Abstract
Human immunodeficiency virus (HIV)-specific T-cell responses are thought to play a key role in viral load decline during primary infection and in determining the subsequent viral load set point. The requirements for this effect are unknown, partly because comprehensive analysis of total HIV-specific CD4+ and CD8+ T-cell responses to all HIV-encoded epitopes has not been accomplished. To assess these responses, we used cytokine flow cytometry and overlapping peptide pools encompassing all products of the HIV-1 genome to study total HIV-specific T-cell responses in 23 highly active antiretroviral therapy naïve HIV-infected patients. HIV-specific CD8+ T-cell responses were detectable in all patients, ranging between 1.6 and 18.4% of total CD8+ T cells. HIV-specific CD4+ T-cell responses were present in 21 of 23 patients, although the responses were lower (0.2 to 2.94%). Contrary to previous reports, a positive correlation was identified between the plasma viral load and the total HIV-, Env-, and Nef-specific CD8+ T-cell frequency. No correlation was found either between viral load and total or Gag-specific CD4+ T-cell response or between the frequency of HIV-specific CD4+ and CD8+ T cells. These results suggest that overall frequencies of HIV-specific T cells are not the sole determinant of immune-mediated protection in HIV-infection.
Although infection by human immunodeficiency virus (HIV) and its simian counterpart, simian immunodeficiency virus (SIV), is persistent and ultimately progressive in the vast majority of untreated hosts, there is increasing evidence that HIV- or SIV-specific cellular immune responses play a major role in determining the tempo of viral replication and thus the clinical outcome of infection. The best evidence for this protective immune function derives from the rhesus macaque model of SIV infection, where it has been shown that (i) interference with CD8+ T-cell function with a depleting monoclonal antibody significantly enhances viral replication (19, 27, 41) and (ii) CD8+ cytotoxic T lymphocytes (CTL) are capable of exerting significant selective pressure on the viral genome, as evidenced by the rapid appearance of specific escape mutations in epitope-encoding sequences (1, 12). Moreover, recent studies have demonstrated that vaccine strategies capable of eliciting viral specific CD8+ T-cell responses can control viral replication and prevent the onset of disease (3, 5, 42).
In humans, evidence supporting a protective effect of cellular immune responses in HIV infection is less direct. The initial appearance of HIV-specific CD8+ T cells is closely associated with the drop in plasma viremia that occurs during acute infection (26), and the loss of HIV-specific CD8+ T cells has been linked to rapid progression to AIDS (23). HIV-specific CD8+ T cells elicit potent selective pressure on the virus, in many cases resulting in the appearance of epitope escape mutations (8, 18, 38). Long-term nonprogressive infection has been associated with both strong virus-specific CTL and with robust gag p24-specific CD4+ T-cell proliferative responses (23, 35, 37, 40). Indeed, some studies have reported a direct inverse correlation between viral load and HIV-specific T-cell responses in untreated HIV-infected subjects. Specifically, using two different HIV peptide-major histocompatibility complex (MHC) class I tetramers, Ogg et al. demonstrated such an association between viral load and HIV-specific CD8+ T-cell immunity (33). Additionally, a similar inverse relationship was observed between HIV Gag-specific CD4+ T-cell proliferative responses and viral load (40). However, subsequent studies analyzing cytokine production by HIV-specific T cells in response to a larger, but still limited, array of potential epitopes have not been able to confirm these relationships (14, 15, 37).
To date, studies correlating the HIV-specific immune response and parameters of viral infection have been restricted to the T-cell responses against single epitopes (33), panels of selected epitopes (6, 14), or selected HIV proteins (15). This approach implies that such selected responses give an accurate representation of the total HIV-specific immune response. We have recently provided evidence that the correlation of particular restricting HLA alleles and epitope immunodominance is not absolute (6), a finding that contradicts the assumption that such selected responses are representative of the total response. Thus, an appreciation of the overall immune response to HIV and its relationship to parameters of viral infection would best be truly achieved by examining the response to every potential HIV epitope in the absence of assumptions of immunodominance.
We have therefore adopted the strategy of using overlapping peptide panels that span every encoded HIV protein to examine the total T-cell response to all potential epitopes in a group of 23 untreated HIV-infected patients. Our results show that high levels of HIV-specific CD4+ and CD8+ T cells are present in nearly all chronically HIV-infected patients. We find no significant correlation between the frequency of HIV-specific CD4+ T cells and HIV-specific CD8+ T cells, or between HIV-specific CD4+ T-cell frequency and HIV plasma viral load. Interestingly, however, our data indicate that the total frequency of HIV-specific CD8+ T cells is positively correlated with viral load in the absence of therapy. These results suggest that the high frequency of HIV-specific CD8+ T cells is in fact the result of high antigen load and that the absolute magnitude of either the CD8+ or CD4+ T-cell response to HIV is not by itself an adequate predictor of the ability of the immune system to control HIV infection.
MATERIALS AND METHODS
Patient cohort.
Twenty-three HIV-1 infected patients were recruited into this study at the Amelia Court HIV Clinic at the University of Texas Southwestern Medical Center. This cohort consists of both men and women and various ethnic groups, including Caucasian, Hispanic, and African American subjects. These patients were recruited on the basis of having no previous history of antiretroviral therapy, although after study samples were obtained, many of the patients began highly active antiretroviral therapy (HAART). As detailed in Table 1, this cohort included patients who were recently infected, chronically infected, or long-term nonprogressors. The viral loads in these patients varied from undetectable (<50 viral RNA copies/ml) to 635,000 viral RNA copies/ml. Viral loads were determined using either the Roche Amplicor Monitor assay or the Roche Ultradirect assay. The patients all gave informed consent prior to entry into this study.
TABLE 1.
Patient cohort characteristics at time of analysis
| Patient | Classificationa | CD4 count | Viral loadb |
|---|---|---|---|
| 1 | Chronic HIV | 345 | 634,854 |
| 2 | Chronic HIV | 254 | 14,428 |
| 3 | Chronic HIV | 471 | 92,497 |
| 4 | RS | 430 | 85,935 |
| 5 | Chronic HIV | 345 | 131,518 |
| 6 | Chronic HIV | 414 | 71,889 |
| 7 | RS | 534 | 535,913 |
| 8 | RS | 478 | 102,347 |
| 9 | Chronic HIV | 396 | 72,005 |
| 10 | Chronic HIV | 1,158 | 676 |
| 11 | Chronic HIV | 501 | 14,596 |
| 12 | Chronic HIV | 702 | 2,098 |
| 13 | Chronic HIV | 865 | 1,669 |
| 14 | Chronic HIV | 794 | 4,814 |
| 15 | Chronic HIV | 644 | 7,107 |
| 16 | Chronic HIV | 561 | 13,282 |
| 17 | Chronic HIV | 798 | 2,870 |
| 18 | RS | 434 | 1,598 |
| 19 | Chronic HIV | 983 | 3,244 |
| 20 | Chronic HIV | 506 | 399 |
| 21 | AIDS | 181 | 71,712 |
| 22 | LTNP | 862 | 49 |
| 23 | Chronic HIV | 594 | 5,160 |
RS, recent seroconverter (<1 year); LTNP, long-term nonprogressor.
