Abstract
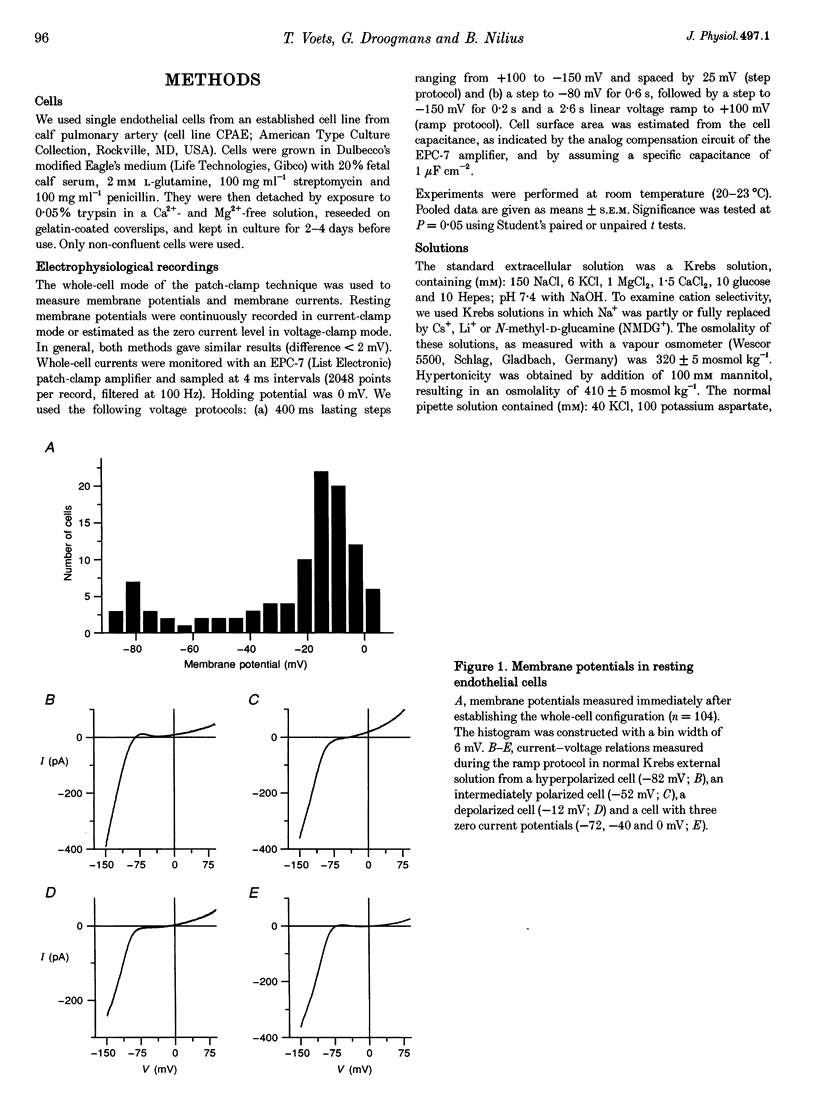
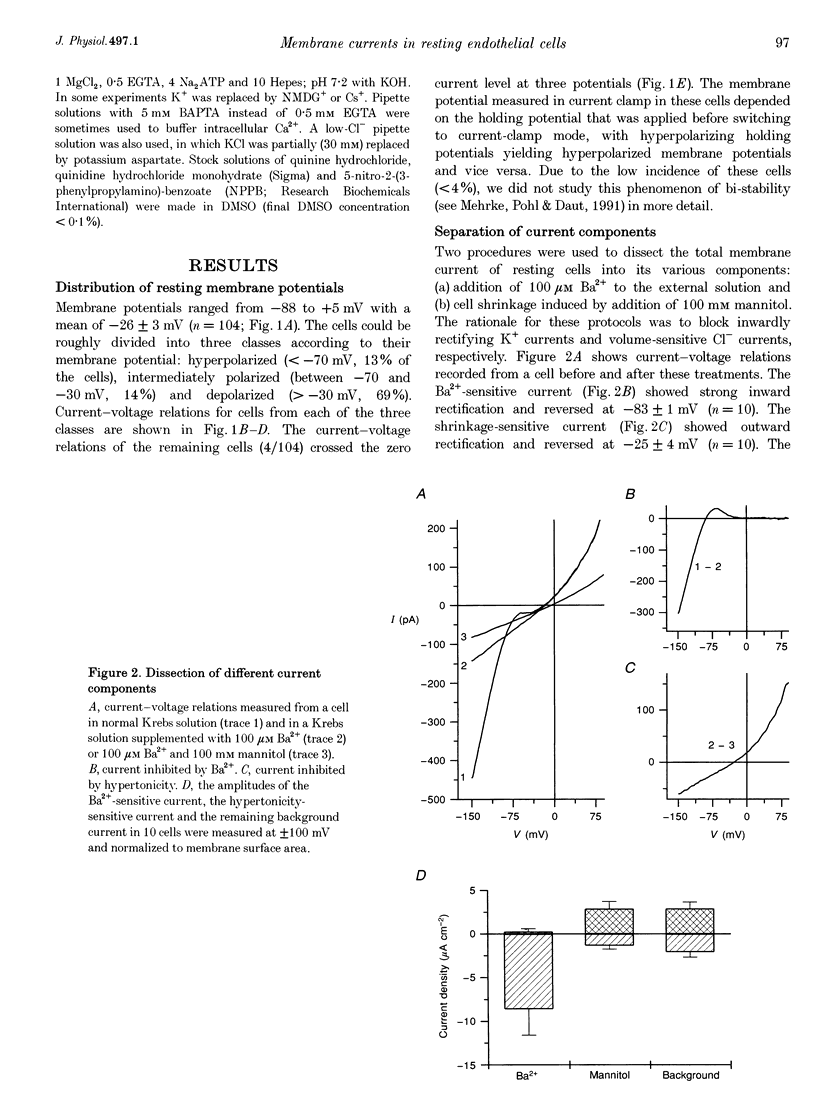
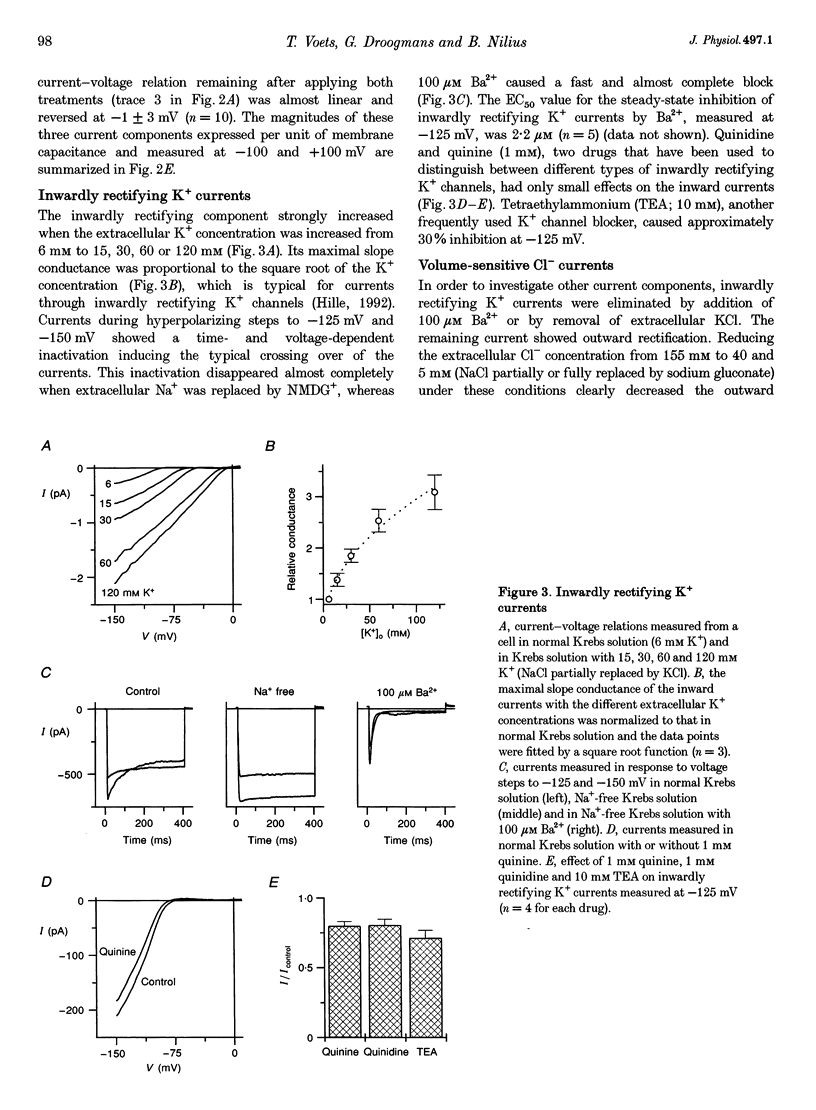
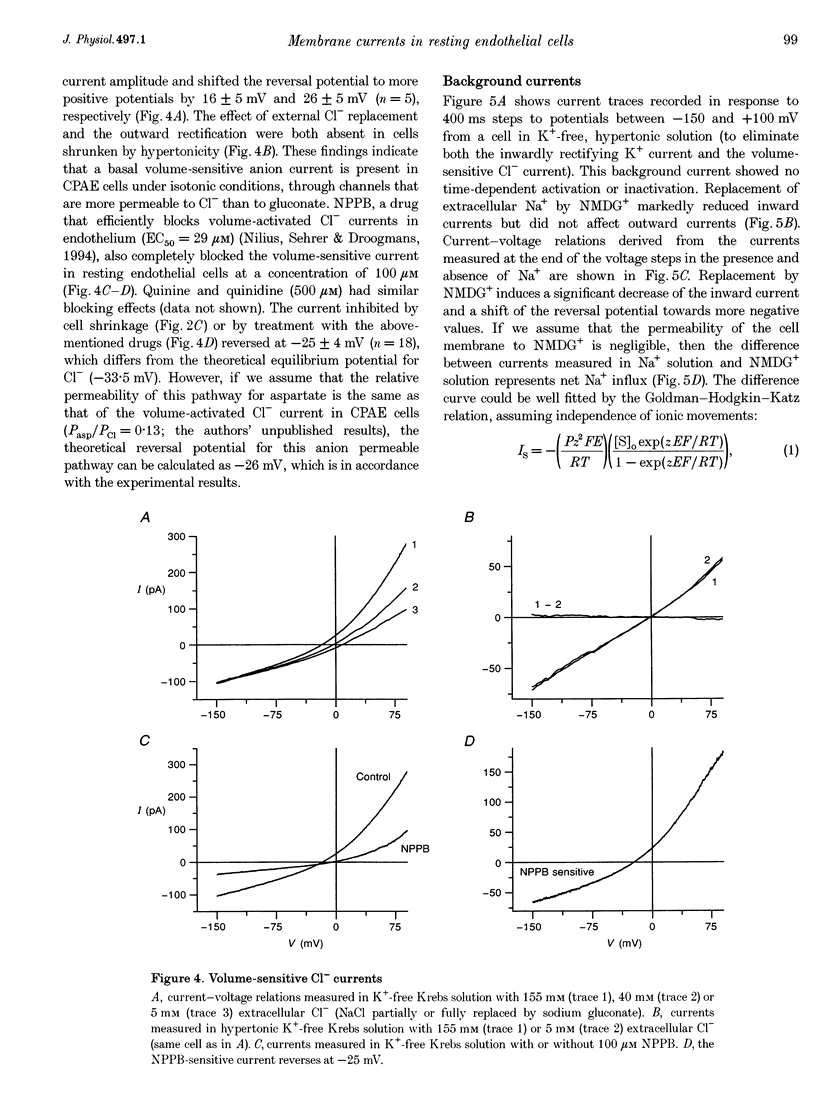
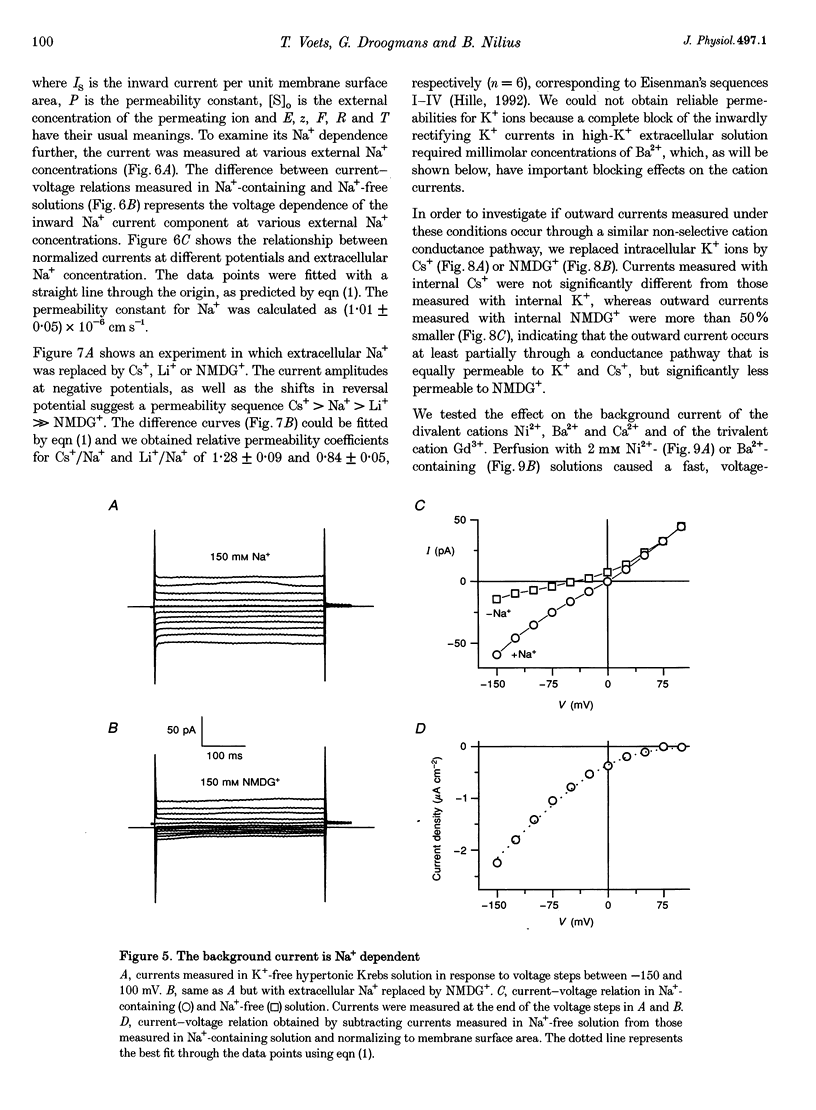
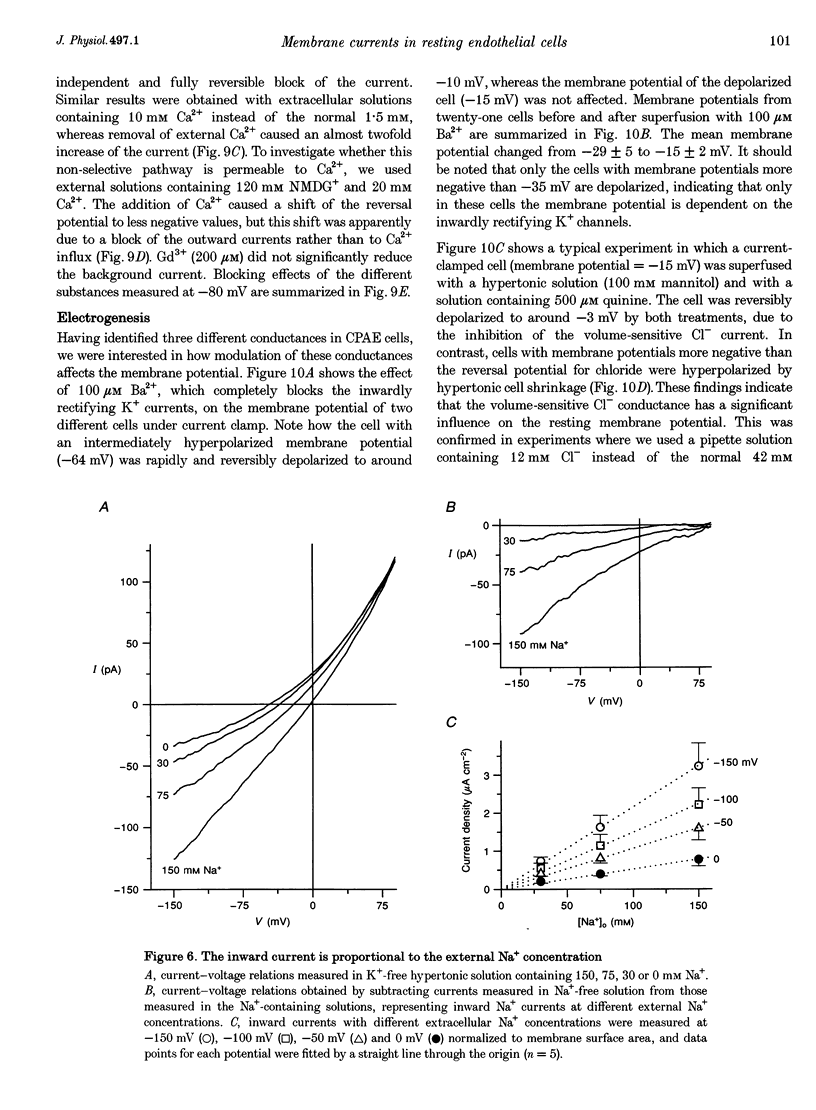
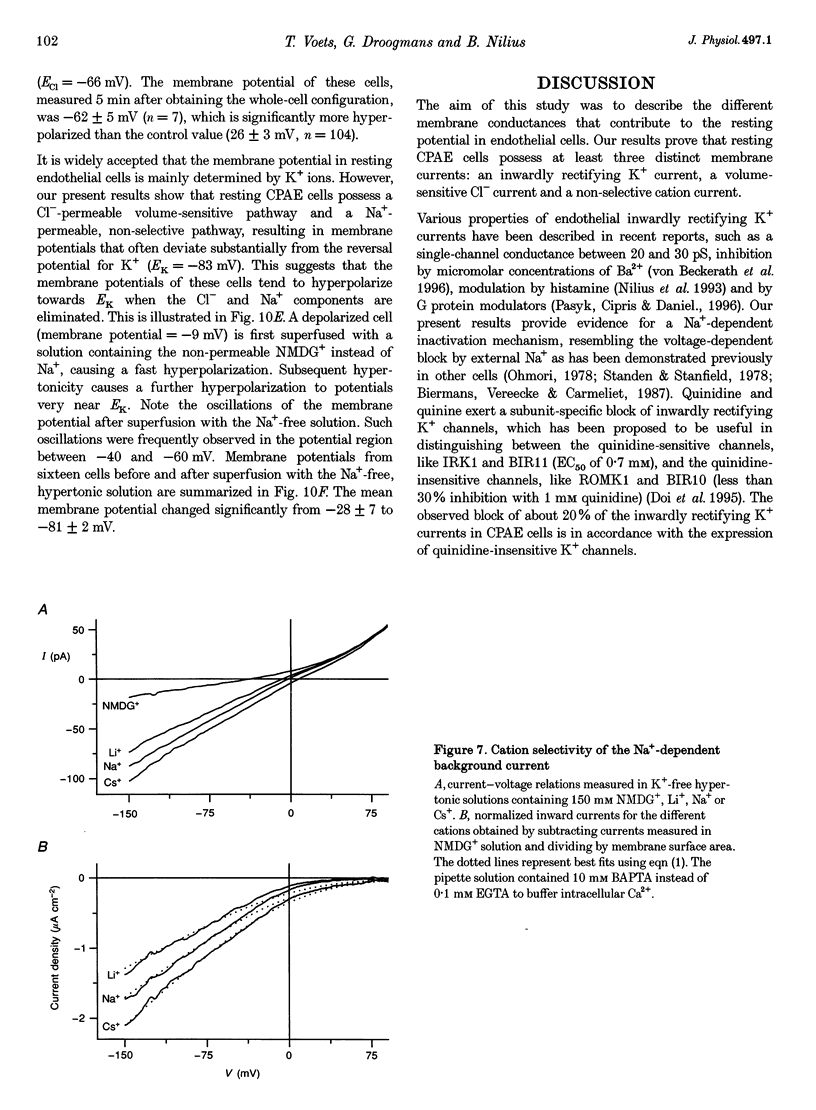
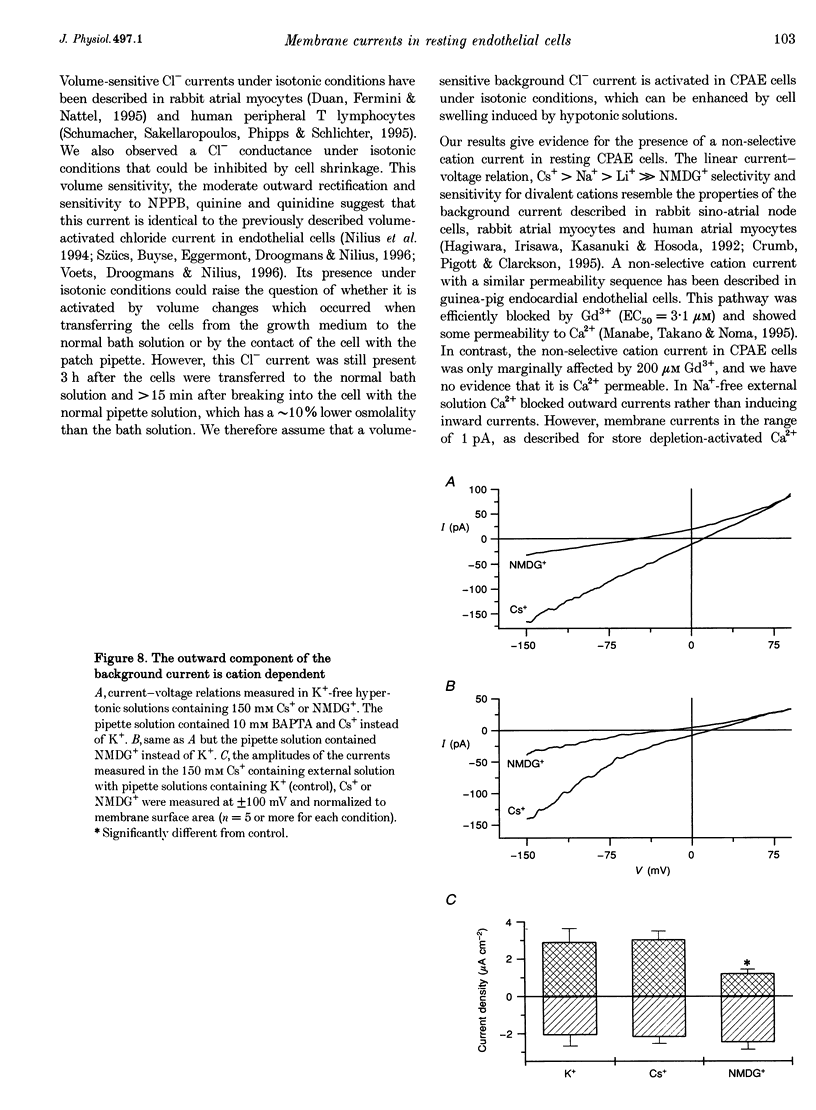
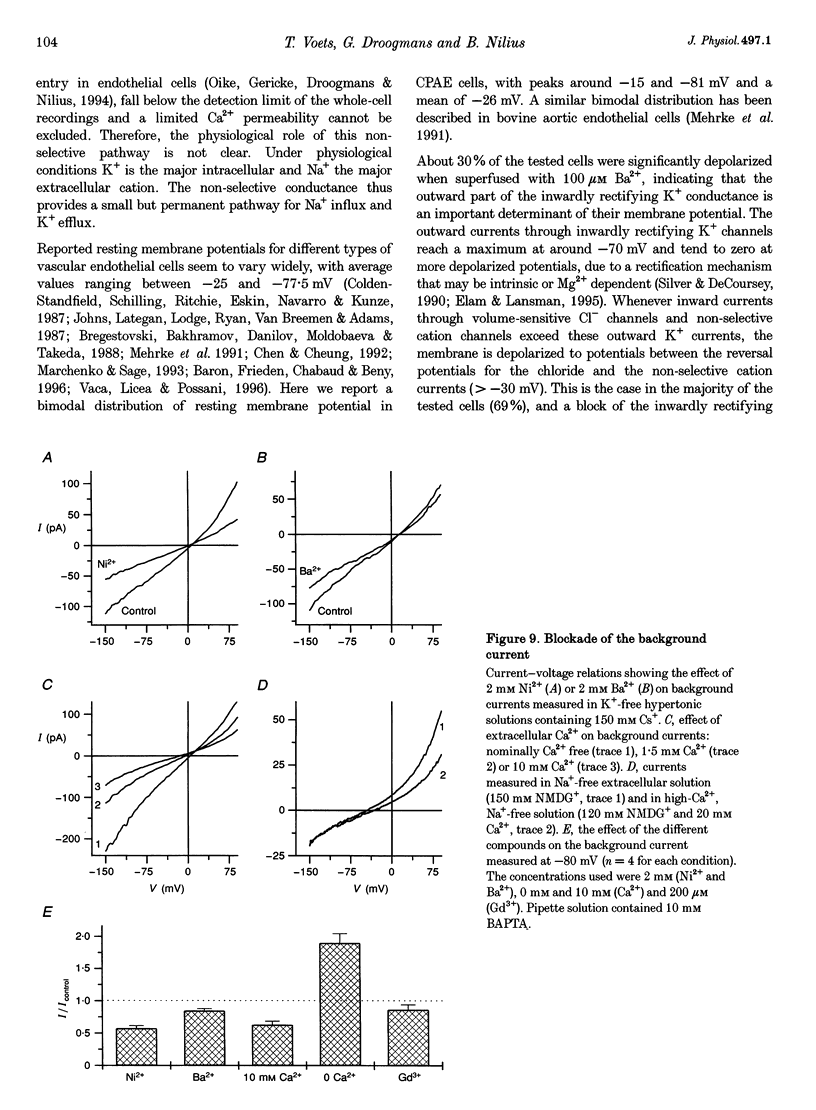
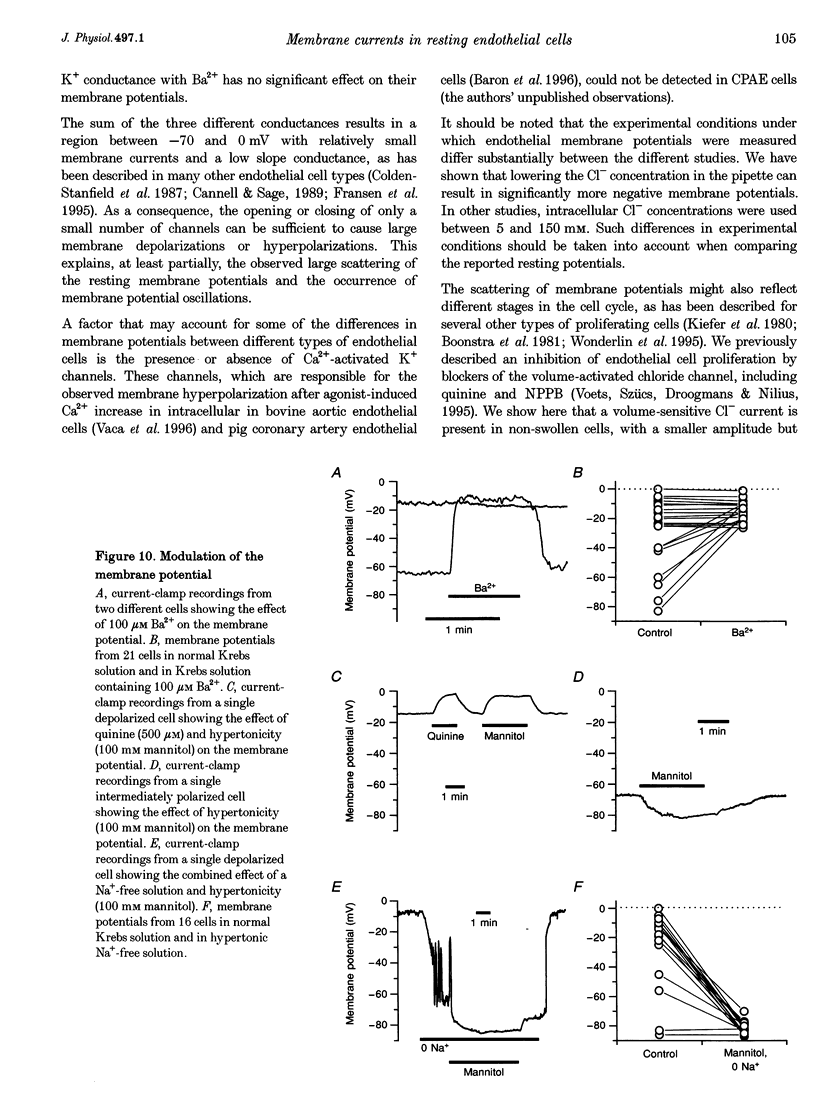
1. We have used the whole-cell patch-clamp technique to characterize the ionic conductances that determine the resting membrane potential in cultured endothelial cells from calf pulmonary artery (CPAE cells). 2. Resting membrane potentials were scattered between -88 and +5 mV with a mean +/- S.E.M. of -26 +/- 3 mV (n = 104). 3. The most prominent membrane current in resting cells was an inwardly rectifying K+ current. This current showed Na(+)-dependent inactivation and was efficiently blocked by external Ba2+ (EC50 = 2.2 microM), but was relatively insensitive to quinine, quinidine and TEA. 4. Hypertonic cell shrinkage inhibited an outwardly rectifying Cl- current, which was also efficiently blocked by 5-nitro-2-(3-phenylpropylamino)-benzoate (NPPB; 100 microM), quinine (500 microM) and quinidine (500 microM). 5. A linear, time-independent background current remained after elimination of these two currents. This current was dependent on extracellular monovalent cations with a permeability sequence of Cs+ > Na+ > Li+ >> N-methyl-D-glucamine. It was partially blocked by millimolar concentrations of the divalent cations Ca2+, Ni2+ and Ba2+. Gd3+ (200 microM) had no significant effect on this background current. 