Abstract
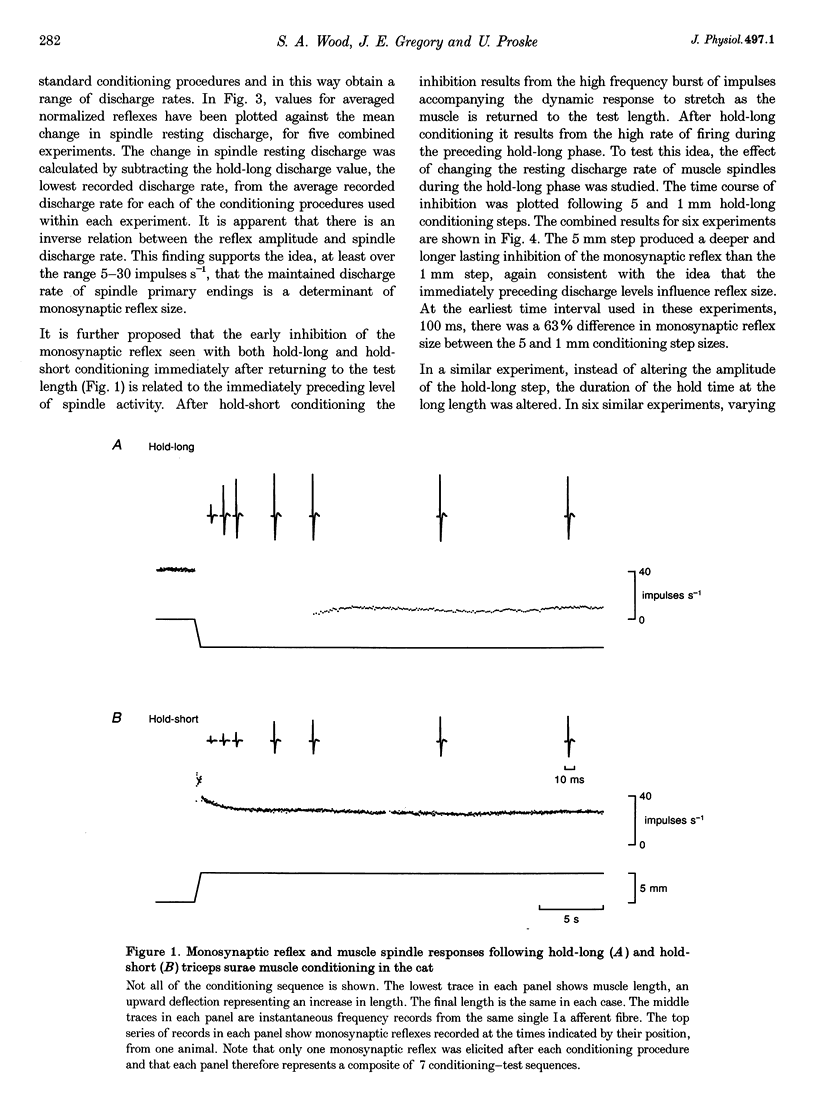
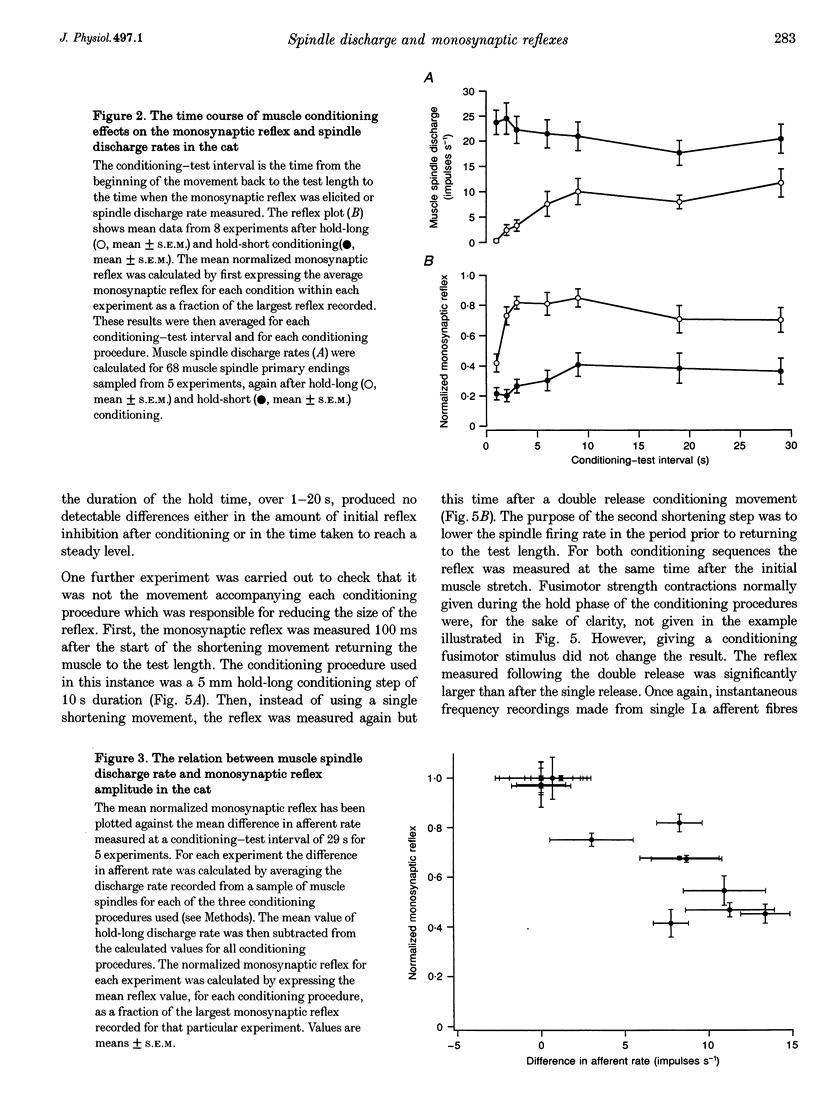
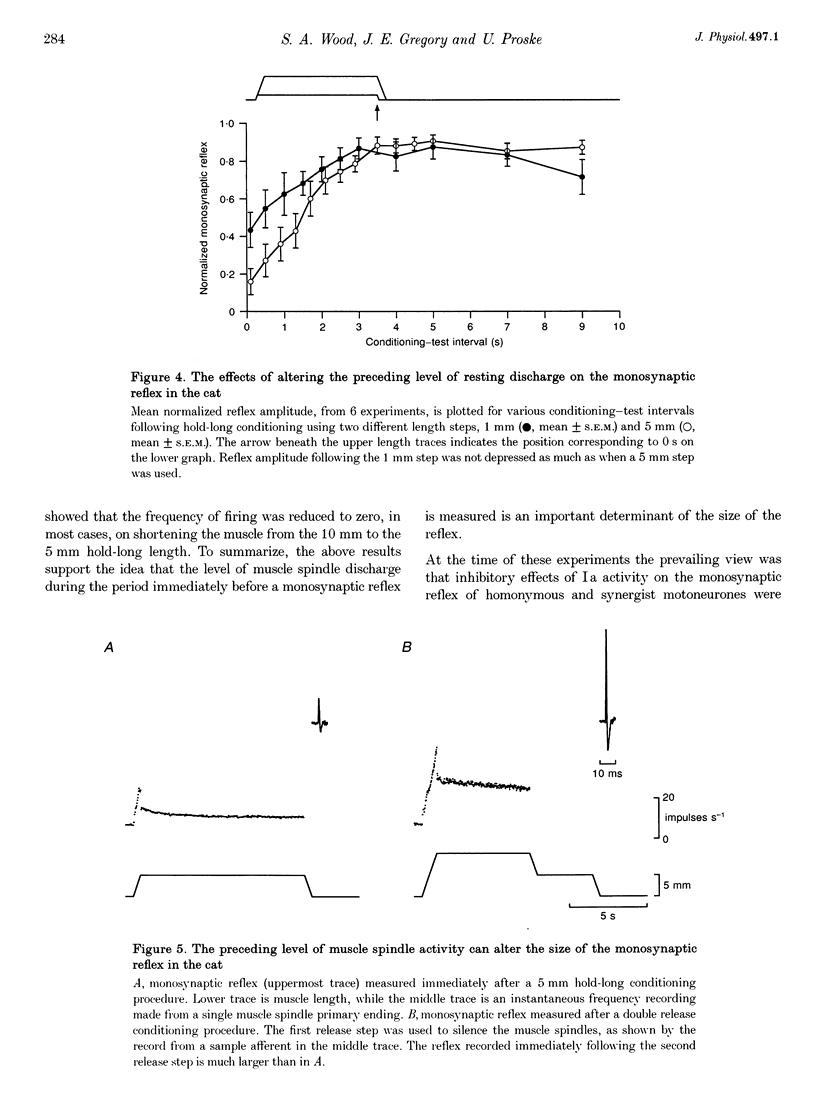
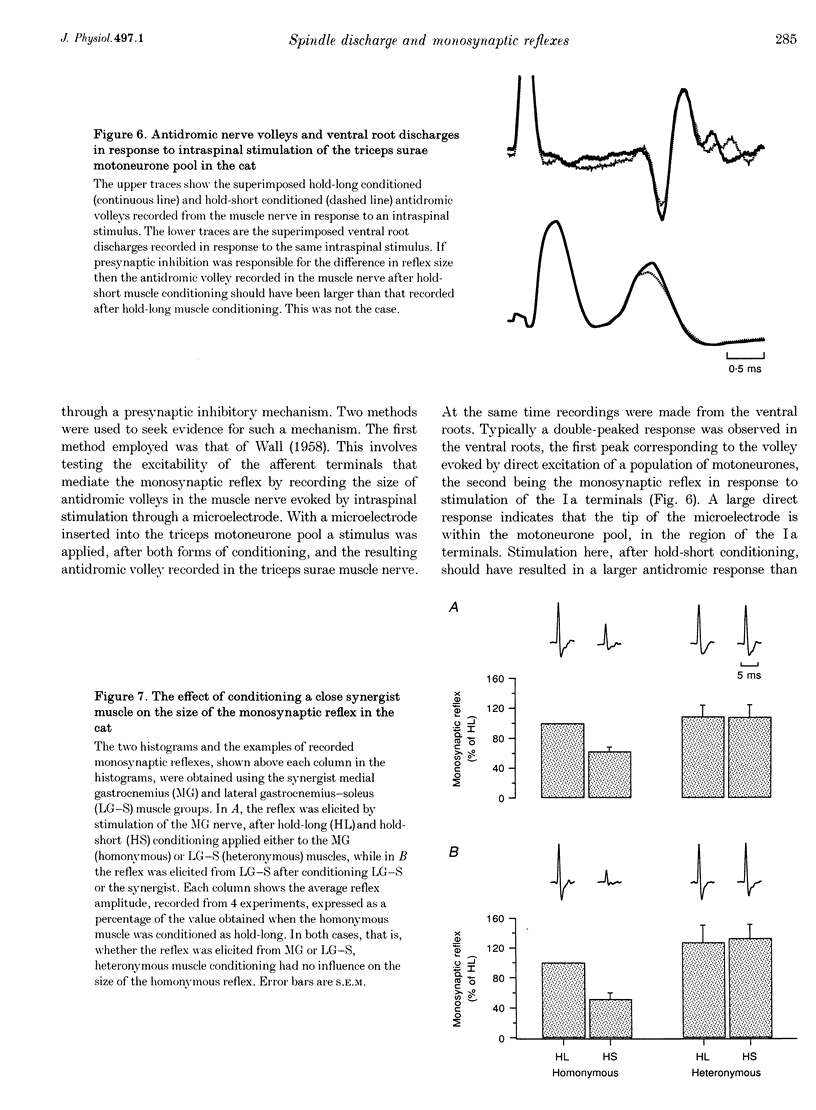
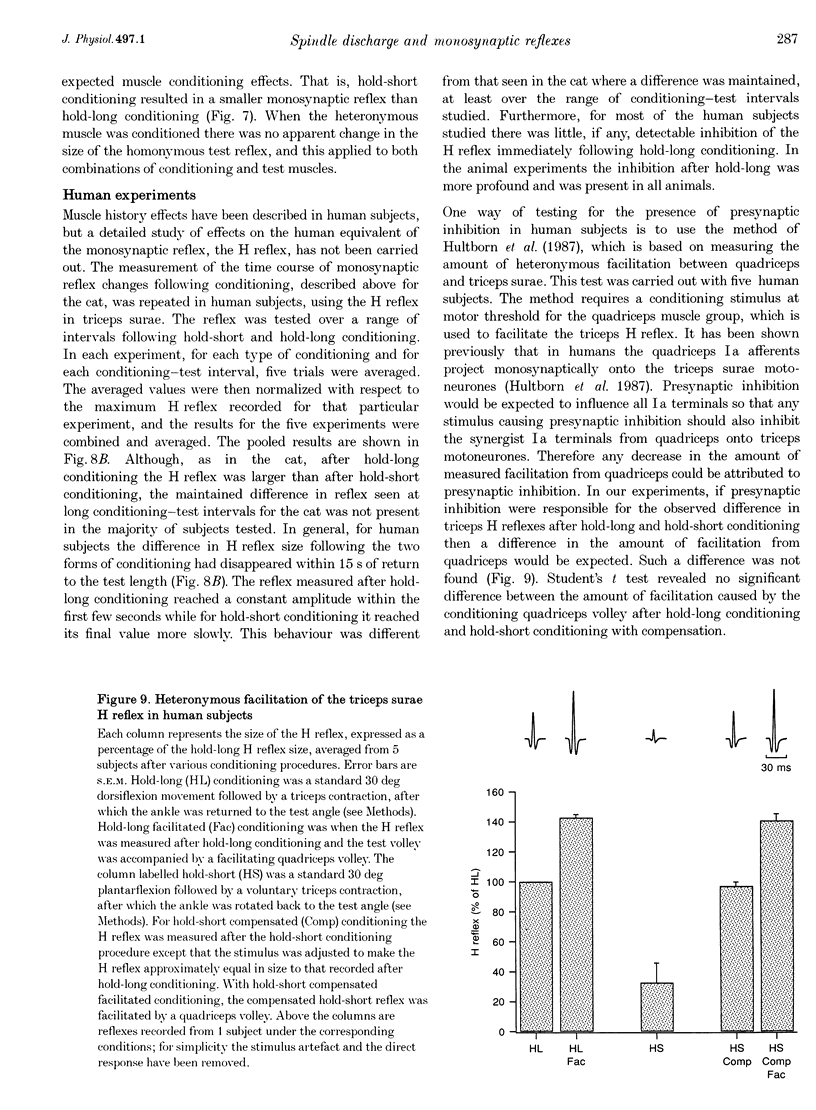
1. Experiments were carried out to test the effect of changes in spindle resting discharge on the size of monosynaptic reflexes in the cat and on the H reflex in humans. Resting discharge was altered by contracting the triceps surae muscle at longer (hold-long) or shorter (hold-short) lengths than that at which the reflex was tested. 2. The reflex in the cat was larger after hold-long than after hold-short conditioning, and the difference, after an initial decline, was well maintained. For the human H reflex a similar pattern was observed except that 15 s after muscle conditioning the difference in reflex size had disappeared. 3. Monosynaptic reflex depression immediately after hold-long conditioning, when most of the muscle spindles are silent, was attributed to the high level of spindle discharge during the immediately preceding hold-long period. The time course of this inhibition was too long to be accounted for by presynaptic inhibition. 