Abstract
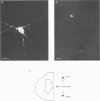
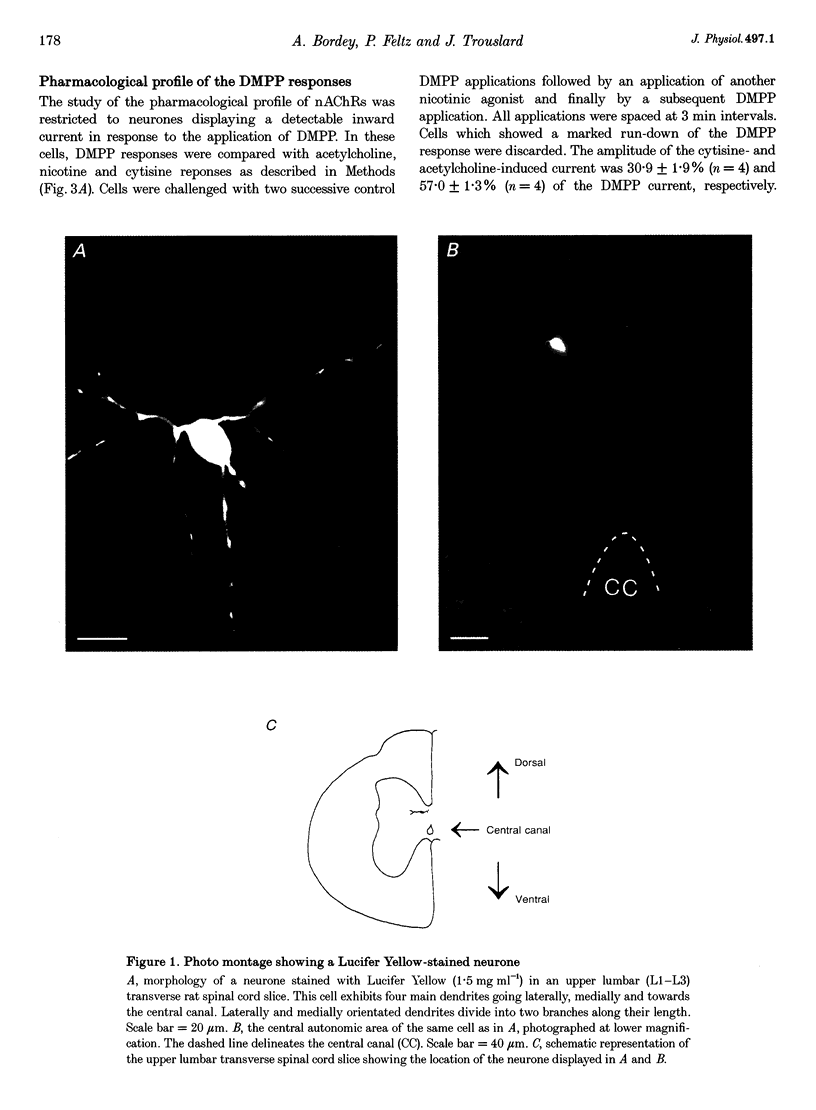
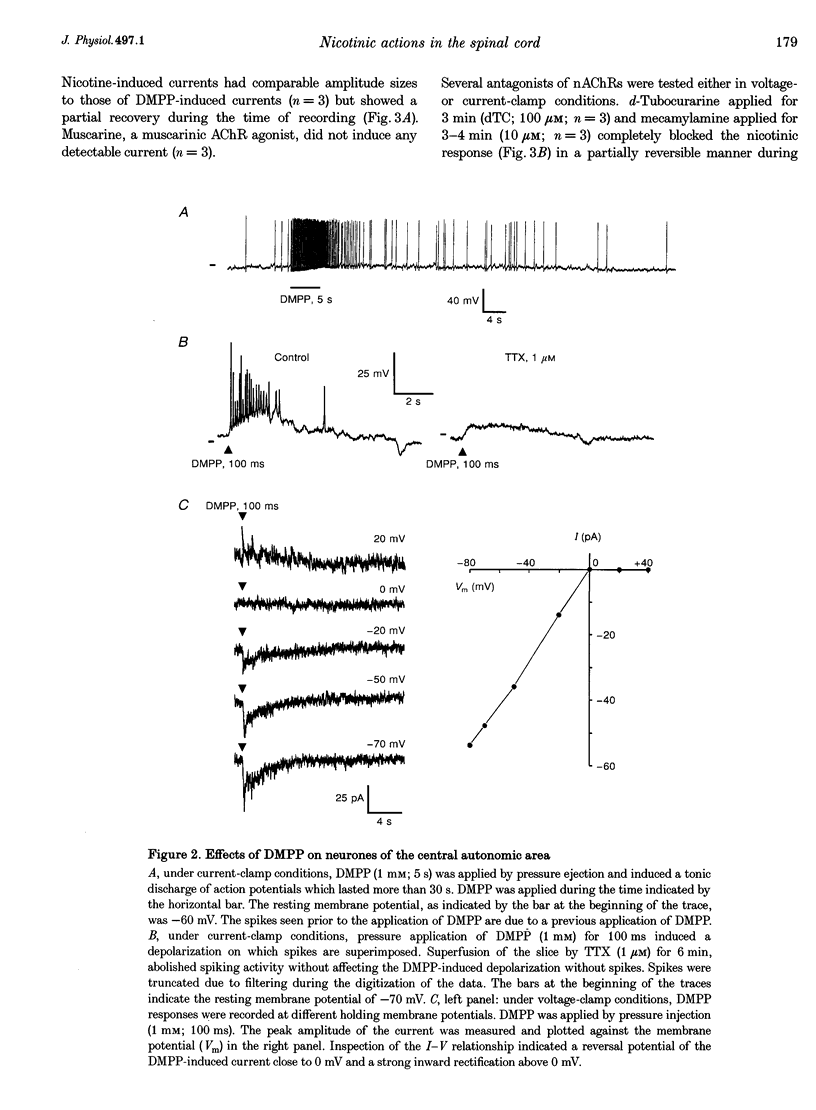
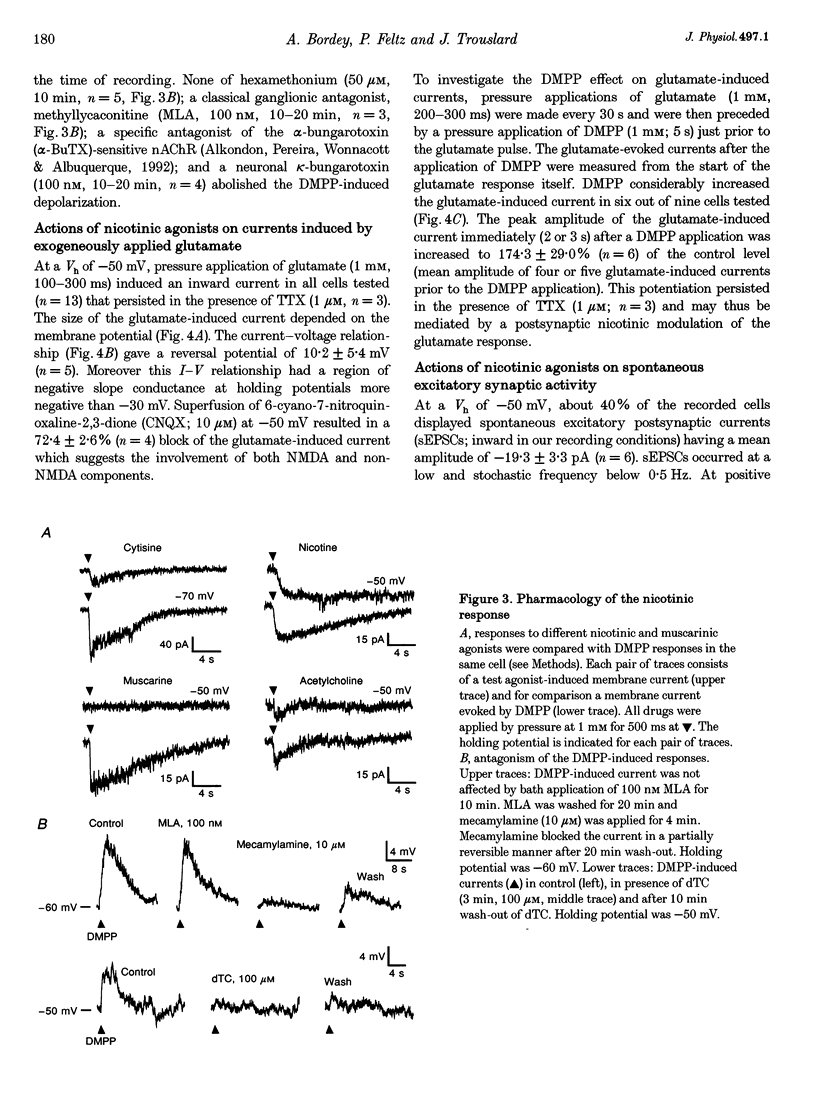
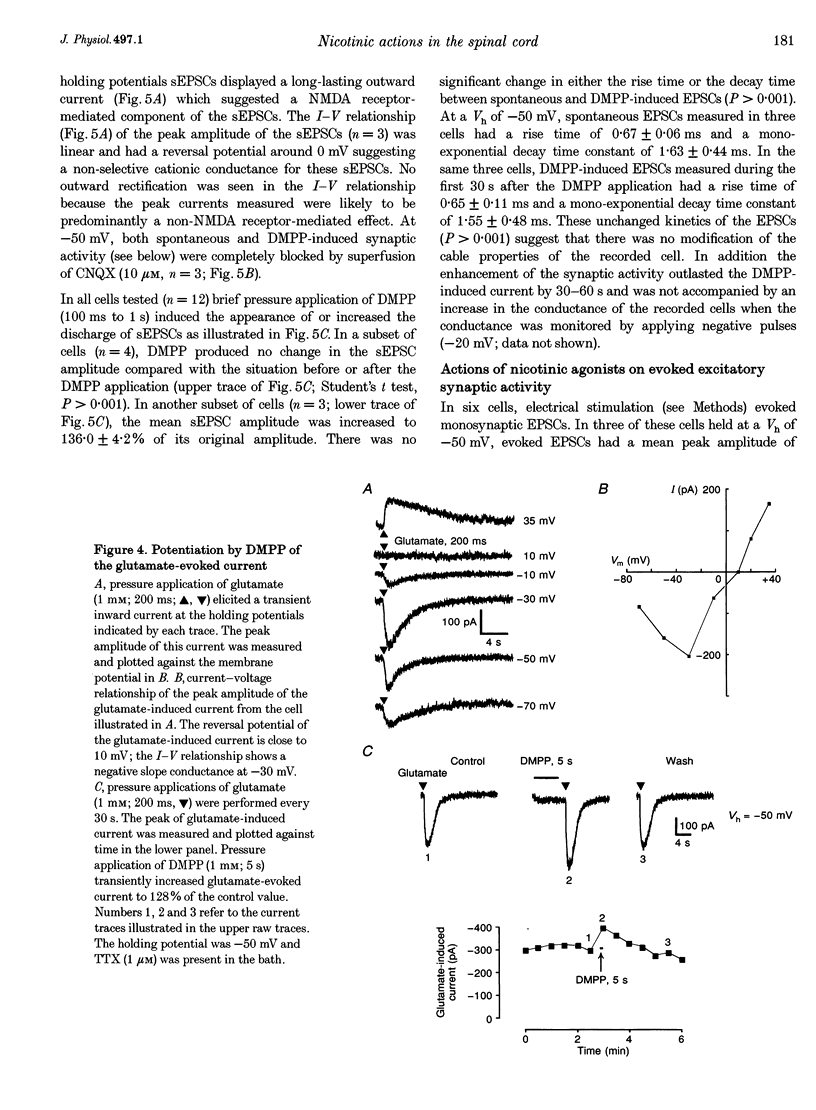
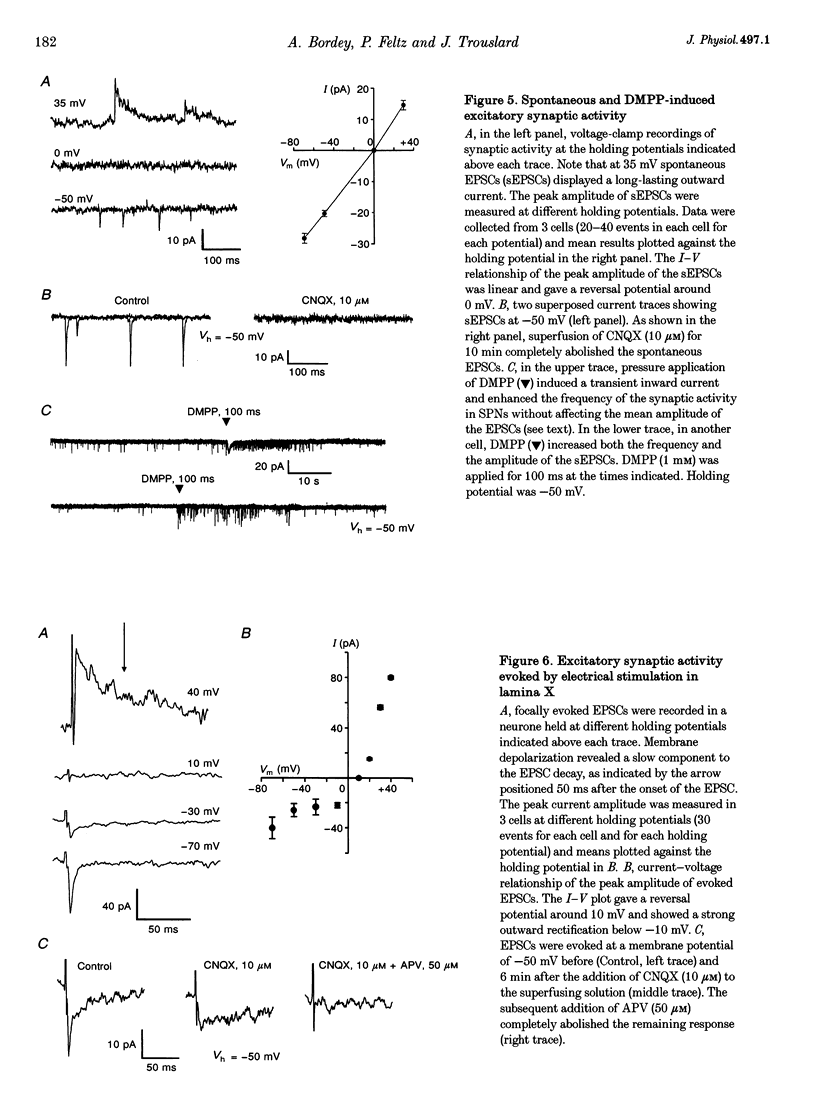
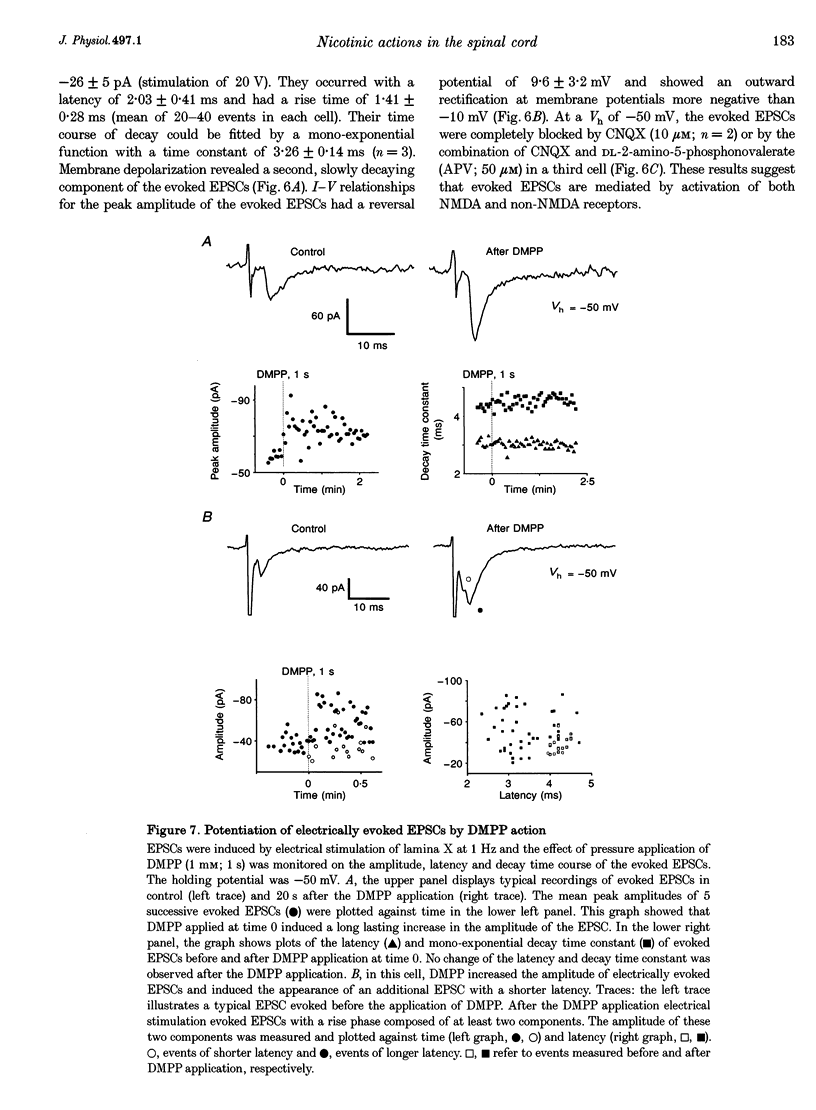
1. Nicotinic responses and actions on excitatory synaptic activity were studied in eighty-four neurones in the region dorsal to the central canal (lamina X) in transverse thoracolumbar spinal cord slices of neonate (P2-P10) rats by using the whole-cell patch-clamp technique. 2. Neurones (n = 15) labelled with Lucifer Yellow, showed the typical morphology of sympathetic preganglionic neurones (SPNs) in the central autonomic area (CA). Unlabelled neurones of comparable morphology were visually identified and recorded. 3. All neurones recorded responded to the nicotinic acetylcholine receptor (nAChR) agonist, DMPP. Under current-clamp conditions, pressure ejections of DMPP depolarized cells and induced the discharge of action potentials. Tetrodotoxin suppressed action potentials but not DMPP-induced depolarization. 4. Under voltage-clamp conditions at a holding potential (Vh) of -50 mV, DMPP induced a transient inward current (which reversed around 0 mV) and an increase in membrane current noise in 50% of the recorded neurones. In the others, DMPP increased membrane current noise without measurable inward current. The current-voltage relationship showed strong inward rectification at holding potentials more positive than 0 mV. 5. In neurones displaying a detectable current response to DMPP, the following agonist rank order potency could be established: DMPP = nicotine > cytisine > ACh. The DMPP response could be blocked by mecamylamine but was insensitive to methyllycaconite. 