6. Continuous measurements of the membrane potential confirm that the three described conductances are the major determinants of the membrane potential. Due to the low slope conductance in the region between -70 and 0 mV, small changes in one of the current components can evoke large depolarizations or hyperpolarizations, which explains the large scattering of the resting membrane potentials.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. J., Barakeh J., Laskey R., Van Breemen C. Ion channels and regulation of intracellular calcium in vascular endothelial cells. FASEB J. 1989 Oct;3(12):2389–2400. doi: 10.1096/fasebj.3.12.2477294. [DOI] [PubMed] [Google Scholar]
- Baron A., Frieden M., Chabaud F., Bény J. L. Ca(2+)-dependent non-selective cation and potassium channels activated by bradykinin in pig coronary artery endothelial cells. J Physiol. 1996 Jun 15;493(Pt 3):691–706. doi: 10.1113/jphysiol.1996.sp021415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biermans G., Vereecke J., Carmeliet E. The mechanism of the inactivation of the inward-rectifying K current during hyperpolarizing steps in guinea-pig ventricular myocytes. Pflugers Arch. 1987 Dec;410(6):604–613. doi: 10.1007/BF00581320. [DOI] [PubMed] [Google Scholar]
- Boonstra J., Mummery C. L., Tertoolen L. G., Van Der Saag P. T., De Laat S. W. Cation transport and growth regulation in neuroblastoma cells. Modulations of K+ transport and electrical membrane properties during the cell cycle. J Cell Physiol. 1981 Apr;107(1):75–83. doi: 10.1002/jcp.1041070110. [DOI] [PubMed] [Google Scholar]
- Bregestovski P., Bakhramov A., Danilov S., Moldobaeva A., Takeda K. Histamine-induced inward currents in cultured endothelial cells from human umbilical vein. Br J Pharmacol. 1988 Oct;95(2):429–436. doi: 10.1111/j.1476-5381.1988.tb11663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bény J. L., Pacicca C. Bidirectional electrical communication between smooth muscle and endothelial cells in the pig coronary artery. Am J Physiol. 1994 Apr;266(4 Pt 2):H1465–H1472. doi: 10.1152/ajpheart.1994.266.4.H1465. [DOI] [PubMed] [Google Scholar]
- Cannell M. B., Sage S. O. Bradykinin-evoked changes in cytosolic calcium and membrane currents in cultured bovine pulmonary artery endothelial cells. J Physiol. 1989 Dec;419:555–568. doi: 10.1113/jphysiol.1989.sp017886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G. F., Cheung D. W. Characterization of acetylcholine-induced membrane hyperpolarization in endothelial cells. Circ Res. 1992 Feb;70(2):257–263. doi: 10.1161/01.res.70.2.257. [DOI] [PubMed] [Google Scholar]
