Abstract
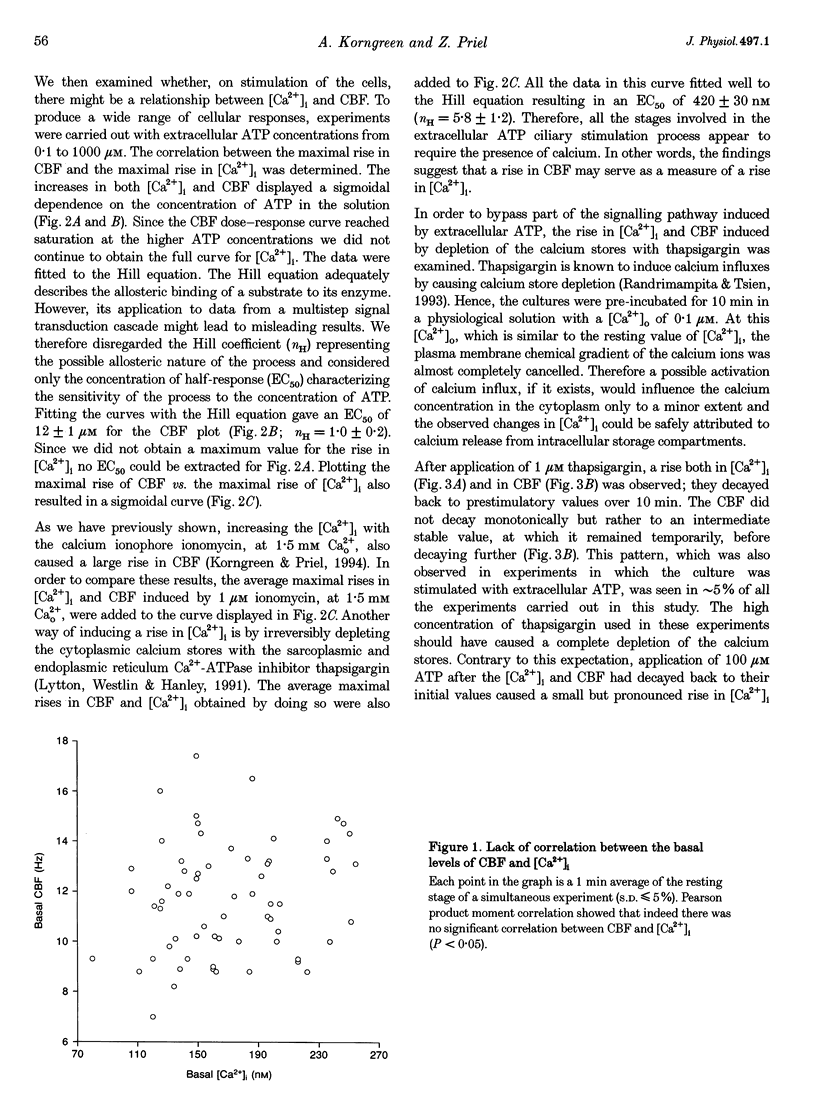
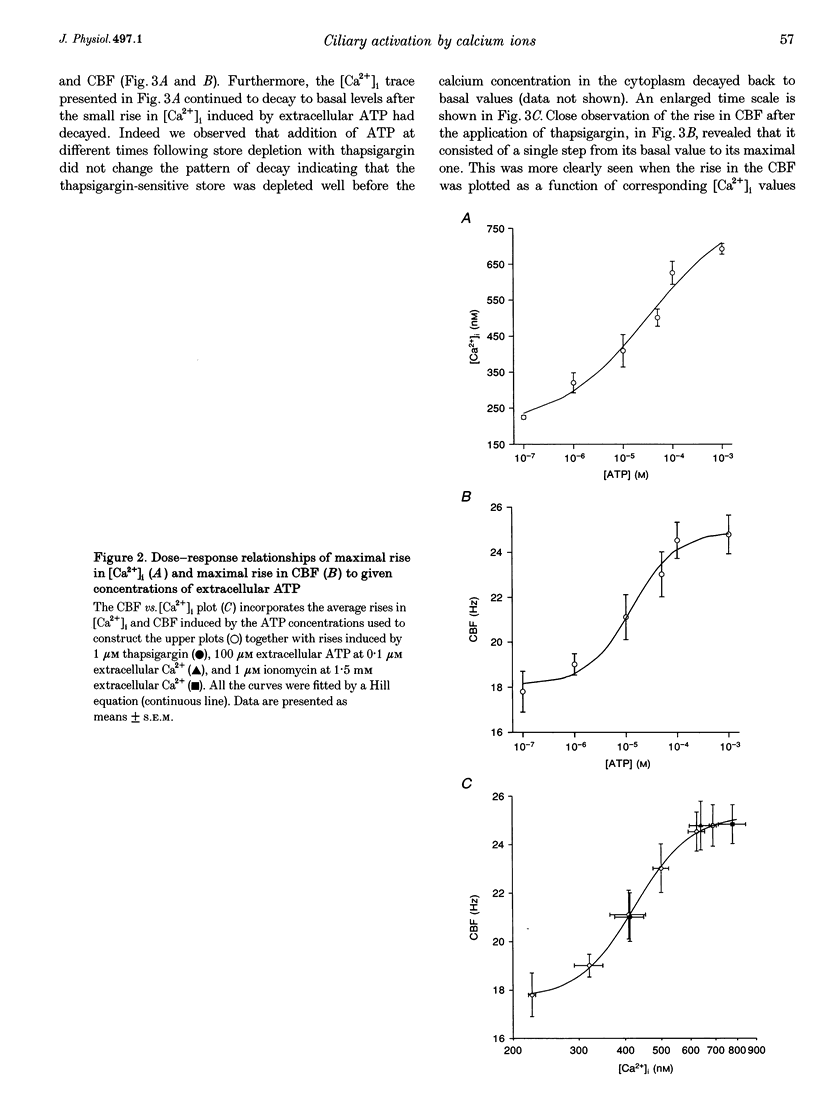
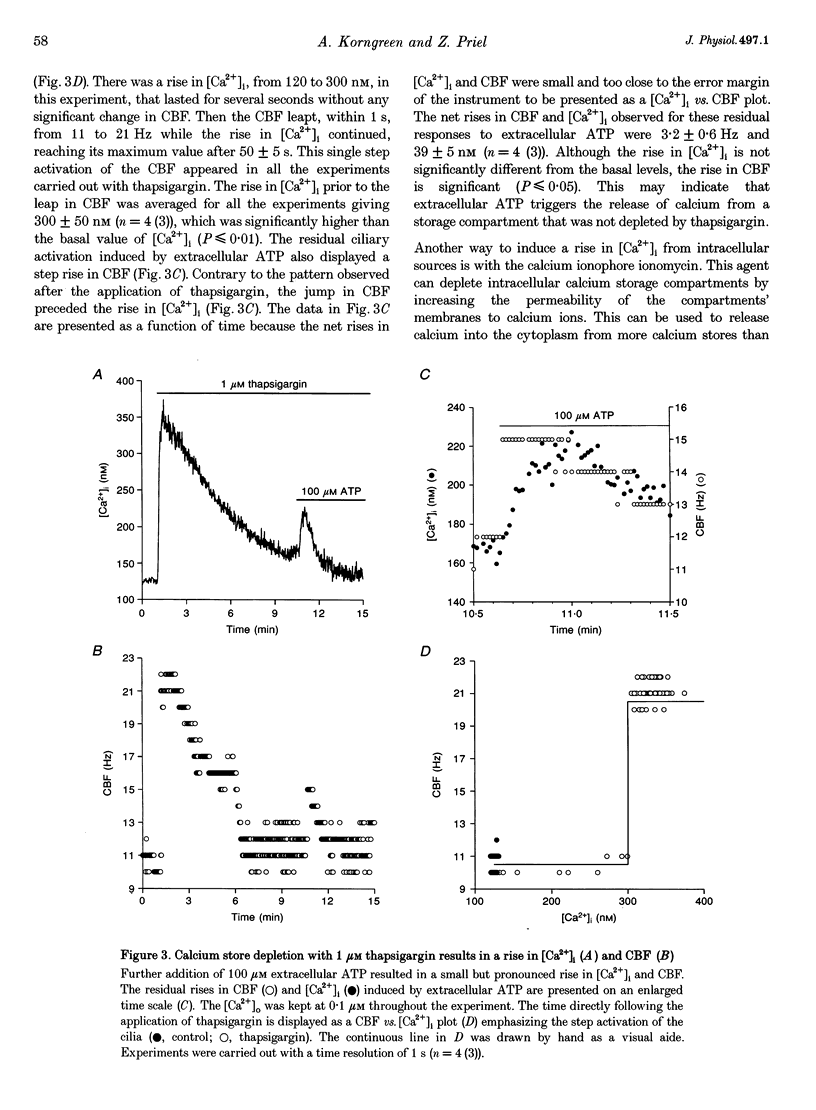
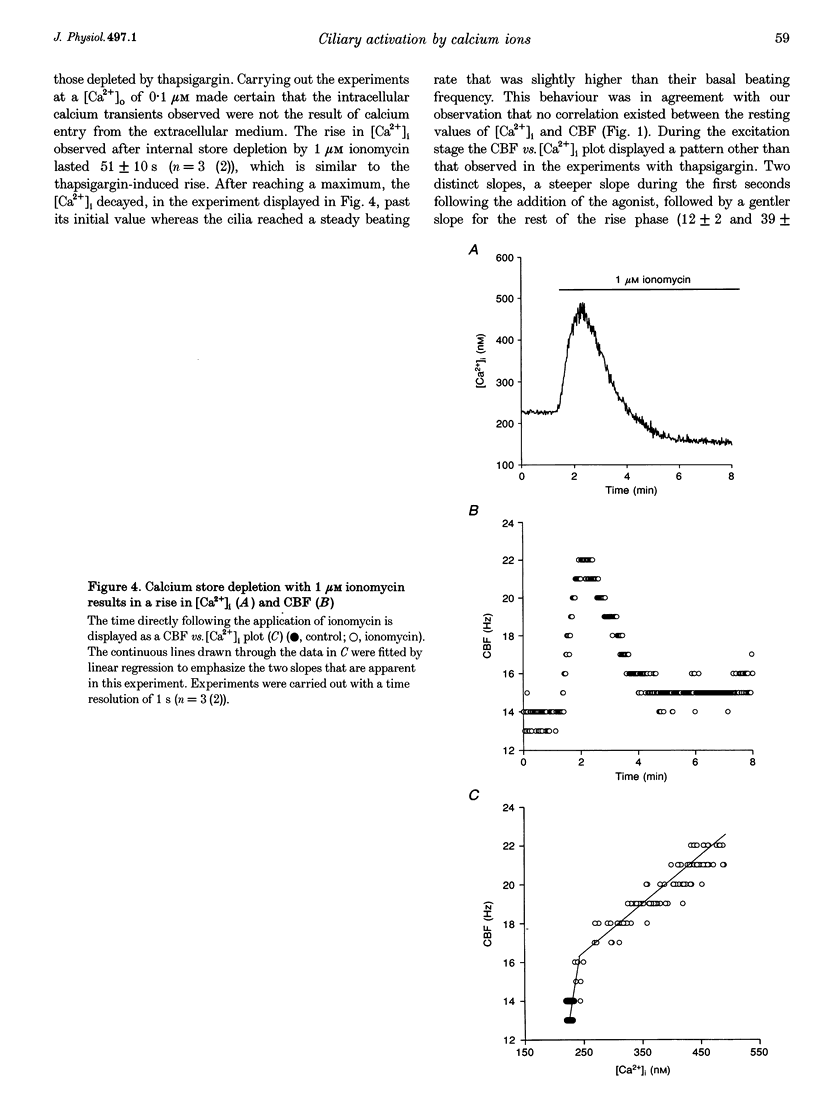
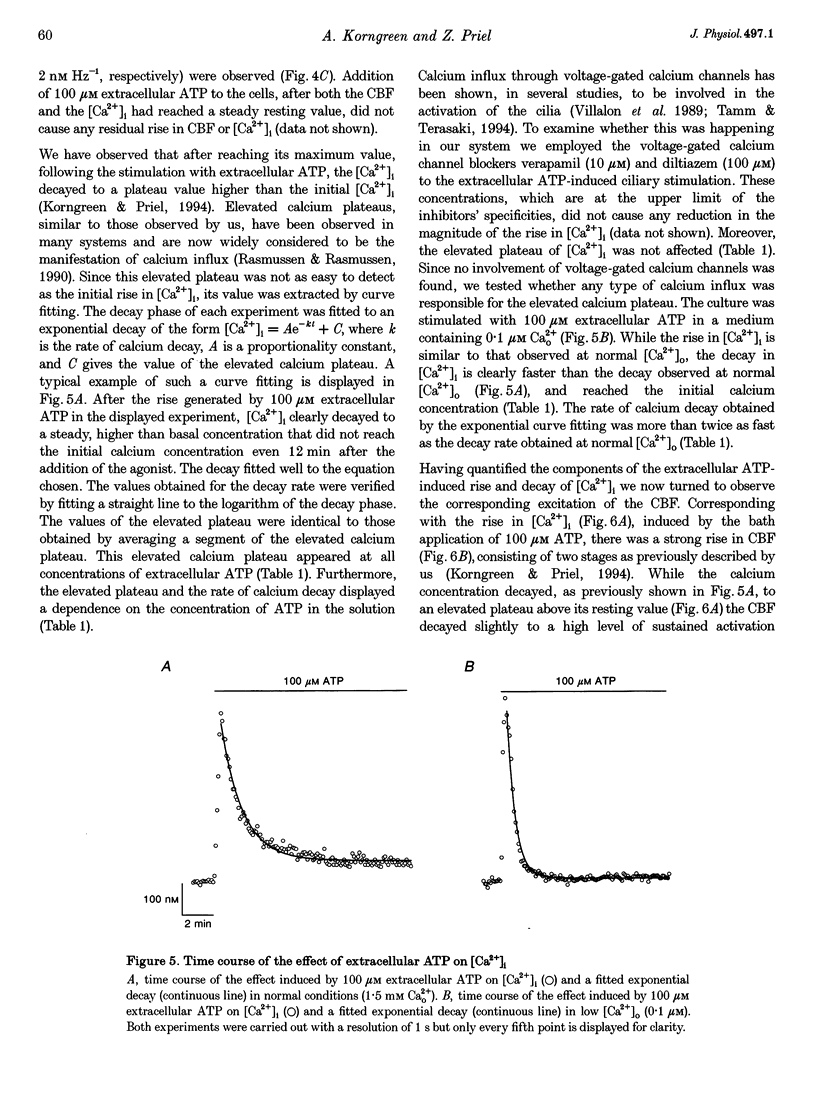
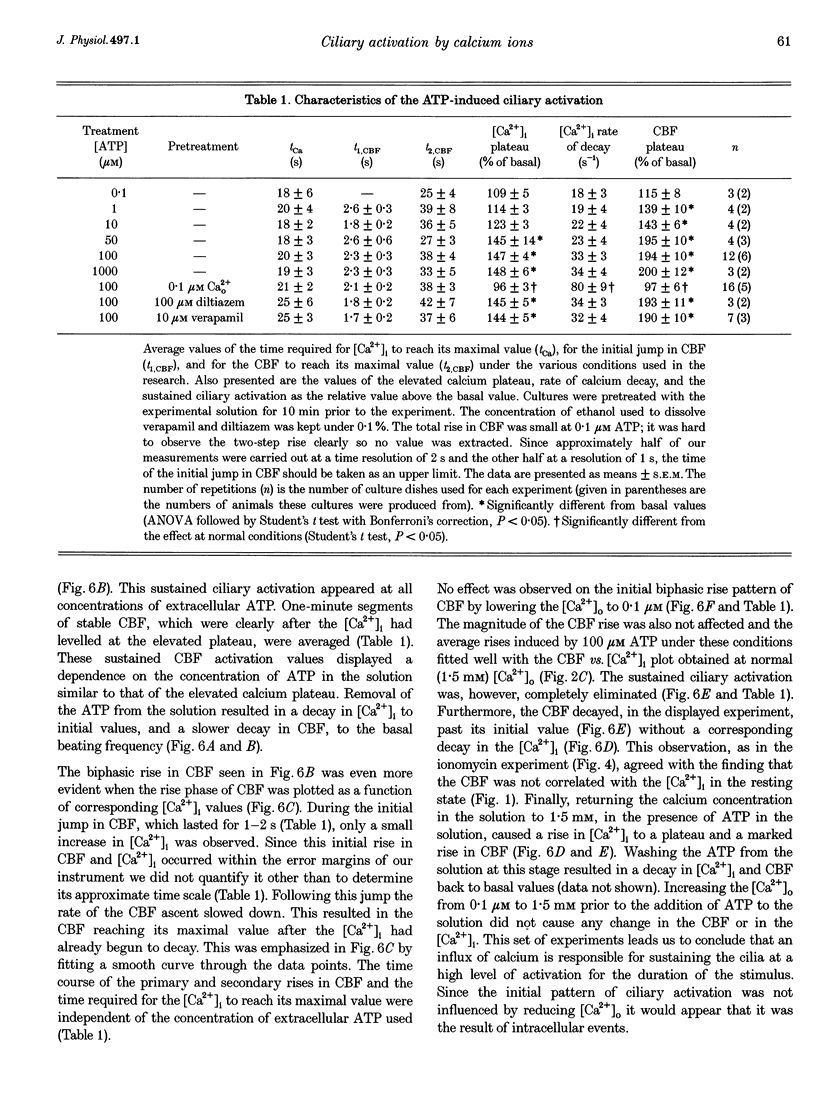
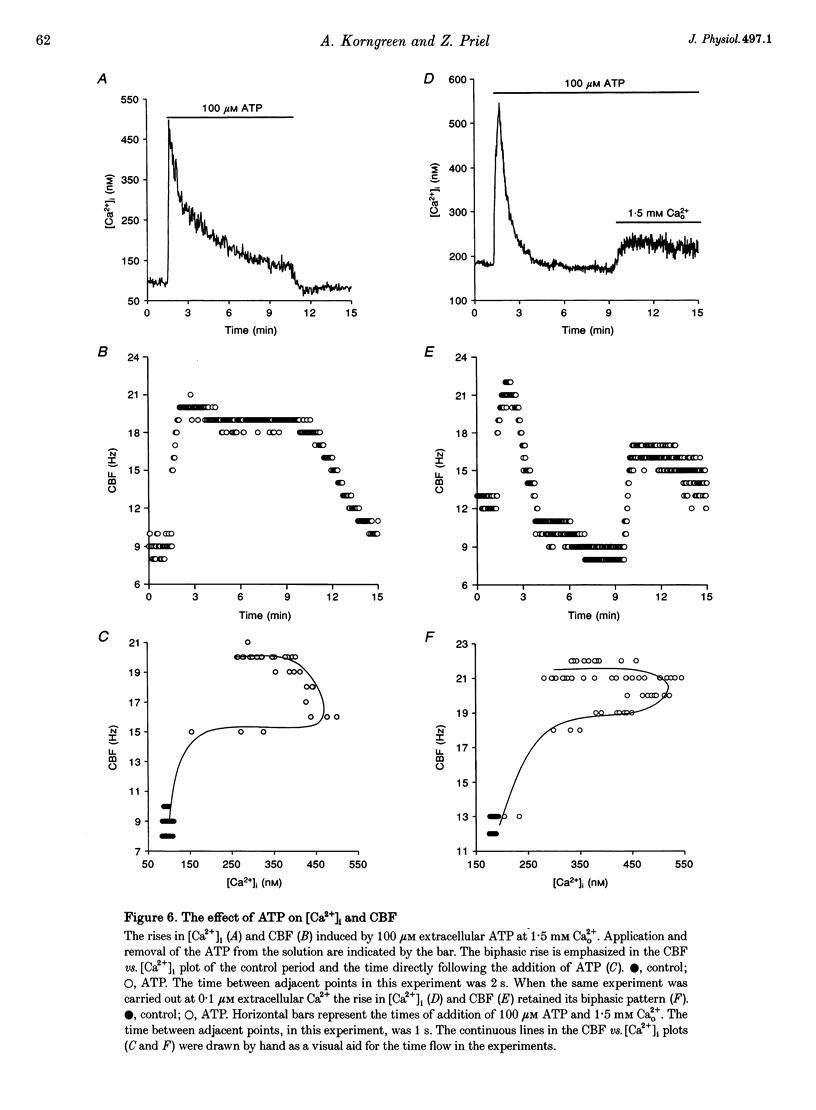
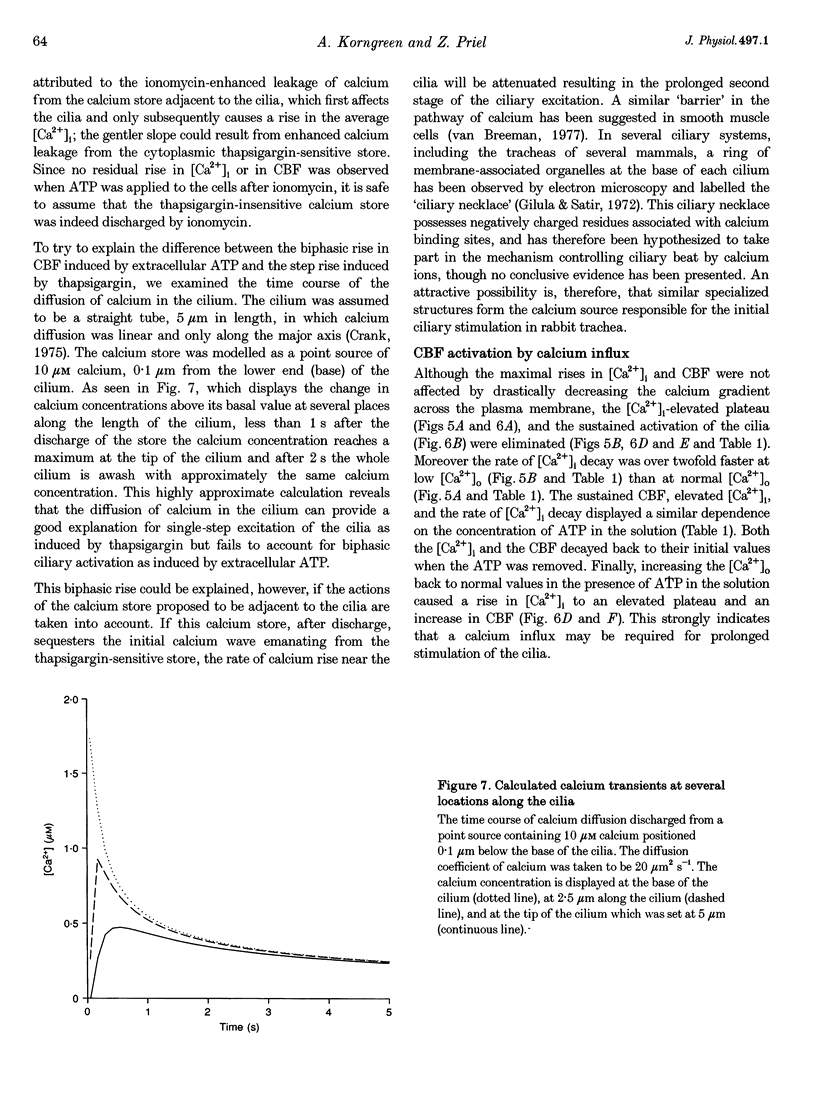
1. Simultaneous measurements of average intracellular calcium concentration ([Ca2+]i) and ciliary beat frequency (CBF) were carried out on ciliated rabbit tracheal cells in order to determine quantitatively the role of calcium in the regulation of mucus-transporting cilia. 2. Extracellular ATP caused a rapid increase in both [Ca2+]i and CBF in the 0.1-1000 microM concentration range. The rise in [Ca2+]i levelled off to an elevated [Ca2+]i plateau while the cilia remained in a high activation state. The magnitude of the rise in [Ca2+]i and CBF as well as the value of the elevated [Ca2+]i plateau and the value of the sustained CBF were dependent on the concentration of ATP in the solution. 