Abstract
The Mini Mental State Examination (MMSE), Montreal Cognitive Assessment (MoCA), and Frontal Assessment Battery are screening tests for dementia. The go/no-go task offers an alternative approach for evaluating dementia patients. However, its role in screening for dementia remains unclear. We aimed to explore the feasibility of using the go/no-go task as a screening test for dementia via a cross-sectional design. Twenty-four Japanese individuals were evaluated using the go/no-go task, the MMSE, and the MoCA. The total MMSE and MoCA scores were correlated with the total number of errors in the go/no-go task (r=-0.699, p < 0.01; r=-0.756, p < 0.01). Moreover, When the MoCA cutoff value was 25 for MCI, the optimal cutoff score for the total number of error in the go/no-go task to detect MCI was 2, with an Area Under curve (AUC) of 0.98, a sensitivity of 0.94. When the MMSE cutoff value was 27 for MCI, the optimal cutoff score for the total number of error in the go/no-go task to detect MCI was 6, with an AUC of 0.89, a sensitivity of 0.76, showed respectively values close to 1. The go/no-go task is possible a practical screening test for dementia.
Keywords: Alzheimer’s disease, Dementia, Frontal Assessment Battery, Go/no-go task
Subject terms: Cognitive ageing, Adaptive clinical trial, Cognitive control, Human behaviour
Introduction
The prevalence of noncommunicable diseases, including dementia, is increasing in an ageing society. In 2017, there were 35.6 million people with dementia worldwide, which is expected to double by 2030 and more than triple by 20501. All individuals have the potential to develop dementia and can progress from a healthy mental state to mild cognitive impairment (MCI) and dementia2–4.
Dementia is a disease of the brain that is usually characterized by progressive global deterioration in intellect, including memory, learning, orientation, language, comprehension, and judgement. Dementia is associated with multiple underlying brain pathologies, including Alzheimer’s disease (AD), vascular dementia, dementia with Lewy bodies, and frontotemporal dementia. The boundaries between these subtypes are unclear, and mixed dementia may be the norm5.
Diabetes, high blood pressure, heart disease, current smoking status, and arteriosclerosis are possible risk factors for Alzheimer’s disease6–10.
However, fundamental treatments and preventive drugs for AD are still being developed2,11–14. MCI is an intermediate stage between a healthy mental state and dementia2,3. The annual rate of MCI progression to dementia ranges from 10–30%15. However, it has been reported that recovery from MCI is possible through improvements in cognitive abilities16–18. For example, multicomponent exercise improves cognitive function in individuals with MCI19. Therefore, since early intervention in individuals with MCI plays an important role, there is a need for a dementia screening test to distinguish among healthy individuals, individuals with MCI, and patients with dementia. The Mini-Mental State Examination (MMSE) is a screening test for dementia that examines cognitive aspects of mental function and is a scored form in which participants answer questions that assess mood and unusual mental experiences20. However, it has been reported that age and years of education affect MMSE test scores21,22, resulting in lower sensitivity for assessing MCI and dementia23–25. The Montreal Cognitive Assessment (MoCA) was developed as a screening test for dementia to improve these conditions26. The MoCA, like the MMSE, is administered in a format in which participants answer questions on paper; however, the go/no-go task can be performed in a game-like manner27. The go/no-go task is part of the Frontal Assessment Battery (FAB), a screening test for dementia28,29. However, the FAS go/no-go inhibitory control task involves randomly repeating the task five times: when the examiner claps his hands once with both hands, the participant claps once; and when the examiner claps twice, the participant claps twice. This task is inconsistent with the go/no-go task in this study. Previous studies have reported that people with Alzheimer’s disease and dementia have slower reaction times and commit more errors than healthy subjects30,31. However, it is unclear whether the go/no-go task alone can be used as a screening test for dementia. The utility of the MoCA has been investigated by comparing it with the MMSE. Therefore, we aimed to clarify the possibility that the go/no-go task can be used as a screening test for dementia by comparing the MMSE and MoCA scores with the scores of the go/no-go task.
Methods
Participants
Twenty-four Japanese people were included as participants. Eighteen participants from a specific elderly nursing home, aged 86.8 ± 5.7 years (mean, standard deviation [SD]), including two men aged 87 ± 7 years (Education history: 1, Junior high school; 1, college) and 16 women aged 86.8 ± 5.5 years (Education history: 2, Junior high school; 13, high school; 1, college), were included. To confirm the reliability of the MMES, MoCA, and go/no-go tasks, six healthy participants from the hospital, aged 32 ± 11.3 years, also participated. Three males were 24.7 ± 3.1 years old (Education history: 3, college), and three females were 39.3 ± 11.8 years old (Education history: 1, high school; 2, college) (Table 1).
Table 1.
Participant characteristics.
| Number | Age | Range | |
|---|---|---|---|
| Participants from a special elderly nursing home | 18 | 86.8 ± 5.7 | 73–96 |
| Male | 2 | 87 ± 7.0 | 80–94 |
| Female | 16 | 86.8 ± 5.5 | 73–96 |
| Participants from the hospital | 6 | 32 ± 11.3 | 22–54 |
| Male | 3 | 24.7 ± 3.1 | 22–29 |
| Female | 3 | 39.3 ± 11.8 | 25–54 |
| Total | 24 | 73.1 ± 25.4 | 22–96 |
Data of age are presented as mean ± standard deviation (SD).
