1992; Koinberg 2004; McKegney 1981; Rawl 2002; Ritz 2000; Skrutkowski 2008). Thirteen studies targeted informational and management continuity of care, nine using a shared model of care (Bonnema 1998; de Wit 2001; Grunfeld 1996; Grunfeld 2006; Jefford 2008; Kousgaard 2003; McWhinney 1994; Wattchow 2006; Wells 2004) and four using an interdisciplinary model of care (Boyes 2006; Hanks 2002; Hughes 1992; Rao 2005). Seven studies targeted relational continuity only, and used a case management model of care (Giesler 2005; Given 2002; Liu 2006; McArdle 1996; McCorkle 1989; Mor 1995; Schumacher 2002). Three studies targeted management continuity exclusively and either used case management (one study: Wells 2003), shared care (one study: Mitchell 2008) or interdisciplinary team (one study: Kane 1984) models of care. One study only targeted informational continuity, and used a shared caremodel (Luker 2000).
Included studies targeted different types of behaviour and used a diverse range of organisational (structural and/or provider‐oriented) and professional strategies. Studies that tested a case management model of care targeted various types of behaviour (Table 2) but they mainly used strategies consisting in staff organisation, arrangement for follow‐up, and coordination of assessment and treatment. Interventions that tested shared care (Table 3) generally targeted a change in referrals or procedures, and used provider‐oriented organisational strategies, such as arrangement for follow‐up, transmission of comprehensive treatment summaries between providers, and the implementation of care protocols, directives and guidelines. Educational materials were distributed to healthcare providers for some of these interventions (Grunfeld 1996; Grunfeld 2006; Jefford 2008; Kousgaard 2003; Luker 2000; Rutherford 2001; Wattchow 2006). Studies evaluating interdisciplinary teams (Table 4) used organisational strategies such as staff organisation and the creation of teams of healthcare professionals working together to care for patients. These interventions also used local consensus processes, formal integration of services, arrangement for follow‐up, coordination of assessment and treatment, and implementation of follow‐up care plans.
Nine studies included more than two experimental groups (Johansson 1999; King 2009; McArdle 1996; McCorkle 1989; McDonald 2005; Oleske 1988; Rao 2005; Rutherford 2001; Wells 2003). To avoid unit‐of‐analysis errors, only results from two of the experimental groups were included in the analysis, with one group representing the control condition and the other representing the intervention (as opposed to an active control). The intervention group was first selected based on whether it would meet the criteria for inclusion in this review. If more than two intervention groups were included based on this, then only the most intensive intervention with respect to improving continuity of care was included in the review.
Description of the outcomes
Diverse patient, provider, and process outcomes were reported across the 51 included studies of this review. Several studies reported patient health‐related measures, such as physical and psychological status, quality of life, and satisfaction (Table 6), whereas fewer studies reported providers' quality of life and psychological status. Among the processes of healthcare services, included studies measured: utilization of healthcare services; care coordination; accessibility to care; and availability and transfer of information between providers. Time to detection of recurrence, survival and place of death were also reported in a limited number of studies.
Risk of bias in included studies
The biases most often identified in the included studies were inadequate allocation concealment, inadequate management of incomplete data, and contamination between experimental groups (see Characteristics of included studies; Figure 1; Figure 2). Also, blinding of study participants was not found in most studies.
1.
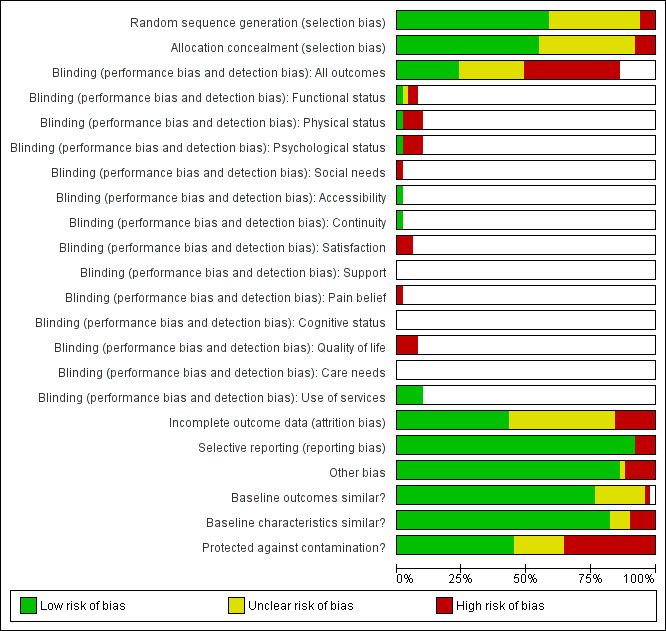
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
2.
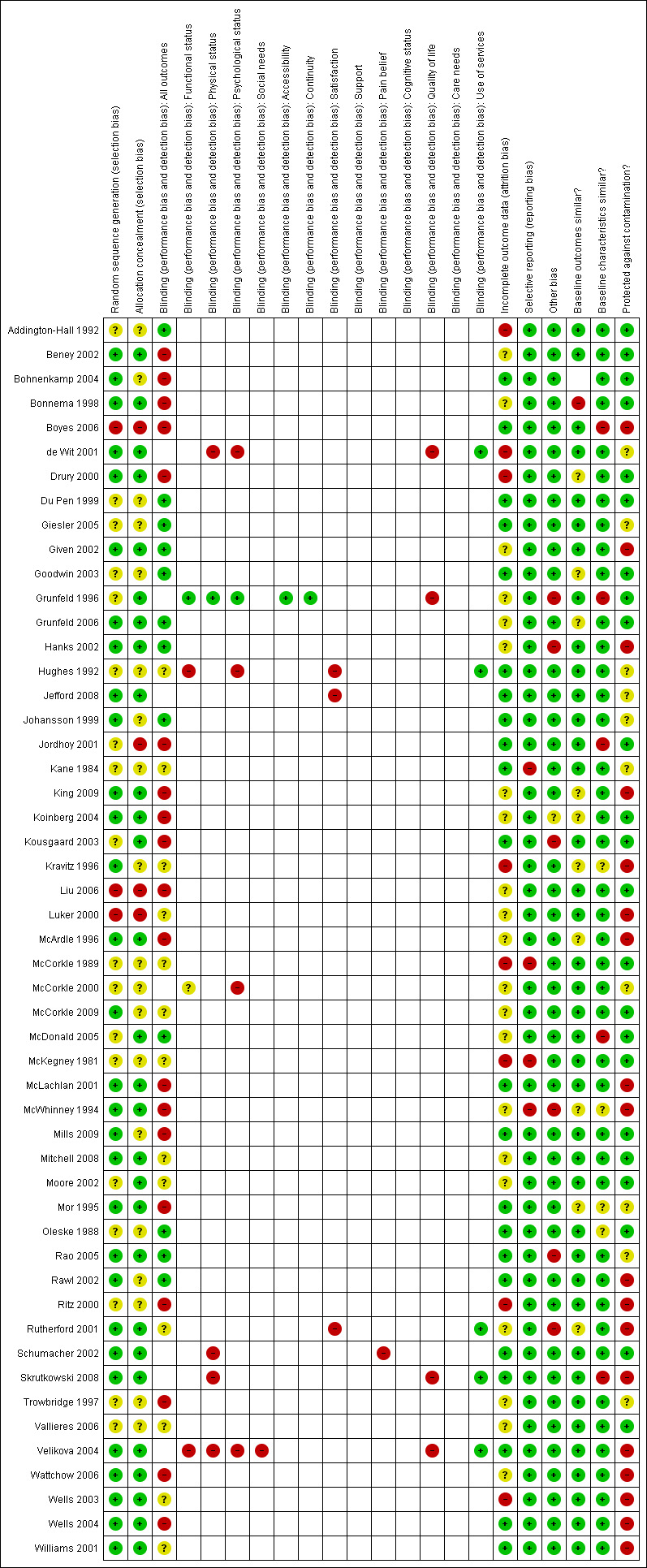
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Among the 51 studies included in this review, more than half (N = 28) adequately concealed the allocation of participants or clusters. For most of the remaining studies (N = 19) the process of allocation concealment was rated as unclear whereas allocation was not explicitly concealed for four studies (Boyes 2006; Jordhoy 2001; Liu 2006; Luker 2000).
Blinding
Participants were blinded in only 12 studies (see Risk of bias tables within Characteristics of included studies).
Incomplete outcome data
Of the 51 studies included in this review, 22 adequately addressed incomplete data (see Risk of bias tables within Characteristics of included studies).
Selective reporting
Most included studies were free of selective outcome reporting (N = 47). However, four studies did not meet that criteria because at least one of the outcomes described in the methods section was not reported in the results of the published article (Kane 1984; McCorkle 1989; McKegney 1981) or because the study results were not presented in the published article (McWhinney 1994).
Other potential sources of bias
For most included studies (N = 45), outcome values and participant characteristics were similar at baseline. A single study reported a significant difference in outcomes between the intervention and control groups at baseline (Bonnema 1998) whereas five studies reported some differences in participant characteristics between the intervention and control groups at baseline (Boyes 2006; Grunfeld 1996; Jordhoy 2001; McDonald 2005; Skrutkowski 2008).
Of the 51 included studies, 23 used a design that prevented contamination between experimental groups. In the remaining studies, contamination between experimental groups was either unclear or possible, either because patients were the unit of randomisation (King 2009; Ritz 2000; Rutherford 2001; Skrutkowski 2008; Velikova 2004; Wells 2004; Williams 2001) or no stratification was done between healthcare professionals (de Wit 2001; King 2009; Given 2002), or because patients in the various groups were followed in the same setting (Ritz 2000; Rutherford 2001; Skrutkowski 2008; Wells 2003), or by the same healthcare professionals (Boyes 2006; Hanks 2002; Kravitz 1996; Luker 2000; McArdle 1996; McLachlan 2001; McWhinney 1994).
Effects of interventions
See: Table 1
Patient health outcomes
Median change in patient outcomes and 95% non‐parametric bootstrap confidence intervals (95% CI) are presented for the ninecomparisons of either studies regrouped according to the type of continuity targeted, the model of care or the interventional strategy used versus usual care (Table 7 and Figures 3 to 29). The stratification of studies according to the cancer phase (treatment phase, after discharge, palliative phase and any phase) was ineffective at reducing heterogeneity and it produced similar results as for the global analyses, except for studies conducted in the palliative phase. Therefore, only results from studies on patients in palliative care are presented (Figure 3; Figure 4; Figure 5) in addition to overall results.
6. Effectiveness of intervention by subgroup.
| Comparisons | Outcomes | Number of studies | Number of participants (total) | Standard Median Effect Sizes across studies (percent) | Confidence Intervals (bootstrap method) | Forest Plots |
| 1. Interventions designed to improve any type of continuity of care versus usual care | Functional status | 16 | 3966 | 0 | ‐1.7 ; 2.7 | Figure 6 |
| Physical status | 25 | 5069 | 0 | ‐0.5 ; 0.5 | Figure 7 | |
| Psychological status | 20 | 4633 | ‐0.2 | ‐3.0 ; 0.4 | Figure 8 | |
| Social status | 8 | 1277 | ‐0.7 | ‐7.0 ; ‐0.01 | Figure 9 | |
| Global Quality of life | 10 | 2622 | 2.1 | ‐0.1 ; 2.1 | Figure 10 | |
| 2. Interventions designed to improve simultaneously the three types of continuity of care versus usual care | Physical status | 4 | 815 | ‐0.5 | ‐2.4 ; 0 | Figure 11 |
| Psychological status | 4 | 1408 | ‐1.1 | ‐3.0 ; 13.1 | Figure 12 | |
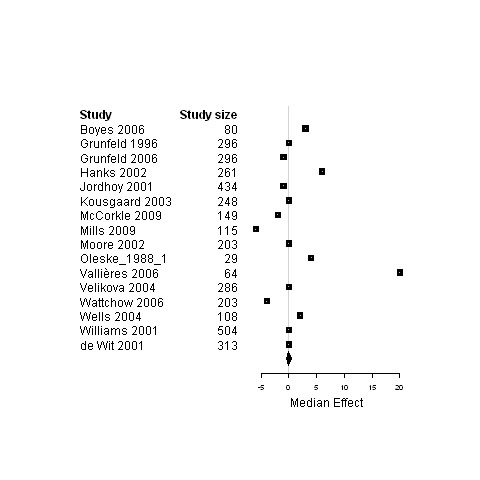
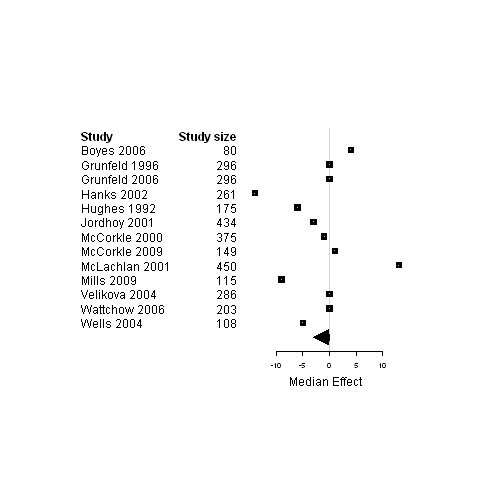
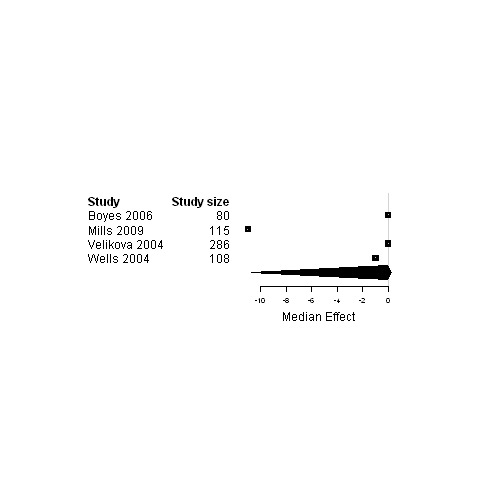
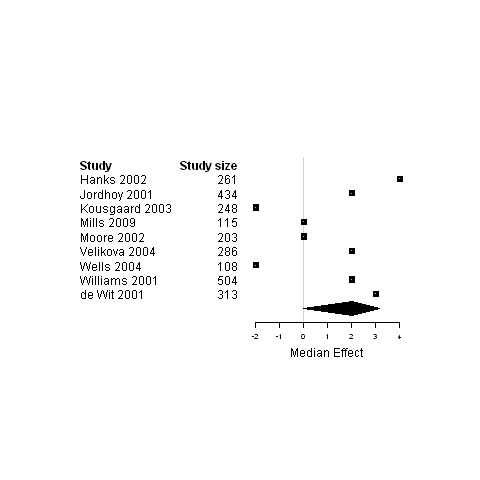
| 3. Interventions designed to improve informational continuity of care versus usual care | Functional status | 11 | 3057 | 0 | ‐3.4 ; 2.7 | Figure 13 |
| Physical status | 16 | 3589 | 0 | ‐0.5 ; 0.5 | Figure 14 | |
| Psychological status | 13 | 3228 | ‐0.24 | ‐3.0 ; 0.02 | Figure 15 | |
| Social status | 4 | 589 | ‐0.01 | ‐10.7 ; 0.3 | Figure 16 | |
| Global Quality of life | 9 | 2472 | 2.0 | ‐0.03 ; 3.2 | Figure 17 | |
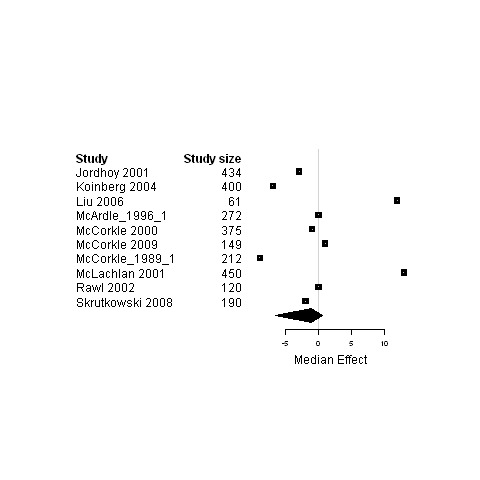
| 4. Interventions designed to relational continuity of care versus usual care | Functional status | 7 | 1771 | 0 | ‐3.4 ; 6.9 | Figure 18 |
| Physical status | 10 | 1985 | ‐0.5 | ‐4.9 ; 12.5 | Figure 19 | |
| Psychological status | 10 | 2663 | ‐1.1 | ‐6.7 ; 0.6 | Figure 20 | |
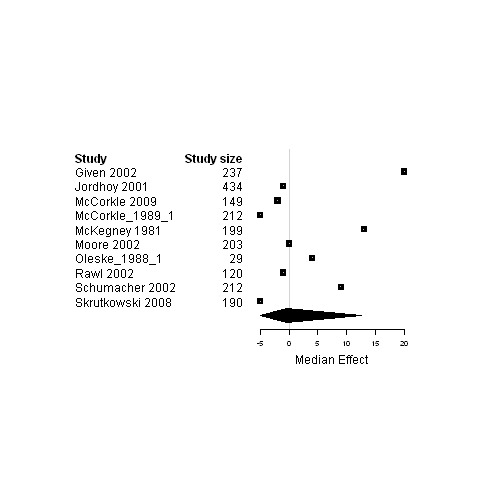
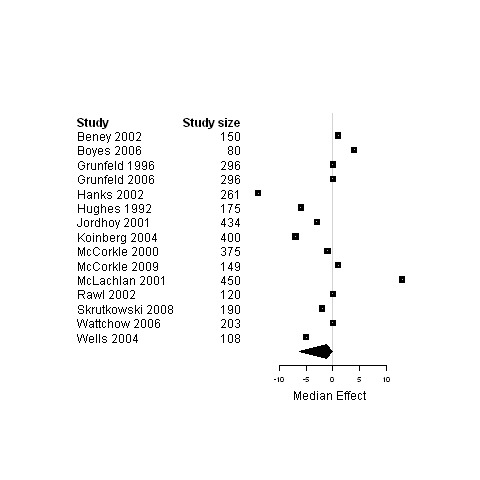
| 5. Interventions designed to improve management continuity of care versus usual care | Functional status | 11 | 2612 | 0 | ‐3.4 ; 2 | Figure 21 |
| Physical status | 18 | 3439 | 0 | ‐0.5 ; 0.03 | Figure 22 | |
| Psychological status | 15 | 3687 | ‐1.1 | ‐6.3 ; 0 | Figure 23 | |
| Social status | 4 | 528 | ‐0.7 | ‐7.0 ; 0.3 | Figure 24 | |
| Global Quality of life | 7 | 1717 | 2.0 | ‐1.9 ; 3.2 | Figure 25 | |
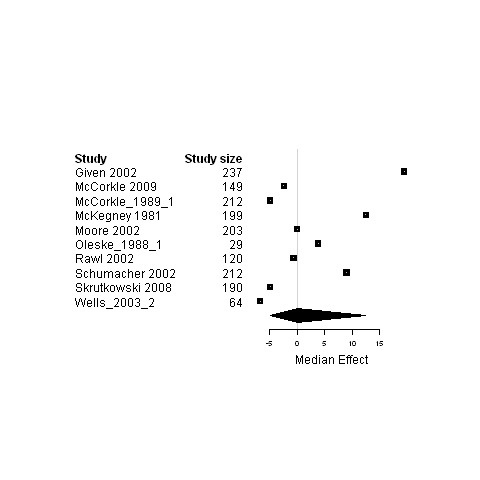
| 6. Interventions using a case management model of care versus usual care | Functional status | 6 | 1377 | ‐0.9 | ‐6.4 ; 18.0 | Figure 26 |
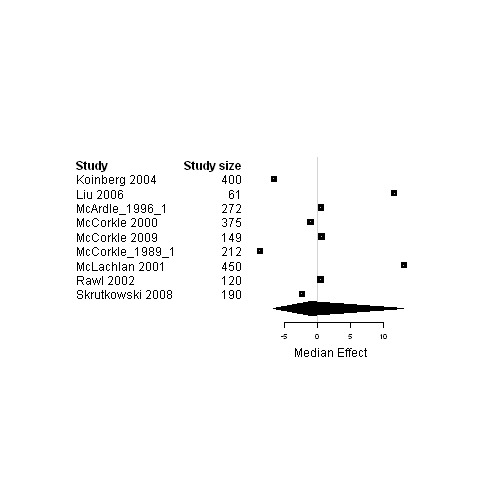
| Physical status | 10 | 1615 | 0 | ‐4.9 ; 12.5 | Figure 27 | |
| Psychological status | 9 | 2229 | ‐1.12 | ‐6.7 ; 13.1 | Figure 28 | |
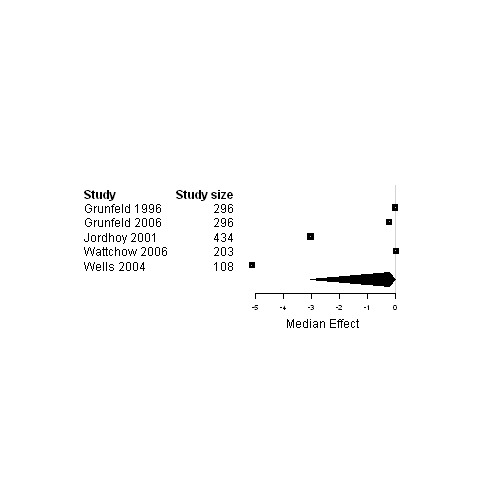
| 7. Interventions using a shared care model versus usual care | Functional status | 5 | 1399 | 2.0 | ‐2.1 ; 2.7 | Figure 29 |
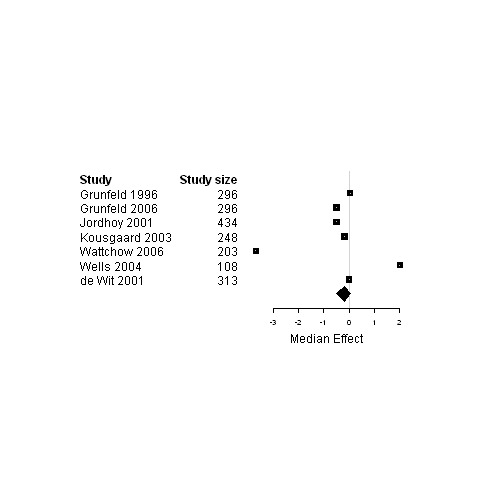
| Physical status | 7 | 1898 | ‐0.2 | ‐0.5 ; 0.03 | Figure 30 | |
| Psychological status | 5 | 1337 | ‐0.2 | ‐3.0 ; 0 | Figure 31 | |
| Global Quality of life | 4 | 1103 | 2.0 | ‐2.2 ; 3.2 | Figure 32 |
The standard median effect size estimates across studies were calculated for patient health measures when a minimum of 4 studies were included in the analyses.
3.
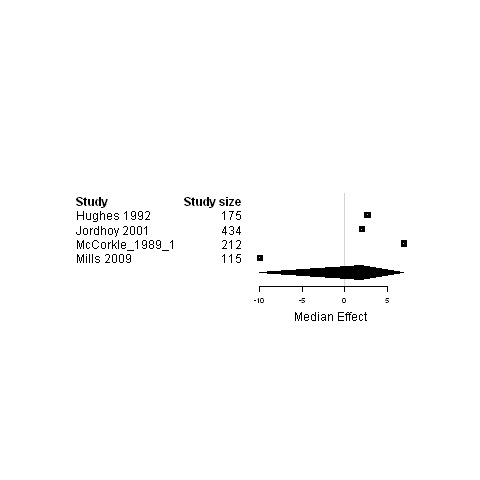
Subgroup analysis of patients in palliative care ‐ Forest plot for the functional status of patients assigned to interventions designed to improve any type of continuity versus usual care.
4.
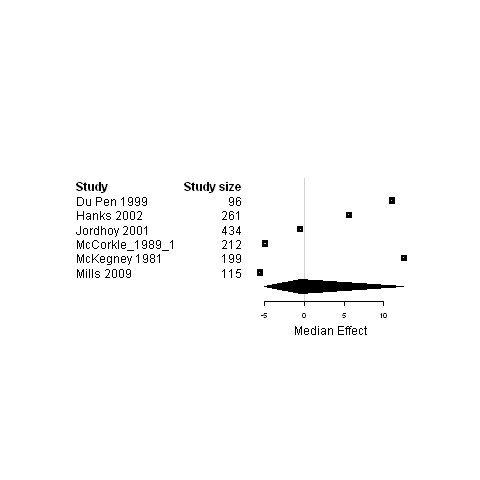
Subgroup analysis of patients in palliative care ‐ Forest plot for the physical status of patients assigned to interventions designed to improve any type of continuity versus usual care.
5.
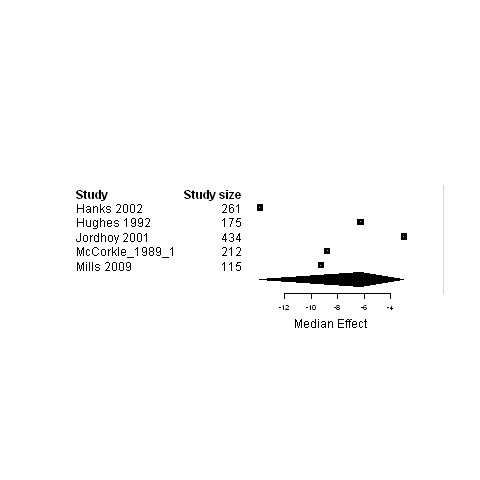
Subgroup analysis of patients in palliative care ‐ Forest plot for the psychological status of patients assigned to interventions designed to improve any type of continuity versus usual care.
6.
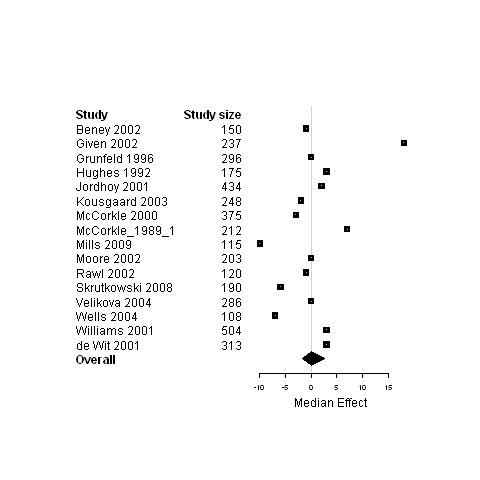
Forest plot for the functional status of patients assigned to interventions designed to improve any type of continuity versus usual care.
7.
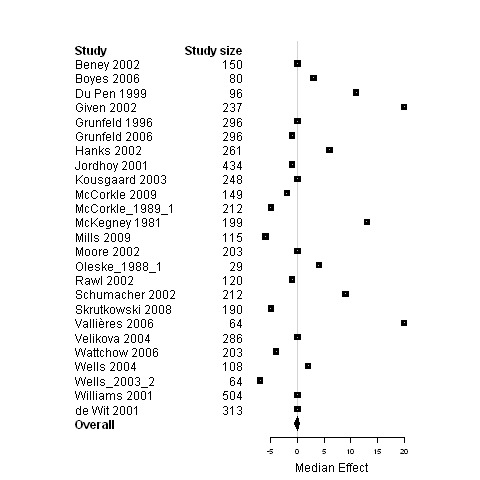
Forest plot for the physical status of patients assigned to interventions designed to improve any type of continuity versus usual care.
8.
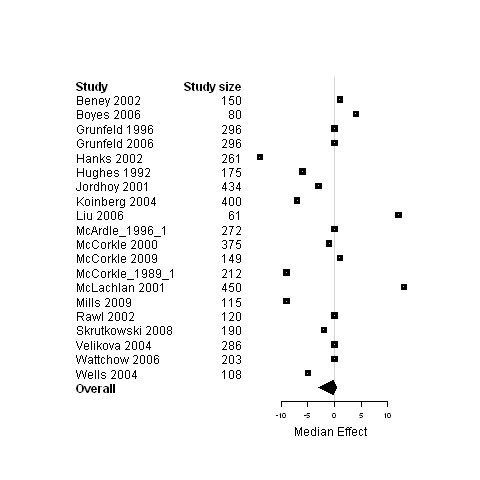
Forest plot for the psychological status of patients assigned to interventions designed to improve any type of continuity versus usual care.
9.
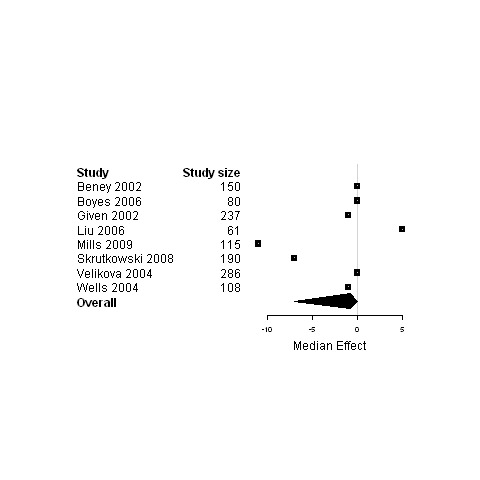
Forest plot for the social status of patients assigned to interventions designed to improve any type of continuity versus usual care.
10.
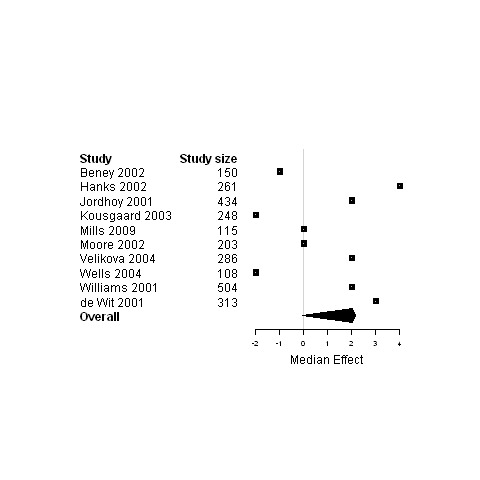
Forest plot for the quality of life of patients assigned to interventions designed to improve any type of continuity versus usual care.
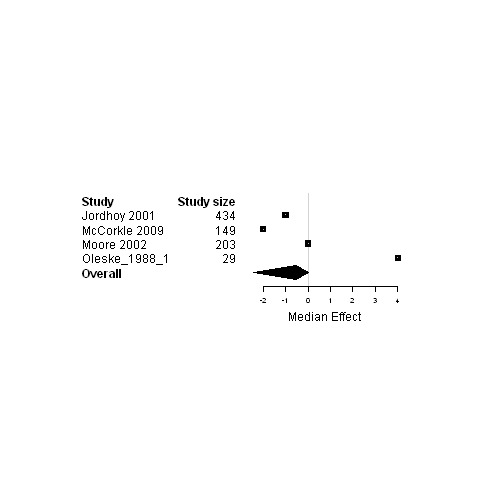
11.
Forest plot for the physical status of patients assigned to interventions designed to improve the three types of continuity versus usual care.
12.

Forest plot for the psychological status of patients assigned to interventions designed to improve the three types of continuity versus usual care.
13.
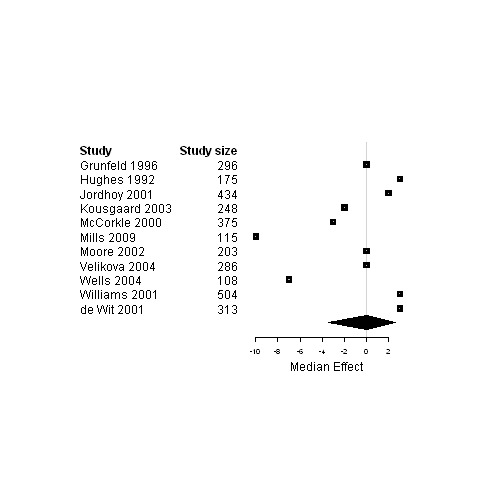
Forest plot for the functional status of patients assigned to interventions designed to improve informational continuity versus usual care.
14.
Forest plot for the physical status of patients assigned to interventions designed to improve informational continuity versus usual care.
15.
Forest plot for the psychological status of patients assigned to interventions designed to improve informational continuity versus usual care.
16.
Forest plot for the social status of patients assigned to interventions designed to improve informational continuity versus usual care.
17.
Forest plot for the quality of life of patients assigned to interventions designed to improve informational continuity versus usual care.
18.
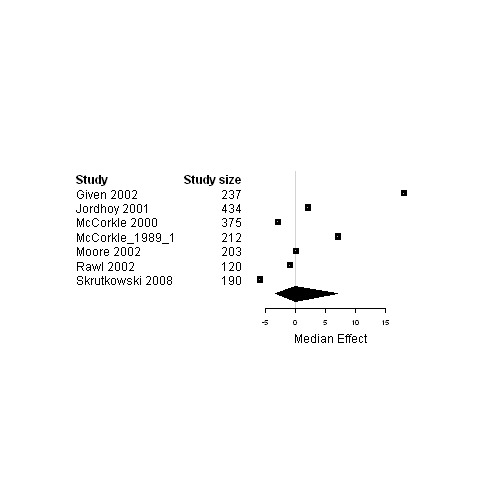
Forest plot for the functional status of patients assigned to interventions designed to improve relational continuity versus usual care.
19.
Forest plot for the physical status of patients assigned to interventions designed to improve relational continuity versus usual care.
20.
Forest plot for the psychological status of patients assigned to interventions designed to improve relational continuity versus usual care.
21.
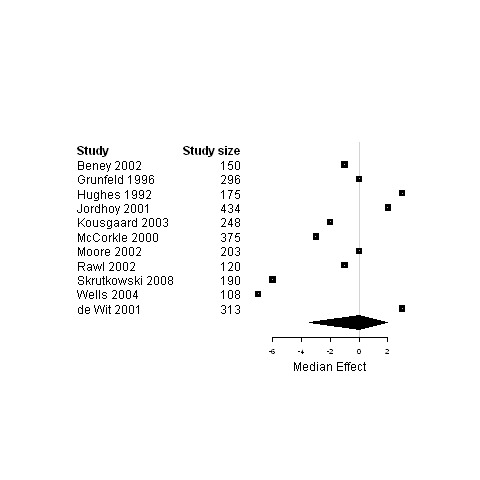
Forest plot for the functional status of patients assigned to interventions designed to improve management continuity versus usual care.
22.
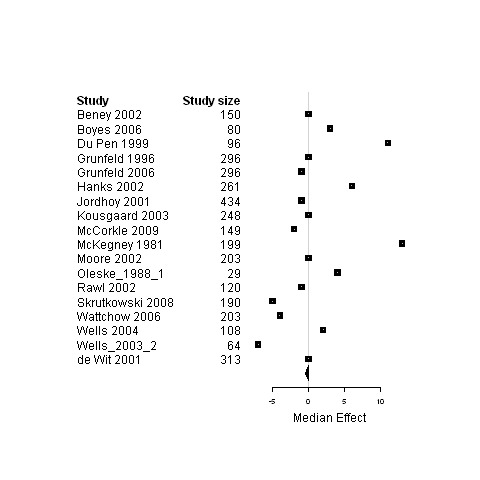
Forest plot for the physical status of patients assigned to interventions designed to improve management continuity versus usual care.
23.
Forest plot for the psychological status of patients assigned to interventions designed to improve management continuity versus usual care.
24.
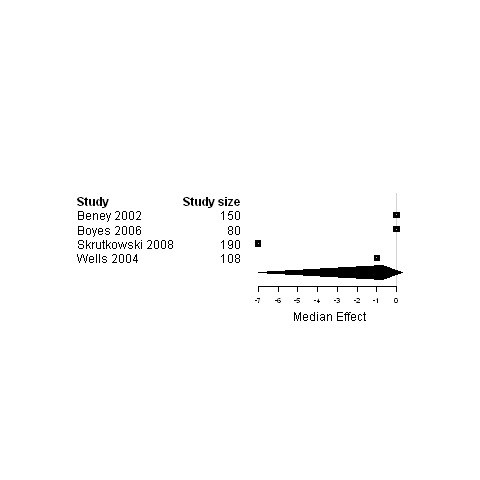
Forest plot for the social status of patients assigned to interventions designed to improve management continuity versus usual care.
25.
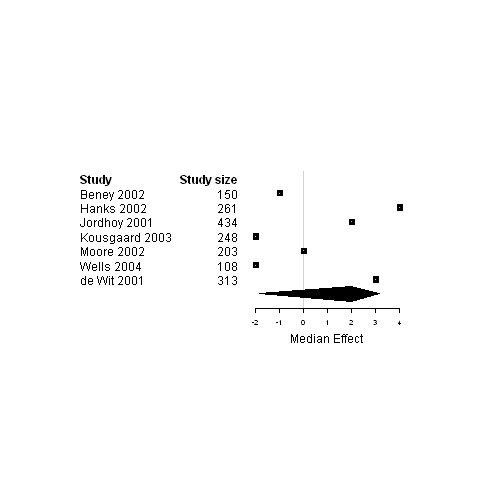
Forest plot for the quality of life of patients assigned to interventions designed to improve management continuity versus usual care.
26.
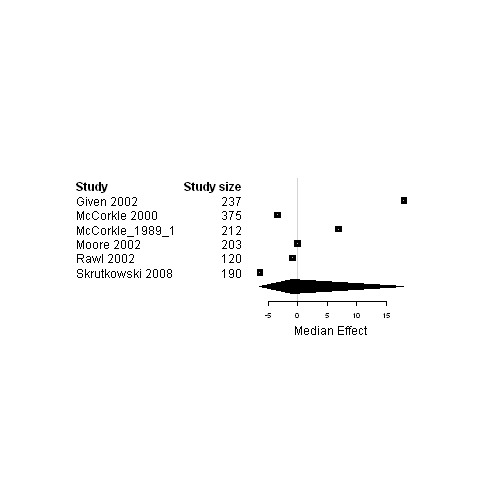
Forest plot for the functional status of patients assigned to interventions using a case management model of care versus usual care.
27.
Forest plot for the physical status of patients assigned to interventions using a case management model of care versus usual care.
28.
Forest plot for the psychological status of patients assigned to interventions using a case management model of care versus usual care.
29.
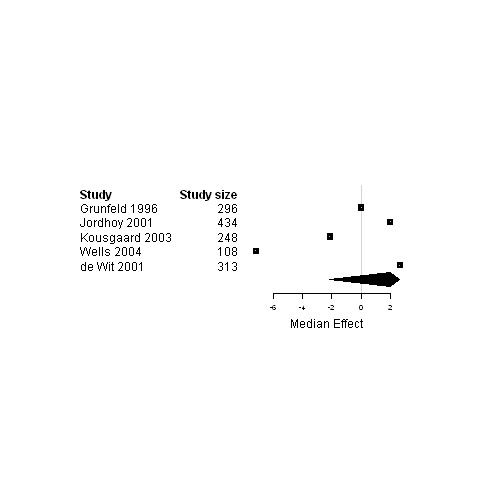
Forest plot for the functional status of patients assigned to interventions using a shared care model versus usual care.
30.
Forest plot for the physical status of patients assigned to interventions using a shared care model versus usual care.
31.
Forest plot for the psychological status of patients assigned to interventions using a shared care model versus usual care.
32.
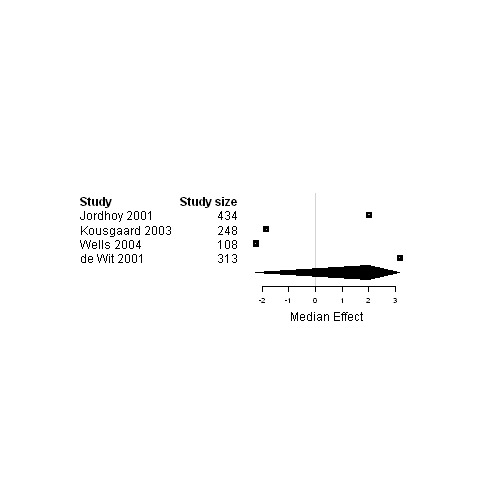
Forest plot for the quality of life of patients assigned to interventions using a shared care model versus usual care.
No difference was found when each subscale of the quality of life instruments was considered separately or when they were considered as a whole in comparisons by type of models of care (data not shown). Similarly, there was no difference when physical symptoms were considered either independently or as a whole within the physical functioning subclass in comparisons by type of models of care (data not shown).
Interventions using strategies other than those included in comparisons
Five strategies of intervention could not be pooled due to a limited number of studies included.
One study tested a telephone follow‐up (Beney 2002) 48 to 72 hours after hospital discharge for patients with cancer. No difference was found for the physical well‐being dimension of health‐related quality of life between patients who received the telephone follow‐up and those who did not (Beney 2002).
Two studies tested communication and case discussion between distant health professionals such as tele‐nursing (Bohnenkamp 2004) or email (McDonald 2005). Bohnenkamp et al. (Bohnenkamp 2004) reported that patients followed with tele‐nursing were more satisfied than patients followed with traditional home visits. McDonald et al. (McDonald 2005) found that patients assigned to a group receiving an email reminder with provider prompts, patient education material, and clinical nurse specialist outreach had significant improvement in ratings of worst pain intensity compared to patients assigned to the control group.
Two studies tested a change in medical record system that aimed to improve pain management, and they used bedside charting of pain level (Kravitz 1996) or a summary of pain assessment included in clinical charts (Trowbridge 1997). Kravitz et al. (Kravitz 1996) reported no significant difference in pain control, sleep, cancer‐related symptoms and analgesic dosing between the intervention group and the control group, whereas Trowbridge et al. (Trowbridge 1997) reported a significant change in prescription pattern and a significant reduction in the pain incidence in the intervention group.
A single study tested the distribution of a care protocol (Du Pen 1999) which consisted in a treatment algorithm for cancer pain management. This study showed that patients assigned to the pain algorithm group had a significant reduction in usual pain intensity compared to patients assigned to standard care, but no other significant difference was observed between the two groups for other symptoms nor quality of life outcomes (Du Pen 1999).