3. No correlation was found between the mean values of [Ca2+]i and CBF at rest but a sigmoidal relationship was found to exist between the maximal rises of these parameters following excitation with extracellular ATP. This sigmoidal correlation incorporated the experiments where [Ca2+]i rise was induced by depletion of internal calcium stores with thapsigargin or by entry of calcium induced by ionomycin. 4. Extracellular ATP caused both the release of calcium from internal stores and calcium influx from the extracellular solution. The release of calcium was identified as originating from a thapsigargin-sensitive and a thapsigargin-insensitive calcium store. It is suggested that the release of calcium from these stores induces the initial rise in CBF. 5. The sustained activation of the cilia and elevated calcium plateau were found to be the result of the extracellular ATP-induced calcium influx. This calcium influx was insensitive to the voltage-gated calcium channel inhibitors verapamil and diltiazem, but was completely eliminated by lowering the extracellular calcium concentration to 0.1 microM. 6. We propose that the initial jump in the CBF is mediated by the calcium released from a thapsigargin-insensitive calcium store adjacent to the cilia, while the later, and longer, rise in CBF is the result of the calcium emanating from the thapsigargin-sensitive store which is positioned further away from the cilia within the cell cytoplasm. The calcium influx that follows is responsible for sustaining the cilia at a high level of excitation.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Di Benedetto G., Magnus C. J., Gray P. T., Mehta A. Calcium regulation of ciliary beat frequency in human respiratory epithelium in vitro. J Physiol. 1991 Aug;439:103–113. doi: 10.1113/jphysiol.1991.sp018659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirksen E. R., Sanderson M. J. Regulation of ciliary activity in the mammalian respiratory tract. Biorheology. 1990;27(3-4):533–545. doi: 10.3233/bir-1990-273-432. [DOI] [PubMed] [Google Scholar]
- Dubyak G. R., el-Moatassim C. Signal transduction via P2-purinergic receptors for extracellular ATP and other nucleotides. Am J Physiol. 1993 Sep;265(3 Pt 1):C577–C606. doi: 10.1152/ajpcell.1993.265.3.C577. [DOI] [PubMed] [Google Scholar]