4. In the cat heteronymous muscle conditioning was used to test whether presynaptic inhibition could be responsible for reflex depression using the synergist muscle pair lateral gastrocnemius-soleus and medial gastrocnemius. Conditioning one of the pair did not affect the reflex in the other, the opposite result to that expected with presynaptic inhibition. A similar experiment in which the triceps H reflex in human subjects was facilitated by a quadriceps volley gave the same result. 5. Thus this study presents evidence that monosynaptic reflexes are depressed by the on-going discharge of muscle spindles in the homonymous muscle, but that this depression does not appear to involve "classical' presynaptic inhibition.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnes C. D., Pompeiano O. Presynaptic and postsynaptic effects in the monosyaptic reflex pathway to extensor motoneurons folowing vibration of synergic muscles. Arch Ital Biol. 1970 Apr;108(2):259–294. [PubMed] [Google Scholar]
- COOK W. A., Jr, NEILSON D. R., Jr, BROOKHART J. M. PRIMARY AFFERENT DEPOLARIZATION AND MONOSYNAPTIC REFLEX DEPRESSION FOLLOWING SUCCINYLCHOLINE ADMINISTRATION. J Neurophysiol. 1965 Mar;28:290–311. doi: 10.1152/jn.1965.28.2.290. [DOI] [PubMed] [Google Scholar]