All participants completed the MMSE, MoCA, and go/no-go tasks as dementia screening tests over two weeks at a nursing home in Numazu, Japan (Fig. 1).
Fig. 1.
Screening test implementation method.
Participants were excluded if, at the discretion of the attending physician at the clinic co-located with the nursing home, they were excluded if they were unable to walk indoors without assistance for at least 3 min, had neurological disorders (e.g., Parkinson’s or Huntington’s disease), had undergone orthopaedic surgery within the last two years, had a history of stroke or psychiatric disorders, or were unable to understand the instructions. Three participants had a history of suspected AD; however, none had severe MMSE scores below 10, except for one with a MoCA score below 10.
All individuals voluntarily participated in this study and were not adversely affected by nonparticipation. Even after agreeing to participate, they could withdraw from the study at any time. We ensured that all personal and medical information was anonymized.
All participants were informed about the potential experimental risks and informed consent was obtained according to the policies for human participants at Shinshu University. Written informed consent was obtained in accordance with the policies for human participants at Shinshu University and in accordance with the Declaration of Helsinki. The Ethics Committee of Shinshu University approved the study protocol (IRB-2018-209, 25/06/2018).
Go/no-go task
The go/no-go task used in this study was developed by Masaki and Moriyama27 based on Luria’s32 experiments on higher-order neural activity. The go/no-go task lasted 8 to 10 min and involved three experimental stages: (1) formation, (2) differentiation, and (3) reverse differentiation. In a standardized pre-task training session during the formation stage, a red light was presented to the participants, and the participants were instructed to grasp the rubber ball in response to that light. Then, the formation stage started, and the participants grasped a rubber ball in response to a red light. This stage comprised five trials. In a standardized pre-task training session during the differentiation stage for the participants, a red light was presented, and the participants were instructed to grasp the rubber ball in response to that light; moreover, they were told that if a yellow light was presented, they should not grasp the rubber ball. After the differentiation stage started, the participants grasped the rubber ball in response to a red light but not in response to a yellow light. In a standardized pre-task training session during the reverse differentiation stage for the participants, a yellow light was presented, and the participants were instructed to grasp the rubber ball in response; however, a red light was presented, and the participants were instructed to not grasp the rubber ball in response. Then, the reverse differentiation stage started, with the participants grasping a rubber ball in response to a yellow light but not in response to a red light. In each stage of differentiation and reverse differentiation, participants completed 20 trials. Red and yellow lights were randomly displayed ten times each. The participants were repeatedly trained until they mastered the task in standardized pre-task training sessions and attending less than five training sessions was deemed insufficient for the experiment.
The stimulus duration was 200–1100 ms, and the interstimulus interval was 1300–7500 ms. Device calibration, environmental conditions, response time measurements and ensuring reproducibility of those times on the basis of the task setting for the go/no-go task were conducted following the research methods of Masaki and Moriyama [27].
In this article, an incorrect response is referred to as a “miss” when participants did not grasp the rubber ball when they should have (rubber ball pressure must be less than 3 mmHg). An incorrect response is referred to as a “mistake” when participants grasped the rubber ball when they should not have (rubber ball pressure must be 3 mmHg or more). Furthermore, the term “error” refers to the total number of misses and mistakes.
MMSE
The MMSE is a 30-point, paper-based test with a set of questions developed by Folstein et al.20 in 1975 for the diagnosis of dementia and takes 6 to 10 min to complete. The MMSE evaluation items included the following: ① time orientation (5 points), ② place orientation (5 points), ③ immediate recall (3 points), ④ attention and numeracy (5 points), ⑤ delayed recall (3 points), ⑥ object name (2 points), ⑦ reading/repetition (1 point), ⑧ language comprehension (3 points), ⑨ sentence comprehension (1 point), ⑩ sentence structure (1 point), and ⑪ graphical ability (1 point). A score of 30 − 28 is considered normal, 27 − 24 indicates mild dementia, and 23 or less indicates dementia.
MoCA
The MoCA is a 30-point test with a set of questions developed by Nasreddine et al.26. in 2005, and the test takes approximately 10 min to complete. The MoCA evaluation items included the following: ① visuospatial/executive function (5 points), ② naming (3 points), ③ memory (0 points), ④ attention (6 points), ⑤ language (3 points), ⑥ abstract concepts (2 points), ⑦ delayed recall (5 points) and ⑧ orientation (6 points), with 1 point added if the number of years of education is 12 years or less. A score of 30 − 26 is considered normal, 25 − 18 indicates mild dementia, and 17 − 10 indicates dementia.
Statistical analysis
The correlation coefficients between the go/no-go task score and the MMSE and MoCA scores were calculated for all participants. MMSE and MoCA scores were evaluated for normality separately and assessed using the Pearson correlation coefficient or nonparametric Spearman test. Statistical significance was set at p < 0.05. In addition, the MoCA cut-off score for MCI was 2526,33 and the MMSE cut-off score was 2734,35, which was assessed using a receiver operating characteristic (ROC) curve for the cut-off score of the go/no-go task. Statistical analyses were performed using SPSS Statistical Version 26.