- Colden-Stanfield M., Schilling W. P., Ritchie A. K., Eskin S. G., Navarro L. T., Kunze D. L. Bradykinin-induced increases in cytosolic calcium and ionic currents in cultured bovine aortic endothelial cells. Circ Res. 1987 Nov;61(5):632–640. doi: 10.1161/01.res.61.5.632. [DOI] [PubMed] [Google Scholar]
- Crumb W. J., Jr, Pigott J. D., Clarkson C. W. Description of a nonselective cation current in human atrium. Circ Res. 1995 Nov;77(5):950–956. doi: 10.1161/01.res.77.5.950. [DOI] [PubMed] [Google Scholar]
- Daut J., Standen N. B., Nelson M. T. The role of the membrane potential of endothelial and smooth muscle cells in the regulation of coronary blood flow. J Cardiovasc Electrophysiol. 1994 Feb;5(2):154–181. doi: 10.1111/j.1540-8167.1994.tb01156.x. [DOI] [PubMed] [Google Scholar]
- Davies P. F., Olesen S. P., Clapham D. E., Morrel E. M., Schoen F. J. Endothelial communication. State of the art lecture. Hypertension. 1988 Jun;11(6 Pt 2):563–572. doi: 10.1161/01.hyp.11.6.563.a. [DOI] [PubMed] [Google Scholar]
- Doi T., Fakler B., Schultz J. H., Ehmke H., Brändle U., Zenner H. P., Süssbrich H., Lang F., Ruppersberg J. P., Busch A. E. Subunit-specific inhibition of inward-rectifier K+ channels by quinidine. FEBS Lett. 1995 Nov 20;375(3):193–196. doi: 10.1016/0014-5793(95)01182-e. [DOI] [PubMed] [Google Scholar]
- Duan D., Fermini B., Nattel S. Alpha-adrenergic control of volume-regulated Cl- currents in rabbit atrial myocytes. Characterization of a novel ionic regulatory mechanism. Circ Res. 1995 Aug;77(2):379–393. doi: 10.1161/01.res.77.2.379. [DOI] [PubMed] [Google Scholar]