- CURTIS D. R., ECCLES J. C. Synaptic action during and after repetitive stimulation. J Physiol. 1960 Feb;150:374–398. doi: 10.1113/jphysiol.1960.sp006393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone C., Nielsen J. Methodological implications of the post activation depression of the soleus H-reflex in man. Exp Brain Res. 1989;78(1):28–32. doi: 10.1007/BF00230683. [DOI] [PubMed] [Google Scholar]
- ECCLES J. C., SCHMIDT R. F., WILLIS W. D. Presynaptic inhibition of the spinal monosynaptic reflex pathway. J Physiol. 1962 May;161:282–297. doi: 10.1113/jphysiol.1962.sp006886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FUJIMORI B., ELDRED E. Central effects of succinylcholine and decamethonium on monosynaptic reflexes. Am J Physiol. 1961 Apr;200:699–702. doi: 10.1152/ajplegacy.1961.200.4.699. [DOI] [PubMed] [Google Scholar]
- Gandevia S. C., Wilson L., Cordo P. J., Burke D. Fusimotor reflexes in relaxed forearm muscles produced by cutaneous afferents from the human hand. J Physiol. 1994 Sep 15;479(Pt 3):499–508. doi: 10.1113/jphysiol.1994.sp020313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerilovsky L., Tsvetinov P., Trenkova G. Peripheral effects on the amplitude of monopolar and bipolar H-reflex potentials from the soleus muscle. Exp Brain Res. 1989;76(1):173–181. doi: 10.1007/BF00253634. [DOI] [PubMed] [Google Scholar]