Two studies evaluated the coordination of assessments and treatment, the assessment being either of continuity (King 2009) or quality of life (Velikova 2004). The study by Velikova et al. (Velikova 2004) showed an improvement in health‐related quality of life for patients assigned to the intervention group whereas in the study by King et al. (King 2009), no significant difference was found between the intervention group and the control group.
Provider and informal carer outcome measures
A limited number of studies (N = 18) reported psychological health or satisfaction of providers or informal carers. They could not be regrouped to calculate median effect size estimates because of the high heterogeneity among studies.
Two studies reported an improvement in caregiver outcomes in the intervention group compared to the usual care group. The purpose of the study by Wells et al. (Wells 2003) was to determine if continued access to information following a baseline pain education program increased knowledge and positive beliefs about cancer pain management. In this study, a significant improvement in pain beliefs of the caregiver was found in the intervention group compared to the usual care group (Wells 2003). The study by Hughes et al. (Hughes 1992) assessed the cost‐effectiveness of a Veteran Affairs Hospital‐based Home Care Program for terminally ill patients with informal caregivers. A significant increase in caregiver satisfaction with care was found in the intervention group at one month follow‐up compared to the customary care (Hughes 1992).
Two studies reported significantly increased provider satisfaction in the intervention group compared to the standard care group. The study by de Wit et al. (de Wit 2001) examined the effectiveness of a Pain Education Program offered by nurses to cancer patients with chronic pain. District nurses of patients from the intervention group were significantly more satisfied with patient's pain treatment than nurses of patients from the usual care group (de Wit 2001). The study by Jefford et al. (Jefford 2008) assessed the impact of sending information to general practitioners about their patient's chemotherapy regimen. General practitioners assigned to the intervention group reported a significant increase in satisfaction and greater levels of confidence in treating those patients with chemotherapy adverse effects at follow‐up compared to general practitioners receiving the usual correspondence (Jefford 2008).
Process of care outcome measures
Accessibility to care and continuity of care
Patient satisfaction with service delivery, consultation and continuity of care was assessed with an instrument developed in the United Kingdom by the College of Health, in a study by Grunfeld et al. (Grunfeld 1996). This study aimed to evaluate the effect on patient satisfaction of transferring the primary responsibility for follow‐up of women with breast cancer in remission from hospital outpatient clinics to general practice. Patients assigned to the general practice group indicated greater satisfaction than did patients in the hospital group. Notably, more patients in the general practice group than in the hospital group could see the doctor on the same day for urgent problems and had enough time to discuss problems with their doctor. Furthermore, almost 90% of patients in the general practice group saw a doctor who knew them well at their follow‐up visit, compared to approximately 50% of patients in the hospital group. Lastly, there was a significant increase in the proportion of patients who were satisfied with continuity of care in the general practice group (Grunfeld 1996).
Place of death
Only four studies (Hughes 1992; Jordhoy 2001; Kane 1984; Moore 2002) reported the place of death of patients with cancer. The study by Hughes et al. (Hughes 1992) assessed the cost‐effectiveness of a Veteran Affairs Hospital‐based Home Care program for terminally ill patients. In the study by Kane et al. (Kane 1984) terminally ill cancer patients were randomly assigned to receive hospice care provided both in a special inpatient unit and at home or conventional care (Kane 1984). The study by Jordhoy et al. (Jordhoy 2001) evaluated a comprehensive palliative care program in patients who had incurable malignant disease and an expected survival of two to nine months. The study by Moore et al. (Moore 2002) assessed the effectiveness of a nurse‐led follow‐up in the management of patients with lung cancer who had completed their initial treatment and were expected to survive for at least three months. For two of these studies, deaths occurred significantly more frequently at home in the intervention group than in the control group (Jordhoy 2001; Moore 2002) whereas in one study, patients assigned to the intervention group spent 3.5 fewer days in the hospital prior to their death, compared to the control group patients (Hughes 1992). In the study by Kane et al. (Kane 1984), no significant difference was observed between the intervention and control groups in the number of deaths at home and at the hospital.
Other process of care outcome measures
Overall, very few studies reported process of care measures at baseline and during the follow‐up period. No pooled analysis was performed for process of care measures because of a high heterogeneity among studies, few process of care data available and context‐dependent measures. Only three studies reported a significant difference between the intervention and the control groups for process of care measures. The study by Hanks et al. (Hanks 2002) assessed the effectiveness of a hospital Palliative Care Team in the setting of a teaching hospital trust in England. In this study, the advice and support provided by a multidisciplinary specialist Palliative Care Team was compared with limited telephone advice. Within the study period, 48% of patients were discharged from hospital to their home. The patients in the complete Palliative Care Program received significantly more general practitioner visits during the period of time spent at home, compared to patients in the limited telephone advice group (Hanks 2002). The study by Jordhoy et al. (Jordhoy 2001) assessed the impact of comprehensive palliative care on patient's quality of life. The intervention was based on cooperation between a palliative medicine unit and the community services to enable patients to spend more time at home and to die there if they preferred. In that study, the time spent in nursing homes during the entire observation period and in the last month before death was less for patients in the intervention group, compared to those in the control group (Jordhoy 2001). The purpose of the study by Oleske et al. (Oleske 1988) was to determine the impact of modest changes in the home health system on patients with cancer. Patients from two certified home health agencies in two regions of Illinois (USA) were either assigned to oncology nurse specialists with continuing education on cancer, to continuing education on cancer alone or to observation only. The mean number of nurse visits to cancer patients during the two year duration of the study significantly declined in the groups who received the intervention and increased in the observation only group (Oleske 1988).
Descriptive analysis of single intervention on the improvement of patient health‐related outcomes
The significant improvements in one or more classes of patient health‐related outcomes are reported by type of model of care for all 51 studies included in this review.
Case management
Twelve out of the 20 studies assessing case management models of care (Table 2) reported significant improvements in one or more classes of patient health‐related outcomes, during the study follow‐up period.
Functional status
The study by Given et al. (Given 2002) was conducted in chemotherapy clinics of two comprehensive and two community cancer centres and evaluated a nursing intervention in patients undergoing an initial course of chemotherapy who reported pain and fatigue at baseline. Patients assigned to the intervention group reported a significant improvement in social functioning compared to those assigned to the usual care group (Given 2002). One study by McCorkle et al. (McCorkle 1989) assessed the effect of home nursing care for patients with progressive lung cancer. The home nursing care group had significantly less distress and greater independence six weeks longer compared to the office care group (McCorkle 1989). The study by Giesler et al. (Giesler 2005) evaluated a nurse‐driven cancer care intervention based on an interactive computer program to help patients with prostate carcinoma identify their quality of life related needs and provide education and support according to their identified needs. Patients who received the intervention experienced significant long‐term improvements in quality of life outcomes related to sexual functioning compared to patients who received standard care (Giesler 2005). The study by Moore et al. (Moore 2002) tested the effectiveness of a nurse‐led follow‐up in the management of patients with lung cancer who had completed their initial treatment and were expected to survive for at least three months. Patients assigned to the intervention group had significantly better scores for emotional functioning compared to those assigned to the conventional care group (Moore 2002).
Physical status
The study by Given et al. (Given 2002) evaluated a nursing intervention in patients undergoing an initial course of chemotherapy who reported pain and fatigue at baseline. Patients who received the intervention reported a significant reduction in physical role impact and symptom counts compared to those in the conventional care group (Given 2002). The study by McKegney et al. (McKegney 1981) evaluated a model of home visits by a nurse practitioner acting as an extension of a multidisciplinary team for patients with incurable cancer with an estimated prognosis of three months to one year. Patients who were visited at home by nurse practitioners had improved pain control over the last 90 days of life, compared to patients in the usual care group (McKegney 1981). The study by Schumacher et al. (Schumacher 2002) tested the effectiveness of the PRO‐SELF Pain Control Program in oncology outpatients with pain associated with bone metastasis. Patients in the PRO‐SELF group were: a) seen by specially trained intervention nurses and received a psycho‐educational intervention, b) taught how to use a pillbox, and c) given written instructions on how to communicate with their physician for unrelieved pain and the need to adjust their analgesic prescriptions. The PRO‐SELF group showed significant reductions in pain intensity (least, average and worst pain) from baseline compared to the standard care group (Schumacher 2002). The study by Goodwin et al. (Goodwin 2003) evaluated the effect of a nurse case management on the treatment of women aged 65 and older newly diagnosed with breast cancer. A significantly higher percentage of women assigned to the case manager group had normal arm function two months after surgery compared to women assigned to the control group (Goodwin 2003). The study by Moore et al. (Moore 2002) tested the effectiveness of a nurse‐led follow‐up in the management of patients with lung cancer who had completed their initial treatment and were expected to survive for at least three months. Patients assigned to the intervention group had significantly less severe dyspnoea and peripheral neuropathy compared to patients assigned to the conventional care group (Moore 2002).
Psychological status
The purpose of the study by McLachlan et al. (McLachlan 2001) was to determine the impact of using standardised questionnaires via a touch‐screen computer to make patient cancer related needs, quality of life and psychosocial information available to the healthcare team. There was a significant reduction in depression at six months of follow‐up for the subgroup of patients who were moderately or severely depressed at baseline in the intervention group compared to the control group (McLachlan 2001). The study by Ritz et al. (Ritz 2000) evaluated an advanced practice nurse intervention based on Brooten's cost‐quality model and the Oncology Nursing Society's standards of advanced practice in oncology nursing, in women diagnosed with breast cancer aged between 30 to 85 years. At one, three and six months after surgery, uncertainty was found to decrease significantly more from baseline in patients assigned to the intervention group compared to those assigned to the control group (Ritz 2000). The study by Giesler et al. (Giesler 2005) evaluated a nurse‐driven cancer care intervention based on an interactive computer program to help patients with prostate carcinoma identify their quality of life related needs and provide education and support according to their identified needs. Men who received the intervention had significant reductions in their cancer worries, such as anxiety about disease recurrence and treatment effectiveness compared to men who received conventional care (Giesler 2005). The study by Liu et al. (Liu 2006) investigated the role of continuing supportive care in reducing the perceived uncertainty among women younger than 60 years, newly diagnosed with breast cancer and undergoing surgery in Taiwan. Women assigned to the intervention group had significantly lower disease uncertainty at one month after surgery and three months after diagnosis compared to women assigned to the standard care group (Liu 2006). One study by McCorkle et al. (McCorkle 2009) evaluated specialised care by an advanced practice nurse in women with gynaecological cancers after their hospital discharge. Women assigned to the intervention group had significantly less uncertainty at six months post‐surgery than did women assigned to the control group (McCorkle 2009). The study by McArdle et al. (McArdle 1996) assessed the effect of support from a nurse specialist in breast cancer and a voluntary support organisation after surgery in patients with breast cancer. Patients supported by breast care nurses tended to have reduced psychological morbidity compared to those assigned to the other groups (McArdle 1996).
Social status, support and care needs
The study by Liu et al. (Liu 2006) investigated the role of continuing supportive care provided by a trained registered nurse for 3 months in increasing social support among women younger than 60 years, newly diagnosed with breast cancer and undergoing surgery in Taiwan. The nurse mainly provided information, emotional and psychological support, appropriate referral, and continual follow‐up. Patients in the intervention group reported higher support by family and friends as well as a significant increase in the overall social support and nurse/physician social support compared to patients assigned to the usual care group (Liu 2006).
Satisfaction
The study by Moore et al. (Moore 2002) tested the effectiveness of a nurse‐led follow‐up in the management of patients with lung cancer who had completed their initial treatment and were expected to survive for at least three months. Patients assigned to the intervention group reported significantly higher satisfaction with care at three, six and 12 months of follow‐up compared to patients assigned to the conventional care group (Moore 2002).
Shared care
Three out of the 14 studies testing a shared care model (Table 3) reported a significant improvement in two classes of patient health‐related outcomes during the study follow‐up period.
Physical status
The study by de Wit et al. (de Wit 2001) assessed the effectiveness of a Pain Education Program offered by nurses in cancer patients with chronic pain. Patients assigned to the intervention group without district nursing at home had a significant decrease in pain intensity compared to patients assigned to the control group without district nursing (de Wit 2001).
Satisfaction
In the study by Bonnema et al. (Bonnema 1998), 96% of patients assigned to the early hospital discharge after surgery for breast cancer were highly satisfied at one and four months after surgery and recommended short hospital stay (four days) (Bonnema 1998). One study by Grunfeld et al. (Grunfeld 1996) assessed the effect of transferring the primary responsibility for follow‐up of women with breast cancer in remission from hospital outpatient clinics to general practice. Women assigned to the general practice group were significantly more satisfied with service delivery and continuity of care at mid‐trial than women assigned to the hospital outpatient clinic group (Grunfeld 1996).
Interdisciplinary team
Three out of the five studies assessing interdisciplinary team models of care (Table 4) reported significant improvements in one or more classes of patient health‐related outcomes during the study follow‐up period.
Physical status
The study by Hanks et al. (Hanks 2002) evaluated the effectiveness of a hospital Palliative Care Team who provided advice and support to new inpatient referrals in the setting of a teaching hospital in England. Patients supported by the multidisciplinary specialist Palliative Care Team had significant improvements in scores of symptom severity at one and four weeks of follow‐up, compared to patients assigned to the control group (Hanks 2002).
Psychological status
The same study by Hanks et al. (Hanks 2002) showed that patients supported by the multidisciplinary specialist Palliative Care Team had a significantly better mood and were less bothered by emotional problems at one and four weeks of follow‐up, compared to patients assigned to the control group (Hanks 2002).
Quality of life
Similarly, the study by Hanks et al. (Hanks 2002) found that patients supported by the multidisciplinary specialist Palliative Care Team had significantly better quality of life scores at one and four weeks of follow‐up, compared to patients assigned to the control group (Hanks 2002).
Satisfaction
In the study by Kane et al. (Kane 1984), terminally ill cancer patients were randomly assigned to receive conventional care or hospice care in a special inpatient unit and at home, whereas terminally ill patients in the study by Hughes et al. (Hughes 1992) participated in a Veteran Affairs Hospital‐based Home Care program. Patients assigned to the intervention groups of these two studies testing an interdisciplinary team model of care had significantly higher satisfaction with care compared to patients assigned to the usual care groups.
Interventions not encompassed within the main models of care identified
Six out of ten studies assessing interventions that could not be encompassed within the main models of care identified in this review (Table 5) reported significant improvements in at least one class of patient health‐related outcomes during the study follow‐up period.
Patient‐held record
Physical status
The purpose of the study by Vallieres et al. (Vallieres 2006) was to determine the effectiveness of a multi‐component clinical intervention to reduce pain in outpatients with cancer. This intervention included an information session, the use of a pain diary, and the possibility to contact a physician to adjust the pain medication. After three weeks, the scores for average pain and worst pain experienced by patients assigned to the intervention group were significantly lower than those of patients in the usual care group (Vallieres 2006).
Implementation of a care protocol
Physical status
The study by Du Pen et al. (Du Pen 1999) evaluated the implementation of clinical guidelines for cancer pain management in the community setting. Patients assigned to the pain algorithm group had a significant reduction in usual pain intensity compared to the standard community practice (Du Pen 1999).
Communication technologies
Physical status
The study by McDonald et al. (McDonald 2005) tested the effectiveness of an email‐based intervention to increase home care nurses' adherence to pain assessment and management guidelines, and to improve patient outcomes. The basic intervention consisted of a one‐time email reminder highlighting six pain specific clinical recommendations, whereas the augmented intervention supplemented this initial email reminder with provider prompts, patient education material, and clinical nurse specialist outreach. Patients in the augmented home care group had significantly lower ratings of worst pain intensity, compared to patients in the basic intervention group (McDonald 2005).
Satisfaction
The study by Bohnenkamp et al. (Bohnenkamp 2004) evaluated the impact of home health with telenursing on patients discharged with ostomies resulting from cancer treatment. Patients followed with telenursing reported being more satisfied than patients followed with traditional home visits.
Changes in medical record system
Physical status
The study by Trowbridge et al. (Trowbridge 1997) tested the effectiveness of a clinical‐practice intervention to improve pain control in outpatients with cancer. The clinical chart of the intervention group contained a summary of the completed pain scales and oncologists who treated these patients were instructed to review the summary sheet prior to their evaluation. A significant reduction in the incidence of pain described as more intense than life's usual aches and pains was found in the intervention group, compared to the usual care group (Trowbridge 1997).
Assessments and feedbacks
Global quality of life
The study by Velikova et al. (Velikova 2004) examined the effects of regular collection and use of health‐related quality of life data in oncology practice on process of care and patients' well‐being. The intervention consisted in regular completion of the European Organization for Research and Treatment of Cancer‐Core Quality of Life Questionnaire version 3.0, and the Hospital Anxiety and Depression Scale on touch‐screen computers in the clinic and feedback of results to physicians. Patients in the intervention groups had significantly better health‐related quality of life scores than patients in the control group (Velikova 2004).
Discussion
Summary of main results
1. Overview of studies
Among the 51 included studies of interventions to improve continuity of care for patients with cancer, only one specified improvement in continuity of care as one of their objectives. The other studies were nevertheless included because the tested interventions had already been identified in the literature as solutions to discontinuity in health care, such as shared care and case management. The included studies had sample sizes ranging from 28 to 1388 participants at the time of randomisation; they were performed in various healthcare settings; they originated from nine countries; and their follow‐up periods ranged from five days to five years. The number of studies and the overall sample size of the present review constitute a solid foundation on which to build a preliminary picture of the types of interventions that could possibly improve continuity of care. However, a high heterogeneity among included studies, related to study designs, settings and outcome measures, limits the extent of the conclusions that can be drawn on the effectiveness of the various models of care aiming to improve continuity on patient, healthcare provider or process of care outcomes.
2. Description and classification of interventions
Throughout the search and identification of studies, an iterative process was undertaken to describe and classify the retrieved interventions, in order to sort out the interventions having a potential to improve continuity of care. The definition of continuity of care proposed by Reid et al (Reid 2002) was useful to guide this process: "how one patient experiences care over time, as coherent and linked; continuity being the result of good information flow, good interpersonal skills, and good coordination of care".
Three models of care used in the included studies showed some potential to improve continuity of care, namely case management, shared care, and interdisciplinary teams. Interestingly, these models have also been identified as promising for delivering survivorship care by the Institute of Medicine (Institute of Medicine 2006).
Case management was the care modality most consistently described as improving relational continuity for patients with cancer. Case management interventions also targeted management continuity in addition to relational continuity.
Most studies using a shared care modality included strategies to improve informational and management continuity.
In this review, five studies assessed an interdisciplinary team as a model of care in the follow‐up of patients with cancer and, as for shared care interventions, these interdisciplinary team interventions were mostly designed to improve informational and management continuity. Teamwork involving multiple disciplines is increasingly promoted in health care and interdisciplinary teams are particularly important in specialised health care, such as palliative care.
Six interventional strategies were used outside the models of care described above and could be identified as having some potential to improve the continuity of care per se. These strategies are (1) patient‐held record, (2) telephone follow‐up, (3) communication and case discussion between distant healthcare professionals, (4) change in medical record system, (5) care protocols, directives and guidelines, and (6) coordination of assessments and treatment.
Three of these strategies typically target healthcare professionals: the distribution of care protocols, directives and guidelines, the coordination of assessments and treatment, and patient‐held records. Patient‐held record can be described as a dynamic tool used bypatients and healthcare professionals, and it can either be a print‐out from the patient’s medical record or a general information sheet (Gysels 2007). Patient‐held record is not described as a type of intervention by the EPOC Group (EPOC), but it falls within patient‐mediated interventions. Interventions using this strategy (4 studies) were included only if the record was used by healthcare professionals meeting with the patient to improve communication and information exchange, and not by the patient alone (Gysels 2007).
Two strategies used to improve continuity were organisational interventions oriented towards professionals: telephone follow‐up, which lies within the category of "arrangement for follow‐up", and the implementation of communication technology, which allows communication and case discussion between distant health professionals. Communication technologies were only used in two instances among the included studies, but these types of interventions should be tested more often in the future because of their cost‐effectiveness.
One of the strategies used to improve continuity could be categorised as a structural intervention (EPOC): the intervention to change the medical record system. Changes in the medical record system were used in 25 of the included interventions, and were mainly designed to improve informational continuity, with few interventions also designed to improve management continuity.
This list of models of care or interventional strategies targeting an improvement in continuity of care is not exhaustive, but it provides a broad picture of some of the strategies tested so far. It is an attempt at identifying and describing which interventions might improve continuity of care and benefit to patients, healthcare providers or in the processes of care. Thus, it provides a preliminary framework to work with when new interventions are developed to improve continuity of care in the follow‐up of patients with cancer.
3. Effectiveness of interventions
The second objective of this review was to determine the effectiveness of tested interventions aiming to improve continuity of care onpatient, healthcare provider and process of care outcomes. Evidence is lacking for most of the studied outcomes, so we cannot conclude on which interventions are the most effective to improve continuity of care in patients with cancer. Continuity of care should not be considered as an aim in itself, but as one attribute of quality of care. The included studies did not directly assess continuity of care, but a variety of primary and secondary outcomes, mainly related to patient outcomes, such as physical and mental health. Our results show that such outcomes may not be the best measures to assess continuity of care in the follow‐up of patients with cancer. Indeed, in this population facing severe disabilities and ultimately death, even seamless care may not result in improved patient outcomes. Provider, informal caregiver, and process of care measures were examined in a limited number of studies and these outcomes also failed to provide strong evidence supporting the identification of the most effective interventions to improve continuity of care.
Thirty‐one of the 51 studies included in this review contributed to patient health data used in the analyses. Based on the median effect size estimates and the 95% BCI, no significant difference in patient health measures was found between the intervention and the control groups, in the analyses by type of continuity of care or by type of model of care. However, according to our descriptive analyses of single interventions on the improvement of patient health‐related outcomes, case management and interdisciplinary teams seemed to be the most favourable models of care to improve one or more classes of patient outcomes. However, an important heterogeneity between studies precludes any conclusion on the effectiveness of interventions included in this review.
Among the few studies reporting provider and informal caregiver outcomes, satisfaction was the outcome most often examined. Mental and physical health of informal caregivers were rarely reported. Outcomes related to health services are difficult to interpret since very few studies reported measures on process of care, and due to their specific context and setting, it is almost impossible to regroup them for analysis. In two of the four studies reporting place of death as an outcome, death occurred at home significantly more frequently in the intervention groups than in the control groups (Jordhoy 2001; Moore 2002). This is considered as a favourable outcome, since home is generally the preferred place of death by patients with cancer. Approximately two‐thirds of cancer patients prefer to die in their own home (Tang 2001). Also, in one study (Hughes 1992), time spent in the hospital before death was shorter in the intervention group compared to the control group. These studies with positive outcomes related to place of death included interventions that improved management and informational continuity, but they used different models of care, so it is difficult to conclude if one model of care is more effective than the other.
Continuity of care in the follow‐up of patients with cancer was directly measured in only one study (King 2009) where the authors developed an instrument based on the following theoretical framework: (1) experience of continuity by a patient is an outcome of service delivery that can be facilitated rather than provided by professionals; (2) patients should be enabled to take control of their continuity and it should be ensured that they feel supported and "in contact" with services between appointments; and (3) staff should receive feedback from patients on their experience of continuity, so that they can respond in whatever clinical manner is fitting with their knowledge, experience and training. In all other studies, continuity was rather inferred through the measurement of related concepts and it was not measured with an instrument intended to assess continuity of care. For example, Grunfeld and colleagues (Grunfeld 1996) evaluated patient satisfaction with service delivery, consultation and continuity of care using a 15‐statement instrument developed by the College of Medicine in the UK. Based on the present review, continuity of care is mainly considered as an intermediate outcome by study authors, as patient health outcomes were generally used as primary outcomes. However, as mentioned earlier, only few improvements in functioning, physical, psychological and social status of patients with cancer are possible when their health deteriorates over time. Therefore, we agree with previous reports (Reid 2002; Sparbel 2000b) that there is a need to develop and validate instruments to directly evaluate continuity of cancer care. This concept is affected by environmental influences, communication, patient, professional, and system factors (Sparbel 2000a). Some continuity measures are available, but most of them focused on only one disease, unrelated to cancer (Dolovich 2004; Gulliford 2006; Hadjistavropoulos 2008; Kowalyk 2004; Wei 2008). Recently, two instruments were developed to assess continuity of care in a comprehensive way, regardless of morbidity and across multiple care settings, the Continuity of Care Across Care Levels Questionnaire (CCAENA) (Letelier 2010) and the Nijmegen Continuity Questionnaire (NCQ) (Uijen 2011a; Uijen 2011b). Interestingly, the CCAENA may be helpful to identify specific elements of continuity of care in transitions across primary and secondary care, but it takes more than 30 minutes to be completed. The NCQ seems more practical since it can be completed in 5 to 10 minutes. It has been validated with patients diagnosed with various chronic diseases, but less than 5% of the study participants had cancer, so it may not completely capture the continuity of care experienced by cancer patients. Even though cancer is recognised as a chronic disease, perception of continuity of care may differ in patients with cancer compared to those with other conditions, such as diabetes or cardiovascular disease. There are important differences between cancer and these diseases regarding their evolution or the intensity of care needed at certain phases of the care trajectory (Murray 2005).
The heterogeneous results observed among the included studies are likely due to the complexity of interventions evaluated in this review, thus making it difficult to isolate the active components to improve continuity of care in patients with cancer. Furthermore, it was difficult to conclude on whether interventions were successfully implemented in clinical practice. Therefore, the results of this review do not allow us to draw firm conclusions regarding the effectiveness of interventions designed to improve continuity of care in the follow‐up of patients with cancer.
Overall completeness and applicability of evidence
Studies included in this review were published between 1981 and 2009, with only four studies published before 1992 and the majority of studies published between 2000 and 2009. Therefore, included studies are relevant to current clinical practice. However, almost all these studies were conducted in high‐income countries, including the United States of America, the United Kingdom, Australia and Canada. Thus, the available evidence from this review may be generalised to high‐income countries, but it may not apply to low‐ and middle‐income countries, since organization of care may differ in these countries.
The relatively large number of studies and participants and the diversity of settings included in this review should have warranted a solid profile of the impact of interventions designed to improve continuity of care, in the follow‐up of patients with cancer. However, disparities among studies made comparisons and analyses of study findings very difficult. More research is needed to clarify which specific components should be included in interventions to assure continuity of care in the follow‐up of patients with cancer. Understanding the implementation of interventions should also be the focus of future research, since very few of the included studies reported specific data on the implementation of the intervention and its uptake and sustainability over time in clinical practice.
Quality of the evidence
The GRADE system rates the quality of evidence and the strength of recommendations (Atkins 2004). In the context of a systematic review, the quality of evidence reflects the extent of confidence that an estimate of effect is correct (Guyatt 2008). We used the GRADE approach to assess the quality of the evidence in each of our analyses and rated all of them as "very low quality", due to inconsistent results and high heterogeneity among studies, especially regarding participants, interventions and outcomes. Thus, we decided not to present the results obtained using GRADE. This high heterogeneity is likely due to our broad inclusion criteria that led to a diversity of study designs, interventions, participants, patients' phases of care, measured outcomes, healthcare settings, and lengths of follow‐up.
Almost all studies included in this review were randomised controlled trials, except for two studies that used a controlled clinical trial design (Liu 2006; Luker 2000). However, several studies had major limitations, such as inadequate equence generation and/or allocation concealment. A small number of studies also failed to report some outcomes, whereas other studies failed to report baseline characteristics for patient, provider, and informal caregiver outcomes. In an effort to provide the most complete analysis and to include all available data, several authors were contacted for missing information on reported outcomes and risk of bias. Unfortunately, less than one‐third of the contacted authors provided the requested information.
Potential biases in the review process
Most studies included in this review did not explicitly aim to improve continuity of care. Consequently, some of the reported effects might be due to study procedures (e.g. type of intervention, data collection) designed for other purposes than improvement in continuity of care.
Assessment of continuity of care in the follow‐up of patients with cancer is complex and challenging. there is a lack of consensus on the definition and measurement of continuity. Therefore, the interpretation of these study findings is difficult and a consensus on the definitions of continuity of cancer care and of the various types of model of care is needed.
Several relating concepts are entangled with continuity of care, like transitional care or integrated care (Holland 2007; Uijen 2011b). Although our search strategy attempted to cover most concepts overlapping with continuity of care, omission of the keyword "coordination of care" in our search could be seen as a limitation. However, we included other related keywords, like "seamless care", "transmural care" and "collaborative care" which reduces the probability of having missed major trials corresponding to our inclusion criteria.
For this review, risk of bias was assessed without blinding to authorship or journal of publication.
Agreements and disagreements with other studies or reviews
A number of systematic reviews have already been undertaken to evaluate the effectiveness of interventions to improve cancer care. Some have focused on specific aspects of these patients' care, such as the reviews by Bruinvels et al. (Bruinvels 1994) and Collins et al. (Collins 2004), which evaluated how the intensity of follow‐up care influenced survival and psychological distress. Alvarez and Agra (Alvarez 2006) also reviewed an aspect of care not directly related to continuity: their study examined how primary care physician education could improve opioid prescription.
Other reviews covered broader subjects related to some aspects of continuity of care, such as the review by Cox and Wilson (Cox 2003) which found significant evidence that nurse‐led telephone follow‐up satisfied patients' need for psychological support and information. Thus, this review only covered relational continuity aspects of cancer care (Cox 2003), without considering informational or management continuity aspects, which are also essential for a cross‐boundary type of care, such as cancer care. Gysels et al. (Gysels 2007) reviewed studies evaluating the impact of a patient‐held record as a means to improve informational continuity aspects of care for patients with cancer. They found no evidence that such an instrument improved patient outcomes nor communication and information exchange amongst healthcare practitioners.
Two reviews covered relational, informational and management continuity of care for patients with cancer, but either they limited their search to some types of interventions, or they included studies on patients with conditions other than cancer. Sussman et al. (Sussman 2004) evaluated the effectiveness of case management interventions to improve care coordination and patient outcomes. They found considerable heterogeneity in the results, which they attributed to the various case management models tested, and concluded that case management interventions seemed only effective for newly diagnosed patients. O'Hare et al. (O'Hare 1993) covered a wider array of interventions aiming to improve discharge planning for many types of participants, including patients with cancer. They found fourteen experimental studies that covered early discharge programs, comprehensive discharge protocols, patient education programs and home care, but only eight among these had been designed for cancer patients. These authors were able to synthesize their findings in a list of recommended strategies, such as the need for a multifaceted approach and for communication linkage across agencies to improve continuity of care.
Similar to this review, all preceding published reviews were unable to gather sufficient evidence to recommend specific interventions or strategies to improve continuity of care. With its broad scope, the present review adds a more comprehensive scheme for analysing pertinent interventions in the future.
Authors' conclusions
Implications for practice.
Cancer is a complex disease characterised by diverse clinical features, treatment phases and outcomes. In its nature, cancer care is thus fragmented. The present review sheds a perspective that encompasses all aspects of cancer care, and although it does not address any specific discipline, it could help all healthcare providers of patients with cancer to consider the overall care trajectory of their patients in their day‐to‐day care. Owing to its broad scope, the present review proposes a comprehensive set of strategies and interventions to improve continuity, which cover most types of healthcare settings, numerous types of healthcare providers, and patients in all phases of their cancer care trajectory. Although we could not find enough evidence on the effectiveness of these strategies, the descriptions that we provide herein will be useful to all stakeholders, either to implement new strategies in their care settings, or to add new components inspired by the presented interventions to improve strategies that already exist in their setting. Also, by identifying a few measures and proxy measures of continuity of care, we propose targets that can be used by care providers, administrators of healthcare settings and policy makers to improve cancer care in their institutions.
Implications for research.
The meta‐analysis method used in this review was useful to pool studies with diverse outcomes. By providing bootstrap confidence intervals around the calculated medians, this approach should allow us to draw clearer conclusions on the effectiveness of complex interventions to improve healthcare.
However, the results of this review do not allow us to conclude on which interventions are the most effective to improve continuity of care in the follow‐up of patients with cancer. Critical issues need to be addressed to improve the delivery of healthcare and continuity of care in patients with cancer. First, future research must address the specific needs of cancer patients and their providers all along the different phases of their cancer care trajectory. Evaluation of the effectiveness of interventions to improve continuity of care in patients with cancer should involve measurement of both patient and process of care outcomes and it should ideally involve validated instruments to directly measure continuity of care. Therefore, we suggest the following objectives for future research:
to describe any new intervention developed to improve continuity of care for patients with cancer, using the two classifications proposed in the present review, first on the type of continuity and second on the model of care and intervention strategy;
to evaluate which patient, provider and process of care outcomes are the most sensitive to change and the most meaningful regarding continuity of care;
to develop and validate instruments specifically designed to measure continuity of cancer care.
Acknowledgements
This study was funded by the Canadian Institutes of Health Research (KRS‐94302). We would like to acknowledge the help of Ms. Nadine Tremblay and Ms. Marie Fortier in data collection. We would also like to thank all authors who responded to our enquiries about their study and the editorial staff of the Cochrane Effective Practive and Organisation of Care Group.
Appendices
Appendix 1. Search strategy ‐ CINAHL database
MH "Palliative Care"
MH "Terminal Care"
MH "Neoplasms+"
MH "Oncology"
MH "Oncology Care Units"
AB"Neoplasms" OR TI"Neoplasms" OR AB"Palliative" OR TI"Palliative" OR AB"Oncolog*" OR TI"Oncolog*" OR AB"Cancer" OR TI"Cancer"
OR/1‐6
MH "Continuity of Patient Care+"
MH "Case Management "
MH "Case Managers"
MH "Nurse Liaison"
MH "Multidisciplinary Care Team"
MH "Patient Care Plans"
MH "Patient Discharge"
MH "Health Care Delivery, Integrated"
MH "Shared Services, Health Care"
TI"continuity" OR AB"continuity" OR TI"Care continuum" OR AB"Care continuum" OR TI"Collaborative practice*" OR AB"Collaborative practice*" OR TI"Collaborative care" OR AB"Collaborative care" OR TI"multidisciplinary team" OR AB"multidisciplinary team" OR TI"interdisciplinary team" OR B"interdisciplinary team" OR TI"interdisciplinary care" OR AB"interdisciplinary care" OR AB"Patient Care Team" OR TI"Patient Care Team" OR TI"Team care" OR AB"Team care" OR TI"Shared care" OR AB"Shared care" OR TI"Shared services" OR AB"Shared services" OR TI"shared notes" OR AB"shared notes" OR TI"Transmural care" OR AB"Transmural care" OR TI"Integrated care" OR AB"Integrated care" OR TI"Service* integration" OR AB"Service* integration" OR TI"Patient‐held record" OR AB"Patient‐held record" OR TI"Discharge planning" OR AB"Discharge planning" OR AB"Patient Discharge" OR TI"Patient Discharge" OR AB"hospital discharge" OR TI"hospital discharge" OR TI"discharg* plan*" OR AB"discharg* plan*" OR TI"Case management" OR AB"Case management" OR TI"Liaison nurse" OR AB"Liaison nurse" OR AB"Nurse‐led follow‐up" OR TI"Nurse‐led follow‐up" OR AB"Cooperative Behavior" OR TI"Cooperative Behavior" OR TI"Patient care planning" OR AB"Patient care planning" OR TI"Multi agency working" OR AB"Multi agency working" OR TI"Seamless care" OR AB"Seamless care" OR TI"Inter agency working" OR AB"Inter agency working" OR TI"Multi professional working" OR AB"Multi professional working" OR TI"Interprofessional working" OR AB"Interprofessional working" OR TI"care management" OR AB"care management"
OR/8‐17
MH "Clinical Trials+"
TX control*
TX random*
MH "Comparative Studies"
TX experiment*
TX "time W4 series"
TX impact
TX intervention*
TX evaluat*
TX effect*
MH "Pretest‐Posttest Design+"
MH "Quasi‐Experimental Studies+"
OR/19‐30
MH"Adult+" OR TI"Adult*" OR AB"Adult*"
AB"Child*" OR TI"child*" OR MH"Child+"
32 NOT (32 AND 33)
7 and 18 and 31 and 34
Appendix 2. Search strategy ‐ EMBASE database
neoplasm/exp/mj
"palliative therapy"/exp/mj
oncology/exp/mj
Neoplasm*:tiab OR "Palliative Care":tiab OR "Oncologic Nursing":tiab OR "Oncology Service":tiab OR "Oncology Services":tiab OR "Cancer":tiab
or/1‐4
("patient care planning"/mj OR "patient care planning":ti,ab)
("case management"/exp/mj OR "case management":ti,ab)
("patient referral"/exp/mj OR "patient referral":ti,ab)
("hospital discharge"/exp/mj OR "hospital discharge":ti,ab)
("integrated health care system"/exp/mj OR "integrated *2 care":ti,ab)
("cooperation"/exp/mj OR "cooperation":ti,ab)
("interdisciplinary communication"/exp OR "interdisciplinary communication": ti,ab)
"health care management"/de OR "health care management": ti,ab OR "health care planning"/de OR "health care planning":ti,ab OR "Cooperative Behavior":ti,ab OR "Community Health Planning":ti,ab OR "Integrated Care":ti,ab OR "Service integration":ti,ab OR "Services integration":ti,ab OR "Professional‐Patient Relations":ti,ab OR "Patient‐centered care":ti,ab OR "Professional‐Family Relations":ti,ab OR "continuity *4 care":ab,ti OR "Care continuity" :ti,ab OR "Continuum of care":ti,ab OR "Care continuum":ti,ab OR "Interpersonal continuity":ti,ab OR "Discharge planning" :ti,ab OR "Patient Discharge":ti,ab OR "discharge plan":ti,ab OR "discharge plans":ti,ab OR "Patient‐held record" :ti,ab OR "Shared care":ti,ab OR "Shared service":ti,ab OR "Shared services":ti,ab OR "shared notes":ti,ab OR "Transmural care":ti,ab OR "Collaborative practice":ti,ab OR "Collaborative practices":ti,ab OR "Collaborative care":ti,ab OR "Nurse‐led follow‐up":ti,ab OR "Liaison nurse":ti,ab OR "Liaison nurses":ti,ab OR "telephone follow‐up":ti,ab OR "Interdisciplinary care":ti,ab OR "Interdisciplinary team":ti,ab OR "Interdisciplinary teams":ti,ab OR "Multi professional working":ti,ab OR "Interprofessional working":ti,ab OR "Interprofessional Relations":ti,ab OR "multidisciplinary *2 team":ti,ab OR "Patient Care Team":ti,ab OR "Team care":ti,ab OR "Multi agency working":ti,ab OR "Inter agency working":ti,ab OR "Seamless care":ti,ab OR "Care management":ti,ab
or/6‐13
"Randomized controlled trial"/de
(random*:ti,ab)
(experiment*:ti,ab)
("time adj series":ti,ab)
(pre test or pretest or post test or posttest):ti,ab
impact:ti,ab
intervention*:ti,ab
chang*:ti,ab
evaluat*:ti,ab
effect?:ti,ab
compar*:ti,ab
control*:ti,ab
or/15‐26
Nonhuman/de
27 not 28
5 and 14 and 29
Appendix 3. Search strategy ‐ PsycINFO
("neoplasms" or "benign neoplasms" or "breast neoplasms" or "endocrine neoplasms" or "leukemias" or "nervous system neoplasms" or "brain neoplasms" or "terminal cancer"):Index Terms
(Oncolog* OR "Palliative care" OR Cancer OR Carcinoma* OR Neoplasm* OR Sarcoma* OR Tumor*): Title
(Oncolog* OR "Palliative care" OR Cancer OR Carcinoma* OR Neoplasm* OR Sarcoma* OR Tumor*): Abstract
#1 OR #2 OR #3
("cooperation" or "collaboration" or "aftercare" or "case management" or "continuum of care" or "discharge planning" or "integrated services" or "interdisciplinary treatment approach" or "partial hospitalization"):Index Terms
("continuity of patient care" OR "continuity of care" OR "Care continuum" OR "Continuum of care" OR "Care continuity" OR "Interpersonal continuity" OR "Collaborative practice*" OR "Collaborative care" OR "multidisciplinary team" OR "interdisciplinary team" OR "Patient Care Team" OR "Team care" OR "interdisciplinary care" OR "Shared care" OR "Shared service*" OR "shared notes" OR "Integrated care" OR "Service* integration" OR "Patient‐held record" OR "Liaison nurse" OR "Nurse‐led follow‐up" OR "telephone follow‐up" OR "Discharge planning" OR "Patient care planning" OR "Patient Discharge" OR "hospital discharge" OR "discharg* plan*" OR "Case management" OR "Transmural care" OR "Patient‐held record" OR "Multi agency working" OR "Seamless care" OR "Inter agency working" OR "Multi professional working" OR "Interprofessional working" OR "care management"): Abstract
("continuity of patient care" OR "continuity of care" OR "Care continuum" OR "Continuum of care" OR "Care continuity" OR "Interpersonal continuity" OR "Collaborative practice*" OR "Collaborative care" OR "multidisciplinary team" OR "interdisciplinary team" OR "Patient Care Team" OR "Team care" OR "interdisciplinary care" OR "Shared care" OR "Shared service*" OR "shared notes" OR "Integrated care" OR "Service* integration" OR "Patient‐held record" OR "Liaison nurse" OR "Nurse‐led follow‐up" OR "telephone follow‐up" OR "Discharge planning" OR "Patient care planning" OR "Patient Discharge" OR "hospital discharge" OR "discharg* plan*" OR "Case management" OR "Transmural care" OR "Patient‐held record" OR "Multi agency working" OR "Seamless care" OR "Inter agency working" OR "Multi professional working" OR "Interprofessional working" OR "care management"): Title
#5 OR #6 OR #7
("experimental design" or "between groups design" or "clinical trials" or "cohort analysis" or "followup studies" or "hypothesis testing" or "null hypothesis testing" or "longitudinal studies" or "prospective studies" or "repeated measures" or "experimental methods" or "quasi experimental methods" or "posttesting" or "pretesting" or "program evaluation" or "educational program evaluation" or "quasi experimental methods" or "random sampling"): Index Terms
(pre test or pretest or post test or posttest OR random* OR control* OR intervention* OR evaluat* OR random* OR experiment* OR impact OR effect*): Title
(pre test or pretest or post test or posttest OR random* OR control* OR intervention* OR evaluat* OR random* OR experiment* OR impact OR effect*): Abstract
#9 OR #10 OR #11
#4 AND #8 AND #12
Appendix 4. Search strategy ‐ Cochrane Central Register of Controlled Trials (CENTRAL)
"Neoplasms"[Mesh] OR "Palliative Care"[Mesh] OR "Medical Oncology"[Mesh] OR "Oncologic Nursing"[Mesh] OR "Oncology Service, Hospital"[Mesh] OR "Cancer Care Facilities"[Mesh] or (Neoplasms OR "Palliative Care" OR "Oncologic Nursing" OR "Oncology Service" OR "Oncology Services" OR "Cancer"):ti,ab,kw in Cochrane Reviews, Other Reviews and Clinical Trials
"Cooperative Behavior"[Mesh] OR "Patient Care Team"[Mesh] OR "Continuity of Patient Care"[Mesh] OR "Case Management"[Mesh] OR "Patient Discharge"[Mesh] OR "Patient Care Planning"[MeSH] OR "Community Health Planning/organization and administration"[Mesh] OR "Delivery of Health Care, Integrated"[Mesh] OR "Professional‐Patient Relations"[MeSH] OR "Interprofessional Relations"[MeSH] OR "Patient‐centered care"[Mesh] OR "Professional‐Family Relations"[MeSH] or "Continuity of patient care" OR "Continuity of care" OR "Care continuity" OR "Continuum of care" OR "Care continuum" OR "Interpersonal continuity" OR "Discharge planning" OR "Patient Discharge" OR "hospital discharge" OR "Discharging plan" OR "Discharging plans" OR "Patient‐held record" OR "Shared care" OR "Shared service" OR "Shared services" OR "Shared notes" OR "Case management" OR "Liaison nurse*" OR "Transmural care" OR "Collaborative practice" OR "Collaborative practices" OR "Collaborative care" OR "Nurse‐led follow‐up" OR "telephone follow‐up" OR "Interdisciplinary care" OR "Interdisciplinary team*" OR "Service integration" OR "Services integration" OR "Integrated care" OR "Patient Care Team" or "Team care" OR "Patient care planning" OR "Multi agency working" OR "Seamless care" OR "Inter agency working" OR "Multi professional working" OR "Interprofessional working" OR "care management":ti,ab,kw in Cochrane Reviews, Other Reviews and Clinical Trials
"Randomized controlled trial":pt or "Random*" OR "Control*" OR "Intervention*" OR "Evaluat*" in Cochrane Reviews, Other Reviews and Clinical Trials
"Animals"[Mesh] not "Animals"[Mesh] and "Humans"[Mesh] in Cochrane Reviews, Other Reviews and Clinical Trials
(( #1 AND #2 AND #3 ) AND NOT #4)
Appendix 5. Search strategy ‐ EPOC register
{after care} OR {follow‐up} OR {aftercare} OR {continuity} OR {continuous} OR {continuum} OR {following} OR {continuing}OR {community} OR {home} OR {after‐treatment*} OR {post‐treatment*}
Appendix 6. Search strategy ‐ PubMed
Neoplasms [Mesh]
"Palliative Care" [Mesh]
"Medical Oncology" [Mesh]
"Oncologic Nursing" [Mesh]
"Oncology Service, Hospital" [Mesh]
"Cancer Care Facilities" [Mesh]
Neoplasms[tiab] OR "Palliative Care"[tiab] OR "Oncologic Nursing" [tiab] OR "Oncology Service" [tiab] OR "Oncology Services" [tiab] OR Cancer [tiab]
#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7
"Cooperative Behavior" [Mesh]
"Patient Care Team" [Mesh]
"Continuity of Patient Care" [Mesh]
"Case Management" [Mesh]
"Patient Discharge" [Mesh]
"Patient Care Planning" [MeSH:NoExp]
"Community Health Planning/organization and administration" [Mesh]
"Delivery of Health Care, Integrated" [Mesh:NoExp]
"Professional‐Patient Relations"[MeSH]
"Interprofessional Relations"[MeSH]
"Patient‐centered care"[Mesh]
"Professional‐Family Relations"[MeSH]
"Continuity of patient care" [tiab] OR "Continuity of care" [tiab] OR "Care continuity" [tiab] OR "Continuum of care" [tiab] OR "Care continuum" [tiab] OR "Interpersonal continuity" [tiab]
"Discharge planning" [tiab] OR "Patient Discharge"[tiab] OR "Hospital discharge"[tiab] OR "Discharging plan"[tiab] OR "Discharging plans"[tiab]
"Patient‐held record"[tiab]
"Shared care" [tiab] OR "Shared service"[tiab] OR "Shared services"[tiab] OR "Shared notes" [tiab]
"Case management" [tiab]
"Liaison nurse*"[tiab]
"Transmural care"[tiab]
"Collaborative practice"[tiab] OR "Collaborative practices"[tiab] OR "Collaborative care"[tiab]
"Nurse‐led follow‐up" [tiab] OR "telephone follow‐up" [tiab]
"Interdisciplinary care" [tiab] OR "Interdisciplinary team*" [tiab]
"Service integration"[tiab] OR "Services integration"[tiab] OR "Integrated care" [tiab]
"Patient Care Team" [tiab] or "Team care" [tiab]
"Patient care planning" [tiab]
"Multi agency working" [tiab] OR "Seamless care" [tiab] OR "Inter agency working" [tiab] OR "Multi professional working" [tiab] OR "Interprofessional working" [tiab] OR "care management" [tiab]
#9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34
Randomized controlled trial [PT]
Random* [TW]
Control* [TW]
Intervention* [TW]
Evaluat* [TW]
#36 OR #37 OR #38 OR #39 OR #40
Animals [Mesh]
Humans [Mesh]
#42 not (#42 and #43)
(#8 and #35 and #41) not #4
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Addington‐Hall 1992.
