Abstract
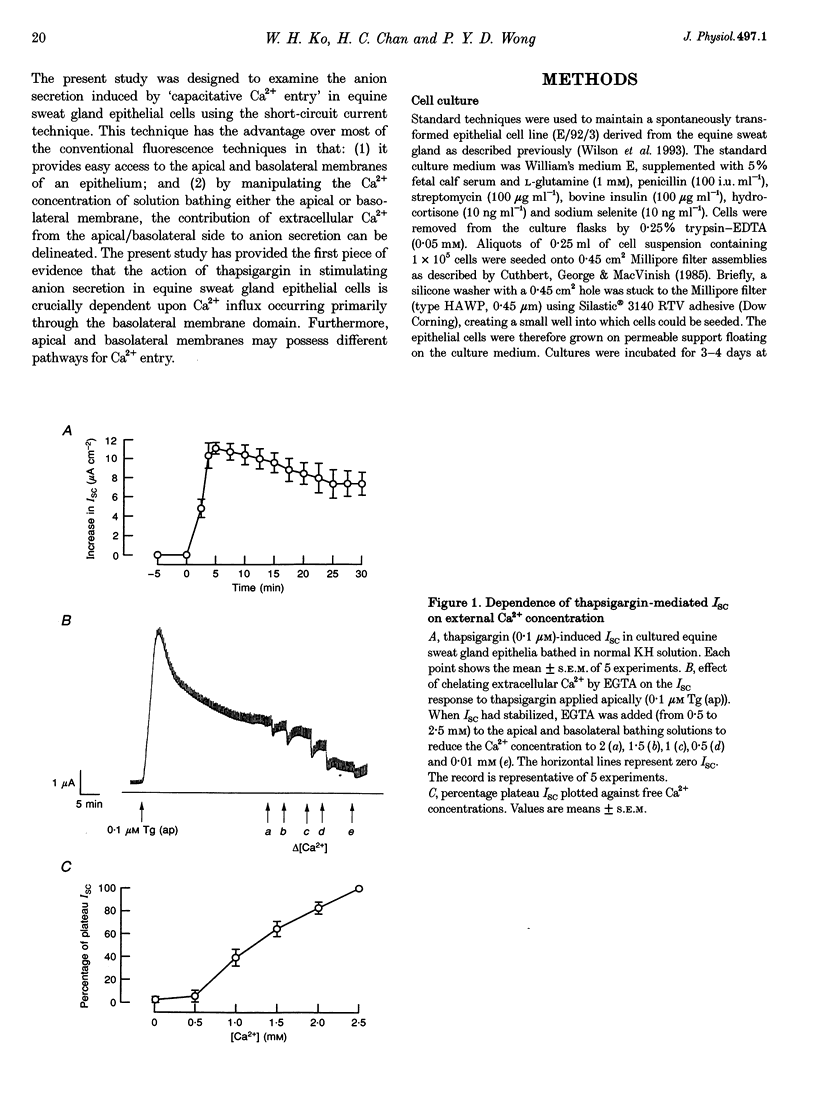
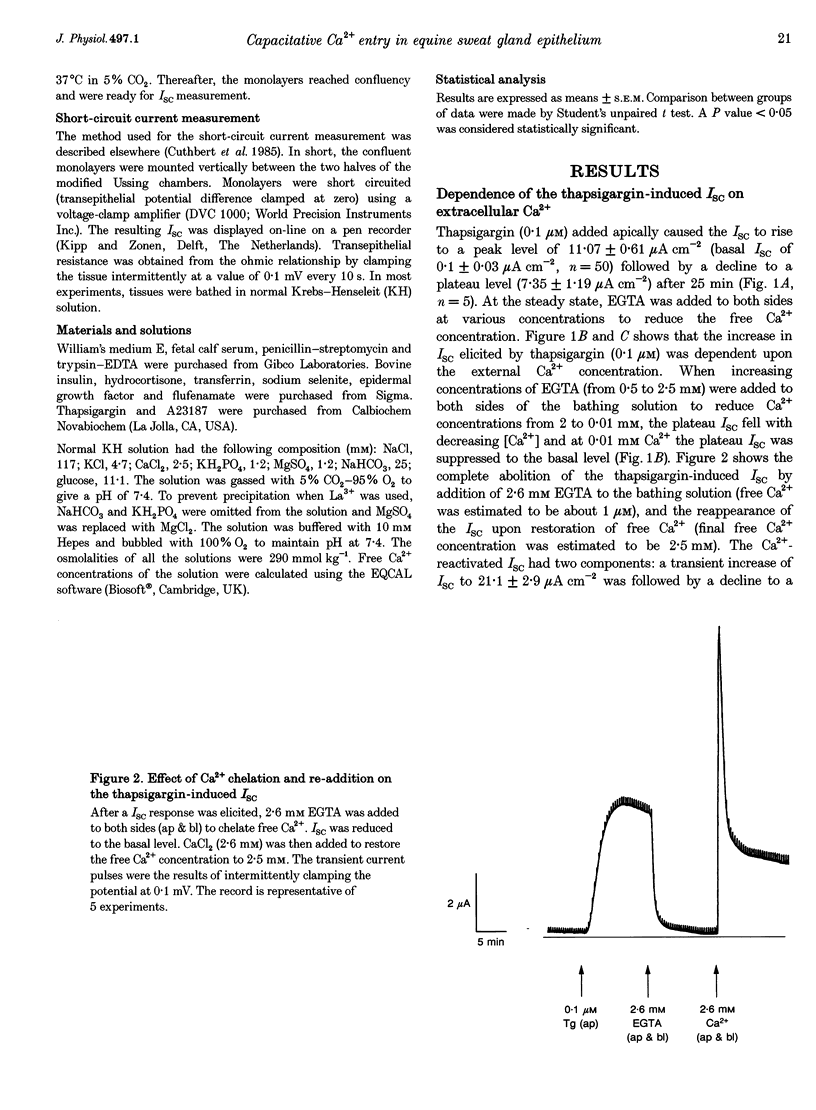
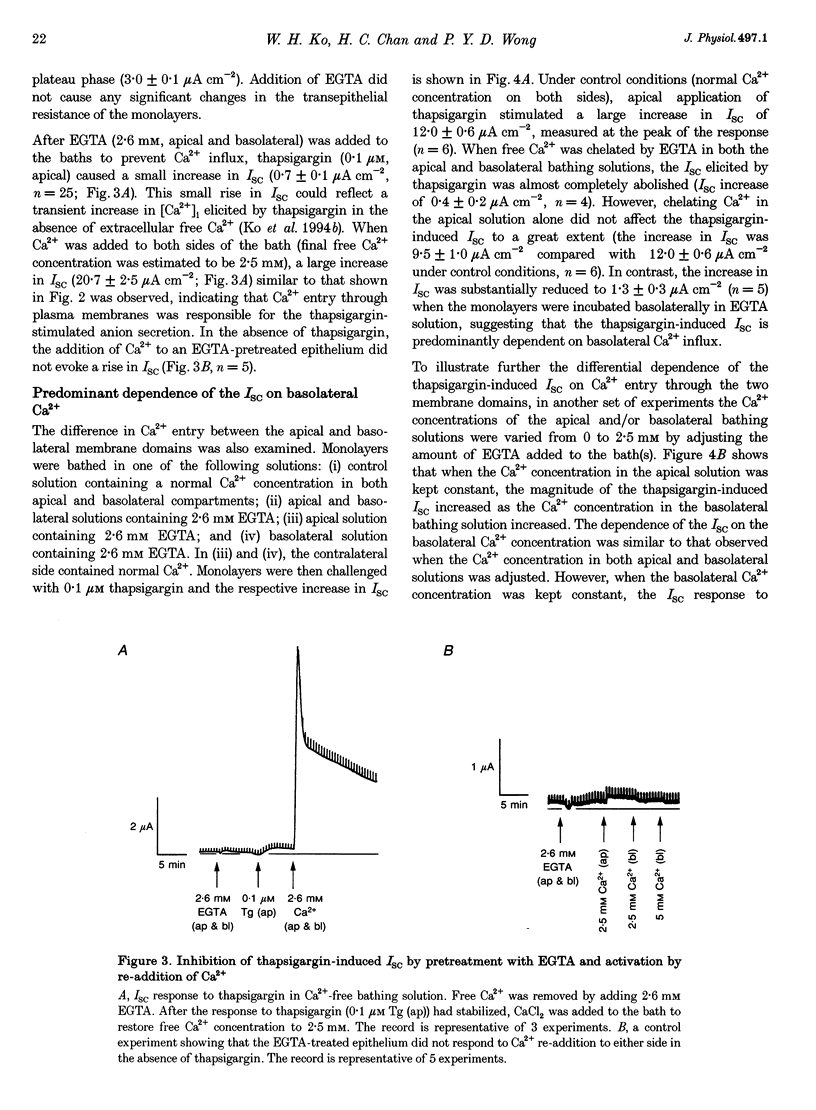
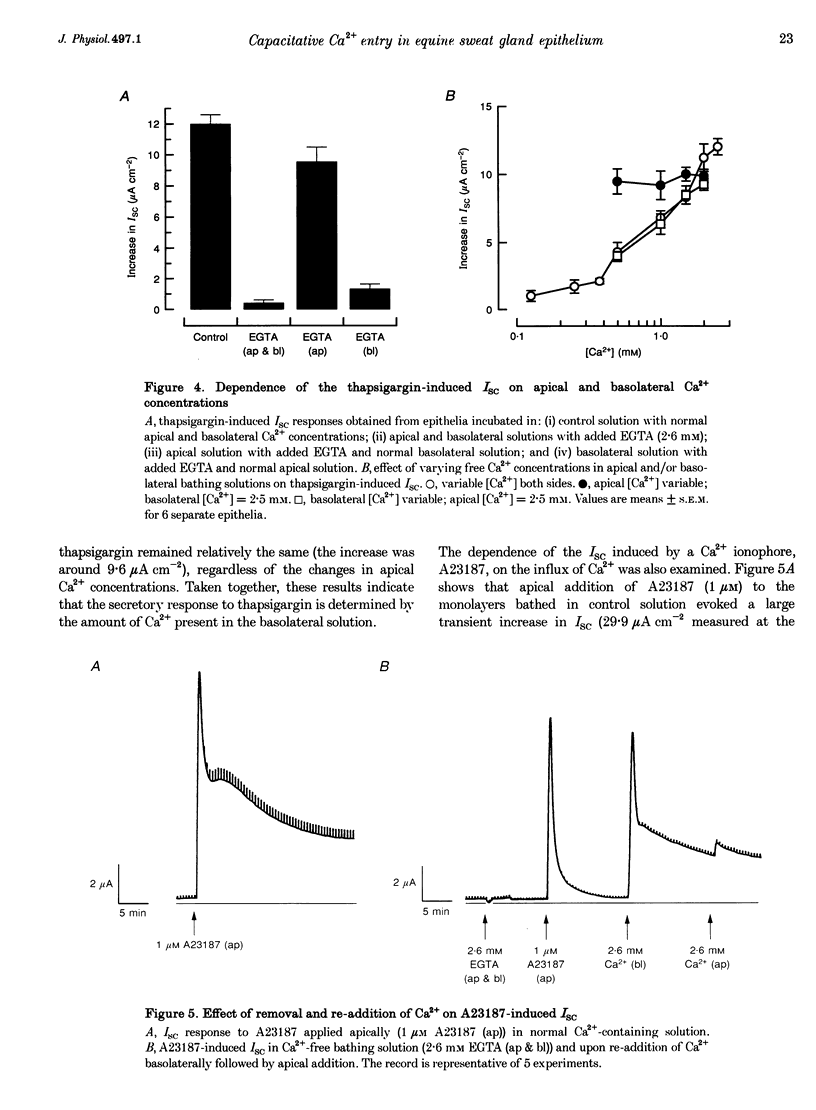
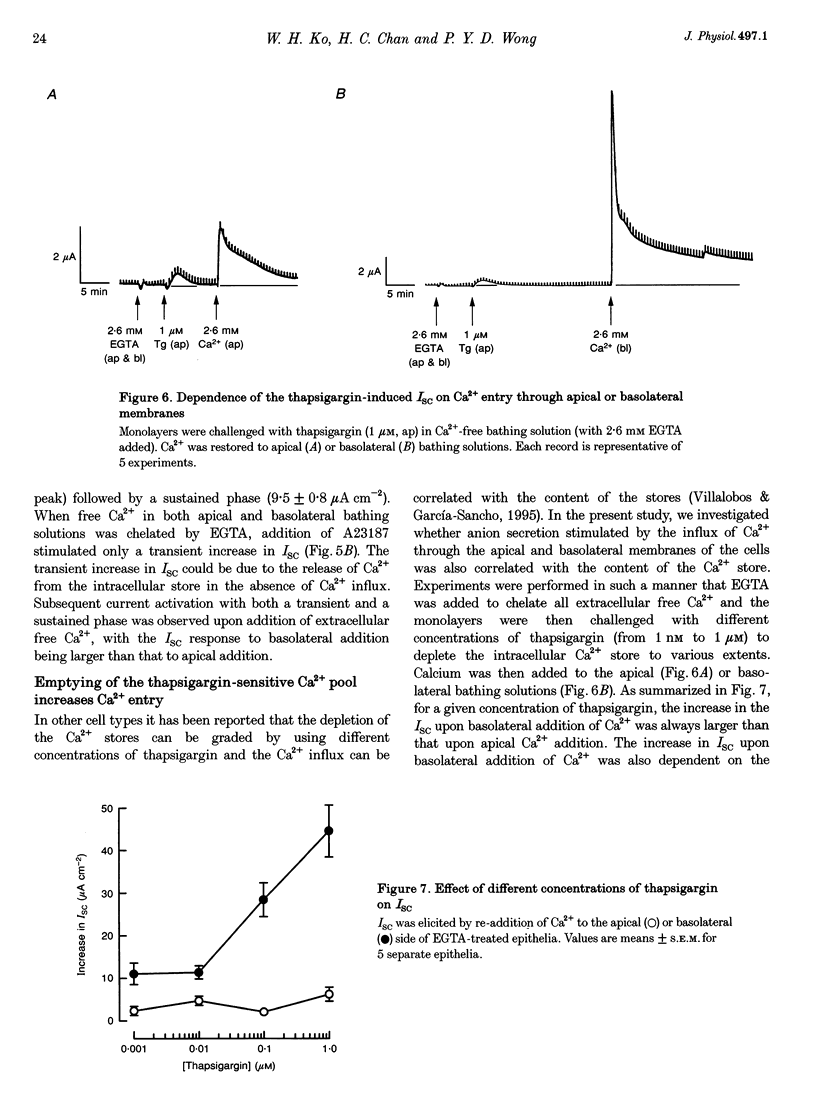
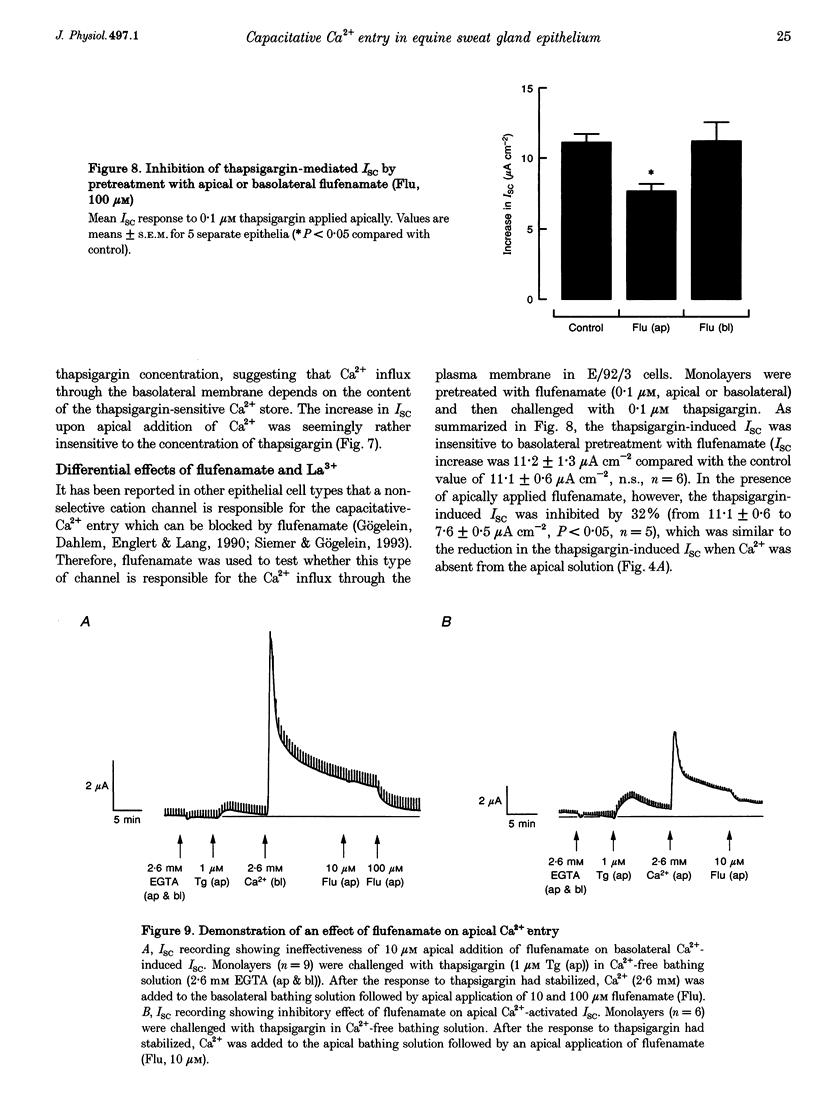
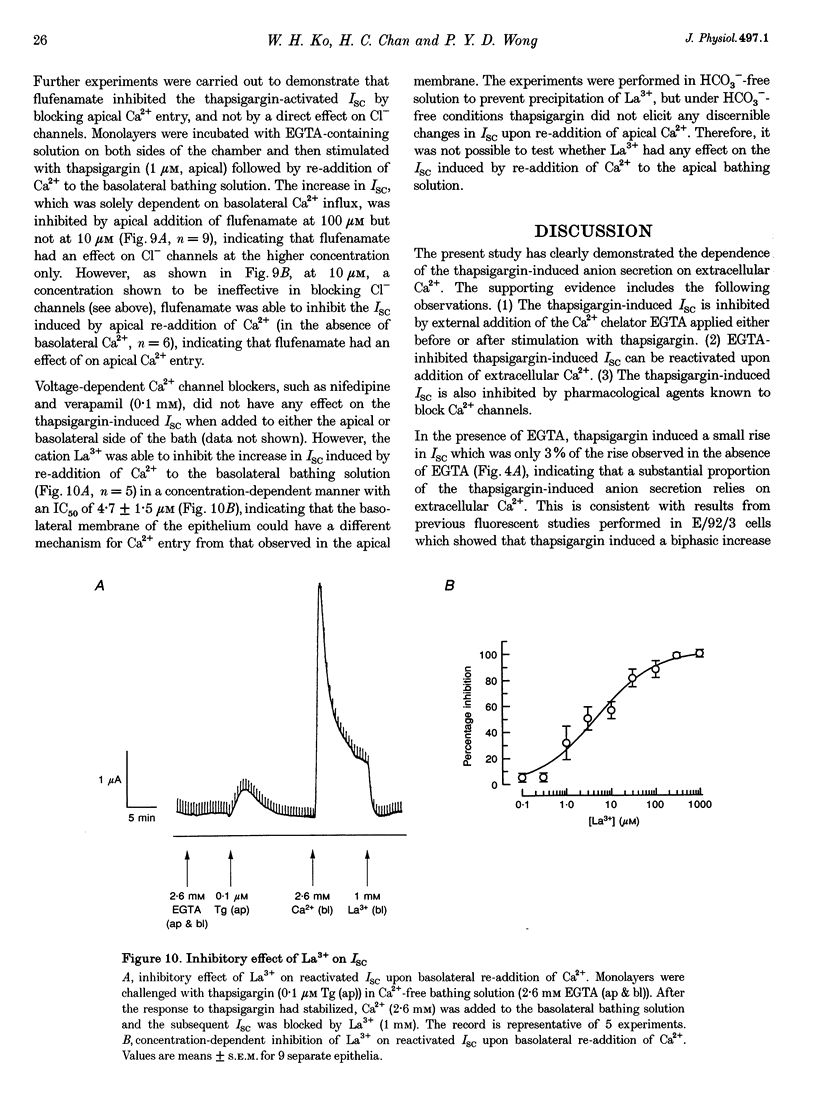
1. Anion secretion induced by capacitative Ca2+ entry through apical and basolateral membranes of cultured equine sweat gland epithelium was studied using the short-circuit current (Isc) technique. 2. Thapsigargin induced an increase in Isc that could be inhibited when external Ca2+ was chelated by EGTA. 3. The inhibition of the thapsigargin-induced Isc could be reversed by re-addition of Ca2+ to apical or basolateral solutions. The magnitude of the reactivated Isc depended predominantly on basolateral Ca2+ concentration. 4. The magnitude of the reactivated Isc upon basolateral Ca2+ addition increased with the thapsigargin concentration, indicating its dependence on the emptied state of the Ca2+ store induced by thapsigargin. 