- Eckert R. Bioelectric control of ciliary activity. Science. 1972 May 5;176(4034):473–481. doi: 10.1126/science.176.4034.473. [DOI] [PubMed] [Google Scholar]
- Fabiato A. Computer programs for calculating total from specified free or free from specified total ionic concentrations in aqueous solutions containing multiple metals and ligands. Methods Enzymol. 1988;157:378–417. doi: 10.1016/0076-6879(88)57093-3. [DOI] [PubMed] [Google Scholar]
- Gheber L., Priel Z., Aflalo C., Shoshan-Barmatz V. Extracellular ATP binding proteins as potential receptors in mucociliary epithelium: characterization using [32P]3'-O-(4-benzoyl)benzoyl ATP, a photoaffinity label. J Membr Biol. 1995 Sep;147(1):83–93. doi: 10.1007/BF00235399. [DOI] [PubMed] [Google Scholar]
- Gheber L., Priel Z. Metachronal activity of cultured mucociliary epithelium under normal and stimulated conditions. Cell Motil Cytoskeleton. 1994;28(4):333–345. doi: 10.1002/cm.970280407. [DOI] [PubMed] [Google Scholar]
- Gilula N. B., Satir P. The ciliary necklace. A ciliary membrane specialization. J Cell Biol. 1972 May;53(2):494–509. doi: 10.1083/jcb.53.2.