| Methods | Cluster‐RCT; Unit of allocation: General practice; Stratified by: Number of GP partners in the practice, postal district | |
| Participants | Cancer patients expected to live for less than a year Setting / country: Inner London health district / UK Type of cancer: Any type Phase of care: Palliative care Sample size at randomisation: 554 |
|
| Interventions | Community‐based nurse coordinator: The coordinators' role was to ensure that patients received appropriate and well coordinated services, tailored to their individual needs and circumstances. They introduced themselves to patients as nurses who provided a link between the hospital, the general practitioner, and community services. They acted as "brokers" of services and their role was to assess need for services from agencies in the NHS, local authority, and voluntary sector; to offer advice on how to obtain these services and to contact the agencies themselves if necessary; to ensure that services were provided and well coordinated; and to stay in regular contact to monitor the changing needs of the patient and family for services. Patients were encouraged to contact the coordinators if they needed help or advice. The coordinators did not themselves provide practical nursing care, specialist palliative care advice, or counselling; instead they liaised with district nurses and hospice or Macmillan nurses, as appropriate, when patients required this type of support. Control: Routinely available services. |
|
| Outcomes | Patient: Symptoms, QoL, anxiety and depression (distress), satisfaction with social support, survival Informal carer: Anxiety and depression Process: Use of services, medication use, sources of help |
|
| Notes | Length of follow‐up: Until death (min. 0.5; max. 27) months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote from reference #1: "general practices in the district were randomly allocated to the coordination or the control group, stratified by number of partners and postal district." |
| Allocation concealment (selection bias) | Unclear risk | See quote above. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote from reference #1: "Independent interviewers, who were not informed which group the patients were in, interviewed patients at home on entry to the trial (baseline interview) and at intervals ranging from two weeks to six months until death or the end of the trial (follow up interviews)." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote from reference #1: "Information from the interview nearest death (or nearest the end of the study, for patients alive at this point) and from the interview after bereavement were used to measure the effects of the coordinating service on patients and families." Comment: There was a low retention rate in the 2 groups (coordination = 33%; control = 42%). Missing outcomes were not perfectly balanced in the 2 groups, with more deaths in the coordination group (147) compared to the control (98). Potentially inappropriate application of simple imputation: last observation carried forward. |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes in the methods section are reported in the results section. |
| Other bias | Low risk | Quotes from reference #1: "By September 1987 it was apparent that too few patients were entering the coordination group to keep the nurse coordinators fully employed. Thirteen randomly selected control group practices were therefore transferred to the coordination group. This change in randomisation has been allowed for in the analysis." Comment: The selection of patients to be switched from one group to the other was apparently performed at random. |
| Baseline outcomes similar? | Low risk | Quote from reference #1: "Scores at the baseline interview were controlled for with the Mantel‐Haenszel test for categorical data and regression analysis for interval data." Comment: No results at baseline are presented, but the authors mention that there was some imbalance. An appropriate analysis was performed to adjust outcomes for baseline imbalance. |
| Baseline characteristics similar? | Low risk | See Table 1. Comment: No characteristics are presented but differences appear to be non‐significant. |
| Protected against contamination? | Low risk | Quote from reference #1: "To prevent the contamination that could occur if patients of the same general practice had been allocated to different groups, general practices in the district were randomly allocated to the coordination or the control group, stratified by number of partners and postal district." Comment: Allocation was performed by practice. |
Beney 2002.
| Methods | RCT; Unit of allocation: Patient | |
| Participants | Patients having haematological or solid tumour malignancies, receiving chemotherapy and discharged home after completing a chemotherapy cycle. Setting / country: Public teaching hospital (University of California San Francisco) / USA Type of cancer: Any type Phase of care: Discharge, surveillance Sample size at randomisation: 150 |
|
| Interventions | Telephone follow‐up: A comprehensive and detailed operations manual reinforcing standardised study methodology, procedures, and data collection forms was created and distributed to each clinical pharmacist. Patients received a telephone follow‐up call from the clinical pharmacist 48 to 72 hours after hospital discharge. Information was solicited regarding drug‐related and non‐drug‐related problems. Problems were addressed, and advice and support were given. Drug‐related questions addressed concerns about access to drugs and adverse drug events. Adequate understanding about and adherence to drug regimens were assessed. When appropriate, patients were given advice, support, and reinforcement of education provided at the time of discharge. Non‐drug‐related problems were triaged, and appropriate follow‐up recommended. Control: No telephone follow‐up after discharge from the oncology service. |
|
| Outcomes | Patient: QoL, symptoms including psychological status (distress) | |
| Notes | Length of follow‐up: 0.25 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "After the patient was discharged, the clinical pharmacist obtained a subject number and study assignment from the investigational drug pharmacist. Patients were randomised using a spreadsheet with a block size of four. This approach guaranteed that patient allocation would not influence the discharge process." Quote from the author e‐mail: "The allocation sequence was generated electronically (bloc of 4) in the centralised pharmacy." |
| Allocation concealment (selection bias) | Low risk | Quote: "After the patient was discharged, the clinical pharmacist obtained a subject number and study assignment from the investigational drug pharmacist." Comment: The investigator was the person responsible for giving assignment. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Comment: The first questionnaire seems to have been administered in person (no mention of blinding of outcome assessors), whereas it was mailed at follow‐up (patients could not have been blinded for follow‐up measures). |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "A total of 161 patients were randomised; of these, 11 were excluded after randomisation because of protocol violations. Of the remaining 150 patients, 76 were assigned to the call‐back group and 74 to the control group, who received no telephone follow‐up. In the control group, 17 patients did not return the survey; therefore, 57 control patients were included in the analyses. Of the 76 patients assigned to call back, three could not be reached after three attempts, two were not called because of logistical problems, and one refused further discussion. We used an intent‐to‐treat approach and therefore included patients assigned to the call‐back group who did not actually receive telephone follow‐up. Of the 76 patients in the call‐back group, 10 did not return the survey; therefore, 66 call‐back patients were included in the analyses." Comment: There were more missing outcomes in the intervention group (77% retention) than in the control group (87% retention). Reasons for attrition are not reported. The statistical analysis only included patients that completed follow‐up (no imputation was used). It is impossible to judge if missing data could have an impact on the effect estimate because the type of variability measures presented in the Table 2 was not specified. |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes in the methods section are reported in the results section. |
| Other bias | Low risk | No evidence of other bias. |
| Baseline outcomes similar? | Low risk | Comment: No significant difference at baseline in primary and secondary outcomes between groups (Table 2). |
| Baseline characteristics similar? | Low risk | Comment: No significant difference in baseline characteristics between the 2 groups at baseline (Table 1). |
| Protected against contamination? | Low risk | It is unlikely that the clinical pharmacists provided the call‐back intervention to patients allocated to the control group since a detailed manual on methodology and procedures was provided to them. |
Bohnenkamp 2004.
| Methods | RCT; Unit of allocation: Patient | |
| Participants | Patients with ostomies resulting from treatment for a cancer diagnosis and discharged from hospital after surgery. Setting / country: Large tertiary care centre (South Western university teaching hospital) / USA Type of cancer: Bladder, colorectal, cervical / ovarian Phase of care: Discharge, surveillance Sample size at randomisation: 28 |
|
| Interventions | Home health visits plus tele‐nursing contact: Tele‐nursing aims to improve patient access to nurses who specialise in ostomy care. Nurses who specialise in ostomy care are extremely important to the continuum of care for patients with ostomies and their families. These nurses determine the proper equipment, educate the patient and family member, and provide supportive counselling. These activities cannot be accomplished only during hospital stay and the specialised nurses are integral to patients' follow‐up care and education. The telenursing group received twice weekly contacts by an ostomy clinical nurse specialist until patients or family members were competent with the care of the ostomy. All patients in this group were supplied with a home health 8" x 8" monitor and equipment for connecting to a television. Instructions and a demonstration regarding the equipment were done with the patient and family either prior to discharge or in their home after discharge, whichever was easier for the patient. Patients in this group had home health nursing visits as per routine plus twice‐weekly telenursing visits. Control: Traditional home health visits only: this group received home health visitation by a nurse who continued evaluation and education according to current management protocols. The enterostomal therapy nurse was available for consultation as needed. |
|
| Outcomes | Patient: Delay to independence with pouch change, readjustment after having an ostomy Process: Number of home health visits, number of tele‐nursing contacts, amount of supplies used |
|
| Notes | Length of follow‐up: 3 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "After discharge from the hospital, patients were assigned to one of two groups: (a) home health visits only or (b) home health plus tele‐nursing contact." Quote from author email message: "The patients were randomised using the flip of the coin process". |
| Allocation concealment (selection bias) | Unclear risk | Quote from author email‐message: " The study was not blinded. It is hard to conceal if a patient received telemedicine or not." Comment: The author did not get exactly what was meant by allocation concealment. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Comment: Only a single subjective outcome (readjustment after having an ostomy) was assessed using a postal survey. Since the patients could not be blinded and the patients were the assessors, than assessors could not have been blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no missing data. |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes in the methods section are reported in the results section. |
| Other bias | Low risk | No evidence of other bias. |
| Baseline characteristics similar? | Low risk | Quote: "No statistically significant differences were evident between the two groups." Comment: This quote relates to patients demographics (Table 1). Quote: "No statistically significant differences existed between the two groups on type of cancer, LOS, or support source." |
| Protected against contamination? | Low risk | Because of the nature of the intervention (very heavy organization), it is unlikely that control group participants received intervention. |
Bonnema 1998.
| Methods | RCT; Unit of allocation: Patient | |
| Participants | Women with operable breast cancer (stage I or II) eligible for modified radical mastectomy or lumpectomy with axillary dissection. Setting / country: General hospital and cancer clinic at a university hospital in Rotterdam / Netherlands Type of cancer: Breast Phase of care: Discharge, Surveillance Sample size at randomisation: 139 |
|
| Interventions | An early discharge protocol was developed to guarantee continuity of care. Discharge was performed on the morning of day 4 after surgery. The protocol included structured patient education provided by the breast cancer nurse and also available in written form, referral to a community health nurse, provision of an emergency telephone number, the scheduling of follow‐up visits, and an information letter being sent to the general practitioner. Drain removal was performed at home or in the outpatient clinic. Control: Long stay after surgery: usually 9‐12 days. Drain was removed before discharge. |
|
| Outcomes | Patient: Complications, uncertainty, state and trait anxiety, object anxiety, loneliness, depression, sleep disturbance, feeling of loss of control, self‐esteem, cancer locus of control, symptoms, communication about the disease Process: Length of hospital stay |
|
| Notes | Length of follow‐up: 4 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A randomisation list was prepared by the statistician (PIMS) using a program for the generation of random numbers and assignment into two groups with a prespecified size of blocks. The size of the blocks (8 patients) was not known by the investigators, and no stratification was applied. The randomisation list was accessible only to the data managers of the central trial office at the Daniel den Hoed Cancer Center. The patient was informed of her diagnosis, treatment plan, and the design of the study by her surgeon. The patient's home situation was subsequently assessed by a breast cancer nurse. Surgeons telephoned the trial office to discover each eligible patient's randomisation before admission." |
| Allocation concealment (selection bias) | Low risk | See quote first item. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Quotes: "Clinical study end points were recorded in the diaries and patients' files by the doctors and nurses." "Three questionnaires were used to assess psychosocial variables and record demographic characteristics. The first was distributed at admission and completed the same day; the second questionnaire was distributed 1 month after surgery, and the third 3 months later, during outpatient visits." Comment: Complications were recorded by nurses and doctors who could not have been blinded to patient experimental group. Psychosocial variable seem to have been collected via self‐report questionnaires. Because the patient could not have been blinded then the assessors could not be blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: There are some missing data in the Table 1 presenting complications, with only results from 120 of the initial 125 patients presented. No details are given on numbers allocated in each groups or in number or reasons for loss of follow‐up. |
| Selective reporting (reporting bias) | Low risk | All outcomes in the methods section are reported in the results section. |
| Other bias | Low risk | No evidence of other bias. |
| Baseline outcomes similar? | High risk | Quote: "Before surgery short stay patients scored higher than long stay patients on scales measuring depression (score 10.3 v 8.9, P = 0.03; minimum score 6, maximum score 24)."
"A shorter stay in hospital seemed to influence the extent to which the disease could be discussed within the patient's family. Before surgery there were no differences between the two groups, but at 1 and 4 months after surgery short stay patients were more likely to discuss their disease with their family ..." Comment: Score "NO" for Depression Score "UNCLEAR" for all other psychosocial variables. Score "Yes" for Communication about the disease and Complication and use of hospital services could not possibly be assessed at baseline. |
| Baseline characteristics similar? | Low risk | Quote: "The two groups were comparable in tumour stage, type of treatment, age, marital status, family income, and educational level (data available on the internet at www.bmj.com)." |
| Protected against contamination? | Low risk | Quote from ref #1: "Women randomised to short stay treatment were discharged on the morning of the fourth day after surgery, with the axillary drain in situ. Women randomised to long stay treatment were discharged after their drain had been removed." Comment: Because of the nature of the intervention, it is impossible that a patient from the controlled group received the intervention. |
Boyes 2006.
| Methods | RCT; Unit of allocation: Patient | |
| Participants | People diagnosed with cancer attending the oncology outpatient clinic and confirmed to receive active treatment. Setting / country: Medical oncology outpatient clinic at one major public cancer treatment centre in the state of New South Wales / Australia Type of cancer: Any type Phase of care: Treatment Sample size at randomisation: 80 |
|
| Interventions | Routine assessment and real‐time feedback: Patients' psychosocial well‐being was repeatedly collected over the course of treatment using a touch screen computer survey. Each time an intervention group patient completed the survey, the computer software immediately scored his/her answers and printed a feedback report, which was placed in the patient's file for consideration during his/her consultation with the oncologist. The feedback report listed the physical symptoms the patient reported as debilitating, a graphical representation of anxiety and depression scores, and the supportive care needs items that the patient reported. Suggested strategies for managing each identified issue were also included. The feedback report and recommended management strategies were developed in consultation with the treatment team including representatives from medical oncology, social work, occupational therapy, nursing, nutrition and dietetics and pastoral care. Control: Participants allocated to the control group underwent a usual consultation and their survey results were not made available to their oncologist. |
|
| Outcomes | Patient: Anxiety and depression (distress), perceived needs, symptoms. | |
| Notes | Length of follow‐up: During 3 visits at outpatient clinic months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "When first completing the computer survey, participants were alternately allocated, by the computer, to either the intervention or the control group." Comment: This is a quasi‐randomised allocation method. |
| Allocation concealment (selection bias) | High risk | Comment: Allocation could not have been completely concealed since one could predict the different groups from alternation. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Comment: Self‐report questionnaire were used and patients were not blinded to group allocation, so blinding of assessors was not possible. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "There was no significant difference in the dropout rate between the two groups (60% of both groups completed the third follow‐up)." "Importantly, the rate of attrition was similar in both groups, as were the reasons for attrition. Therefore, it is unlikely that missing data due to attrition had any impact on the results." |
| Selective reporting (reporting bias) | Low risk | All outcomes in the methods section are reported in the results section. |
| Other bias | Low risk | No evidence of other bias. |
| Baseline outcomes similar? | Low risk | Quotes: "Trends in the mean level of anxiety and depression were compared between the control and intervention groups using random effects models. Trends in the proportion of patients with a moderate or high level of need were compared between the control and intervention groups using generalised estimating equations" Comment: There were some differences in depression and in one of the need domain at baseline but no statistical tests were presented to compare these scores. Statistical analysis, however, took into account baseline value to detect trend in outcome during follow‐up. |
| Baseline characteristics similar? | High risk | Quote: "Table 1 summarizes the demographic, disease and treatment characteristics of participants at baseline. Patient characteristics were well balanced between the two groups although the intervention group had a significantly larger proportion of older patients (60 to 79 years) and patients diagnosed more than 1 year prior to the study." Comment: These potential confounding variables were not taken into account in analysis. |
| Protected against contamination? | High risk | Quote from discussion: "In addition to the limitations of sample size and eligibility criteria, there was the potential for contamination between the intervention and control groups as all medical oncologists saw patients in both groups. We believe that the impact of this is likely to be minimal as there is considerable evidence that medical oncologists have poor awareness of the issues faced by their patients (Newell et al. 1998; Fallowfield et al. 2001)." Allocation was not by practice or professional, and the same professionals were in contact with control and experimental groups patients. |
de Wit 2001.
| Methods | RCT; Unit of allocation: Patient; Stratified by: With/without district nursing, and by gender, age, metastatic sites | |
| Participants | Patients experiencing pain related to cancer, cancer therapy, or illness, admitted to a hospital, and expected to live for at least 3 months. Setting / country: A 180‐bed cancer centre (Antoni van Leeuwenhoek Hospital) / Netherlands Type of cancer: Any type Phase of care: Any phase Sample size at randomisation: 313 |
|
| Interventions | Pain Education Program (PEP): The Pain Education Program included the use of multiple teaching methods, which were provided both in the hospital and post‐discharge by telephone. The PEP was started in the hospital and consisted of pain information and instruction that was tailored to the needs and abilities of the individual patient. The purposes of the pain education program for patients were: (1) to improve knowledge of their pain and pain treatment; (2) to enhance motivation to adhere to the drug regimen; (3) to monitor pain daily by means of a pain diary; and (4) to stimulate help‐seeking behaviour (how to communicate about pain and how to contact healthcare providers). Topics discussed included: the definition of pain, pharmacological pain management, side‐effects, myths and misconceptions related to pain management, non‐adherence, use of non‐pharmacological pain treatment and pain assessment. The verbal instruction, which was provided in the hospital, was audio‐taped on a cassette so that it could be listened to at home. Patients were called at home at three and seven days postdischarge by the same nurse to determine whether the pain information and instruction provided in the hospital was fully understood, and to offer the opportunity to ask questions. Shared care: The second part of the intervention consisted of informing district nurses about the PEP that patients received. District nurses of intervention group patients received additional information about patients' pain complaints by telephone and by means of a written summary. By informing district nurses about patients' pain treatment, the purpose of the PEP was to improve their knowledge and understanding regarding patients' pain experience, to enhance their involvement in the pain treatment, and to ensure optimal continuity of care. Control: Regular pain treatment provided to patients; district nurses of control group patients received no additional information and instruction. |
|
| Outcomes | Patient: QoL, pain, pain knowledge, pain cognition, pain experience, nurses estimation of patient's pain intensity, nurses assessment of patient's pain relief Professional: Pain management, nurse satisfaction with the pain treatment Process: Number of visits at home to the patients by the district nurses (after discharge), number of district nurses who contacted another healthcare provider, frequency of contacts with the general practitioner, extent of information provided by hospital nurse |
|
| Notes | Length of follow‐up: 2 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from reference #2: "Patients were randomised by an independent trial office by means of a computer." |
| Allocation concealment (selection bias) | Low risk | See quote first item. |
| Blinding (performance bias and detection bias) Physical status | High risk | |
| Blinding (performance bias and detection bias) Psychological status | High risk | |
| Blinding (performance bias and detection bias) Quality of life | High risk | |
| Blinding (performance bias and detection bias) Use of services | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | High risk | |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes in the methods section are reported in the results section. |
| Other bias | Low risk | No evidence of other bias. |
| Baseline outcomes similar? | Low risk | Quote from reference #1: "Finally, pretest differences were found between the control and intervention groups with district nursing regarding the use of pain medication, patient’s cognitive functioning, and patient’s physical functioning. To correct for this latter imbalance, BMDP’s unbalanced repeated measures analysis of covariance (5V) was used (Jennrich and Schluchter, 1986)." Comment: BMDP is a general purpose statistics package for Bio‐medical Data Processing, including complex ANOVA designs, non‐linear and stepwise regression, time series, survival analysis, maximum likelihood estimation, various multivariate analyses. |
| Baseline characteristics similar? | Low risk | Quote from reference #1: "There were no differences with respect to demographic variables between the two control and two intervention groups, and between the separated control and intervention groups with and without district nursing." |
| Protected against contamination? | Unclear risk | Comment: Allocation was performed at the patient level. Additional professionals (nurses) were providing the intervention but did not have any contact with control group patients. If some of the district nurses were with control and intervention group patients, then they might have caused some contamination. In the intervention group, nurses received more information on their patients by telephone and by means of a written summary. |
Drury 2000.
| Methods | RCT; Unit of allocation: Patient | |
| Participants | Patients with cancer attending radiotherapy clinics. Setting / country: Radiotherapy clinics run by the Oxford Radcliffe National Health Service (NHS) Trust / UK Type of cancer: Any type Phase of care: Treatment Sample size at randomisation: 650 |
|
| Interventions | Patient‐held record: Patient‐held record consisted of A4‐size plastic wallet containing communication/diary sheets for use by the patient, their family, health professionals and carers, as well as pages for appointments, medication, addresses and telephone numbers. The study nurse explained the use of the record as a means of communication and as an aide memoire. Patients were encouraged to read it and write in it and to show it to anyone concerned with their care. The record explicitly invited carers to use it as an aid for communication. It was explained to record holders that the supplementary record was intended to improve communication with health professionals and act as a reminder. Control: Normal care |
|
| Outcomes | Patient: QoL Process: number of contacts with health professionals, clinic attendance |
|
| Notes | Length of follow‐up: 6 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The allocations, generated using random numbers and in blocks of 10, were in sealed, numbered, opaque envelopes, which were opened sequentially." |
| Allocation concealment (selection bias) | Low risk | See quote first item. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Quote: "Patients in both groups received an information sheet about the trial." Comment: Questionnaire were mailed, so the patient becomes the assessor, and patients were not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "After three months, the response rate to the questionnaire was much lower among the record holders than the normal care group, and record holders more often failed to complete all the questions, particularly those concerning the record itself." Quote: "574 patients were sent the three month questionnaire, of whom 450 responded: 206/284 (72.5%) in the RH group and 244/290 (84.1%) in the NC group, a difference of 11.6% (95% confidence interval = 4.9 to 18.3; P = 0.001)." Comment: Missing outcome data do not balance across the 2 groups, no imputation of missing data was done and no results were adjusted. However, missing data were found not have an impact on the observed effect size (constipation) (Cochrane Handbook; Section 8.12.2.1.). |
| Selective reporting (reporting bias) | Low risk | Relevant outcome (QoL) in the methods section is reported in the results section. |
| Other bias | Low risk | No evidence of any other bias. |
| Baseline outcomes similar? | Unclear risk | No baseline measure of outcomes. |
| Baseline characteristics similar? | Low risk | Quote: "There were no statistically significant differences in age, sex, or diagnosis between those who responded to the questionnaire and those who did not, and these characteristics were comparable in the two groups of responders (Table 2)." |
| Protected against contamination? | Low risk | It is not possible that control patients were contaminated, since the control patient simply did not receive the record. |
Du Pen 1999.
| Methods | Cluster‐RCT; Unit of allocation: Physicians' practice | |
| Participants | Ambulatory patients with diagnostic evidence of locally invasive or metastatic solid tumours and with at least a 6‐month life expectancy and a screening pain score of at least 3 on a scale of 0 to 10. Setting / country: Practices of 13 Western Washington oncology physicians / USA Type of cancer: Any type Phase of care: Palliative care Sample size at randomisation: 96 |
|
| Interventions | Treatment algorithms for cancer pain management in the community setting: The algorithm was based on the Agency for Health Care Policy and Research Guidelines for Cancer Pain Management. The process is operationalised with a set of tools, starting from the initial assessment. A clinic flow sheet is used to document the intensity of the pain, note the presence of any neuropathic pain character, and note the presence of any pain‐ or analgesic‐related side effects. A bulleted set of analgesic guiding principles for opioids, nonsteroidal antiinflammatory drugs, tricyclic antidepressants, and anticonvulsants is available for the oncology clinic staff for reference. The algorithm decision tree directs the oncologist/oncology nurse to comprehensive side effect protocols, equianalgesic conversion charts, and a primer for intractable pain. A flow sheet for each patient's chart was created to monitor significant pain and symptom indicators against their analgesic therapy. All of these tools were designed with the goal of maximum ease of use in the outpatient oncology setting. Patients had an initial clinic visit with the pain algorithm physician, at which time the intervention was initiated. The study nurse facilitated the assessment of pain and side effects as outlined by the algorithm and titrated medications under the direction of the algorithm physician. Patients were instructed regarding their role in the algorithmic process, and the importance of reporting increased and/or unrelieved pain or side effects was stressed. The pain intensity represented the first level of algorithmic treatment decision making, the pain character represented the second level. The algorithm also drove routine reassessment. The most recent pain intensity score determined frequency of contact. Control: Standard‐practice: Pain management by patients' community oncologists, who used their usual pain management and side effect strategies and documented in their usual fashion. |
|
| Outcomes | Patient: Pain, symptoms, QoL, satisfaction with pain management, pain Professional: Pain management |
|
| Notes | Length of follow‐up: 3 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The patients were randomised within referring physicians’ practices in permuted blocks such that an approximate balance between treatment arms and the treatment assignment of each patient was not predictable." |
| Allocation concealment (selection bias) | Unclear risk | See quote, first item. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: A data collection nurse who recorded outcome data, but was blinded to patient treatment randomisation, collected data for both the algorithm and standard practice groups." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: (See Table 1) Similar proportion of drop out in the 2 groups with similar reasons for dropping out. |
| Selective reporting (reporting bias) | Low risk | All outcomes in the methods section are reported in the results section. |
| Other bias | Low risk | No evidence of any other bias. |
| Baseline outcomes similar? | Low risk | Quotes: "There were no significant differences between the two groups on any of the baseline variables" "The algorithm treatment was the main effect influencing usual pain reduction, even when the two strongest confounders (chemotherapy and patient adherence) were introduced using analysis of covariance techniques." "Two‐way analysis of variance, using patient adherence as a covariate, indicated a significant confounding effect of non adherence on worst pain reduction (P < 0.02), whereas reduction in usual pain was statistically correlated with primary treatment effect (P < 0.02), despite the introduction of the adherence effect." "There was no statistically significant difference between the treatment groups in type of chemotherapy administered." "When chemotherapy was factored out, patients in the algorithm group had significantly lower worst pain scores than patients in the standard‐treatment group in both early (t = ‐2.70, P < 0.008) and late (t = ‐2.2, P < 0.04) phases of the study". Comment: The authors do not present baseline data for all the outcomes listed in Methods Section, although they state they are not different at baseline. See description of each outcome. They also acknowledge the influence of 2 confounding variables (chemotherapy and patient adherence) which they took into account in statistical analysis. |
| Baseline characteristics similar? | Low risk | Quote: "Baseline demographic and descriptive data were similar for patients in the algorithm and standard‐practice groups (Table 2). There were no significant differences between the two groups on any of the baseline variables." |
| Protected against contamination? | Low risk | Patients were cluster randomised by practice so contamination was prevented. |
Giesler 2005.
| Methods | RCT; Unit of allocation: Patient‐spouse dyad; Stratified by: Recruitment site, treatment modality | |
| Participants | Patient with a diagnosis of Stage T1a‐T2c prostate carcinoma and scheduled to undergo or to have undergone surgery, external beam radiation, or brachytherapy. Setting / country: Indiana University Cancer Center, the West Michigan Cancer Center, Ingham Hospital, and the Sparrow Radiation Clinic (Lansing, MI); and the Veterans Administration Medical Center (Louisville, KY) / USA Type of cancer: Prostate Phase of care: Pre‐treatment, surveillance Sample size at randomisation: 99 |
|
| Interventions | Nurse‐driven cancer care: After the conclusion of treatment for prostate carcinoma, dyads (patient/partner) in the intervention arm met once each month for 6 months with a nurse intervener (twice in person and 4 times by telephone). The nurse intervener identified and tracked quality‐of‐life problems using an assessment program developed for the cancer care intervention that was run from a laptop computer (the proximal‐distal continuum, a framework that is advocated frequently by quality‐of‐life investigators). For each problem, evidence‐based strategies were considered; and a mutually agreed upon, tailored plan of care then was developed and implemented by the nurse and the dyad. During each visit, the menu‐driven computer program provided standardised questions and response formats that the nurse intervener used to elicit and document information concerning quality‐of‐life problems. If a participants score exceeded a pre‐specified threshold for a problem, the program prompted the nurse to assess the problem in greater detail and helped identify strategies for that problem. After the first intervener visit, the program also was used to record whether previously identified problems had resolved or persisted and whether prior strategies should be continued, adjusted in terms of intensity or frequency, or halted. After the first visit, participants were provided with a videotape to view at home which showed couples discussing how cancer had affected their sexuality and relationship, and a binder or tool kit, which contained tabbed pages with information related to managing the symptoms and side effects commonly experienced by patients with prostate carcinoma. Control: Standard care. |
|
| Outcomes | Patient: QoL, dyadic adjustment, depression | |
| Notes | Length of follow‐up: 12 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "After the baseline interview, participants were randomised to the intervention arm or the control arm, stratified by recruitment site and treatment modality." |
| Allocation concealment (selection bias) | Unclear risk | See quote first item. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "Interviewers were blind to the group assignment of participants." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "By Wave 4, 85 of the original consenting dyads remained in the study, and 14 dyads had dropped out. The primary reason stated for dropping out was inconvenience. Attrition rates across the intervention and standard‐care groups were nearly identical, and drop‐outs did not differ from those who completed the study on any demographic, clinical, or baseline quality‐of‐life variables, with the exception that drop‐outs had marginally worse role‐emotional functioning at baseline (P < 0.07)." Comment: The proportions of missing data in each group were similar, but there is an incomplete reporting of reasons for missing outcomes. For the 2 outcomes that turned out to be significantly changed after the intervention (sexual limitation and cancer worry, Table 2), we replaced missing data with hypothetical results that showed no effect of the intervention. We then observed that the final results were only slightly modified, so we evaluated the risk of bias from missing data to be small. |
| Selective reporting (reporting bias) | Low risk | All outcomes in the methods section are reported in the results section. |
| Other bias | Low risk | No evidence of any other bias. |
| Baseline outcomes similar? | Low risk | Quote: "In regression analyses, interactions between the group variable and other potentially confounding variables also were tested. If an interaction was significant (P < 0.05), then we reported and tested the means (adjusted for age) for the intervention group and the control group separately for each level of the interacting variable. We observed that only baseline depression interacted with group. To describe this interaction, we dichotomised baseline depression into a low group and a high group based on the median (≥ 5 vs. < 5)." Comment: No baseline outcome measures are provided, but the regression analysis performed takes into account differences at baseline. A sensitivity analysis was performed to evaluate the impact of depression on primary outcomes. |
| Baseline characteristics similar? | Low risk | Quote: "We adjusted for age in all regression models, because intervention and control group participants differed on age (P < 0.001) but not on other characteristics (P < 0.25), including baseline quality‐of‐life variables." Comment: Age was significantly different between groups, but it was adjusted for in all analysis. |
| Protected against contamination? | Unclear risk | Dyads were the unit of randomisation. There are no details on the control group intervention. We do not know if the nurse involved in the intervention was also involved with control group patients. |
Given 2002.
| Methods | RCT; Unit of allocation: Patient‐caregiver dyad; Stratified by: Cancer site | |
| Participants | Patients with a diagnosis of a solid tumour undergoing a first course of chemotherapy and having a family caregiver who agreed to participate in the study. Setting / country: Six outpatient cancer treatment sites in Michigan / USA Type of cancer: Any type Phase of care: Treatment Sample size at randomisation: 237 |
|
| Interventions | Nursing intervention plus conventional care: The nursing intervention was based on a cognitive‐behavioural framework that focused on problem solving, information acquisition, self‐care management for symptoms, and emotional and social support for patients. It consisted of 10 contacts, 6 in person and 4 via telephone, occurring at 2‐week intervals over a 20‐week period. In‐person meetings with the patient‐caregiver dyad lasted approximately one hour; telephone encounters lasted 20 minutes, on average, and were conducted independently for the patient and caregiver. Patients were queried at each contact regarding the severity and impact on dimensions of their quality of life of 15 symptoms, as well as functional limitations and emotional distress. Severity of the symptoms was rated by patients on a 10‐point scale ranging from 1 (barely noticeable), to a 10 (worst possible). When severity was rated as 5 or higher, that symptom was transferred to the plan of care (using a computer assisted protocol) for problem resolution. If patients reported that a symptom reached a threshold of 3 or higher on a 5‐point scale for any one of the quality‐of‐life dimensions, it was transferred to the plan of care. Interventions were tailored to the patients' problems and categorised as teaching, counselling and support, coordination, and communication. At each intervention encounter, the nurse would also ask the patient to evaluate the efficacy of the intervention strategies identified previously and the status of the problem resolution. Intervention strategies then were modified, changed, or deleted depending on the result. Revisions to the plan of care were made as necessary to resolve the problem. Interventions were tailored to the patients problems and categorised as teaching, counselling and support, coordination, and communication. Each intervention nurse had the same cancer‐nursing intervention software loaded onto a laptop computer. This software housed problem‐specific, evidence‐based intervention strategies that the nurse and patient could mutually select for the patient to implement on his or her own behalf to move the problem toward resolution. Every screen for each patient‐caregiver dyad was reviewed by the nurse coordinator on a monthly basis. Control: Conventional care |
|
| Outcomes | Patient: Symptoms, QoL ‐ functioning | |
| Notes | Length of follow‐up: 5 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from author email: "This was a minimization approach (Taves 1986) where patients were allocated by site of cancer by location in order to balance the design between the experimental and control group. Persons in the settings (oncologists and nurses ) were not aware of the group assignments." Comment: The minimisation approach is an approach recognised as valid for generating the allocation sequence. |
| Allocation concealment (selection bias) | Low risk | Quote from author email: "Trial nurses at the respective sites informed prospective patient participants, obtained their informed consent and forwarded their information to a central location where trained interviewers contacted participants by telephone to collect data." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote from author email message: "Further, interviewers were unaware of the allocation (at intake because allocation had not occurred) and later because no information as to study arm was provided them and they had no access to this information." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote from ref #2: "Retention at 10 weeks by site or stage of cancer did not differ between patients in the experimental and control groups." Quote from ref #2: "Comparing score at baseline, no statistically significant differences were found by group for those lost and retained at 20 weeks. However, although not significant, some differences were noted." Quote from ref #2: "Because of differences in the number of observations after 10 and 20 weeks owing to death or withdrawal, a repeated measures analysis of variance design was not used. Instead, a GLM was used to test separate group effects at 10 and 20 weeks. By testing for group effects at both 10 and 20 weeks, we could determine whether and when the intervention might affect symptom severity." Comment: Proportion of missing data is equal in experimental and controls groups (27%). Reasons for attrition were, however, not explained in the article. No imputation was used. |
| Selective reporting (reporting bias) | Low risk | All outcomes in the methods section are reported in the results section. |
| Other bias | Low risk | No evidence of other bias. |
| Baseline outcomes similar? | Low risk | Quote from ref #1: "The baseline measure for each dependent variable was entered as a covariate, as was the site of cancer by group interaction term." Quote from ref #2: "Tests for differences in site and stage of cancer, severity, number of symptoms, total number of supportive medications, and between community and comprehensive sites revealed no differences in symptom severity at baseline at the 5% level of significance." |
| Baseline characteristics similar? | Low risk | Quote from ref #1: "The tests for equivalency at baseline between the experimental and control groups regarding demographic, independent, and dependent variables are presented in Table 1. No significant differences between the groups at baseline were found." |
| Protected against contamination? | High risk | Quote from ref#2: Thus 237 patients and their family caregivers completed the intake interview and were randomly assigned to either the 10‐contact, 20‐week experimental intervention or to conventional care. Conventional care was the usual practice for each setting. Comment: Patients from each of the groups were present in each setting and were not stratified by practitioner. Even if the family caregivers were randomised, they were not the one providing the intervention, so a risk of contamination between practitioners was possible. |
Goodwin 2003.