5. The thapsigargin-induced Isc, as well as the Ca(2+)-dependent reactivation of Isc in EGTA-treated epithelia, was inhibitable by apical, but not basolateral, addition of flufenamate, and by basolateral addition of La3+. Other Ca2+ channel blockers, verapamil and nifedipine, had no effect when applied to either membrane. 6. The results suggest that thapsigargin-induced anion secretion by the equine sweat gland epithelial cells is crucially dependent upon the Ca2+ influx occurring primarily through the basolateral membrane, and that apical and basolateral membranes may possess different pathways for Ca2+ entry.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berridge M. J. Inositol trisphosphate and calcium signalling. Nature. 1993 Jan 28;361(6410):315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- Brayden D. J., Hanley M. R., Thastrup O., Cuthbert A. W. Thapsigargin, a new calcium-dependent epithelial anion secretagogue. Br J Pharmacol. 1989 Nov;98(3):809–816. doi: 10.1111/j.1476-5381.1989.tb14609.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbert A. W., George A. M., MacVinish L. Kinin effects on electrogenic ion transport in primary cultures of pig renal papillary collecting tubule cells. Am J Physiol. 1985 Sep;249(3 Pt 2):F439–F447. doi: 10.1152/ajprenal.1985.249.3.F439. [DOI] [PubMed] [Google Scholar]
