Abstract
Whether or not vascular endothelial growth factor pathway inhibitors (VPIs) increase the risk of artery dissection is still unknown. This study aimed to quantitatively evaluate the possibility of artery dissection as a class effect of VPIs using nationwide real‐world data. This cohort study was conducted based on the National Database of Health Insurance Claims and Specific Health Checkups of Japan (NDB), which spans nearly the entire Japanese population of over 100 million individuals. We included the patients prescribed with 12 types of VPIs between 2012 and 2020. The incidence rate (IR) ratio of artery dissection for each VPI were estimated in comparison with bevacizumab, the only VPI in Japan with artery dissection listed in the package insert. Artery dissection as an outcome was targeted for acute artery dissection requiring hospitalization (including dissecting aneurysm). As a reference, a natural IR standardized by sex and age of bevacizumab‐prescribed patients was also estimated using the direct method for the general population of NDB. Of 503,342 patients, the IR of artery dissection for bevacizumab was 44.4 (/100,000 person‐years), and the adjusted IR ratios for each VPI compared with bevacizumab were consistently similar to or >1.0. The IRs for each VPI were also higher than the crude natural IR (1.66/100,000 person‐years; 95% CI: 1.59–1.73) and the standardized natural IR (2.18/100,000 person‐years; 95% CI: 1.86–2.50). Real‐world evidence suggests the risk of artery dissection as a class effect of VPIs. More attention on this risk will be necessary when using VPIs in clinical practice.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
Whether or not VPIs increase the risk of artery dissection is still unknown.
WHAT QUESTION DID THIS STUDY ADDRESS?
Does VPIs increase the risk of artery dissection as a class effect?
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
This nationwide cohort study of 503,342 patients based on the National Database of Health Insurance Claims and Specific Health Checkups of Japan (NDB) suggests a class effect of VPIs on the risk of artery dissection, with adjusted incidence rate ratios consistently equal to or greater than 1.0 compared with bevacizumab.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
More attention to this risk will be necessary when using VPIs in clinical practice.
INTRODUCTION
The engagement of vascular endothelial growth factor (VEGF) with VEGF receptors (VEGFR) on endothelial cells results in heightened proliferation, migration, degeneration, and permeability of normal blood vessel. 1 Targeting the VEGF pathway for pharmacological inhibition of angiogenesis is a crucial therapeutic objective, aiming to impede tumor growth and the development of metastases. 2 Bevacizumab, the world's first anticancer drug that inhibits the VEGF pathway, received approval from the U.S. Food and Drug Administration (FDA) in February 2004. 3 In Japan, this drug was approved in April 2007 for the treatment of metastatic colorectal cancer, 4 followed by the approval of indications for seven areas of oncology as of February 2024. 5
Accumulating case reports have linked the use of VEGF pathway inhibitors (VPIs) to abnormal structural changes in the arterial wall, leading to aortic dissection. 6 , 7 , 8 At the cellular and molecular levels, inhibition of the VEGF pathway is suggested to lead to overexpression of matrix metalloproteinase 9 (MMP9), which in turn degrades the extracellular matrix, possibly causing degeneration of the aortic media; however, the mechanism remains to be fully elucidated. 9 , 10 In August 2019, the European Medicines Agency (EMA), based on assessments utilizing EudraVigilance and discussions with manufacturers, recommended the addition of artery aneurysm and artery dissection to the “Special warnings and precautions for use” section of the Summary of Product Characteristics for systemically administered VEGF/VEGFR inhibitors. 11 Subsequently, the signals were detected in the FDA Adverse Event Reporting System (FAERS) from January to March 2020 regarding the possible association of artery aneurysm and artery dissection with VEGF/VEGFR inhibitors, and the United States Prescribing Information updated the “Adverse Reactions” section to include artery aneurysm and artery dissection. 12
In Japan, considering the accumulation of multiple domestic cases in the adverse reaction reports for bevacizumab, artery dissection was added to the “Clinically Significant Adverse Reactions” section of the bevacizumab's package insert in June 2020. 5 However, the risk of artery dissection by VPIs other than bevacizumab remains unclear. Sufficient data substantiating this association were not available because of (i) difficulty in distinguishing between whether the occurrence was related to background factors such as hypertension or to VPIs; (ii) previous studies have primarily relied on disproportionality analyses based on spontaneous adverse drug reaction reports 13 , 14 , 15 or series of case reports 16 and; (iii) no nationwide cohort study having been reported at the planning stage of this study.
The purpose of this cohort study was to quantitatively evaluate the possibility of artery dissection as a class effect of VPIs by examining the incidence rate (IR) of artery dissection for each VPI in patients prescribed VPIs based on a nationwide medical information database in Japan.