Plasma viral loads of <400 are shown as 399; viral loads of <50 are shown as 49. Values are the number of RNA copies per milliliter.
Peptides.
Three different sets of HIV peptides were utilized in these experiments: (i) optimally defined epitopes from 8 to 11 amino acids in length derived from HIV Gag, Pol, Env, and Nef, as described in the Los Alamos Molecular Immunology Database (24); (ii) 15-mer peptides overlapping by 11 amino acids corresponding to sequences of chimeric HIV strain HXBc2/Bal R5 (Gag, Pol, Env, or Nef) or HIV strain SF2 (Tat, Rev, Vif, Vpr, and Vpu); and (iii) 20-mer peptides overlapping by 10 amino acids corresponding to strain HXB2 Gag and strain MN Env proteins (obtained through the NIH AIDS Research and Reference Reagent Program). The peptides were synthesized as free acids and were more than 80% pure. Lyophilized peptides were resuspended in dimethyl sulfoxide (DMSO; Sigma, St. Louis, Mo.) at 100 mg/ml for peptide mixtures. The addition of small amounts of DMSO (0.5 to 1% final concentration) does not affect antigen-specific CD4+ or CD8+ T-cell responses (data not shown). Four different pools of the optimally defined 8 to 11 amino acid peptides were made corresponding to peptide origin (37 Gag, 18 Pol, 20 Env, and 20 Nef peptides). The overlapping 15-mers were also grouped together in pools corresponding to antigen, and for HIV Pol and Env, the peptides were grouped into two separate pools (122 Gag, 248 Pol, 211 Env, 49 Nef, 27 Rev, 23 Tat, 46 Vif, 22 Vpr, and 7 Vpu peptides; total, 746). The overlapping 20-mer peptides were grouped into a Gag pool (49 peptides) and an Env pool (80 peptides). The concentration of each single peptide within a pool was 400 μg/ml. The final concentration of any single peptide was 2 μg/106 cells in all experiments.
Cell stimulation.
Peripheral blood mononuclear cells (PBMC) were isolated using Ficoll-Hypaque (Pharmacia, Uppsala, Sweden) density centrifugation. In some instances PBMC were frozen (90% fetal calf serum–10% DMSO) at −140°C until use. Stimulation was performed with fresh or frozen PBMC as described elsewhere (6, 37). Briefly, 106 PBMC were incubated with 1 μg each of costimulatory antibodies against CD28 and CD49d and 5 μl of each peptide mixture (final concentration, 2 μg/ml/peptide). In every experiment a negative control (anti-CD28-CD49d) was included to control for spontaneous production of gamma interferon (IFN-γ), as well as a positive control (Staphylococcus enterotoxin B; final concentration, 1 μg/ml) to ensure that the cells were responsive. The cultures were incubated for 1 h at 37°C in a 5% CO2 incubator, followed by an additional 5 h in the presence of the secretion inhibitor Brefeldin A (10 μg/ml; Sigma). The cells were than placed at 4°C overnight and stained the next day.
Immunofluorescent staining.
Stimulated PBMC were washed once (1,200 rpm for 5 min) with FACS buffer (1% bovine serum albumin, 0.1% sodium azide). The cells were then surface stained for 20 min on ice with antibodies directly conjugated to CD3 and CD8. The cells were washed as above and then fixed and permeabilized using 750 μl of 2× fixation-permeablization solution (Becton Dickinson Immunocytometry Systems, San Jose, Calif.). After a 10-min incubation (25°C in darkness), the cells were washed twice in FACS buffer (1,800 rpm for 8 min) and stained intracellularly with antibodies directly conjugated to CD69 and IFN-γ. The cells were washed a final time (1,800 rpm for 8 min) and resuspended in 1% paraformaldehyde (Electron Microscopy Systems, Fort Washington, Pa.) in phosphate-buffered saline.
Flow cytometric analysis.
Six-parameter flow cytometric analysis was preformed using a FACScalibur flow cytometer (Becton Dickinson Immunocytometry Systems). Fluorescein isothiocyanate, phycoerythrin, peridinin-chlorophyll protein, and allophycocyanin (APC) were used as the fluorescent parameters. Between 100,000 and 130,000 live CD3+ lymphocytes were collected. The list-mode data files were analyzed with PAINT-A-GATE software (Becton Dickinson Immunocytometry Systems). In all data shown, the percentages represent the number of IFN-γ+ CD69+CD3+CD8+ or CD8− cells that responded to each peptide or control. In general, the background (anti CD28-CD49d alone) was less than 0.1% of total T cells (for exceptions, see the legend to Fig. 2).
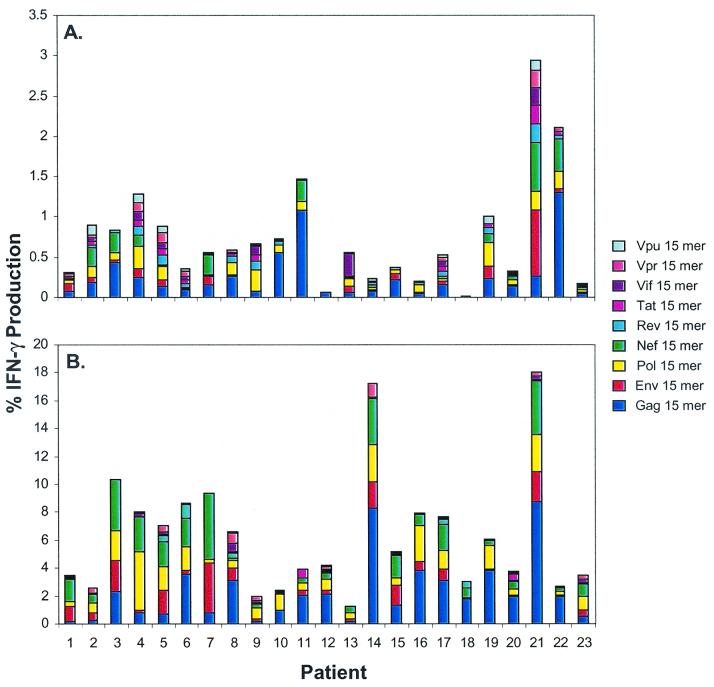
FIG. 2.
Total HIV-specific CD4+ (A) and CD8+ (B) T-cell responses in samples from 23 untreated HIV-infected patients. PBMC isolated from patients 1 to 23 (see Table 1) were incubated with peptide mixtures containing 15-mers overlapping by 11 amino acids derived from HIV Gag, Pol, Env, Nef, Tat, Rev, Vif, Vpr, and Vpu in the presence of costimulatory antibodies as described in Materials and Methods. The bars in panel A represent the percentage of CD3+CD8−CD69+ IFN-γ+ cells that responded to each peptide mixture with background (CD28-CD49d alone) IFN-γ production from the CD3+CD8+ population removed. The bars in panel B represent the percent of CD3+CD8+CD69+ IFN-γ+ cells that responded to each peptide mixture with background IFN-γ production removed. Background IFN-γ production from CD3+CD8+ cells was ≤0.1% in all patients except for patients 15 (0.15%), 17 (0.37%), and 22 (0.2%). Background IFN-γ production in CD3+CD8− cells was ≤0.1% in all patients except for patients 10 (0.16%), 15 (0.16%), and 21 (0.17%).