- Gögelein H., Dahlem D., Englert H. C., Lang H. J. Flufenamic acid, mefenamic acid and niflumic acid inhibit single nonselective cation channels in the rat exocrine pancreas. FEBS Lett. 1990 Jul 30;268(1):79–82. doi: 10.1016/0014-5793(90)80977-q. [DOI] [PubMed] [Google Scholar]
- Illek B., Fischer H., Machen T. E. Intracellular Ca2+ signalling is modulated by K+ channel blockers in colonic epithelial cells (HT-29/B6). Pflugers Arch. 1992 Oct;422(1):48–54. doi: 10.1007/BF00381512. [DOI] [PubMed] [Google Scholar]
- Kerst G., Fischer K. G., Normann C., Kramer A., Leipziger J., Greger R. Ca2+ influx induced by store release and cytosolic Ca2+ chelation in Ht29 colonic carcinoma cells. Pflugers Arch. 1995 Sep;430(5):653–665. doi: 10.1007/BF00386159. [DOI] [PubMed] [Google Scholar]
- Ko W. H., Chan H. C., Chew S. B., Wong P. Y. Ionic mechanisms of Ca(2+)-dependent electrolyte transport across equine sweat gland epithelium. J Physiol. 1996 Jun 15;493(Pt 3):885–894. doi: 10.1113/jphysiol.1996.sp021431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko W. H., O'Dowd J. J., Pediani J. D., Bovell D. L., Elder H. Y., Jenkinson D. M., Wilson S. M. Extracellular ATP can activate autonomic signal transduction pathways in cultured equine sweat gland epithelial cells. J Exp Biol. 1994 May;190:239–252. doi: 10.1242/jeb.190.1.239. [DOI] [PubMed] [Google Scholar]
- Ko W. H., Pediani J. D., Bovell D. L., Wilson S. M. Sr2+ can become incorporated into an agonist-sensitive, cytoplasmic Ca2+ store in a cell line derived from the equine sweat gland epithelium. Experientia. 1995 Aug 16;51(8):804–808. doi: 10.1007/BF01922434. [DOI] [PubMed] [Google Scholar]
- Kwan C. Y., Putney J. W., Jr Uptake and intracellular sequestration of divalent cations in resting and methacholine-stimulated mouse lacrimal acinar cells. Dissociation by Sr2+ and Ba2+ of agonist-stimulated divalent cation entry from the refilling of the agonist-sensitive intracellular pool. J Biol Chem. 1990 Jan 15;265(2):678–684. [PubMed] [Google Scholar]