METHODS
Study design and setting
We conducted a cohort study, utilizing the National Database of Health Insurance Claims and Specific Health Checkups of Japan (NDB). The NDB was selected because of the following points: (i) NDB stands as one of the largest health‐related databases, encompassing comprehensive electronic health insurance claims and specific health check‐up data obtained from most medical facilities including hospitals, clinics, pharmacies, and dental clinics, 17 (ii) NDB spans the medical records of nearly the entire Japanese population, exceeding 100 million individuals, and (iii) NDB allows for tracking of patients as they move from the hospital where they were treated, ensuring robust data for analysis. 18 The study period (data period available for this study) was from August 1, 2010 to March 31, 2020.
Since this study was conducted as an official activity of the Pharmaceuticals and Medical Devices Agency (PMDA) under the PMDA Law Article 15–5–(c) and (f), 19 it was not subject to review by institutional review boards. 20 This study was reported in line with the Strengthening the Reporting of Observational Studies in Epidemiology guidelines. 21
Target population
For the primary analysis, the patients who were prescribed at least one VPI (aflibercept beta, axitinib, bevacizumab, lenvatinib, nintedanib, pazopanib, ramucirumab, regorafenib, sorafenib, sunitinib, or vandetanib) between April 1, 2012 and March 31, 2020 (“Prescription identification period”) were included. Notably, the present study included multi‐kinase inhibitors (excluding topical formulations), such as sunitinib and vandetanib, since our aim was to target VPIs that have been approved in Japan. If a patient had received prescriptions for multiple different VPIs during the prescription identification period, they were entered into a cohort for each VPI, resulting in a possibility of the same patient being entered into two or more cohorts. This strategy was adopted to collect information on VPIs prescribed as the second line or the subsequent line of chemotherapy. The earliest prescription date of VPIs triggered for cohort entry of a patient was defined as “t 0.” Patients with 0 days of follow‐up or patients prescribed the same VPI as t 0 between August 1, 2010 and March 31, 2012 (a period before the prescription identification period) were excluded for focusing on new users of a particular VPI.
For the secondary analysis in comparison with historical control, patients who prescribed docetaxel or ramucirumab (the earliest prescription date set as “t 0”) for lung cancer were included (see Figure S1). Based on the type of regimen at t 0, the patients were categorized into the combination therapy of docetaxel and ramucirumab as the exposure group, or the docetaxel monotherapy as the historical control group. A historical control group was selected because of the possibility that patients prescribed docetaxel monotherapy in the period after the ramucirumab approval (June 2016) 22 may have a worse general condition, potentially leading to confounding by indication.
For the additional analysis to calculate the natural IR of artery dissection, the broader population (general population) was identified by including patients with at least one claims data between August 1, 2010 and December 31, 2017 and excluding patients with death and artery dissection between August 1, 2010 and December 31, 2018.
Follow‐up period
For the primary analysis, the follow‐up period continued with the prescription of the same VPIs, starting on the day after t 0 (“start date”) and ending at the earliest date according to the following: (i) the date of occurrence of atrial dissection, (ii) 30 days after the prescription end date of VPI, or (iii) the end date of the study period. The prescription period comprised the start date and duration until the latest prescription end date and an additional 30 days. The prescription end date was set as 20 days after the latest prescription date for intravenous drugs, and the number of prescription days minus 1 day for capsules or tablets.
For the secondary analysis, the prescription period comprised the start date and duration to the latest prescription end date. If the gap between the end date of the preceding prescription and the start date of the subsequent prescription was within 90 days, we considered it a continuous prescription period for the patient. The censoring criteria were basically the same as the primary analysis except with one additional criterion: (iv) switching to the different group from t 0 (i.e., exposure or historical control groups).
For the additional analysis, the follow‐up period of this population started on January 1, 2019 and ended at the earliest date of the following: (i) date of the occurrence of artery dissection, (ii) date of the death record, or (iii) December 31, 2019.
Outcome definitions
The primary outcome was the occurrence of “artery dissection” targeting “acute artery dissection requiring hospitalization (including dissecting aneurysm)” during the follow‐up period. The cases of artery dissection were defined when either algorithm A or algorithm B as described below was met (also see Table S1). Algorithm A was defined based on observations from at least one of the following therapeutic interventions, that is, nicardipine drip, thoracic endovascular aortic repair, or aortic aneurysmectomy on the day or day after the date of admission for artery dissection. Algorithm B was defined based on discharge on the same day or the next day of admission for artery dissection (intended to capture cases of discharge due to death without treatment being performed). These definitions were based on results of the outcome validation study (unpublished) for artery dissection events by utilizing data from MID‐NET®, another medical information database in Japan, 23 with few minor modifications applied to the original definitions to ensure that data categories fitted to the data source of this study (i.e, NDB). We considered these definitions can be applied to this study and clinically plausible because artery dissection is mainly treated in a large hospitals such as university hospitals and regional core hospitals, which are the cooperative hospitals of the MID‐NET®.
