Abstract
Objective
To investigate the clinical effect and safety of Implantable Collamer Lens (ICL) implantation in myopic patients with relatively shallow anterior chamber depth (ACD).
Methods
Retrospective analysis, comparative, non-interventional case series. Patients with myopia who underwent ICL implantation were included in the study and were categorized into two groups: one with a relatively shallow ACD (2.60 mm ≤ ACD < 2.80 mm) and the other with a normal ACD (ACD ≥2.80 mm). Each group comprised 51 eyes and were reviewed for at least 6 months. To evaluate preoperative and postoperative visual acuity, intraocular pressure (IOP), central vault, ACD, endothelial cell density (ECD) and anterior chamber angle parameters [ trabecular iris angle (TIA), angle opening distance at 500 μm and 750 μm (AOD500, AOD750), trabecular iris space area at 500 μm and 750 μm (TISA500, TISA750) ]. Analyze the trend of postoperative data changes of 1 week, 1 month, 3 months, and at least 6 months. Patients in relatively shallow ACD with vault <100 μm or >1000 μm were performed detailed follow-up.
Results
The postoperative uncorrected distance visual acuity (UDVA) and best corrected visual acuity (BCVA) surpassed the preoperative BCVA in two groups. The group differences of effectiveness index (EI) was p = 0.409 and of safety index (SI) was p = 0.563. The EI were 1.07 and 1.09, SI were1.10 and 1.21. Postoperative vision and refractive error were effectively corrected. The preoperative and postoperative IOP and ECD values of the two groups were within the normal range, and the preoperative and postoperative differences and the group differences of IOP and ECD were p = 0.649, p = 0.501, p = 0.222, p = 0.276. The ACD in two groups were reduced by approximately 25 % and 26 %, the nasal and temporal TIA were reduced to 45 % and 50 % approximately. The nasal and temporal AOD500, AOD750, TISA500, and TISA750 in the relatively shallow ACD group were reduced 40 % approximately and in the normal ACD group were reduced to 30 % approximately. All the preoperative and postoperative differences and the group differences of anterior segment parameters were statistically significant (all p < 0.01), with more significant anterior segment changes in normal ACD group.The postoperative data in two groups showed that when the follow-up time was prolonged, the central vault was decreasing, and the postoperative ACD was increasing, the postoperative TIA increased slowly in relatively shallow ACD group and decreased slowly in normal ACD group, temporal TIA were greater than nasal TIA, with no significant variation. There were four eyes in the relatively shallow ACD group in the remarkable abnormal postoperative central vault. Three eyes had second operation: one eye had an ICL alignment, and two eyes had ICL replacement.
Conclusion
Patients with relatively shallow ACD who underwent ICL implantation had fine postoperative clinical efficacy and safety.
Keywords: Relatively shallow anterior chamber depth, Implantable Collamer Lens, Clinical efficacy, Safety
1. Background
Myopic refractive surgery is categorized into keratorefractive surgery and intraocular refraction surgery. Phakic posterior chamber intraocular lens (Implantable Collamer lens, ICL) is one of the most common intraocular refractive surgeries in clinical application. As a kind of intraocular surgery in which the posterior chamber intraocular lens haptics are fixed in the ciliary sulcus without destroying the morphology of the eye, ICL implantation has refractive stability, low corneal risk, better visual quality, and comfort. It retains lens accommodation and has surgical reversibility and has been chosen as a preferred operation method for an increasing number of myopic patients in recent years [1,2]. ICL implantation is also the only operation method for refractive correction for patients with over-high myopia, thin corneal thickness or corneal curvature abnormality (either too low or too high), and corneal biomechanical abnormality. It is generally recognized worldwide that ICL implantation has good operation safety in patients with anterior chamber depth (ACD) ≥ 2.80 mm and open anterior chamber angle (ACA). For safe ICL implantation, the US FDA recommends patients having ACD ≥3.00 mm [3], and the European clinical guidelines for refractive surgery requires ordinary myopia patients having ACD ≥2.80 mm and hyperopia patients having ACD ≥3.00 mm [4]. While the Chinese consensus for intraocular refraction surgery suggests that patients undergoing intraocular refractive surgery (intraocular lens of anterior chamber,iris-clamped, posterior chamber) having ACD ≥2.80 mm, and patients of PRL (Phakic Refractive Lens, which is currently under improvement and not commercially available) implantation having ACD ≥2.60 mm [5]. For patients with relatively shallow ACD (2.60 mm ≤ ACD ≤2.80 mm), there have been relevant reports on the safety of PRL implantation, but there is a lack of relevant data evaluation for ICL implantation both domestically and internationally, and there are also few reports on the efficacy and safety of ICL implantation. Sufficient ACD and ACA openness can provide an ideal postoperative vault and trabecular iris angle (TIA), which can effectively avoid complicated cataracts caused by low vault, secondary glaucoma caused by shallow TIA or ACA closure, corneal endothelial damage, etc [[6], [7], [8]]. Observation of changes to postoperative intraocular pressure(IOP), vault, ACD, ACA status, and corneal endothelial cell density (ECD) can evaluate the safety and stability of ICL implantation [9,10]. Therefore, we selected the patients with relatively shallow ACD having ICL implantation in our hospital to compare the postoperative outcomes and safety in early and midterm postoperative time.
2. Objects and methods
2.1. Objects
Retrospective analysis. Patients having ICL implantation in Daping Hospital from January 2020 to March 2021 were selected, who were divided into two groups: relatively shallow ACD group (31 people and 51 eyes) and normal ACD group (26 people and 51 eyes). All patients were informed preoperatively of the risks of ICL implantation for normal and relatively shallow ACD, and all they expressed their understanding and agreed to the surgery by signing the surgery informed consent form. All patients were informed about the use of their preoperative and postoperative data for the study, and they expressed their agreement and signed the informed consent form. The study was approved by the hospital ethics committee [Medical Research and Ethical Review (2022) No.182].
2.1.1. Inclusion criteria
(1) willingness to undergo myopic surgery (2) 18–45 years old (3) diopter remains stable for ≥2 years, with an increase of <0.5D within 2 years (4) indications for ICL implantation and inability to undergo keratorefractive surgery (5) relatively shallow ACD group: 2.60 mm ≤ ACD <2.80 mm; normal ACD group: ACD ≥2.80 mm (6) ECD ≥2000 cells/mm2 (7) Follow-up time ≥6 months.
2.1.2. Exclusion criteria
(1) cataract (2) ciliary body cysts (3) stenosis or closure of ACA (4) ECD <2000 cells/mm2 (5) follow-up time < 6months and incomplete medical records.
2.2. Methods
2.2.1. Preoperative examination
All patients were examined by slit lamp for anterior segment, slit lamp combined with indirect funduscopy for fundus examination, standard logarithmic visual acuity chart for visual acuity examination, non-contact tonometer for IOP examination, auto kerato-refractometer and phoroptor to record optometry results. Visante's anterior segment OCT (Carl Zeiss, Germany) for anterior segment condition measurements, corneal endothelial counting for ECD measurements and UBM for ACA and ciliary sulcus morphology measurements. Visual acuity, IOP, computerized and comprehensive optometry, ACD, ACA parameters TIA, angle opening distance at 500 μm and 750 μm (AOD500, AOD750), trabecular iris space area at 500 μm and 750 μm (TISA500, TISA750) and ECD were recorded.
2.2.2. ICL lens selection
ICL sizes were available in four varieties: 12.1, 12.6, 13.2, and 13.7 mm, with optical diameters spanning from 4.9 to 5.8 mm. By using an online calculation formula provided by the STAAR company, the ICL lens size was determined by the refractive error, corneal curvature, ACD, white-to-white (WTW), scleral spur-to-scleral spur (STS), and the shape of the ciliary sulcus, which were referred to corneal topography, anterior segment OCT, and UBM [11]. The rules for size selection are consistent with those for eyes with ACD greater than 2.80 mm.