Results
Correlations between MMSE scores and go/no-go task scores
Table 2 summarizes the correlation between MMSE scores and go/no-go task scores. We removed MMSE items ③ immediate recall, ⑨ sentence comprehension, and ⑪ graphical ability because all the answers were correct. Some MMSE items had correlation coefficients with go/no-go task items higher than 0.4 and had significant correlations with five or more items.
Table 2.
Correlations between the MMSE and go/no-go task scores.
| MMSE | ||||
|---|---|---|---|---|
| Orientation of date | Orientation of place | Delayed recall task | Total score | |
| The go/no-go task | ||||
| Response | ||||
| Formation response time | − 0.71** | − 0.54* | − 0.67** | − 0.86** |
| Differentiation response time | − 0.34 | − 0.45* | − 0.39 | − 0.56** |
| Reverse differentiation response time | − 0.21 | − 0.14 | 0.07 | − 0.49* |
| Mean response time | − 0.57** | − 0.53* | − 0.47* | − 0.77** |
| Times | ||||
| Formation misses | − 0.39 | − 0.28 | − 0.30 | − 0.57** |
| Differentiation misses | − 0.57** | − 0.33 | − 0.48* | − 0.58** |
| Reverse differentiation misses | − 0.69** | − 0.45* | − 0.50* | − 0.73** |
| Total misses | − 0.62** | − 0.40 | − 0.50* | − 0.74** |
| Differentiation mistakes | − 0.08 | − 0.35 | − 0.008 | − 0.12 |
| Reverse differentiation mistakes | − 0.34 | − 0.38 | 0.07 | − 0.32 |
| Total mistakes | − 0.25 | − 0.44* | − 0.01 | − 0.28 |
| Total error | − 0.64** | − 0.44* | − 0.46* | − 0.70** |
*Indicates a significant correlation, *: p < 0.05, **: p < 0.01.
MMSE, Mini Mental State Examination.
The time orientation score of the MMSE was correlated with the formation response time (r=-0.71, p < 0.01), mean response time (r=-0.57, p < 0.01), differentiation misses (r=-0.57, p < 0.01), reverse differentiation misses (r=-0.69, p < 0.01), total misses (r=-0.62, p < 0.01), and total error (r=-0.64, p < 0.01) of the go/no-go task. The place orientation score was correlated with the formation response time (r=-0.54, p < 0.05), differentiation response time (r=-0.45, p < 0.05), mean response time (r=-0.53, p < 0.05), reverse differentiation misses (r=-0.45, p < 0.05), and total errors (r=-0.44, p < 0.05) of the go/no-go task. The delayed-recall score correlated with the formation response time (r=-0.67, p < 0.01), mean response time (r=-0.47, p < 0.05), differentiation misses (r=-0.48, p < 0.05), reverse differentiation misses (r=-0.50, p < 0.05), total misses (r=-0.50, p < 0.05), and total error (r=-0.46, p < 0.05) in the go/no-go task. The total MMSE score was correlated with the formation response time (r=-0.86, p < 0.01), differentiation response time (r=-0.56, p < 0.01), reverse differentiation response time (r=-0.49, p < 0.05), mean response time (r=-0.77, p < 0.01), formation misses (r=-0.57, p < 0.01), differentiation misses (r=-0.58, p < 0.01), reverse differentiation misses (r=-0.73, p < 0.01), total misses (r=-0.74, p < 0.01), and total error (r=-0.70, p < 0.01) in the go/no-go task (Table 2).
Correlations between the MoCA and go/no-go task scores
Table 3 summarizes the correlation between the MoCA and the go/no-go task scores. Some items of the MoCA had correlation coefficients greater than 0.4 with go/no-go task items and had significant correlations with five or more items.
Table 3.
Correlations between the MoCA and go/no-go task scores.
| MoCA | |||||||
|---|---|---|---|---|---|---|---|
| Trail making | Digit span | Serial 7’s | Abstraction | Delayed recall of five words | Orientation | Total score | |
| The go/no-go task | |||||||
| Response | |||||||
| Formation response time | − 0.58* | − 0.68** | − 0.76** | − 0.50* | − 0.80** | − 0.76** | − 0.73** |
| Differentiation response time | − 0.42 | − 0.47* | − 0.48* | − 0.25 | − 0.56** | − 0.54* | − 0.69** |
| Reverse differentiation response time | − 0.39 | − 0.42 | − 0.41 | − 0.05 | − 0.43 | − 0.31 | − 0.54* |
| Mean response time | − 0.63** | − 0.57** | − 0.75** | − 0.40 | − 0.72** | − 0.67** | − 0.82** |
| Times | |||||||
| Formation misses | − 0.34 | − 0.69** | − 0.33 | − 0.47* | − 0.70** | − 0.34 | − 0.64** |
| Differentiation misses | − 0.38 | − 0.44* | − 0.35 | − 0.55** | − 0.75** | − 0.32 | − 0.53** |
| Reverse differentiation misses | − 0.42* | − 0.58** | − 0.39 | − 0.37 | − 0.75** | − 0.43* | − 0.67** |
| Total misses | − 0.42* | − 0.73** | − 0.45* | − 0.47* | − 0.83** | − 0.46* | − 0.72** |
| Differentiation mistakes | − 0.29 | − 0.24 | − 0.33 | − 0.32 | − 0.27 | − 0.44* | − 0.41* |
| Reverse differentiation mistakes | − 0.29 | − 0.24 | − 0.33 | − 0.32 | − 0.27 | − 0.44* | − 0.41* |
| Total mistakes | − 0.33 | − 0.16 | − 0.19 | − 0.24 | − 0.22 | − 0.40 | − 0.34 |
| Total error | − 0.61** | − 0.66** | − 0.45* | − 0.48* | − 0.81** | − 0.45* | − 0.76** |
*Indicates a significant correlation, *: p < 0.05, **: p < 0.01.