- Elam T. R., Lansman J. B. The role of Mg2+ in the inactivation of inwardly rectifying K+ channels in aortic endothelial cells. J Gen Physiol. 1995 Apr;105(4):463–484. doi: 10.1085/jgp.105.4.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransen P. F., Demolder M. J., Brutsaert D. L. Whole cell membrane currents in cultured pig endocardial endothelial cells. Am J Physiol. 1995 May;268(5 Pt 2):H2036–H2047. doi: 10.1152/ajpheart.1995.268.5.H2036. [DOI] [PubMed] [Google Scholar]
- Hagiwara N., Irisawa H., Kasanuki H., Hosoda S. Background current in sino-atrial node cells of the rabbit heart. J Physiol. 1992 Mar;448:53–72. doi: 10.1113/jphysiol.1992.sp019029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns A., Lategan T. W., Lodge N. J., Ryan U. S., Van Breemen C., Adams D. J. Calcium entry through receptor-operated channels in bovine pulmonary artery endothelial cells. Tissue Cell. 1987;19(6):733–745. doi: 10.1016/0040-8166(87)90015-2. [DOI] [PubMed] [Google Scholar]
- Kiefer H., Blume A. J., Kaback H. R. Membrane potential changes during mitogenic stimulation of mouse spleen lymphocytes. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2200–2204. doi: 10.1073/pnas.77.4.2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manabe K., Takano M., Noma A. Non-selective cation current of guinea-pig endocardial endothelial cells. J Physiol. 