Antibodies.
Unconjugated mouse anti-human CD28, unconjugated mouse anti-human CD49d, fluorescein isothiocyanate-conjugated mouse anti-human IFN-γ, phycoerythrin-conjugated mouse anti-human CD69, peridinin-chlorophyll protein-conjugated mouse-anti-human CD3, and APC-conjugated mouse anti-human CD8 monoclonal antibodies were obtained from Becton Dickinson Immunocytometry Systems.
RESULTS
Use of overlapping peptides in the intracellular cytokine assay.
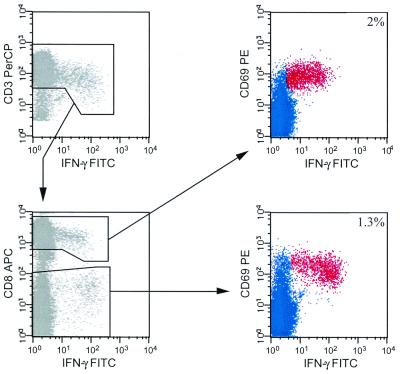
To assess total CD4+ and CD8+ T-cell responses to HIV by measuring intracellular cytokine production, we first had to establish a method to determine these responses in the most efficient manner, using a minimum number of PBMC while examining a maximum number of potential peptides. We examined whether a mixture of peptides 15 amino acids in length, overlapping by 11 amino acids, and derived from HIV-1 Gag (122 peptides) could be recognized simultaneously by both CD4+ and CD8+ T cells, as depicted in Fig. 1. To quantify Gag-specific T-cell responses, we examined IFN-γ production in PBMC from CD3+CD8+ and CD3+CD8− (hereafter referred to as CD4+) T cells. While examining CD4+ responses in this manner may theoretically underestimate the total CD4+ T-cell response, comparative analysis of responding CD3+CD8− and CD3+CD4+ T-cell populations to the HIV-gag 15-mer mixture shows that they are directly equivalent (data not shown). To determine if the 15-mer peptides were of optimal length for both CD4+ and CD8+ T-cell responses, we compared the CD4+ and CD8+ T-cell responses to different HIV Gag peptide mixtures (optimized 8- to 11-mers, overlapping 15-mers, and overlapping 20-mers; data not shown). The CD4+ T-cell responses to the overlapping 15-mer and 20-mer Gag peptide mixtures were nearly identical, while more CD8+ T cells recognized the Gag 15-mer peptide mixture than the Gag 20-mer mixture in samples from 10 patients examined. CD8+ T-cell responses to the optimized Gag 8- to 11-mers and the Gag 15-mers, however, were more disparate, potentially due to a number of factors, including the location of the recognized sequence within the 15-mer (i.e., peptide overhang length and location) as well as sequence differences (data not shown). This discrepancy suggests that while the 15-mer peptides are recognized, they may underestimate the CD8+ T-cell response slightly in some patients. However, since the optimal Gag peptide mixture only contains 37 different epitopes that bind to a limited array of HLA alleles, it is unlikely to encompass the entire repertoire of Gag peptides recognized in every patient. Because the overlapping 15-mers theoretically contain every possible T-cell epitope (patient sequence variation notwithstanding), they can be used to quantify total T-cell responses independently of the patient's MHC haplotype.
FIG. 1.
Simultaneous measurement of HIV-specific CD8+ and CD4+ T-cell responses. PBMC from patient 22 were stimulated with overlapping 15-mer peptides from Gag and stained as described in Materials and Methods. Cells were gated initially on small, viable, resting lymphocytes and then gated for CD3+ IFN-γ+ cells (top left panel). CD3+ IFN-γ+ lymphocytes were then gated based on CD8 and IFN-γ expression (bottom left panel). Quantification of CD8−-mediated HIV-specific responses (top right panel) was determined by gating on CD8+CD69+ IFN-γ+ cells. Quantification of CD4-mediated HIV-specific responses (bottom right panel) was determined by gating on CD8−CD69+ IFN-γ+ cells. HIV Gag-specific cells are highlighted in red, and the percent response from each cell type is indicated.
Quantification of total HIV-specific T-cell responses using overlapping peptide mixtures.
Having established that the 15-mer peptides provided the best assessment of both CD4+ and CD8+ T-cell responses, we used the overlapping 15-mer peptide mixtures to quantify the total HIV-specific T-cell responses in the 23 untreated HIV-positive patients (Fig. 2). Significant IFN-γ production (≥0.05% above background) to the various HIV 15-mer peptide mixtures was detected in the CD4+ T lymphocyte subset in 21 of 23 patients (Fig. 2A), varying from 0.2 to 2.94% (mean frequency, 0.74%) of total CD4+ T lymphocytes. Responses to the Gag peptide mixture were observed in most patients (21 of 23) and ranged between 0.06 and 1.3% of total CD4+ T lymphocytes, comparable to previous findings (37). Although detected in most of the patients, HIV Gag-specific CD4+ T-cell responses were only dominant in 11 of 21 responding patients. CD4+ T-cell responses specific for other HIV-peptide mixtures besides Gag were found in every responding patient (Pol, 11 of 21; Env, 11 of 21, Nef, 8 of 23; Tat, 6 of 21; Rev, 8 of 21; Vif, 4 of 21; Vpr, 6 of 21; Vpu, 4 of 21). These results indicate that any HIV protein can be the target of CD4+ T cells, although responses directed at HIV Gag, Pol, and Env are the most common. Furthermore, in agreement with previous studies, substantial numbers of HIV-specific CD4+ T cells are present in most of the HIV-infected patients regardless of disease progression status (37). Because longitudinal information on the rates of disease progression were unavailable for most of the patients in this cohort, we were unable to examine the relationship between progression and the frequency of responding HIV-specific CD4+ T cells. There was no correlation between absolute CD4+ T-cell count and the total HIV-specific CD4+ T-cell response or the response to any individual HIV protein (data not shown). Interestingly, patient 21, with a CD4+ T-cell count of 181 cells/mm3, had the highest total CD4+ T-cell response detected, indicating that substantially strong CD4+ T-cell responses can be present even in individuals with advanced disease.
High frequencies (1.3 to 18.05%; mean frequency, 6.31%) of HIV-specific CD8+ T cells were detected in all 23 patients (Fig. 2B). Each patient responded to at least four different peptide mixtures, indicating that most HIV-infected patients elicit a broad response directed at several different HIV proteins. HIV Gag-specific responses were detected in samples from every patient (0.19 to 8.76% of total CD8+ T cells) but were dominant in only 12 of 23 patients. The Pol, Env, and Nef 15-mer mixtures were recognized by most patients and in some patients with relatively high frequency (Pol, 22 of 23, 0.27 to 4.21%; Env, 19 of 23, 0.17 to 3.63%; Nef 22 of 23, 0.11 to 4.68%). CD8+ T-cell responses directed at the HIV accessory proteins Tat, Rev, Vif, and Vpr were also common, although the frequency was often considerably lower (Tat, 5 of 23, 0.06 to 0.44%; Rev, 7 of 23, 0.05 to 0.91%; Vif, 6 of 23, 0.06 to 0.63%; Vpr, 11 of 23, 0.06 to 0.97%). The Vpu peptide mixture was only recognized by patient 8, and at a very low frequency (0.08%) of total CD8+ T cells. At least four different 15-mer mixtures were recognized in every patient, demonstrating the diversity of the HIV-specific CD8+ T-cell response in these patients. Comparison of the total CD8+ T cell 15-mer response with the response to the 95 optimized peptides, by Wilcoxon signed-rank test, indicated that the 15-mer responses were significantly stronger in this cohort (P = 0.01). These data confirm previous findings that the response to a single HIV epitope is not predictive of the entire response within an infected patient (6), since multiple HIV proteins are recognized in every HIV-infected patient.
