Abstract
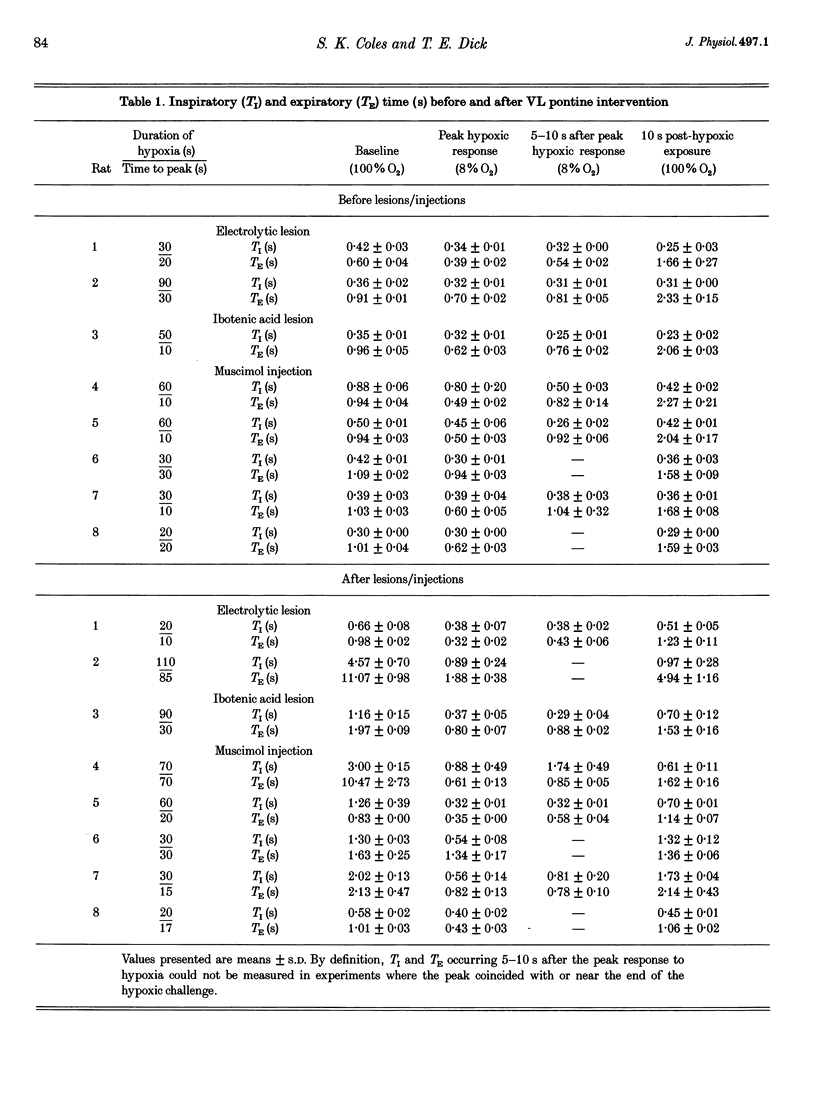
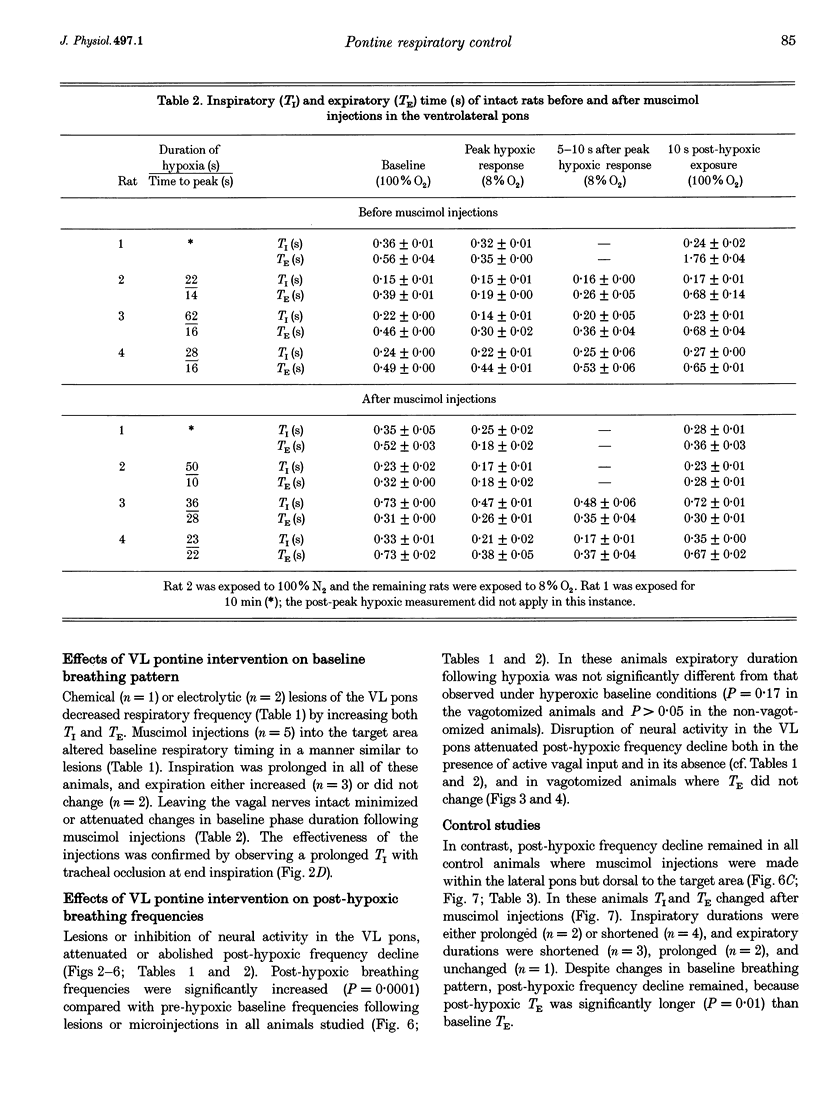
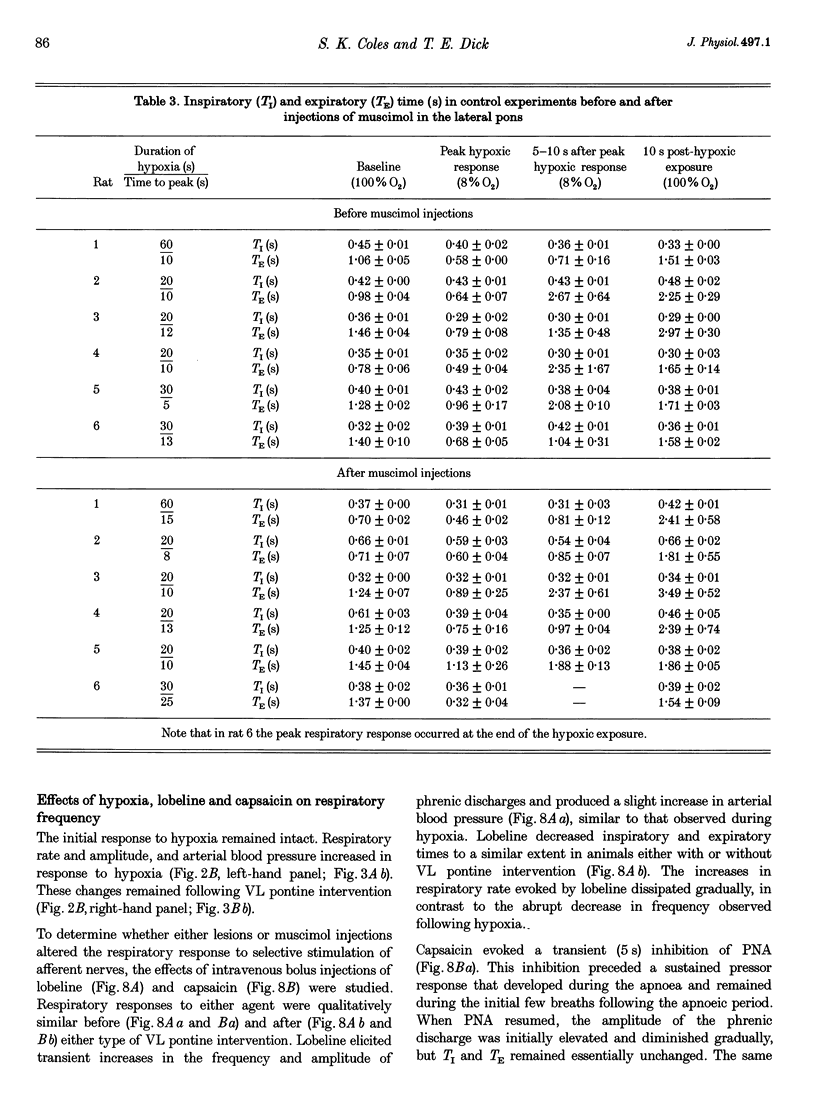
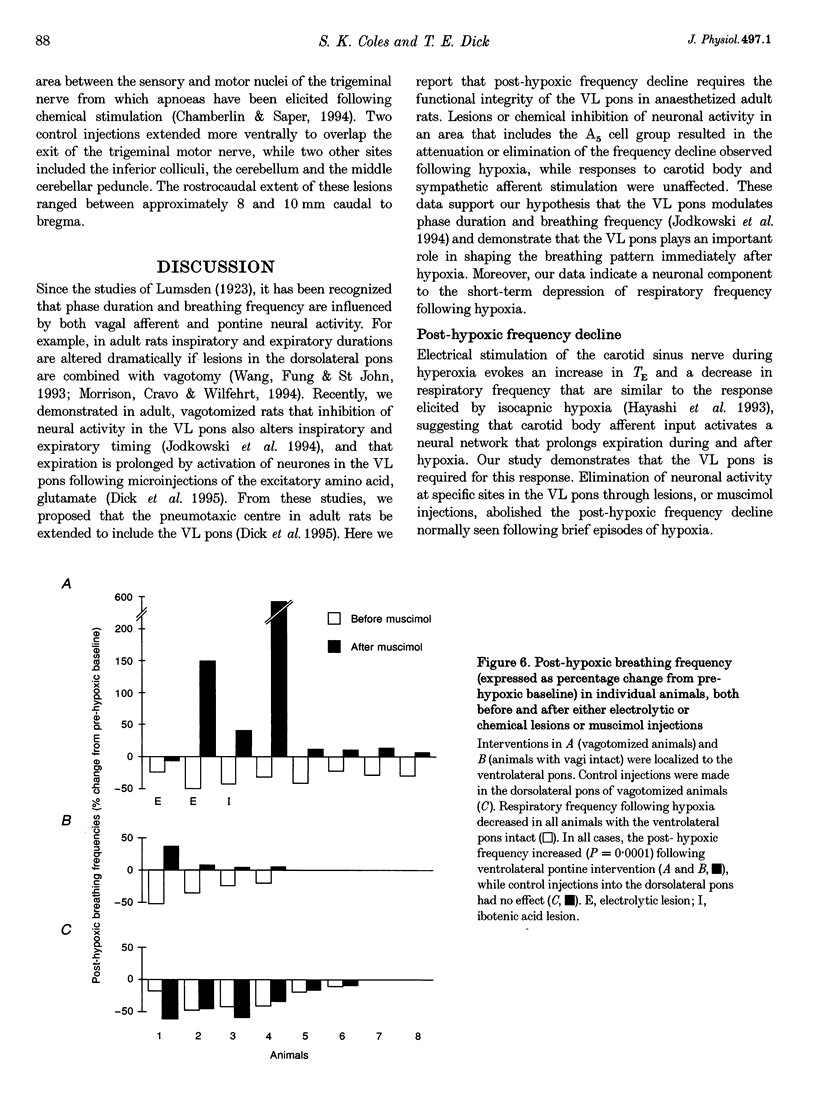
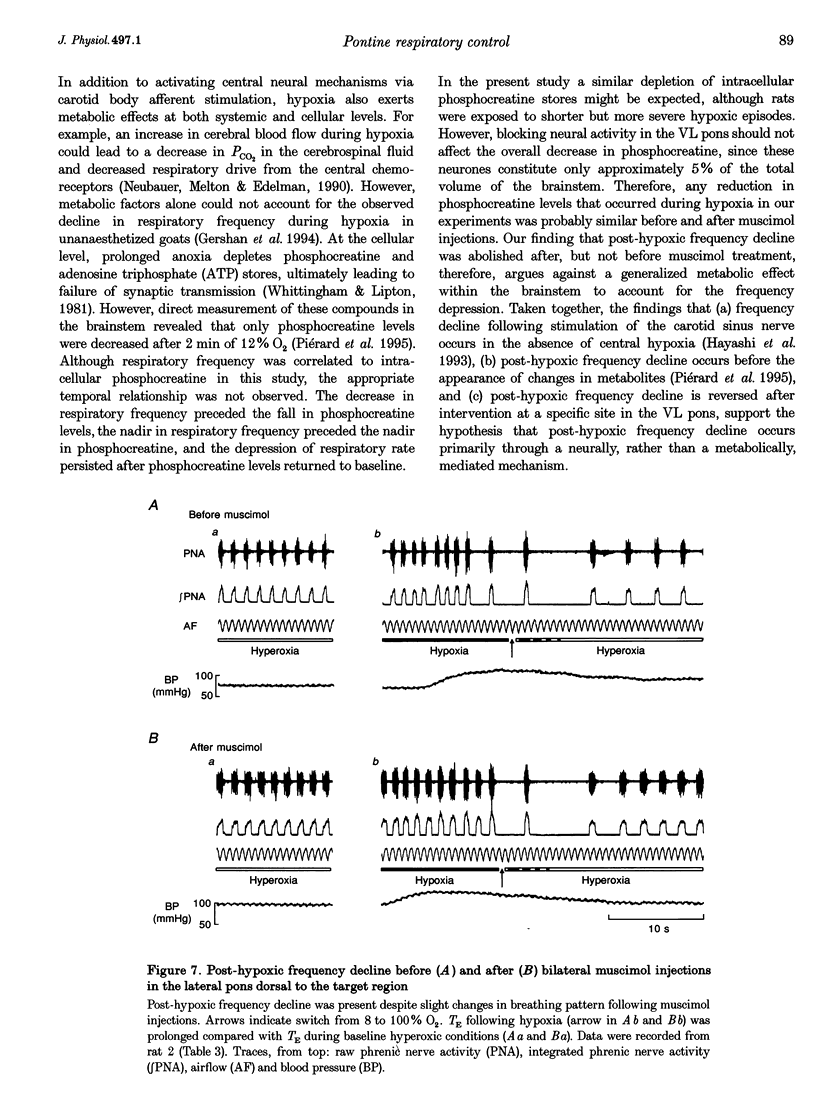
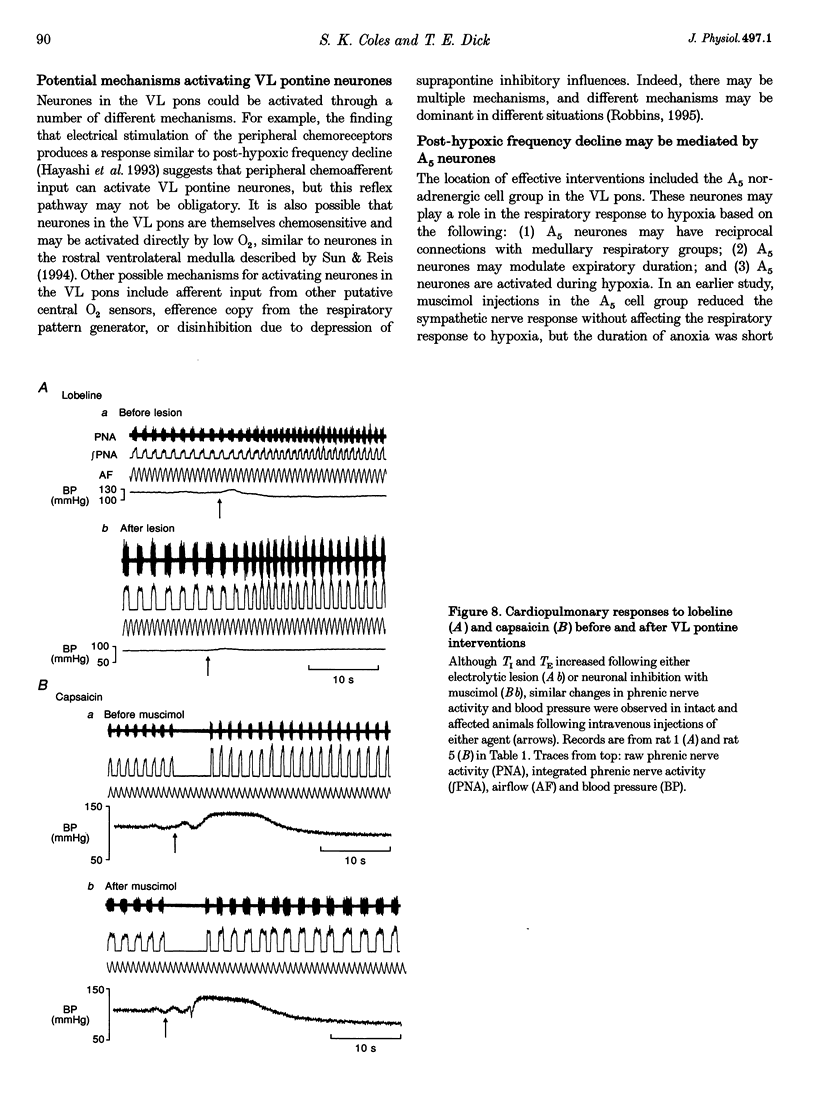
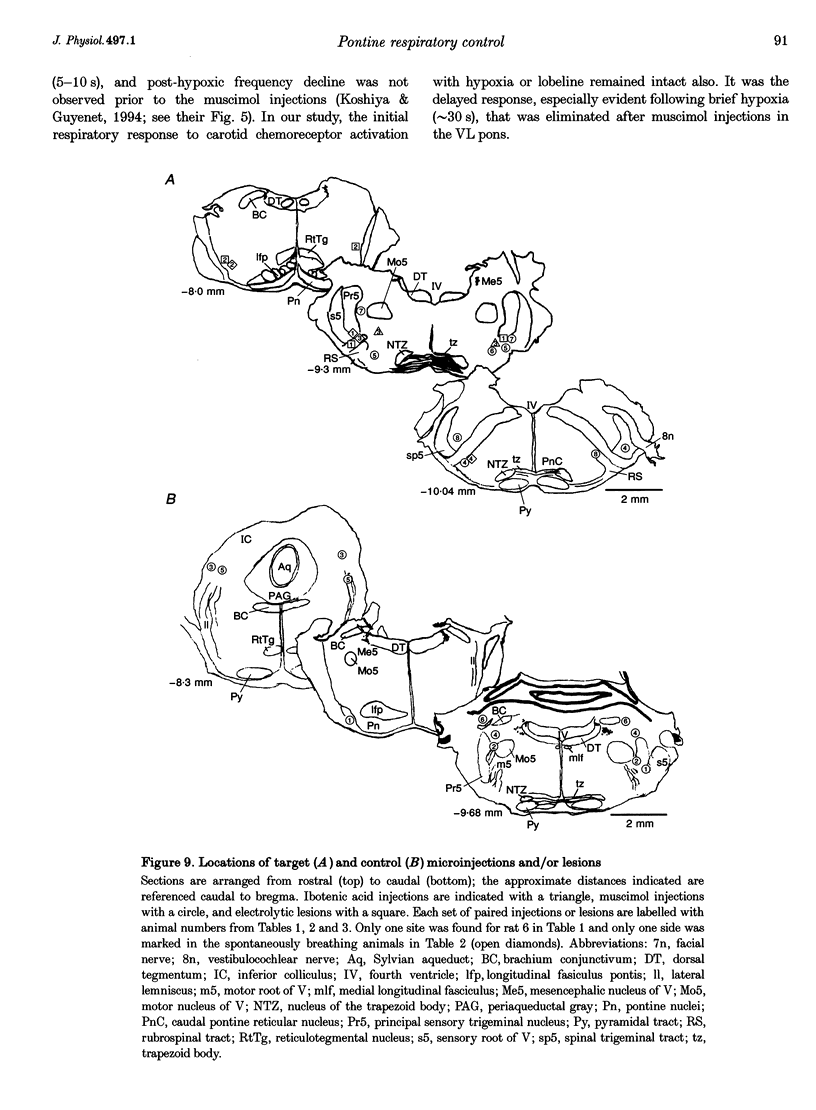
1. The breathing pattern following acute hypoxia (arterial O2 pressure (Pa,O2), 27.4 +/- 7.7 mmHg) was measured in intact, anaesthetized and spontaneously breathing adult rats (n = 4) and in anaesthetized, vagotomized, paralysed and ventilated animals (n = 14). Measurements were made both before and after bilateral lesions or chemical inactivation of neurones in the lateral pons. Respiratory motor activity was recorded as an index of the respiratory cycle. We tested the hypothesis that the ventrolateral pons is required for expression of post-hypoxic frequency decline, defined as a decrease in respiratory frequency below steady-state baseline levels following brief exposures to hypoxia. 2. We identified an area in the ventrolateral pons where brief (1 ms) low current (< or = 20 microA) pulses evoked a short-latency inhibitor of phrenic nerve activity. At this site, bilateral electrical or chemical lesions (n = 3) were performed, or neural activity was inhibited by focal injections of 10 mM muscimol (n = 9). In six control animals, neural