2.2.3. Surgical methods
All patients were given antibiotic eye drops for three days to prevent infection preoperatively. They received adequate pupil dilation 1 h preoperatively and a temporal transparent corneal incision with 3 mm width was made under surface anesthesia. The ICL implantation was performed by the same physician who is qualified to perform this procedure, implanting EVO (V4c) Lens. Patients were given routine care including the use of antibiotics, corticosteroids, nonsteroidal anti-inflammatory drugs, and artificial tear eye drops postoperatively.
2.2.4. Follow-up observation
All patients were followed up for at least half a year, postoperative data in time of 1 week, 1 month, 3 months, and 6 months were incorporated into the study, and postoperative data in time of >6 months were counted as a 6-month data. Patients with vault ≤100 μm or ≥1000 μm were followed up in detail. For each review, visual acuity, IOP, optometry, slit lamp, anterior segment OCT and ECD were included, and examination methods were the same as preoperative.
All data were used to statistically analyze by SPSS19.0 and to statistical descriptions by mean ± SD. As the data satisfied normal distribution, independent sample t-test was used to test the difference between the relatively shallow ACD group and the normal ACD group data, paired t-test was used to test the differences between the preoperative and postoperative >6 months data, repeated measures analysis of variance was used to analyze the differences at different postoperative times, LSD method and Bonferroni correction (when p < 0.05) for pairwise comparison was used to analyze the differences that differed within the group. Otherwise, Wilcoxon signed-rank test would be used.The statistical results were considered significant if p < 0.05.
3. Results
3.1. Comparison of preoperative characteristics of patients having ICL implantation with ACD <2.80 mm and ACD ≥2.80 mm (Table 1)
Table 1.
Preoperative characteristics of eyes with ACD<2.80 mm and ACD≥2.80 mm.
| Variables | n | Age (y) | Sph (D) | Cyl (D) | SE (D) | IOP (mmHg) | ECD (cells/mm2) | ACD (mm) | WTW (mm) | ATA (mm) | ICL size (mm) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| ACD<2.80 mm | 51 | 28.33 ± 5.30 | −8.33 ± 2.57 | −1.11 ± 0.87 | −8.89 ± 2.64 | 15.7 ± 3.0 | 2611 ± 215 | 2.72 ± 0.06 | 11.22 ± 0.33 | 11.54 ± 0.38 | 12.5 ± 0.3 |
| ACD≥2.80 mm | 51 | 28.61 ± 5.94 | −7.78 ± 1.58 | −1.14 ± 0.82 | −8.36 ± 1.78 | 15.5 ± 2.8 | 2661 ± 196 | 3.25 ± 0.22 | 11.55 ± 0.40 | 11.89 ± 0.41 | 12.7 ± 0.3 |
| t value | −0.246 | −1.288 | 0.176 | −1.186 | 0.457 | −1.229 | −16.764 | −4.661 | −4.583 | −3.549 | |
| p value | 0.806 | 0.201 | 0.861 | 0.238 | 0.649 | 0.222 | <0.01 | <0.01 | <0.01 | <0.01 |
Sph = Spherical refractive erro; Cyl = Cylindrical refractive erro; SE=Spherical equivalent; D = diopter; IOP=Intraocular pressure; ECD = Endothelial cell count; ACD = Anterior chamber depth; WTW=White-to-white; ATA = Angle to angle; Data are mean ± SD unless otherwise indicated.
There were no statistically significant differences in the number of eyes, age, diopter, IOP, and ECD between the two groups.
3.2. Comparison of preoperative and postoperative >6 months variations of patients having ICL implantation with ACD <2.80 mm and ACD ≥2.80 mm in early and midterm time (Table 2 to Table 4)
Table 2.
Preoperative and postoperative(≥6m) vision and diopter of eyes with ACD<2.80 mm and ACD≥2.80 mm.
| Variables | Vision(LogMAR) |
Sph/D |
Cyl/D |
SE/D |
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre-op |
Post-op |
t value | p value | EI | SI | Pre-op | Post-op | t value | p value | Pre-op | Post-op | t value | p value | Pre-op | Post-op | t value | p value | ||||
| BCVA | UDVA | BCVA | |||||||||||||||||||
| ACD<2.80 mm | 0.050 ± 0.052 | 0.020 ± 0.068 | 0.002 ± 0.014 | 2.858 | 6.607 | 0.006 | <0.01 | 1.07 | 1.10 | −8.33 ± 2.57 | 0.05 ± 0.52 | −23.749 | <0.01 | −1.11 ± 0.87 | −0.58 ± 0.36 | −3.718 | 0.001 | −8.89 ± 2.64 | −0.24 ± 0.57 | −24.284 | <0.01 |
| ACD≥2.80 mm | 0.090 ± 0.040 | 0.011 ± 0.045 | 0.004 ± 0.020 | 8.601 | 13.932 | <0.01 | <0.01 | 1.19 | 1.21 | −7.78 ± 1.58 | 0.14 ± 0.48 | −34.477 | <0.01 | −1.14 ± 0.82 | −0.55 ± 0.36 | −5.045 | <0.01 | −8.36 ± 1.78 | −0.13 ± 0.48 | −31.708 | <0.01 |
| t value | −4.3 | 0.828 | −0.581 | −1.288 | −0.888 | 0.176 | −0.479 | −1.186 | −1.021 | ||||||||||||
| p value | <0.01 | 0.409 | 0.563 | 0.201 | 0.377 | 0.861 | 0.633 | 0.238 | 0.31 | ||||||||||||
Data are mean ± SD unless otherwise indicated.
Table 4.
Preoperative and postoperative (≥6m) anterior segment parameters of 51 eyes with ACD<2.80 mm and ACD≥2.80 mm.
| Variables | ACD<2.80 mm |
ACD≥2.80 mm |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Pre-op | Post-op | post/pre(%) | p value | Pre-op | Post-op | post/pre(%) | p value | ||
| ACD (mm) | 2.73 ± 0.06 | 2.07 ± 0.27 | 75.82 | <0.01 | 3.25 ± 0.22 | 2.39 ± 0.19 | 73.54 | <0.01 | |
| p value | <0.01 | <0.01 | 0.112 | ||||||
| TIA (°) | T | 48.7 ± 8.5 | 26.4 ± 6.5 | 54.21 | <0.01 | 58.4 ± 10.3 | 29.7 ± 6.2 | 50.86 | <0.01 |
| N | 46.4 ± 7.9 | 25.5 ± 6.6 | 54.96 | <0.01 | 56.1 ± 9.6 | 27.4 ± 6.4 | 48.84 | <0.01 | |
| p value | T | <0.01 | 0.011 | 0.284 | |||||
| N | <0.01 | 0.138 | 0.025 | ||||||
| AOD500 (mm) | T | 0.610 ± 0.204 | 0.253 ± 0.072 | 41.48 | <0.01 | 0.910 ± 0.353 | 0.289 ± 0.073 | 31.76 | <0.01 |
| N | 0.554 ± 0.163 | 0.243 ± 0.073 | 43.86 | <0.01 | 0.808 ± 0.287 | 0.265 ± 0.073 | 32.80 | <0.01 | |
| p value | T | <0.01 | 0.015 | 0.023 | |||||
| N | <0.01 | 0.142 | 0.002 | ||||||
| AOD750 (mm) | T | 0.829 ± 0.219 | 0.360 ± 0.111 | 43.43 | <0.01 | 1.202 ± 0.376 | 0.424 ± 0.091 | 35.27 | <0.01 |
| N | 0.746 ± 0.184 | 0.343 ± 0.102 | 45.98 | <0.01 | 1.057 ± 0.308 | 0.380 ± 0.100 | 35.95 | <0.01 | |
| p value | T | <0.01 | 0.002 | 0.029 | |||||
| N | <0.01 | 0.068 | 0.030 | ||||||
| TISA500 (mm2) | T | 0.202 ± 0.071 | 0.086 ± 0.029 | 42.57 | <0.01 | 0.314 ± 0.148 | 0.091 ± 0.025 | 28.98 | <0.01 |
| N | 0.186 ± 0.056 | 0.079 ± 0.027 | 42.47 | <0.01 | 0.278 ± 0.105 | 0.083 ± 0.027 | 29.86 | <0.01 | |
| p value | T | <0.01 | 0.298 | 0.007 | |||||
| N | <0.01 | 0.389 | 0.001 | ||||||
| TISA750 (mm2) | T | 0.379 ± 0.118 | 0.162 ± 0.048 | 42.74 | <0.01 | 0.576 ± 0.235 | 0.181 ± 0.043 | 31.42 | <0.01 |
| N | 0.347 ± 0.096 | 0.151 ± 0.045 | 43.52 | <0.01 | 0.511 ± 0.177 | 0.163 ± 0.046 | 31.90 | <0.01 | |
| p value | T | <0.01 | 0.043 | 0.009 | |||||
| N | <0.01 | 0.168 | 0.001 | ||||||
T = Temporal; N=Nasal; TIA = Trabecular iris angle; AOD500 = Angle opening distance at 500 μm; AOD750 = Angle opening distance at 750 μm; TISA500 = Trabecular iris space area at 500 μm; TISA750 = Trabecular iris space area at 750 μm; Data are mean ± SD unless otherwise indicated.