6. Pressure application of glutamate induced inward currents in all cells tested at a Vh of -50 mV. This response reversed at 10 mV, displayed a region of negative slope conductance at Vh more negative than -30 mV and was partially blocked by CNQX. Pressure application of DMPP transiently increased the amplitude of the glutamate-induced current in six out of nine cells tested. This potentiation persisted in the presence of tetrodotoxin. 7. Forty per cent of the recorded neurones displayed spontaneous excitatory postsynaptic currents (sEPSCs). At a Vh of -50 mV the sEPSCs had a mean amplitude of -19.3 pA and occurred at a frequency below 0.5 Hz. sEPSCs were blocked by CNQX and inverted around 0 mV. Brief application of DMPP increased the discharge frequency of sEPSCs without affecting their kinetics. Additionally, in some cells DMPP increased mean sEPSC amplitude. 8. Focal electrically evoked EPSCs reversed close to 10 mV and were sensitive to CNQX. They occurred with a constant latency, rise time and a mono-exponential decay time. Application of DMPP decreased the percentage of stimulation failures and increased the amplitude of evoked EPSCs, in all cells tested. 9. It is concluded that neurones in the CA, presumed to be SPNs, have functional nAChRs with activation having two distinct effects: firstly, a direct depolarization of the postsynaptic membrane; and secondly, a facilitation of the excitatory transmission onto these cells. This second effect is achieved by an increase of the size of the glutamate-induced current at the postsynaptic level as well as by an enhancement of the presynaptic release of glutamate.