activity was inhibited by muscimol injections into the lateral pons, dorsal to the target site. 3. Prior to pontine intervention, respiratory frequency decreased below baseline levels following 20-110 s of 8% O2. The decrease in frequency resulted from a prolongation of expiration (up to 276%), which gradually returned to baseline levels (tau = 45 s). 4. Following lesions or inhibition of neural activity in the ventrolateral pons, baseline inspiratory (TI) and expiratory (TE) durations were altered, albeit minimally, in the animals with intact vagus nerves. Expiratory duration following hypoxia was not different from baseline levels either in vagotomized (P = 0.18) or intact (P > 0.05) animals. In contrast, injections of muscimol at more dorsal sites did not alter the decrease in frequency normally seen following hypoxia. 5. Histological examination revealed that effective lesion or injection sites were within the lateral pontine tegmental field and included portions of the noradrenergic A5 cell group. 6. We conclude that the mechanism responsible for post-hypoxic frequency decline involves an active neural process that depends on the integrity of the ventrolateral pons.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boddy K., Dawes G. S., Fisher R., Pinter S., Robinson J. S. Foetal respiratory movements, electrocortical and cardiovascular responses to hypoxaemia and hypercapnia in sheep. J Physiol. 1974 Dec;243(3):599–618. doi: 10.1113/jphysiol.1974.sp010768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrum C. E., Guyenet P. G. Afferent and efferent connections of the A5 noradrenergic cell group in the rat. J Comp Neurol. 1987 Jul 22;261(4):529–542. doi: 10.1002/cne.902610406. [DOI] [PubMed] [Google Scholar]
- Chamberlin N. L., Saper C. B. Topographic organization of respiratory responses to glutamate microstimulation of the parabrachial nucleus in the rat. J Neurosci. 1994 Nov;14(11 Pt 1):6500–6510. doi: 10.1523/JNEUROSCI.14-11-06500.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawes G. S., Gardner W. N., Johnston B. M., Walker D. W. Breathing in fetal lambs: the effect of brain stem section. J Physiol. 1983 Feb;335:535–553. doi: 10.1113/jphysiol.1983.sp014549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbins E. G., Feldman J. L. Brainstem network controlling descending drive to phrenic motoneurons in rat. J Comp Neurol. 1994 Sep 1;347(1):64–86. doi: 10.1002/cne.903470106. [DOI] [PubMed] [Google Scholar]
- Ellenberger H. H., Feldman J. L. Brainstem connections of the rostral ventral respiratory group of the rat. Brain Res. 1990 Apr 9;513(1):35–42. doi: 10.1016/0006-8993(90)91086-v. [DOI] [PubMed] [Google Scholar]