| Methods | Cluster‐RCT; Unit of allocation: Surgeon; Stratified by: Size of breast cancer practice | |
| Participants | Women aged 65 and older newly diagnosed with breast cancer identified within 2 months of diagnosis. Setting / country: 13 community and 2 public hospitals in southeast Texas / USA Type of cancer: Breast Phase of care: Any phase Sample size at randomisation: 335 |
|
| Interventions | Community‐based nurse case management plus patient education: Over the period of intervention, the nurse case manager interacted with the client through a combination of home visits, telephone conversations, being present at physician appointments, visits to client if she was hospitalised and contacts made at other community locations. The case manager roles were to educate, counsel, advocate for the patient and coordinate patient care. The model for the case management intervention was based on previous literature and consists of four stages of activities: assessment, planning, implementation, and evaluation. The planning phase included goal setting, decision making, advocacy, and planning with the patient, family, and healthcare professionals. The implementation phase included interventions such as managing symptoms, offering emotional support, teaching, enlisting social support, coordinating care, providing referrals, and accompanying patients to physician visits. In the evaluation phase, the intervention included monitoring progress and documenting follow‐up. The case managers did not advocate for a specific treatment (e.g. breast‐conserving surgery vs mastectomy). Rather, the goal was to ensure that the patient was fully informed of her options and that the surgeon and other providers were aware of all matters relevant to ensuring a successful outcome. The three case managers in this study were baccalaureate‐degree registered nurses with previous experience with case management in other settings. Each received 40 hours of training from advanced practice nurses in oncology and geriatrics on treatment and complications of breast cancer, availability of community resources, assessment of older patients, and methods of communicating with treating physicians. They were educated in the evaluation and treatment guidelines promulgated by The National Cancer Institute and were given patient‐education brochures produced by the American Cancer Society and the National Cancer Institute. The case management services were provided for 12 months from first contact with the client. Patient need determined the frequency of contact, although minimum contact during the intervention period included at least one in‐person assessment and monthly telephone calls. A checklist outlining the steps in the case management process and the specific activities under each step served as a prompt (available by request). The case manager also employed a number of standard assessment instruments, including activity of daily living scale,instrumental activity of daily living scale, Mini‐Mental State Examination, Geriatric Depression Scale, short form, Comprehensive Functional Assessment, and a Home Safety Checklist. These assessments were usually completed during the first two encounters with the patient. This information was used by the nurse case manager to assess patient needs and was not used or analysed by the investigators. Control: No details provided |
|
| Outcomes | Patient: Arm function Process: Receipt of appropriate treatment, treatment received in the first 6 months after diagnosis, proportion of patients receiving evaluation during follow‐up |
|
| Notes | Length of follow‐up: 12 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The surgeons were stratified by total number of new breast cancer patients seen in the previous year (≤ 5, 6 to 24, 25 to 39, and ≥ 40 patients) and then randomised into intervention and control groups. Randomisation was done at the level of the surgeon to reduce the chance of contamination of the control group from the case management intervention. Size of breast cancer practice was chosen as a stratification variable because of previous findings that breast cancer patient volume was a determinant of the extent of evaluation and appropriateness of treatment. Within each stratum, randomisation was performed in blocks of four to ensure balance in the number of surgeons assigned to each group. Surgeons in solo practice (n = 39) were randomised as individuals, whereas surgeons in group practice (21 surgeons in six groups) were randomised by group. The six surgeons at the two public hospitals were treated as two groups and stratified separately so that one public hospital was in the intervention group and one in the control group." |
| Allocation concealment (selection bias) | Unclear risk | See quote first item. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quotes from reference #1: "Bilingual interviewers, who were blinded to the purpose or structure of the study, interviewed control and intervention subjects at 2 and 12 months after diagnosis at home." "Six months after diagnosis, a trained data abstractor blinded to the purposes of the study abstracted the hospital and surgeons’ medical records for dates of diagnosis and treatment, cancer stage and size, histology, hormone receptor status, diagnostic tests obtained, type of surgery, other treatments recommended or prescribed, and consultations obtained (abstracting forms available on request)" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "The nurse case managers made a total of 4,049 individual contacts with 169 women in the intervention group in the year after diagnosis of breast cancer. Of these 169 subjects, 14 received no contacts from the nurse case manager because they (n = 11) or their surgeon (n = 3) refused permission to participate, but these women were included in the analyses of outcome, which were by intention to treat." Comment: No missing outcome data. |
| Selective reporting (reporting bias) | Low risk | Quote from reference #2: "In addition to demographic characteristics (age, education, income, race, living alone, ADL assistance, stage of cancer, attending a support group), participants were assessed for the presence of depressive symptomatology us |
| Other bias | Low risk | No evidence of any other bias. |
| Baseline outcomes similar? | Unclear risk | Comment: Primary outcomes could not be assessed at baseline since they evaluate the intervention (treatment received, receipt of appropriate therapy, evaluation process, satisfaction with decision‐making process). However, arm function results at baseline are not provided. |
| Baseline characteristics similar? | Low risk | Quote from reference #1: "The characteristics of the 335 participating women with breast cancer are described in Table 1. There were no significant differences between the intervention and control groups." |
| Protected against contamination? | Low risk | Quote from reference #1: "Randomisation was done at the level of the surgeon to reduce the chance of contamination of the control group from the case management intervention." |
Grunfeld 1996.
| Methods | RCT; Unit of allocation: Patient | |
| Participants | Women with initial stage I, II, or III breast cancer and no distant metastases, in remission, and receiving regular follow‐up care. Setting / country: Two district general hospitals in England / UK Type of cancer: Breast Phase of care: Discharge, surveillance Sample size at randomisation: 296 |
|
| Interventions | Routine follow‐up from general practitioners (GP): Discharge letter sent from hospital to general practitioner to transfer primary responsibility for follow‐up of women with breast cancer in remission from hospital to general practice. The discharge letter outlined the patient's breast cancer history, described the follow‐up routine recommended and assured the GP that rapid referral was possible if problems developed. Letter was accompanied by an educational handbook on breast cancer follow‐up care. Control: Continued routine follow‐up in outpatient clinics according to usual practice. |
|
| Outcomes | Patient: Satisfaction, QoL, anxiety and depression (distress), QoL, number of women with recurrence, survival Process: Time to diagnosis of recurrence, frequency of follow‐up visits, time for appointment |
|
| Notes | Length of follow‐up: 18 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote from reference #3: "Follow‐up groups were assigned by a telephone call to the trial coordination centre in Oxford. Random allocation was in blocks of eight." |
| Allocation concealment (selection bias) | Low risk | See quote first item. |
| Blinding (performance bias and detection bias) Functional status | Low risk | |
| Blinding (performance bias and detection bias) Physical status | Low risk | |
| Blinding (performance bias and detection bias) Psychological status | Low risk | |
| Blinding (performance bias and detection bias) Accessibility | Low risk | |
| Blinding (performance bias and detection bias) Continuity | Low risk | |
| Blinding (performance bias and detection bias) Quality of life | High risk | Quality of life was assessed using 3 self administered instruments; the assessor being the patient in these cases, he was not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote from reference #3: "the denominator was adjusted for patients who had died or gone away," Comment: Proportion of missing data are similar in intervention (5%) and control groups (3%) at the onset of the intervention. However, sample sizes presented in the Table 4 suggest more attrition and remain unexplained. There seems to have been some imputation, but it is unclear. |
| Selective reporting (reporting bias) | Low risk | All outcomes in the methods section are reported in the results section. |
| Other bias | High risk | Quote from reference #3: "However, the trial has one important limitation which must be recognised: as there was no common clinical examination at the end of the trial, it could be argued that there were unrecognised cases of recurrence in the general practice group which would have been elicited by examination at the hospital. With respect to local recurrence, women in both groups were receiving mammography and there were more cases of locoregional recurrence detected in the general practice group than the hospital group. With respect to metastatic recurrence, most distant recurrences are symptomatic at the time of diagnosis, although the possibility that hospital clinics are better at eliciting metastatic symptoms cannot be excluded by our design." Quote from reference #3: "Of the 148 participants randomised to the general practice group, 141 (95.7%) received the intervention as allocated, 5 (3.4%) referred themselves back to hospital, and 2 (1.4%) were registered with general practitioners who had refused to provide follow up. Of the 148 participants randomised to the hospital group, 5 (3.4%) requested discharge to general practice follow up so that 143 (96.6%) received the intervention as allocated." Comment: Analysis "per treatment" performed. Patients that switched from control to intervention group after randomisation were included in the intervention group. |
| Baseline outcomes similar? | Low risk | Quote from reference #3: "The general practice group was younger at diagnosis (mean age 55.6 v 59.0 years) and at entry to the study (mean age 59.1 v 62.4 years). There were more stage I patients in the hospital group (50.3% v 40.4%). Otherwise the two groups were very similar in clinical characteristics and in baseline scores on all sub‐scales of the quality of life instruments." Comment: In addition to the above quote, baseline outcome measures are presented in the Table 4. |
| Baseline characteristics similar? | High risk | Quote from reference #3: "The general practice group was younger at diagnosis (mean age 55.6 v 59.0 years) and at entry to the study (mean age 59.1 v 62.4 years). There were more stage I patients in the hospital group (50.3% v 40.4%). Otherwise the two groups were very similar in clinical characteristics and in baseline scores on all sub‐scales of the quality of life instruments." Comment: These differences were not taken into account in the analysis. Recurrence might be affected by the staging of cancer, with an increased risk of recurrence in the group with later cancer stage (control group). |
| Protected against contamination? | Low risk | Comments: there were no possibility for contamination from healthcare professionals since the settings were different in control and intervention groups. |
Grunfeld 2006.
| Methods | RCT; Unit of allocation: Patient; Stratified by: N/A | |
| Participants | Women with initial stage I, II, or III breast cancer and no distant metastases, in remission, and receiving regular follow‐up care. Setting / country: Two district general hospitals in England / UK Type of cancer: Breast Phase of care: Discharge, surveillance Sample size at randomisation: 296 |
|
| Interventions | Routine follow‐up from general practitioners (GP): Discharge letter sent from hospital to general practitioner to transfer primary responsibility for follow‐up of women with breast cancer in remission from hospital to general practice. The discharge letter outlined the patient's breast cancer history, described the follow‐up routine recommended and assured the GP that rapid referral was possible if problems developed. Letter was accompanied by an educational handbook on breast cancer follow‐up care. Control: Continued routine follow‐up in outpatient clinics according to usual practice. |
|
| Outcomes | Patient: QoL, anxiety and depression (distress), incidence rate of recurrence‐related serious clinical events, survival, number of women with recurrence and nature of recurrence. | |
| Notes | Length of follow‐up: 18 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from reference #1: "After patients provided informed consent, they were randomly allocated to treatment groups by a telephone call to the trial coordinating centre of the Ontario Clinical Oncology Group. Randomisation was conducted using a computer‐generated centre‐specific schedule." |
| Allocation concealment (selection bias) | Low risk | See quote first item |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote from reference #1 "All SCEs were adjudicated by a committee, the members of which were unaware of treatment allocation." Comment: No details on the other outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quotes: "All available data were included without imputation. Polynomial growth curve models as a function of the actual time from randomisation to assessment were fit to the data." "The MIXED procedure in SAS version 9.1 (SAS Institute, Cary,NC) was used. Using this approach, missing data were assumed to be missing at random." Comment: No details on the proportion of missing data, except for SF‐36 (Table 3). |
| Selective reporting (reporting bias) | Low risk | All outcomes in the methods section are reported in the results section. |
| Other bias | Low risk | No evidence of any other bias |
| Baseline outcomes similar? | Unclear risk | No details provided |
| Baseline characteristics similar? | Low risk | Quote: "The two groups were reasonably well balanced for baseline characteristics (Table 1)." Comment: Statistical analysis were not performed but baseline characteristics seem well‐balanced between groups. |
| Protected against contamination? | Low risk | Unit of randomisation was the patient, but there is no contamination possible since the 2 groups are followed in distinct settings by distinct care providers. |
Hanks 2002.
| Methods | RCT; Unit of allocation: Patient; Stratified by: Hospital site, and within each hospital setting, by cancer and non‐cancer patients | |
| Participants | Inpatients newly referred to the Palliative Care Team. Setting / country: Teaching hospital trust (United Bristol Healthcare Trust) in England / UK Type of cancer: Any type Phase of care: Palliative care Sample size at randomisation: 261 |
|
| Interventions | Full‐palliative care team (PCT): the multidisciplinary specialist PCT was the usual service and comprised 2 clinical academic consultants, one specialist registrar, and 3 clinical nurse specialists (2.5 full‐time equivalent). The PCT has close link with a clinical psychologist, a local hospice and community‐based palliative care services and access to social workers, rehabilitation staff and the chaplaincy in the hospital. Initial assessment of patients was undertaken by a specialist doctor or specialist nurse, either alone or together, and detailed advice about any problems identified was written in the patients case notes and communicated to the patients medical and nursing team personally or by telephone. Appropriate follow‐up was then instituted which usually involved both telephone and in‐person consultations with the patient, their family and the medical and nursing staff caring for the patient by one of the specialist nurses or the registrar. All patients were reviewed at least weekly by one of the consultants. For patients who were discharged from hospital, the PCT also provided liaison with community based health professionals and outpatient follow‐up in the Palliative Care clinic if appropriate. Control: Telephone‐PCT, limited telephone advice: A more limited form of intervention was devised as a control. This involved no direct contact between the PCT and the patient or their family. Instead, within one working day of referral, a telephone consultation took place between a senior medical member of the PCT and the referring doctor and also between a PCT nurse specialist and a member of the ward nursing staff directly involved with the patient. A second telephone consultation could be made if necessary but thereafter no further follow‐up or advice was given. Such a telephone advisory service commonly forms a part of the operational policy of specialist palliative care teams. |
|
| Outcomes | Patient: Emotional bother, QoL, satisfaction, mood Informal carer: Satisfaction, anxiety and depression (distress) Process: Length of hospital stay and rates of readmission, use of hospital resources, use of primary care services |
|
| Notes | Length of follow‐up: Until death (min. 0.25; max. 1) months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomisation schedule was prepared by generating random numbers on a computer (within Microsoft Access) in permuted blocks of three to ensure equality of randomisation between the strata." |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomisation details were recorded on adhesive labels placed in opaque non‐resealable envelopes. Randomisation was undertaken by a non‐clinical administrator with no involvement in patient recruitment or assessment." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "The researchers who undertook the assessments were blind to the group allocation." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "The randomised groups were compared on an intention‐to‐treat basis, including the use of confidence intervals. All analyses therefore included individuals in the group to which they were randomised, regardless of whether they subsequently switched groups." Comment: Proportions of missing data in the control (43%) and in the intervention groups (33%) were similar. Reason for attrition was death in all cases. No imputation was used. |
| Selective reporting (reporting bias) | Low risk | All outcomes in the methods section are reported in the results section. |
| Other bias | High risk | Quote: "However, there was a highly significant difference in the number of patients who switched intervention groups, in favour of the ‘full‐PCT’. It seems clear from these data that patients in both groups were being well managed. This poses the question as to whether either or both interventions contributed to this good management." |
| Baseline outcomes similar? | Low risk | Quote: "The prevalence of the most bothersome symptom volunteered by patients at the baseline assessment was also similar in the two allocated groups (Table 5)." + table 4 "The primary analyses involved regression models comparing the allocated groups in respect of outcomes at follow‐up, adjusting for baseline scores as covariates." |
| Baseline characteristics similar? | Low risk | Quote: "The allocated groups were similar in baseline characteristics except in gender distribution (Table 4)." Comment: The small difference in gender between group is unlikely to have biased study results, since there are no indication that gender would affect outcome measurements. |
| Protected against contamination? | High risk | Patients were the units of allocation and the intervention was delivered by the same team (Palliative care team). |
Hughes 1992.
| Methods | RCT; Unit of allocation: Patient; Stratified by: N/A | |
| Participants | Terminal ill patients admitted to medicine, surgery, and neurology, and their primary informal caregivers. Setting / country: Department of Edward Hines, Jr. Veteran affairs (VA) Hospital, Illinois / USA Type of cancer: Not mentioned Phase of care: Palliative care Sample size at randomisation: 175 patients and their primary informal caregivers |
|
| Interventions | Hospital‐based home care program (HBHC): this home based palliative care program encompass an interdisciplinary team that is led by a physician and includes nurses, a social worker, a physical therapist, a dietitian, and health technicians. The Hines HBHC program develops goal‐oriented, interdisciplinary patient care plans at team meetings, and schedules visits according to individual patient needs. The HBHC physician is also able to manage HBHC patients both in and out of the hospital. The HBHC model of care emphasizes the provision of care to high‐risk patients, the provision of comprehensive services based on need, the importance of timely communication about patients across team members, and the instruction and involvement of informal caregivers in patient care to the maximum extent possible. To improve subject recall, patients were provided with a healthcare diary and were asked to record in the diary all home healthcare visits, clinic visits, and admissions to healthcare facilities for the six‐month period of their participation in the study. Control: Customary care within or outside the VA (i.e. in community home care or in Hospice care) with the exception of access to HBHC. |
|
| Outcomes | Patient: Survival, patient emotional adjustment, satisfaction, cognitive functioning, functional status Informal carer: Emotional adjustment, caregiver satisfaction Process: Use of hospital and community services, use of non VA‐services, length of hospital stay, place of death |
|
| Notes | Length of follow‐up: 6 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "This study used a randomised pretest‐multiple posttest experimental design." Quote: "Prior to the patient's discharge, the patient was randomised to treatment or control group status." |
| Allocation concealment (selection bias) | Unclear risk | See quote first item. |
| Blinding (performance bias and detection bias) Functional status | High risk | Quote "If a patient died between baseline and the one‐month follow‐up, only the one‐month interview was conducted with the caregiver." |
| Blinding (performance bias and detection bias) Psychological status | High risk | Quote "If a patient died between baseline and the one‐month follow‐up, only the one‐month interview was conducted with the caregiver." |
| Blinding (performance bias and detection bias) Satisfaction | High risk | Quote "If a patient died between baseline and the one‐month follow‐up, only the one‐month interview was conducted with the caregiver." |
| Blinding (performance bias and detection bias) Use of services | Low risk | Quote: "VA services were tracked through existing records, files, and computer data bases. Use of healthcare services outside the VA was monitored by participants. To improve subject recall, patients were provided with a healthcare diary and were asked to record in the diary all home healthcare visits, clinic visits, and admissions to healthcare facilities for the six‐month period of their participation in the study. Patients were contacted monthly by research staff to retrieve the diary information, and diaries were also examined by research staff in patients' homes during the one‐month and six‐month interviews." Comment: Such information is objective in nature so there is no need for the assessor to be blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "The major reason for attrition from posttest measurement was mortality, at 79 percent in the HBHC group and 78 percent in the control group. An examination of survival days indicated no group differences: 76.2 days, s.d. = 67.1 in the HBHC group versus 83.1 days, s.d. = 68.1, (n.s.) in the control group for all study subjects and 48.0 days, s.d. = 43.3 versus 54.5 days, s.d. = 47.7 days for decedents." Comment: Similar proportion of missing data in the 2 study groups. |
| Selective reporting (reporting bias) | Low risk | All outcomes in the methods section are reported in the results section. |
| Other bias | Low risk | No evidence of other bias. |
| Baseline outcomes similar? | Low risk | Quote: "Baseline functional and cognitive status, morale, and satisfaction with care scores for control and HBHC groups (also shown in Table 1) indicate that the study groups were very similar to each other at the time of randomisation" |
| Baseline characteristics similar? | Low risk | Quote: "Baseline characteristics of HBHC and control group patients displayed in Table 1 indicate that the HBHC group was slightly older (P < 0.10) and the mean age for retirement was also slightly higher in the HBHC group versus for the control sample (P < 0.10)." Comment: This difference is unlikely to lead to bias. |
| Protected against contamination? | Unclear risk | The home care intervention program encompass an interdisciplinary team and an informal caregiver. There are no evidence that the same healthcare providers are accessible to intervention and control group participants. The HBHC is a program in the VA Hospital. Control group patients were able to access customary care within or outside the VA with the exception of access to Hines HBHC. |
Jefford 2008.
| Methods | Cluster‐RCT; Unit of allocation: General practitioner | |
| Participants | General practitioners patients receiving chemotherapy. Setting / country: Peter MacCallum Cancer Centre / Australia Type of cancer: Not mentioned Phase of care: Treatment, discharge, surveillance Sample size at randomisation: 97 |
|
| Interventions | Tailored chemotherapy information faxed to general practitioners (GPs): In addition to usual correspondence, a cover letter and a chemotherapy information sheet relevant to their patients' regimen were faxed to the patients' GP. The GP practice was then contacted to confirm receipt of information and asked to file it in the patient's record. The cover letter was generic but contained several patient‐specific fields: name of the patient, name of treating doctor, type of cancer, treatment intent (to cure the disease, to increase the chance of long‐term, disease‐free survival [adjuvant treatment], or to palliate symptoms/improve quality‐of‐life/extend survival), and type of CT. The sheet also included the telephone number of the drug information service and listed a number of relevant, reputable Internet sites. The chemotherapy sheets were developed for 23 CT regimens, used to treat haematological and solid tumours. Each sheet named component drugs, explained the treatment cycle, listed common adverse effects, suggestions for management and advice about when to call the cancer centre, how to contact relevant staff, and had a further information section. They were developed by a medical oncologist and behavioural scientist in collaboration with pharmacy staff following a focus group of 10 GPs and following a review by medical, nursing and pharmacy staff. Control: Usual correspondence to GPs from their patient's oncologist |
|
| Outcomes | Professional: Satisfaction with communication received from the treatment centre, perceived confidence in managing chemotherapy adverse effects Process: Perceptions on the utility of correspondence |
|
| Notes | Length of follow‐up: 0.25 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation lists were developed by biased coin procedure." |
| Allocation concealment (selection bias) | Low risk | Quote: "Sequentially numbered, opaque envelopes concealed experimental group allocation from the research assistant until after baseline data collection." |
| Blinding (performance bias and detection bias) Satisfaction | High risk | Self‐report measure |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Participants who withdrew from the study, reported not receiving information, or did not read the information, had their last observation carried forward for any analysis that compared baseline and follow‐up scores." The proportions of missing data were different between experimental (21%) and control (12%) groups. Reasons of withdrawal are not described. Imputation was not appropriate. However, missing data were found not have an impact on the observed effect size (confidence or satisfaction), i.e. if missing data for outcomes found to be significant (confidence or satisfaction) were replaced with the same values found in the control group, and they remained significant (Cochrane Handbook; Section 8.12.2.1.). This highlights that there exists only a small chance of bias from missing values. |
| Selective reporting (reporting bias) | Low risk | All outcomes in the methods section are reported in the results section. |
| Other bias | Low risk | No evidence of other sources of bias were found. |
| Baseline outcomes similar? | Low risk | Quotes: "Similarly, no significant differences were observed for any of the major outcome variables at baseline." "All reported means, SEs, and 95% CIs for the major outcome variables were adjusted for the effect of baseline as a covariate." |
| Baseline characteristics similar? | Low risk | Quotes: "Overall, there were no significant differences between the two groups with respect to age, sex, overall experience, or oncology caseload (Table1)." "Comparisons of follow‐up scores were tested by an analysis of covariance with baseline added as a covariate." |
| Protected against contamination? | Unclear risk | Allocation was by GP so it is impossible that a patient received the wrong treatment. There is a risk that some GPs in the two groups also practiced in the same clinic. |
Johansson 1999.
| Methods | RCT; Unit of allocation: Patient; Stratified by: Diagnosis, stage of disease | |
| Participants | Patients newly diagnosed with breast cancer or under examination for a suspected breast cancer or diagnosed with colorectal, gastric or prostate cancer. Setting / country: Primary healthcare services in Uppsala county / Sweden Type of cancer: Gastric, breast, prostate, colorectal Phase of care: Any phase Sample size at randomisation: 527 |
|
| Interventions | (1) Individual support (IS) by intensified primary healthcare services (IPC): Each patient was referred by project staff to ordinary home‐care nurse. Patients GP was informed of cancer diagnosis and of referral to home‐care nurse. The home‐care nurse contacted the patient and suggested follow‐up contacts during the period of primary treatment and rehabilitation or palliative care. GP and home‐care nurse received copies of medical record each time patient was discharged from hospital, or at each visit to specialist clinics. Education and supervision in cancer care was also provided for general practitioners and home‐care nurses (12 seminars). Home‐care nurses were offered supervision by an oncology team (dietitian, psychologist, physiotherapist, urotherapist, specialist nurse). The supervision aimed to support the nurse in dealing with patient problems. A further aim was to ensure that the nurse was informed about the patient's diagnosis, treatments, impairments, etc. The nurses were invited to participate in open supervision groups regularly. An oncology nurse led the groups and was assisted by members of the oncology team. In total, 55 supervision meetings were arranged during the project period. Nurses who did not participate in the supervision meetings were regularly contacted by telephone. All home‐care nurses also had the possibility to contact the oncology team as needed. The individual support intervention comprised psychological support and nutritional support for patients with GI cancer. (2) Group rehabilitation (R) a group intervention starting approximately 3 months after diagnosis; (3) Combination of (1) and (2) Control: Standard care (SC) |
|
| Outcomes | Process: Situation about continuing/discontinuing contact with the home‐care nurse 6 months after diagnosis, number of follow‐up contacts with home‐care nurse, psychologist and/or dietician, utilization of inpatient specialist care | |
| Notes | Length of follow‐up: 6 or 3 (group Rehabilitation only) months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from ref #2: "Patients were randomised (computer‐generated allocation schedule) to one of four alternatives: (1) individual support (IS), starting at diagnosis; (2) group rehabilitation (GR), starting 3 months after diagnosis; (3) a combination of individual support and group rehabilitation (ISGR), and (4) standard care (SC)." |
| Allocation concealment (selection bias) | Unclear risk | No details provided. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Comment: Only reported objective measures will be included in the reviewed so there are no outcome assessors. Patients' satisfaction was evaluated with a questionnaire that was not validated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote from reference #1: "100 patients (19%) failed to complete the measures (Figure 1). Comparisons between these patients and those who are included in the present analysis shows that the former group has a higher mean age (69 vs. 65 years.) and a larger portion of patients with advanced disease (40% vs. 18%). However, these patients are equally distributed between the comparison groups (controls, n = 52 vs. IPC, n = 48)." Comment: Similar proportions of missing data and reasons for attrition in control group (79%) and individual support intervention group (84%). Only subjects that completed the study were analysed (no imputation was used). |
| Selective reporting (reporting bias) | Low risk | All outcomes described in Methods are reported in Results. |
| Other bias | Low risk | No evidence of any other bias. |
| Baseline outcomes similar? | Low risk | Comment: Outcomes could not be assessed at baseline (use of services). |
| Baseline characteristics similar? | Low risk | Quote from ref #1: "Table 1 summarizes the demographic and medical characteristics of the patients included in the present analysis. There were no statistically significant differences (chi‐square test) between patient's randomised to IPC and patients randomised to controls regarding these variables." |
| Protected against contamination? | Unclear risk | The intensified primary care program seems to have been done by a different group of healthcare professionals than the standard care program, so contamination seems unlikely, but no details were provided by the authors on this issue. |
Jordhoy 2001.
| Methods | Cluster‐RCT; Unit of allocation: Community healthcare districts of living; Stratified by: Pairs of districts, Inhabitants' age, type of area represented | |
| Participants | Advanced cancer patients referred to the hospital Palliative Medical Unit (PMU) by healthcare districts. Setting / country: Norwegian Public Health Service (Hospital of Trondheim and community care close to the PMU (8 districts) / Norway Type of cancer: Any type Phase of care: Palliative care Sample size at randomisation: 434 patients (312 close family members) |
|
| Interventions | Comprehensive palliative care: In this home based palliative care program, the patients general practitioner (GP) and a community nurse were defined as the main professional caregivers. Treatment plans were set up in a meeting of the patient, the informal caregiver, the GP, the community nurse and the nurse or physician from the Palliative Medical Unit (PMU). Hospital service was offered on request, always at the PMU. The PMU has 12 inpatient beds, an outpatient clinic, and a multidisciplinary consultant team that works in and out of the hospital. The PMU consultant team organised the follow‐up. Predefined guidelines were used to keep the interaction at an optimum between services. The educational program for community professionals included bedside training and 6‐12 hours of lecture every 6 months. Follow‐up consultations by community staff were set up as routine. Multidisciplinary staff meetings were arranged weekly. For referrals and admission to nursing homes, conventional routines were followed. Control: Conventional care (without well‐defined follow‐up routines). |
|
| Outcomes | Patient: Psychologic distress, QoL Informal carer: Satisfaction Process: Place of death, use of hospital services, use of nursing home services, use of hospital services, proportion of readmission time in institutions (nursing homes and hospital) |
|
| Notes | Length of follow‐up: Until death (min: 2; max: 24) months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote from ref #1: "Before opening of the trial, three clusters were allocated to intervention and three to conventional care (control). Eligible patients were assigned treatment according to the district (cluster) in which they lived." |
| Allocation concealment (selection bias) | High risk | Quote from reference #1: "A difference in distribution of diagnostic groups was probably related to lack of concealment of individual patient allocation, because the treatment assignment of individual patients could be identified from their address." |
| Blinding (performance bias and detection bias) All outcomes | High risk | Quote from ref #1: "All questionnaires, except the baseline forms, were distributed by mail." Comment: Since the patients could not be blinded to group assignment, and since they were responsible for completing the questionnaire, then the assessors could not be blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote from ref #1: "If data from one assessment point were missing, then the mean of the two adjacent ones was used. HRQL scores were assumed to be zero for the time after death. For the patients who withdrew or dropped out before death during the first 4 months, the last value carried forward was used to impute the missing subsequent values. The latter approach might, however, introduce a bias if the main reason for drop‐out was deterioration. Hence, the analyses were repeated imputing worse possible scale/item score for the missing ones. The results were consistent with those that are presented." Quote from ref #1: Missing items were imputed for the EORTC QLQ‐C30 and the IES multi‐item scales, using the method advocated by the EORTC Quality‐of‐Life Study Group. If at least half of the items from a scale were completed, the values of missing ones were imputed as the mean value of the completed items. For the IES, which had a higher number of missing items, the analyses were made both with and without using imputation; imputation had a minor impact on the group means and did not alter the results concerning the comparisons between treatment groups. Comment: Sensitivity analysis was used both for missing items and missing participants to minimize the risk of bias. Such a procedure is valid. |
| Selective reporting (reporting bias) | Low risk | All outcomes described in Methods are reported in Results. |
| Other bias | Low risk | No evidence of any other bias. |
| Baseline outcomes similar? | Low risk | Quote from reference #1: "the treatment groups were comparable on a wide range of baseline data, including HRQL scores" See table 4. Quote from ref #1: "To adjust for possible baseline differences, the AUC calculation for each patient was based on changes from baseline (actual score ‐ baseline score), i.e., on the improvement or deterioration at 1 to 4 months compared with trial entry." |
| Baseline characteristics similar? | High risk | Quote from references #1 and #2: "Diagnoses were classified by traditional sharing of treatment responsibility among the departments at the University Hospital of Trondheim (groups A‐C, table 1). The distribution of patients to these groups differed significantly between the treatment groups. There was also a difference in the time from diagnosis to inclusion." Quote from ref #1: "At baseline, the groups differed for housing, access to informal help, home care nursing, and, slightly, for living situation (Table 2)." Comment: No mention of any statistical adjustment for baseline differences in patient characteristics was found. |
| Protected against contamination? | Low risk | Quote from ref #1: "Thus, to minimize the exposure of the control group to the experimental effect, a cluster randomised design was chosen." Comment: Cluster‐randomisation by community healthcare districts prevents contamination. |
Kane 1984.
| Methods | RCT; Unit of allocation: Patient | |
| Participants | Patients admitted to the hospital with a terminal prognosis of two weeks to six months and informed of this prognosis. Setting / country: Veterans Administration Wadsworth Medical Center, West Los Angeles, a university‐affiliated teaching hospital / USA Type of cancer: Any type Phase of care: Palliative care Sample size at randomisation: 246 (or 247?) |
|
| Interventions | Hospice care: The hospice included: (a) an 11‐bed inpatient unit team staffed by 2 physicians, 19 nurses, a social worker, a chaplain, and about 30 volunteers, which sought to spend more time with the patients and their family to help them cope more effectively with impending death, (b) a home care program serving about 25 patients at any given time and (c) a consultation service for patients awaiting admission to the hospice inpatient unit or needing emergency hospital care when no hospice inpatients bed were available. The last modality (consultation) was used when all the beds in the hospice unit were assigned, because all hospice patients receiving home care were guaranteed admission on demand. Although patients on the consultation service were regarded as hospice patients, they remained in the care of a family physician and hospice staff provided limited help and advice for the patient and his familial caregiver during this time. Control: Conventional care usually located in the intermediate care unit (chronic care ward) but may also be in the surgery, ENT, or haematology/oncology wards of the hospital. |
|
| Outcomes | Patient: Pain, symptoms, depression, anxiety, satisfaction, survival, functional status Informal carer: Anxiety, satisfaction Process: Place of death, use of institution services (hospital and nursing home), surgical procedures, radiation, chemotherapy |
|
| Notes | Length of follow‐up: Until death or the maximum number of interviews completed (N = 6) (min. 1.5; max. 4.5 months) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "After informed consent was received from patients and their FCGs, patients were randomly assigned to receive hospice or conventional care; the sampling proportion was deliberately weighted to favour hospice care." Quote from email message from the author: "I do not remember." |
| Allocation concealment (selection bias) | Unclear risk | See quote first item |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote from ref #2: "Interviews to assess the reliability of the various scales... were with the four interviewers conducting the data collection." Comment: Data were collected by means of interviews performed by 4 interviewers, but no other details are presented. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quotes: "Of the 263 patients eligible for the study, only 17 (6%) declined to participate. Another 10 patients withdrew after enrolment." "The survival curves for the hospice and control groups were essentially the same." Comment: The proportion of participants who withdrew in each group was not specified, and neither were reasons of withdrawal. However, because the authors used survival analysis, they took into account individuals lost to follow‐up. |
| Selective reporting (reporting bias) | High risk | Results from the functional status described in Methods was not reported in Results or elsewhere. |
| Other bias | Low risk | No evidence of any other bias. |
| Baseline outcomes similar? | Low risk | Quote: "Repeated‐measures analyses of covariance, with baseline score as a covariate, were performed for symptoms, affect, satisfaction, activities of daily living, and involvement‐with‐care measures to determine whether there were differences between hospice and control subjects." |
| Baseline characteristics similar? | Low risk | Quote from ref #1: "t‐tests on baseline data revealed no significant differences between hospice and control subjects on any measures." Comment: Baseline characteristics presented in Table 1 are similar in the 2 experimental groups. |
| Protected against contamination? | Unclear risk | Experimental and control group patients were located in separated units of the same hospital, but it is unclear whether there was some link between professionals in these units. |
King 2009.
| Methods | RCT; Unit of allocation: Patient | |
| Participants | Patients having breast, lung or colorectal cancer and having reached the end of first treatment. Setting / country: Breast, lung and colorectal cancer services at 4 North London NHS Trusts; North London Marie Curie Hospice / UK Type of cancer: Breast, lung, colorectal Phase of care: Treatment, discharge, surveillance Sample size at randomisation: 93 |
|
| Interventions | (1) FULL INTERVENTION: Usual care + continuity assessment + feedback to the clinical nurse specialist (CNS). The patient completed a 17‐item continuity assessment. After each item, patients ticked a box if they wished to discuss the issue further with a clinical team member. Four boxes were given at the end of the questionnaire in which participants could also write any additional information they wanted to give on up to four items. Patients responses were fed back to CNS who were expected to take action as necessary in any areas highlighted by patients for further attention. This could involve discussion with patients and/or discussion between members of the team. How or when actions should be taken was not indicated, but was rather left to the CNSs expertise. Nurses were asked to complete a clinical feedback form briefly detailing any action they had taken in response. (2) PARTIAL INTERVENTION: Usual care + continuity assessment (partial intervention). Control: Usual care |
|
| Outcomes | Patient: Perceived needs Process: Patient experience of continuity |
|
| Notes | Length of follow‐up: 1.5 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from ref #2: "An independent statistician used a blocked randomised design to achieve equal numbers in each trial arm. Researchers telephoned the trial centre to receive each participants allocation from an administrator independent of the trial." |
| Allocation concealment (selection bias) | Low risk | See quote first item. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Quote from ref #1: "Patients in all three arms completed the continuity assessment (primary outcome), and the needs assessment and satisfaction rating (secondary outcomes) by post after 6 weeks." Comment: Patients filled the self‐report instruments, so they were the assessors and could not be blinded to treatment allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote from reference #1: "Attrition was highest in the 32 patients randomised to the partial intervention arm only (arm 2, Figures 1 and 2). Most (81%) of those lost to follow‐up were patients who failed to respond. A small number died, and two refused to complete the follow‐up questionnaires. Patients in the intervention groups were significantly more likely to drop out than those in arm 1 (arm 2: OR: 6.25, P < 0.004, arm 3: OR: 3.75, P < 0.042)." Comment: There was a less important proportion of missing data in arm 1 (14%) than in arm 2 (50%) or arm 3 (38%). Reasons for dropping out are not explained. No imputation was used for missing data. |
| Selective reporting (reporting bias) | Low risk | All outcomes described in Methods are reported in Results. |
| Other bias | Low risk | No evidence of other sources of bias were found. |
| Baseline outcomes similar? | Unclear risk | Quotes from ref #1: "Patients in trial arm 1 completed no assessments at baseline. Patients in trial arms 2 and 3 completed a need assessment (the supportive care needs survey, (SCNS), which covers psychological, physical, sexuality, patient care and health system domains (Bonevski et al, 2000). They also completed the same visual analogue scales to measure satisfaction that we had used in our earlier cohort study (King et al, 2008)." Comment: Results of the baseline outcome assessment are lacking for one of the trial arms and are not presented in the publication for the 2 other trial arms. However, results were adjusted for the covariates for these 2 arms. Lack of baseline assessment in one of the trial arms justifies the evaluation made of this item. |
| Baseline characteristics similar? | Low risk | Quote from reference #1: "There were no significant differences in patient characteristics between the three arms of the trial." Quote from reference #3: "There were no demographic differences between patients in the five treatment phases at baseline (Table 1)." |
| Protected against contamination? | High risk | Comment: Patients were the one randomised and randomisation was not stratified according to providers. Since there were 29 clinics and 93 participants, then contamination was possible. |
Koinberg 2004.
| Methods | RCT; Unit of allocation: Patient; Stratified by: Centre | |
| Participants | Women with newly diagnosed breast cancers (UICC classification: p‐TNM stage I or stage II) receiving radiotherapy. Setting / country: Three Swedish hospitals / Sweden Type of cancer: Breast Phase of care: Surveillance Sample size at randomisation: 400 (n = 264 in main analyses, see text) |
|
| Interventions | Nurse‐led follow‐up on demand: approximately 3 months after surgery, the patient met with an experienced nurse and received information about how to recognize a recurrence in breast, skin, axilla and scar. The nurse also arranged mammography at 1‐year intervals. The nurse gave advice on aspects of self‐care (medication, breast self‐examination) and provided time for talking about the patient's psycho social situation. The patient could contact the nurse any time for questions or symptoms related to the breast cancer. The nurse coordinated the healthcare resources and had rapid access to specialists in surgery and /or oncology within her own hospital, if needed. The nurse informed patient of mammography results by telephone or letter. After 3 years, the patients were referred back to the routine mammography‐screening program. Control: Physician‐led follow‐up: routine medical follow‐up by the physician. A specialist in oncology or surgery examined the patients four times per year during the first 2 years after surgery, followed by biannual examinations for up to 5 years, and yearly after 5 years. At the follow‐up visits, the examination included history taking concerning symptoms that could signal a loco‐regional relapse or distant metastases as well as a clinical examination of the breasts, chest wall and regional lymph nodes. Mammography was carried out at 1‐year intervals. Blood tests, chest X‐ray or other imaging techniques were only performed on clinical indication. |
|
| Outcomes | Patient: Anxiety and depression (distress), satisfaction, number of loco‐regional recurrences to distant metastases or any first breast cancer recurrence, survival Process: Number of physician and nurse contacts, number of evaluations |
|
| Notes | Length of follow‐up: 60 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from ref #1: "Randomisation was achieved by means of telephone contact with an external secretariat. The random selection was computer‐generated and stratified by centre. The block size was unknown to the study co‐ordinators at the centres." |
| Allocation concealment (selection bias) | Low risk | See quote first item. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Comment: Data were collected via self‐report questionnaire, so the assessor (i.e. the patient) could not be blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: Proportion of missing data in the whole sample reached 32% for some measures. Proportion in each group were not reported. There is no mention in the publications of any type of imputation used. Because we do not know if the proportions of missing data were similar in the 2 groups, then we cannot judge if there is a risk of bias due to incomplete data. |
| Selective reporting (reporting bias) | Low risk | All outcomes described in Methods are reported in Results. |
| Other bias | Unclear risk | Quote from ref #1: In the monitoring process of the study, we found that, at one of the centres, the women randomised to the nurse based system were scheduled to see a surgeon or oncologist each year in conjunction with the mammography. Before the data were scrutinised and any analyses undertaken, the 135 women from this clinic were excluded, since we deemed the two study arms at this centre to be too similar. However after all analyses and our first interpretation of data, we ran all analyses with the third centre included. One woman taking part in the study had experienced a recurrence of breast cancer before randomisation and was thus excluded. Finally, in the main analysis based on two centres 131 patients had routine follow‐up visits to a physician (PG), and 133 patients had contact with a nurse (NG) on demand. Comment: The authors do not mention if the results from the excluded centre were different from the other ones before re‐including it in the analyses. |
| Baseline outcomes similar? | Unclear risk | Quote from ref #2: "The participants were asked to answer the Hospitality and Depression Scale (HAD) and Satisfaction and Accessibility (SaaC) scales at baseline and twice a year over a period of five years." Comment: The outcomes were measured at baseline, but results are not presented. |
| Baseline characteristics similar? | Low risk | Quote from ref #1: "Marital status and age group were very similar in the study groups. The distributions of patients according to the UICC classification and treatment received were also similar in the two groups. About 8% of the women in NG stated that they had no support person available, whereas all women in PG had persons to confide in (Table 1)." Comment: No statistical analysis performed. All baseline characteristics appear similar except for the "axillary metastases present". We retested for differences between groups using the difference of proportions test and found a significant difference between groups (P = 0.04). However, the clinical significance of this difference seems small. |
| Protected against contamination? | Low risk | Comment: Randomisation was stratified by centre. |
Kousgaard 2003.