1995 Sep 1;487(Pt 2):407–419. doi: 10.1113/jphysiol.1995.sp020889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchenko S. M., Sage S. O. Electrical properties of resting and acetylcholine-stimulated endothelium in intact rat aorta. J Physiol. 1993 Mar;462:735–751. doi: 10.1113/jphysiol.1993.sp019579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehrke G., Pohl U., Daut J. Effects of vasoactive agonists on the membrane potential of cultured bovine aortic and guinea-pig coronary endothelium. J Physiol. 1991 Aug;439:277–299. doi: 10.1113/jphysiol.1991.sp018667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilius B., Schwarz G., Droogmans G. Modulation by histamine of an inwardly rectifying potassium channel in human endothelial cells. J Physiol. 1993 Dec;472:359–371. doi: 10.1113/jphysiol.1993.sp019951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilius B., Sehrer J., Droogmans G. Permeation properties and modulation of volume-activated Cl(-)-currents in human endothelial cells. Br J Pharmacol. 1994 Aug;112(4):1049–1056. doi: 10.1111/j.1476-5381.1994.tb13189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohmori H. Inactivation kinetics and steady-state current noise in the anomalous rectifier of tunicate egg cell membranes. J Physiol. 1978 Aug;281:77–99. doi: 10.1113/jphysiol.1978.sp012410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oike M., Gericke M., Droogmans G., Nilius B. Calcium entry activated by store depletion in human umbilical vein endothelial cells. Cell Calcium. 1994 Nov;16(5):367–376. doi: 10.1016/0143-4160(94)90030-2. [DOI] [PubMed] [Google Scholar]