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard P. R., Kennedy J. R. Calcium regulation of ciliary activity in rabbit tracheal epithelial explants and outgrowth. Eur J Cell Biol. 1986 Apr;40(2):203–209. [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hansen M., Boitano S., Dirksen E. R., Sanderson M. J. A role for phospholipase C activity but not ryanodine receptors in the initiation and propagation of intercellular calcium waves. J Cell Sci. 1995 Jul;108(Pt 7):2583–2590. doi: 10.1242/jcs.108.7.2583. [DOI] [PubMed] [Google Scholar]
- Hansen M., Boitano S., Dirksen E. R., Sanderson M. J. Intercellular calcium signaling induced by extracellular adenosine 5'-triphosphate and mechanical stimulation in airway epithelial cells. J Cell Sci. 1993 Dec;106(Pt 4):995–1004. doi: 10.1242/jcs.106.4.995. [DOI] [PubMed] [Google Scholar]
- Korngreen A., Priel Z. Simultaneous measurement of ciliary beating and intracellular calcium. Biophys J. 1994 Jul;67(1):377–380. doi: 10.1016/S0006-3495(94)80492-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lytton J., Westlin M., Hanley M. R. Thapsigargin inhibits the sarcoplasmic or endoplasmic reticulum Ca-ATPase family of calcium pumps. J Biol Chem. 1991 Sep 15;266(26):17067–17071. [PubMed] [Google Scholar]