- Gillies J. D., Lance J. W., Neilson P. D., Tassinari C. A. Presynaptic inhibition of the monosynaptic reflex by vibration. J Physiol. 1969 Nov;205(2):329–339. doi: 10.1113/jphysiol.1969.sp008968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Serratos H. Inward spread of activation in vertebrate muscle fibres. J Physiol. 1971 Feb;212(3):777–799. doi: 10.1113/jphysiol.1971.sp009356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory J. E., Mark R. F., Morgan D. L., Patak A., Polus B., Proske U. Effects of muscle history on the stretch reflex in cat and man. J Physiol. 1990 May;424:93–107. doi: 10.1113/jphysiol.1990.sp018057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory J. E., Morgan D. L., Proske U. Aftereffects in the responses of cat muscle spindles. J Neurophysiol. 1986 Aug;56(2):451–461. doi: 10.1152/jn.1986.56.2.451. [DOI] [PubMed] [Google Scholar]
- Gregory J. E., Morgan D. L., Proske U. Changes in size of the stretch reflex of cat and man attributed to aftereffects in muscle spindles. J Neurophysiol. 1987 Sep;58(3):628–640. doi: 10.1152/jn.1987.58.3.628. [DOI] [PubMed] [Google Scholar]
- HUGON M., PAILLARD J. Dépression durable du réflexe achillén, consécutif à l'allongement du triceps sural, chez l'homme. J Physiol (Paris) 1955;47(1):193–196. [PubMed] [Google Scholar]