- Pasyk E. A., Cipris S., Daniel E. E. A G protein, not cyclic AMP, mediates effects of VIP on the inwardly rectifying K+ channels in endothelial cells. J Pharmacol Exp Ther. 1996 Feb;276(2):690–696. [PubMed] [Google Scholar]
- Rhodin J. A. The ultrastructure of mammalian arterioles and precapillary sphincters. J Ultrastruct Res. 1967 Apr;18(1):181–223. doi: 10.1016/s0022-5320(67)80239-9. [DOI] [PubMed] [Google Scholar]
- Schumacher P. A., Sakellaropoulos G., Phipps D. J., Schlichter L. C. Small-conductance chloride channels in human peripheral T lymphocytes. J Membr Biol. 1995 Jun;145(3):217–232. doi: 10.1007/BF00232714. [DOI] [PubMed] [Google Scholar]
- Silver M. R., DeCoursey T. E. Intrinsic gating of inward rectifier in bovine pulmonary artery endothelial cells in the presence or absence of internal Mg2+. J Gen Physiol. 1990 Jul;96(1):109–133. doi: 10.1085/jgp.96.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standen N. B., Stanfield P. R. A potential- and time-dependent blockade of inward rectification in frog skeletal muscle fibres by barium and strontium ions. J Physiol. 1978 Jul;280:169–191. doi: 10.1113/jphysiol.1978.sp012379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szücs G., Buyse G., Eggermont J., Droogmans G., Nilius B. Characterization of volume-activated chloride currents in endothelial cells from bovine pulmonary artery. J Membr Biol. 1996 Feb;149(3):189–197. doi: 10.1007/s002329900019. [DOI] [PubMed] [Google Scholar]