MoCA, Montreal Cognitive Assessment.
In the MoCA, the trail making score was correlated with the formation response time (r=-0.58, p < 0.05), mean response time (r=-0.63, p < 0.01), reverse differentiation misses (r=-0.42, p < 0.05), total misses (r=-0.42, p < 0.05), and total error (r=-0.61, p < 0.01) of the go/no-go task. The digit span score was correlated with the formation response time (r=-0.68, p < 0.01), differentiation response time (r=-0.47, p < 0.05), mean response time (r=-0.57, p < 0.01), formation misses (r=-0.69, p < 0.01), differentiation misses (r=-0.44, p < 0.05), reverse differentiation misses (r=-0.58, p < 0.01), total misses (r=-0.73 p < 0.01), and total error (r=-0.66, p < 0.01) in the go/no-go task. The scores on the serial 7s task were correlated with the formation response time (r=-0.76, p < 0.01), differentiation response time (r=-0.48, p < 0.05), mean response time (r=-0.75, p < 0.01), total misses (r=-0.45, p < 0.05), and total error (r=-0.45, p < 0.05) in the go/no-go task. The abstraction score correlated with the formation response time (r=-0.50, p < 0.05), formation misses (r=-0.47, p < 0.05), differentiation misses (r=-0.55, p < 0.01), total misses (r=-0.47, p < 0.05), and total error (r=-0.48, p < 0.01) of the go/no-go task. The scores for delayed recall of five words was correlated with the formation response time (r=-0.80, p < 0.01), differentiation response time (r=-0.56, p < 0.01), mean response time (r=-0.72, p < 0.01), formation misses (r=-0.70, p < 0.01), differentiation misses (r=-0.75, p < 0.01), reverse differentiation misses (r=-0.75, p < 0.01), total misses (r=-0.83, p < 0.01), and total error (r=-0.81, p < 0.01) in the go/no-go task. The orientation score was correlated with the formation response time (r=-0.76, p < 0.01), differentiation response time (r=-0.54, p < 0.05), mean response time (r=-0.67, p < 0.01), reverse differentiation misses (r=-0.43, p < 0.05), total misses (r=-0.46, p < 0.05), differentiation mistakes (r=-0.44, p < 0.05), reverse differentiation mistakes (r=-0.44, p < 0.05), and total error (r=-0.45, p < 0.05) in the go/no-go task. The total MoCA score was correlated with the formation response time (r=-0.73, p < 0.01), differentiation response time (r=-0.69, p < 0.01), reverse differentiation response time (r=-0.54, p < 0.05), mean response time (r=-0.82, p < 0.01), formation misses (r=-0.64, p < 0.01), differentiation misses (r=-0.53, p < 0.01), reverse differentiation misses (r=-0.67, p < 0.01), total misses (r=-0.72, p < 0.01), differentiation mistakes (r=-0.41, p < 0.05), reverse differentiation mistakes (r=-0.41, p < 0.05), and total error (r=-0.76, p < 0.01) of the go/no-go task (Table 3).
Correlations between the total scores of the MoCA and MMSE and the go/no-go task
Table 4 summarizes the correlations between the total scores of the MoCA, MMSE, and go/no-go tasks. The mean response time of the go/no-go task correlated with the total misses of the go/no-go task (r = 0.56, p < 0.01), the total error of the go/no-go task (r = 0.50, p < 0.01), the MMSE total score (r=-0.77, p < 0.01), and the MoCA total score (r=-0.82, p < 0.01).
Table 4.
Correlations between the total scores of the MoCA and MMSE and go/no-go task.
| The go/no-go task | MMSE | MoCA | ||||
|---|---|---|---|---|---|---|
| Mean response time | Total misses | Total mistakes | Total error | Total score | Total score | |
| The go/no-go task | ||||||
| Response | ||||||
| Mean response time | – | |||||
| Times | ||||||
| Total misses | 0.56** | – | ||||
| Total mistakes | 0.22 | 0.05 | – | |||
| Total error | 0.50* | 0.87** | 0.43* | – | ||
| Cognitive function test | ||||||
| MMSE total score | − 0.77** | − 0.74** | − 0.27 | − 0.70** | – | |
| MoCA total score | − 0.82** | − 0.72** | − 0.34 | − 0.76** | 0.86** | – |
* Indicates a significant correlation, *: p < 0.05, **: p < 0.01.