- Lückhoff A., Clapham D. E. Inositol 1,3,4,5-tetrakisphosphate activates an endothelial Ca(2+)-permeable channel. Nature. 1992 Jan 23;355(6358):356–358. doi: 10.1038/355356a0. [DOI] [PubMed] [Google Scholar]
- Naito Y., Kaneko H. Reactivated triton-extracted models o paramecium: modification of ciliary movement by calcium ions. Science. 1972 May 5;176(4034):523–524. doi: 10.1126/science.176.4034.523. [DOI] [PubMed] [Google Scholar]
- Ovadyahu D., Eshel D., Priel Z. Intensification of ciliary motility by extracellular ATP. Biorheology. 1988;25(3):489–501. doi: 10.3233/bir-1988-25309. [DOI] [PubMed] [Google Scholar]
- Pozzan T., Rizzuto R., Volpe P., Meldolesi J. Molecular and cellular physiology of intracellular calcium stores. Physiol Rev. 1994 Jul;74(3):595–636. doi: 10.1152/physrev.1994.74.3.595. [DOI] [PubMed] [Google Scholar]
- Randriamampita C., Tsien R. Y. Emptying of intracellular Ca2+ stores releases a novel small messenger that stimulates Ca2+ influx. Nature. 1993 Aug 26;364(6440):809–814. doi: 10.1038/364809a0. [DOI] [PubMed] [Google Scholar]