Statistical analysis
As the primary analysis, for each VPI, the total follow‐up period, the number of patients with artery dissection, and IR of artery dissection with a 95% confidence interval (CI) were estimated. Crude and adjusted IR ratios (IRRs) of each VPI compared with bevacizumab were estimated using the multivariable Poisson model with the following adjusted factors: age group (over or under 65 years old), sex, the presence of diseases and corresponding treatments (cardiovascular events [including cerebral infarction, cerebral hemorrhage, and acute coronary syndrome], hypertension, diabetes mellitus, dyslipidemia; see Table S2 for more details of covariate definitions), and past medical history of artery dissection (see “Outcome definitions” section above). Bevacizumab was used as a reference because it is the only VPI for which artery dissection is listed under “Clinically Significant Adverse Reactions” in the package insert in Japan. As subgroup analyses, the IR and IRR of artery dissection were also calculated in the population without each covariate described above.
For the secondary analysis, the total follow‐up period, the number of patients with artery dissection, and IR of artery dissection with 95% CI were calculated for each group. Calculation of crude and adjusted hazard ratios using the multivariable Cox regression model with the adjusted factors listed above in addition to the history of G‐CSF (Granulocyte‐colony stimulating factor) prescription was planned but was impossible to carry out due to no cases in the exposure group (see “Results”).
Finally, as an additional analysis, we calculated the crude natural IR of artery dissection in the general population by dividing the number of people who developed the artery dissection by the follow‐up period, and its 95% CI was estimated. A natural IR standardized with the sex and age of the bevacizumab‐prescribed cohort was also estimated using the direct method for the general population. All analyses were conducted using the SAS statistical software (version 9.4; SAS Institute, Cary, NC, USA). No tests of statistical difference were conducted.
RESULTS
In total, 503,342 patients were included in the primary analysis after applying all inclusion and exclusion criteria (Figure 1). The most prescribed VPI was bevacizumab (n = 278,722), followed by ramucirumab (n = 73,593), and sorafenib (n = 33,849). It should be noted that since no patients were identified for cabozantinib in this study, further analysis was omitted for this drug. As shown in Table 1, in all VPIs, patients were predominantly aged 50 and above and generally had hypertension, diabetes mellitus, or hyperlipidemia in their medical history. VPIs were usually prescribed more for males, but a relatively higher percentage of females were prescribed bevacizumab, probably as a treatment for breast, ovarian, or cervical cancer.
FIGURE 1.
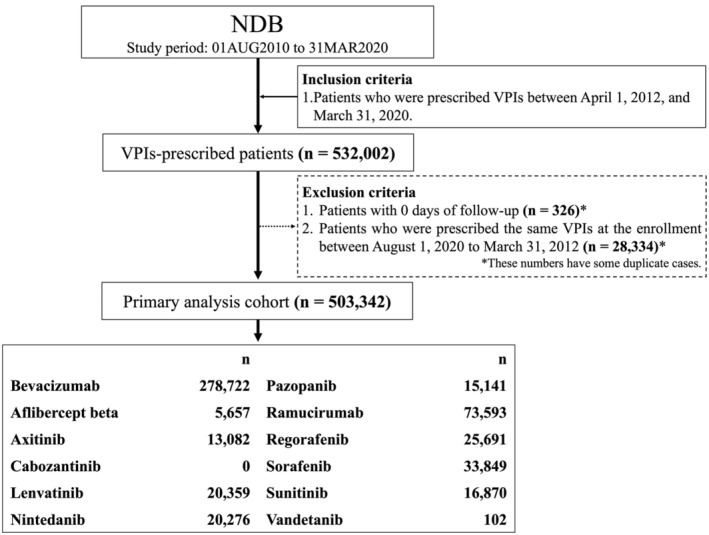
Study flowchart for the cohort identification in the primary analysis. NDB, the National Database of Health Insurance Claims and Specific Health Checkups of Japan; VPI, VEGF pathway inhibitor.
TABLE 1.
Patient background of the primary analysis.