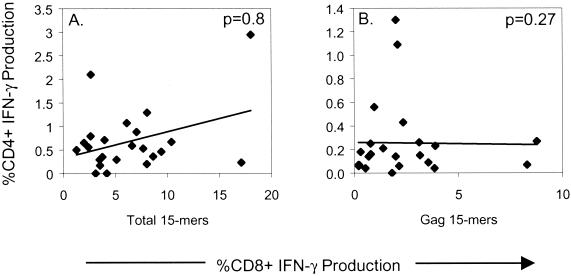
A previous study has suggested that HIV Gag-specific CD8+ T-cell precursor frequency and HIV p24-specific CD4+ T helper cell lymphoproliferative responses are positively correlated in chronically infected untreated HIV patients (20). Therefore, we examined the relationship between total HIV and Gag-specific CD4+ and CD8+ T-cell responses (Fig. 3). There was no significant relationship between the total responding HIV-specific CD4+ and CD8+ T-cell frequency (P = 0.8, Spearman rank correlation) (Fig. 3A). Likewise, there was no correlation between responding HIV Gag-specific CD4+ and CD8+ T-cell frequencies (P = 0.27) (Fig. 3B). Notwithstanding the fact that the assays being compared are essentially different, our data nevertheless show that the frequency of responding HIV-specific CD8+ T cells is not directly related to the frequency of HIV-specific CD4+ T cells.
FIG. 3.
Correlation between HIV-specific CD4+ and CD8+ T-cell frequency. Total HIV-specific (A) and Gag-specific (B) CD4+ and CD8+ T-cell frequencies were determined with the indicated overlapping 15-mer peptide mixtures. P values, as determined by the Spearman rank correlation, are as follows: (A) P = 0.8; (B) P = 0.27. The solid line represents a regression line.
Correlation between viral load and responding HIV-specific CD4+ and CD8+ T-cell frequency.
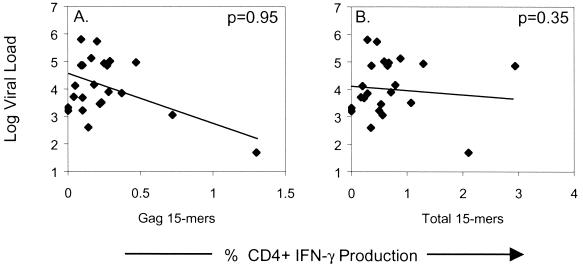
Previous studies have suggested that there is an inverse correlation between HIV Gag-specific CD4+ T-cell lymphoproliferative activity and HIV viral load (20, 40), a finding not confirmed by another study where the HIV gag p55-specific CD4+ T-cell frequency and viral load were compared (37). Our results also suggest that there is no correlation between the frequency of responding HIV Gag-specific CD4+ T cells and viral load (Fig. 4A). It is possible that there is no direct correlation between the CD4+ T-cell response to a single HIV protein, since the CD4+ T-cell response to HIV is specific for multiple HIV proteins in most people (Fig. 2A). We therefore examined the relationship between the total HIV-specific CD4+ T-cell frequency and viral load, as these may be closely related. Our results, however, failed to indicate a direct correlation between the total frequency of responding HIV-specific CD4+ T cells and viral load (Fig. 4B).
FIG. 4.
Correlation between viral load and HIV-specific CD4+ T-cell frequency. Plasma viral load (RNA copies/ml) was determined in each patient from the same time point in which HIV-specific CD4+ T-cell responses were quantified. P values shown in each figure are determined by the Spearman rank correlation. The solid line represents a regression line.
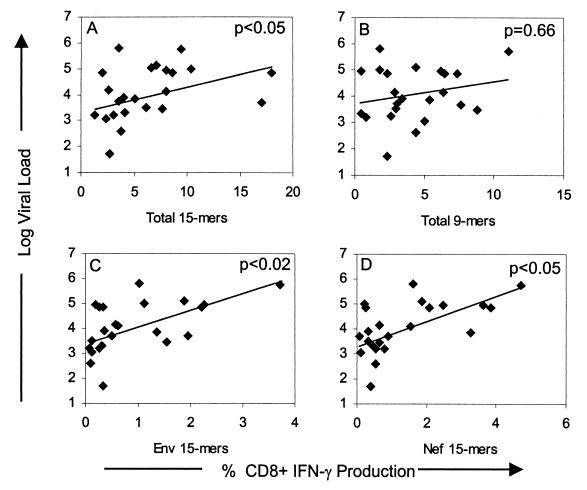
In contrast to previous reports that demonstrated an inverse correlation between the HIV-specific CD8+ T-cell frequency and viral load (7, 30, 33), we found a positive correlation between viral load and the total responding HIV-specific CD8+ T-cell frequency (Fig. 5A) (P < 0.05, Spearman rank correlation), Env-specific CD8+ T-cell frequency (Fig. 5C) (P < 0.02), and the Nef-specific CD8+ T-cell frequency (Fig. 5D) (P < 0.05). No correlation between viral load and the CD8+ T-cell response to the optimized 9-mer mixtures (Fig. 5B) was observed. Likewise, no significant correlation was found between plasma viral load and the frequency of CD8+ T cells responding to any other single HIV proteins, as well as the combination of the Gag-Pol or Rev-Tat 15-mer mixtures (data not shown), contrary to previous reports (33, 43).
FIG. 5.
Correlation between viral load and HIV-specific CD8+ T-cell frequency. Plasma viral load (RNA copies/ml) was determined in each patient from the same time point that HIV-specific CD8+ T-cell responses were quantified. P values shown in each figure are determined by the Spearman rank correlation. The solid line represents a regression line.
DISCUSSION
Recent developments in the ability to accurately quantify antigen-specific T cells have led to significant advancements in our understanding of the strength and specificity of T-cell responses to HIV. Here we have utilized one such technique, intracellular cytokine staining, to quantify the total CD4+ and CD8+ T-cell response to HIV. Rather than focus on single peptides or combinations of epitopes as a representation of the HIV-specific response, we have examined the T-cell response to a panel of overlapping 15-mer peptides that cover every HIV protein. This system has a considerable advantage over existing tetramer technologies in that it examines the response to all potential HIV epitopes, regardless of the HLA haplotype of the infected individual. There is a strong correlation between the frequency of peptide-specific IFN-γ-producing CD8+ T cells and tetramer-positive CD8+ T cells (17, 29), suggesting that these two assays examine the same cell populations and that therefore either can be used to accurately quantify antigen-specific T-cell responses to single peptides. Using intracellular cytokine staining, we have found that markedly high frequencies of CD4+ and CD8+ T cells specific for multiple different HIV proteins are present in nearly every untreated HIV-infected individual. Our results are novel in that this is the first report to examine both the CD4+ and CD8+ T-cell response to every potential HIV epitope. Previous attempts to quantify the total HIV-specific CD8+ T-cell response to HIV only examined the responses to the HIV Gag, Pol, Env, and Nef proteins (15) or panels of selected epitopes (14). Although the overlapping 15-mer panels may slightly underestimate the total HIV-specific response due to the effects of amino acid overlaps and potential sequence differences between peptides and autologous virus, our results nevertheless provide the most comprehensive assessment of the total T-cell response to HIV performed to date.