- Rasmussen H., Rasmussen J. E. Calcium as intracellular messenger: from simplicity to complexity. Curr Top Cell Regul. 1990;31:1–109. doi: 10.1016/b978-0-12-152831-7.50003-2. [DOI] [PubMed] [Google Scholar]
- Rozengurt E., Heppel L. A., Friedberg I. Effect of exogenous ATP on the permeability properties of transformed cultures of mouse cell lines. J Biol Chem. 1977 Jul 10;252(13):4584–4590. [PubMed] [Google Scholar]
- Salathe M., Bookman R. J. Coupling of [Ca2+]i and ciliary beating in cultured tracheal epithelial cells. J Cell Sci. 1995 Feb;108(Pt 2):431–440. doi: 10.1242/jcs.108.2.431. [DOI] [PubMed] [Google Scholar]
- Sanderson M. J., Chow I., Dirksen E. R. Intercellular communication between ciliated cells in culture. Am J Physiol. 1988 Jan;254(1 Pt 1):C63–C74. doi: 10.1152/ajpcell.1988.254.1.C63. [DOI] [PubMed] [Google Scholar]
- Sleigh M. A., Blake J. R., Liron N. The propulsion of mucus by cilia. Am Rev Respir Dis. 1988 Mar;137(3):726–741. doi: 10.1164/ajrccm/137.3.726. [DOI] [PubMed] [Google Scholar]
