Abstract
Low levels and function of natural killer (NK) cells are associated with increased coronavirus disease 2019 (COVID-19) severity. NK cell immunotherapy may improve immune function to reduce infection severity. We conducted a first-in-human, open-label, phase 1, dose-escalating (100 × 106, 300 × 106, or 900 × 106 cells) study of a single dose of DVX201, a cord-blood-derived allogeneic NK cell therapy, in hospitalized patients with COVID-19. Participants were followed for 28 days. The maximum allowed steroid dose for eligibility was up to 0.5 mg/kg prednisone (or equivalent) daily. We enrolled nine participants, 3 per dose level. Eight participants had ≥1 comorbidity associated with increased COVID-19 severity, three of whom had a hematologic malignancy. Infusions were well tolerated, with no treatment-related adverse events. There was no evidence of inflammatory complications related to infusions. Peripheral blood NK cells generally increased after infusion, peaking by day 7. The median time from infusion to discharge was 2 days (range: 1–13). Two patients (both with acute lymphoblastic leukemia) were readmitted with recurrent COVID-19. This trial demonstrates the safety of allogeneic NK cell immunotherapy as a potential antiviral. Larger controlled trials are needed to establish efficacy.
Keywords: COVID-19, natural killer cell therapy, allogeneic, immunotherapy, SARS-CoV-2, T cell therapy, cellular therapy, NK cell
Graphical abstract
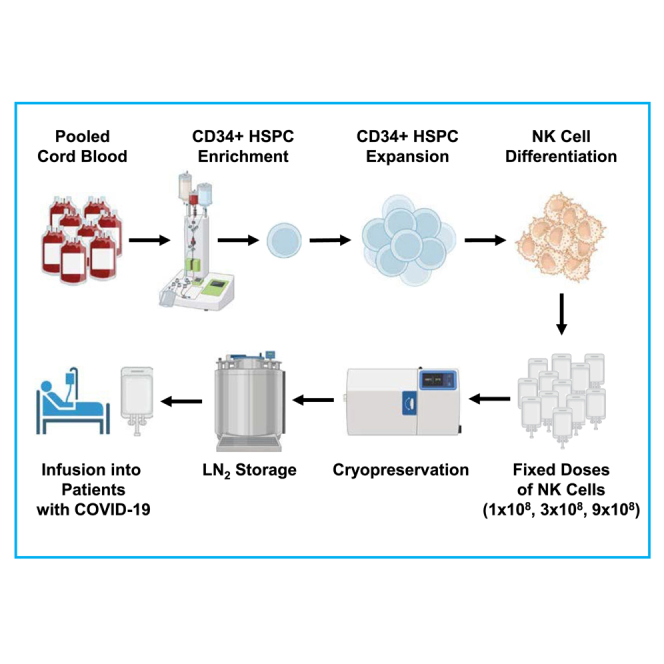
In this open-label, first-in-human, phase 1, dose-escalating trial of cord-blood-derived allogeneic NK cell therapy in patients hospitalized for COVID-19, Hill and colleagues demonstrate the safety of this treatment as a potential complementary therapy for COVID-19 in patients at risk for severe or protracted infection.