Vision was measured using best corrected visual acuity (BCVA) and uncorrected distance visual acuity (UDVA). Effectiveness index (EI) = postoperative UDVA/preoperative BCVA; Safety index (SI) = postoperative BCVA/preoperative BCVA. The postoperative UDVA and BCVA surpassed the preoperative BCVA. The EI and SI were both greater than 1.00 (see Table 2). Postoperative vision and refractive error were effectively corrected, with no impact from ACD on postoperative vision and refractive error.
The preoperative and postoperative IOP and ECD values of the two groups were within the normal range, and the preoperative and postoperative differences and the group differences of IOP and ECD all were p > 0.05 (see Table 3), indicating that ICL implantation had no significant effect on postoperative IOP and ECD in early and midterm postoperative time, with no impact from ACD on IOP and ECD.
Table 3.
Preoperative and postoperative(≥6m) IOP and ECD with ACD<2.80 mm and ACD≥2.80 mm.
| Variables | IOP (mmHg) |
ECD (cells/mm2) |
||||||
|---|---|---|---|---|---|---|---|---|
| Pre-op | Post-op | t value | p value | Pre-op | Post-op | t value | p value | |
| ACD<2.80 mm | 15.7 ± 3.0 | 15.7 ± 2.8 | 0.286 | 0.776 | 2611 ± 215 | 2615 ± 256 | 0.649 | 0.519 |
| ACD≥2.80 mm | 15.5 ± 2.8 | 15.3 ± 2.8 | −0.107 | 0.915 | 2661 ± 196 | 2666 ± 217 | −0.143 | 0.887 |
| t value | 0.457 | 0.676 | −1.229 | −1.094 | ||||
| p value | 0.649 | 0.501 | 0.222 | 0.276 | ||||
Data are mean ± SD unless otherwise indicated.
The relatively shallow ACD group: the ACD were reduced by approximately 25 %, the nasal and temporal TIA were reduced to 45 % approximately, the AOD500, AOD750, TISA500, and TISA750 were reduced to 40%–45 % approximately. The normal ACD group: the ACD were reduced by approximately 26 %, the nasal and temporal TIA were reduced to 50 % approximately, the AOD500, AOD750, TISA500, and TISA750 were reduced to 25%–35 % approximately. All the postoperative anterior segment parameters differences were statistically significant (all p < 0.01), with more significant anterior segment changes in normal ACD group (see Table 4). It indicated that ICL implantation significantly alters the anterior segment structure and results in a smaller anterior chamber space.
3.3. Postoperative variations of patients having ICL implantation with ACD <2.80 mm and ACD ≥2.80 mm in early and midterm time (Table 5)
Table 5.
Postoperative variations of eyes with ACD<2.80 mm and ACD≥2.80 mm.
| Variables | 1w | 1m | 3m | ≥6m | p | p1 | p2 | p3 | p4 | p5 | p6 | Cor p | Cor p1 | Cor p2 | Cor p3 | Cor p4 | Cor p5 | Cor p6 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IOP (mmHg) | ACD<2.80 mm | 20.7 ± 5.7 | 15.3 ± 2.6 | 15.5 ± 2.5 | 15.7 ± 2.8 | <0.001 | <0.001 | <0.001 | <0.001 | 0.554 | 0.288 | 0.526 | <0.001 | <0.001 | <0.001 | <0.001 | 1.000 | 1.000 | 1.000 |
| ACD≥2.80 mm | 17.4 ± 2.8 | 14.7 ± 2.7 | 14.8 ± 2.6 | 15.3 ± 2.8 | <0.001 | <0.001 | <0.001 | <0.001 | 0.738 | 0.065 | 0.167 | <0.001 | <0.001 | <0.001 | <0.001 | 1.000 | 0.391 | 1.000 | |
| ECD (cells/mm2) | ACD<2.80 mm | 2625 ± 197 | 2607 ± 229 | 2643 ± 209 | 2615 ± 256 | 0.173 | 0.236 | 0.247 | 0.631 | 0.007 | 0.669 | 0.069 | 0.173 | 1.000 | 1.000 | 1.000 | 0.043 | 1.000 | 0.413 |
| ACD≥2.80 mm | 2705 ± 192 | 2663 ± 186 | 2732 ± 193 | 2666 ± 217 | 0.015 | 0.052 | 0.234 | 0.186 | 0.003 | 0.908 | 0.024 | 0.015 | 0.311 | 1.000 | 1.000 | 0.016 | 1.000 | 0.142 | |
| ACD (mm) | ACD<2.80 mm | 1.96 ± 0.21 | 1.99 ± 0.25 | 2.02 ± 0.25 | 2.07 ± 0.27 | <0.001 | 0.106 | 0.001 | <0.001 | 0.009 | <0.001 | <0.001 | <0.001 | 0.633 | 0.004 | <0.001 | 0.056 | <0.001 | 0.002 |
| ACD≥2.80 mm | 2.31 ± 0.21 | 2.34 ± 0.20 | 2.38 ± 0.26 | 2.39 ± 0.19 | 0.069 | 0.074 | 0.096 | <0.001 | 0.257 | <0.001 | 0.859 | 0.069 | 0.447 | 0.576 | <0.001 | 1.000 | 0.001 | 1.000 | |
| vault (μm) | ACD<2.80 mm | 504.12 ± 212.36 | 483.92 ± 200.59 | 457.25 ± 216.87 | 407.12 ± 222.63 | <0.001 | 0.013 | <0.001 | <0.001 | 0.002 | <0.001 | <0.001 | <0.001 | 0.075 | 0.001 | <0.001 | 0.011 | <0.001 | <0.001 |
| ACD≥2.80 mm | 612.75 ± 172.80 | 595.49 ± 170.66 | 585.88 ± 208.41 | 546.67 ± 155.86 | 0.013 | 0.078 | 0.263 | <0.001 | 0.675 | <0.001 | 0.064 | 0.013 | 0.465 | 1.000 | <0.001 | 1.000 | <0.001 | 0.383 | |
| T TIA (°) | ACD<2.80 mm | 22.8 ± 7.0 | 23.9 ± 7.0 | 23.8 ± 6.8 | 26.3 ± 6.7 | 0.003 | 0.325 | 0.259 | <0.001 | 0.995 | 0.010 | <0.001 | 0.003 | 1.000 | 1.000 | 0.003 | 1.000 | 0.058 | <0.001 |
| ACD≥2.80 mm | 32.0 ± 9.0 | 31.9 ± 7.1 | 28.3 ± 7.0 | 29.7 ± 6.2 | <0.001 | 0.915 | <0.001 | 0.041 | <0.001 | 0.005 | 0.071 | <0.001 | 1.000 | 0.002 | 0.245 | 0.001 | 0.033 | 0.428 | |
| N TIA (°) | ACD<2.80 mm | 23.1 ± 7.3 | 23.2 ± 6.4 | 22.1 ± 6.9 | 25.4 ± 6.6 | 0.009 | 0.932 | 0.326 | 0.027 | 0.188 | 0.036 | <0.001 | 0.009 | 1.000 | 1.000 | 0.164 | 1.000 | 0.216 | <0.001 |
| ACD≥2.80 mm | 30.0 ± 8.9 | 29.5 ± 8.0 | 26.3 ± 7.3 | 27.4 ± 6.3 | <0.001 | 0.490 | <0.001 | 0.012 | 0.001 | 0.024 | 0.114 | <0.001 | 1.000 | 0.001 | 0.074 | 0.003 | 0.147 | 0.687 | |
T = Temporal; N=Nasal; p1 = 1w vs 1m; p2 = 1w vs 3m; p3 = 1w vs 6m; p4 = 1m vs 3m; p5 = 1m vs 6m; p6 = 3m vs 6m; Cor = Corrected; p1-6 are calculated using the LSD method (when p < 0.05, Bonferromi correction were uesd by Cor p1-6); Data are mean ± SD unless otherwise indicated.