| Bevacizumab | Aflibercept beta | Axitinib | Lenvatinib | Nintedanib | Pazopanib | Ramucirumab | Regorafenib | Sorafenib | Sunitinib | Vandetanib | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of patients | 278,722 | 5657 | 13,082 | 20,359 | 20,276 | 15,141 | 73,593 | 25,691 | 33,849 | 16,870 | 102 | |||||||||||
| Age category | ||||||||||||||||||||||
| 0–9 | 347 | 0.12% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 31 | 0.20% | <10 | <0.01% | 0 | 0% | 29 | 0.09% | <10 | <0.06% | 0 | 0% |
| 10–19 | 386 | 0.14% | 0 | 0% | 10 | 0.08% | <10 | <0.05% | <10 | <0.05% | 181 | 1.20% | <10 | <0.01% | 15 | 0.06% | 59 | 0.17% | <20 | <0.12% | <10 | <9.80% |
| 20–29 | 1150 | 0.41% | <20 | <0.35% | 26 | 0.20% | <30 | <0.15% | <20 | <0.10% | 290 | 1.92% | 139 | 0.19% | 51 | 0.20% | 45 | 0.13% | 71 | 0.42% | <10 | <9.80% |
| 30–39 | 6579 | 2.36% | 108 | 1.91% | 108 | 0.83% | 82 | 0.40% | 55 | 0.27% | 514 | 3.39% | 986 | 1.34% | 360 | 1.40% | 154 | 0.45% | 239 | 1.42% | <10 | <9.80% |
| 40–49 | 23140 | 8.30% | 386 | 6.82% | 651 | 4.98% | 441 | 2.17% | 210 | 1.04% | 1171 | 7.73% | 3732 | 5.07% | 1456 | 5.67% | 763 | 2.25% | 1076 | 6.38% | 13 | 12.75% |
| 50–59 | 46,357 | 16.63% | 932 | 16.48% | 1764 | 13.48% | 1400 | 6.88% | 1055 | 5.20% | 2117 | 13.98% | 8861 | 12.04% | 3771 | 14.68% | 2689 | 7.94% | 2416 | 14.32% | 17 | 16.67% |
| 60–69 | 95,495 | 34.26% | 2037 | 36.01% | 4434 | 33.89% | 4937 | 24.25% | 5265 | 25.97% | 4342 | 28.68% | 25,848 | 35.12% | 9104 | 35.44% | 9441 | 27.89% | 5840 | 34.62% | 34 | 33.33% |
| 70–79 | 85,335 | 30.62% | 1900 | 33.59% | 4587 | 35.06% | 8658 | 42.53% | 10,215 | 50.38% | 4618 | 30.50% | 28,271 | 38.42% | 8869 | 34.52% | 13,795 | 40.75% | 5512 | 32.67% | 22 | 21.57% |
| 80–89 | 19,558 | 7.02% | 278 | 4.91% | 1475 | 11.28% | 4606 | 22.62% | 3368 | 16.61% | 1807 | 11.93% | 5661 | 7.69% | 2033 | 7.91% | 6695 | 19.78% | 1657 | 9.82% | <10 | <9.80% |
| 90‐ | 375 | 0.13% | <10 | <0.18% | 27 | 0.21% | 204 | 1.00% | 90 | 0.44% | 70 | 0.46% | 86 | 0.12% | 32 | 0.12% | 179 | 0.53% | 43 | 0.25% | <10 | <9.80% |
| Sex | ||||||||||||||||||||||
| Female | 143,338 | 51.43% | 2325 | 41.10% | 3357 | 25.66% | 5805 | 28.51% | 4856 | 23.95% | 5756 | 38.02% | 22,818 | 31.01% | 9455 | 36.80% | 7475 | 22.08% | 4682 | 27.75% | 39 | 38.24% |
| Male | 135,384 | 48.57% | 3332 | 58.90% | 9725 | 74.34% | 14,554 | 71.49% | 15,420 | 76.05% | 9385 | 61.98% | 50,775 | 68.99% | 16,236 | 63.20% | 26,374 | 77.92% | 12,188 | 72.25% | 63 | 61.76% |
| Past medical history | ||||||||||||||||||||||
| Artery dissection | 215 | 0.08% | 10 | 0.18% | 40 | 0.31% | 40 | 0.20% | 30 | 0.15% | 39 | 0.26% | 100 | 0.14% | 55 | 0.21% | 61 | 0.18% | 24 | 0.14% | 0 | 0% |
| Cardiovascular event | 2064 | 0.74% | 58 | 1.03% | 293 | 2.24% | 518 | 2.54% | 758 | 3.74% | 338 | 2.23% | 1131 | 1.54% | 301 | 1.17% | 640 | 1.89% | 237 | 1.40% | <10 | <9.80% |
| Hypertension | 81,269 | 29.16% | 2629 | 46.47% | 7351 | 56.19% | 12,094 | 59.40% | 8549 | 42.16% | 7311 | 48.29% | 28,622 | 38.89% | 12,699 | 49.43% | 17,574 | 51.92% | 7166 | 42.48% | 37 | 36.27% |
| Diabetes mellitus | 33,487 | 12.01% | 919 | 16.25% | 2408 | 18.41% | 6580 | 32.32% | 5109 | 25.20% | 2617 | 17.28% | 11,902 | 16.17% | 4814 | 18.74% | 9270 | 27.39% | 2815 | 16.69% | 18 | 17.65% |
| Hyperlipidemia | 54,662 | 19.61% | 1423 | 25.15% | 3646 | 27.87% | 5159 | 25.34% | 7610 | 37.53% | 4322 | 28.55% | 17,390 | 23.63% | 5683 | 22.12% | 6130 | 18.11% | 3944 | 23.38% | 16 | 15.69% |
| Types of cancer | ||||||||||||||||||||||
| Unknown | 11,020 | 3.95% | 48 | 0.85% | 364 | 2.78% | 151 | 0.74% | 234 | 1.15% | 1743 | 11.51% | 3613 | 4.91% | 542 | 2.11% | 607 | 1.79% | 1086 | 6.44% | <10 | <9.80% |
| Lung cancer | 47,114 | 16.90% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 13,695 | 18.61% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% |
| Gastric cancer | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 41,802 | 56.80% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% |
| Breast cancer | 32,932 | 11.82% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% |