Introduction
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), can result in a spectrum of clinical manifestations, ranging from asymptomatic infection to severe disease, multisystem organ failure, and post-acute sequelae of SARS-CoV-2 infection.1,2,3 Therapeutic strategies for COVID-19 include the direct inhibition of SARS-CoV-2 replication,4,5,6 establishment of immunity through active and passive immunization,7,8 and anti-inflammatory treatments.9,10,11,12,13 Despite these advances, COVID-19 still results in substantial morbidity in certain patient populations, underscoring the ongoing need for additional therapeutic options, particularly among immunocompromised individuals.14
Adoptive cellular immunotherapy for the treatment of COVID-19 is a promising approach as a complementary treatment for patients with (or at high-risk for) severe or protracted disease. Immunocompromised patients are at higher risk for morbidity, mortality, and protracted infections and often exhibit impaired vaccine responses, highlighting the need for additional therapeutic strategies.15,16 A few trials to date have reported the use of cellular immunotherapies for COVID-19. Allogeneic SARS-CoV-2-specific T cell infusions were analyzed in six immunocompromised patients with persistent infection and shown to be safe and potentially beneficial in decreasing viral loads.17 In a randomized trial of 45 patients receiving placebo or multiple doses of cord-blood-derived, allogeneic, non-human leukocyte antigen (HLA)-matched regulatory T cells for acute respiratory distress syndrome (ARDS) due to COVID-19, the authors found the treatment to be safe.18 A randomized trial of mesenchymal stromal cells in 222 mechanically ventilated patients with COVID-19 found the infusions to be safe but was stopped early due to lack of evidence of improvement in survival or ventilator-free days.19 Lastly, a phase 1/2 trial of an allogeneic, ex-vivo-expanded, donor-unrestricted invariant natural killer T (iNKT) cell product in 21 patients with ARDS due to COVID-19 found the product to be safe and potentially associated with improved 30-day survival.20
There has been similar emerging interest in the use of NK cells for the treatment of infectious diseases and certain cancers. NK cells are integral to the innate immune system and the first responders against transformed cells, including virally infected and malignant cells. Their function is mediated though both direct cytolytic mechanisms and indirect cytokine/chemokine-mediated effects on adaptive immune responses.21,22 Multiple NK cell receptors are involved in the recognition of infected cells by binding common stress ligands or pathogen-associated molecules.23 Upon ligand recognition, NK cells kill their target cells by releasing cytotoxic molecules such as perforin and granzymes and via death-receptor-mediated apoptosis, as previously documented in the context of cytomegalovirus (CMV) infections.24 Further, NK cells secrete cytokines, including tumor necrosis factor alpha (TNF-α) and interferon gamma (IFN-γ), and chemokines, such as MIP-1α/CCL3, MIP-1β/CCL4, and interleukin (IL)-8, to recruit additional immune effector cells and enhance adaptive immune responses that ultimately orchestrate the complex coordination of adaptive and innate immune responses.25,26
In patients with COVID-19, low levels and poor function of lymphocytes and NK cells are correlated with risk for disease progression.1,27,28 NK cell exhaustion is primarily caused by the cytokine storm associated with severe COVID-19, where elevated levels of IL-6, IL-10, and transforming growth factor β (TGF-β) suppress NK cell function and their ability to produce IFN-γ and granzyme B. The upregulation of inhibitory receptors further hinders their capacity to eliminate infected cells. Varying levels of these cytokines may both influence the severity of COVID-19 and serve as markers of NK cell function.29 In-depth characterization of NK cells from the peripheral blood of patients with COVID-19 has demonstrated the upregulation of NK exhaustion, migration, and apoptosis markers.30 These observations have provided the rationale for using adoptive NK cell immunotherapy as a means to boost functional innate immunity in patients with COVID-19.31
Though NK cell therapies leveraging our improved knowledge of cancer immunology have proven safe and effective in a variety of oncologic applications, limited data currently exist on their use as treatment for infections.32,33 To the authors’ knowledge, there are no currently published results of NK cell therapy for COVID-19. Here, we report the results of an investigator-initiated phase 1 trial (ClinicalTrials.gov: NCT04900454) of an off-the-shelf, allogeneic, unmodified NK cell product (DVX201) for the treatment of patients hospitalized with COVID-19.
Results
Patient characteristics
We enrolled nine participants with a median age of 45 years (interquartile range [IQR]: 39–74.5) (Table 1). Three participants (33%) had a hematologic malignancy, and 8 (89%) had at least one comorbidity associated with increased COVID-19 severity. Three participants were fully vaccinated, one received casirivimab/imdevimab (Regeneron) prior to infusion, and none received prior tixagevimab/cilgavimab (Evusheld) for prophylaxis. The median time from symptom onset to DVX201 infusion was 10 days (IQR: 6–38). Two of the participants with hematologic malignancies (DVX201-008 and DVX201-009) had protracted infection prior to enrollment with ongoing respiratory symptoms for 64 and 87 days prior to DVX201 infusion; DVX201-009 had negative SARS-CoV-2 testing from the upper respiratory tract but detection in the lower respiratory tract. On the day of infusion, four (44%) participants were receiving supplemental oxygen ranging from 2 to 6 L by nasal cannula. Eight participants were receiving treatment with remdesivir at the time of enrollment, six of whom were also receiving dexamethasone (Table 2; Figure S1).