- Honig M. G., Collins W. F., 3rd, Mendell L. M. Alpha-motoneuron EPSPs exhibit different frequency sensitivities to single Ia-afferent fiber stimulation. J Neurophysiol. 1983 Apr;49(4):886–901. doi: 10.1152/jn.1983.49.4.886. [DOI] [PubMed] [Google Scholar]
- Houk J., Henneman E. Responses of Golgi tendon organs to active contractions of the soleus muscle of the cat. J Neurophysiol. 1967 May;30(3):466–481. doi: 10.1152/jn.1967.30.3.466. [DOI] [PubMed] [Google Scholar]
- Hultborn H., Illert M., Nielsen J., Paul A., Ballegaard M., Wiese H. On the mechanism of the post-activation depression of the H-reflex in human subjects. Exp Brain Res. 1996 Mar;108(3):450–462. doi: 10.1007/BF00227268. [DOI] [PubMed] [Google Scholar]
- Hultborn H., Meunier S., Morin C., Pierrot-Deseilligny E. Assessing changes in presynaptic inhibition of I a fibres: a study in man and the cat. J Physiol. 1987 Aug;389:729–756. doi: 10.1113/jphysiol.1987.sp016680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz R., Morin C., Pierrot-Deseilligny E., Hibino R. Conditioning of H reflex by a preceding subthreshold tendon reflex stimulus. J Neurol Neurosurg Psychiatry. 1977 Jun;40(6):575–580. doi: 10.1136/jnnp.40.6.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mark R. F., Coquery J. M., Paillard J. Autogenetic reflex effects of slow or steady stretch of the calf muscles in man. Exp Brain Res. 1968;6(2):130–145. doi: 10.1007/BF00239167. [DOI] [PubMed] [Google Scholar]