MMSE, Mini Mental State Examination; MoCA, Montreal Cognitive Assessment.
The total number of misses in the go/no-go task was correlated with the total number of errors in the go/no-go task (r = 0.87, p < 0.01), the total MMSE score (r=-0.74, p < 0.01), and the total MoCA score (r=-0.72, p < 0.01). The total number of mistakes in the go/no-go task was correlated with the total number of errors in the go/no-go task (r = 0.43, p < 0.01). The total number of errors in the go/no-go task correlated with the MMSE (r=-0.70, p < 0.01) and the MoCA total scores (r=-0.76, p < 0.01). The total MMSE score correlated with the total MoCA score (r = 0.86, p < 0.01; Table 4).
Receiver operating characteristic (ROC) curves for the go/no-go task from the MoCA and MMSE
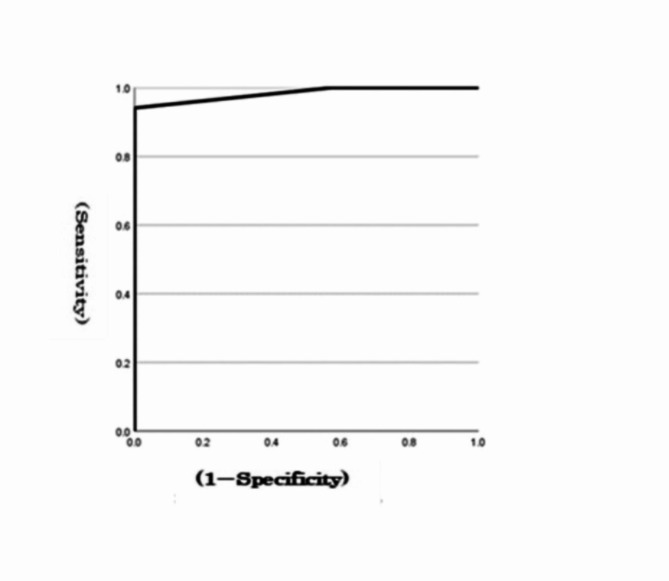
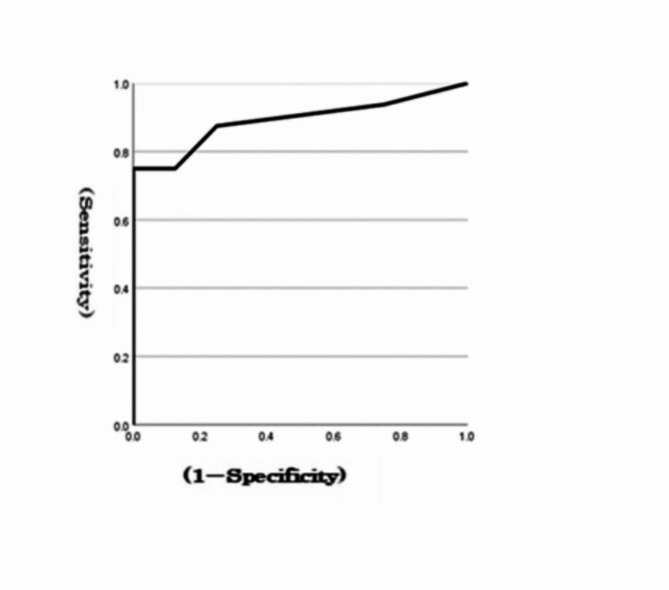
At a MoCA cut-off value of 25 for MCI, the optimal cut-off score for the total errors in the go/no-go task to detect MCI was 2, with an area under the curve (AUC) of 0.98 and a sensitivity of 0.94 (Fig. 2). When the MMSE cut-off value was 27 for MCI, the optimal cut-off score for the total errors in the go/no-go task to detect MCI was 6, with an AUC of 0.89 and a sensitivity of 0.75 (Fig. 3). The AUC and sensitivity values of the go/no-go task for the MoCA (0.98, 0.94) and MMSE (0.89, 0.75) were both close to 1.
Fig. 2.
ROC curves for a go/no-go task from the MoCA.
Fig. 3.
ROC curves for a go/no-go task from the MMSE.
The results revealed that 9 out of the 12 items in the go/no-go task were significantly correlated, with a correlation coefficient of 0.4 or higher for the total MMSE score and 11 items for the total MoCA score. In addition, the total error in the go/no-go task was significantly correlated with the MMSE total score (correlation coefficient of 0.70) and the MoCA total score (correlation coefficient of 0.76). The AUC and sensitivity values of the MoCA (0.98, 0.94) and MMSE (0.89, 0.75) go/no-go tasks based on the ROC curves were both close to 1.
Discussion
This study aimed to investigate the possibility of using the go/no-go task as a screening test for dementia by assessing the associations among MMSE scores, MoCA scores and go/no-go task scores. The total MMSE and MoCA scores were strongly correlated with the mean reaction time in the go/no-go task.
