Abstract
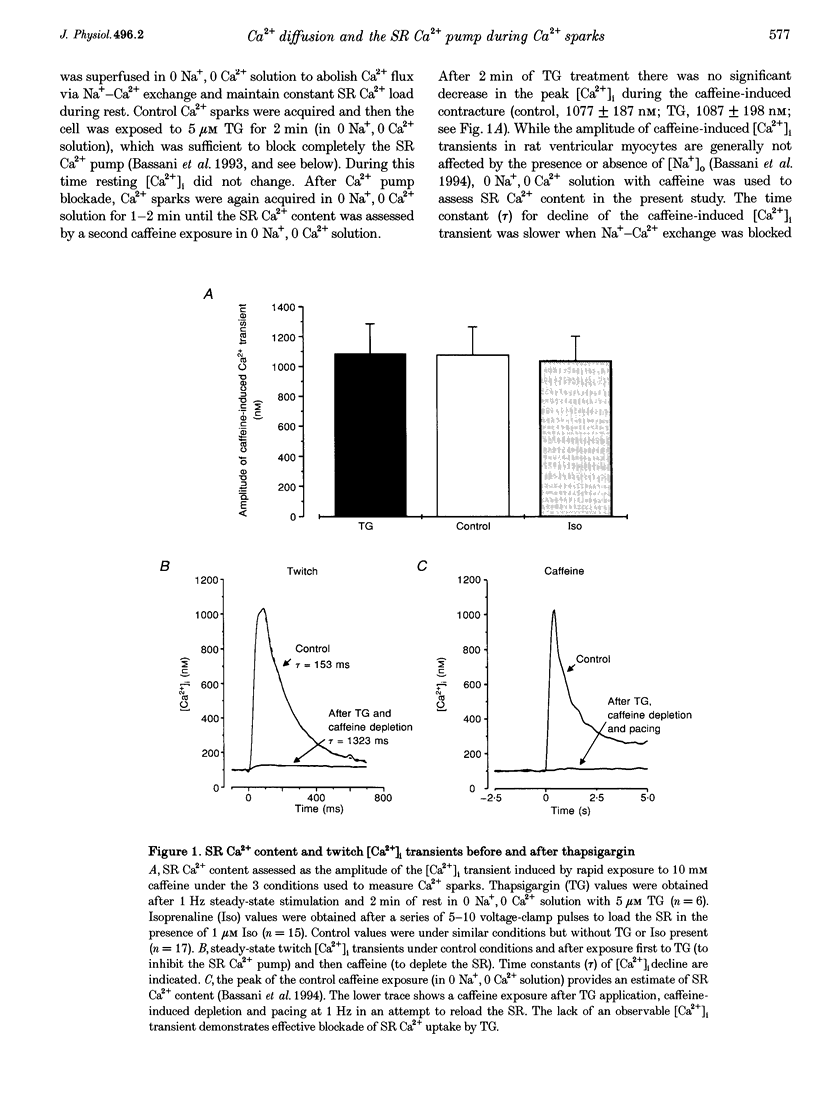
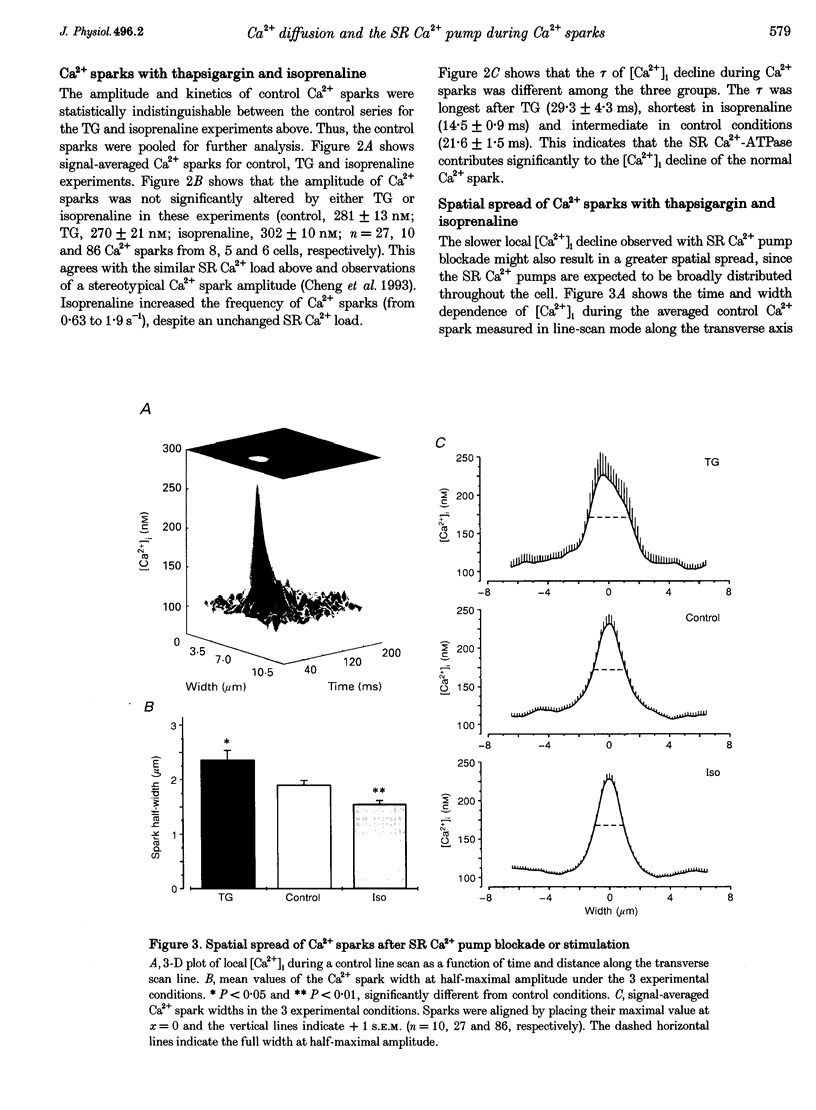
1. We sought to evaluate the contribution of the sarcoplasmic reticulum (SR) Ca2+ pump (vs. diffusion) to the kinetics of [Ca24]i decline during Ca2+ sparks, which are due to spontaneous local SR Ca2+ release, in isolated rat ventricular myocytes measured using fluo-3 and laser scanning confocal microscopy. 2. Resting Ca2+ sparks were compared before (control) and after the SR Ca2(+)-ATPase was either completely blocked by 5 microM thapsigargin (TG) or stimulated by isoprenaline. Na(+)-Ca2+ exchange was blocked using Na(+)-free, Ca(2+)-free solution (0 Na+, O Ca2+) and conditions were arranged so that the SR Ca2+ content was the same under all conditions when Ca2+ sparks were measured. 3. The control Ca2+ spark amplitude (281 +/- 13 nM) was not changed by TG (270 +/- 21 nM) or isoprenaline (302 +/- 10 nM). However, the time constant of [Ca2+]i decline was significantly slower in the presence of TG (29.3 +/- 4.3 ms) compared with control (21.6 +/- 1.5 ms) and faster with isoprenaline (14.5 +/- 0.9 ms), but in all cases was much faster than the global [Ca2+]i decline during a control twitch (177 +/- 10 ms). 4. The spatial spread of Ca2+ during the Ca2+ spark was also influenced by the SR Ca2+ pump. The apparent 'space constant' of the Ca2+ sparks was longest when the SR Ca2+ pump was blocked, intermediate in control and shortest with isoprenaline. 5. We conclude that while Ca2+ diffusion from the source of Ca2+ release is the dominant process in local [Ca2+]i decline during the Ca2+ spark, Ca2+ transport by the SR contributes significantly to both the kinetics and spatial distribution of [Ca2+]i during the Ca2+ spark.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bassani J. W., Bassani R. A., Bers D. M. Relaxation in rabbit and rat cardiac cells: species-dependent differences in cellular mechanisms. J Physiol. 1994 Apr 15;476(2):279–293. doi: 10.1113/jphysiol.1994.sp020130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassani J. W., Bassani R. A., Bers D. M. Twitch-dependent SR Ca accumulation and release in rabbit ventricular myocytes. Am J Physiol. 1993 Aug;265(2 Pt 1):C533–C540. doi: 10.1152/ajpcell.1993.265.2.C533. [DOI] [PubMed] [Google Scholar]