- Erickson J. T., Millhorn D. E. Hypoxia and electrical stimulation of the carotid sinus nerve induce Fos-like immunoreactivity within catecholaminergic and serotoninergic neurons of the rat brainstem. J Comp Neurol. 1994 Oct 8;348(2):161–182. doi: 10.1002/cne.903480202. [DOI] [PubMed] [Google Scholar]
- Errchidi S., Monteau R., Hilaire G. Noradrenergic modulation of the medullary respiratory rhythm generator in the newborn rat: an in vitro study. J Physiol. 1991 Nov;443:477–498. doi: 10.1113/jphysiol.1991.sp018846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershan W. M., Forster H. V., Lowry T. F., Korducki M. J., Forster A. L., Forster M. A., Ohtake P. J., Aaron E. A., Garber A. K. Effect of metabolic rate on ventilatory roll-off during hypoxia. J Appl Physiol (1985) 1994 Jun;76(6):2310–2314. doi: 10.1152/jappl.1994.76.6.2310. [DOI] [PubMed] [Google Scholar]
- Gluckman P. D., Johnston B. M. Lesions in the upper lateral pons abolish the hypoxic depression of breathing in unanaesthetized fetal lambs in utero. J Physiol. 1987 Jan;382:373–383. doi: 10.1113/jphysiol.1987.sp016372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet P. G., Koshiya N., Huangfu D., Verberne A. J., Riley T. A. Central respiratory control of A5 and A6 pontine noradrenergic neurons. Am J Physiol. 1993 Jun;264(6 Pt 2):R1035–R1044. doi: 10.1152/ajpregu.1993.264.6.R1035. [DOI] [PubMed] [Google Scholar]