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alkondon M., Pereira E. F., Wonnacott S., Albuquerque E. X. Blockade of nicotinic currents in hippocampal neurons defines methyllycaconitine as a potent and specific receptor antagonist. Mol Pharmacol. 1992 Apr;41(4):802–808. [PubMed] [Google Scholar]
- Barber R. P., Phelps P. E., Houser C. R., Crawford G. D., Salvaterra P. M., Vaughn J. E. The morphology and distribution of neurons containing choline acetyltransferase in the adult rat spinal cord: an immunocytochemical study. J Comp Neurol. 1984 Nov 1;229(3):329–346. doi: 10.1002/cne.902290305. [DOI] [PubMed] [Google Scholar]
- Bordey A., Feltz P., Trouslard J. Patch-clamp characterization of nicotinic receptors in a subpopulation of lamina X neurones in rat spinal cord slices. J Physiol. 1996 Feb 1;490(Pt 3):673–678. doi: 10.1113/jphysiol.1996.sp021176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borges L. F., Iversen S. D. Topography of choline acetyltransferase immunoreactive neurons and fibers in the rat spinal cord. Brain Res. 1986 Jan 1;362(1):140–148. doi: 10.1016/0006-8993(86)91407-1. [DOI] [PubMed] [Google Scholar]
- Brown D. A., Docherty R. J., Halliwell J. V. The action of cholinomimetic substances on impulse conduction in the habenulointerpeduncular pathway of the rat in vitro. J Physiol. 1984 Aug;353:101–109. doi: 10.1113/jphysiol.1984.sp015325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brussaard A. B., Yang X., Doyle J. P., Huck S., Role L. W. Developmental regulation of multiple nicotinic AChR channel subtypes in embryonic chick habenula neurons: contributions of both the alpha 2 and alpha 4 subunit genes. Pflugers Arch. 1994 Nov;429(1):27–43. doi: 10.1007/BF02584027. [DOI] [PubMed] [Google Scholar]