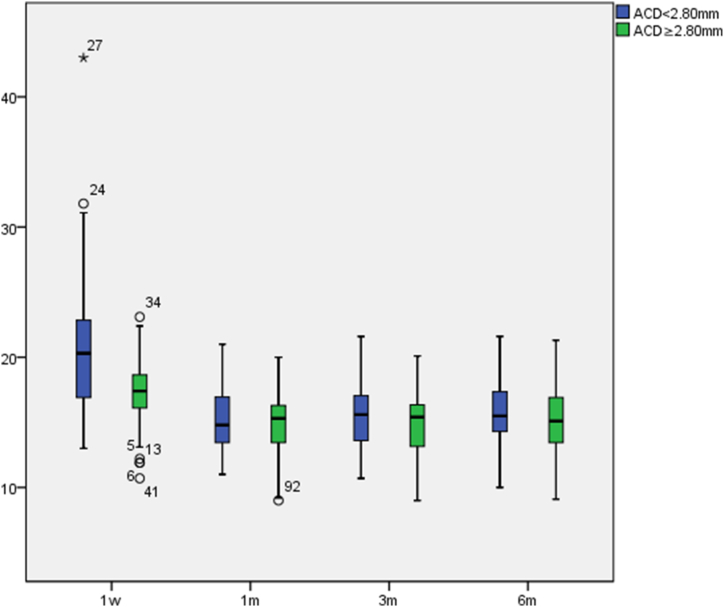
The postoperative IOP in both groups were all within normal values (see Fig. 1), indicating no significant increase due to the narrowness of the anterior chamber angle.There were significant differences in IOP at different postoperative times (p < 0.001). In pairwise comparisons, the IOP in both groups at 1 week postoperative compared to 1 month postoperative, 3 months postoperative, and postoperative >6 months had significant differences (p1 < 0.001, p2 < 0.001, p3 < 0.001). There were no significant differences in IOP at other times.
Fig. 1.
Postoperative IOP variations(mmHg).
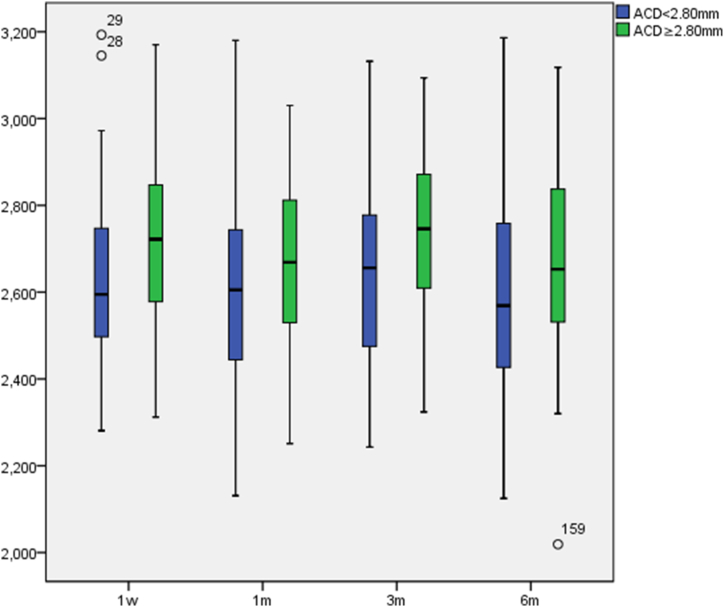
The postoperative ECD in both groups were all within normal values (see Fig. 2), with no significant differences in the relatively shallow ACD group (p = 0.173) and significant differences in normalACD group (p = 0.015) in ECD at different postoperative times. In pairwise comparisons, the ECD in both groups at 1 month postoperative compared to 3 months postoperative had significant differences (p4 = 0.007 and 0.003, Cor p4 = 0.043 and 0.016), with no significant differences at Other times.
Fig. 2.
Postoperative ECD variations(cells/mm2).
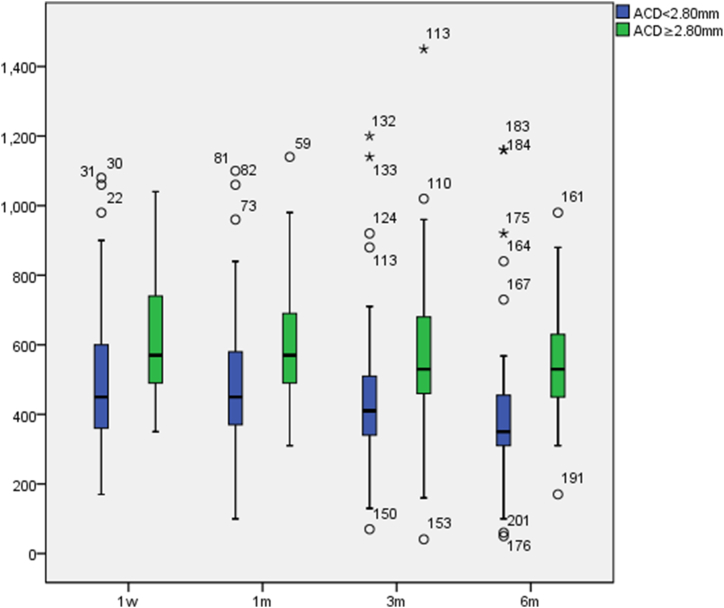
The vault in the normal ACD group were greater than in the relatively shallow ACD group. In the relatively shallow ACD group, the minimum value was 50 μm and the maximum value was 1160 μm; in the normal ACD group, the minimum value was 160 μm and the maximum value was 1450 μm. Most vaults were within the ideal range, which provided good surgical safety. Postoperative vaults in both groups decreased gradually with postoperative time (see Fig. 3), with significant differences in vaults at different times: the relatively shallow ACD group (p < 0.001), the normal ACD group (p = 0.013). The pairwise comparison differences are presented in Table 5.
Fig. 3.
Postoperative vault variations(mm).
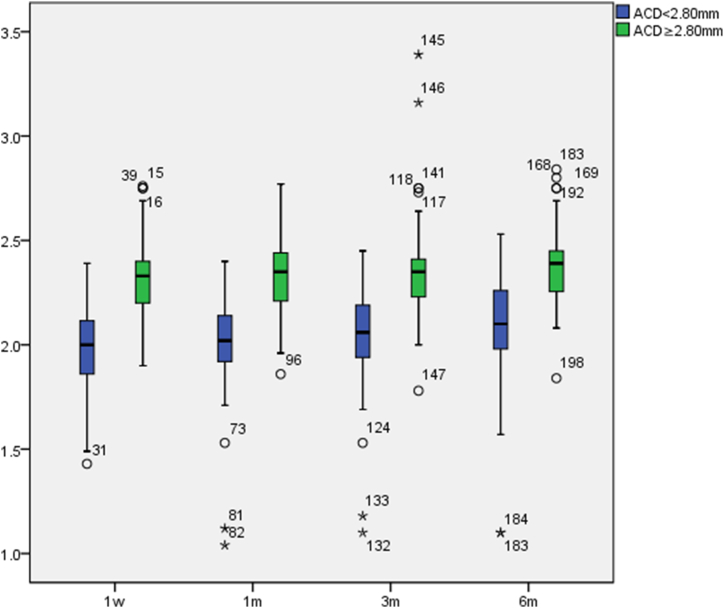
The postoperative ACD in the normal ACD group were greater than in the relatively shallow ACD group. Postoperative ACD values in both groups gradually increased with postoperative time (see Fig. 4) and were negatively correlated with vault (p = −0.859, p = −0.521). There were significant differences in the relatively shallow ACD group (p < 0.001) and no significant differences in the normal ACD group (p = 0.069) at different times. The pairwise comparison differences are presented in Table 5.