- Bassani J. W., Yuan W., Bers D. M. Fractional SR Ca release is regulated by trigger Ca and SR Ca content in cardiac myocytes. Am J Physiol. 1995 May;268(5 Pt 1):C1313–C1319. doi: 10.1152/ajpcell.1995.268.5.C1313. [DOI] [PubMed] [Google Scholar]
- Bassani R. A., Bassani J. W., Bers D. M. Mitochondrial and sarcolemmal Ca2+ transport reduce [Ca2+]i during caffeine contractures in rabbit cardiac myocytes. J Physiol. 1992;453:591–608. doi: 10.1113/jphysiol.1992.sp019246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bers D. M., Berlin J. R. Kinetics of [Ca]i decline in cardiac myocytes depend on peak [Ca]i. Am J Physiol. 1995 Jan;268(1 Pt 1):C271–C277. doi: 10.1152/ajpcell.1995.268.1.C271. [DOI] [PubMed] [Google Scholar]
- Cannell M. B., Cheng H., Lederer W. J. Spatial non-uniformities in [Ca2+]i during excitation-contraction coupling in cardiac myocytes. Biophys J. 1994 Nov;67(5):1942–1956. doi: 10.1016/S0006-3495(94)80677-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannell M. B., Cheng H., Lederer W. J. The control of calcium release in heart muscle. Science. 1995 May 19;268(5213):1045–1049. doi: 10.1126/science.7754384. [DOI] [PubMed] [Google Scholar]
- Cheng H., Cannell M. B., Lederer W. J. Partial inhibition of Ca2+ current by methoxyverapamil (D600) reveals spatial nonuniformities in [Ca2+]i during excitation-contraction coupling in cardiac myocytes. Circ Res. 1995 Feb;76(2):236–241. doi: 10.1161/01.res.76.2.236. [DOI] [PubMed] [Google Scholar]
- Cheng H., Lederer W. J., Cannell M. B. Calcium sparks: elementary events underlying excitation-contraction coupling in heart muscle. Science. 1993 Oct 29;262(5134):740–744. doi: 10.1126/science.8235594. [DOI] [PubMed] [Google Scholar]
- Hove-Madsen L., Bers D. M. Passive Ca buffering and SR Ca uptake in permeabilized rabbit ventricular myocytes. Am J Physiol. 1993 Mar;264(3 Pt 1):C677–C686. doi: 10.1152/ajpcell.1993.264.3.C677. [DOI] [PubMed] [Google Scholar]
- Klein M. G., Cheng H., Santana L. F., Jiang Y. H., Lederer W. J., Schneider M. F. Two mechanisms of quantized calcium release in skeletal muscle. Nature. 1996 Feb 1;379(6564):455–458. doi: 10.1038/379455a0. [DOI] [PubMed] [Google Scholar]