- Conroy W. G., Berg D. K. Neurons can maintain multiple classes of nicotinic acetylcholine receptors distinguished by different subunit compositions. J Biol Chem. 1995 Mar 3;270(9):4424–4431. doi: 10.1074/jbc.270.9.4424. [DOI] [PubMed] [Google Scholar]
- Coote J. H., Macleod V. H., Fleetwood-Walker S., Gilbey M. P. The response of individual sympathetic preganglionic neurones to microelectrophoretically applied endogenous monoamines. Brain Res. 1981 Jun 29;215(1-2):135–145. doi: 10.1016/0006-8993(81)90497-2. [DOI] [PubMed] [Google Scholar]
- Coote J. H. The organisation of cardiovascular neurons in the spinal cord. Rev Physiol Biochem Pharmacol. 1988;110:147–285. doi: 10.1007/BFb0027531. [DOI] [PubMed] [Google Scholar]
- Dineley-Miller K., Patrick J. Gene transcripts for the nicotinic acetylcholine receptor subunit, beta4, are distributed in multiple areas of the rat central nervous system. Brain Res Mol Brain Res. 1992 Dec;16(3-4):339–344. doi: 10.1016/0169-328x(92)90244-6. [DOI] [PubMed] [Google Scholar]
- ECCLES J. C., FATT P., KOKETSU K. Cholinergic and inhibitory synapses in a pathway from motor-axon collaterals to motoneurones. J Physiol. 1954 Dec 10;126(3):524–562. doi: 10.1113/jphysiol.1954.sp005226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmonds B., Gibb A. J., Colquhoun D. Mechanisms of activation of glutamate receptors and the time course of excitatory synaptic currents. Annu Rev Physiol. 1995;57:495–519. doi: 10.1146/annurev.ph.57.030195.002431. [DOI] [PubMed] [Google Scholar]