Murata et al.36. reported a rapid reaction time and a greater number of mistakes in the first stage of the go/no-go task. Then, the reaction time increased and the number of mistakes decreased substantially with improved frontal cortex function. However, this improvement considerably decreased the reaction time and number of mistakes in another study36. These findings showed that the improved performance in the go/no-go tasks could manifest as good results with a slow reaction time or good results with a fast reaction time, suggesting that dementia cannot be detected based only on the reaction time in go/no-go tasks. We intend to determine the correlation between rapid and slow reaction times in the go/no-go task and the total scores of the MMSE and MoCA in the future.
Delayed recall and the repetition and writing of sentences on the MMSE are significantly correlated with the go/no-go task31. We identified a significant correlation between the go/no-go task score and MMSE score.
Previous studies targeting older adults have reported significant associations between low total MoCA scores and decreased total brain volume, frontal lobe volume, total hippocampal volume, and atrophy of the third ventricle37,38. In addition, low total MMSE scores are significantly associated with hippocampal atrophy and white matter hyperintensities39,40. Similarly, hippocampal atrophy has been correlated with decreased MMSE and MoCA scores.
Compared to younger adults, older adults exhibit decreased cortical activity in the middle temporal gyrus (MTG) and superior temporal gyrus, as well as delayed activation in the MTG, prefrontal cortex, and presupplementary motor area, during evaluation via the go/no-go task using magnetoencephalography recordings41–43. Furthermore, lower go/no-go task scores are supposedly related to hippocampal atrophy44. However, few studies on patients with dementia have performed go/no-go tasks, and the go/no-go task alone has not been used to screen for dementia.
In the future, we will assess whether the anatomical evaluation of the MMSE and MoCA scores matches that of a go/no-go task in patients with dementia. The advantages of the go/no-go task include an examination time of approximately 10 min and the ability to assess a maximum of 50 participants simultaneously. Cognitive assessment tests, such as the MoCA and MMSE, were administered to participants by a health care professional or trained tester. Participants were required to be tested passively. Therefore, participants may experience a sense of mission and responsibility, both for their own benefit and for the well-being of others, motivating them to undergo testing. In contrast, go/no-go tasks can be computer-based and performed by the participant alone, without requiring an examiner. Go/no-go tasks reduce the burden on the participants and allow the evaluation of cognitive functions in a game-like manner.
In the future, we would like to clarify the usefulness of the go/no-go task, which can be widely used for people with disabilities and patients with AD who have slight deterioration of the frontal lobe45.
Conclusion.
With the ageing of society, the number of patients with dementia is increasing. The MMSE, MoCA, and go/no-go tasks are used to detect dementia. However, it is unclear whether the go/no-go task alone can be used as a screening test for dementia. The purpose of this study was to clarify the feasibility of using the go/no-go task as a screening test for dementia by comparing the MMSE and MoCA scores with the scores of the go/no-go task. The results revealed that 9 out of the 12 items in the go/no-go task were significantly correlated, with a correlation coefficient of 0.4 or higher for the MMSE total score and 11 items in the MoCA total score. In addition, the total error in the go/no-go task was significantly correlated with the MMSE total score (correlation coefficient of 0.70) and the MoCA total score (correlation coefficient of 0.76). The AUC and sensitivity values of the MoCA (0.98, 0.94) and MMSE (0.89, 0.75) go/no-go tasks based on the ROC curves were both close to 1. Our results revealed that the go/no-go task could be a useful screening test for dementia. Further studies using larger samples are warranted to test its feasibility and true performance.
Limitations.
The generalizability of the results is limited owing to the small and homogeneous number of participants. In particular, the disproportionate proportions of older and younger people and the effects of age and sex and their interactions may have led to bias. Therefore, it is difficult to generalize these results scientifically. It is necessary to increase the number of participants and equalize the age and male/female ratio to increase the statistical reliability and usefulness of this study. In the future, we must clarify the usefulness of the go/no-go task, which can be widely used for people with disabilities and patients with AD who have slight deterioration of the frontal lobe.
The go/no-go task does not involve a passive examination requiring participants to respond to questions such as the MMSE and MoCA. The task consisted of a test in which participants actively responded to the stimulus of the task. According to the study methodology, we could not assess these relationships in participants who were unable to complete fewer than five training sessions. A disadvantage of the go/no-go task was its inability to assess dementia grades in cases where the participants did not understand the task rules. However, there were no such participants in this study, as this situation was only possible for the participants who understood the rules. Continued research on these go/no-go tasks and a detailed evaluation of participants’ misses and mistakes, as well as the time between stimuli and reactions may be necessary to understand the participants’ level of recognition. However, we must consider introducing different difficulty levels to the go/no-go task by adjusting stimulus timing or complexity. This could allow for a more nuanced understanding of the effects of dementia severity on cognitive function and provide deeper insights into the relationship between task performance and cognitive decline. Furthermore, we must consider incorporating a brief post-task questionnaire to assess the participants’ comprehension.
Acknowledgements
The authors would like to thank the participants and the staff of the Yuaikai Medical Corporation.