- Missiaen L., De Smedt H., Pary J. B., Oike M., Casteels R. Kinetics of empty store-activated Ca2+ influx in HeLa cells. J Biol Chem. 1994 Feb 25;269(8):5817–5823. [PubMed] [Google Scholar]
- Negulescu P. A., Machen T. E. La3+ and pH sensitivity of Ca2+ entry and intracellular store filling in gastric parietal cells. Am J Physiol. 1995 Nov;269(5 Pt 1):G770–G778. doi: 10.1152/ajpgi.1995.269.5.G770. [DOI] [PubMed] [Google Scholar]
- Nitschke R., Leipziger J., Greger R. Agonist-induced intracellular Ca2+ transients in HT29 cells. Pflugers Arch. 1993 Jun;423(5-6):519–526. doi: 10.1007/BF00374950. [DOI] [PubMed] [Google Scholar]
- Paradiso A. M., Mason S. J., Lazarowski E. R., Boucher R. C. Membrane-restricted regulation of Ca2+ release and influx in polarized epithelia. Nature. 1995 Oct 19;377(6550):643–646. doi: 10.1038/377643a0. [DOI] [PubMed] [Google Scholar]
- Petersen O. H. Stimulus-secretion coupling: cytoplasmic calcium signals and the control of ion channels in exocrine acinar cells. J Physiol. 1992 Mar;448:1–51. doi: 10.1113/jphysiol.1992.sp019028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putney J. W., Jr, Bird G. S. The inositol phosphate-calcium signaling system in nonexcitable cells. Endocr Rev. 1993 Oct;14(5):610–631. doi: 10.1210/edrv-14-5-610. [DOI] [PubMed] [Google Scholar]