| Colorectal cancer | 158,149 | 56.74% | 5609 | 99.15% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 15,954 | 21.68% | 21,426 | 83% | 0 | 0% | 0 | 0% | 0 | 0% |
| Renal cell carcinoma | 0 | 0% | 0 | 0% | 12,718 | 97.22% | 0 | 0% | 0 | 0% | 8988 | 59.36% | 0 | 0% | 0 | 0% | 3851 | 11.38% | 12,780 | 75.76% | 0 | 0% |
| Hepatocellular carcinoma | 1158 | 0.42% | 0 | 0% | 0 | 0% | 15,934 | 78.27% | 0 | 0% | 0 | 0% | 1215 | 1.65% | 3052 | 11.88% | 28,373 | 83.82% | 0 | 0% | 0 | 0% |
| PNET | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 1108 | 6.57% | 0 | 0% |
| Cervical cancer | 5283 | 1.90% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% |
| Ovarian cancer | 17,706 | 6.35% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% |
| Thyroid cancer | 0 | 0% | 0 | 0% | 0 | 0% | 4481 | 22.01% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 1194 | 3.53% | 0 | 0% | <102 | <100% |
| Gastrointestinal stromal tumor | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 1089 | 4.24% | 0 | 0% | 2056 | 12.19% | 0 | 0% |
| Malignant soft tissue tumor | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 4616 | 30.49% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% |
| Malignant glioma | 12,664 | 4.54% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% |
| Interstitial lung disease | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 20,042 | 98.85% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% |
Note: Age category and sex were collected at t 0. Past medical history and types of cancer were collected from the start date of the study period to t 0.
Abbreviation: PNET, pancreatic neuroendocrine tumor.
The results of the primary analysis are shown in Figure 2. The IR of artery dissection for bevacizumab was 44.4 (/100,000 person‐years). The adjusted incidence rate ratio (aIRR) of artery dissection for each VPI compared with bevacizumab was consistently similar to or >1.0. The highest aIRR was observed for vandetanib, but the point estimate had a wide CI due to the inclusion of fewer patients compared with other VPIs. The consistently higher aIRRs were also observed in the subgroup analyses limited to patients without each covariate (Tables S1) and to incident new users (Table S8). Kaplan–Meier curve for the entire population cohort is shown in Figure S2. In order to investigate changes in IRR depending on the timing of outcome onset, we divided the follow‐up period into two or four intervals (separated by the median date of outcome onset for the entire population, and separated by the period considered clinically important) and estimated the IRR for each VPI compared with bevacizumab. The results showed that as in the primary analysis, the IRR was consistently similar to or >1.0, except for some intervals for ramucirumab (Figures S3 and S4).
FIGURE 2.
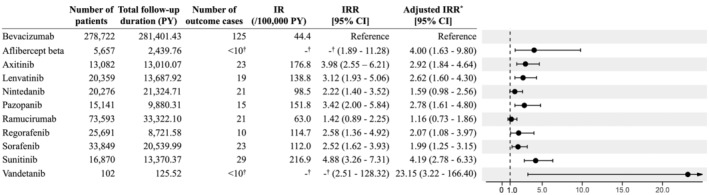
Risk of artery dissection in patients prescribed VEGF pathway inhibitors in the primary analysis. CI, confidence interval; IR, incidence rate; IRR, incidence rate ratio; PY, person‐year. *Adjusted covariates: Sex, age category (<65 years old or ≥65 years old), the past medical history of diseases and their treatment (artery dissection, cardiovascular events, hypertension, diabetes mellitus, and dyslipidemia). †Based on NDB publication standards, aggregated values <10 were masked so that they could not be identified.
In the secondary analysis, 12,583 patients were eligible for the exposure group, while 17,867 patients were placed in the control group (Figure S1). The backgrounds of patients in the exposure and control groups were not significantly different (Table 2a). The median and interquartile range (IQR) of a follow‐up period were 0.252 years (IQR: 0.164–0.427) for the exposure group and 0.222 years (IQR: 0.164–0.375) for the control group. As a result, the number of patients with artery dissection was 0 in the exposure group and <10 in the control group, making it impossible to conduct a comparative analysis (Table 2b).
TABLE 2.