- Grace A. A., Llinás R. Morphological artifacts induced in intracellularly stained neurons by dehydration: circumvention using rapid dimethyl sulfoxide clearing. Neuroscience. 1985 Oct;16(2):461–475. doi: 10.1016/0306-4522(85)90018-1. [DOI] [PubMed] [Google Scholar]
- Inokuchi H., Masuko S., Chiba T., Yoshimura M., Polosa C., Nishi S. Membrane properties and dendritic arborization of the intermediolateral nucleus neurons in the guinea-pig thoracic spinal cord in vitro. J Auton Nerv Syst. 1993 May;43(2):97–106. doi: 10.1016/0165-1838(93)90346-v. [DOI] [PubMed] [Google Scholar]
- Khan I. M., Taylor P., Yaksh T. L. Cardiovascular and behavioral responses to nicotinic agents administered intrathecally. J Pharmacol Exp Ther. 1994 Jul;270(1):150–158. [PubMed] [Google Scholar]
- Khan I. M., Yaksh T. L., Taylor P. Ligand specificity of nicotinic acetylcholine receptors in rat spinal cord: studies with nicotine and cytisine. J Pharmacol Exp Ther. 1994 Jul;270(1):159–166. [PubMed] [Google Scholar]
- Krupp J., Feltz P. Excitatory postsynaptic currents and glutamate receptors in neonatal rat sympathetic preganglionic neurons in vitro. J Neurophysiol. 1995 Apr;73(4):1503–1512. doi: 10.1152/jn.1995.73.4.1503. [DOI] [PubMed] [Google Scholar]
- Krupp J., Feltz P. Synaptic- and agonist-induced chloride currents in neonatal rat sympathetic preganglionic neurones in vitro. J Physiol. 1993 Nov;471:729–748. doi: 10.1113/jphysiol.1993.sp019925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llano I., Marty A., Armstrong C. M., Konnerth A. Synaptic- and agonist-induced excitatory currents of Purkinje cells in rat cerebellar slices. J Physiol. 1991 Mar;434:183–213. doi: 10.1113/jphysiol.1991.sp018465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Léna C., Changeux J. P., Mulle C. Evidence for "preterminal" nicotinic receptors on GABAergic axons in the rat interpeduncular nucleus. J Neurosci. 