- Tamm S. L., Terasaki M. Visualization of calcium transients controlling orientation of ciliary beat. J Cell Biol. 1994 Jun;125(5):1127–1135. doi: 10.1083/jcb.125.5.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarasiuk A., Bar-Shimon M., Gheber L., Korngreen A., Grossman Y., Priel Z. Extracellular ATP induces hyperpolarization and motility stimulation of ciliary cells. Biophys J. 1995 Mar;68(3):1163–1169. doi: 10.1016/S0006-3495(95)80292-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdugo P. Ca2+-dependent hormonal stimulation of ciliary activity. Nature. 1980 Feb 21;283(5749):764–765. doi: 10.1038/283764a0. [DOI] [PubMed] [Google Scholar]
- Villalón M., Hinds T. R., Verdugo P. Stimulus-response coupling in mammalian ciliated cells. Demonstration of two mechanisms of control for cytosolic [Ca2+]. Biophys J. 1989 Dec;56(6):1255–1258. doi: 10.1016/S0006-3495(89)82772-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss T., Gheber L., Shoshan-Barmatz V., Priel Z. Possible mechanism of ciliary stimulation by extracellular ATP: involvement of calcium-dependent potassium channels and exogenous Ca2+. J Membr Biol. 1992 May;127(3):185–193. doi: 10.1007/BF00231506. [DOI] [PubMed] [Google Scholar]