| Methods | Cluster‐RCT; Unit of allocation: General practitioner | |
| Participants | Cancer patients newly referred to the department of oncology and scheduled for treatment or attendance for control. Setting / country: Department of Oncology of Aarhus University Hospital / Denmark Type of cancer: Any type Phase of care: Pre‐treatment, treatment, discharge, surveillance, recurrence, Second‐primary‐cancer Sample size at randomisation: 199 GPs (248 patients) |
|
| Interventions | Shared care program: The patient was instructed to see his/her own GP about questions and problems. A discharge summary letter was written for the GP by the department of oncology in accordance with specially developed guidelines. The discharge summary included specific information on the disease and its treatment, general information about chemotherapy, radiotherapy, pain treatment, information about treatment of induced nausea and sickness and information about some acute oncological conditions (knowledge transfer). It also stated names and phone numbers of doctors and nurses responsible for the patient in the discharge summary letter to the GPs (improved communication channels). It also aimed to improve patient involvement in their own care by providing patients with oral as well as written information about the information package to their GP, and by encouraging patients to contact their GP when facing problems they assumed could be solved in this setting. Control: Normal procedure which included no procedure for informing the GP about newly diagnosed patients. The participating practitioner received the traditional information from the department, i.e. the discharge letter of an extract from the hospital record. |
|
| Outcomes | Patient: Performance status, QoL, attitudes of patients towards contacts with the GP Process: Patient perception of cooperation within the healthcare system, number of contacts with GP (patient interview), number of contacts with patient (GP interview) |
|
| Notes | Length of follow‐up: 6 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote from ref #2: "A project secretary outside the hospital premises kept a list of numbers from 1 to 250 randomly arranged into two groups. After obtaining written informed consent from the patients, the investigator opened an envelope with a random number of 1 to 250. This number was communicated to the project secretary who informed the investigator of the group to which this number (patient) belonged. Once a patient was randomised to a particular group, any further patients from the same general practice were automatically assigned to the same group." Comment: From the quote above, it is not clear how the 250 numbers were randomised into 2 groups. |
| Allocation concealment (selection bias) | Low risk | See quote above. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Quote from ref #2: "The study was unblinded. Patients in both groups were informed of the group to which they had been assigned as active involvement of the patients in the intervention group was part of the strategy". Comment: Data were collected though a self‐report questionnaire so assessors could not have been blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: Proportions of attrition were similar in control (26%) and experimental (33%) groups. Reasons for attrition also. |
| Selective reporting (reporting bias) | Low risk | All outcomes described in Methods are reported in Results. |
| Other bias | High risk | Quote from ref #2: "We used random allocation without blinding because we wanted to involve the patients by informing them about their possibilities. This implies a risk of information bias by the GP which may have influenced the time 0 scores. Patients in the intervention group may have had more positive expectations of their GP, knowing that they would be better informed about the disease and its treatment, and this may explain why the next two assessments by the GP were relatively less positive." Quote from ref #2: "The absence of regular baseline data is a problem as the patients actually received information about the intervention before answering the first questionnaire. They had 14 days to answer the questions at time 0. We had not foreseen this bias due to positive expectations in the intervention group. It would have been a real baseline if the patients had answered the time 0 questionnaires before randomisation, but we had a practical problem with time." Comment: Baseline assessments might have been biased, but not more than the other assessments made later during follow‐up, because of unblinded trial. However, this bias might have been prevented or adjusted for using statistical adjustments. |
| Baseline outcomes similar? | Low risk | Patients attitude towards their GP (many items)‐global assessment: NO No. of contacts with their GP: YES EORTC: YES Performance: YES |
| Baseline characteristics similar? | Low risk | Quote from ref #2: "Randomisation yielded an almost equal number of patients and an almost equal distribution according to disease and sociodemographic parameters in each group (table 1). However, the randomisation produced an imbalance in age with more young patients (18 to 49 years) in the intervention group." Comment: Sensitivity analysis were performed to see if results were different between younger and older patients. |
| Protected against contamination? | Low risk | Comment: Patients were randomised by GP. |
Kravitz 1996.
| Methods | RCT; Unit of allocation: Patient | |
| Participants | Patients with cancer admitted to the hospital within the past 48 hr with a diagnosis of malignancy and having at least "moderate pain" during the baseline assessment. Setting / country: University hospital in southern California and its affiliated VA Medical Center / USA Type of cancer: Any type Phase of care: Treatment Sample size at randomisation: 87 |
|
| Interventions | Bedside charting of pain level: Patients had daily pain assessments by study staff who graphically recorded their reported pain intensity levels on bedside wall charts. The sheet was placed behind patients' vital signs on a clipboard in their hospital room. Both nurses and physicians were able to review the information during their daily rounds and incorporate the information into their pain management plans. "Current pain" (dots) and "worst pain" (Xs) were charted in red ink on a 0‐10 scale each day. To highlight trends in symptomatology, dots and Xs were connected by solid lines. The display sheet also contained space to record patients' estimates of the number of hours sleep obtained during the past 24 hr, a short outline of pain management guidelines and an equianalgesic dosing table. Control: Patients had periodic pain assessments by study staff but did not have this information displayed. |
|
| Outcomes | Patient: Pain, sleep duration, symptoms, QoL, sleep latency Professional: Pain management |
|
| Notes | Length of follow‐up: 5 days | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: " Patients with either current pain or worst pain were asked for informed consent and then randomised to either the intervention or control group using a random numbers table." |
| Allocation concealment (selection bias) | Unclear risk | No mention of allocation concealment in the paper |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Research assistants performed the assessment but there is no mention of blinding in the paper. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Measurements were not available for some patients on days 3 and 5 because of early discharge, weekend or holiday schedules, or absence from the wards for diagnostic testing. For these patients, we substituted measures taken on day 2 (instead of day 3) or day 4 (instead of day 5). We could not measure day 5 outcomes for 28 patients due to their having been discharged before day 4." Comment: From Table 1 we can infer that the was a proportion of 36% missing data (28/78), but there is no data on the proportion in each experimental group. Imputation used was not acceptable. There are not enough data to verify if missing data could change study results. |
| Selective reporting (reporting bias) | Low risk | All outcomes described in Methods are reported in Results. |
| Other bias | Low risk | No evidence of any other bias |
| Baseline outcomes similar? | Unclear risk | Current pain, worst pain, Quality of life, Opioid analgesic score : YES Sleep duration, Sleep latency, Symptom scale: UNCLEAR |
| Baseline characteristics similar? | Unclear risk | Comment: Only 3 patient characteristics were presented: age, gender and white/non‐white. There are no description of the type of malignancy, prognosis, or other. |
| Protected against contamination? | High risk | Quote: "The intervention was intended to affect pain control primarily by influencing physician prescribing behaviour. In the two teaching hospitals where the study was conducted, physicians in their first postgraduate year of internal medicine training were responsible for writing all medication orders and were therefore the main target of the intervention. Because some interns cared for both intervention and control‐group patients, their exposure to intervention patients might have carried over into their care of control patients; this would tend to reduce the observed benefit of the intervention." |
Liu 2006.
| Methods | CCT; Unit of allocation: Patient; Stratified by: Day of visit to the hospital | |
| Participants | Women newly diagnosed as having breast cancer and scheduled to have a breast operation within a few days. Setting / country: Two urban teaching hospitals in Taipei /Taiwan Type of cancer: Breast Phase of care: Pre‐treatment, treatment, discharge, surveillance Sample size at randomisation: 61 |
|
| Interventions | Education and continuing supportive care (CSC): A follow‐up care plan was implemented. Through it, patients were sequentially provided with psychological support and health education on care of the breast cancer by a trained registered nurse. With this program, a senior nurse served as a coordinator to organise all the important information and to provide the proper teaching time scheduler. In this study, a senior nurse not only provided teaching and emotional support to both the subjects and their primary caregivers but also followed up the outcome step‐by‐step. The main contents of the continuing supportive care (CSC) were comprehensive, including provision of information, emotional and psychological support, appropriate referral, and continuous follow‐up (see the Appendix published with the article for more details). Control: Routine care provided by nurses from different units of hospital. Nurses would decide how and when to teach the subjects based on their own experience, and educational contents were not organised. Emotional support and education provided by nurses were not continuous but occasional throughout the whole disease progress. |
|
| Outcomes | Patient: Social support, uncertainty | |
| Notes | Length of follow‐up: 3 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "We used a quasi‐experimental research design in which study participants were not randomly assigned to the comparison groups. (...) Instead, we referred patients who came to the hospital on the same day to the same group." Comment: This trial used a quasi‐experimental design. |
| Allocation concealment (selection bias) | High risk | See quote first item. |
| Blinding (performance bias and detection bias) All outcomes | High risk | The outcomes were evaluated with self‐administered questionnaires, and patients were not blinded, so assessment could not possibly be blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "Five patients failed to complete the follow‐up over the 3‐month period: 4 in the experimental group and 1 in the control group. Reasons for dropping out included emigration soon after surgery was completed (n = 1) and inability to respond on time at 3 months after surgery (n = 4)." Comment: Reasons for attrition in each group are not described. No imputation seems to have been used. |
| Selective reporting (reporting bias) | Low risk | All outcomes described in Methods are reported in Results. |
| Other bias | Low risk | No evidence of any other bias. |
| Baseline outcomes similar? | Low risk | Table 2 shows that patients had similar outcomes at baseline. |
| Baseline characteristics similar? | Low risk | Table 1 shows that patients had similar characteristics at baseline. |
| Protected against contamination? | Low risk | Quote: "We decided not to perform random assignment of individual patients into groups for comparison to avoid a possible contamination bias that could be caused by 2 or more patients visiting the clinic on the same day likely discovering the contents of the nursing care received by the other person, so that those assigned to the control group would get information from their experiment counterparts on a later day." |
Luker 2000.
| Methods | CCT; Unit of allocation: Patient; Stratified by: Week in which women attended the breast specialist unit | |
| Participants | Women newly diagnosed with breast cancer. Setting / country: Hospital‐based specialist service / UK Type of cancer: Breast Phase of care: Pre‐treatment, treatment, discharge, surveillance Sample size at randomisation: 76 |
|
| Interventions | Services from a breast care nurse (same as control) + information cards: Eleven information cards were developed by breast specialist secondary care professionals for members of the primary healthcare team. Women with breast cancer were asked to take the information cards to their own general practitioner (GP) practice. They covered information on the rationale for a specific treatment, prognostic indicators, complications and side‐effects, suggestions for dealing with side‐effects and indicators for referral back to specialist services. Women were given cards corresponding to their treatment and the number and type of cards given to each woman was determined by the treatment received. Control: Services of a breast care nurse who offered home visits prior to admission for surgery and written patient information leaflets on a variety of treatment regimes. |
|
| Outcomes | Process: Number of contacts with GP and district nurse | |
| Notes | Length of follow‐up: 4 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "Study participants were allocated to one of two study groups: intervention or non‐intervention. Allocation was determined by the week in which women attended the breast specialist unit, alternate weeks being classed as intervention or non‐intervention weeks." Comment: This is a quasi‐experimental design trial. |
| Allocation concealment (selection bias) | High risk | See quote first item. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Comment: No details provided concerning the blinding of the persons who made interviews or analysis. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The proportions of dropouts in each group are presented (11% in intervention and 2% in control) but not the reasons for dropping out. Numbers of participants reported in the tables do not concur with the text regarding attrition, so it was impossible to judge if missing data could impact the observed effect size. |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes (objective measures) were reported in Results. |
| Other bias | Low risk | No evidence of any other bias |
| Baseline outcomes similar? | Low risk | Only the proportion of patients that had a contact with breast care nurse could be compared at baseline, since the other measures could not possibly be assessed at baseline. See Table 1 for comparison of no. of contacts with breast care nurse. |
| Baseline characteristics similar? | Low risk | Quote: "Patients in the intervention and non‐intervention groups appeared to be well matched on a number of demographic variables (Table 1)." |
| Protected against contamination? | High risk | Quote: "13/42 practices had women in the intervention and non‐intervention groups." Comment: The same professionals were in charge of control and experimental patients, so contamination was possible. |
McArdle 1996.
| Methods | RCT; Unit of allocation: Patient | |
| Participants | Patients undergoing breast cancer surgery. Setting / country: Three teaching hospitals in Glasgow / UK Type of cancer: Breast Phase of care: Treatment, discharge, surveillance Sample size at randomisation: 272 |
|
| Interventions | (1) Routine support from ward staff + support from breast care nurse: The support from breast care nurse included information about surgery, symptoms, treatments, and the option of a joint interview with husband or other relatives. The nurse emphasised that the patients would be seen again at their subsequent clinic visits and that they could make an appointment to see her at any time. The patients were given a contact telephone number. (2) Routine support from ward staff + support from voluntary organization (Tak Tent). Tak Tent support consisted in an introductory leaflet and subsequent contact by one of the counsellors after discharge from hospital. The individual counsellor was left to decide the level of support required: maintaining contact by telephone or post, arranging one to one meetings for counselling, and encouraging attendance at Tak Tent group meetings with fellow cancer sufferers. The counselling was based on the transactional analysis theory. (3) Routine support from ward staff + support from the breast care nurse + support from voluntary organization (Tak Tent). Control: Routine support from ward staff + an information booklet on breast cancer: Routine support consisted in care from three consultant surgeons with a strong interest in the management of breast cancer. The extent of surgery and the choice of adjuvant treatment were defined by a standard joint protocol. |
|
| Outcomes | Patient: Psychological morbidity, anxiety and depression (distress) | |
| Notes | Length of follow‐up: 12 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Before surgery, patients were randomised by telephone to one of four groups:..." Quote from author email message: "The allocation sequence was generated by using random numbers from a computer, with a range from 1 to 4 corresponding to the four treatment options. A list of the random allocations was printed out and kept secure by a secretary in the Royal Infirmary. Other than keeping the list, the secretary had nothing to do with the study. The study was carried out in three Glasgow hospitals with established breast clinics (Royal Infirmary, Western Infirmary and Victoria Infirmary). The first author (June McArdle) went round the three hospitals to identify eligible patients for the study. On identifying each eligible patient, she phoned the secretary at the Royal Infirmary who told her the next treatment allocation on the list. She was the only person who phoned the secretary to get treatment allocations. Since June McArdle never saw the list, she had no means of juggling the patients so as to get a particular treatment for a particular patient." |
| Allocation concealment (selection bias) | Low risk | See quote first item |
| Blinding (performance bias and detection bias) All outcomes | High risk | The outcomes were evaluated with self‐administered questionnaires, and patients were not blinded, so assessment could not possibly be blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: It is difficult to understand if attrition was similar in the four groups from the way data are presented. |
| Selective reporting (reporting bias) | Low risk | All outcomes described in Methods are reported in Results. |
| Other bias | Low risk | No evidence of any other bias |
| Baseline outcomes similar? | Unclear risk | Comment: No baseline data collection was performed. Breast care nurse met with patients before surgery whereas the first baseline test was done 1 month after surgery. |
| Baseline characteristics similar? | Low risk | Quote: "The overall number of patients and the number in each institution were similar in each group, and the groups were well matched for the various baseline characteristics recorded." Comment: Table 1 presents the baseline characteristics. No statistics were performed but the groups seem to be well matched. |
| Protected against contamination? | High risk | Comment: Allocation was not by institution. It is possible the booklet became available to the other groups, or that the practitioners involved modified their practice when aware of the intervention. |
McCorkle 1989.
| Methods | RCT; Unit of allocation: Patient | |
| Participants | Homebound subjects with newly diagnosed lung cancer (stage II or higher) and their spouses. Setting / country: Medical centres, hospitals and radiation outpatient facility in King County, Washington / USA Type of cancer: Lung Phase of care: Treatment, discharge, surveillance, palliative care Sample size at randomisation: 166 + 46 patient‐spouse dyads |
|
| Interventions | (1) Oncology home care group (OHC): Received care from oncology home care nurses trained to give personalised care to persons with advanced cancer and to their families. The advanced training background included: knowledge of symptom management, cancer treatments, pain management, physical assessment, psycho social assessment, grief and mourning theory, communications systems, community resources and agencies, systems analysis, self support, professional role development, pathophysiology of death, and research theory and methodology. Specialised services by other disciplines were called upon as needed. (2) Standard home care group (SHC): received care from an interdisciplinary team of health professionals (comprising registered nurses, physical therapist, home health aides, medical social work, occupational therapist, speech pathologist) that discussed treatment and case management plans, coordination of visits, length and intensity of services, need for consultation, coordination with physician, family and community resources. Control: Traditional treatment (referred to as an office care group (OC) in the paper): patients received whatever care they needed except for home care. This program was provided by the patients physician. |
|
| Outcomes | Patient: Symptoms, current concerns, perception of health, mood state, social dependency, pain Informal carer: Psychological distress during bereavement Process: Use of hospital services, number of visits to physician within the last 6 weeks |
|
| Notes | Length of follow‐up: Until death or the end of follow‐up (min. < 1; max. 6), but bereaved spouses continued to receive follow‐up for 25 months after the patient's death | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote from Ref #1: "Subjects were assigned randomly to one of the three treatment groups after the initial interview was completed. The project director contacted the appropriate agency and made a referral for home care services for the assigned patient." |
| Allocation concealment (selection bias) | Unclear risk | See quote first item. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Comment: No mention was made on the way the interviews were performed. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote from Ref #1:"78 subjects who completed four interviews were used to complete the substantive analyses, for which complete data (i.e. with no attrition) were required. The fifth occasion data were not included because of the small sample size at that data collection interval." Comment: Number of losses and reasons for attrition are reported in the whole sample but not in each study group. The way chosen to deal with missing data seems unacceptable. |
| Selective reporting (reporting bias) | High risk | Comment: Two measures listed in Methods were not reported in the Results section: pain and mood state. |
| Other bias | Low risk | No evidence of any other bias. |
| Baseline outcomes similar? | Low risk | Quotes from Ref #1: "The three groups did not differ significantly with respect to McGill‐Melzack Pain Questionnaire, ICC, and POMS scores. There were significant differences found on the Symptom Distress Scale, the Enforced Social Dependency Scale, and the Health Perceptions Questionnaire". "When the means for the core measures were plotted by occasion, it was apparent that the groups differed notably on the first occasion, with the oncology home care group tending to do better on most of the variables. See Figure I as an illustration of this tendency. This is unusual because randomisation did not occur until after Occasion 1. Because group assignment was nonexistent at Occasion 1, the study was effectively double‐blind at this point, and there was no way group assignment could have had an effect on the outcome measures" "In an attempt to adjust for this problem, the following analyses treat data from Occasion 1 as covariates in predicting scores on Occasions 2,3, and 4. Thus, the principal analyses pertain to three levels of Occasion adjusted for initial level at Occasion 1, as if the groups had in fact been matched at Occasion 1. Although this kind of adjustment can be questionable when there is reason to believe that the covariate and treatment are confounded, the current conditions are precisely those that minimize this danger: Since Occasion 1 preceded group assignment, there is every reason to believe that the initial differences were due to chance sampling error." Comment: Significant differences were found at baseline on three outcome variables, but analysis was adjusted consequently, by using results at baseline as covariates. |
| Baseline characteristics similar? | Low risk | Quote from Ref #1: "Chi‐square tests indicated no statistically significant group differences on demographic variables indicating that randomisation resulted in equal distribution of potentially confounding variables across treatment groups." |
| Protected against contamination? | Low risk | Patients were assigned to different healthcare providers when they were assigned to the various treatment groups, so contamination is unlikely. |
McCorkle 2000.
| Methods | RCT; Unit of allocation: Patient | |
| Participants | Post‐surgical cancer patients aged 60 or older diagnosed with a solid tumour and given a survival prognosis of 6 months or greater after the surgery. Setting / country: Comprehensive Cancer Center in south eastern Pennsylvania / USA Type of cancer: Any type Phase of care: Discharge, surveillance, palliative care Sample size at randomisation: 375 |
|
| Interventions | Specialised home care provided by advanced practice nurses (APNs): The APN telephoned the patients within 24 h after discharge to schedule a meeting. The intervention consisted in standard assessment and management of post‐surgical guidelines, doses of instructional content, and schedules of contacts. It lasted 4 weeks and consisted of three home visits and five telephone contacts provided by APNs. Both the patients and their family caregivers received comprehensive clinical assessments, monitoring, and teaching, including skills training. The APNs followed specific guidelines to assess and monitor the physical, emotional, and functional status of patients, provide direct care when needed, assist in obtaining services or other resources from the community, and provide teaching, counselling, and support during the period of recovery. Nurses also functioned as a liaison to healthcare settings and providers, as well as to patients and families, in the provision of technical and psychological support. If complications arose, the APNs consulted with physicians and intervened immediately. APNs were available on a 24‐hour basis using a paging system. Control: Standard postoperative care in the hospital and routine follow‐up in outpatient clinics upon discharge. |
|
| Outcomes | Patient: Survival | |
| Notes | Length of follow‐up: 44 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: " Once Wave 1 data were obtained, subjects were randomised using the sealed opaque envelope technique" |
| Allocation concealment (selection bias) | Unclear risk | See quote first item. |
| Blinding (performance bias and detection bias) Functional status | Unclear risk | Functional status was "reported by the patient and rated by the interviewer". No details on blinding of the interviewer. |
| Blinding (performance bias and detection bias) Psychological status | High risk | Symptom distress and depressive symptom were self‐reported. Since the patients were not blinded than the assessor could not be blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "Reduction in sample size was caused primarily by death." Comment: Survival being an end‐point, it should not be addressed as missing data. However, the other causes of attrition should have been reported in each group since they could have bias the results. |
| Selective reporting (reporting bias) | Low risk | All outcomes described in Methods are reported in Results. |
| Other bias | Low risk | No evidence of any other bias. |
| Baseline outcomes similar? | Low risk | Comment: The three psychosocial measures were similar in the experimental groups at baseline (Table 2). |
| Baseline characteristics similar? | Low risk | Quotes: "Despite randomisation, there were differences between the two groups on stage at diagnosis, with the intervention group having more late stage patients (38%) compared with the control group (26%)." "Since the two groups differed on stage of disease post‐randomisation, stratified log‐rank test was used to compare them." |
| Protected against contamination? | Unclear risk | Comment: Patients were the unit of randomisation. There is no information on the setting through which the APN nurses in charge of patients in the intervention group operated. If they operated through the same outpatient clinics than nurses in charge of control group patients, then there would be a risk of contamination. |
McCorkle 2009.
| Methods | RCT; Unit of allocation: Patient | |
| Participants | Post‐surgical women suspected of having a primary diagnosis of ovarian cancer following abdominal surgery. Setting / country: A North eastern teaching hospital associated with a comprehensive cancer centre in State of Connecticut /USA Type of cancer: Cervical / Ovarian Phase of care: Surveillance, palliative care Sample size at randomisation: 149 |
|
| Interventions | Specialised care by an advanced practice nurse (APN): APN activities included symptom management and monitoring, emotional support, patient education, coordination of resources, referrals, and direct nursing care. Services included 18 patient contacts during the first 6 months after hospital discharge (home visits, telephone calls, clinic visits). The plan of care and intervention strategies were individually tailored to each patient's needs and personal priorities and were determined jointly by the nurse and patient. Women in high distress were evaluated and monitored by a psychiatric consultation‐liaison nurse (PCLN) as recommended by the National Comprehensive Cancer Network guidelines. In addition, patients received the Symptom Management Toolkit (SMT), a manual containing information on 16 symptoms commonly experienced post‐surgically or with chemotherapy. Control: Attention control group: patients in this group also received the Symptom Management Toolkit (SMT). An initial contact with a research assistant took place at patients' homes where instruction on the use of the SMT was given. At subsequent contacts, research assistants inquired about the presence of symptoms and the utility of the proposed strategies in the SMT in managing the symptom. Patients who had questions outside the content of the SMT were encouraged to call their oncologists. Services included 1 home visit and 3 weekly telephone calls during the first month after hospital discharge and monthly telephone calls for the remaining 5 months of the intervention period. |
|
| Outcomes | Patient: QoL, depression, uncertainty, symptom distress | |
| Notes | Length of follow‐up: 6 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from ref #1: "After baseline data were obtained, consented patients were randomised into the intervention or attention control group using the sealed envelope technique." |
| Allocation concealment (selection bias) | Unclear risk | See quote first item. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote from ref #1: "Subsequent data collection visits were completed as in‐person interviews by trained research assistants in the patient’s home." Comment: Outcomes were collected via questionnaire administered to patients. The authors presented the study as a single‐blind randomised clinical trial, but no details are provided on who was blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Proportions of attrition in the 2 study groups were not reported. Overall, there was 15% attrition in the 2 groups. |
| Selective reporting (reporting bias) | Low risk | All outcomes described in Methods are reported in Results. |
| Other bias | Low risk | No evidence of any other bias. |
| Baseline outcomes similar? | Low risk | Quote from ref #1: "At baseline, there were significant differences between the two groups on three outcome variables (CES‐D, uncertainty, and SF‐12‐mental), with the nursing intervention group reporting poorer QOL. However, baseline scores for both groups were adjusted for model testing and were consistent with reports in the literature documenting high psychological and physical impact in high‐risk populations." |
| Baseline characteristics similar? | Low risk | Quotes from ref #1: "Patients in the intervention and attention control groups did not differ significantly in terms of demographic and clinical characteristics, including stage, primary ovarian site, and new or recurrent disease" "All applicable covariate variables (age less than 60 or greater than and equal to 60, White race or not, recurrent cancer or not, education less than or equal to high school or greater than high school, early or late stage, married or not, number of co‐morbidities, combined income less than or equal to $30 000 or greater than $30 000, emotional distress score equal to or greater than 4 or not, PCLN or not, and adjusted QOL baseline scores) identified in the preliminary analyses were included as well as their interactions with time." |
| Protected against contamination? | Low risk | Comment: Patients were not randomised by clinics, or practice, but healthcare providers were in charge of the intervention group whereas a research assistant was in charge of the control. The risk of contamination between the two appears to be small. |
McDonald 2005.
| Methods | Cluster‐RCT; Unit of allocation: Nurse | |
| Participants | Patients admitted with a primary diagnosis of cancer, self‐reporting daily or constant pain at admission and their home health nurses. Setting / country: A large urban, non‐profit home care organisation / USA Type of cancer: Any type Phase of care: Any phase Sample size at randomisation: 336 (673 patients) |
|
| Interventions | (1) Basic home care: consisted of a patient‐specific, one‐time e‐mail reminder sent to nurses within 10 days of each new eligible patient's admission to home care. The e‐mail identified the patient by name, indicated that the patient reported pain at admission, and highlighted six pain‐specific clinical recommendations; the first letter of each practice spelling out the acronym "RELIEF" for: R Reassess pain E Eliminate Barriers L Learn more about analgesics I Intervene to limit side effects E Encourage the use of complementary (non‐medication) therapies F Follow up with MD/Nurse practitioner if pain is not relieved; (2) Augmented home care: In addition to the basic e‐mail, it included a laminated pocket card that directed the nurses how to complete a comprehensive pain assessment, including a 0 to 10 visual scale to use with patients, a prompter card to help nurses improve communication with physicians, a self‐care guide to review with patients and open a dialogue about pain control, and follow‐up by an oncology Clinical Nurse Specialist (CNS) who served as an expert peer. The CNS was employed by the agency and available to all staff requesting a consultation. The augmented intervention outreach was a more pro‐active approach. It consisted of an e‐mail sent by the CNS to the nurse a week after the first e‐mail and reminded the augmented group nurse that the CNS was available for consultation. Control: Usual care: the control group nurses did not receive any intervention materials and provided usual care. |
|
| Outcomes | Patient: pain, QoL, patient‐related barriers to pain management, nurses estimation of patients pain intensity Professional: pain management |
|
| Notes | Length of follow‐up: 18 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Each nurse was randomly assigned to either the control group or one of two treatment groups (basic intervention or augmented intervention) the first time s/he began caring for an eligible patient." "Although nurses were randomly assigned to treatment or control groups, random assignment of patients to nurses was not feasible. Patients referred to the study agency, however, are assigned to a specific nurse based primarily on where the patient lives and the nurse's overall caseload." |
| Allocation concealment (selection bias) | Low risk | Quote: "Furthermore, agency staff responsible for assigning patients to nurses were blinded to the study." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quotes: "Record abstractions were completed by trained nurse reviewers who were blinded to the intervention group assignment of study nurses and their patients." "All interviews, which took place over an 18‐month period, were conducted by trained interviewers blinded to the study groups." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "Two conditions are necessary for the intervention estimates to be biased: (i) the pattern of attrition differs by treatments and controls and (ii) attrition is correlated with the outcome measure being examined. Because there was evidence of differential attrition among the groups in the study, we followed the traditional econometric approach of estimating outcome models jointly with a sample retention equation to produce attrition‐corrected estimates of the interventions on nurse process measures and patient outcomes. Specifically, a bivariate probit specification was used to model process and outcome measures that were binary in nature (yes/no) while a two‐stage Heckman selection correction specification was used to model continuous outcome variables". Comment: The authors seem to have used quite sophisticated statistical methods to take into account missing outcomes, but we were not able to judge whether they were appropriate. |
| Selective reporting (reporting bias) | Low risk | All outcomes described in Methods are reported in Results. |
| Other bias | Low risk | No evidence of any other bias. |
| Baseline outcomes similar? | Low risk | Quote: "In addition to the main variables of interest ‐ membership in the basic or augmented treatment group ‐ all regressions included pre‐intervention, aggregate measures of pain frequency and intensity, age, level of disability, and number of comorbid chronic conditions of each nurses patients; the nurses age, sex, race/ethnicity, educational level, experience, employment status, and overall caseload; and county of practice." |
| Baseline characteristics similar? | High risk | Quotes: "There were no statistically significant differences between control and basic intervention nurses or between control and augmented intervention nurses" "The groups differed, however, with respect to whether the patient had surgery immediately prior to home care admission and time since cancer diagnosis." "In particular, we controlled for patient‐level baseline measures of health and functional status assessed by the nurse during the initial visit, including frequency and intensity of pain, ADL and IADL limitations, limitations in cognitive functioning, and the presence and number of certain pre‐existing medical conditions; the patients demographic characteristics, including age, sex, race/ethnicity, marital status, education, expected payment source, and baseline measures of social support; the provider nurses baseline characteristics; and an indicator of the reimbursement environment (pre‐Prospective Payment System or otherwise). Finally, measures of the nurses caseload at the time of patient assignment and county of practice were also included to control for factors simultaneously affecting patient assignment to a specific nurse and patient outcomes. Comment: There is no mention that the results were adjusted to take into account baseline differences in surgery prior/after home care admission or time since cancer diagnosis. |
| Protected against contamination? | Low risk | Quote: "A nurses initial random assignment to a specific group (usual care, basic intervention, or augmented intervention) determined the status for all new patients allocated to that particular nurses care for the duration of the study." Comment; Because nurses worked in the patient homes, then the risk of contamination between nurses was negligible. |
McKegney 1981.
| Methods | Cluster‐RCT; Unit of allocation: Counties in Vermont that were paired on the basis of population density, distance from the Medical Centre, socio‐economic status, local medical facilities, referral patterns, and local social services resources. | |
| Participants | Patients receiving palliative radiation and/or chemotherapy having an expected survival of greater than 3 months but less than 1 year. Setting / country: Medical Center Hospital of Vermont / USA Type of cancer: Any type Phase of care: Treatment, palliative care Sample size at randomisation: 199 |
|
| Interventions | Home visits by trained oncology nurse practitioners + multidisciplinary care: Nurse practitioners with extensive experience in care of the patients with advance cancer were selected. The home visit by the nurses was primarily focused on attending to the needs of the patient, and interactions with family members were incidental to that task. In addition to providing physical care, much of the nurses time was spent in talking with the patient about their illness and its implications. The nurse frequently mobilised family and other social resources to meet the patients needs and also coordinated with the patients local physician. These nurses thus served in the well‐known public health, or visiting nurse role, with the difference that the project nurse had the benefit of a multidisciplinary healthcare team back‐up resource. A Protocol for Management of Pain was developed by the team and used by the nurses as part of their wide range of physical treatments and psychosocial interventions. This protocol was based upon sound pharmacological principles, many of which are often ignored (additional details provided in the article). The patients also received multidisciplinary care at the Medical Center Hospital of Vermont (MCHV) and/or from their private physicians. Regular participants in the multidisciplinary team consisted of medical and radiation oncologists, psychiatrists, social workers, physical therapists, nutritionists, occupational therapists, enterostomal therapists, and clergymen. Patients with an expected survival of less than 3 months were visited by nurses biweekly and those expected to live longer were visited monthly. Control: Multidisciplinary care alone: patients in this group were not visited at home by nurses but received multidisciplinary care at the hospital and care was otherwise the same as that of the intensive group. |
|
| Outcomes | Patient: Psychological symptoms, internal ‐ external expectation of control, pain, health status | |
| Notes | Length of follow‐up: 48 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote from ref #1:"Counties in Vermont were paired on the basis of population density, distance from the Medical Center, socioeconomic status, local medical facilities, referral patterns, and local social services resources. The paired counties were randomly separated into two groups, with one designated intensive and the other non‐intensive. |
| Allocation concealment (selection bias) | Unclear risk | See quote first item. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: "Follow‐up data bases were gathered from both intensive and non‐intensive patients by trained independent raters, using structured interviews in the patients’ homes, done at the same frequency as the nurses’ visits, which were based upon the patients’ prognosis." Comment: Although the raters are described as independent, there is no mention that they were blind to treatment allocation. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quotes from ref #1: "A total of 199 patients, 98 in the intensive and 101 in the non‐intensive groups, were followed; at the close of the four‐year study 139 had died." "Of the 139 patients who died during the study, 38 intensive and 45 non‐intensive patients had a sufficient number of pain ratings (three or more) to compare the effectiveness of pain management over time in the two groups." Comment: This study only uses data collected from the patients that died. Among those that died, they only kept the ones that had 3 or more assessments made before their deaths. The number of patients who died in each group are not presented, we only know the amount of patients who died AND had enough data collected in each group. We thus cannot infer the proportion of missing data in each group. The choice made to use only data from the patients with 3 or more assessments appears unacceptable. |
| Selective reporting (reporting bias) | High risk | No results of the KPS at follow‐up were presented. |
| Other bias | Low risk | Quote: "Another major problem in this study involved the trained observers who gathered follow‐up data from patients in their homes. These observers were continually instructed to limit their activities to asking questions, observing behavior, and recording data. Early in the study, it became apparent that these home observers could no their needs became apparent in the process of data gathering." Comment: This bias might have led to an underestimation of treatment effect. Because the intervention had a significant effect on pain, then we consider that the bias was either small or that treatment effect was considerable, The bias could not have led the observation of a wrong effect. |
| Baseline outcomes similar? | Low risk | Quotes: "The initial, on‐study scores on the CMI, 1‐E, and KIS did not differ significantly between the intensive and non‐intensive groups." "It should be briefly noted that the intensive and non‐intensive patients did not differ in terms of length of survival, nor did these two groups differ in several other quality of life outcomes such as physical activity, nutrition, optimism, or overall health status as defined by the KPS (7)." |
| Baseline characteristics similar? | Low risk | Quote from ref #1: "A comparison of patient characteristics for these two groups demonstrated similarities in cancer diagnosis, sex, age, social class, and religious preference (Table 1)." |
| Protected against contamination? | Low risk | Comment: Randomisation was clustered by counties. |
McLachlan 2001.
| Methods | RCT; Unit of allocation: Patient; Stratified by: Clinic of origin | |
| Participants | Patients having a diagnosis of cancer attending the ambulatory clinics at Peter MacCallum Cancer Institute. Setting / country: Peter MacCallum Cancer Institute, Melbourne / Australia Type of cancer: Any type Phase of care: Any phase Sample size at randomisation: 450 |
|
| Interventions | Coordinated psychosocial care based on patient self‐assessment: patients completed a self‐report questionnaire about their cancer needs, quality of life and psychosocial information via a touch screen computer. A computer‐generated one‐page summary of the questionnaire results was then made available immediately for consideration during the consultation with the doctor, where the coordination nurse was also present. After discussion with the patient and doctor, the coordination nurse formulated an individualised management plan based on the issues raised in the summary report, and pre‐specified psychosocial guidelines formulated by a group of multidisciplinary experts. The guidelines were developed to be linear single pathways broadening to multiple options, but the coordination nurse was encouraged to apply her clinical expertise in prioritising and negotiating referrals. The nurses were responsible to implement the plan and involve other members of the healthcare team as appropriate. Control: Patients underwent a conventional clinical encounter, and the self‐reported information was not made available to the healthcare professionals at any time. However, for ethical reasons, if a control group patient reported a serious concern (e.g. suicidal ideation), then the care coordination nurse was allowed to inform the appropriate health professionals. |
|
| Outcomes | Patient: Cancer needs, QoL, depression Process: Number of services offered and accepted by patients |
|
| Notes | Length of follow‐up: 6 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Before randomisation, patients were stratified by clinic of origin (lung, gynaecology, medical oncology, head and neck cancer, or skin cancer). Computer‐generated randomisation charts were prepared for each clinic and held in the statistical office. The probability of a patient being assigned to a particular arm depended on the imbalance in the number of preceding patients assigned to the two arms in that clinic." |
| Allocation concealment (selection bias) | Low risk | See quote first item. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Quote: "Patients completed self‐reported questionnaires via a touch‐screen computer (see Measures)." Comment: The outcomes were evaluated with self‐administered questionnaires, and patients were not blinded, so assessment could not possibly be blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Overall, 385 patients (86%) completed the 2‐month questionnaires and 318 (71%) completed the 6‐month questionnaires. The percentage of patients completing the questionnaires was very similar in both arms: 84% of control patients compared with 86% of intervention patients at 2 months (P < 0.48) and 69% compared with 72% respectively at 6 months (P < 0.59). The reasons for not completing the questionnaires were also similar and included death, patient refusal, poor health, and lost to follow‐up." |
| Selective reporting (reporting bias) | Low risk | All outcomes described in Methods are reported in Results. |
| Other bias | Low risk | No evidence of any other bias |
| Baseline outcomes similar? | Low risk | Quote: "Patients in the intervention arm tended to have more psychologic needs, physical and daily living needs, and patient care and support needs as measured by the CNQ, but a similar proportion were moderately or severely depressed on the BDI (Table 3). Both groups had very similar functional scores and global health status/QOL scores on the QLQ‐C30 (data not shown)." "Each primary outcome variable was analysed using a linear model for the change from baseline, where the model also included the baseline value as a factor. Other changes from baseline were analysed in the same way." |
| Baseline characteristics similar? | Low risk | Quote: "Patient demographics were well balanced in the two arms (Table 2)." |
| Protected against contamination? | High risk | Quote: "Another possible design limitation of this study was the potential for contamination between the groups. Doctors and clinic nurses were involved in seeing both intervention and control patients in the ambulatory care clinics. The health professionals behavior may have changed as a result of a heightened awareness of the study purposes and issues raised by patients in the intervention group. The usual care patients could then have benefited from this shift, resulting in the high levels of satisfaction in both groups" |
McWhinney 1994.
| Methods | RCT; Unit of allocation: Patient; Stratified by: N/A | |
| Participants | Patients having symptomatic cancer which had metastasised or spread to surrounding tissues, expected to survive for two months, and being cared for at home by an eligible care giver. Setting / country: A home care service and about 200 general practitioners, most of whom provided home care: London, Ontario / Canada Type of cancer: Any type Phase of care: Palliative care Sample size at randomisation: 146 |
|
| Interventions | Palliative care home support team: the team was based in on a 14‐bed palliative care unit, and consisted of 2 experienced palliative care nurses, one physician, and a part time social worker. Because of the range of home care services available already, the team was planned to be a consulting and support service for family physicians and home care nurses. Within 3 days of referral by a family doctor or nurse (with family doctor's agreement) one of the team nurses carried out a full assessment of the patient in his/her home. The nurse's assessment and recommendations were discussed with the team physician, then sent to the family physician with copies to the visiting nurse and home care case manager. A consultation by the team physician was available on request. All new and active cases discussed at the weekly team meeting. Involvement of the team following initial assessment depended on the wishes of the patient and family and on negotiation with the family physician and home care nurse. There was either no further contact with the patient or caregivers, progress monitored through telephone calls, or periodic visits made to the home. Patients were given a number to call one of the team nurse if their home nurse or family physician could not be reached. Patients received the intervention immediately after enrolment. Control: Waiting list: patients received the same intervention as the intervention group one month after enrolment. Emergency consultation by the team physician was made available for patients in the waiting list group if requested by the family physician. |
|
| Outcomes | Patient: Pain, symptom ‐ nausea | |
| Notes | Length of follow‐up: 18 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The project coordinator assessed eligibility and conducted randomisation using a computer generated table of random numbers. A research assistant, who was blind to the assignment, visited the home to give more details of the study; obtain written consent;and explain the questionnaires, including the Melzack three day nausea and pain diary, and leave them with the patient and care giver. The assistant visited again after three days to collect the questionnaires then notified the coordinator that baseline data collection was completed. Data collection was repeated at one and two months, one month being the main comparison point." |
| Allocation concealment (selection bias) | Low risk | See quote above. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Quote: "A research assistant, who was blind to the assignment, visited the home to give more details of the study, obtain written consent, and explain the questionnaires to complete. Data collection was repeated at one and two months." Measures were collected through self‐report questionnaires, and the patients were not blinded, so the assessors could not have been blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "Of the 146 randomised, 53 were lost to follow up before one month, 36 because of early death and 14 because of failure to complete the one month questionnaires. Only 74 care givers completed the questionnaires." Comment: No results were presented. |
| Selective reporting (reporting bias) | High risk | No results were presented. |
| Other bias | High risk | Quote: "Admission of patients in the control group to the palliative care unit exposed them to a standard of palliative care equivalent to that offered by the palliative care home support team." |
| Baseline outcomes similar? | Unclear risk | Comment: No results were presented because of an important attrition due to death among participants. |
| Baseline characteristics similar? | Unclear risk | Comment: No results were presented because of an important attrition due to death among participants. |
| Protected against contamination? | High risk | Quote: "Admission of patients in the control group to the palliative care unit exposed them to a standard of palliative care equivalent to that offered by the palliative care home support team." Comment: The same care providers were in charge of emergency care for control group participants and for intervention group participants. Some possibilities of contamination therefore existed. |
Mills 2009.