1993 Jun;13(6):2680–2688. doi: 10.1523/JNEUROSCI.13-06-02680.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGehee D. S., Role L. W. Physiological diversity of nicotinic acetylcholine receptors expressed by vertebrate neurons. Annu Rev Physiol. 1995;57:521–546. doi: 10.1146/annurev.ph.57.030195.002513. [DOI] [PubMed] [Google Scholar]
- McMahon L. L., Yoon K. W., Chiappinelli V. A. Nicotinic receptor activation facilitates GABAergic neurotransmission in the avian lateral spiriform nucleus. Neuroscience. 1994 Apr;59(3):689–698. doi: 10.1016/0306-4522(94)90187-2. [DOI] [PubMed] [Google Scholar]
- Miura M., Takayama K., Okada J. Distribution of glutamate- and GABA-immunoreactive neurons projecting to the cardioacceleratory center of the intermediolateral nucleus of the thoracic cord of SHR and WKY rats: a double-labeling study. Brain Res. 1994 Feb 28;638(1-2):139–150. doi: 10.1016/0006-8993(94)90643-2. [DOI] [PubMed] [Google Scholar]
- Mtui E. P., Anwar M., Gomez R., Reis D. J., Ruggiero D. A. Projections from the nucleus tractus solitarii to the spinal cord. J Comp Neurol. 1993 Nov 8;337(2):231–252. doi: 10.1002/cne.903370205. [DOI] [PubMed] [Google Scholar]
- Myslinski N. R., Randić M. Responses of identified spinal neurones to acetylcholine applied by micro-electrophoresis. J Physiol. 1977 Jul;269(1):195–219. doi: 10.1113/jphysiol.1977.sp011899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahin R. L., Madsen A. M., Giesler G. J., Jr Anatomical and physiological studies of the gray matter surrounding the spinal cord central canal. J Comp Neurol. 1983 Nov 1;220(3):321–335. doi: 10.1002/cne.902200306. [DOI] [PubMed] [Google Scholar]