Author contributions
N. T. K. and M. planned the experiment and collected the data. N. T. M. K. H. F. and S. performed the data analysis. N. T. M. and K. composed the article. All authors read and approved the final manuscript. All authors have approved the study and manuscript, warrant that it is factual, have agreed to its submission, and have the right to publish.
Data availability
The data that support the findings of this study are not openly available due to reasons of sensitivity and are available from the corresponding author upon reasonable request. Data are located in controlled access data storage at Shinshu university.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.World Health Organization. Global Action Plan on the Public Health Response to Dementia 2017–2025 (World Health Organization, 2017).
- 2.Kim, A. Y., Jerdi, A., MacDonald, S., Triggle, C. R. & R. & Alzheimer’s disease and its treatment-yesterday, today, and tomorrow. Front. Pharmacol.15, 1399121 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Petersen, R. C. et al. Current concepts in mild cognitive impairment. Arch. Neurol.58, 1985–1992 (2001). [DOI] [PubMed] [Google Scholar]
- 4.Trevisan, K., Cristina-Pereira, R. & Silva-Amaral, D. & Aversi-Ferreira, T. A. Theories of aging and the prevalence of Alzheimer’s disease. BioMed Res. Int.2019, 9171424 (2019). [DOI] [PMC free article] [PubMed]
- 5.Alzheimer’s Disease International. World Alzheimer Report 2009: the Global Prevalence of Dementia. https://www.alzint.org/resource/world-alzheimer-report-2009/ (2009).
- 6.Arvanitakis, Z., Capuano, A. W., Leurgans, S. E., Bennett, D. A. & Schneider, J. A. Relation of cerebral vessel disease to Alzheimer’s disease dementia and cognitive function in elderly people: a cross-sectional study. Lancet Neurol.15, 934–943 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hofman, A. et al. Atherosclerosis, apolipoprotein E, and prevalence of dementia and Alzheimer’s disease in the Rotterdam study. Lancet349, 151–154 (1997). [DOI] [PubMed] [Google Scholar]
- 8.Luchsinger, J. A. et al. Aggregation of vascular risk factors and risk of incident Alzheimer disease. Neurology65, 545–551 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Skoog, I. et al. 15-year longitudinal study of blood pressure and dementia. Lancet347, 1141–1145 (1996). [DOI] [PubMed] [Google Scholar]
- 10.Austin, T. R. et al. Association of brain volumes and white matter injury with race, ethnicity, and cardiovascular risk factors: the multi-ethnic study of atherosclerosis. J. Am. Heart Assoc.11, e023159 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Birks, J. S. & Harvey, R. J. Donepezil for dementia due to Alzheimer’s disease. Cochrane Database Syst. Rev.6, CD001190 (2018). [DOI] [PubMed] [Google Scholar]
- 12.Casserly, I. & Topol, E. J. Convergence of atherosclerosis and Alzheimer’s disease: inflammation, cholesterol, and misfolded proteins. Lancet363, 1139–1146 (2004). [DOI] [PubMed] [Google Scholar]
- 13.McGuinness, B., Craig, D., Bullock, R. & Passmore, P. Statins for the prevention of dementia. Cochrane Database Syst. Rev.2016, CD003160 (2016). [DOI] [PMC free article] [PubMed]
- 14.Batko, J. et al. Chaperones-a new class of potential therapeutic targets in Alzheimer’s disease. Int. J. Mol. Sci.25, 3401 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bruscoli, M. & Lovestone, S. Is MCI really just early dementia? A systematic review of conversion studies. Int. Psychogeriatr.16, 129–140 (2004). [DOI] [PubMed] [Google Scholar]
- 16.Ishikawa, T. & Ikeda, M. Mild cognitive impairment in a population-based epidemiological study. Psychogeriatrics7, 104–108 (2007). [Google Scholar]
- 17.Shimada, H., Doi, T., Lee, S. & Makizako, H. Reversible predictors of reversion from mild cognitive impairment to normal cognition: a 4-year longitudinal study. Alzheimers Res. Ther.11, 24–24 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De la Rosa, A. et al. Physical exercise in the prevention and treatment of Alzheimer’s disease. J. Sport Health Sci.9, 394–404 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suzuki, T. et al. A randomized controlled trial of multicomponent exercise in older adults with mild cognitive impairment. PLOS ONE. 8, e61483 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Folstein, M. F., Folstein, S. E. & McHugh, P. R. Mini-mental state. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr Res.12, 189–198 (1975). [DOI] [PubMed] [Google Scholar]
- 21.Fratiglioni, L. et al. Predicting dementia from the Mini-mental State examination in an elderly population: the role of education. J. Clin. Epidemiol.46, 281–287 (1993). [DOI] [PubMed] [Google Scholar]