Table 1.
Participant demographics and clinical characteristics
| Participant ID | Age, years | Sex | Race | Comorbidities | mRNA SARS-CoV-2 vaccination history | Days of symptoms before infusion | Supplemental oxygen at infusion (L) | Infusion dose |
|---|---|---|---|---|---|---|---|---|
| DVX201-001 | 63 | M | White | coronary artery disease, hypertension, obesity, type 2 diabetes | 1 dose | 10 | 0 | 100 × 106 |
| DVX201-002 | 25 | M | White | acute lymphoblastic leukemia (ALL), obesity | none | 4 | 0 | 100 × 106 |
| DVX201-003 | 72 | M | Asian | hypertension, type 2 diabetes | 1 dose | 6 | 0 | 100 × 106 |
| DVX201-004 | 42 | M | Latino | obesity | 2 doses | 12 | 6 | 300 × 106 |
| DVX201-006 | 42 | M | Asian | none | none | 12 | 3 | 300 × 106 |
| DVX201-007 | 36 | M | Latino | hypertension, metastatic cholangiocarcinoma, obesity | none | 6 | 5 | 300 × 106 |
| DVX201-008 | 45 | M | Latino | ALL, hypertension, obesity | none | 64 | 2 | 900 × 106 |
| DVX201-009a | 77 | F | White | bronchiectasis, chronic lymphocytic leukemia (CLL) | 3 doses | 87 | 0 | 900 × 106 |
| DVX201-010 | 77 | M | White | hypertension, hyperlipidemia, type 2 diabetes | 2 doses | 6 | 0 | 900 × 106 |
M, male; F, female.
Negative SARS-CoV-2 PCR from nasopharyngeal swab but positive in bronchoalveolar lavage sample.
Table 2.
Participant clinical courses
| Participant ID | Diagnosis date time framea | SARS-CoV-2 therapies administered during the same hospitalization | Clinical course | Days to negative SARS-CoV-2 PCR from infusion | Days to discharge from infusion | Change in pulmonary radiographic findings |
|---|---|---|---|---|---|---|
| DVX201-001 | 9/2021–11/2021 | remdesivir, dexamethasone | discharged home without complications | 7 | 1 | post-imaging not performed |
| DVX201-002 | 10/2021–12/2021 | remdesivir, casirivimab and imdevimab (Regeneron), dexamethasone | started chemotherapy 5 days after infusion and discharged; readmitted for hypoxemic respiratory failure attributed to persistent COVID-19 | 53 | 13 | improvement with subsequent worsening |
| DVX201-003 | 10/2021–12/2021 | remdesivir, dexamethasone | discharged home without complications | 7 | 1 | improved |
| DVX201-004 | 11/2021–1/2022 | remdesivir, dexamethasone | improved and discharged home off oxygen | N/Ab | 3 | post-imaging not performed |
| DVX201-006 | 1/2022–3/2022 | remdesivir, dexamethasone | improved and discharged home off oxygen | 0 | 2 | post-imaging not performed |
| DVX201-007 | 1/2022–3/2022 | remdesivir, dexamethasone | improved and discharged home off oxygen but with residual dyspnea with exertion | 1 | 6 | improved |
| DVX201-008 | 3/2022–5/2022 | remdesivir, dexamethasone, nirmatrelvir-ritonavir | improved and discharged on 2 L oxygen; readmitted with persistent COVID-19 | 24 | 2 | improvement with subsequent worsening |
| DVX201-009 | 3/2022–5/2022 | remdesivir, dexamethasone | discharged home without complications | N/Ac | 1 | post-imaging not performed |
| DVX201-010 | 5/2022–7/2022 | remdesivir | improved and discharged home | 27 | 8 | post-imaging not performed |
General time periods are provided to protect participant privacy.
Last positive test was on day 14 and indeterminate; participant refused subsequent testing.
Only positive from bronchoalveolar lavage prior to enrollment and not retested. Nasopharyngeal swabs were negative.