| Methods | RCT; Unit of allocation: Patient | |
| Participants | Patients newly diagnosed of inoperable lung cancer. Setting / country: Three hospitals in Northern Ireland / UK Type of cancer: Lung Phase of care: Palliative care Sample size at randomisation: 115 |
|
| Interventions | Patient‐held quality‐of‐life diary: The intervention involved the weekly completion by the patient of a QoL questionnaire in a diary format. The questionnaire was the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire C30 and the related lung cancer module LC13. Patients kept their diary at home and were requested to complete it at a regular time each week and to share the information with any health professional involved in their care. A new diary was posted to the patient each month with a self‐addressed envelope in which the previous diary could be returned to the researcher via mail or at their next hospital appointment. Relevant healthcare professionals were informed about the study and that patients may wish to share their diary information during consultations. The hospital team received basic training sessions from the researcher on the content and layout of the diary, whereas the primary care team received this information in written form via mail. Control: Standard care: patients did not receive QOL questionnaires in a diary format. |
|
| Outcomes | Patient: QoL, satisfaction Process: Discussion of patient problems |
|
| Notes | Length of follow‐up: 4 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from ref #1: "Patients providing written informed consent were assigned randomly to the diary group or standard care using block randomisation with computer‐generated random numbers." |
| Allocation concealment (selection bias) | Unclear risk | See quote first item. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Comment: Outcomes were measured using self‐report questionnaire. Because the patients were not blinded and they were the ones performing the assessment, then the assessors were not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: Proportions of missing data were similar in control group (53%) and in intervention group (47%). Reasons for dropping out were also similar. |
| Selective reporting (reporting bias) | Low risk | All outcomes described in Methods are reported in Results. |
| Other bias | Low risk | No evidence of any other bias. |
| Baseline outcomes similar? | Low risk | Quote from ref #1: "Groups were comparable regarding age, sex, diagnosis, WHO performance status, treatment received, and baseline QOL scores." |
| Baseline characteristics similar? | Low risk | See quote item G. |
| Protected against contamination? | Low risk | Comment: Allocation was not performed by practice or by care professionals. However, since the intervention did not require any particular action of the care professionals involved, but to check patients' diary of participants in the intervention group, then we judged the risk of contamination was minimal. |
Mitchell 2008.
| Methods | RCT; Unit of allocation: Patient | |
| Participants | Patients having a life expectancy of at least 1 month and referred to three specialist palliative care units (note from author: 92.5% patients with cancer). Setting / country: An inner urban, an outer metropolitan and a regional general hospital in Queensland / Australia Type of cancer: Any type Phase of care: Palliative care Sample size at randomisation: 159 (101 GPs) |
|
| Interventions | Case conferences between the patient's GPs and a specialist team: the intervention case conference was intended to provide an opportunity for negotiating a treatment plan for the patient, with the GP playing an active part. The case conference was conducted by teleconference, with the GP phoning in to a routine specialist team meeting. At two of the participating services, representatives of domiciliary nursing services routinely attended these meetings. Subsequent communication followed normal practice (i.e. faxed or posted letters, and telephone communication between family physician and specialist, or domiciliary nurses present at specialist team meetings acting as an intermediary). Control: Standard care: case review by the specialist team, with routine communication with the GP thereafter (i.e. faxed or posted letters, and telephone communication between family physician and specialist, or domiciliary nurses present at specialist team meetings acting as an intermediary). |
|
| Outcomes | Patient: QoL Informal carer: Carer burden Process: Number of case conference completed |
|
| Notes | Length of follow‐up: Until death or the end of the study (min. 1; max. 22) months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from ref #1: "The unit of randomisation was the GP?patient dyad: these were randomised by an administrative officer off‐site using a computer‐generated random number list and block randomisation. Subsequent patients of a previously randomised GP were allocated to the same arm as the first patient. We achieved concealment of allocation from both patient and GP by explaining that different means of communication between GPs and palliative care teams were being tested without informing them of the details. Neither the palliative care team nor the research officer at each site could be blinded to the allocation." |
| Allocation concealment (selection bias) | Low risk | See quote first item. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: "Neither the palliative care team nor the research officer at each site could be blinded to the allocation." Comment: Patients were interviewed for data collection. From the above citation, it seems that interviews were conducted by a research officer, but it cannot be ascertained. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: Proportions of participants who withdrew from the study because of death were similar between intervention (84%) and control groups (89%). Details of the reasons of the other withdrawals are not given for each study group, but for all participants instead. No details on the way the researchers have dealt with withdrawals other than death are provided. Because number of participants in each group used for analysis are not detailed, we could not verify if missing data could have changed the observed effect size. |
| Selective reporting (reporting bias) | Low risk | All outcomes described in Methods are reported in Results. |
| Other bias | Low risk | No evidence of other bias. |
| Baseline outcomes similar? | Low risk | Quotes from ref #1: "The control group had a few QoL subscale outcomes that were better than the intervention group at baseline, and others over the following 3 weeks. (Table 4)" Relative to carer burden: "There were no differences between the groups at baseline." "Two analytical approaches were planned a priori. The first used measures of change from baseline. The second used death as the fixed point and grouped interview data by time from the data collection interviews to death. The purpose of this analysis was to account for differing degrees of severity of illness present at recruitment into the study." Comment: The first analytical approach used takes into account baseline differences in measurements. |
| Baseline characteristics similar? | Low risk | Quotes from ref #1: "The demographic characteristics of the two groups were similar, except for age on admission (the median intervention group age was 6.6 years older) (Table 2). Colorectal cancer was over‐represented in the control group (24% vs 14%), and prostate cancer over‐represented in the intervention group (14% vs 8%)." "The intervention and control GPs were similar, Table 2." Comment: Few characteristic differed between treatment groups, and these differences in age seem unlikely to bias study results. |
| Protected against contamination? | Low risk | Comment: The GPs were allocated so contamination was prevented |
Moore 2002.
| Methods | RCT; Unit of allocation: Patient; Stratified by: Hospital and treatment intent | |
| Participants | Patients with lung cancer having completed their initial treatment and expected to survive for at least 3 months. Setting / country: Specialist cancer hospital and three local cancer units in south‐eastern England / UK Type of cancer: Lung Phase of care: Surveillance, palliative care Sample size at randomisation: 203 |
|
| Interventions | Nurse‐led follow‐up: Patients were allocated to one of two clinical nurse specialists in lung cancer and were assessed monthly by protocol over the telephone or in a nurse‐led clinic to identify signs of disease progression, symptoms warranting intervention, or serious complication. Additional contacts were made as necessary: patients had access to the clinical nurse specialists in the nurse‐led clinic or by telephone without an appointment. Nurses role was to provide information and support, coordinate input from other agencies or services. The clinical nurse specialist was responsible for the entire care of patients unless the patient needed further treatment. The emphasis was on rapid and comprehensive communication with general practitioner and primary healthcare team by telephone, fax, or letter, as appropriate. Regular discussion was made with and referral to medical team on detection of any new symptom or rapid worsening of condition. Also, documentation from nurse‐led clinic was held in notes and sent to general practitioner, home care team or hospice, if applicable, and consultant in charge of patient. Medical consultants gave regular clinical supervision sessions for the clinical nurse specialist. Control: Conventional medical follow up: routine outpatient appointments (one post‐treatment appointment, then appointments at two or three month intervals) for medical assessment and investigations to monitor disease progression. Patients were also seen on the basis of need. |
|
| Outcomes | Patient: QoL, satisfaction, survival, symptom‐free survival, progression‐free survival Process: Use of hospital services, place of death |
|
| Notes | Length of follow‐up: 12 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "For randomisation, patients were stratified according to hospital and treatment intent." Comment: No details are provided regarding sequence generation. |
| Allocation concealment (selection bias) | Low risk | Quote: "An independent trials office was responsible for randomisation of patients to either conventional medical follow up or nurse led follow up." |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No details on the way the outcomes were measure are provided in the article. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: Proportions of attrition and reasons for attrition are generally similar between treatment group, with slight difference at 6 months for the proportion of of non‐compliant (13% vs 3%) and at 12 months for the number of deaths (29% vs 17%). No imputation was used. Because numbers of participants presented in the tables differed for the flow chart numbers, we could not verify if missing data had an impact on the observed effect size. |
| Selective reporting (reporting bias) | Low risk | All outcomes described in Methods are reported in Results. |
| Other bias | Low risk | No evidence of other bias |
| Baseline outcomes similar? | Low risk | Quote: "The clinical characteristics at baseline were similar between groups (table 1), as were scores for quality of life and patient satisfaction at baseline." |
| Baseline characteristics similar? | Low risk | See quote from item G. |
| Protected against contamination? | Low risk | Nurse specialists from three local cancer units were responsible for patients in the intervention group whereas physician in an outpatient unit of the hospital were responsible for control group participants. The use of different settings and specialists prevents contamination. |
Mor 1995.
| Methods | RCT; Unit of allocation: Patient; Stratified by: Unmet need status, gender | |
| Participants | Cancer patients initiating a new course of chemotherapy. Setting / country: One of two hospital based chemotherapy clinics and eight private medical oncology practices in Rhode Island / USA Type of cancer: Any type Phase of care: Treatment, discharge, surveillance Sample size at randomisation: 257 |
|
| Interventions | Short‐term case management intervention: The intervention consisted in two visits from the case manager and intervening telephone calls accompanied by individualised information about possibly needed services and their availability in the community. Specifically, it comprised an initial needs assessment, the development of an intervention plan, a follow up phase, and a termination visit. The initial home visit allowed the case manager and patient to discuss the nature and length of the intervention and their mutual expectations. The case manager conducted a clinical assessment and presented the patient with preliminary educational material focusing on needs documented in the baseline interview. A specific intervention plan was devised for each need identified in the clinical assessment. the case manager provided information on the service resources needed by the patient that were located near the patient's home. The first follow‐up contact occurred via telephone two to three days after the initial visit to reinforce the patient/case manager relationship and to address any new questions or concerns that developed. The case manager then telephoned patients at two‐week intervals to assess new unmet needs requiring intervention and to monitor the progress of previously implemented interventions. The patient could contact the case manager for assistance at any time during the follow‐up period. At the last visit, the case manager reviewed the patients' unmet need status and earlier instructions about how to solve selected types of unmet needs that might arise in the future. The case manager also presented each patient with a generic "termination" package, containing further information on community resources. A resource database was created especially for the project in order to organize information about statewide community service agencies and cancer‐specific disease and treatment information. This provided the case manager with general resource material, and allowed her to generate customised information packets for each experimental patient. Control: No intervention from the case manager. |
|
| Outcomes | Patient: Unmet needs, symptom severity, mood state, QoL Process: Use of hospital services, use of home care services |
|
| Notes | Length of follow‐up: 6 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The study was a stratified, random assignment trial. Random assignment to the Case Manager or Control groups occurred within six strata based upon unmet needs status and gender." Quote from author email message: "Among those who met BOTH eligibility criteria and who consented to the RCT, an old fashioned table of random numbers was used to randomly assigned patients into the intervention and control groups by blocks of 10 persons per block." |
| Allocation concealment (selection bias) | Low risk | Quotes from author email message: "This was an RCT done outside a clinical practice setting; the PI (me) is not a clinician and the intervention staff and the measurement, data collection staff were different and did not really know one another." |
| Blinding (performance bias and detection bias) All outcomes | High risk | Quote from author email message: "We tried to retain “Blindness” of the research data collection at the post‐tests, but often the respondents would describe a person who came to visit them. But the lack of blindness clearly did not bias the results which were negative on the a priori designated outcomes." Comment: The interviewers were supposed to be blinded, but because the patients were not blinded, they often described the care they went through so the interviewer could identify their experimental group. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: " There were no differences in the attrition pattern observed among experimental and control patients." "Attrition from follow‐up was largely attributed to patient death." Comment: Proportions of attrition at 3 and 6 months were similar between control (3 months: 16%; 6 months: 29%) and intervention groups (3 months 16%; 6 months: 28%). There are no further details on the proportions and reasons for attrition in each treatment group, but the first quote above suggests that there are no important imbalances. |
| Selective reporting (reporting bias) | Low risk | All outcomes described in Methods are reported in Results. |
| Other bias | Low risk | No evidence of any other bias |
| Baseline outcomes similar? | Unclear risk | YES: Need status UNCLEAR: Other outcomes |
| Baseline characteristics similar? | Unclear risk | Comment: Baseline characteristics are presented in Table 1 and are similar in the 2 treatment groups. |
| Protected against contamination? | Unclear risk | Comment: The allocation was not performed by clusters. There are very few details on the control group treatment. The case manager was available to intervention patients only, in their home, but no details on the setting where the case manager was based are available. |
Oleske 1988.
| Methods | Cluster‐RCT; Unit of allocation: Home health agency; Stratified by: Cancer patient volume per year, size of RN staff, organisation type | |
| Participants | Cancer patients referred to home health agencies. Setting / country: Medicare‐certified home health agencies in two health planning regions of Illinois / USA Type of cancer: Any type Phase of care: Any phase Sample size at randomisation: 29 |
|
| Interventions | (1) Oncology nurse specialist + continuing education on cancer: a new nursing personnel called "Areawide Oncology Nurse Coordinators" (AONC) was added to home care. One AONC was assigned to each region to serve the home health agencies. The AONCs were professional nurses who had completed at least some graduate education, had advanced training in oncology, and were experienced in follow‐up care of the cancer patient. AONC's functions were multifaceted, but their primary function was to serve as a role model and consultant to home health nurses on the care of the cancer patients and their families. AONC nurses spent approximately 60% of their time in consultant‐practitioner activities, 20% in education and 20% in community activities and coordination of resources. Consultant‐practitioner activities consisted in receiving referrals from the agency nursing staff. Each AONC attempted to see all patients referred to assess patient/family needs and problems, to propose nursing interventions and goals, using forms. This visit was performed together with the agency nurse, where the AONC assist the agency nurse in assessing the patient, family and environment, and in developing a plan of care. Once filled, these forms became part of the patient's agency chart, and a copy was routinely sent to the patient's physician(s). Social workers or discharge coordinators also received a copy if they had specifically requested oncology nursing consultation. Subsequent visits by the AONC to the patient were also made with the agency nurse and were scheduled on an individual basis depending on the needs and/or problems existing. An exchange of all consultation forms between the two AONC's and the principal investigator of the project provided a means for peer review. The agency nurse remained responsible for communicating specific patient problems and/or needs to the physician, and for requesting medical orders. On occasion, after consultation with the agency nurse, the AONC may have contacted the physician to discuss problems or observations specific to the patient's disease process, treatment or other specific procedure. Educational activities comprised the provision of consultation to home health nurses in the field. Continuing education on cancer was also offered as didactic sessions over a two and one‐half year period to home health nurses. Community activities and coordinating resources comprised carrying on outreach and liaison activities aimed at cancer patients and health professionals to increase rates of utilization and the acceptability of home services. (2) Continuing education on cancer: continuing education on cancer was offered as didactic sessions to home health nurses. Control: "Observation only" |
|
| Outcomes | Patient: Physiologic complications Process: Duration of care, number of visits by the home health nurse, number of episodes of hospitalisation, referral rate to home care, status at the last nurse contact, use of home care services |
|
| Notes | Length of follow‐up: 36 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Of the remaining agencies within each Region, home health agencies were stratified according to cancer patient volume per year, size of RN staff, and organization type, and then randomly assigned to one of three intervention groups: (1) oncology nurse specialist plus continuing education on cancer, (2) continuing education on cancer alone, and (3) observation only." Comment: Agencies were the unit randomised, but the patients were not the same at baseline and follow‐up. The professionals were the same between baseline and follow up, so all process measures followed a RCT design. However, the patient measures do not follow a RCT design. |
| Allocation concealment (selection bias) | Unclear risk | See quote first item. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "All the data reported here were abstracted from the participating home health agency. To abstract this information, research assistants were trained by the principal investigator (D.M.O.) on location at a home health agency." Comment: No details are given relative to blinding. However, because of the objective nature of the results the risk of bias appears to be small. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: " Excluded from the computation of the referral rates are a total of 54 individuals who resided out of the study regions, but who received their care through one of the participating agencies." Comment: None of the participating agency withdrew from the study. |
| Selective reporting (reporting bias) | Low risk | All outcomes described in Methods are reported in Results. |
| Other bias | Low risk | No evidence of any other bias. |
| Baseline outcomes similar? | Low risk | Quotes: "The data concerning nurse performance (number of home health nurse visits and duration on agency caseload) were evaluated by analysis of variance using the software package, SAS; the variables, physiologic complications, disposition at discharge and hospitalisation rates, were evaluated with log‐linear analyses using BMDP." "Our primary statistical question is whether the observed change from 1980 to 1982 of a specified outcome differed by intervention group, i.e. we were looking for an intervention group by year interaction. Comment: All baseline outcome values are presented in the article, but not the between‐groups statistical comparison. However, the statistics used took into account baseline outcome values. |
| Baseline characteristics similar? | Unclear risk | Quote: "Except for physiologic complications, all our analyses of variance and log‐linear analyses included region and the patient's disability level at referral." Comment: Patient characteristics are presented for the whole sample or by region, but not for the study groups. |
| Protected against contamination? | Low risk | The home health agency were the units of randomisation. |
Rao 2005.
| Methods | RCT; Unit of allocation: Patient; Stratified by: Site (Veterans Affairs) and patient's functional status | |
| Participants | Hospitalised frail patients, 65 years of age or older, with a diagnosis of cancer. Setting / country: Veterans Affairs medical centres / USA Type of cancer: Any type Phase of care: Any phase Sample size at randomisation: 1388 |
|
| Interventions | Enrolled patients were randomly assigned to receive inpatient care in a geriatric evaluation and management unit (GEMU) or usual inpatient care (UCIP), followed by outpatient care in a geriatric evaluation and management clinic (GEMC) or usual outpatient care (UCOP). Four combinations of care were thus studied: (1) GEMU + UCOP (2) UCIP + GEMC (3) GEMU + GEMC Inpatient geriatric evaluation and management unit (GEMU) or Outpatient geriatric evaluation and management clinic (GEMC): The inpatient and outpatient intervention teams, each consisting of a geriatrician, a social worker, and a nurse, followed their standard protocols for geriatric evaluation and management, with specific instructions to complete the history taking and physical examination, including screening for geriatric syndromes such as incontinence or falls (within three days for patients assigned to the geriatric evaluation and management unit); develop a list of problems; assess the patient's functional, cognitive, affective, and nutritional status; evaluate the caregiver's capabilities; and assess the patient's social situation. A plan of care was developed, and the team on the geriatric evaluation and management unit met at least twice a week to discuss the plan. Preventive and management services (e.g. dietetics, physical and occupational therapy, and clinical pharmacy) were coordinated to address the problems identified, with a general emphasis on maintaining the patients functional status. Control: Usual inpatient (UCIP) or outpatient care (UCOP): Inpatients who were assigned to receive usual care received all appropriate hospital services except for those provided by the team on the geriatric evaluation and management unit. Outpatients assigned to receive usual care were provided with at least one follow‐up appointment in an appropriate clinic. After the initial site visits, the process of care was evaluated with the use of annual questionnaires, as well as a specific checklist for each part of the intervention, in order to ensure compliance with the study protocol. |
|
| Outcomes | Patient: QoL, functional status, physical performance Process: Use of hospital services |
|
| Notes | Length of follow‐up: 12 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from ref #2: "Randomisation was performed with the use of a computer program at the coordinating centre. The randomisation codes were generated according to a two‐by‐two factorial design, with stratification according to the centre and the patients functional status (high or low), with the use of permuted blocks of eight patients for the four treatment groups." |
| Allocation concealment (selection bias) | Low risk | See quote first item. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "All follow‐up data, except for PPT results, were gathered via a telephone call to the patient by a centralised research assistant, blinded to the patient’s study group status, who recorded all answers to the survey questions."... and was unaware of the treatment assignments." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "All of these 99 cancer patients were followed successfully for 1 year or until death. Over 99% of all total potential follow‐up interviews at 6 and 12 months were obtained successfully by telephone interview." |
| Selective reporting (reporting bias) | Low risk | All outcomes described in Methods are reported in Results. |
| Other bias | High risk | Quote: "Second, we also have no information on the 99 cancer patients in terms of stage of cancer, active treatment, length of disease, and response to therapy, all factors that affect quality of life." |
| Baseline outcomes similar? | Low risk | Quote from ref #2: "None of the variables differed significantly among the 4 treatment groups." This quote refers to baseline characteristics and outcomes presented in Table 1 (ref #2). Quote: "Values are mean changes in scores (adjusted for length of stay) from randomisation to either discharge (D/C) or follow‐up at 12 months (12 M)." |
| Baseline characteristics similar? | Low risk | Quotes from ref#1: "There was no difference in baseline demographics of the cancer patients among the different randomisation groups." "Of note, 15 patients carried a diagnosis of secondary cancer with bony metastasis; these patients were evenly distributed among the different treatment groups." See quote item G. |
| Protected against contamination? | Unclear risk | Comment: Patients were the ones that were randomised. It is not clear if the same health practitioners were in charge of more than one treatment groups. It is possible since the units where the interventions took place were within the same medical centres. |
Rawl 2002.
| Methods | RCT; Unit of allocation: Patient‐caregiver dyad; Stratified by: Site of recruitment, site of the patients' cancer, and caregivers' employment status. | |
| Participants | Patients newly diagnosed with breast, colorectal, or lung cancer receiving chemotherapy and having an identified caregivers. Setting / country: A large, urban, mid‐western, tertiary‐cancer centre and a community‐based cancer centre in a medium‐sized mid‐western city / USA Type of cancer: Breast, lung, colorectal Phase of care: Treatment Sample size at randomisation: 120 |
|
| Interventions | Computer‐based nursing intervention: A master's‐prepared oncology nurse specialist contacted each patient on nine occasions, five in person and four by telephone, every two weeks. The intervention was computer‐based to guide the nurse's clinical assessment, problem identification, selection of interventions, and measurement of outcomes. The computer‐based nursing‐intervention program was loaded on laptop computers, which allowed nurse specialists to input quantified assessments of patients physical and psychosocial functioning (including anxiety and depression) and symptom experiences. Nurses asked patients to rate on a four‐point scale the frequency, intensity, limitations, and degree of bother or distress caused by each symptom or problem. From the symptom assessment protocol, a computerised plan of care was developed in collaboration with the patient and caregiver, tailored specifically to address the identified patient needs. In all subsequent encounters, whether in person or via telephone, nurses evaluated the effectiveness of interventions, assessed new problems, and provided concrete, objective information about disease and treatment, symptom management, and availability of community resources. In addition, nurses provided emotional support and counseling to patients and caregivers at each visit. Intervention nurse training: Nurse specialists were trained intensively, focusing on delivery of the intervention protocol and use of the computer‐based nursing intervention system. Researchers developed an intervention manual that outlined policies and procedures and detailed all elements of the protocol to be delivered. A clinical nurse manager conducted training sessions onsite and prepared simulated cases to facilitate development of skills in problem assessment, implementation of appropriate interventions, and evaluation of intervention outcomes. Participants were encouraged to telephone the intervention nurses between scheduled meetings if questions of concerns arose. Control: Conventional cancer care (any education normally delivered during chemotherapy but no attention outside of medical visits). Standard care consisted of verbally telling the patients about what they might expect from chemotherapy and symptoms that should be reported to the doctor. |
|
| Outcomes | Patient: QoL, depression, state‐trait anxiety | |
| Notes | Length of follow‐up: 6 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "After completion of a baseline telephone interview, patients were assigned randomly to receive the computer‐based nursing intervention or conventional cancer care (control group). Group assignment was generated via computer and stratified according to (a) site of recruitment, (b) site of the patients cancer, and (c) caregivers employment status." |
| Allocation concealment (selection bias) | Unclear risk | See quote first item. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "Each interview took approximately one hour to complete, and interviewers were blind to respondents’ group assignments." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quotes: "About the same number of dyads dropped out at each site (15 and 16); however, attrition from the intervention group was twice that of the standard care group (21 versus 10)." "Because the attrition or dropout rate was higher in the intervention group than in the control group, baseline differences between patients who dropped out and those who completed the study were examined. Attrition status was defined as those who left the study for whatever reason at time 2 and time 3. Two‐way ANOVA were run to compare baseline SF‐36 sub‐scale scores, anxiety, and depression scores. Significant main effects were found for attrition on depression scores (F = 5.34, P = 0.02), the SF‐36 vitality sub‐scale scores (F = 10.64, P = 0.001), and SF‐36 social functioning sub‐scale scores (F = 4.13, P = 0.04). Patients who left the study had significantly higher depression scores at baseline (X = 14.3) than those who completed the study (X = 10.6). Similarly, those who left the study had lower SF‐36 vitality scores (X = 36.9) than those who completed the study (X = 51.5), and lower SF‐36 social functioning scores (X = 61.5) than those who completed the study (X = 73.0), indicating that those who left the study had worse functioning at baseline. Researchers observed no main effects for group, and although more patients were lost from the intervention group, researchers found no group‐by‐attrition status interactions." Comment: Proportion of attrition was different between control (19%) and intervention groups (38%). The statistical analysis allowed to demonstrate that there were no differences in outcomes scores for the participants who left between groups. |
| Selective reporting (reporting bias) | Low risk | All outcomes described in Methods are reported in Results. |
| Other bias | Low risk | No evidence of any other bias. |
| Baseline outcomes similar? | Low risk | Quote: Repeated measures analyses of variance (ANOVA) were used to analyze the effect of the treatment over time on each of the following outcomes: (a) SF‐36 psychosocial functioning sub‐scales of vitality, social functioning, role emotional, and mental health, (b) depression, and (c) anxiety." Comment: Baseline outcomes measures are presented and seem to be similar (no statistics performed). Statistical analysis chosen takes into account baseline differences in outcomes. |
| Baseline characteristics similar? | Low risk | Quote: "Researchers compared demographic characteristics of the standard care (n = 54) and intervention groups (n = 55) using t tests and chi‐square analyses and found no significant differences." |
| Protected against contamination? | High risk | Quote: "Another limitation of the study relates to the possibility of diffusion of the intervention to the standard care group. After the study had been completed, one resourceful participant who had been randomised to the standard care group confessed that she had actively sought information about the intervention from patients who were receiving it. After learning what the intervention entailed, she hired an oncology clinical nurse specialist to provide similar services. The researchers do not know how many other patients or caregivers in the standard care group may have been as resourceful." |
Ritz 2000.
| Methods | RCT; Unit of allocation: Patient | |
| Participants | Women with newly diagnosed breast cancer. Setting / country: Integrated healthcare system in a large mid‐western suburban community / USA Type of cancer: Breast Phase of care: Treatment, discharge, pre‐treatment, surveillance Sample size at randomisation: 210 |
|
| Interventions | Advanced nursing care + standard medical care: Brootens cost quality model and ONSs advanced standards of practice were used as conceptual framework. Advanced Practice Nurse (APN) interventions included assessments, diagnosis, outcome identification, planning, care coordination, symptom management, patient education, consultation and referrals. Follow‐up APN care was provided during clinic, hospital, telephone, and home care visits. Contacts were based on need as determined by the patient, family, and APNs. One APN was on call 8 am to 8 pm every week day and from 8 to noon on week ends. Control: Standard medical care |
|
| Outcomes | Patient: QoL‐ uncertainty, mood state, QoL ‐ well‐being Process: Number of inpatient visits, use of services (hospital, community and home care) |
|
| Notes | Length of follow‐up: 24 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: " After eligibility criteria were verified and informed consents obtained, the women were assigned randomly to one of two groups: women in the control group received standard medical care, and women in the intervention group received standard medical care plus APN care." |
| Allocation concealment (selection bias) | Unclear risk | See quote first item. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Comment: Outcomes were measured using self‐administered questionnaires. Because the patients were not blinded to treatment allocation, than assessors could not be blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: Proportions of attrition were different between control (24% at baseline; 48% at 24 months) and intervention groups (5% at baseline; 24% at 24 months). The authors chose not to use the 24 months data because of the important attrition. No details on imputation of QOL data were given in the article. |
| Selective reporting (reporting bias) | Low risk | All outcomes described in Methods are reported in Results. |
| Other bias | Low risk | No evidence of other bias. |
| Baseline outcomes similar? | Low risk | Quote: "Intervention and control groups did not differ significantly on any of the QOL scales at baseline" Comment: In addition, the statistical analysis took into account baseline outcomes differences. |
| Baseline characteristics similar? | Low risk | Quote: " The randomisation process produced intervention and control groups that were similar demographically and in characteristics of disease at diagnosis and treatment (see Table 2) with two exceptions: women in the intervention group were significantly more likely to have a lower histology (P = 0.04) and to receive adjuvant hormone therapy (P = 0.03) than women in the control group." "Other factors were included as covariates if they affected the QOL scale being analysed." "The intervention group showed significantly less uncertainty than the control group (P = 0.043) after adjustment for baseline, extent of disease, and hormone therapy." Comment: There were differences at baseline, but these were taken into account in the analysis. |
| Protected against contamination? | High risk | Comment: Patients were the unit of allocation. Professionals in charge of control and intervention groups were located in the same setting so contamination cannot be overruled. |
Rutherford 2001.
| Methods | RCT; Unit of allocation: Patient‐GP dyad. | |
| Participants | Patients admitted to the hospital for major surgery and having a GP or agreeing to be referred to a GP in living area. Setting / country: Royal Women's Hospital oncology unit / Australia Type of cancer: Endometrial, cervical / ovarian Phase of care: Discharge, surveillance Sample size at randomisation: 200 |
|
| Interventions | Increased general practitioners (GPs) contacts with hospital: GPs were invited to contact patients in the hospital by either personal visit or telephone call, to assist with discharge planning and continuity of care. Payment was available for visiting (150 AUSD) or telephoning (75 AUSD). Discharge summary (DS) for the patient: The discharge summary was collated by the research nurse and comprised diagnosis and management plans with input from allied health, information on the specific gynaecological cancer for each patient, educational materials on chemotherapy and radiotherapy. It was either given to the patient on her discharge or mailed to her 1‐2 days after discharge. Combination studied included: (1) GPs not invited + DS (2) GPs invited + DS (3) GPs invited + No DS Control: Routine hospital discharge summary without any invitation to contact the hospital and no reception of cancer specific discharge summary. |
|
| Outcomes | Process: Number of GP contacts during admission to hospital and after discharge | |
| Notes | Length of follow‐up: 10 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "She (the study nurse) then contacted an independent third party who allocated the patient to one of four groups using a randomisation schedule supplied by the statistical consultant." |
| Allocation concealment (selection bias) | Low risk | See quote first item. |
| Blinding (performance bias and detection bias) Satisfaction | High risk | |
| Blinding (performance bias and detection bias) Use of services | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "Contact was successfully made with 94 of 100 GPs: 47% on the first call, 42% on the second call and 10 % on the third call." Comment: Proportions of patient attrition were important but differed only slightly between treatment groups, with 40% attrition in ‐C+DS group, 52% in ‐C‐DS group, 32% in +C+DS group and 30% in +C‐DS group. Reasons for not responding to mailed survey were not detailed. The quote above indicates some GP attrition as well but no details were provided on which group it was in. |
| Selective reporting (reporting bias) | Low risk | All outcomes described in Methods are reported in Results. |
| Other bias | High risk | Quote: "An important unforeseen finding was the large number of patients referred to the RWH from rural areas: 52% of GPs were more than 21 km and/or more than 30 minutes travel time from the hospital. This halved the number of GPs who were in a position to provide a hospital visit, and consequently reduced the statistical power of the study" Comment: Since one of the primary outcome was the number of GPs responding to the invitation, then the proximity of the GP becomes an important confounding variable and the samples should at least have been stratified accordingly. |
| Baseline outcomes similar? | Unclear risk | NO: Patient confidence in GP management of future problems; Patient satisfaction; GP confidence in management of patients' future problems. They were not reported at baseline. YES: Number of GPs responding to invitation to visit the hospital; Rate of GP hospital visit; Rates of GP telephone calls; Rate of patient contact with GPs after discharge (all objective outcomes). They cannot be measured at baseline. |
| Baseline characteristics similar? | Low risk | Quote: "There were no significant differences between patients in the four study groups in age or rate of cancer diagnosis." "No significant differences between GPs in the four study groups in age, sex, years post‐graduation, practice size and sessions worked were found." Comment: There are not many baseline patient characteristics presented. The GP characteristics are well‐detailed. |
| Protected against contamination? | High risk | Comment: Patient was the unit randomised, and the intervention affected the patient and his/her GP. All patients were recruited in the same hospital. No details on the possibility that the same GP could follow patients from different study groups. |
Schumacher 2002.
| Methods | RCT; Unit of allocation: Patient; Stratified by: Site and on the basis of whether they participated by themselves or with a family caregiver | |
| Participants | Oncology outpatients with pain from bone metastasis. Setting / country: Outpatient settings in Northern California: university‐based cancer centre, community‐based oncology practices, health maintenance organisation, outpatient radiation therapy centre, veteran's affairs facility, military hospital / USA Type of cancer: Not mentioned Phase of care: Any phase Sample size at randomisation: 212 |
|
| Interventions | PRO‐SELF Pain Control Program: patients were seen by specially trained intervention nurses and received a psycho educational intervention, were taught how to use a pillbox, and were given instructions on how to communicate with their physician about unrelieved pain and the need for changes in their analgesic prescriptions. Patients were coached during two follow‐up home visits and three phone calls on how to improve their cancer pain management. At the week 1 visit, the PRO‐SELF nurse conducted the academic detailing session with the patient and family caregiver. The nurse identified the specific areas of knowledge deficit and focused the education in these areas. During weeks 2, 4, 5, the PRO‐SELF nurse contacted patients by phone and reviewed their pain intensity scores and pain medication intake. The PRO‐SELF nurse made home visits during weeks 3 and 6. Previous teaching was reinforced and patients were coached about how to make changes in their pain management plan. Control: Standard care: patients were seen by a research nurse three times (at weeks 1, 3, 6) and were called three times by phone between the home visits (at weeks 2, 4, 5). These patients received the patient version of the Cancer Pain Guideline published by the Agency for Health Care Policy and Research (AHCPR). The focus of the visits and phone calls was on monitoring patients' level of adherence with completing the diary. |
|
| Outcomes | Patient: Pain, mood state, QoL, pains level of interference with function, pain knowledge Professional: Pain management, pain management |
|
| Notes | Length of follow‐up: 1.5 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from ref #8: "Patients were stratified by site and on the basis of whether they participated by themselves or with a family caregiver. Both patients and clinicians at the study sites were blinded to the patients group assignment. Patients were randomly assigned to either the PRO‐SELF© or the standard care group." Quote from author email message: "Identification numbers (IDs) were assigned to prospective patients in each of the unique strata before the patients were recruited. Using the SPSS (Statistical Package for the Social Sciences) computer program, that has the ability to select true random samples, each identification number was randomly assigned to correspond to one of the two study groups. Envelopes were created for each identification number with the ID number on the outside of the envelope and the study group assignment on a folded paper inside the envelope. As patients were recruited into the study and assigned ID numbers appropriate to their strata, the research nurse was able to open the envelope specific to that patients ID number and discover to which study group that patient had been randomly assigned." Other email: "Yes, the envelopes were opaque. They were that buff colour that you cannot see through." |
| Allocation concealment (selection bias) | Low risk | See quote first item. |
| Blinding (performance bias and detection bias) Physical status | High risk | |
| Blinding (performance bias and detection bias) Pain belief | High risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quotes from ref #8: "Thirty‐eight patients (i.e. 22 in the PRO‐SELF© group and 16 in the standard care group) did not complete the entire study for a variety of reasons, including: increased severity of illness or intervening cancer treatments that required hospitalisation (n = 28; 16 in the PRO‐SELF© group and 12 in the standard care group) and death (n = 10; six in the PRO‐SELF© group and four in the standard care group). No differences were found in any of the demographic, disease, or baseline pain characteristics between patients who did and did not complete the study. The percentage of patients who did not complete the entire study did not differ significantly by treatment group (i.e. 19% in the PRO‐SELF© group and 17% in the standard care group)." "All calculations used actual values. Adjustments were not made for missing data." Comment: Proportions and reasons for attrition are similar in the 2 treatment groups. |
| Selective reporting (reporting bias) | Low risk | All outcomes described in Methods are reported in Results. |
| Other bias | Low risk | No evidence of any other bias. |
| Baseline outcomes similar? | Low risk | Comment ref #8: All outcomes were controlled for with scores at baseline. Quote from ref #7: "As shown in Table 2, no significant differences in any of the baseline pain characteristics or analgesic prescriptions were found between patients in the PRO‐SELF and the standard care groups." Quote from ref #9: "No significant differences were found in any of the baseline pain characteristics among patients in the PRO‐SELF© and standard care groups (see Table 3). All of the participants experienced moderate to severe pain from bone metastasis that lasted almost half of the day." |
| Baseline characteristics similar? | Low risk | Quote from ref #8: "No significant differences were found in any of the demographic or disease characteristics between patients in the standard care and PRO‐SELF© groups or among the patients in the three responder groups." |
| Protected against contamination? | Low risk | Quote from ref #8: "Both patients and clinicians at the study sites were blinded to the patients group assignment. " Comment: The patients were the units of randomisation, but the clinicians were blinded. |
Skrutkowski 2008.
| Methods | RCT; Unit of allocation: Patient | |
| Participants | Patients with breast or lung cancer receiving treatment in the ambulatory oncology settings. Setting / country: Three outpatient ambulatory oncology clinics in a large university health centre in Quebec / Canada Type of cancer: Breast, lung Phase of care: Any phase Sample size at randomisation: 190 |
|
| Interventions | Pivot nurse in oncology (PNO) + usual care by clinic nurses: patients and their informal caregiver (if present) met the PNO in the ambulatory setting. The PNO was a baccalaureate‐prepared, experienced palliative care nurse who had received additional training in cancer symptom management and the SMM. The PNO reviewed understanding of the diagnosis, expected side effects of treatment, available resources with the patient. The PNO also identified potential sources of support for the patient by creating a genogram and ecomap. The genogram identified family members and the relationships between them, and the ecomap outlined significant people, agencies, or institutions and their relationships to the family. The PNO assessed patients needs and coping skills, taught specific ways to identify and cope with symptoms, and offered additional education and support as needed. The PNO also coordinated care across treatment modalities and the disease continuum. The PNO particularly advocated for patients during interdisciplinary rounds and developed care plans with referrals to specialised services when needed. The PNO initiated follow‐up telephone calls as needed to provide support, information, coaching, or active listening to patients. Control: Usual care by clinic nurses included symptom assessment and teaching management but was not organised in a formally coordinated model. Patients may not have seen the same nurse at each appointment. Follow‐up by telephone usually was limited to patient‐initiated phone calls. |
|
| Outcomes | Patient: Symptom distress, symptoms ‐ fatigue, QoL Process: Use of hospital services, number of clinic appointments |
|
| Notes | Length of follow‐up: 6 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "When patients consented, the research assistant contacted one of the investigators for randomisation and assigned the patients to groups. Randomisation was done using a computer‐generated list of numbers that only three of the investigators could access." |
| Allocation concealment (selection bias) | Low risk | Comment: From the quote reported in the first item's description, we can suppose that not all investigators had access to the computer‐generated list of numbers to conceal allocation to the persons more involved in the trial. However, this was not explicitly stated. |
| Blinding (performance bias and detection bias) Physical status | High risk | |
| Blinding (performance bias and detection bias) Quality of life | High risk | |
| Blinding (performance bias and detection bias) Use of services | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Twenty‐five of 28 patients who died during the study had lung cancer (Chi‐squared[1] = 12.11, P = 0.001). Another 32 patients (14 with breast cancer and 18 with lung cancer) withdrew. More patients in the usual care group (n = 23) versus the intervention group (n = 9) withdrew (Chi‐squared[1] = 6.68, P = 0.01)." "All analyses were by intention‐to‐treat, meaning all participants data were included, whether or not they provided survey data at each assessment period or died before completing the study." "Repeated measures analyses of variance using linear mixed models were conducted to determine whether the scores in the intervention and usual care groups varied over time and across groups. All analyses were done using Proc Mixed procedure from SAS version 8 (SAS Institute Inc., 1999)." Comment: Reasons for attrition differed between groups. The authors imputed missing data using an appropriate method. |
| Selective reporting (reporting bias) | Low risk | All outcomes described in Methods are reported in Results. |
| Other bias | Low risk | No evidence of other bias. |
| Baseline outcomes similar? | Low risk | Quote: "Repeated measures analyses of variance using linear mixed models were conducted to determine whether the scores in the intervention and usual care groups varied over time and across groups." Comment: Baseline outcome values are not presented but the statistical analysis used takes into account baseline differences. |
| Baseline characteristics similar? | High risk | Comment: baseline characteristics of participants are presented in Table 1. No statistics were presented but all characteristics appeared to be similar in the 2 study groups, except for proportion of cancer stage III or IV. We tested for differences in cancer stage III or IV proportions between groups using the difference of proportions test and found a significant difference (P = 0.02). |
| Protected against contamination? | High risk | Comment: Patients were the units of randomisation. Healthcare professionals in charge of patients seem to deliver care within the same setting (ambulatory clinics), but this is not clear. A nurse could have provided care to persons of the two groups. |
Trowbridge 1997.