- Hamada O., Garcia-Rill E., Skinner R. D. Electrical activation and inhibition of respiration in vitro. Neurosci Res. 1994 Mar;19(2):131–142. doi: 10.1016/0168-0102(94)90136-8. [DOI] [PubMed] [Google Scholar]
- Hayashi F., Coles S. K., Bach K. B., Mitchell G. S., McCrimmon D. R. Time-dependent phrenic nerve responses to carotid afferent activation: intact vs. decerebellate rats. Am J Physiol. 1993 Oct;265(4 Pt 2):R811–R819. doi: 10.1152/ajpregu.1993.265.4.R811. [DOI] [PubMed] [Google Scholar]
- Hedrick M. S., Ryan M. L., Pizarro J., Bisgard G. E. Modulation of respiratory rhythm by alpha 2-adrenoceptors in awake and anesthetized goats. J Appl Physiol (1985) 1994 Aug;77(2):742–750. doi: 10.1152/jappl.1994.77.2.742. [DOI] [PubMed] [Google Scholar]
- Hilaire G., Monteau R., Errchidi S. Possible modulation of the medullary respiratory rhythm generator by the noradrenergic A5 area: an in vitro study in the newborn rat. Brain Res. 1989 Apr 24;485(2):325–332. doi: 10.1016/0006-8993(89)90577-5. [DOI] [PubMed] [Google Scholar]
- Jodkowski J. S., Coles S. K., Dick T. E. A 'pneumotaxic centre' in rats. Neurosci Lett. 1994 May 19;172(1-2):67–72. doi: 10.1016/0304-3940(94)90664-5. [DOI] [PubMed] [Google Scholar]
- Koshiya N., Guyenet P. G. Role of the pons in the carotid sympathetic chemoreflex. Am J Physiol. 1994 Aug;267(2 Pt 2):R508–R518. doi: 10.1152/ajpregu.1994.267.2.R508. [DOI] [PubMed] [Google Scholar]