Safety
No dose limiting toxicities (DLTs) were reported at any dose level. Thus, dose escalation continued up to 900 × 106 cells. DVX201 infusions were well tolerated, with no infusion-related events, graft-versus-host disease (GvHD), or cytokine release syndrome (CRS). Adverse events (AEs) are summarized in Tables S1 and S2. There were no AEs assessed as related to DVX201 infusion. There were three severe AEs (SAEs) in two participants with active hematologic malignancies; one participant had sepsis with bacteremia and recurrent COVID-19, and another participant had recurrent COVID-19 in the context of weaning off corticosteroids. No deaths occurred during the study period.
Clinical outcomes
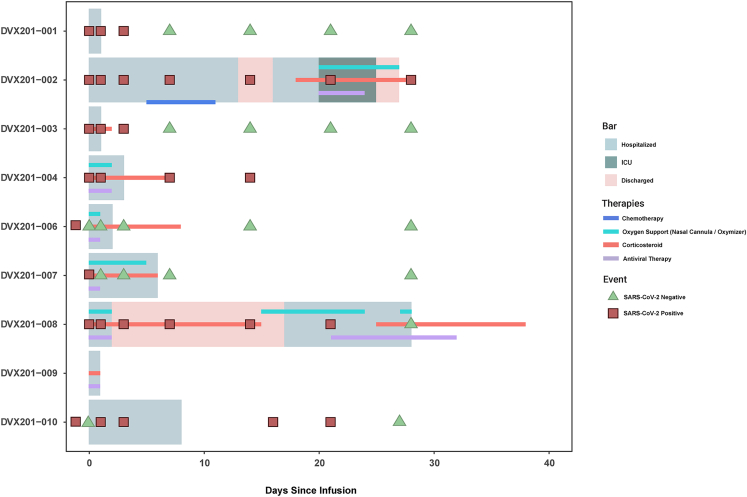
A Swimmer plot depicting each participant’s clinical course is provided in Figure 1. Corticosteroid use is plotted in Figure S1. Eight participants were discharged without supplemental oxygen at a median of 2 days (IQR: 1–7) after DVX201 infusion. Among the four participants requiring supplemental oxygen during hospitalization but prior to study infusion, oxygen was discontinued in three at a median of 2.5 days (IQR: 2–4.5) after infusion (Figure S2). All four patients with chest radiographic imaging before and after DVX201 infusion demonstrated improvement in pulmonary infiltrates (Table 2).
Figure 1.
Clinical course of each participant
Swimmer plot depicting the clinical course of each participant. Participant DVX201-002 received lymphodepleting chemotherapy 5 days after DVX201 infusion. Participant DVX201-009 had persistent SARS-CoV-2 detection in the lower respiratory tract but not in nasopharyngeal swabs (data not shown on the plot).
Of the three patients with a hematologic malignancy, DVX201-008 clinically improved but remained on 2 L of supplemental oxygen by nasal cannula at discharge and was readmitted 15 days later for worsening respiratory symptoms attributed to COVID-19. DVX201-002 was discharged with clinical improvement but then readmitted 16 days after infusion for bacteremia and hypoxemic respiratory failure with concern for persistent COVID-19 and a positive nasopharyngeal (NP) swab for SARS-CoV-2; notably, this individual started chemotherapy for acute lymphoblastic leukemia (ALL) 5 days after DVX201 infusion. The third participant, DVX201-009, had a protracted course of COVID-19 for 2 months prior to infusion with several prior hospitalizations and had clinical and radiological improvement with complete resolution of symptoms 8 days after DVX201 infusion. However, the patient also received concurrent treatment with remdesivir and dexamethasone.