- Nielsen J., Petersen N., Ballegaard M., Biering-Sørensen F., Kiehn O. H-reflexes are less depressed following muscle stretch in spastic spinal cord injured patients than in healthy subjects. Exp Brain Res. 1993;97(1):173–176. doi: 10.1007/BF00228827. [DOI] [PubMed] [Google Scholar]
- Schieppati M., Crenna P. From activity to rest: gating of excitatory autogenetic afferences from the relaxing muscle in man. Exp Brain Res. 1984;56(3):448–457. doi: 10.1007/BF00237985. [DOI] [PubMed] [Google Scholar]
- Táboríková H., Sax D. S. Conditioning of H-reflexes by a preceding subthreshold H-reflex stimulus. Brain. 1969 Mar;92(1):203–212. doi: 10.1093/brain/92.1.203. [DOI] [PubMed] [Google Scholar]
- WALL P. D. Excitability changes in afferent fibre terminations and their relation to slow potentials. J Physiol. 1958 Jun 18;142(1):1–21. doi: 10.1113/jphysiol.1958.sp005997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson L. R., Gandevia S. C., Burke D. Increased resting discharge of human spindle afferents following voluntary contractions. J Physiol. 1995 Nov 1;488(Pt 3):833–840. doi: 10.1113/jphysiol.1995.sp021015. [DOI] [PMC free article] [PubMed] [Google Scholar]