We found that CD4+ T cells specific for multiple HIV proteins are readily detectable in the majority of chronically infected patients, in agreement with a previous report that examined HIV p55-specific CD4+ T-cell responses (37). These results indicate that HIV-specific CD4+ T cells are generated and persist during ongoing virus replication in untreated individuals. Previous evidence has suggested that there is a positive correlation between CD4+ T-cell proliferative responses and the frequency of HIV-specific CD8+ T cells (20), as well as a negative correlation between CD4+ T-cell proliferative responses and viral load (40). We failed to find these relationships by intracellular cytokine staining to directly quantify total HIV-specific CD4+ and CD8+ T-cell frequencies. This discrepancy can be explained by a number of factors. First, proliferative assays do not precisely quantify responder cell frequencies but provide an overall “bulk” view of a complex cellular response that depends on a number of factors, including absolute numbers of CD4+ T cells, responding cell frequency and/or proliferative ability, APC function, and assay culture conditions. Indeed, recent data suggest that there is inconsistency between HIV-specific CD4+ T-cell proliferative responses and the frequency of IFN-γ-producing CD4+ T cells (44). Other studies have demonstrated that in vitro proliferative responses to HIV antigens are abrogated during viremia, yet HIV-specific IFN-γ-producing CD4+ T cells persist, calling into question whether the absence of these responses should be used as a proxy for absent virus-specific T cell help in HIV infection (M. Connors, personal communication). Thus, proliferation assays may correlate with viral load via an indirect association between viral replication, hyperactivation, and in vitro apoptosis and not through a direct connection with the number or frequency of functional HIV-specific CD4+ T cells. Animal models have established the importance of virus-specific CD4+ T cells in initiating and maintaining CD8+ T-cell effector function (46), but our data suggest that in HIV infection this relationship is more complex than a simple linear correlation between the frequencies of HIV-specific CD4+ and either CD8+ T cells or viral load.
Our results show that a positive correlation exists between the total HIV-specific CD8+ T-cell response and viral load. This is in contrast to a previous report that identified an inverse correlation between the frequency of CD8+ T cells specific for two individual HIV epitopes restricted by HLA-A2 (as measured by tetramer analysis) and plasma viral load (33). As was recently shown, a limited examination of the HIV-specific CD8+ response in HLA-A2-positive patients is not likely to be representative of the entire HIV-specific CD8+ T-cell response (6, 16). Other studies utilizing precursor frequency analysis and standard 51Cr release assays have also demonstrated an inverse correlation between viral load and HIV-specific CD8+ T-cell activity (7, 30). However, because precursor frequency assays often underestimate CD8+ T-cell responses (17, 29) and 51Cr release assays are at best semiquantitative, the validity of these findings is somewhat questionable. In fact, two recent studies that examined a panel of optimized HIV epitopes or selected vaccinia virus-expressed HIV proteins by intracellular cytokine staining were unable to find an inverse correlation between HIV-specific CD8+ T-cell frequency and viral load (14, 15).
A massive expansion of HIV-specific CD8+ T cells in response to increasing HIV load has been shown to occur during acute HIV infection (26). This expansion of antigen-specific CD8+ T cells occurs in other viral infection as well, for example in lymphocytic choriomeningitis virus infection in mice (29) and Epstein-Barr virus infection in humans (9). Although evidence suggests that HIV-specific CD8+ T cells play a significant role in delaying progression to AIDS (19, 23, 39, 41), it is clear that in the vast majority of infected individuals, HIV-specific CD8+ T-cell responses are by themselves insufficient to effectively contain viral replication. Because of the complex feedback between HIV-specific CD8+ T cells and viral load, the interpretation of correlations between these two quantities is difficult and controversial. While it has been argued that a negative correlation is indicative of control of virus replication by the CD8+ T-cell response (33), it has also been reported on theoretical grounds that a negative correlation indicates that the virus actively impairs the immune response (45). Additional support for a positive correlation between HIV load and HIV-specific CD8+ T cells comes from HAART-treated patients. Shortly after therapy initiation, the viral titer in these individuals rapidly decreases, accompanied by a rapid decrease in the frequency of HIV-specific CD8+ T cells (32, 34). Interestingly, after the viral load drops below detection limits in HAART-treated individuals, at which time the decay rate of the viral reservoir becomes lower (13, 36), the HIV-specific CD8+ T-cell frequency also appears to decay at a much lower rate (10), again reflecting the positive relationship between viral load and HIV-specific CD8+ T-cell frequency.
Taken together, these results suggest that the relationship between HIV-1-specific CD8+ T-cell frequencies and viral load is more reflective of viral replication driving T-cell expansion than of CTL controlling viral replication. This implies that the frequency of HIV-specific CD8+ T cells is not the sole predictor of the ability of the immune system to control HIV replication. Given the ample evidence of the antiviral activity of CD8+ T cells—most critically, that removal of CD8+ T cells from SIV-infected macaques leads to increased viral replication and rapid progression (19, 41)—this observation does not invalidate a role for CD8+ CTL in viral control but does suggest that the functional ability of HIV-specific CD8+ T cells to control viral replication must be linked to parameters other than frequency alone. One possibility is that the diversity of HIV peptides recognized by CD8+ T cells may be linked to control of viral replication (2). The enormous rate of viral replication, in concert with a high incidence of tolerable mutations endows HIV with the ability to rapidly escape from immunological pressure, or the so-called epitope escape (18, 38). Recognition of a broad array of viral epitopes might prevent such escape, both by providing backup recognition if mutations nullify one or two responses and by providing a higher likelihood of immune recognition of obligate wild-type epitopes (viral protein sequences critical to function and nontolerant of mutation). However, given the capacity of HIV to make compensatory mutations, it is likely that even relatively conserved areas can tolerate mutations (22). Given the associations between rapid progression or nonprogression and HLA haplotype (21, 31), it is also possible that recognition of particular HIV epitopes in context of certain HLA molecules plays a significant role in the ability of CD8+ T cells to control viral replication (28).
Other parameters of the CD8 response may also be relevant to viral control. In addition to which epitopes are recognized, the number of distinct T-cell receptor-defined clonotypes recognizing each epitope may be pertinent. A broad array of clonotypes might provide resistance to escape mutation, as well as a higher likelihood of including high avidity responses, which might enhance function by allowing recognition of epitopes with a lower level of MHC class I affinity or decreased expression levels. It has also been suggested that the functional differentiation of HIV-specific CD8+ T cells may be impaired in HIV infection, such that the cytokine-producing cells present lack efficient cytolytic function (4, 11). Since the CD27 phenotype has been associated with functionally efficient CTL, it is possible that frequencies of HIV-specific CTL with this phenotype show a different relationship with viral load (4, 25).