Fig. 4.
Postoperative ACD variations(mm).
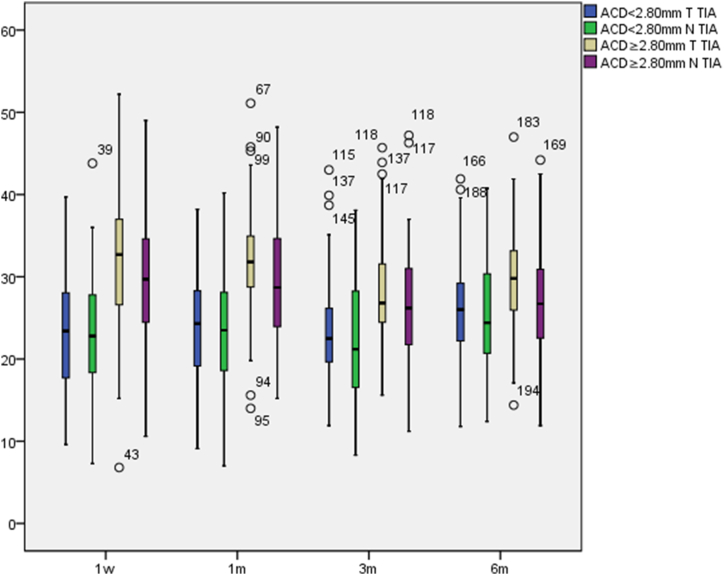
The postoperative TIA in the normal ACD group were greater than in the relatively shallow ACD group, temporal TIA were greater than nasal TIA (see Fig. 5). At different postoperative times, the postoperative TIA increased slowly in the relatively shallow ACD group and decreased slowly in the normal ACD group, with no significant variation. There were significant differences in temporal and nasal TIA in the relatively shallow ACD group (p = 0.003, p = 0.009) and the normal ACD group (p < 0.001, p < 0.001) at different times. The pairwise comparison differences are presented in Table 5.
Fig. 5.
Postoperative TIA variations(°).
3.4. Postoperative specialties of patients having ICL implantation with ACD <2.80 mm
The 51 cases in relatively shallow ACD group were followed up for at least 6 months, vaults of 39 eyes were 250–750 μm (76.47 %), vaults of 2 eyes were ≥1000 μm, and vaults of 2 eyes were ≤100 μm (see Table 6 for details). In postoperative specialties, there is no anterior chamber angle closure and all ICL haptics are located in the ciliary sulcus with UBM examination. Cases of vault ≥1000 μm showed no significant decrease in vault and normalized IOP in follow-up time, the postoperative ACD ≥1.10 mm and 15° ≤ TIA ≤20°, were given the treatments of 1 eye alignment and 1 eye replacement. Cases of vault ≤100 μm, in follow-up time the vaults were stabilized at 50–100 μm, and the postoperative ACD ≥2.50 mm, TIA ≥15°, were given the treatments of 1 eye (case of vault 50 μm) replacement and 1 eye (case of vault 60 μm) observation. For observation eye, the vaults were basically stabilized, the postoperative ACD ≥2.00 mm and TIA ≥20°, without high IOP or anterior subcapsular cataract.
Table 6.
Postoperative specialties conditions of patients having ICL implantation with ACD<2.80 mm(vault≥1000 or vault ≤100).
| Preoperative |
ICL |
Postoperative |
Solution | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ACD | WTW | STS | ATA | TIA(T/N) | Size | Location | time | Vault | ACD | TIA(T/N) | ||
| Case1 | 2.71 | 10.9 | 11.02 | 11.41 | 54.2/48.9 | I12.6 | horizontal | 14m | 1160 | 1.1 | 21.0/19.7 | ICL alignment |
| horizontal→vertical | ||||||||||||
| Case2 | 2.71 | 11 | 11.2 | 11.43 | 49.5/38.7 | T12.6 | horizontal | 14m | 1160 | 1.1 | 16.1/16.9 | ICL replacement |
| 12.6 → 12.1 | ||||||||||||
| Case3 | 2.79 | 11.2 | 10.92 | 11.01 | 48.4/47.3 | I12.1 | horizontal | 7m | 50 | 2.53 | 20.3/14.3 | ICL replacement |
| 12.1 → 12.6 | ||||||||||||
| Case4 | 2.75 | 11.2 | 11.69 | 11.91 | 60.4/50.8 | T12.1 | horizontal | 12m | 60 | 2.37 | 25.7/24.4 | observation |
ATA = angle-to-angle.
STS = scleral spur-to-scleral spur.
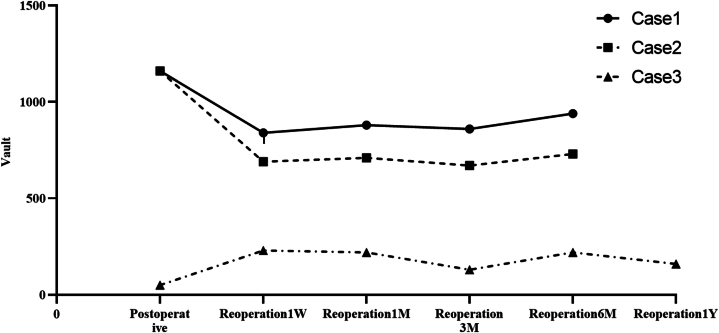
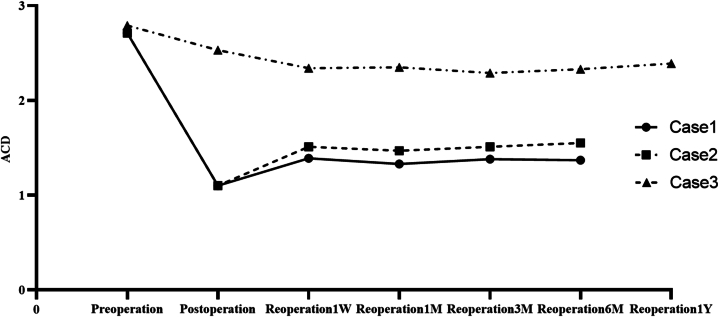
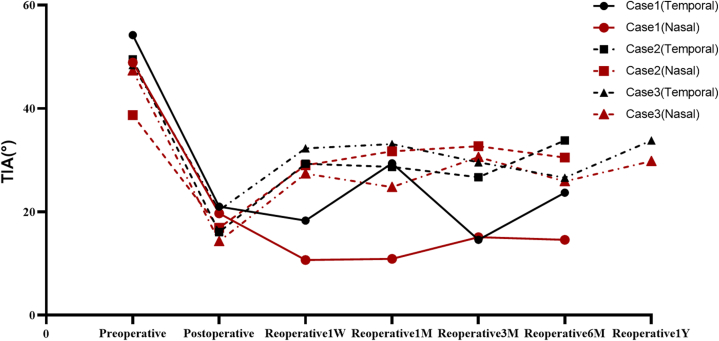
With follow-up of 3 reoperation eyes from 6 months to 1 year (see Table 7, Fig. 6, Fig. 7, Fig. 8), the eye with low vault having ICL replacement had good reoperation results: reoperation vault was stabilized at 300 μm, reoperation ACD≥2.00 mm and TIA ≥20°. The eye with a high vault having ICL alignment also had good reoperation outcomes: reoperation vault was stabilized at 850 μm, reoperation ACD ≥1.50 mm, and TIA ≥15°. The eye with a high vault having ICL replacement still had ideal reoperation effects: reoperation vault was stabilized at 700 μm, postoperative ACD ≥1.5 mm, and TIA ≥25°. There were better reoperation vault, ACD, and TIA, and there were steady changes after reoperation, without complications such as complicated cataract and secondary glaucoma.