- Li Y. W., Wesselingh S. L., Blessing W. W. Projections from rabbit caudal medulla to C1 and A5 sympathetic premotor neurons, demonstrated with phaseolus leucoagglutinin and herpes simplex virus. J Comp Neurol. 1992 Mar 22;317(4):379–395. doi: 10.1002/cne.903170405. [DOI] [PubMed] [Google Scholar]
- Lumsden T. Observations on the respiratory centres. J Physiol. 1923 Aug 16;57(6):354–367. doi: 10.1113/jphysiol.1923.sp002073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg J. M., Brodin E., Saria A. Effects and distribution of vagal capsaicin-sensitive substance P neurons with special reference to the trachea and lungs. Acta Physiol Scand. 1983 Nov;119(3):243–252. doi: 10.1111/j.1748-1716.1983.tb07334.x. [DOI] [PubMed] [Google Scholar]
- Martin-Body R. L., Johnston B. M. Central origin of the hypoxic depression of breathing in the newborn. Respir Physiol. 1988 Jan;71(1):25–32. doi: 10.1016/0034-5687(88)90112-0. [DOI] [PubMed] [Google Scholar]
- Monteau R., Errchidi S., Gauthier P., Hilaire G., Rega P. Pneumotaxic centre and apneustic breathing: interspecies differences between rat and cat. Neurosci Lett. 1989 May 8;99(3):311–316. doi: 10.1016/0304-3940(89)90465-5. [DOI] [PubMed] [Google Scholar]