Of course, any or all of these mechanisms could presumably operate, making determination of protective thresholds complex. Moreover, immunological protection is a dynamic process where the frequencies, specificities, and functional activities of CD8+ T-cell clonotypes change in concert with evolution of viral species during the course of infection. In reaction to mutations at dominant epitopes, the immune system could mount a response (i) with the same CD8+ T-cell clones, (ii) with newly elicited clones to such mutant epitopes, or (iii) with newly elicited clones to previously cryptic or subdominant clones. This would likely cause expansion in the overall breadth and frequency of the CD8+ T-cell response, which would, in turn, result in further immunological pressure on the virus and subsequent epitope escape. The overall effect would be one of action and reaction: viral expansion driving CD8+ T-cell expansion, driving viral mutation, and driving further CD8+ T-cell expansion. Thus, a positive correlation between CD8+ T-cell frequency and viral load would ensue until sufficient depletion of CD4+ T cells by the action of virus and/or CD8+ T cells rendered the CD8+ T cells ineffective (46). In addition to the concept of action and reaction, one can envision a situation in which the specificity of certain CD8+ T-cell clones is against viral epitopes that cannot tolerate mutation, possibly for reasons of viral fitness. In this situation one might find a genuine negative correlation between viral load and CD8+ T-cell response. Thus, determination of protective correlates in HIV infection will likely require quantitative measurement of various parameters of CD8+ T-cell-mediated immune responses (patterns of epitope recognition, clonotypic complexity, phenotype, and function) and of viral infection over the course of untreated early infection. Approaches outlined in this report, particularly the use of cytokine flow cytometry and overlapping pan-genome peptides, will allow such dissection of the response, and will constitute a powerful tool in this effort.
ACKNOWLEDGMENTS
We thank Gary Nabel for providing the overlapping HIV 15-mer peptide panels.
This work was supported by the following grants: AI 47603 (R.A.K.), AI 43638 (R.A.K.), AI 35522 (R.A.K.), and AI 47606 (L.J.P.). S.B. is supported by a grant from the Swiss National Science Foundation, no. 631-62898.
REFERENCES
- 1.Allen T M, O'Connor D H, Jing P, Dzuris J L, Mothe B R, Vogel T U, Dunphy E, Liebl M E, Emerson C, Wilson N, Kunstman K J, Wang X, Allison D B, Hughes A L, Desrosiers R C, Altman J D, Wolinsky S M, Sette A, Watkins D I. Tat-specific cytotoxic T lymphocytes select for SIV escape variants during resolution of primary viraemia. Nature. 2000;407:386–390. doi: 10.1038/35030124. [DOI] [PubMed] [Google Scholar]
- 2.Altfeld M, Rosenberg E S, Shankarappa R, Mukherjee J S, Hecht F M, Eldridge R L, Addo M M, Poon S H, Phillips M N, Robbins G K, Sax P E, Boswell S, Kahn J O, Brander C, Goulder P J, Levy J A, Mullins J I, Walker B D. Cellular immune responses and viral diversity in individuals treated during acute and early HIV-1 infection. J Exp Med. 2001;193:169–180. doi: 10.1084/jem.193.2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amara R R, Villinger F, Altman J, Lydy S L, O'Neil S P, Staprans S I, Montefiori D C, Xu Y, Herndon J G, Wyatt L S, Candido M A, Kozyr N L, Earl P L, Smith J M, Ma H-L, Grimm B D, Hulsey M L, Miller J, McClure H M, McNicholl J M, Moss B, Robinson H L. Control of a mucosal challenge and prevention of AIDS by a multiprotein DNA/MVA vaccine. Science. 2001;292:69–74. doi: 10.1126/science.1058915. [DOI] [PubMed] [Google Scholar]
- 4.Appay V, Nixon D F, Donahoe S M, Gillespie G M, Dong T, King A, Ogg G S, Spiegel H M, Conlon C, Spina C A, Havlir D V, Richman D D, Waters A, Easterbrook P, McMichael A J, Rowland-Jones S L. HIV-specific CD8(+) T cells produce antiviral cytokines but are impaired in cytolytic function. J Exp Med. 2000;192:63–75. doi: 10.1084/jem.192.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barouch D H, Santra S, Schmitz J E, Kuroda M J, Fu T M, Wagner W, Bilska M, Craiu A, Zheng X X, Krivulka G R, Beaudry K, Lifton M A, Nickerson C E, Trigona W L, Punt K, Freed D C, Guan L, Dubey S, Casimiro D, Simon A, Davies M E, Chastain M, Strom T B, Gelman R S, Montefiori D C, Lewis M G. Control of viremia and prevention of clinical AIDS in rhesus monkeys by cytokine-augmented DNA vaccination. Science. 2000;290:486–492. doi: 10.1126/science.290.5491.486. [DOI] [PubMed] [Google Scholar]
- 6.Betts M R, Casazza J P, Patterson B A, Waldrop S, Trigona W, Fu T-M, Kern F, Picker L J, Koup R A. Putative immunodominant human immunodeficiency virus-specific CD8+ T-cell responses cannot be predicted by major histocompatibility complex class I haplotype. J Virol. 2000;74:9144–9151. doi: 10.1128/jvi.74.19.9144-9151.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Betts M R, Krowka J F, Kepler T B, Davidian M, Christopherson C, Kwok S, Louie L, Eron J, Sheppard H, Frelinger J A. Human immunodeficiency virus type 1-specific cytotoxic T lymphocyte activity is inversely correlated with HIV type 1 viral load in HIV type 1-infected long-term survivors. AIDS Res Hum Retrovir. 1999;15:1219–1228. doi: 10.1089/088922299310313. [DOI] [PubMed] [Google Scholar]
- 8.Borrow P, Lewicki H, Wei X P, Horwitz M S, Peffer N, Meyers H, Nelson J A, Gairin J E, Hahn B H, Oldstone M B A, Shaw G M. Antiviral pressure exerted by HIV-1-specific cytotoxic T lymphocytes (CTLs) during primary infection demonstrated by rapid selection of CTL escape virus. Nat Med. 1997;3:205–211. doi: 10.1038/nm0297-205. [DOI] [PubMed] [Google Scholar]
- 9.Callan M F, Steven N, Krausa P, Wilson J D, Moss P A, Gillespie G M, Bell J I, Rickinson A B, McMichael A J. Large clonal expansions of CD8+ T cells in acute infectious mononucleosis. Nat Med. 1996;2:906–911. doi: 10.1038/nm0896-906. [DOI] [PubMed] [Google Scholar]
- 10.Casazza J P, Betts M R, Picker L J, Koup R A. Decay kinetics of HIV-specific CD8+ T cells in peripheral blood after initiation of highly active antiretroviral therapy. J Virol. 2001;75:6508–6516. doi: 10.1128/JVI.75.14.6508-6516.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Champagne P, Ogg G S, King A S, Knabenhans C, Ellefsen K, Nobile M, Appay V, Rizzardi G P, Fleury S, Lipp M, Forster R, Rowland-Jones S, Sekaly R P, McMichael A J, Pantaleo G. Skewed maturation of memory HIV-specific CD8 T lymphocytes. Nature. 2001;410:106–111. doi: 10.1038/35065118. [DOI] [PubMed] [Google Scholar]