Table 7.
Reoperative specialties conditions of patients having ICL implantation with ACD<2.80 mm.
| Solution | Case1 |
Case2 |
Case3 |
|
|---|---|---|---|---|
| ICL alignment | horizontal→vertical | ICL replacement | ||
| Reoperation1W | Vault | 840 | 690 | 230 |
| ACD | 1.39 | 1.51 | 2.34 | |
| TIA(Temporal) | 18.3 | 29.3 | 32.3 | |
| TIA(Nasal) | 10.7 | 29.0 | 27.4 | |
| Reoperation1M | Vault | 880 | 710 | 220 |
| ACD | 1.33 | 1.47 | 2.35 | |
| TIA(Temporal) | 29.4 | 28.7 | 33.1 | |
| TIA(Nasal) | 10.9 | 31.7 | 24.8 | |
| Reoperation3M | Vault | 880 | 710 | 130 |
| ACD | 1.33 | 1.47 | 2.29 | |
| TIA(Temporal) | 29.4 | 28.7 | 29.6 | |
| TIA(Nasal) | 10.9 | 31.7 | 30.6 | |
| Reoperation6M | Vault | 880 | 710 | 220 |
| ACD | 1.33 | 1.47 | 2.33 | |
| TIA(Temporal) | 29.4 | 28.7 | 26.6 | |
| TIA(Nasal) | 10.9 | 31.7 | 25.9 | |
| Reoperation1Y | Vault | 160 | ||
| ACD | 2.39 | |||
| TIA(Temporal) | 33.8 | |||
| TIA(Nasal) | 29.8 | |||
Fig. 6.
Reoperation vault variations.
Fig. 7.
Reoperation ACD variations.
Fig. 8.
Reoperation TIA variations.
4. Discussion
Evolution Implantable Collamer Lens(EVO-ICL, V4c)is a posterior chamber IOL commonly used in clinical operation, and the central hole design can avoid intraocular structure damages caused by iridectomy, which establishes the postoperative aqueous circulation state closer to the physiological state [7,11]. Appropriate the vault, ACD, and opening status of ACA are important indicators for evaluating postoperative safety, and the ACD can reflect the volume of the anterior chamber and the opening degree of ACA, and observation of IOP and ECD can also corroborate postoperative safety. ICL haptics are located in the ciliary sulcus, when with the small size ICL, the opisthocoelous iris, the obvious lens swelling, and the wide ciliary sulcus results in the ICL haptics slipping down or not being able to stabilize in the ciliary sulcus, the vault may be lower and ICL may close to the anterior capsule of the lens, causing anterior subcapsular cataracts [2,12,13]. When with the large size ICL, the naturally dilated pupil, the antedisplacement of iris and the narrow ciliary sulcus, the vault may be higher and the iris moves forward resulting in a narrow ACA and a shallower anterior chamber and affecting the aqueous circulation of ACA, also the friction increases between the posterior surface of iris and the anterior surface of ICL, leading to iris depigmentation and chances of secondary closed-angle and pigment-disseminated glaucoma increase [14]. The postoperative results and safety of ICL implantation of patients with ACD ≥2.80 mm have been great accurately demonstrated [15,16], however, as a result of a preoperative relative narrow ACD and compact ACA, the postoperative vault, ACD and opening status of ACA of patients with relatively shallow ACD tend to be at or below the safe limit range easily, so preoperative evaluation and postoperative observation are particularly necessary and important.
In our study, the postoperative UDVA and BCVA surpassed the preoperative BCVA. The EI and SI were both greater than 1.00. Both patients in relatively shallow ACD group and normal ACD group had effectively corrected postoperative vision and refractive error, with no statistically significant group differences., It suggested that patients with relatively shallow ACD had the same perfect surgical outcomes as patients with normal ACD [1,[17], [18], [19], [20], [21], [22], [23]] As the same findings in our study, Xingtao Zhou [9].ect showed that the postoperative visual acuity of patients with relatively shallow ACD was basically in accordance with the preoperative expected visual acuity, and there was no significant relationship between the postoperative outcome and preoperative ACD. There was no significant decrease in visual acuity during the six-month follow-up period, which suggested that postoperative outcomes have perfect stability. The efficacy of ICL implantation is correlated with the accuracy of preoperative optometry and ICL degree choice [11].
Postoperative IOP and opening status of ACA laterally reflect aqueous humor circulation and function of ACA, and abnormalities of them may increase the probability of postoperative secondary glaucoma, which is particularly important in the mid-and long-term postoperative observation. IOP of patients with relatively shallow ACD were elevated in the early postoperative period (1 postoperative week), while within the normal range at other times, which was considered to be related to the hormonal ocular hypertension caused by topical steroid use, the relative inadequate drainage of aqueous humor when the status of ACA narrowed and the generation and drainage of aqueous humor had not yet reached a dynamic balance in the early period [17,24]. When steroid use was ceased for 1 postoperative week, IOP reached the normal values and no longer ascended in follow-up time, indicating that within the opening status of ACA, the possibility of postoperative long-term ocular hypertension of patients with relatively shallow ACD of ICL implantation was lower, and secondary glaucoma after ICL implantation has rarely been reported. Dynamic analysis of postoperative changes in ACA parameters (TIA, AOD, TISA, etc.) showed an approximate 50 % reduction in postoperative TIA, and an approximate 60 % reduction in postoperative AOD and TISA, similar to the results of Xingtao Zhou [9]. These changes were related to the peripheral iris expanding forward, the overall anterior chamber area decrease and the opening status of ACA becoming narrow caused by ICL haptics located in the ciliary sulcus. Changes in ACA were particularly significant within 1 postoperative month and stabilized at 3 postoperative months, which is generally consistent with the changes in patients with ACD ≥2.80 mm [22,25]. Postoperative status of ACA of patients with relatively shallow ACD remained open, and there was a negative correlation between the degree of ACA openness and vault. There were no obvious changes in ACA narrowing, progressive decreasing, or closure during the long-term follow-up, which indicated that under the premise of ACA openness, ACA narrowness would not cause inadequate drainage of aqueous humor and IOP fluctuation. The morphology of ACAdid not change significantly with the prolongation of the follow-up time, which further supported the safety of ICLimplantation for patients with relatively shallow ACD.
Vault and postoperative ACD are also indicators of postoperative safety of ICL, which can reflect the influence of ICL position on the anterior surface of the lens and the entire anterior chamber space. Shallow or excessive vaults are not complications in and of themselves but may increase the risk of complications [11]. The manufacturer suggests an ideal postoperative vault of 250–900 μm or 50 %–180 % of corneal thickness [26]. However, some recommend a range of 250–750 μm [27], while others argue that an even wider range, from 90 μm to 1000 μm or more, could be sufficient if there are no clinical issues [[28], [29], [30]]. The postoperative vault gradually decreases over time, with a significant decrease in 3 months, a slower decrease in 1 year, and a general stabilization in 3 years. The higher the postoperative vault in early time, the more pronounced the vault decrease over time [8,10,25,[31], [32], [33]]. Postoperative ACD is negatively correlated with vault, which are affected by pupil size and ocular accommodation [34]. Spasm of accommodation may lead to curvature changes on the anterior surface of the lens and pupil narrowing in bright light, and iris flattening leads to the low vault and high ACD [32], so changes of the pupil to the vault should be considered and the patient with enlarged pupil needs to pay attention to preoperative and postoperative vault and ACA changes in a dark environment. Our study is similar to Xingtao Zhou's, patients with relatively shallow ACD had ideal vault and ACD in early postoperative time, suggesting high safety. The vault was mildly decreased in prolonged follow-up time, but the decrease was not significant, suggesting well postoperative stability. There were no complications such as complicated cataract, secondary glaucoma, and massive depigmentation of iris during the follow-up time, which indicated that patients with relatively shallow ACD who had ICL implantation have consistent safety with patients with ACD ≥2.80 mm.