- Perrins R., Roberts A. Nicotinic and muscarinic ACh receptors in rhythmically active spinal neurones in the Xenopus laevis embryo. J Physiol. 1994 Jul 15;478(Pt 2):221–228. doi: 10.1113/jphysiol.1994.sp020244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche K. W., Tingley W. G., Huganir R. L. Glutamate receptor phosphorylation and synaptic plasticity. Curr Opin Neurobiol. 1994 Jun;4(3):383–388. doi: 10.1016/0959-4388(94)90100-7. [DOI] [PubMed] [Google Scholar]
- Ross C. A., Ruggiero D. A., Joh T. H., Park D. H., Reis D. J. Rostral ventrolateral medulla: selective projections to the thoracic autonomic cell column from the region containing C1 adrenaline neurons. J Comp Neurol. 1984 Sep 10;228(2):168–185. doi: 10.1002/cne.902280204. [DOI] [PubMed] [Google Scholar]
- Sherriff F. E., Henderson Z. A cholinergic propriospinal innervation of the rat spinal cord. Brain Res. 1994 Jan 14;634(1):150–154. doi: 10.1016/0006-8993(94)90268-2. [DOI] [PubMed] [Google Scholar]
- Silver R. A., Traynelis S. F., Cull-Candy S. G. Rapid-time-course miniature and evoked excitatory currents at cerebellar synapses in situ. Nature. 1992 Jan 9;355(6356):163–166. doi: 10.1038/355163a0. [DOI] [PubMed] [Google Scholar]
- Strack A. M., Sawyer W. B., Marubio L. M., Loewy A. D. Spinal origin of sympathetic preganglionic neurons in the rat. Brain Res. 1988 Jul 5;455(1):187–191. doi: 10.1016/0006-8993(88)90132-1. [DOI] [PubMed] [Google Scholar]