- 12.Chen Z W, Craiu A, Shen L, Kuroda M J, Iroku U C, Watkins D I, Voss G, Letvin N L. Simian immunodeficiency virus evades a dominant epitope-specific cytotoxic T lymphocyte response through a mutation resulting in the accelerated dissociation of viral peptide and MHC class I. J Immunol. 2000;164:6474–6479. doi: 10.4049/jimmunol.164.12.6474. [DOI] [PubMed] [Google Scholar]
- 13.Chun T W, Carruth L, Finzi D, Shen X, DiGiuseppe J A, Taylor H, Hermankova M, Chadwick K, Margolick J, Quinn T C, Kuo Y H, Brookmeyer R, Zeiger M A, Barditch-Crovo P, Siliciano R F. Quantification of latent tissue reservoirs and total body viral load in HIV-1 infection. Nature. 1997;387:183–188. doi: 10.1038/387183a0. [DOI] [PubMed] [Google Scholar]
- 14.Dalod M, Dupuis M, Deschemin J C, Sicard D, Salmon D, Delfraissy J F, Venet A, Sinet M, Guillet J G. Broad, intense anti-human immunodeficiency virus (HIV) ex vivo CD8+ responses in HIV type 1-infected patients: comparison with anti-Epstein-Barr virus responses and changes during antiretroviral therapy. J Virol. 1999;73:7108–7116. doi: 10.1128/jvi.73.9.7108-7116.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gea-Banacloche J C, Migueles S A, Martino L, Shupert W L, McNeil A C, Sabbaghian M S, Ehler L, Prussin C, Stevens R, Lambert L, Altman J, Hallahan C W, de Quiros J C, Connors M. Maintenance of large numbers of virus-specific CD8+ T cells in HIV-infected progressors and long-term nonprogressors. J Immunol. 2000;165:1082–1092. doi: 10.4049/jimmunol.165.2.1082. [DOI] [PubMed] [Google Scholar]
- 16.Goulder P J, Altfeld M A, Rosenberg E S, Nguyen T, Tang Y, Eldridge R L, Addo M M, He S, Mukherjee J S, Phillips M N, Bunce M, Kalams S A, Sekaly R P, Walker B D, Brander C. Substantial differences in specificity of HIV-specific cytotoxic T cells in acute and chronic HIV infection. J Exp Med. 2001;193:181–194. doi: 10.1084/jem.193.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goulder P J, Tang Y, Brander C, Betts M R, Altfeld M, Annamalai K, Trocha A, He S, Rosenberg E S, Ogg G, O'Callaghan C A, Kalams S A, McKinney R E, Mayer K, Koup R A, Pelton S I, Burchett S K, McIntosh K, Walker B D. Functionally inert HIV-specific cytotoxic T lymphocytes do not play a major role in chronically infected adults and children. J Exp Med. 2000;192:1819–1832. doi: 10.1084/jem.192.12.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goulder P J R, Phillips R E, Colbert R A, McAdam S, Ogg G, Nowak M A, Giangrande P, Luzzi G, Morgan B, Edwards A, McMichael A J, Rowland-Jones S. Late escape from an immunodominant cytotoxic T lymphocytes response associated with progression to AIDS. Nat Med. 1997;3:212–217. doi: 10.1038/nm0297-212. [DOI] [PubMed] [Google Scholar]
- 19.Jin X, Bauer D E, Tuttleton S E, Lewin S, Gettie A, Blanchard J, Irwin C E, Safrit J T, Mittler J, Weinberger L, Kostrikis L G, Zhang L, Perelson A S, Ho D D. Dramatic rise in plasma viremia after CD8(+) T cell depletion in simian immunodeficiency virus-infected macaques. J Exp Med. 1999;189:991–998. doi: 10.1084/jem.189.6.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kalams S A, Buchbinder S P, Rosenberg E S, Billingsley J M, Colbert D S, Jones N G, Shea A K, Trocha A K, Walker B D. Association between virus-specific cytotoxic T-lymphocyte and helper responses in human immunodeficiency virus type 1 infection. J Virol. 1999;73:6715–6720. doi: 10.1128/jvi.73.8.6715-6720.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaslow R A, Carrington M, Apple R, Park L, Munoz A, Saah A J, Goedert J J, Winkler C, O'Brien S J, Rinaldo C, Detels R, Blattner W, Phair J, Erlich H, Mann D L. Influence of combinations of human major histocompatibility complex genes on the course of HIV-1 infection. Nat Med. 1996;2:405–411. doi: 10.1038/nm0496-405. [DOI] [PubMed] [Google Scholar]
- 22.Kelleher A D, Long C, Holmes E C, Allen R L, Wilson J, Conlon C, Workman C, Shaunak S, Olson K, Goulder P, Brander C, Ogg G, Sullivan J S, Dyer W, Jones I I, McMichael A J, Rowland-Jones S, Phillips R E. Clustered mutations in HIV-1 gag are consistently required for escape from HLA-B27-restricted cytotoxic T lymphocyte responses. J Exp Med. 2001;193:375–386. doi: 10.1084/jem.193.3.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klein M R, van Baalen C A, Holwerda A M, Kerkhof Garde S R, Bende R J, Keet I P M, Eeftinck-Schattenkerk J-K M, Osterhaus A D M E, Schuitemaker H, Miedema F. Kinetics of gag-specific cytotoxic T lymphocyte responses during the clinical course of HIV-1 infection: a longitudinal analysis of rapid progressors and long-term asymptomatics. J Exp Med. 1995;181:1365–1372. doi: 10.1084/jem.181.4.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Korber B T M, Brander C, Walker B D, Koup R A, Moore J P, Haynes B F, Myers G. HIV molecular immunology database. Los Alamos, N.Mex: Los Alamos National Laboratory; 1998. [Google Scholar]
- 25.Kostense S, Ogg G S, Manting E H, Gillespie G, Joling J, Vandenberghe K, Veenhof E Z, van Baarle D, Jurriaans S, Klein M R, Miedema F. High viral burden in the presence of major HIV-specific CD8(+) T cell expansions: evidence for impaired CTL effector function. Eur J Immunol. 2001;31:677–686. doi: 10.1002/1521-4141(200103)31:3<677::aid-immu677>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 26.Koup R A, Safrit J T, Cao Y, Andrews C A, Wu Y, McLeod G, Borkowsky W, Farthing C, Ho D D. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J Virol. 1994;68:4650–4655. doi: 10.1128/jvi.68.7.4650-4655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Metzner K J, Jin X, Lee F V, Gettie A, Bauer D E, Di Mascio M, Perelson A S, Marx P A, Ho D D, Kostrikis L G, Connor R I. Effects of in vivo CD8(+) T cell depletion on virus replication in rhesus macaques immunized with a live, attenuated simian immunodeficiency virus vaccine. J Exp Med. 2000;191:1921–1931. doi: 10.1084/jem.191.11.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Migueles S A, Sabbaghian M S, Shupert W L, Bettinotti M P, Marincola F M, Martino L, Hallahan C W, Selig S M, Schwartz D, Sullivan J, Connors M. HLA B*5701 is highly associated with restriction of virus replication in a subgroup of HIV-infected long term nonprogressors. Proc Natl Acad Sci USA. 2000;97:2709–2714. doi: 10.1073/pnas.050567397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murali-Krishna K, Altman J D, Suresh M, Sourdive D J, Zajac A J, Miller J D, Slansky J, Ahmed R. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity. 1998;8:177–187. doi: 10.1016/s1074-7613(00)80470-7. [DOI] [PubMed] [Google Scholar]