| Methods | RCT; Unit of allocation: Patient | |
| Participants | Outpatients with a pathologic diagnosis of carcinoma or sarcoma and having recurrent or metastatic disease. Setting / country: 23 clinics in Indiana / USA Type of cancer: Any type Phase of care: Recurrence Sample size at randomisation: 510 |
|
| Interventions | Summary of pain assessment included in clinical charts: Patients completed assessments of average and worst pain in the previous seven days, satisfaction with their current pain regimen and degrees of relief received at baseline and four weeks later. A summary sheet of these evaluations was included in the patients' clinical charts. Oncologists who treated these patients were instructed to review the summary sheet prior to an evaluation. Control: Patients completed assessments of average and worst pain in the previous seven days, satisfaction with their current pain regimen and degrees of relief received at baseline and four weeks later, but the summary was not available for the oncologists. |
|
| Outcomes | Patient: Pain Professional: Pain management |
|
| Notes | Length of follow‐up: 1 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Patients completed survey by mail and could not be blinded to assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No details on attrition were presented. |
| Selective reporting (reporting bias) | Low risk | All outcomes described in Methods are reported in Results. |
| Other bias | Low risk | No evidence of any other bias. |
| Baseline outcomes similar? | Low risk | Quote: "No other significant difference was found between the groups in their assessments of their pain, pain regimens, and relief received at baseline and at the four‐week follow‐up." |
| Baseline characteristics similar? | Low risk | Quote: "The two groups were similar with respect to cancer sites and performance status" Comment: Gender and age were similar as well. They were all entered in analysis as covariates. However, values were not reported. |
| Protected against contamination? | Unclear risk | Quote: "The ten oncologists treating these patients [intervention] were instructed to review the summary sheet prior to an evaluation. Such summaries were not available to the 12 oncologists treating the control patients." The patient was the unit of allocation, and treating oncologists were in the same facility. An oncologist may have treated control and intervention group patients. From the quote, it seems that different oncologists treated the patients in two groups, but this is not explicit. |
Vallieres 2006.
| Methods | RCT; Unit of allocation: Patient | |
| Participants | Patients with cancer experiencing pain or taking analgesics and being treated by radiotherapy for more than 1 week (> 5 fractions) on an outpatient basis. Setting / country: Radiation Oncology Department of Centre Hospitalier Universitaire de Québec (CHUQ‐L'Hôtel‐Dieu de Québec Hospital, a tertiary care centre affiliated to Laval University / Canada Type of cancer: Any type Phase of care: Treatment Sample size at randomisation: 64 |
|
| Interventions | Multi component clinical intervention to reduce pain: the clinical intervention included a patient education session, a patient pain diary, and the possibility to contact a physician to adjust the pain medication. The educational component consisted of giving patients an information brochure which covered the general principles and philosophy of analgesic treatment as well as the major myths and misconceptions surrounding the use of opioid analgesics to relieve cancer pain. The patient pain diary was developed and validated in Quebec City for French‐speaking patients. Patients had to record (a) the intensity of the pain they experienced, twice a day and (b) the number of rescue doses of prescribed analgesics taken in 24 h. The patients recorded this information by themselves and were asked to bring the diary to the investigating radiation oncologist at each visit to the radiation therapy centre, or when meeting any of their regular care providers (nurse, family doctor, and regular staff oncologist). Patients were recommended to call or visit the investigating radiation oncologist or any of their other healthcare providers if their pain level reached 2/5 twice in a row or if they took at least three rescue doses of prescribed analgesics in 24 h. Adjustments of the analgesics regimen was based on WHO guidelines after consultation of the patient's pain diary. Control: Usual treatment of pain by the staff radiation oncologist, i.e. pain treatment at the discretion of the staff, without a systematic pain assessment or any patient education. |
|
| Outcomes | Patient: Pain | |
| Notes | Length of follow‐up: 0.75 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Eligible patients who agreed to participate were then randomly assigned to the experimental (intervention) or control group and referred to the investigating physician (or research nurse) for the baseline visit." Comment: Method of random allocation not specified |
| Allocation concealment (selection bias) | Unclear risk | See quote first item. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: "Measures Information on patients’ pain levels was collected by the investigating radiation oncologist at baseline and then after 2 and 3 weeks of follow‐up." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: Losses to follow‐up were not specified. |
| Selective reporting (reporting bias) | Low risk | Comment: Main outcomes are reported. |
| Other bias | Low risk | Comment: There is no evidence of other bias. |
| Baseline outcomes similar? | Low risk | Quote: "At baseline, there were no significant differences in average, worst, weakest, and “at present” pain levels (Table 2)." |
| Baseline characteristics similar? | Low risk | Quote: "There were a few differences between control and experimental groups, but none were statistically significant (Table 1)." |
| Protected against contamination? | Low risk | Comment: It is unlikely that the control group received the intervention |
Velikova 2004.
| Methods | RCT; Unit of allocation: Patient; Stratified by: Site of cancer | |
| Participants | Outpatients attending the oncology clinic to start cytotoxic or biologic treatment and expected to attend the clinic at least three times. Setting / country: Leeds Cancer Centre Medical Oncology Clinic at St James's Hospital ‐ Leeds / UK Type of cancer: Any type Phase of care: Treatment Sample size at randomisation: 286 |
|
| Interventions | Regular quality‐of‐life (HRQL) assessments and feedback to physicians: touch‐screen questionnaires were completed by the patients in the waiting room before every medical encounter. The intervention questionnaires used were the European Organisation for Research and Treatment of Cancer‐Core Quality of Life Questionnaire, version 3.0 (EORTC QLQ‐C30), and the Hospital Anxiety and Depression Scale (HADS). Results were fed back to physicians in a graphic printout form. The physicians were trained in interpretation of the questionnaires. A manual was prepared, with description of scales, interpretation of scores, and explanation of the graphs. Structured meetings were conducted individually, with each physician to discuss the study and review examples of HRQL and clinical details of real patients. Posters with interpretative information were displayed in clinics. The physicians were asked to review and use the HRQL results during all intervention encounters, unless totally inappropriate. Control: (1) No touch‐screen measurement of HRQL before clinic encounters. (2) Attention‐control group: completion of HRQL questionnaires on touch‐screen computer, but no feedback to physicians. |
|
| Outcomes | Patient: QoL Process: Length of encounters with physician, medical decisions, non medical actions |
|
| Notes | Length of follow‐up: 6 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The random assignment was unbalanced 2:1:1 in favour of the intervention group, and stratified by site of cancer in random permuted blocks (block size was 8). Random assignment was carried out by telephone, by the Administrative Office at Cancer Research UK Centre (Leeds)." Quote from author e‐mail message: "The randomisation was carried out using randomised permuted blocks, stratified by site of cancer. The randomisation list for each stratum was generated in advance by a statistician using STATA. At the time of randomisation for each patient, the oncologist telephoned the independent randomisation line and the patients were allocated to the next available treatment on the appropriate list." |
| Allocation concealment (selection bias) | Low risk | See quote first item. |
| Blinding (performance bias and detection bias) Functional status | High risk | |
| Blinding (performance bias and detection bias) Physical status | High risk | |
| Blinding (performance bias and detection bias) Psychological status | High risk | |
| Blinding (performance bias and detection bias) Social needs | High risk | |
| Blinding (performance bias and detection bias) Quality of life | High risk | |
| Blinding (performance bias and detection bias) Use of services | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Characteristics of non respondents were compared with respondents using Chi‐2 and t tests." Comment: Proportions of patients that completed the 6 months study were similar between groups (Arm #1= 65%; #2 = 57%; #3=65%). Reasons for attrition were also similar (Fig. 2) |
| Selective reporting (reporting bias) | Low risk | All outcomes described in Methods are reported in Results. |
| Other bias | Low risk | No evidence of other bias. |
| Baseline outcomes similar? | Low risk | Quote: "The model included FACT‐G scores over time as the outcome variable; baseline FACT‐G score as a covariate; performance status, time, study arm, and study arm X time as fixed effects; and patient and patient X time as random effects." Comment: Table 1 demonstrate that baseline outcomes measures were similar between groups. For the FACT‐G measure, the time effect was evaluated so baseline difference were taken into account. |
| Baseline characteristics similar? | Low risk | Quote: "Table 1 presents the baseline patients and encounters characteristics, demonstrating a good balance of baseline variables between the study arms" Comment: Statistical tests were not performed to compare baseline characteristics between groups but results appeared fairly similar. |
| Protected against contamination? | High risk | Quote: "An optimal experimental design for the study was difficult to achieve for several reasons. The study was conducted in a natural environment (oncology clinics), with two groups of subjects (physicians and patients) who were in continuous complex interactions. The experimental intervention was both at patient level (completion of intervention questionnaires) and physician level (feeding back of HRQL information). Random assignment of physicians was considered, but rejected due to practical limitations. In the Cancer Centre, Leeds (similar to many large oncology practices in the United Kingdom), patient care is provided by teams consisting of four to seven physicians, and over time, patients usually see several different physicians who, if physicians were randomly assigned, might happen to be either in the experimental or the control group. If different clinics were randomly assigned instead of individual physicians, definite differences between patients would result, as the clinics were specialised by cancer site. Therefore, patients were chosen as the units of random assignment, with an analysis of possible physician‐sensitizing effect planned at the design stage." |
Wattchow 2006.
| Methods | RCT; Unit of allocation: Patient | |
| Participants | Patients having had surgery for colon cancer with histologic grade Dukes stage A, B or C (cases of disseminated cancer were excluded) and having completed post‐surgical chemotherapy. Setting / country: Hospitals in South Australia, Victoria, Western Australia and Northern Territory / Australia Type of cancer: Colorectal Phase of care: Surveillance Sample size at randomisation: 203 |
|
| Interventions | Follow‐up by general practitioners (GPs): Follow‐up guidance, based on current clinical practice and guidance was provided, and inserted into the patients GP records. The recommended follow‐up regimen (over 5 years) comprised: review of the patient 3 monthly for the first 2 years postoperatively and then 6 monthly for the next 3 years; patient history; physical examination; diagnostic tests. In accordance with the study pragmatic design, there was no compulsion for clinicians in either setting to adhere to the guidance. Participating clinicians received regular study information from contact with the study researcher and a newsletter. Patients could be referred back to surgical clinics at any point. Control: Follow‐up by surgeons: Follow‐up guidance concerning timing of physical exams and diagnostic tests (based on current clinical practice and guidance) was provided and inserted in surgeon/hospital record. The recommended follow‐up regimen (over 5 years) comprised: review of the patient 3 monthly for the first 2 years postoperatively and then 6 monthly for the next 3 years; patient history; physical examination; diagnostic tests. In accordance with the study's pragmatic design, there was no compulsion for clinicians to adhere to the guidance. Participating clinicians received regular study information from contact with the study researcher and a newsletter. Patients could consult their GP at any point. |
|
| Outcomes | Patient: QoL, anxiety and depression (distress), satisfaction, number and time to detection of recurrences, death rate Process: Number and type of investigations |
|
| Notes | Length of follow‐up: 24 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Consenting patients were then randomly allocated to either GP‐led or surgeon‐led follow‐up using an Excel random number generator. Randomisation was conducted by the study researchers, who were not involved in the design of the study or the clinical care of the patients, and was concealed until the interventions were assigned. The study was single‐blinded. Researchers at all times were unaware of the patient allocation until after the randomisation process." |
| Allocation concealment (selection bias) | Low risk | See quote first item. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Self‐report questionnaires were used, and patients could not be blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: Proportions of patients who completed follow‐up were similar in the control (76%) and intervention groups (78%). The reasons for attrition were equivalent for deaths, but other reasons for withdrawal are not mentioned. Because non‐parametric analyses were used, it is not possible to evaluate if missing values could have had an impact on intervention effect estimate. |
| Selective reporting (reporting bias) | Low risk | All outcomes described in Methods are reported in Results. |
| Other bias | Low risk | No evidence of any other bias. |
| Baseline outcomes similar? | Low risk | Quote: "Comparisons adjusting for baseline values were undertaken using analysis of covariance on ranks." Comment: According to table 3, the two groups were similar at baseline for the two outcome measures; no significant difference was observed in unadjusted or adjusted data for baseline values. In addition, analysis were adjusted for baseline differences. |
| Baseline characteristics similar? | Low risk | Quote: "Table 2 shows the characteristics of the trial participants at baseline. Of patients, 70% were recruited in SA. Groups had similar characteristics with the exception of education, where there was a trend towards higher levels of education in the surgeon follow‐up group. To examine external validity of our sample we compared age, sex and Dukes staging with SA Cancer Registry data (Cancer Council of SA, 2001) (included in Table 2) using Chi2‐tests. Study participants did not differ significantly compared with registry patients with respect to gender (P = 0.53) and Dukes staging (P = 0.12), but had a slightly narrower age distribution (P = 0.05)." Comment : Education likely unaffected study results. |
| Protected against contamination? | High risk | Quote: "Patients allocated to 'GP‐led' follow‐up could be referred back to surgical clinics at any point in the study; similarly, patients in the 'surgeon‐led' follow‐up group could consult their GP at any time during the course of the study. Comment: Patients from the 2 groups were followed generally in different settings, but a possibility for contamination existed. |
Wells 2003.
| Methods | RCT; Unit of allocation: Patient‐primary caregiver dyad | |
| Participants | Patients with cancer‐related pain and their primary caregivers. Setting / country: Comprehensive cancer centre and a cancer clinic located in a Veterans' Administration Medical Center / USA Type of cancer: Any type Phase of care: Any phase Sample size at randomisation: 64 |
|
| Interventions | Baseline pain education program for patient and family: The pain education program included structured and tailored components. The structured component was a 15‐minute videotape (Taking Charge of Your Pain, Purdue Frederick) that included information about pain, methods to control pain, and emphasised the importance of communicating pain to providers. It also discussed the low risk of addiction to opioids used to control cancer pain and the variety of medications available to manage side effects. Information in the videotape was presented both by experts (e.g. physician and nurse) and by patients. The tailored component consisted of individualised consultation regarding the videotape, written information about analgesics and side effect management, and discussion of the patients present pain regimen. Patients kept the printed materials, which were written at an 8th grade level, for future use at home. This education program took 20 to 30 minutes. Two types of follow‐up care were tested: (1) Baseline pain education program + access to a pain hotline: The participants in the pain hotline group received a toll‐free number they could call with questions or concerns about pain control. These patients were encouraged to call the hot line from the clinic to ensure that they were familiar with using the service. Patients were free to call their oncologist with questions. (2) Baseline pain education program + weekly telephone calls: The weekly calls group received four telephone calls over the month following the education program from an oncology nurse specialist. The oncology nurse assessed the patients' understanding of their prescribed analgesic regimens, probed for any difficulties attributed to the analgesics, and encouraged patients to contact the treating oncologist if problems were identified. The study nurse did not alter opioid prescriptions or adjust medications. This was left to the treating physicians and their staff. Patients were free to call their oncologist with questions. Control: A baseline pain education program + usual care: the usual care group received no additional follow‐up information after the pain education program. Patients were free to call their oncologist with questions. |
|
| Outcomes | Patient: Pain, pain relief, pain interference, patients beliefs Informal carer: Pain beliefs and experience Professional: Pain management Process: Number of patient‐initiated telephone calls |
|
| Notes | Length of follow‐up: 6 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from author e‐mail message: "In the patient and family education study we began with a table of random numbers and made group assignment from that table. The subject ID number was tied to the random assignment and we kept them in sealed envelopes (this was a while ago ‐ seems antiquated now). The envelope had the subject ID on the outside and the group assignment on the inside. The person doing the recruiting and consent process was unaware of group assignment until the consent process was finished". Comment: the author confirmed in an further message that the envelopes were opaque. |
| Allocation concealment (selection bias) | Low risk | See quote first item |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: "Follow‐up data collection began 1 month after the pain education program. Follow‐up data were collected with monthly telephone calls using an interview format." Comment: Data were collected via interviews but no details were provided about the interviewer. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Many patients had one or more missing follow‐up data points. Therefore, a decision was made to include patients with a minimum of three (baseline + 2 follow‐ups) in the analyses. Slopes of the pain outcomes were computed for individual patients who had three or more data points. All available data points from each patient were included in the calculation of slopes. Of the 64 patients enrolled in the study, 54 (82%) had at least three data points. Approximately half of each of the intervention groups completed five or six follow‐up data points, and all available data points were included in the calculation of each subject’s slope. Additionally, between 10% (hotline) to 25% (control) of patients completed four follow‐up data points. Four patients each were eliminated from the hotline and usual care groups, and two from the weekly calls group because they had less than three data points. Of the 10 patients who did not complete three data points, 8 died over the six months of enrolment in the study. Patients who did not complete three data points were not significantly different from patients who did by group or on the demographic variables of age, education, race, and work status. There was a trend toward more women and single patients to complete less than three data points and, therefore, these were eliminated from the analyses (Ps = 0.06). There were no differences between patients who did and did not complete three or more data points on any clinical variable. Comment: We don't know the attrition in each of the three groups, neither the reasons for withdrawals. The study researchers took the decision to restrict analyses to patients who had three or more data points collection. |
| Selective reporting (reporting bias) | Low risk | All main outcomes are reported. |
| Other bias | Low risk | No evidence of any other bias. |
| Baseline outcomes similar? | Low risk | Quote: "Using slopes controls for differences in baseline values and provides an indication of improvement or decline in outcomes measured." Comment: According to table 5, no statistical differences between outcomes of patients in the 3 study groups at baseline were significant. |
| Baseline characteristics similar? | Low risk | Quote: "The information groups were comparable on all demographic variables except current work status. Patients in the weekly calls group were more likely to be working than patients in the other groups (Table 3). Students t‐tests indicated, however, that work status was not related to any outcome variable (Ps > 0.05)." |
| Protected against contamination? | High risk | All patients had access to professional within the same oncology clinic. |
Wells 2004.
| Methods | RCT; Unit of allocation: Patient; Stratified by: Breast operation | |
| Participants | Patients with breast cancer requiring axillary clearance surgery (level 1, 2, and 3). Setting / country: Teaching hospital / UK Type of cancer: Breast Phase of care: Discharge, treatment, pre‐treatment, surveillance Sample size at randomisation: 108 patients and 86 carers |
|
| Interventions | Nurse‐led early discharge after surgery: discharge was done within 36 h of surgery, with wound drains still in situ. The essential components of the nurse‐led model of care were: (a) preoperative assessment, information and education about wound drain care and recognizing complications; (b) preoperative liaison with primary care (in particular community nurses) to negotiate postoperative involvement; (c) faxed discharge summary to primary healthcare team; (d) patients held records and care protocols to be shared with primary care staff; (e) joint home visit by designated breast care nurse and community nurse (if available) the day after discharge from hospital; (f) daily telephone assessment by breast care nurse until day after drain removal, including systematic assessment of symptoms, wound drainage and condition of wound; (g) negotiated home visits by breast care nurse or community nurse depending on needs; (h) removal of wound drain when 24 h drainage < 50 ml or at 5 days post‐operation; (i) 24 h access to breast care nurse via mobile phone, during supported early discharge period; (j) hospital review by breast care nurse for seroma aspiration, discussion of any problems or concerns. Control: Conventional hospital stay following surgery until wound drains were removed (approximately 6 days). |
|
| Outcomes | Patient: QoL, arm morbidity, satisfaction, wound healing, nursing dependency Informal carer: Carer burden Process: Use of home care and community services, hospital stay duration, surgical cancellations |
|
| Notes | Length of follow‐up: 12 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A central telephone service provided by the Scottish Cancer Therapy Network Trials Office randomised consenting patients using a block randomisation technique." Quote from author email: "The research nurse telephoned the SCTN randomisation hotline and provided details of whether the participant was scheduled for a mastectomy or wide local excision (our stratification factors). SCTN then used a computerised block randomisation technique and allocated the participant to one of 2 groups. This allocation was then provided to the research nurse. Neither the research nurse nor the patient could be blinded to the allocation, because it was obvious whether the patient was discharged the day after surgery or not. However, the randomisation process itself was not influenced in any way by the research nurse or the research team." |
| Allocation concealment (selection bias) | Low risk | See quote first item. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Quote: "Owing to the nature of the intervention, it was not possible to blind participants, researchers or staff involved". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Proportion of completed questionnaires and patient attrition are similar between groups presented in Figure 1. |
| Selective reporting (reporting bias) | Low risk | All outcomes described in Methods are reported in Results. |
| Other bias | Low risk | No evidence of any other bias. |
| Baseline outcomes similar? | Low risk | Baseline outcomes are presented in Table 1 and are not different between treatment groups. |
| Baseline characteristics similar? | Low risk | Quote: "Baseline characteristics of patients (n = 108) and carers (n = 86) were similar for both groups (Table 1)." |
| Protected against contamination? | High risk | Comment: Patients were the unit randomised. From what is presented in the paper, the breast care nurse seem to be available to both groups of patients, so a risk of contamination from this provider is cannot be ruled out. |
Williams 2001.
| Methods | RCT; Unit of allocation: Patient; Stratified by: N/A | |
| Participants | Patients under care of the Department of Oncology. Setting / country: Singleton Hospital, Swansea in south west Wales / UK Type of cancer: Except basal cell carcinoma of the skin Phase of care: Any phase Sample size at randomisation: 504 |
|
| Interventions | Patient held record (PHR) used by the patient and healthcare professionals. The PHR contained instructions for its use printed inside the front cover. The PHR was A6 size with four different coloured sections for (i) free text entries by the patient, (ii) free text entries by health professionals, (iii) details of medications, and (iv) dates of appointments. The patients could use it to note questions they wanted to ask, all current medication, problems with changes of medication, anything else they felt important as a memory aid. The patients were invited to bring the booklet to any hospital, to surgery or to show it to doctors or nurses that visited their home. Control: No details provided |
|
| Outcomes | Patient: QoL Process: Number of contacts with health professionals, booklet use |
|
| Notes | Length of follow‐up: 6 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation after consent and study registration was by an independent computer randomised schedule." |
| Allocation concealment (selection bias) | Low risk | See quote first item. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Outcomes were collected by interview, but no details on blinding are provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: Attrition and reasons for attrition were comparable between groups. |
| Selective reporting (reporting bias) | Low risk | All outcomes described in Methods are reported in Results. |
| Other bias | Low risk | No evidence of any other bias. |
| Baseline outcomes similar? | Low risk | Quotes: "There was no difference between the two groups in baseline demographic data or diagnoses (table 1) or in quality of life, except for the nausea and vomiting sub‐scale of the EORTC (mean score patient held record group 10.09; control group 14.20; p = 0.03; table 2)." "To counteract the effect of possible differences in baseline health related quality of life scores, changes in individual score from baseline were analysed using t tests." |
| Baseline characteristics similar? | Low risk | See quotes item G. |
| Protected against contamination? | High risk | Patients were the unit randomised. Intervention seemed to be taking place in general practice, but no details available to judge if professionals treating control and intervention group patient could be in contact. |
Characteristics of ongoing studies [ordered by study ID]
Abernethy 2006.
| Trial name or title | |
| Methods | Cluster‐RTC; Unit of allocation: General practice; Stratified by: GP‐practice size |
| Participants | Patients in palliative care and their GP Setting / country: Southern Adelaide Palliative Services / Australia Type of cancer: All patients with advanced life‐limited illness Phase of care: Palliative care Planned sample size: 461 |
| Interventions | (1) General practitioner (GP) educational outreach visiting: Educational sessions for GPs about palliative care pain management. Evidence‐based key messages derived from a structured literature review and focus on knowledge and attitude deficits. Trained educator conducts two 20‐30 minute sessions with GP, within 2 weeks of randomisation and 2‐4 weeks later. Educational sessions take place in GP’s office.
(2) Structured patient and caregiver educational outreach visiting: derived from a blend of “patient coaching” and “educational outreach visiting”. Evidence‐based key messages about palliative care pain management were derived from structured literature review. Key messages focus on knowledge and attitude deficits. Trained educator conducts two 30‐40 minute sessions with patient, with or without their caregivers. Educational sessions take place in a place chosen by the patient (e.g. home).
(3) Case conferencing: uses the case conferencing model funded through the Enhanced Primary Care Medicare Benefits Schedule (EPC) items in Australia. Minimally included the GP, patient and/or caregiver, and palliative care nurse. Other participants based upon patient’s needs. Organised by the palliative care nurse. Conferences to be conducted within 28 days of randomisation. Patients and caregivers set the agenda for the case conference by identifying functional, physical, or emotional goals and concerns. GP's remuneration for participation ranges from 48‐105 AUSD (35‐79 USD) based on level and time of participation. Control: Standard palliative care: Consultative medical and nursing support to GPs and other clinicians who provide the majority of clinical care for people at the end of life; Other services included social work, inpatient care, community and outpatient visits, home care, nursing home consultations, a bereavement program, volunteers, and complementary care; Nurses may or may not provide some basic education on pain as part of usual clinical encounters; Formal EPC‐based case conferences rarely occur; Mean length of stay in program is 119 days with median 47. |
| Outcomes | Patient: Symptoms, pain, QoL, daily medication, side‐effect diaries, satisfaction with care Informal carer: Satisfaction with care, palliative resources available Process: Hospitalisation rates after an intervention at a single time point (case conferencing) |
| Starting date | |
| Contact information | |
| Notes | Planned follow‐up length: Until death (min. 0.5; max. 32.5 months) |
Augestad 2008.
| Trial name or title | |
| Methods | Multi‐centre RCT study; Unit of allocation: Patient; Stratified by: Dukes's staging and whether there is a stoma |
| Participants | Patients undergoing surgery for colon cancer. Setting / country: Three hospital trusts and one university hospital / Norway Type of cancer: Colorectal Phase of care: Surveillance, treatment, discharge Planned sample size at randomisation: 170 |
| Interventions | Patients randomised to GP follow‐up (intervention group) will be referred to their GP. This referral will contain information about the surgery and any complications, Dukes's staging, guidelines for follow‐up and behavioural strategy in the case of a Serious Clinical Events (SCE). The regular check‐ups will be performed at three‐month intervals for the first two years and then every six months. All patients with elevated CEA prior to surgery will be requested to undergo this test at every postoperative clinical examination. Chest x‐ray and ultrasound will be performed on a regular basis. Colonoscopy will be performed twice during the follow‐up period. The follow‐up guideline will be similar in both arms Control: Regular follow‐up will take place at the hospital's surgical outpatient clinic. This follow‐up will be performed by consultants or internship doctors in digestive surgery. |
| Outcomes | Patient: QoL, SCE, costs of follow‐up: travelling/transportation, production losses, co‐payments and other patient/family expenses Process: Costs of follow‐up: outpatient visits, GP visits, laboratory tests, radiographs/ultrasound, examinations due to suspected relapse, treatment of relapse |
| Starting date | |
| Contact information | |
| Notes | Planned study duration: 60 months |
Hebert 2004.
| Trial name or title | |
| Methods | RCT; Unit of allocation: Patient; Stratified by: Initial score on the Palliative Performance Scale |
| Participants | Palliative home care adult patients Setting / country: Home care offices (most located in hospitals) / Canada Phase of care: Palliative care Planned sample size at randomisation: 320 |
| Interventions | Combination of traditional and tele‐care (video‐phone) visits. 48 video‐phones were allocated to each health region (in Alberta). Patients in the intervention group will receive care via video‐phones (all or partially?) for 8 weeks. Control: Traditional palliative home care visit. One or more contacts each week, assistance with respect to pain and symptom management. |
| Outcomes | Patient: Symptom management, QoL, readiness to use technology Process: Costs |
| Starting date | |
| Contact information | |
| Notes | Planned study duration: 2 months |
Kimman 2007.
| Trial name or title | |
| Methods | RTC; Unit of allocation: Patient; Stratified by: Treatment modalities and hospital |
| Participants | Breast cancer patients within 6 weeks after treatment. Setting / country: Seven hospitals and two radiotherapy clinics / Netherlands Type of cancer: Breast Phase of care: Surveillance Planned sample size at randomisation: 320 |
| Interventions | 1) Nurse‐led telephone follow‐up; a mammography at one year combined with an outpatient clinic visit, and telephone interviews by a breast care nurse (BCN) or nurse practitioner (NP) at the same time points as during the usual follow‐up (i.e. 3, 6, 9 and 18 months). 2) Nurse‐led telephone follow‐up (same as condition 1 above) plus short educational group program (EGP). The EGP consists of two interactive group sessions of 2.5 hours. It is held at cancer information centres, and provides support to cancer patients and their relatives (partner, friend or family member). 2) Usual follow‐up (see control) plus EGP. Control: Usual follow‐up; 5 outpatient clinic visits in the first 18 months (at 3, 6, 9, 12, and 18 months), with a mammography at one year. |
| Outcomes | Patient: Cancer specific QoL, perceived behavioral control, anxiety, satisfaction with follow‐up/care, costs Process: Costs |
| Starting date | |
| Contact information | |
| Notes | Planned study duration: 18 months |
Meier 2004.
| Trial name or title | |
| Methods | Cluster RCT; Unit of allocation: Care coordinator nurse |
| Participants | Patients in their end‐of‐life's phase Setting / country: Franklin Health's offices in New Jersey (headquarters) and locally health units in communities (in all the 50 states) / United States Phase of care: Palliative care Planned sample size at randomisation: 321 |
| Interventions | Usual complex case management + palliative care assessment and feedback (CCM +): training for care coordinator nurses, clinical account managers, and physician managers on: (1) formal symptom assessment, (2) use of computer‐based treatment protocols and care pathways, (3) communication skills for advance care planning and bad news discussions and (4) feedback to treating physicians. Control: Usual care complex case management (CCM) (initial visit, signed consent, initial assessment, identification of issues and goals, work with patient/family/providers to achieve goals/monitor status, measure of impacts, summary reports). |
| Outcomes | Patient: Pain, symptoms, QoL, communication about treatment preferences, satisfaction, medication prescribed for symptom control Informal carer: Satisfaction Professional: Evaluation of acceptability, feasibility, utility and benefits of the intervention for the patients Process: Hospital days, intensive care unit days, emergency department use, physician visits, length of stay in complex care management, physician inpatient and outpatient relative value units, hospice referral rate, home care services used, analgesic/anxiolytic/antidepressant prescribing, and site of death |
| Starting date | |
| Contact information | |
| Notes | Planned study duration: 6‐8 weeks (or until death) for symptoms; 9‐16 weeks (or until death) for patient and family satisfaction |
Senn 2007.
| Trial name or title | |
| Methods | RCT; Unit of allocation: Patient |
| Participants | Cancer patients who went through initial treatment Setting / country: Health Network / France and United Kingdom Type of cancer: Prostate, breast, colorectal Phase of care: Surveillance Planned sample size at randomisation: 1200 |
| Interventions | GP follow‐up: a trained GP will be responsible for follow‐up with possible referral to the specialist physician (and its team) when requested. The procedure of surveillance is exactly the same used in the control group. The GP and the specialist give relevant information to each other within the 15 days following each consultation Control: Usual follow‐up by the specialist physician (and their team). |
| Outcomes | Patient: Satisfaction, QoL, iatrogenic effects Professional: Physician's (SG and specialist) perception or the surveillance performed in the study, satisfaction Process: Adequacy between the reference protocol and the carried‐out surveillance (performed exams, date of exam versus forecast schedule), presence of relevant information according to the surveillance, costs |
| Starting date | |
| Contact information | |
| Notes | Planned study duration: 24 months |
Differences between protocol and review
There is no major difference between the protocol and this review.
Contributions of authors
All authors reviewed the manuscript and approved its final version. MA and AG designed the study and AG coordinated it. AG, MM and MA participated in data collection. PHC, AG, MM and MA participated in data analysis. All authors participated in the interpretation of results. MM and AG wrote the first draft. MA is the guarantor.
Sources of support
Internal sources
No sources of support supplied
External sources
-
CIHR, Canada.
This study was funded by the Canadian Institute of Health Research (KRS‐94302).