- Séguéla P., Wadiche J., Dineley-Miller K., Dani J. A., Patrick J. W. Molecular cloning, functional properties, and distribution of rat brain alpha 7: a nicotinic cation channel highly permeable to calcium. J Neurosci. 1993 Feb;13(2):596–604. doi: 10.1523/JNEUROSCI.13-02-00596.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tölle T. R., Berthele A., Zieglgänsberger W., Seeburg P. H., Wisden W. The differential expression of 16 NMDA and non-NMDA receptor subunits in the rat spinal cord and in periaqueductal gray. J Neurosci. 1993 Dec;13(12):5009–5028. doi: 10.1523/JNEUROSCI.13-12-05009.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban L., Willetts J., Murase K., Randić M. Cholinergic effects on spinal dorsal horn neurons in vitro: an intracellular study. Brain Res. 1989 Oct 23;500(1-2):12–20. doi: 10.1016/0006-8993(89)90294-1. [DOI] [PubMed] [Google Scholar]
- Vidal C., Changeux J. P. Nicotinic and muscarinic modulations of excitatory synaptic transmission in the rat prefrontal cortex in vitro. Neuroscience. 1993 Sep;56(1):23–32. doi: 10.1016/0306-4522(93)90558-w. [DOI] [PubMed] [Google Scholar]
- Wada E., Wada K., Boulter J., Deneris E., Heinemann S., Patrick J., Swanson L. W. Distribution of alpha 2, alpha 3, alpha 4, and beta 2 neuronal nicotinic receptor subunit mRNAs in the central nervous system: a hybridization histochemical study in the rat. J Comp Neurol. 1989 Jun 8;284(2):314–335. doi: 10.1002/cne.902840212. [DOI] [PubMed] [Google Scholar]
- Yoshimura M., Nishi S. Intracellular recordings from lateral horn cells fo the spinal cord in vitro. J Auton Nerv Syst. 1982 Jul;6(1):5–11. doi: 10.1016/0165-1838(82)90017-0. [DOI] [PubMed] [Google Scholar]
- Zhang Z. W., Vijayaraghavan S., Berg D. K. Neuronal acetylcholine receptors that bind alpha-bungarotoxin with high affinity function as ligand-gated ion channels. Neuron. 1994 Jan;12(1):167–177. doi: 10.1016/0896-6273(94)90161-9. [DOI] [PubMed] [Google Scholar]