- 30.Musey L, Hughes J, Schacker T, Shea T, Corey L, McElrath M J. Cytotoxic-T-cell responses, viral load, and disease progression in early human immunodeficiency virus type 1 infection. N Engl J Med. 1997;337:1267–1274. doi: 10.1056/NEJM199710303371803. [DOI] [PubMed] [Google Scholar]
- 31.Nelson G W, Kaslow R, Mann D L. Frequency of HLA allele-specific peptide motifs in HIV-1 proteins correlates with the allele's association with relative rates of disease progression after HIV-1 infection. Proc Natl Acad Sci USA. 1997;94:9802–9807. doi: 10.1073/pnas.94.18.9802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nixon D F, Douek D, Kuebler P J, Jin X, Vesanen M, Bonhoeffer S, Cao Y, Koup R A, Ho D D, Markowitz M. Molecular tracking of an human immunodeficiency virus nef specific cytotoxic T-cell clone shows persistence of clone-specific T-cell receptor DNA but not mRNA following early combination antiretroviral therapy. Immunol Lett. 1999;66:219–228. doi: 10.1016/s0165-2478(98)00162-x. [DOI] [PubMed] [Google Scholar]
- 33.Ogg G S, Jin X, Bonhoeffer S, Dunbar P R, Nowak M A, Monard S, Segal J P, Cao Y, Rowland-Jones S L, Cerundolo V, Hurley A, Markowitz M, Ho D D, Nixon D F, McMichael A J. Quantitation of HIV-1-specific cytotoxic T lymphocytes and plasma load of viral RNA. Science. 1998;279:2103–2106. doi: 10.1126/science.279.5359.2103. [DOI] [PubMed] [Google Scholar]
- 34.Ogg G S, Jin X, Bonhoeffer S, Moss P, Nowak M A, Monard S, Segal J P, Cao Y, Rowland-Jones S L, Hurley A, Markowitz M, Ho D D, McMichael A J, Nixon D F. Decay kinetics of human immunodeficiency virus-specific effector cytotoxic T lymphocytes after combination antiretroviral therapy. J Virol. 1999;73:797–800. doi: 10.1128/jvi.73.1.797-800.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pantaleo G, Menzo S, Vaccarezza M, Graziosi C, Cohen O J, Demarest J F, Montefiori D, Orenstein J M, Fox C, Schrager L K, et al. Studies in subjects with long-term nonprogressive human immunodeficiency virus infection. N Engl J Med. 1995;332:209–216. doi: 10.1056/NEJM199501263320402. [DOI] [PubMed] [Google Scholar]
- 36.Perelson A S, Essunger P, Cao Y, Vesanen M, Hurley A, Saksela K, Markowitz M, Ho D D. Decay characteristics of long-lived HIV-1-infected compartments during combination therapy. Nature. 1997;387:188–191. doi: 10.1038/387188a0. [DOI] [PubMed] [Google Scholar]
- 37.Pitcher C J, Quittner C, Peterson D M, Connors M, Koup R A, Maino V C, Picker L J. HIV-1-specific CD4+ T cells are detectable in most individuals with active HIV-1 infection, but decline with prolonged viral suppression. Nat Med. 1999;5:518–525. doi: 10.1038/8400. [DOI] [PubMed] [Google Scholar]
- 38.Price D A, Goulder P J, Klenerman P, Sewell A K, Easterbrook P J, Troop M, Bangham C R, Phillips R E. Positive selection of HIV-1 cytotoxic T lymphocyte escape variants during primary infection. Proc Natl Acad Sci USA. 1997;94:1890–1895. doi: 10.1073/pnas.94.5.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rinaldo C, Huang X-L, Fan Z, Ding M, Beltz L, Logar A, Panicali D, Mazzara G, Liebmann J, Cottrill M, Gupta P. High levels of anti-human immunodeficiency virus type 1 (HIV-1) memory cytotoxic T-lymphocyte activity and low viral load are associated with lack of disease in HIV-1-infected long-term nonprogressors. J Virol. 1995;69:5838–5842. doi: 10.1128/jvi.69.9.5838-5842.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rosenberg E, Billingsley J, Caliendo A, Boswell S, Sax P, Kalams S, Walker B. Vigorous HIV-1-specific CD4+ T cell responses associated with control of viremia. Science. 1997;278:1447–1450. doi: 10.1126/science.278.5342.1447. [DOI] [PubMed] [Google Scholar]
- 41.Schmitz J E, Kuroda M J, Santra S, Sasseville V G, Simon M A, Lifton M A, Racz P, Tenner-Racz K, Dalesandro M, Scallon B J, Ghrayeb J, Forman M A, Montefiori D C, Rieber E P, Letvin N L, Reimann K A. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science. 1999;283:857–860. doi: 10.1126/science.283.5403.857. [DOI] [PubMed] [Google Scholar]
- 42.Seth A, Ourmanov I, Schmitz J E, Kuroda M J, Lifton M A, Nickerson C E, Wyatt L, Carroll M, Moss B, Venzon D, Letvin N L, Hirsch V M. Immunization with a modified vaccinia virus expressing simian immunodeficiency virus (SIV) Gag-Pol primes for an anamnestic Gag-specific cytotoxic T-lymphocyte response and is associated with reduction of viremia after SIV challenge. J Virol. 2000;74:2502–2509. doi: 10.1128/jvi.74.6.2502-2509.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Baalen C A, Pontesilli O, Huisman R C, Geretti A M, Klein M R, de Wolf F, Miedema F, Gruters R A, Osterhaus A D. Human immunodeficiency virus type 1 Rev- and Tat-specific cytotoxic T lymphocyte frequencies inversely correlate with rapid progression to AIDS. J Gen Virol. 1997;78:1913–1918. doi: 10.1099/0022-1317-78-8-1913. [DOI] [PubMed] [Google Scholar]
- 44.Wilson J D, Imami N, Watkins A, Gill J, Hay P, Gazzard B, Westby M, Gotch F M. Loss of CD4+ T cell proliferative ability but not loss of human immunodeficiency virus type 1 specificity equates with progression to disease. J Infect Dis. 2000;182:792–798. doi: 10.1086/315764. [DOI] [PubMed] [Google Scholar]
- 45.Wodarz D, Hall S E, Usuku K, Osame M, Ogg G S, McMichael A J, Nowak M A, Bangham C R. Cytotoxic T-cell abundance and virus load in human immunodeficiency virus type 1 and human T-cell leukaemia virus type 1. Proc R Soc Biol Sci. 2001;268:1215–1221. doi: 10.1098/rspb.2001.1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zajac A J, Blattman J N, Murali-Krishna K, Sourdive D J, Suresh M, Altman J D, Ahmed R. Viral immune evasion due to persistence of activated T cells without effector function. J Exp Med. 1998;188:2205–2213. doi: 10.1084/jem.188.12.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]