As limited intraocular space for patients with relatively shallow ACD, the probability of complications increases due to abnormal vaults, so the situation needs to be handled on a case-by-case basis. Through longer-term observation of four patients with abnormal vaults, we found that when vault ≥50 μm, postoperative ACD >1.10 mm, and TIA >15° within 1 postoperative year, the probability of postoperative complications and reoperation were little. There was no postoperative IOP increase, ACA closure, or anterior subcapsular cataracts during the period of observation. For abnormal vaults, the one eye with a low vault replaces a larger size ICL, while the two eyes with high vaults choose to adjust the ICL position (horizontal is adjusted to vertical position) and replace a smaller size ICL respectively. The reoperation vault, ACD, and TIA were within the ideal range of postoperative parameters of ICL implantation, which reduced the probability of complications such as complicated cataract and secondary glaucoma. With follow-up for at least 18 months to another eye with a low vault, there was no persistent decline in vault and no clinical complications such as anterior subcapsular cataracts. It suggested that those low vaults should be evaluated in conjunction with postoperative ACD and TIA, early continuous observation did not increase the probability of postoperative complications. When the signs of postoperative complications arise, reoperation such as ICL replacement and alignment should be considered if necessary. For patients with relatively shallow ACD, it was necessary to combine TIA, lens prominence situation, and ciliary sulcus morphology with the joint judgment before ICL implantation to select an appropriate size of ICL, which can reduce replacing times to achieve better postoperative results.
ECD is also one of the indicators of postoperative safety of ICL. Sufficient evidences have suggested that there is no significant correlation between ACD and ECD reduction [15,33], and there is no association between patients with ACD ≥2.80 mm having ICL implantation and ECD reduction [6]. Under limited spatial conditions in patients with relatively shallow ACD, abnormal postoperative vault and excessive narrowing of ACA are more likely to cause IOP fluctuation and iris and corneal endothelium contact, inducing corneal endothelium damage. Similar to the results of Xingtao Zhou [9,35], patients with relatively shallow ACD who had ICL implantation showed that little impact on the corneal endothelium, ACA, and lens, suggesting postoperative reliable safety and well results.
At present, our postoperative observation time of ICL implantation in patients with relatively shallow ACD is too short. As there is a lack of long-term follow-up observation, it needs to follow up on its long-term efficacy for further research.
Generally, research involving both eyes should take into account inter-eye correlation bias [36]. We think that the differences and similarities between both eyes have little impact on the results, so we did not distinguish between binocular/monocular patients. The potential bias for inter-eye correlation may exist in this study and will be gradually improved in subsequent work.
5. Conclusion
In summary, research shows that patients with relatively shallow ACD have shown similar positive clinical outcomes and safety in the early and midterm postoperative periods following ICL implantation, as compared to patients with normal ACD. When patients with shallow ACD have Appropriate vault and postoperative ACD, the postoperative outcomes are positive without IOP increasing, ECD loss, or ACA closure. These findings provide clinicians with valuable information in determining whether ICL implantation is viable for patients with relatively shallow ACD.
CRediT authorship contribution statement
Ting Huang: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Software, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization. Hongyan Zhang: Software, Resources, Investigation, Conceptualization. Ke Li: Writing – review & editing, Methodology, Conceptualization.
Data availability statement
The datasets generated during and analyzed during the current study are available from the corresponding author on reasonable request.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Ting Huang, Email: 376200339@qq.com.
Hongyan Zhang, Email: 1415763681@qq.com.
Ke Li, Email: karenkeke@163.com.
References
- 1.Igarashi A., Shimizu K., Kamiya K. Eight-year follow-up of posterior chamber phakic intraocular lens implantation for moderate to high myopia. Am. J. Ophthalmol. 2014;157:532–539.e1. doi: 10.1016/j.ajo.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 2.Tan W., Wang Z., Zeng Q., Lei X., Pan C., Shu B., Jin L., Chen Q. The influence of iris -ciliary angle (ICA) on the vault after implantation of V4c implantable collamer lens: a chain mediation model of ICL haptic related factors. BMC Ophthalmol. 2023;23:403. doi: 10.1186/s12886-023-03122-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chuck R.S., Jacobs D.S., Lee J.K., Afshari N.A., Vitale S., Shen T.T., Keenan J.D. Refractive errors & refractive surgery preferred practice pattern. Ophthalmology. 2018 Jan;125(1):P1–P104. doi: 10.1016/j.ophtha.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 4.Kohnen T., Neuhann T., Knorz M.C. German Ophthalmology Society and the German Professional Association of Ophthalmologists. Bewertung und Qualitätssicherung refraktiv-chirurgischer Eingriffe durch die Deutsche Ophthalmologische Gesellschaft und den Berufsverband der Augenärzte Deutschlands (Stand: Januar 2014) [Evaluation and quality assurance of refractive surgical interventions by the German Ophthalmology Society and the German Professional Association of Ophthalmologists (status 2014)] Klin Monbl Augenheilkd. 2014;231(6):642–650. doi: 10.1055/s-0034-1368481. [DOI] [PubMed] [Google Scholar]
- 5.Chinese Ophthalmological Society Chinese consensus guidelines of phakic posterior chamber intraocular lens implantation(2019) Chin. J. Ophthalmol. 2019;55(9):652–657. doi: 10.3760/cma.j.issn.0412-4081.2019.09.005. [DOI] [Google Scholar]
- 6.Pechméja J., Guinguet J., Colin J., Binder P.S. Severe endothelial cell loss with anterior chamber phakic intraocular lenses. J. Cataract Refract. Surg. 2012;38:1288–1292. doi: 10.1016/j.jcrs.2012.04.022. [DOI] [PubMed] [Google Scholar]
- 7.Hu Z., Ye J. Clinical advances in posterior chamber phakic intraocular lens implantation for ametropia correction. Chin J Optom Ophthalmol Vis Sci. 2019;21(9):715–720. doi: 10.3760/cma.j.issn.1674-845X.2019.09.012. [DOI] [Google Scholar]
- 8.Fernández-Vega-Cueto L., Lisa C., Esteve-Taboada J.J., Montés-Micó R., Alfonso J. Implantable collamer lens with central hole: 3-year follow-up. Clin. Ophthalmol. 2018;12:2015–2029. doi: 10.2147/OPTH.S171576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Niu L., Miao H., Han T., Ding L., Wang X., Zhou X. Visual outcomes of Visian ICL implantation for high myopia in patients with shallow anterior chamber depth. BMC Ophthalmol. 2019;19:121. doi: 10.1186/s12886-019-1132-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lim D.H., Lee M.G., Chung E.-S., Chung T.-Y. Clinical results of posterior chamber phakic intraocular lens implantation in eyes with low anterior chamber depth. Am. J. Ophthalmol. 2014;158:447–454.e1. doi: 10.1016/j.ajo.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 11.Thompson V., Cummings A., Wang X. Implantable collamer lens procedure planning: a review of global approaches. Clin. Ophthalmol. 2024;18:1033–1043. doi: 10.2147/OPTH.S456397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alfonso J.F., Lisa C., Fernández-Vega L., Almanzar D., Pérez-Vives C., Montés-Micó R. Prevalence of cataract after collagen copolymer phakic intraocular lens implantation for myopia, hyperopia, and astigmatism. J. Cataract Refract. Surg. 2015;41:800–805. doi: 10.1016/j.jcrs.2014.07.039. [DOI] [PubMed] [Google Scholar]
- 13.Schmidinger G., Lackner B., Pieh S., Skorpik C. Long-term changes in posterior chamber phakic intraocular collamer lens vaulting in myopic patients. Ophthalmology. 2010;117:1506–1511. doi: 10.1016/j.ophtha.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 14.Choi J.H., Lim D.H., Nam S.W., Yang C.M., Chung E.S., Chung T.-Y. Ten-year clinical outcomes after implantation of a posterior chamber phakic intraocular lens for myopia. J. Cataract Refract. Surg. 2019;45:1555–1561. doi: 10.1016/j.jcrs.2019.06.015. [DOI] [PubMed] [Google Scholar]
- 15.Kamiya K., Shimizu K., Igarashi A., Kitazawa Y., Kojima T., Nakamura T., Ichikawa K. Posterior chamber phakic intraocular lens implantation in eyes with an anterior chamber depth of less than 3 mm: a multicenter study. Sci. Rep. 2018;8 doi: 10.1038/s41598-018-31782-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guber I., Mouvet V., Bergin C., Perritaz S., Othenin-Girard P., Majo F. Clinical outcomes and cataract formation rates in eyes 10 Years after posterior phakic lens implantation for myopia. JAMA Ophthalmol. 2016;134:487. doi: 10.1001/jamaophthalmol.2016.0078. [DOI] [PubMed] [Google Scholar]
- 17.Kamiya K., Ando W., Tsujisawa T., Takahashi M., Shoji N. Effect of angle opening parameters on corneal endothelial cell density and intraocular pressure after posterior chamber phakic intraocular lens implantation. J. Clin. Med. 2020;9:2704. doi: 10.3390/jcm9092704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Changes of Intraocular Pressure in the Early Stage after Implantable Collamer Lens V4c Implantation and Related factors.Pdf, (n.d.).