- 22.Scazufca, M., Almeida, O. P., Vallada, H. P., Tasse, W. A. & Menezes, P. R. Limitations of the Mini-mental State Examination for screening dementia in a community with low socioeconomic status: results from the Sao Paulo ageing & health study. Eur. Arch. Psychiatry Clin. Neurosci.259, 8–15 (2009). [DOI] [PubMed] [Google Scholar]
- 23.Holsinger, T., Deveau, J., Boustani, M. & Williams, J. W. Does this patient have dementia? JAMA297, 2391–2404 (2007). [DOI] [PubMed] [Google Scholar]
- 24.Ihl, R., Frölich, L., Dierks, T., Martin, E. M. & Maurer, K. Differential validity of psychometric tests in dementia of the Alzheimer type. Psychiatry Res.44, 93–106 (1992). [DOI] [PubMed] [Google Scholar]
- 25.Wind, A. W. et al. Limitations of the mini-mental state examination in diagnosing dementia in general practice. Int. J. Geriatr. Psychiatry. 12, 101–108 (1997). [DOI] [PubMed] [Google Scholar]
- 26.Nasreddine, Z. S. et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc.53, 695–699 (2005). [DOI] [PubMed] [Google Scholar]
- 27.Masaki, T. & Moriyama, G. Study on types of human higher nervous activity. J. Tokyo Sci. Univ.4, 69–81 (1971). [Google Scholar]
- 28.Dubois, B., Slachevsky, A., Litvan, I. & Pillon, B. The FAB: a frontal assessment battery at bedside. Neurology55, 1621–1626 (2000). [DOI] [PubMed] [Google Scholar]
- 29.Kugo, A. et al. Japanese version of the frontal assessment battery for dementia. Psychiatry Res. Japanese Version. 153, 69–75 (2007). [DOI] [PubMed] [Google Scholar]
- 30.Collette, F., van der Linden, M., Delrue, G. & Salmon, E. Frontal hypometabolism does not explain inhibitory dysfunction in Alzheimer disease. Alzheimer Dis. Assoc. Disord. 16, 228–238 (2002). [DOI] [PubMed] [Google Scholar]
- 31.Terasawa, K., Misaki, S. & Murata, Y. Relevance between Alzheimer’s disease patients and normal subjects using the go/no-go task and Alzheimer assessment scores. J. Child. Adolesc. Behav.2, 162 (2014). [Google Scholar]
- 32.Luria, R. Higher Cortical Functions in Man (Consultants Bureau, 1966).
- 33.Fujiwara, Y. et al. Brief screening tool for mild cognitive impairment in older Japanese: validation of the Japanese version of the Montreal Cognitive Assessment. Geriatr. Gerontol. Int.10, 225–232 (2010). [DOI] [PubMed] [Google Scholar]
- 34.Luis, C. A., Keegan, A. P. & Mullan, M. Cross validation of the Montreal Cognitive Assessment in community dwelling older adults residing in the Southeastern US. Int. J. Geriatr. Psychiatry. 24, 197–201 (2009). [DOI] [PubMed] [Google Scholar]
- 35.Saxton, J. et al. Computer assessment of mild cognitive impairment. Postgrad. Med.121, 177–185 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murata, Y. et al. Effect of a two-year health program on brain function, physical fitness and blood chemistry. J. Community Med. Health Educ.5, 349 (2015). [Google Scholar]
- 37.Ashrafi, F. et al. Correlation of MRI findings and cognitive function in multiple sclerosis patients using montreal cognitive assessment test. Med. J. Islam Repub. Iran.30, 357 (2016). [PMC free article] [PubMed] [Google Scholar]
- 38.Paul, R. et al. Neuroimaging signatures and cognitive correlates of the montreal cognitive assessment screen in a nonclinical elderly sample. Arch. Clin. Neuropsychol.26, 454–460 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sakurai, R. et al. MMSE cutoff discriminates hippocampal atrophy: neural evidence for the cutoff of 24 points. J. Am. Geriatr. Soc.69, 839–841 (2021). [DOI] [PubMed] [Google Scholar]
- 40.van der Flier, W. M. et al. Small vessel disease and general cognitive function in nondisabled elderly: the LADIS study. Stroke36, 2116–2120 (2005). [DOI] [PubMed] [Google Scholar]
- 41.Casey, B. J. et al. A developmental functional MRI study of prefrontal activation during performance of a Go-No-Go task. J. Cogn. Neurosci.9, 835–847 (1997). [DOI] [PubMed] [Google Scholar]
- 42.Humberstone, M. et al. Functional magnetic resonance imaging of single motor events reveals human presupplementary motor area. Ann. Neurol.42, 632–637 (1997). [DOI] [PubMed] [Google Scholar]
- 43.Liston, C. et al. Frontostriatal microstructure modulates efficient recruitment of cognitive control. Cereb. Cortex. 16, 553–560 (2006). [DOI] [PubMed] [Google Scholar]
- 44.Nagata, T. et al. Association between executive dysfunction and hippocampal volume in Alzheimer’s disease. Int. Psychogeriatr.23, 764–771 (2011). [DOI] [PubMed] [Google Scholar]
- 45.Ball, S. L. et al. Personality and behaviour changes mark the early stages of Alzheimer’s disease in adults with Down’s syndrome: findings from a prospective population-based study. Int. J. Geriatr. Psychiatry. 21, 661–673 (2006). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are not openly available due to reasons of sensitivity and are available from the corresponding author upon reasonable request. Data are located in controlled access data storage at Shinshu university.