- Putney J. W., Jr Excitement about calcium signaling in inexcitable cells. Science. 1993 Oct 29;262(5134):676–678. doi: 10.1126/science.8235587. [DOI] [PubMed] [Google Scholar]
- Schumann S., Greger R., Leipziger J. Flufenamate and Gd3+ inhibit stimulated Ca2+ influx in the epithelial cell line CFPAC-1. Pflugers Arch. 1994 Oct;428(5-6):583–589. doi: 10.1007/BF00374581. [DOI] [PubMed] [Google Scholar]
- Siemer C., Gögelein H. Effects of forskolin on crypt cells of rat distal colon. Activation of nonselective cation channels in the crypt base and of a chloride conductance pathway in other parts of the crypt. Pflugers Arch. 1993 Aug;424(3-4):321–328. doi: 10.1007/BF00384359. [DOI] [PubMed] [Google Scholar]
- Tojyo Y., Tanimura A., Matsumoto Y., Sugiya H. Staurosporine enhances Ca2+ entry induced by depletion of intracellular Ca2+ stores in rat parotid acinar cells. Cell Calcium. 1995 Jan;17(1):32–40. doi: 10.1016/0143-4160(95)90100-0. [DOI] [PubMed] [Google Scholar]
- Tsunoda Y., Stuenkel E. L., Williams J. A. Characterization of sustained [Ca2+]i increase in pancreatic acinar cells and its relation to amylase secretion. Am J Physiol. 1990 Nov;259(5 Pt 1):G792–G801. doi: 10.1152/ajpgi.1990.259.5.G792. [DOI] [PubMed] [Google Scholar]
- USSING H. H., ZERAHN K. Active transport of sodium as the source of electric current in the short-circuited isolated frog skin. Acta Physiol Scand. 1951 Aug 25;23(2-3):110–127. doi: 10.1111/j.1748-1716.1951.tb00800.x. [DOI] [PubMed] [Google Scholar]
- Villalobos C., García-Sancho J. Capacitative Ca2+ entry contributes to the Ca2+ influx induced by thyrotropin-releasing hormone (TRH) in GH3 pituitary cells. Pflugers Arch. 1995 Oct;430(6):923–935. doi: 10.1007/BF01837406. [DOI] [PubMed] [Google Scholar]
- Wilson S. M., Ko W. H., Pediani J. D., Rakhit S., Nichol J. A., Bovell D. L. Calcium-dependent regulation of membrane ion permeability in a cell line derived from the equine sweat gland epithelium. Comp Biochem Physiol A Physiol. 1995 Jun;111(2):215–221. doi: 10.1016/0300-9629(95)00011-u. [DOI] [PubMed] [Google Scholar]