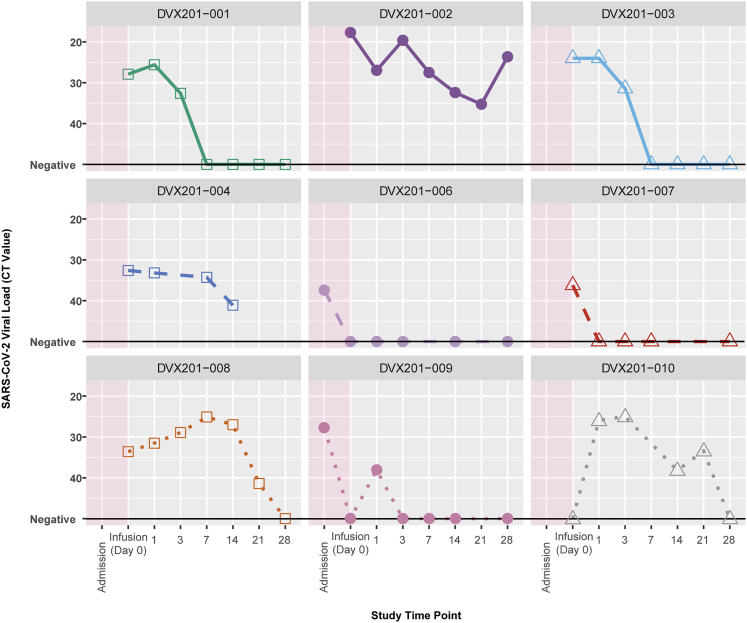
Kinetics of SARS-CoV-2 detection
SARS-CoV-2 viral loads over time are shown in Figure 2, with concurrent therapies and oxygen support overlaid in Figure S3. DVX201-009 had SARS-CoV-2 detection in the lower respiratory tract only at baseline, and two participants had a negative test on the day of infusion; one of these individuals, DVX201-006, had subsequent low-level positive results through the date of last test on day 28. Of the six other participants with upper respiratory tract SARS-CoV-2 detection on the day of infusion, four (67%) cleared the virus within 28 days, with first negative tests at a median of 7 days (IQR: 2–25.5) after infusion. DVX201-002 had persistent detection of SARS-CoV-2 through day 28 in the context of induction chemotherapy for ALL. DVX201-004 refused nasal swabs after day 14 and had an indeterminate test at that time.
Figure 2.
Kinetics of SARS-CoV-2 detection
Spaghetti plot of SARS-CoV-2 viral load per participant via nasopharyngeal swab. CT value indicates the cycle threshold; lower CT value indicates higher viral load. DVX201-010 was missing a CT value from his pre-infusion time point. DVX201-009 had SARS-CoV-2 detected in the bronchoalveolar lavage only at the time of admission. Subsequent data points were from nasopharyngeal swabs. DVX201-010 had a negative PCR test for SARS-CoV-2 on the day of infusion after enrollment. This was likely a false negative, and this participant tested positive on days −5 and −3 prior to infusion, as well as at multiple time points after infusion.
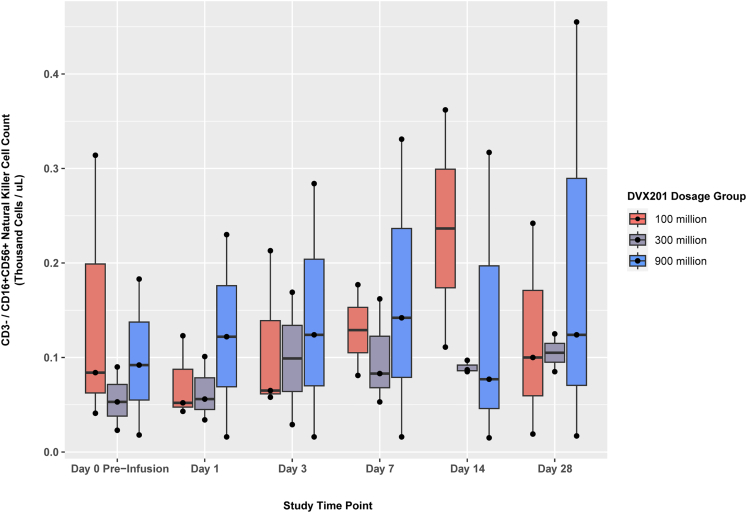
Kinetics of peripheral blood CD3−/CD16+CD56+ NK cells
Changes in CD3−/CD16+/CD56+ NK cells pre- and post-infusion are shown in Figure 3, with participant-level plots in Figure S4. This demonstrated a general but non-statistically significant increase in NK cells over time, despite most of the participants receiving corticosteroids during the study (Table 2).
Figure 3.