- 19.Miao H., Chen X., Tian M., Chen Y., Wang X., Zhou X. Refractive outcomes and optical quality after implantation of posterior chamber phakic implantable collamer lens with a central hole (ICL V4c) BMC Ophthalmol. 2018;18:141. doi: 10.1186/s12886-018-0805-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fu M., Li M., Xian Y., Yu Z., Zhang H., Choi J., Niu L., Wang X., Zhou X. Two-year visual outcomes of evolution implantable collamer lens and small incision lenticule extraction for the correction of low myopia. Front. Med. 2022;9 doi: 10.3389/fmed.2022.780000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aruma A., Li M., Choi J., Miao H., Wei R., Yang D., Yao P., Sun L., Wang X., Zhou X. Visual outcomes after small incision lenticule extraction and implantable collamer lens V4c for moderate myopia: 1-year results. Graefes Arch. Clin. Exp. Ophthalmol. 2021;259:2431–2440. doi: 10.1007/s00417-020-04982-4. [DOI] [PubMed] [Google Scholar]
- 22.Liu F., Xia F., Niu L., Zhao J., Wang X., Zhou X. Early assessment of circumferential anterior segment structures following implantable collamer lens V4c implantation via SS-OCT. Transl. Vis. Sci. Technol. 2022;11:4. doi: 10.1167/tvst.11.11.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wannapanich T., Kasetsuwan N., Reinprayoon U. Intraocular implantable collamer lens with a central hole implantation: safety, efficacy, and patient outcomes. Clin. Ophthalmol. 2023;17:969–980. doi: 10.2147/OPTH.S379856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24.Zhang Y., Zhou J. Zhao X. Changes of intraocular pressure in the early stage after implantable collamer lens V4c implantation and related factors. Chin J Ophthalmol Otorhinol. 2021;21:413–418. doi: 10.14166/j.issn.1671-2420.2021.06.003. [DOI] [Google Scholar]
- 25.Cao X., Tong J., Wang Y., Zhou T., Ye B., Li X., Shen Y. Long-term ultrasound biomicroscopy observation of position changes of a copolymer posterior chamber phakic intraocular lens. J. Cataract Refract. Surg. 2014;40:1454–1461. doi: 10.1016/j.jcrs.2013.12.022. [DOI] [PubMed] [Google Scholar]
- 26.Alfonso J.F., Fernández-Vega L., Lisa C., Fernandes P., Jorge J., Micó R.M. Central vault after phakic intraocular lens implantation: correlation with anterior chamber depth, white-to-white distance, spherical equivalent, and patient age. J. Cataract Refract. Surg. 2012;38:46–53. doi: 10.1016/j.jcrs.2011.07.035. [DOI] [PubMed] [Google Scholar]
- 27.Rocamora L., Orlando J.I., Lwowski C., Kohnen T., Mertens E., Van Keer K. Postoperative vault prediction for phakic implantable collamer lens surgery: LASSO formulas, J. Cataract Refract. Surg. 2023;49:126–132. doi: 10.1097/j.jcrs.0000000000001079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martínez-Plaza E., López-de La Rosa A., López-Miguel A., Holgueras A., Maldonado M.J. EVO/EVO+ Visian Implantable Collamer Lenses for the correction of myopia and myopia with astigmatism. Expert Rev. Med. Devices. 2023;20:75–83. doi: 10.1080/17434440.2023.2174429. [DOI] [PubMed] [Google Scholar]
- 29.Dougherty P.J., Rivera R.P., Schneider D., Lane S.S., Brown D., Vukich J. Improving accuracy of phakic intraocular lens sizing using high-frequency ultrasound biomicroscopy. J. Cataract Refract. Surg. 2011;37:13–18. doi: 10.1016/j.jcrs.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 30.Gonvers M., Bornet C., Othenin-Girard P. Implantable contact lens for moderate to high myopia: relationship of vaulting to cataract formation. J. Cataract Refract. Surg. 2003;29:918–924. doi: 10.1016/S0886-3350(03)00065-8. [DOI] [PubMed] [Google Scholar]
- 31.Benda F., Filipová L., Filipec M. Correction of moderate to high hyperopia with an implantable collamer lens: medium-term results. J. Refract. Surg. 2014;30:526–533. doi: 10.3928/1081597X-20140711-05. [DOI] [PubMed] [Google Scholar]
- 32.Zhu Q.-J., Chen W.-J., Zhu W.-J., Xiao H.-X., Zhu M.-H., Ma L., Yuan Y., Song E. Short‐term changes in and preoperative factors affecting vaulting after posterior chamber phakic Implantable Collamer Lens implantation. BMC Ophthalmol. 2021;21:199. doi: 10.1186/s12886-021-01963-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garcia-De La Rosa G., Olivo-Payne A., Serna-Ojeda J.C., Salazar-Ramos M.S., Lichtinger A., Gomez-Bastar A., Ramirez-Miranda A., Navas A., Graue-Hernandez E.O. Anterior segment optical coherence tomography angle and vault analysis after toric and non-toric implantable collamer lens V4c implantation in patients with high myopia. Br. J. Ophthalmol. 2018;102:544–548. doi: 10.1136/bjophthalmol-2017-310518. [DOI] [PubMed] [Google Scholar]
- 34.Choi H., Kim T., Kim S.J., Sa B.G., Ryu I.H., Lee I.S., Kim J.K., Han E., Kim H.K., Yoo T.K. Predicting postoperative anterior chamber angle for phakic intraocular lens implantation using preoperative anterior segment metrics. Transl. Vis. Sci. Technol. 2023;12:10. doi: 10.1167/tvst.12.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qian T., Du J., Ren R., Zhou H., Li H., Zhang Z., Xu X. Vault-correlated efficacy and safety of implantable collamer lens V4c implantation for myopia in patients with shallow anterior chamber depth. Ophthalmic Res. 2023;66(1):445–456. doi: 10.1159/000528616. Epub 2023 Jan 3. PMID: 36596292. [DOI] [PubMed] [Google Scholar]
- 36.Armstrong R.A. Statistical guidelines for the analysis of data obtained from one or both eyes. Ophthalmic Physiol. Opt. 2013;33:7–14. doi: 10.1111/opo.12009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and analyzed during the current study are available from the corresponding author on reasonable request.