- Morrison S. F., Cravo S. L., Wilfehrt H. M. Pontine lesions produce apneusis in the rat. Brain Res. 1994 Jul 25;652(1):83–86. doi: 10.1016/0006-8993(94)90320-4. [DOI] [PubMed] [Google Scholar]
- Neubauer J. A., Melton J. E., Edelman N. H. Modulation of respiration during brain hypoxia. J Appl Physiol (1985) 1990 Feb;68(2):441–451. doi: 10.1152/jappl.1990.68.2.441. [DOI] [PubMed] [Google Scholar]
- Nicholson C. Diffusion from an injected volume of a substance in brain tissue with arbitrary volume fraction and tortuosity. Brain Res. 1985 May 6;333(2):325–329. doi: 10.1016/0006-8993(85)91586-0. [DOI] [PubMed] [Google Scholar]
- Núez-Abades P. A., Morillo A. M., Pásaro R. Brainstem connections of the rat ventral respiratory subgroups: afferent projections. J Auton Nerv Syst. 1993 Feb;42(2):99–118. doi: 10.1016/0165-1838(93)90042-s. [DOI] [PubMed] [Google Scholar]
- Peterson D. F., Brown A. M. Pressor reflexes produced by stimulation of afferent fibers in the cardiac sympathetic nerves of the cat. Circ Res. 1971 Jun;28(6):605–610. doi: 10.1161/01.res.28.6.605. [DOI] [PubMed] [Google Scholar]
- Piérard C., Champagnat J., Denavit-Saubie M., Gillet B., Beloeil J. C., Guezennec C. Y., Barrère B., Pérès M. Brain stem energy metabolism response to acute hypoxia in anaesthetized rats: a 31P NMR study. Neuroreport. 1995 Dec 29;7(1):281–285. [PubMed] [Google Scholar]