- Vaca L., Licea A., Possani L. D. Modulation of cell membrane potential in cultured vascular endothelium. Am J Physiol. 1996 Mar;270(3 Pt 1):C819–C824. doi: 10.1152/ajpcell.1996.270.3.C819. [DOI] [PubMed] [Google Scholar]
- Voets T., Droogmans G., Nilius B. Potent block of volume-activated chloride currents in endothelial cells by the uncharged form of quinine and quinidine. Br J Pharmacol. 1996 Aug;118(7):1869–1871. doi: 10.1111/j.1476-5381.1996.tb15616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voets T., Szücs G., Droogmans G., Nilius B. Blockers of volume-activated Cl- currents inhibit endothelial cell proliferation. Pflugers Arch. 1995 Nov;431(1):132–134. doi: 10.1007/BF00374387. [DOI] [PubMed] [Google Scholar]
- Wonderlin W. F., Woodfork K. A., Strobl J. S. Changes in membrane potential during the progression of MCF-7 human mammary tumor cells through the cell cycle. J Cell Physiol. 1995 Oct;165(1):177–185. doi: 10.1002/jcp.1041650121. [DOI] [PubMed] [Google Scholar]
- von Beckerath N., Dittrich M., Klieber H. G., Daut J. Inwardly rectifying K+ channels in freshly dissociated coronary endothelial cells from guinea-pig heart. J Physiol. 1996 Mar 1;491(Pt 2):357–365. doi: 10.1113/jphysiol.1996.sp021221. [DOI] [PMC free article] [PubMed] [Google Scholar]