Patient background and results of the secondary analysis: (a) Patient background of the secondary analysis. (b) Results of the secondary analysis.
| Exposure group (combination therapy of docetaxel and ramucirumab) | Control group (docetaxel monotherapy) | |||
|---|---|---|---|---|
| (a) | ||||
| Number of patients | 12,583 | 17,867 | ||
| Age category | ||||
| 0–9 | 0 | 0% | 0 | 0% |
| 10–19 | 0 | 0% | 0 | 0% |
| 20–29 | <10 | <0.08% | <20 | <0.11% |
| 30–39 | 112 | 0.89% | 103 | 0.58% |
| 40–49 | 620 | 4.93% | 521 | 2.92% |
| 50–59 | 1679 | 13.34% | 1840 | 10.30% |
| 60–69 | 4963 | 39.44% | 6217 | 34.80% |
| 70–79 | 4818 | 38.29% | 7344 | 41.10% |
| 80–89 | 382 | 3.04% | 1820 | 10.19% |
| 90‐ | <10 | <0.08% | <10 | <0.06% |
| Sex | ||||
| Female | 3836 | 30.49% | 4476 | 25.05% |
| Male | 8747 | 69.51% | 13,391 | 74.95% |
| Past medical history | ||||
| Artery dissection | 20 | 0.16% | 21 | 0.12% |
| Cardiovascular event | 194 | 1.54% | 263 | 1.47% |
| Hypertension | 5216 | 41.45% | 6197 | 34.68% |
| Diabetes mellitus | 2192 | 17.42% | 2854 | 14.46% |
| Hyperlipidemia | 3498 | 27.80% | 3743 | 20.95% |
| Past prescription history | ||||
| G‐CSF | 3389 | 26.93% | 4623 | 25.87% |
| (b) | ||||
| Number of artery dissection cases | 0 | 0% | <10 | <0.056% |
| Follow‐up duration (year, median) | 0.252 | 0.222 | ||
| Follow‐up duration (year, quartile range) | [0.164–0.427] | [0.164–0.375] | ||
| Total follow‐up duration (PY) | 4212.04 | 5788.21 | ||
| Incidence rate (/PY) | 0 | < 0.00173 | ||
Abbreviations: G‐CSF, granulocyte‐colony stimulating factor; PY, person‐year.
Furthermore, in the additional analysis, the crude natural IR of artery dissection in the general population was 1.66 (/100,000 person‐years; 95% CI: 1.59–1.73), and the standardized IR (sex and age were standardized to the bevacizumab‐prescribed patient population) was 2.18 (/100,000 person‐years; 95% CI: 1.86–2.50).
DISCUSSION
In the present study, we have evaluated the possibility of whether the risk of artery dissection is a class effect of VPIs using nationwide real‐world data (RWD). Among 503,342 patients prescribed with VPIs, the aIRR for each VPI compared with bevacizumab, the only VPI to mention artery dissection under “Clinically Significant Adverse Reactions” in its package insert, was consistently similar to or >1.0, suggesting an increased risk of artery dissection by all VPIs. This observation was supported by the results of the subgroup analysis showing results consistent with the primary analysis, as well as by the additional analysis showing that the IR of artery dissection for each VPI was higher than the standardized natural IR. Although caution may be needed in comparison with the standardized natural IR because of possible differences in patient backgrounds, the incidence of artery dissection according to the Japanese guidelines was reported as ~ 3 per 100,000 people per year in Japan, 24 indicating a similar range to that of the natural IR in this study. Thus, our findings from this study provided an important insight into VPIs, indicating the risk of artery dissection as a class effect.
In the previous studies, an increased safety signal on aneurysms and/or artery dissection by VPIs has been reported based on the disproportionality analyses of data from spontaneous adverse drug reaction reports in the United States, 13 EU, 14 and Japan. 15 However, our finding provides more robust evidence than previous studies through (i) utilization of a nationwide database (NDB), (ii) use of more accurate outcome definition for identifying artery dissection, and (iii) analyzing data by pharmaco‐epidemiological approaches. Therefore, the IRs based on RWD obtained in this study are valuable information for the use of VPIs in clinical practice and the consideration of safety measures required. Recently, Kang et al. reported the incidence rate (IR) of aneurysm and artery dissection in the tyrosine kinase inhibitors targeting the VEGFR (VEGFR‐TKIs) group to be 6.0 per 1000 person‐years in Korean population, which is nearly 10 times of IRs for bevacizumab and ramucirumab discovered in this study. 25 A reason for this may be due to differences in outcome definitions (only on the disease name in the previous study vs. the composite definition considering treatment and a case of aneurysm in this study) or in the analytical methods used (propensity score matching in the previous study vs. general population in this study).
In the secondary analysis, there were no cases of artery dissection in the exposure group. The median follow‐up duration of a patient was 0.252 years, and a similar median time of 94 days from the start of VPI to the incidence of artery aneurysm/dissection has been reported. 26 Therefore, no cases of artery dissection in the exposure group in this study could be due to an insufficient sample size for testing drugs. Further studies with expanded study periods are warranted.