- Lipp P., Niggli E. Submicroscopic calcium signals as fundamental events of excitation--contraction coupling in guinea-pig cardiac myocytes. J Physiol. 1996 Apr 1;492(Pt 1):31–38. doi: 10.1113/jphysiol.1996.sp021286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-López J. R., Shacklock P. S., Balke C. W., Wier W. G. Local calcium transients triggered by single L-type calcium channel currents in cardiac cells. Science. 1995 May 19;268(5213):1042–1045. doi: 10.1126/science.7754383. [DOI] [PubMed] [Google Scholar]
- López-López J. R., Shacklock P. S., Balke C. W., Wier W. G. Local, stochastic release of Ca2+ in voltage-clamped rat heart cells: visualization with confocal microscopy. J Physiol. 1994 Oct 1;480(Pt 1):21–29. doi: 10.1113/jphysiol.1994.sp020337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negretti N., O'Neill S. C., Eisner D. A. The relative contributions of different intracellular and sarcolemmal systems to relaxation in rat ventricular myocytes. Cardiovasc Res. 1993 Oct;27(10):1826–1830. doi: 10.1093/cvr/27.10.1826. [DOI] [PubMed] [Google Scholar]
- Sagara Y., Fernandez-Belda F., de Meis L., Inesi G. Characterization of the inhibition of intracellular Ca2+ transport ATPases by thapsigargin. J Biol Chem. 1992 Jun 25;267(18):12606–12613. [PubMed] [Google Scholar]
- Santana L. F., Cheng H., Gómez A. M., Cannell M. B., Lederer W. J. Relation between the sarcolemmal Ca2+ current and Ca2+ sparks and local control theories for cardiac excitation-contraction coupling. Circ Res. 1996 Jan;78(1):166–171. doi: 10.1161/01.res.78.1.166. [DOI] [PubMed] [Google Scholar]
- Tada M., Kirchberger M. A., Repke D. I., Katz A. M. The stimulation of calcium transport in cardiac sarcoplasmic reticulum by adenosine 3':5'-monophosphate-dependent protein kinase. J Biol Chem. 1974 Oct 10;249(19):6174–6180. [PubMed] [Google Scholar]
- Tinker A., Lindsay A. R., Williams A. J. Cation conduction in the calcium release channel of the cardiac sarcoplasmic reticulum under physiological and pathophysiological conditions. Cardiovasc Res. 1993 Oct;27(10):1820–1825. doi: 10.1093/cvr/27.10.1820. [DOI] [PubMed] [Google Scholar]
- Tsugorka A., Ríos E., Blatter L. A. Imaging elementary events of calcium release in skeletal muscle cells. Science. 1995 Sep 22;269(5231):1723–1726. doi: 10.1126/science.7569901. [DOI] [PubMed] [Google Scholar]