- Robbins P. A. Hypoxic ventilatory decline: site of action. J Appl Physiol (1985) 1995 Aug;79(2):373–374. doi: 10.1152/jappl.1995.79.2.373. [DOI] [PubMed] [Google Scholar]
- Schmid K., Böhmer G., Fallert M. Influence of rubrospinal tract and the adjacent mesencephalic reticular formation on the activity of medullary respiratory neurons and the phrenic nerve discharge in the rabbit. Pflugers Arch. 1988 Nov;413(1):23–31. doi: 10.1007/BF00581224. [DOI] [PubMed] [Google Scholar]
- Sun M. K., Reis D. J. Hypoxia selectively excites vasomotor neurons of rostral ventrolateral medulla in rats. Am J Physiol. 1994 Jan;266(1 Pt 2):R245–R256. doi: 10.1152/ajpregu.1994.266.1.R245. [DOI] [PubMed] [Google Scholar]
- U'Prichard D. C., Bechtel W. D., Rouot B. M., Snyder S. H. Multiple apparent alpha-noradrenergic receptor binding sites in rat brain: effect of 6-hydroxydopamine. Mol Pharmacol. 1979 Jul;16(1):47–60. [PubMed] [Google Scholar]
- Wang W., Fung M. L., St John W. M. Pontile regulation of ventilatory activity in the adult rat. J Appl Physiol (1985) 1993 Jun;74(6):2801–2811. doi: 10.1152/jappl.1993.74.6.2801. [DOI] [PubMed] [Google Scholar]
- Whittingham T. S., Lipton P. Cerebral synaptic transmission during anoxia is protected by creatine. J Neurochem. 1981 Dec;37(6):1618–1621. doi: 10.1111/j.1471-4159.1981.tb06337.x. [DOI] [PubMed] [Google Scholar]