The PMDA conducted a safety assessment on the risk of artery dissection by VPIs based on the present study as a major evidence for review and other available data including adverse drug reaction reports and literature. Finally, the package inserts of all VPIs included in the present study were revised on February 15, 2024 to add a precaution about the risk of artery dissection under “Clinically Significant Adverse Reactions.” 27
Limitations
This study had certain limitations. First, caution may be necessary in interpreting the IRR of VPIs compared with bevacizumab due to a confounding indication between bevacizumab and other VPIs. For this possibility, the secondary analysis in comparison with a historical control group was designed in this study, but no cases were identified in the exposure group as described above. Consistent results showing a similar or higher risk of artery dissection among different VPIs in comparison with bevacizumab may imply that such factors do not have large impacts in interpreting study results. Second, it is difficult to exclude the possibility that unmeasured confounding, such as peripheral vascular disease, previous radiotherapy for malignancy, phenotypes of each patient that influence blood pressure response to drugs, and time‐dependent changes in blood pressure after exposure to drugs may have impacted the results despite sex, age, and five covariates having been taken into consideration in this study.
CONCLUSION
The results of the present study suggest a class effect of VPIs on the risk of artery dissection. Further attention to this risk is needed when VPIs are used in clinical practice, and patients with a priori predictions of high risk should be followed more closely for artery dissection events and be managed for minimizing their risk by optimal cardiovascular care.
AUTHOR CONTRIBUTIONS
J.O., T.W., K.K., S.S., S.W., Y.N., A.K., S.K., K.A., T.I., N.H., and Y.U. wrote the manuscript; J.O., K.K., S.W., Y.N., A.K., S.K., A.K., T.I., N.H., and Y.U. designed the research; J.O., T.W., K.K., and Y.N. performed the research; J.O., T.W., and K.K. analyzed the data.
FUNDING INFORMATION
No funding was received for this work.
CONFLICT OF INTEREST STATEMENT
The authors have no conflict of interest.
Supporting information
Figure S2
Table S1–S8
Figure S2
Figure S3
Figure S4
ACKNOWLEDGMENTS
We sincerely appreciate Haruka Shida, the former member of the Office of Medical Informatics and Epidemiology, and the members of the division of Pharmacoepidemiology at the Pharmaceuticals and Medical Devices Agency for their tremendous help in conducting the present study.
Okui J, Waki T, Kajiyama K, et al. Risk of artery dissection during systemic exposure to vascular endothelial growth factor pathway inhibitors. Clin Transl Sci. 2024;17:e70096. doi: 10.1111/cts.70096
Past presentation on this research: A part of this article was included in an official Pharmaceuticals and Medical Devices Agency (PMDA) report that is available at the PMDA website (https://www.pmda.go.jp/files/000266521.pdf, https://www.pmda.go.jp/files/000266522.pdf).
REFERENCES
- 1. Ferrara N, Gerber H‐P, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669‐676. [DOI] [PubMed] [Google Scholar]
- 2. Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182‐1186. [DOI] [PubMed] [Google Scholar]
- 3. U.S. Food and Drug Administration Drug Approval Package: Avastin (Bevacizumab). NDA #125085. Accessed November 25, 2024. https://www.washihttps://www.accessdata.fda.gov/drugsatfda_docs/nda/2004/STN‐125085_Avastin.cfm
- 4. Ministry of Health, Labour and Welfare, Japan . AVASTIN® (Bevacizumab). Report on the deliberation results. Accessed November 25, 2024.https://www.pmda.go.jp/files/000224437.pdf
- 5. Pharmaceuticals and Medical Devices Agency, Japan . AVASTIN® (Bevacizumab) package insert. Accessed February 01, 2024.https://www.info.pmda.go.jp/go/pack/4291413A1022_1_24/
- 6. Edeline J, Laguerre B, Rolland Y, Patard J‐J. Aortic dissection in a patient treated by sunitinib for metastatic renal cell carcinoma. Ann Oncol. 2010;21:186‐187. [DOI] [PubMed] [Google Scholar]
- 7. Aragon‐Ching JB, Ning Y‐M, Dahut WL. Acute aortic dissection in a hypertensive patient with prostate cancer undergoing chemotherapy containing bevacizumab. Acta Oncol. 2008;47:1600‐1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Niwa N, Nishiyama T, Ozu C, Yagi Y, Saito S. Acute aortic dissection in a patient with metastatic renal cell carcinoma treated with axitinib. Acta Oncol. 2015;54:561‐562. [DOI] [PubMed] [Google Scholar]