Declarations of interest
None known
New
References
References to studies included in this review
Addington‐Hall 1992 {published data only}
- Addington‐Hall JM, MacDonald LD, Anderson HR, Chamberlain J, Freeling P, Bland JM, et al. Randomised controlled trial of effects of coordinating care for terminally ill cancer patients. BMJ 1992;305(6865):1317‐22. [Reference: #1] [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald LD, Addington‐Hall JM, Anderson HR. Acceptability and perceived effectiveness of a district co‐ordinating service for terminal care: implications for quality assurance. Journal of Advanced Nursing 1994;20(2):337‐43. [Reference: #2] [DOI] [PubMed] [Google Scholar]
- Raftery JP, Addington‐Hall JM, MacDonald LD, Anderson HR, Bland JM, Chamberlain J, et al. A randomized controlled trial of the cost‐effectiveness of a district co‐ordinating service for terminally ill cancer patients. Palliative Medicine 1996;10(2):151‐61. [Reference: #3] [DOI] [PubMed] [Google Scholar]
Beney 2002 {published data only}
- Beney J, Devine EB, Chow V, Ignoffo RJ, Mitsunaga L, Shahkarami M, et al. Effect of telephone follow‐up on the physical well‐being dimension of quality of life in patients with cancer. Pharmacotherapy 2002;22(10):1301‐11. [DOI] [PubMed] [Google Scholar]
Bohnenkamp 2004 {published data only}
- Bohnenkamp SK, McDonald P, Lopez AM, Krupinski E, Blackett A. Traditional versus telenursing outpatient management of patients with cancer with new ostomies. Oncology Nursing Forum 2004;31(5):1005‐10. [DOI] [PubMed] [Google Scholar]
Bonnema 1998 {published data only}
- Bonnema J, Wersch AM, Geel AN, Pruyn JF, Schmitz PI, Paul MA, et al. Medical and psychosocial effects of early discharge after surgery for breast cancer: randomised trial. BMJ 1998;316(7140):1267‐71. [Reference: #1] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry S. Early discharge after surgery for breast cancer was safe and well received by patients [commentary on Bonnema J, van Wersch AM, van Geel AN et al. Medical and psychosocial effects of early discharge after surgery for breast cancer: randomised trial. BR MED J 1998 Apr 25;316(7140):1267‐71]. Evidence‐Based Nursing 1999;2(1):16. [Reference: Commentary on study] [DOI] [PMC free article] [PubMed] [Google Scholar]
Boyes 2006 {published data only}
- Boyes A, Newell S, Girgis A, McElduff P, Sanson‐Fisher R. Does routine assessment and real‐time feedback improve cancer patients' psychosocial well‐being?. European Journal of Cancer Care 2006;15(2):163‐71. [DOI] [PubMed] [Google Scholar]
de Wit 2001 {published data only}
- Wit R, Dam F. From hospital to home care: a randomized controlled trial of a Pain Education Programme for cancer patients with chronic pain. Journal of Advanced Nursing 2001;36(6):742‐54. [Reference: #3] [DOI] [PubMed] [Google Scholar]
- Wit R, Dam F. From hospital to home care: a randomized controlled trial of a Pain Education Programme for cancer patients with chronic pain (erratum). Journal of Advanced Nursing 2002;37(4):404. [Reference: #2] [DOI] [PubMed] [Google Scholar]
- Wit R, Dam F, Abu‐Saad HH, Loonstra S, Zandbelt L, Buuren A, et al. Empirical comparison of commonly used measures to evaluate pain treatment in cancer patients with chronic pain. Journal of Clinical Oncology 1999;17(4):1280. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Wit R, Dam F, Hanneman M, Zandbelt L, Buuren A, Heijden K, et al. Evaluation of the use of a pain diary in chronic cancer pain patients at home. Pain 1999;79(1):89‐99. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Wit R, Dam F, Loonstra S, Zandbelt L, Buuren A, Heijden K, et al. Improving the quality of pain treatment by a tailored pain education programme for cancer patients in chronic pain. European Journal of Pain 2001;5(3):241‐56. [Reference: #5] [DOI] [PubMed] [Google Scholar]
- Wit R, Dam F, Loonstra S, Zandbelt L, Buuren A, Heijden K, et al. The Amsterdam Pain Management Index compared to eight frequently used outcome measures to evaluate the adequacy of pain treatment in cancer patients with chronic pain. Pain 2001;91(3):339‐49. [Reference: #6] [DOI] [PubMed] [Google Scholar]
- Wit R, Dam F, Zandbelt L, Buuren A, Heijden K, Leenhouts G, et al. A pain education program for chronic cancer pain patients: follow‐up results from a randomized controlled trial. Pain 1997;73(1):55‐69. [Reference: #1] [DOI] [PubMed] [Google Scholar]
Drury 2000 {published data only}
- Drury M, Yudkin P, Harcourt J, Fitzpatrick R, Jones L, Alcock C, et al. Patients with cancer holding their own records: a randomised controlled trial. British Journal of General Practice 2000;50(451):105‐10. [PMC free article] [PubMed] [Google Scholar]
Du Pen 1999 {published data only}
- Du Pen SL, Du Pen AR, Polissar N, Hansberry J, Kraybill BM, Stillman M, et al. Implementing guidelines for cancer pain management: results of a randomized controlled clinical trial. Journal of Clinical Oncology 1999;17(1):361‐70. [DOI] [PubMed] [Google Scholar]
Giesler 2005 {published data only}
- Giesler RB, Given B, Given CW, Rawl S, Monahan P, Burns D, et al. Improving the quality of life of patients with prostate carcinoma: a randomized trial testing the efficacy of a nurse‐driven intervention. Cancer 2005;104(4):752‐62. [DOI] [PubMed] [Google Scholar]
Given 2002 {published data only}
- Given B, Given CW, McCorkle R, Kozachik S, Cimprich B, Rahbar MH, et al. Pain and fatigue management: results of a nursing randomized clinical trial. Oncology Nursing Forum 2002; Vol. 29, issue 6:949‐56. [Reference: #1] [DOI] [PubMed]
- Given C, Given B, Rahbar M, Jeon S, McCorkle R, Cimprich B, et al. Effect of a cognitive behavioral intervention on reducing symptom severity during chemotherapy. Journal of Clinical Oncology 2004;22(3):507‐16. [Reference: #2] [DOI] [PubMed] [Google Scholar]
Goodwin 2003 {published data only}
- Goodwin JS, Satish S, Anderson ET, Nattinger AB, Freeman JL. Effect of nurse case management on the treatment of older women with breast cancer. Journal of the American Geriatrics Society 2003;51(9):1252‐9. [Reference: #1] [DOI] [PubMed] [Google Scholar]
- Goodwin JS, Satish S, Freeman JL, Anderson E. Effect of nurse case management on outcomes of older women with breast cancer. Journal of the American Geriatrics Society 1996;44(9):P234. [Reference: Meeting abstract] [DOI] [PubMed] [Google Scholar]
- Jennings‐Sanders A, Kuo YF, Anderson ET, Freeman JL. How do nurse case managers care for older women with breast cancer?. Oncology Nursing Forum 2005;32(3):625‐32. [Reference: #2] [DOI] [PubMed] [Google Scholar]
- Solberg SM. A nurse case management intervention improved medical care given to older women with newly diagnosed breast cancer. Evidence Based Nursing 2004;7(2):58. [1367‐6539: (Print); MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Grunfeld 1996 {published data only}
- Grunfeld E, Fitzpatrick R, Mant D, Yudkin P, Adewuyi‐Dalton R, Stewart J, et al. Comparison of breast cancer patient satisfaction with follow‐up in primary care versus specialist care: results from a randomized controlled trial. British Journal of General Practice 1999;49(446):705‐10. [Reference: #1] [PMC free article] [PubMed] [Google Scholar]
- Grunfeld E, Gray A, Mant D, Yudkin P, Adewuyi‐Dalton R, Coyle D, et al. Follow‐up of breast cancer in primary care vs specialist care: results of an economic evaluation. British Journal of Cancer 1999;79(7‐8):1227‐33. [Reference: #2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunfeld E, Mant D, Yudkin P, Adewuyi‐Dalton R, Cole D, Stewart J, et al. Routine follow up of breast cancer in primary care: randomised trial [see comments] 5281. BMJ 1996;313(7058):665‐9. [Reference: #3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Grunfeld 2006 {published data only}
- Grunfeld E, Levine M, Julian J, Pritchard K, Coyle D, Mirsky D, et al. A randomized controlled trial (RCT) of routine follow‐up for early stage breast cancer: A comparison of primary care versus specialist care. Journal of Clinical Oncology 2004;22(14):665. [Reference: #2] [DOI] [PubMed] [Google Scholar]
- Grunfeld E, Levine MN, Julian JA, Coyle D, Szechtman B, Mirsky D, et al. Randomized trial of long‐term follow‐up for early‐stage breast cancer: a comparison of family physician versus specialist care. Journal of Clinical Oncology 2006;24(6):848‐55. [Reference: #1] [DOI] [PubMed] [Google Scholar]
- Khatcheressian JL, Smith TJ. Randomized trial of long‐term follow‐up for early‐stage breast cancer: a comparison of family physician versus specialist care. Journal of Clinical Oncology 2006;24(6):835‐7. [Reference: Commentary] [DOI] [PubMed] [Google Scholar]
Hanks 2002 {published data only}
- Hanks GW, Robbins M, Sharp D, Forbes K, Done K, Peters TJ, et al. The imPaCT study: a randomised controlled trial to evaluate a hospital palliative care team. British Journal of Cancer 2002;87(7):733‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hughes 1992 {published data only}
- Hughes SL, Cummings J, Weaver F, Manheim L, Braun B, Conrad K. A randomized trial of the cost effectiveness of VA hospital‐based home care for the terminally ill. Health Service Research 1992;26(6):801‐17. [PMC free article] [PubMed] [Google Scholar]
Jefford 2008 {published data only}
- Jefford M, Baravelli C, Dudgeon P, Dabscheck A, Evans M, Moloney M, et al. Tailored chemotherapy information faxed to general practitioners improves confidence in managing adverse effects and satisfaction with shared care: results from a randomized controlled trial. Journal of Clinical Oncology 2008;26(14):2272‐7. [DOI] [PubMed] [Google Scholar]
Johansson 1999 {published data only}
- Johansson B, Berglund G, Glimelius B, Holmberg L, Sjoden PO. Intensified primary cancer care: a randomized study of home care nurse contacts. Journal of Advanced Nursing 1999;30(5):1137‐46. [Reference: #1] [DOI] [PubMed] [Google Scholar]
- Johansson B, Glimelius B, Sjoden PO. [Home care for elderly cancer patients. More intensive follow‐up and home services reduce the need of specialist care]. Lakartidningen 2003;100(17):1524‐6, 1529‐31. [Reference: #3] [PubMed] [Google Scholar]
- Johansson B, Holmberg L, Berglund G, Brandberg Y, Hellbom M, Persson C, et al. Reduced utilisation of specialist care among elderly cancer patients: A randomised study of a primary care intervention. European Journal of Cancer 2001;37(17):2161‐8. [Reference: #2] [DOI] [PubMed] [Google Scholar]
Jordhoy 2001 {published data only}
- Jordhoy MS, Fayers P, Loge JH, Ahlner‐Elmqvist M, Kaasa S. Quality of life in palliative cancer care: Results from a cluster randomized trial. Journal of Clinical Oncology 2001;19(18):3884‐94. [Reference: #1] [DOI] [PubMed] [Google Scholar]
- Jordhoy MS, Fayers P, Saltnes T, Ahlner‐Elmqvist M, Jannert M, Kaasa S. A palliative‐care intervention and death at home: a cluster randomised trial. Lancet 2000;356(9233):888‐93. [Reference: #2] [DOI] [PubMed] [Google Scholar]
- Ringdal GI, Jordhoy MS, Kaasa S. Family satisfaction with end‐of‐life care for cancer patients in a cluster randomized trial. Journal of Pain and Symptom Management 2002;24(1):53‐63. [Reference: #3] [DOI] [PubMed] [Google Scholar]
Kane 1984 {published data only}
- Kane RL, Wales J, Bernstein L, Leibowitz A, Kaplan S. A randomised controlled trial of hospice care. Lancet 1984;1(8382):890‐4. [Reference: #1] [DOI] [PubMed] [Google Scholar]
- Wales J, Kane R, Robbins S, Bernstein L, Krasnow R. UCLA hospice evaluation study. Methodology and instrumentation. Medical Care 1983;21(7):734‐44. [Reference: #2] [DOI] [PubMed] [Google Scholar]
King 2009 {published data only}
- King M, Jones L, McCarthy O, Rogers M, Richardson A, Williams R, et al. Development and pilot evaluation of a complex intervention to improve experienced continuity of care in patients with cancer. British Journal of Cancer 2009;100(2):274‐80. [Reference: #1] [DOI] [PMC free article] [PubMed] [Google Scholar]
- King M, Jones L, Nazareth I. Concern and continuity in the care of cancer patients and their carers: a multi‐method approach to enlightened management. National Coordinating Centre for NHS Service Delivery and Organisation R&D. London: National Co‐ordinating Centre for NHS Service Delivery and Organisation R&D (NCCSDO), 2006. [Reference: #2]
- King M, Jones L, Richardson A, Murad S, Irving A, Aslett H, et al. The relationship between patients' experiences of continuity of cancer care and health outcomes: A mixed methods study. British Journal of Cancer 2008;98(3):529‐36. [Reference: #3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Koinberg 2004 {published data only}
- Koinberg I, Engholm GB, Genell A, Holmberg L. A health economic evaluation of follow‐up after breast cancer surgery: Results of an RCT study. Acta Oncologica 2009;48(1):99‐104. [Reference: #2] [DOI] [PubMed] [Google Scholar]
- Koinberg IL, Engholm GB, Genell A, Holmberg L. A health economic evaluation of follow up after breast cancer surgery ‐ result from of an RCT study. European Journal of Cancer Supplements 2008;6(7):167. [Reference: #3] [Google Scholar]
- Koinberg IL, Fridlund B, Engholm GB, Holmberg L. Nurse‐led follow‐up on demand or by a physician after breast cancer surgery: a randomised study. European Journal of Oncological Nursing 2004;8(2):109‐17; discussion 118‐20. [Reference: #1] [DOI] [PubMed] [Google Scholar]
Kousgaard 2003 {published data only}
- Kousgaard KR, Nielsen JD, Olesen F, Jensen AB. General practitioner assessment of structured oncological information accompanying newly referred cancer patients. Scandinavian Journal of Primary Health Care 2003;21(2):110‐4. [Reference: #1] [DOI] [PubMed] [Google Scholar]
- Nielsen JD, Palshof T, Mainz J, Jensen AB, Olesen F. Randomised controlled trial of a shared care programme for newly referred cancer patients: bridging the gap between general practice and hospital. Quality and Safety in Health Care 2003;12(4):263‐72. [Reference: #2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kravitz 1996 {published data only}
- Kravitz RL, Delafield JP, Hays RD, Drazin R, Conolly M. Bedside charting of pain levels in hospitalized patients with cancer: a randomized controlled trial. Journal of Pain and Symptom Management 1996;11(2):81‐7. [DOI] [PubMed] [Google Scholar]
Liu 2006 {published data only}
- Liu LN, Li CY, Tang ST, Huang CS, Chiou AF. Role of continuing supportive cares in increasing social support and reducing perceived uncertainty among women with newly diagnosed breast cancer in Taiwan. Cancer Nursing 2006;29(4):273‐82. [DOI] [PubMed] [Google Scholar]
Luker 2000 {published data only}
- Luker K, Beaver K, Austin L, Leinster SJ. An evaluation of information cards as a means of improving communication between hospital and primary care for women with breast cancer. Journal of Advanced Nursing 2000;31(5):1174‐82. [DOI] [PubMed] [Google Scholar]
McArdle 1996 {published data only}
- McArdle JMC, George WD, McArdle CS, Smith DC, Moodie AR, Hughson AVM, et al. Psychological support for patients undergoing breast cancer surgery: a randomised study. BMJ 1996;312(7034):813‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
McCorkle 1989 {published data only}
- McCorkle R, Benoliel JQ, Donaldson G, Georgiadou F, Moinpour C, Goodell B. A randomized clinical trial of home nursing care for lung cancer patients 2155. Cancer 1989;64(6):1375‐82. [Reference: #1] [DOI] [PubMed] [Google Scholar]
- McCorkle R, Robinson L, Nuamah I, Lev E, Benoliel JQ. The effects of home nursing care for patients during terminal illness on the bereaved's psychological distress. Nursing Research 1998;47(1):2‐10. [Reference: #2] [DOI] [PubMed] [Google Scholar]
McCorkle 2000 {published data only}
- McCorkle R, Strumpf NE, Nuamah IF, Adler DC, Cooley ME, Jepson C, et al. A specialized home care intervention improves survival among older post‐surgical cancer patients. Journal of the American Geriatrics Society 2000;48(12):1707‐13. [DOI] [PubMed] [Google Scholar]
McCorkle 2009 {published data only}
- McCorkle R, Dowd M, Ercolano E, Schulman‐Green D, Williams AL, Siefert ML, et al. Effects of a nursing intervention on quality of life outcomes in post‐surgical women with gynecological cancers. Psycho‐Oncology 2009;18(1):62‐70. [Reference: #1] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulman‐Green D, Ercolano E, Dowd M, Schwartz P, McCorkle R. Quality of life among women after surgery for ovarian cancer. Palliative Support Care 2008;6(3):239‐47. [Reference: #2] [DOI] [PMC free article] [PubMed] [Google Scholar]
McDonald 2005 {published data only}
- McDonald MV, Pezzin LE, Feldman PH, Murtaugh CM, Peng TR. Can just‐in‐time, evidence‐based “reminders” improve pain management among home health care nurses and their patients?. Journal of Pain and Symptom Management 2005;29(5):474‐88. [DOI] [PubMed] [Google Scholar]
McKegney 1981 {published data only}
- McKegney FP, Bailey LR, Yates JW. Prediction and management of pain in patients with advanced cancer. General Hospital Psychiatry 1981;3(2):95‐101. [Reference: #1] [DOI] [PubMed] [Google Scholar]
- Yates J, Stewart C, Hasler G, Hochstein S. Controlled study of home care by nurse‐practitioners in patients with advanced cancer. Annual Meeting of the American Association for Cancer Research (69th), Apr 5‐8, 1978. NTIS Order Number: HRP‐0026397. 1978:22. [Reference: #2]
- Yates JW, McKegney FP, Visco G, Brown GS. Intensive rehabilitative support of the patient with advanced cancer through home health care. Proceedings of the American Association of Cancer Research. 1977; Vol. 18:199. [Reference: #3]
McLachlan 2001 {published data only}
- McLachlan SA, Allenby A, Matthews J, Wirth A, Kissane D, Bishop M, et al. Randomized trial of coordinated psychosocial interventions based on patient self‐assessments versus standard care to improve the psychosocial functioning of patients with cancer. Journal of Clinical Oncology 2001;19(21):4117‐25. [DOI] [PubMed] [Google Scholar]
McWhinney 1994 {published data only}
- McWhinney IR, Bass MJ, Donner A. Evaluation of a palliative care service: problems and pitfalls. BMJ 1994;309(6965):1340‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mills 2009 {published data only}
- Mills ME, Murray LJ, Johnston BT, Cardwell C, Donnelly M. Does a patient‐held quality‐of‐life diary benefit patients with inoperable lung cancer?. Journal of Clinical Oncology 2009;27(1):70‐7. [Reference: #1] [DOI] [PubMed] [Google Scholar]
- Mills ME, Murray LJ, Johnston BT, Donnell M. Feasibility of a standardised quality of life questionnaire in a weekly diary format for inoperable lung cancer patients. European Journal of Oncology Nursing 2008;12(5):457‐63. [Reference: #2] [DOI] [PubMed] [Google Scholar]
Mitchell 2008 {published data only}
- Mitchell G, Cherry M, Kennedy R, Weeden K, Burridge L, Clavarino A, et al. General practitioner, specialist providers case conferences in palliative care‐‐lessons learned from 56 case conferences. Australian Family Physician 2005;34(5):389‐92. [Reference: #3] [PubMed] [Google Scholar]
- Mitchell GK, Abernethy AP. A comparison of methodologies from two longitudinal community‐based randomized controlled trials of similar interventions in palliative care: what worked and what did not?. Journal of Palliative Medicine 2005;8(6):1226‐37. [Reference: #4] [DOI] [PubMed] [Google Scholar]
- Mitchell GK, Jong IC, Mar CB, Clavarino AM, Kennedy R. General practitioner attitudes to case conferences: how can we increase participation and effectiveness?. Medical Journal of Australia 2002;177(2):95‐7. [Reference: #2] [DOI] [PubMed] [Google Scholar]
- Mitchell GK, Mar CB, O'Rourke PK, Clavarino AM. Do case conferences between general practitioners and specialist palliative care services improve quality of life? A randomised controlled trial (ISRCTN 52269003). Palliative Medicine 2008;22(8):904‐12. [Reference: #1] [DOI] [PubMed] [Google Scholar]
Moore 2002 {published data only}
- Faithfull S, Corner J, Meyer L, Huddart R, Dearnaley D. Evaluation of nurse‐led follow up for patients undergoing pelvic radiotherapy. British Journal of Cancer 2001;85(12):1853‐64. [Reference: #2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore S, Corner J, Haviland J, Wells M, Salmon E, Normand C, et al. Nurse led follow up and conventional medical follow up in management of patients with lung cancer: randomised trial. BMJ 2002;325(7373):1145. [Reference: #1] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mor 1995 {published data only}
- Mor V, Wool M, Guadagnoli E, Allen S. The impact of short term case management on cancer patients' concrete needs and quality of life. Advances in Medical Sociology 1995;6:269‐94. [Google Scholar]
Oleske 1988 {published data only}
- May DM, Oleske D, Justo‐Ober PK, Heide E. The role of the area wide oncology nurse coordinator in the home care of cancer patients. Oncology Nursing Forum 1982;9(4):39‐43. [Reference: #2] [PubMed] [Google Scholar]
- Oleske DM, Hauck WW. A population‐based evaluation of the impact of interventions for improving care to cancer patients in the home setting. Home Health Care Services Quarterly 1988;9(1):45‐61. [Reference: #1] [DOI] [PubMed] [Google Scholar]
Rao 2005 {published data only}
- Cohen HJ, Feussner JR, Weinberger M, Carnes M, Hamdy RC, Hsieh F, et al. A controlled trial of inpatient and outpatient geriatric evaluation and management. New England Journal of Medicine 2002;346(12):905‐12. [Reference: #2] [DOI] [PubMed] [Google Scholar]
- Rao AV, Hsieh F, Feussner JR, Cohen HJ. Geriatric evaluation and management units in the care of the frail elderly cancer patient 3874. Journals of Gerontology Series A ‐ Biological Sciences & Medical Sciences 2005;60(6):798‐803. [Reference: #1] [DOI] [PubMed] [Google Scholar]
Rawl 2002 {published data only}
- Rawl SM, Given BA, Given CW, Champion VL, Kozachik SL, Barton D, et al. Intervention to improve psychological functioning for newly diagnosed patients with cancer. Oncology Nursing Forum 2002;29(6):967‐75. [DOI] [PubMed] [Google Scholar]
Ritz 2000 {published data only}
- Ritz LJ, Nissen MJ, Swenson KK, Farrell JB, Sperduto PW, Sladek ML, et al. Effects of advanced nursing care on quality of life and cost outcomes of women diagnosed with breast cancer. Oncology Nursing Forum 2000;27(6):923‐32. [PubMed] [Google Scholar]
Rutherford 2001 {published data only}
- Rutherford A, Burge B. General practitioners and hospitals. Continuity of care. Australian Family Physician 2001;30(11):1101‐7. [PubMed] [Google Scholar]
Schumacher 2002 {published data only}
- Edrington J, Miaskowski C, Dodd M, West C, Paul SM, Schumacher K. The use of responder analysis to identify differences in patient outcomes following a self‐care intervention to improve cancer pain management. Oncology Nursing Forum 2007;34(1):3. [Reference: #11] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edrington JM, Paul S, Dodd M, West C, Facione N, Tripathy D, et al. No evidence for sex differences in the severity and treatment of cancer pain. Journal of Pain and Symptom Management 2004;28(3):225‐32. [Reference: #10] [DOI] [PubMed] [Google Scholar]
- Kim JE, Dodd M, West C, Paul S, Facione N, Schumacher K, et al. The PRO‐SELF pain control program improves patients' knowledge of cancer pain management 5794. Oncology Nursing Forum 2004;31(6):1137‐43. [Reference: #9] [DOI] [PubMed] [Google Scholar]
- Miaskowski C, Dodd M, West C, Paul SM, Schumacher K, Tripathy D, et al. The use of a responder analysis to identify differences in patient outcomes following a self‐care intervention to improve cancer pain management. Pain 2007;129(1‐2):55‐63. [Reference: #8] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miaskowski C, Dodd M, West C, Schumacher K, Paul SM, Tripathy D, et al. Randomized clinical trial of the effectiveness of a self‐care intervention to improve cancer pain management. Journal of Clinical Oncology 2004;22(9):1713‐20. [Reference: #7] [DOI] [PubMed] [Google Scholar]
- Miaskowski C, Dodd MJ, West C, Paul SM, Tripathy D, Koo P, et al. Lack of adherence with the analgesic regimen: a significant barrier to effective cancer pain management. Journal of Clinical Oncology 2001;19(23):4275‐9. [Reference: #13] [DOI] [PubMed] [Google Scholar]
- Miaskowski C, Mack KA, Dodd M, West C, Paul SM, Tripathy D, et al. Oncology outpatients with pain from bone metastasis require more than around‐the‐clock dosing of analgesics to achieve adequate pain control. Journal of Pain 2002;3(1):12‐20. [Reference: #12] [DOI] [PubMed] [Google Scholar]
- Rustoen T, Moum T, Padilla G, Paul S, Miaskowski C. Predictors of quality of life in oncology outpatients with pain from bone metastasis. Journal of Pain and Symptom Management 2005;30(3):234‐42. [Reference: #6] [DOI] [PubMed] [Google Scholar]
- Schumacher KL, Koresawa S, West C, Dodd M, Paul SM, Tripathy D, et al. The usefulness of a daily pain management diary for outpatients with cancer‐related pain. Oncology Nursing Forum 2002;29(9):1304‐13. [Reference: #5] [DOI] [PubMed] [Google Scholar]
- Schumacher KL, Koresawa S, West C, Hawkins C, Johnson C, Wais E, et al. Putting cancer pain management regimens into practice at home. Journal of Pain and Symptom Management 2002;23(5):369‐82. [Reference: #4] [DOI] [PubMed] [Google Scholar]
- Schumacher KL, West C, Dodd M, Paul SM, Tripathy D, Koo P, et al. Pain management autobiographies and reluctance to use opioids for cancer pain management. Cancer Nursing 2002;25(2):125‐33. [Reference: #3] [DOI] [PubMed] [Google Scholar]
- Villars P, Dodd M, West C, Koetters T, Paul SM, Schumacher K, et al. Differences in the prevalence and severity of side effects based on type of analgesic prescription in patients with chronic cancer pain. Journal of Pain and Symptom Management 2007;33(1):67‐77. [Reference: #14] [DOI] [PMC free article] [PubMed] [Google Scholar]
- West CM, Dodd M, Schumacher K, Paul S, Tripathy D, Miaskowski C. Family caregivers' perceptions of patient's level of upset by cancer pain and their effects on family caregiver outcomes. Oncology Nursing Forum 2007;34(1):164. [Reference: #2] [Google Scholar]
- West CM, Dodd MJ, Paul SM, Schumacher K, Tripathy D, Koo P, et al. The PRO‐SELF(c): Pain Control Program‐‐an effective approach for cancer pain management. Oncology Nursing Forum 2003;30(1):65‐73. [Reference: #1] [DOI] [PubMed] [Google Scholar]
Skrutkowski 2008 {published data only}
- Skrutkowski M, Saucier A, Eades M, Swidzinski M, Ritchie J, Marchionni C, et al. Impact of a pivot nurse in oncology on patients with lung or breast cancer: symptom distress, fatigue, quality of life, and use of healthcare resources. Oncology Nursing Forum 2008;35(6):948‐54. [DOI] [PubMed] [Google Scholar]
Trowbridge 1997 {published data only}
- Trowbridge R, Dugan W, Jay SJ, Littrell D, Casebeer LL, Edgerton S, et al. Determining the effectiveness of a clinical‐practice intervention in improving the control of pain in outpatients with cancer. Academic Medicine 1997;72(9):798‐800. [DOI] [PubMed] [Google Scholar]
Vallieres 2006 {published data only}
- Vallieres I, Aubin M, Blondeau L, Simard S, Giguere A. Effectiveness of a clinical intervention in improving pain control in outpatients with cancer treated by radiation therapy. International Journal of Radiation Oncology, Biology, Physics 2006;66(1):234‐7. [DOI] [PubMed] [Google Scholar]
Velikova 2004 {published data only}
- Velikova G, Booth L, Smith AB, Brown PM, Lynch P, Brown JM, et al. Measuring quality of life in routine oncology practice improves communication and patient well‐being: A randomized controlled trial. Journal of Clinical Oncology 2004;22(4):714‐24. [DOI] [PubMed] [Google Scholar]
Wattchow 2006 {published data only}
- Wattchow DA, Weller DP, Esterman A, Pilotto LS, McGorm K, Hammett Z, et al. General practice vs surgical‐based follow‐up for patients with colon cancer: randomised controlled trial. British Journal of Cancer 2006;94(8):1116‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wells 2003 {published data only}
- Wells NHJT. Improving cancer pain management through patient and family education. Journal of Pain and Symptom Management 2003;25(4):344‐56. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Wells 2004 {published data only}
- Wells M, Harrow A, Donnan P, Davey P, Devereux S, Little G, et al. Patient, carer and health service outcomes of nurse‐led early discharge after breast cancer surgery: a randomised controlled trial. British Journal of Cancer 2004;91(4):651‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Williams 2001 {published data only}
- Williams JG, Cheung WY, Chetwynd N, Cohen DR, El‐Sharkawi S, Finlay I, et al. Pragmatic randomised trial to evaluate the use of patient held records for the continuing care of patients with cancer. Quality in Health Care 2001;10(3):159‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
Abernethy 2006 {published data only}
- Abernethy AP, Currow DC, Hunt R, Williams H, Roder‐Allen G, Rowett D, et al. A pragmatic 2 x 2 x 2 factorial cluster randomized controlled trial of educational outreach visiting and case conferencing in palliative care‐methodology of the Palliative Care Trial [ISRCTN 81117481]. Contemp Clin Trials 2006; Vol. 27, issue 1:83‐100. [1551‐7144: (Print); MEDLINE: ] [DOI] [PubMed]
- Abernethy AP, Currow DC, Shelby‐James T, Williams H, Roder‐Allen G, Hunt R, et al. Improving palliative care: A 2x2x2 factorial cluster randomized controlled trial of case conferencing and educational outreach visiting. Journal of Clinical Oncology 2006; Vol. 24, issue 18:8517. [MEDLINE: ; 0732‐183X] [DOI] [PubMed]
- Agar M, Currow D, Plummer J, Seidel R, Carnahan R, Abernethy AP. Changes in anticholinergic load from regular prescribed medications in palliative care as death approaches. Palliat Med 2009; Vol. 23, issue 3:257‐65. [MEDLINE: ] [DOI] [PubMed]
- Currow DC, Abernethy AP, Shelby‐James TM, Phillips PA. The impact of conducting a regional palliative care clinical study. Palliative Medicine 2006; Vol. 20, issue 8:735‐43. [MEDLINE: ; 0269‐2163] [DOI] [PubMed]
Augestad 2008 {published data only}
- Augestad KM, Vonen B, Aspevik R, Nestvold T, Ringberg U, Johnsen R, et al. Should the surgeon or the general practitioner (GP) follow up patients after surgery for colon cancer? A randomized controlled trial protocol focusing on quality of life, cost‐effectiveness and serious clinical events. Bmc Health Services Research 2008; Vol. 8. [MEDLINE: ; 1472‐6963] [DOI] [PMC free article] [PubMed]
Hebert 2004 {published data only}
Kimman 2007 {published data only}
- Kimman ML, Voogd AC, Dirksen CD, Falger P, Hupperets P, Keymeulen K, et al. Improving the quality and efficiency of follow‐up after curative treatment for breast cancer‐‐rationale and study design of the MaCare trial. BMC Cancer 2007; Vol. 7:1. [1471‐2407: (Electronic); MEDLINE: ] [DOI] [PMC free article] [PubMed]
Meier 2004 {published data only}
Senn 2007 {published data only}
- Senn A, Coussens E, Czernichow P. Joint follow‐up of cancer patients: a Franco British clinical trial project. Oncologie 2007; Vol. 9, issue 12:870‐5. [MEDLINE: ; 1292‐3818]
Additional references
Airey 2002
- Airey C, Becher H, Erens B, Fuller E. National Surveys of NHS patients ‐ Cancer: National Overview 1999/2000. London, England: Department of Health, 2002:73. [Google Scholar]
Alvarez 2006
- Alvarez MP, Agra Y. Systematic review of educational interventions in palliative care for primary care physicians. Palliative Medicine 2006;20(7):673‐83. [DOI] [PubMed] [Google Scholar]
Atkins 2004
- Atkins AD, Best D, Briss PA, Eccles M, Falck‐Ytter Y, Flottorp S, et al. Grading quality of evidence and strength of recommendations. BMJ 2004;328:1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
Barnes 2000
- Barnes EA, Hanson J, Neumann CM, Nekolaichuk CL, Bruera E. Communication between primary care physicians and radiation oncologists regarding patients with cancer treated with palliative radiotherapy. Journal of Clinical Oncology 2000;18(15):2902‐7. [DOI] [PubMed] [Google Scholar]
Beddar 1994
- Beddar SM, Aikin JL. Continuity of care: a challenge for ambulatory oncology nursing. Seminars in Oncology Nursing 1994;10(4):254‐63. [DOI] [PubMed] [Google Scholar]
Bickell 2001
- Bickell NA, Young GJ. Coordination of care for early‐stage breast cancer patients. Journal of General Internal Medicine 2001;16(11):737‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bruinvels 1994
- Bruinvels DJ, Stiggelbout AM, Kievit J, Houwelingen HC, Habbema JD, Velde CJ. Follow‐up of patients with colorectal cancer. A meta‐analysis. Annals of Surgery 1994;219(2):174‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Byers 1999
- Byers T, Mouchawar J, Marks J, Cady B, Lins N, Swanson GM, et al. The American Cancer Society challenge goals. How far can cancer rates decline in the U.S. by the year 2015?. Cancer 1999;86(4):715‐27. [PubMed] [Google Scholar]
Choi 2006
- Choi BCK, Pak AWP. Multidisciplinarity, interdisciplinarity and transdisciplinarity in health research, services, education and policy: 1. Definitions, objectives, and evidence of effectiveness. Clinical & Investigational Medicine 2006;29(6):351‐64. [PubMed] [Google Scholar]
Collins 2004
- Collins RF, Bekker HL, Dodwell DJ. Follow‐up care of patients treated for breast cancer: a structured review. Cancer Treatment Reviews 2004;30(1):19‐35. [DOI] [PubMed] [Google Scholar]
Cox 2003
- Cox K, Wilson E. Follow‐up for people with cancer: nurse‐led services and telephone interventions. Journal of Advanced Nursing 2003;43(1):51‐61. [DOI] [PubMed] [Google Scholar]
Dolovich 2004
- Dolovich LR, Nair KM, Ciliska DK, Lee HN, Birch S, Gafni A, et al. The Diabetes Continuity of Care Scale: the development and initial evaluation of a questionnaire that measures continuity of care from the patient perspective. Health Social Care Community 2004;12:475‐87. [DOI] [PubMed] [Google Scholar]
Dudgeon 2007
- Dudgeon D, Vaitonis V, Seow H, King S, Angus H, Sawka C. Ontario, Canada: using networks to integrate palliative care province‐wide. Journal of Pain and Symptom Management 2007;33(5):640‐4. [DOI] [PubMed] [Google Scholar]
Dumont 2005
- Dumont I, Dumont S, Turgeon J. Continuity of care for advanced cancer patients. Journal of Palliative Care 2005;21(1):49‐56. [PubMed] [Google Scholar]
Dworkind 1999
- Dworkind M, Towers A, Murnaghan D, Guibert R, Iverson D. Communication between family physicians and oncologists: qualitative results of an exploratory study. Cancer Prevention and Control 1999;3(2):137‐44. [PubMed] [Google Scholar]
Earle 2004
- Earle CC, Neville BA. Under use of necessary care among cancer survivors. Cancer 2004;101(8):1712‐9. [DOI] [PubMed] [Google Scholar]
Earle 2006
- Earle CC. Failing to plan is planning to fail: improving the quality of care with survivorship care plans. Journal of Clinical Oncology 2006;24(32):5112‐6. [DOI] [PubMed] [Google Scholar]
Edwards 2002
- Edwards BK, Howe HL, Ries LA, Thun MJ, Rosenberg HM, Yancik R, et al. Annual report to the nation on the status of cancer, 1973‐1999, featuring implications of age and aging on U.S. cancer burden. Cancer 2002;94(10):2766‐92. [DOI] [PubMed] [Google Scholar]
Efron 1993
- Efron B, Tibshirani RJ. An Introduction to the Bootstrap. Chapman and Hall, 1993. [Google Scholar]
EPOC
- Cochrane Effective practice and organisation of care review group. Data collection checklist. http://epoc.cochrane.org/sites/epoc.cochrane.org/files/uploads/datacollectionchecklist.pdf.
Etim 2006
- Etim AE, Schellhase KG, Sparapani R, Nattinger AB. Effect of model of care delivery on mammography use among elderly breast cancer survivors. Breast Cancer Research and Treatment 2006;96(3):293‐9. [DOI] [PubMed] [Google Scholar]
Faithfull 2001
- Faithfull S, Corner J, Meyer L, Huddart R, Dearnaley D. Evaluation of nurse‐led follow up for patients undergoing pelvic radiotherapy. The British Journal of Cancer 2001;85(12):1853‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fillion 2006
- Fillion L, Serres M, Lapointe‐Goupil R, Bairati I, Gagnon P, Deschamps M, et al. Implementing the role of patient‐navigator nurse at a university hospital centre. Canadian Oncology Nursing Journal 2006;16(1):5‐17. [DOI] [PubMed] [Google Scholar]
Freeman 2001
- Freeman G, Shepperd S, Robinson I, Ehrich K, Richards S. Continuity of Care: Report of a scoping exercise for the National Co‐ordinating Centre for NHS Service Delivery and Organisation R&D (NCCSDO). http://www.sdo.nihr.ac.uk/files/project/2‐final‐report.pdf 2001:141.
Ganz 2006
- Ganz PA. Monitoring the physical health of cancer survivors: a survivorship‐focused medical history. Journal of Clinical Oncology 2006;24(32):5105‐11. [DOI] [PubMed] [Google Scholar]
Grimshaw 2004
- Grimshaw JM, Thomas RE, MacLennan G, Fraser C, Ramsay CR, Vale L, et al. Effectiveness and efficiency of guidelines dissemination and implementation strategies. Health Technology Assessments 2004;8(6):1‐72. [DOI] [PubMed] [Google Scholar]
Gulliford 2006
- Gulliford MC, Naithani S, Morgan M. Measuring continuity of care in diabetes mellitus: an experience‐based measure. Annals of Family Medicine 2006;4:548‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck‐Ytter Y, Schünemann HJ, for the GRADE Working Group. What is "quality of evidence" and why is it important to clinicians?. BMJ 2008;336:995‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gysels 2007
- Gysels M, Richardson A, Higginson IJ. Does the patient‐held record improve continuity and related outcomes in cancer care: a systematic review. Health Expect 2007; Vol. 10, issue 1:75‐91. [DOI] [PMC free article] [PubMed]
Hack 2005
- Hack TF, Degner LF, Parker PA. The communication goals and needs of cancer patients: a review. Psycho‐Oncology 2005;14(10):831‐45; discussion 846‐7. [DOI] [PubMed] [Google Scholar]
Hadjistavropoulos 2008
- Hadjistavropoulos H, Biem H, Sharpe D, Bourgault‐Fagnou M, Janzen J. Patient perceptions of hospital discharge: reliability and validity of a Patient Continuity of Care Questionnaire. International Journal for Quality in Health Care 2008;20(5):314‐23. [DOI] [PubMed] [Google Scholar]
Haggerty 2003
- Haggerty JL, Reid RJ, Freeman GK, Starfield BH, Adair CE, McKendry R. Continuity of care: a multidisciplinary review. BMJ 2003; Vol. 327, issue 7425:1219‐21. [DOI] [PMC free article] [PubMed]
Hewitt 2003
- Hewitt M, Rowland JH, Yancik R. Cancer survivors in the United States: age, health, and disability. Journal of Gerontology Series A: Biological Sciences and Medical Sciences 2003;58(1):82‐91. [DOI] [PubMed] [Google Scholar]
Hewitt 2007
- Hewitt ME, Bamundo A, Day R, Harvey C. Perspectives on post‐treatment cancer care: qualitative research with survivors, nurses, and physicians. Journal of Clinical Oncology 2007;25(16):2270‐3. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hickman 1994
- Hickman M, Drummond N, Grimshaw J. A taxonomy of shared care for chronic disease. Journal of Public Health Medicine 1994;16(4):447‐54. [DOI] [PubMed] [Google Scholar]
Holland 2007
- Holland DE, Harris MR. Discharge planning, transitional care, coordination of care, and continuity of care: clarifying concepts and terms from the hospital perspective. Home Health Care Services Quarterly 2007;26:3‐19. [DOI] [PubMed] [Google Scholar]
Hopwood 2000
- Hopwood P, Stephens RJ. Depression in patients with lung cancer: prevalence and risk factors derived from quality‐of‐life data. Journal of Clinical Oncology 2000;18(4):893‐903. [DOI] [PubMed] [Google Scholar]
Institute of Medicine 2006
- Institute of Medicine, National Research Council. In: Hewitt M, Greenfield S, Stovall E editor(s). From cancer patient to cancer survivor: lost in transition. Washington, D.C.: The National Academies Press, 2006:506. [Google Scholar]
Jemal 2007
- Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA ‐ A Cancer Journal for Clinicians 2007;57(1):43‐66. [DOI] [PubMed] [Google Scholar]
Johansson 2000
- Johansson B, Berglund G, Hoffman K, Glimelius B, Sjoden PO. The role of the general practitioner in cancer care and the effect of an extended information routine. Scandinavian Journal of Primary Health Care 2000;18(3):143‐8. [DOI] [PubMed] [Google Scholar]
Keating 2005
- Keating NL, Norredam M, Landrum MB, Huskamp HA, Meara E. Physical and mental health status of older long‐term cancer survivors. Journal of the American Geriatrics Society 2005;53(12):2145‐52. [DOI] [PubMed] [Google Scholar]
Keating 2006
- Keating NL, Landrum MB, Guadagnoli E, Winer EP, Ayanian JZ. Factors related to underuse of surveillance mammography among breast cancer survivors. Journal of Clinical Oncology 2006;24(1):85‐94. [DOI] [PubMed] [Google Scholar]
Kowalyk 2004
- Kowalyk KM, Hadjistavropoulos HD, Biem HJ. Measuring continuity of care for cardiac patients: development of a patient self‐report questionnaire. Canadian Journal of Cardiology 2004;20:205‐12. [PubMed] [Google Scholar]
Letelier 2010
- Letelier MJ, Aller MB, Henao D, Sanchez‐Perez I, Vargas I, Coderch de Lassaletta J, et al. Design and validation of a questionnaire to measure continuity between care levels from the user's perspective. Gaceta Sanitaria 2010;24:339‐46. [DOI] [PubMed] [Google Scholar]
Lofmark 2005
- Lofmark R, Nilstun T, Bolmsjo IA. From cure to palliation: staff communication, documentation, and transfer of patient. Journal of Palliative Medicine 2005;8(6):1105‐9. [DOI] [PubMed] [Google Scholar]
Miedema 2003
- Miedema B, MacDonald I, Tatemichi S. Cancer follow‐up care. Patients' perspectives. Canadian Family Physician 2003;49:890‐5. [PMC free article] [PubMed] [Google Scholar]
Moore 2006
- Moore S, Wells M, Plant H, Fuller F, Wright M, Corner J. Nurse specialist led follow‐up in lung cancer: The experience of developing and delivering a new model of care. European Journal of Oncology Nursing 2006;10(5):364‐77. [DOI] [PubMed] [Google Scholar]
Moxley 1989
- Moxley DP. The practice of case management. Sage Publications, 1989. [Google Scholar]
Murray 2005
- Murray SA, Kendall M, Boyd K, Sheikh A. Illness trajectories and palliative care. BMJ 2005;330(7502):1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
O'Hare 1993
- O'Hare PA, Yost LS, McCorkle R. Strategies to improve continuity of care and decrease rehospitalization of cancer patients: a review. Cancer Investigation 1993;11(2):140‐58. [DOI] [PubMed] [Google Scholar]
Oeffinger 2006
- Oeffinger KC, McCabe MS. Models for delivering survivorship care. Journal of Clinical Oncology 2006;24(32):5117‐24. [DOI] [PubMed] [Google Scholar]
Passik 1998
- Passik SD, Dugan W, McDonald MV, Rosenfeld B, Theobald DE, Edgerton S. Oncologists' recognition of depression in their patients with cancer. Journal of Clinical Oncology 1998;16(4):1594‐600. [DOI] [PubMed] [Google Scholar]
R Software [Computer program]
- R Development Core Team. R: A language and environment for statistical computing. Version 2.12.1. Vienna, Austria: R Foundation for Statistical Computing ISBN 3‐900051‐07‐0, URL http://www.R‐project.org/, 2010.
Reid 2002
- Reid R, Haggerty J, McKendry R. Defusing the confusion: concept and measures of continuity of healthcare. Available at http://www.chsrf.ca/Migrated/PDF/ResearchReports/CommissionedResearch/cr_contcare_e.pdf. Ottawa: Canadian Health Services Research Foundation, 2002:1‐50. [MEDLINE: ]
rmeta Package [Computer program]
- Thomas Lumley. rmeta. Version 2.16. R Foundation for Statistical Computing, 2009‐11.
Sant 2003
- Sant M, Aareleid T, Berrino F, Bielska Lasota M, Carli PM, Faivre J, et al. EUROCARE‐3: survival of cancer patients diagnosed 1990‐94‐‐results and commentary. Annals of Oncology 2003;14(Suppl 5):v61‐118. [DOI] [PubMed] [Google Scholar]
SAS Software [Computer program]
- SAS Institute Inc.. SAS software, Version 9.2 of the SAS System for Windows. Cary, North Carolina, USA: SAS Institute Inc., Copyright © 2002‐2008.
Shojania 2009
- Shojania KG, Jennings A, Mayhew A, Ramsay CR, Eccles MP, Grimshaw J. The effects of on‐screen, point of care computer reminders on processes and outcomes of care. Cochrane Database of Systematic Reviews 2010, Issue 1. [DOI: 10.1002/14651858.CD001096.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Smeenk 1998a
- Smeenk FW, Witte LP, Haastregt JC, Schipper RM, Biezemans HP, Crebolder HF. Transmural care of terminal cancer patients: effects on the quality of life of direct caregivers. Nursing Research 1998;47(3):129‐36. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Smeenk 1998b
- Smeenk FW, Witte LP, Haastregt JC, Schipper RM, Biezemans HP, Crebolder HF. Transmural care. A new approach in the care for terminal cancer patients: its effects on re‐hospitalization and quality of life. Patient Education and Counseling 1998;35(3):189‐99. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Smeenk 2000
- Smeenk FW, Witte LP, Nooyen IW, Crebolder HF. Effects of transmural care on coordination and continuity of care. Patient Education and Counseling 2000;41(1):73‐81. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Smith 1999
- Smith SD, Nicol KM, Devereux J, Cornbleet MA. Encounters with doctors: quantity and quality. Palliative Medicine 1999;13(3):217‐23. [DOI] [PubMed] [Google Scholar]
Smith 2009
- Smith SM, Allwright S, O'Dowd T. Effectiveness of shared care across the interface between primary and specialty care in chronic disease management. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI: 10.1002/14651858.CD004910.pub2] [DOI] [PubMed] [Google Scholar]
Sparbel 2000a
- Sparbel KJH, Anderson MA. Integrated literature review of continuity of care: Part 1, Conceptual issues. Journal of Nursing Scholarship 2000;32(1):17‐24. [DOI] [PubMed] [Google Scholar]
Sparbel 2000b
- Sparbel KJH, Anderson MA. A continuity of care integrated literature review, Part 2: Methodological issues. Journal of Nursing Scholarship 2000;32(2):131‐5. [DOI] [PubMed] [Google Scholar]
Steinman 2006
- Steinman MA, Ranji SR, Shojania KG, Gonzales R. Improving antibiotic selection: a systematic review and quantitative analysis of quality improvement strategies. Medical Care 2006;44(7):617‐28. [DOI] [PubMed] [Google Scholar]
Sussman 2004
- Sussman J, Howell D, O’Brien MA, Whelan T. An evaluation of the effectiveness of a specialized nursing case management model in coordinating supportive cancer care in the community. Hamilton: The Supportive Cancer Care Research Unit (SCCRU), Juravinski Cancer Centre, 2004. [Google Scholar]
Tang 2001
- Tang ST, McCorkle R. Determinants of place of death for terminal cancer patients. Cancer Investigation 2001;19(2):165‐80. [DOI] [PubMed] [Google Scholar]
Uijen 2011a
- Uijen AA, Schellevis FG, Bosch WJHM, MokkinkHGA, Weel C, Schers HJ. Nijmegen Continuity Questionnaire: Development and testing of a questionnaire that measures continuity of care. Journal of Clinical Epidemiology 2001;64:1391‐9. [DOI] [PubMed] [Google Scholar]
Uijen 2011b
- Uijen AA, Schers HJ, Schellevis FG, Bosch WJHM. How unique is continuity of care? A review of continuity and related concepts. Family Practice 2011;0:1‐8. [DOI] [PubMed] [Google Scholar]
Walsh 2006
- Walsh JM, McDonald KM, Shojania KG, Sundaram V, Nayak S, Lewis R, et al. Quality improvement strategies for hypertension management: a systematic review. Medical Care 2006;44(7):646‐57. [DOI] [PubMed] [Google Scholar]
Wei 2008
- Wei X, Barnsley J, Zakus D, Cockerill R, Glazier R, Sun X. Assessing continuity of care in a community diabetes program: initial questionnaire development and validation. Journal of Clinical Epidemiology 2008;61:925‐31. [DOI] [PubMed] [Google Scholar]
Yabroff 2004
- Yabroff KR, Lawrence WF, Clauser S, Davis WW, Brown ML. Burden of illness in cancer survivors: findings from a population‐based national sample. Journal of the National Cancer Institute 2004;96(17):1322‐30. [DOI] [PubMed] [Google Scholar]
Yancik 2001
- Yancik R, Ganz PA, Varricchio CG, Conley B. Perspectives on comorbidity and cancer in older patients: approaches to expand the knowledge base. Journal of Clinical Oncology 2001;19(4):1147‐51. [DOI] [PubMed] [Google Scholar]