- 9. Shen YH, Zhang L, Ren P, et al. AKT2 confers protection against aortic aneurysms and dissections. Circ Res. 2013;112:618‐632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Takada M, Yasui T, Oka T, et al. Aortic dissection and cardiac dysfunction emerged coincidentally during the long‐term treatment with angiogenesis inhibitors for metastatic renal cell carcinoma. Int Heart J. 2018;59:1174‐1179. [DOI] [PubMed] [Google Scholar]
- 11. European Medicines Agency . PRAC recommendations on signals adopted at the 8–11 July 2019 PRAC meeting. Accessed November 25, 2024.https://www.ema.europa.eu/en/documents/prac‐recommendation/prac‐recommendations‐signals‐adopted‐8‐11‐july‐2019‐prac‐meeting_en.pdf
- 12. U.S. Food and Drug Administration . Potential signals of serious risks/new safety information identified by the FDA Adverse Event Reporting System (FAERS); January–March 2020. 2020. Accessed November 25, 2024.https://www.fda.gov/drugs/questions‐and‐answers‐fdas‐adverse‐event‐reporting‐system‐faers/january‐march‐2020‐potential‐signals‐serious‐risksnew‐safety‐information‐identified‐fda‐adverse
- 13. Wang S, Chen M, Zhang X, et al. Aneurysm and artery dissection following the use of vascular endothelial growth factor inhibitor: a real‐world analysis using a spontaneous reporting system. J Am Heart Assoc. 2021;10:e020844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Guyon J, Gouverneur A, Maumus‐Robert S, et al. Association between antiangiogenic drugs used for cancer treatment and artery dissections or aneurysms. JAMA Oncol. 2021;7:775‐778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Oshima Y, Tanimoto T, Yuji K, Tojo A. Association between aortic dissection and systemic exposure of vascular endothelial growth factor pathway inhibitors in the Japanese adverse drug event report database. Circulation. 2017;135:815‐817. [DOI] [PubMed] [Google Scholar]
- 16. Dai S, Zhong Y, Cui H, Zhao J, Li S. Aortic dissection induced by vascular endothelial growth factor inhibitors. Front Pharmacol. 2023;14:1189910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yasunaga H. Real world data in Japan: chapter I NDB. Ann Clin Epidemiol. 2019;1:28‐30. [Google Scholar]
- 18. Shida H, Kajiyama K, Sawada S, et al. Use of National Database of health insurance claims and specific health checkups for examining practical utilization and safety signal of a drug to support regulatory assessment on postmarketing drug safety in Japan. Front Med. 2023;10:1096992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Act on the Pharmaceuticals and Medical Devices Agency (Act No. 192 of 2002). Accessed November 25, 2024.https://elaws.e‐gov.go.jp/document?lawid=414AC0000000192
- 20. Pharmaceuticals and Medical Devices Agency, Japan . MIHARI project. Accessed November 25, 2024.https://www.pmda.go.jp/safety/surveillance‐analysis/0045.html
- 21. Vandenbroucke JP, von Elm E, Altman DG, et al. Strengthening the reporting of observational studies in epidemiology (STROBE): explanation and elaboration. PLoS Med. 2007;4:e297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pharmaceuticals and Medical Devices Agency, Japan . Cyramza® (Ramucirumab). Package insert. Accessed November 25, 2024.https://www.pmda.go.jp/PmdaSearch/iyakuDetail/ResultDataSetPDF/530471_4291429A1023_1_17
- 23. Yamaguchi M, Inomata S, Harada S, et al. Establishment of the MID‐NET® medical information database network as a reliable and valuable database for drug safety assessments in Japan. Pharmacoepidemiol Drug Saf. 2019;28:1395‐1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ogino H, Iida O, Akutsu K, et al. JCS/JSCVS/JATS/JSVS 2020 guideline on diagnosis and treatment of aortic aneurysm and aortic dissection. Circ J. 2023;87:1410‐1621. [DOI] [PubMed] [Google Scholar]
- 25. Kang S, Yeon B, Kim MS, Yoo M, Kim B, Yu YM. Aneurysm and artery dissection after Oral VEGFR‐TKI use in adults with cancer. JAMA Netw Open. 2023;6:e2345977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cheng C, Nguyen MN, Nayernama A, et al. Arterial aneurysm and dissection with systemic vascular endothelial growth factor inhibitors: a review of cases reported to the FDA adverse event reporting system and published in the literature. Vasc Med. 2021;26:526‐534. [DOI] [PubMed] [Google Scholar]
- 27. Ministry of Health, Labour and Welfare, Japan Revision of Precautions . Nintedanib ethanesulfonate, Axitinib, Aflibercept beta (genetical recombination), Cabozantinib malate, Sunitinib malate, Sorafenib tosilate, Pazopanib hydrochloride, Vandetanib, Ponatinib hydrochloride, Ramucirumab (genetical recombination), Regorafenib hydrate, Lenvatinib mesilate. 2024. Accessed November 25, 2024.https://www.pmda.go.jp/files/000266902.pdf
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S2
Table S1–S8
Figure S2
Figure S3
Figure S4