Kinetics of CD3−/CD16+CD56+ NK cell counts
Box and whisker plots of NK cell counts at each study time point, grouped by dosage group. Horizontal line inside each box represents the median, the box represents the lower and upper quartiles, and black dots represent individual patient data points. Vertical lines represent the interquartile range. Changes did not reach statistical significance.
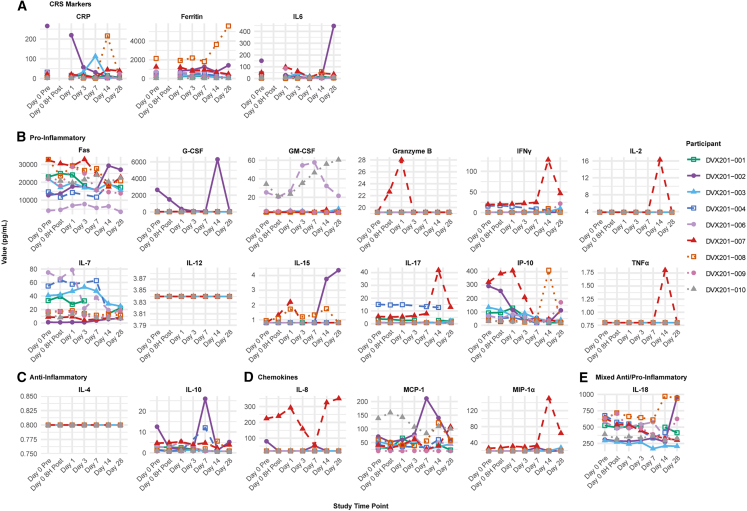
Kinetics of inflammatory markers
Changes in cytokines and inflammatory markers are illustrated in Figures 4 and S5. C-reactive protein (CRP) generally decreased within 7 days of receiving DVX201, although levels appeared to already be down trending in most participants. IL-6 levels were relatively stable, with some participants having an increase at day 1 but an overall decline by day 7. Ferritin remained relatively stable over time. Two participants, DVX201-002 and DVX201-008, were re-hospitalized with worsening symptoms of COVID-19 and had increases in CRP, IL-6, and/or ferritin at that time.
Figure 4.
Spaghetti plots of the absolute values of cytokines and/or inflammatory biomarkers over time
Each line indicates data from a given participant organized by (A) markers of cytokine release syndrome, (B) pro-inflammatory markers, (C) anti-inflammatory markers, (D) chemokines, and (E) mixed anti-/pro-inflammatory. The majority of participants had measurements below the level of detection for GCSF, GM-CSF, granzyme B, IL-2, TNF-⍺, IL-12, IL-4, IL-8, IL-12, IL-15, and MIP-1⍺.
Overall, there were relatively limited changes for most measurable cytokines over time. Notably, there was no spike in pro-inflammatory cytokines within the first 72 h after infusion to indicate that CRS triggered or worsened by DVX201, although a number of these biomarkers had subtle increases over the first 7 days followed by decreases through day 28 (Figures 4 and S5). For example, IL-7, a pro-inflammatory cytokine thought to promote T cell and NK cell survival by increasing anti-apoptotic Bcl-2, generally increased in the first week after infusion followed by a subsequent return to baseline. DVX201-007 had the fastest decline in viral load among participants, and their profile was notable for a general early rise in cytolytic cytokines followed by a delayed rise in Th1/M1 cytokines and additional inflammatory processes. They had a spike on day 1 in IL-15 (promotes NK cell and CD8 T cell expansion/function), IFN-γ-induced protein 10 kDa (IP-10), IL-6, and granzyme B, followed by rapid decreases. Around day 14, they had a second cluster of spikes (highest fold change out of all participants) for IFN-γ, IL-2, IL-17, TNF-α, and MIP-1α. Given the limited number of participants, these data are hypothesis generating.
Discussion
In this phase 1 trial, we assessed adoptive immunotherapy with allogeneic NK cells (DVX201) in nine patients hospitalized with COVID-19 and showed that this therapy was safe and well tolerated. No treatment-related AEs were observed, including no cases of CRS. Although the study was not designed to assess efficacy, our findings underscore the potential utility of NK cell therapy as an additional therapeutic strategy, particularly in immunocompromised patients.
Remdesivir or nirmatrelvir-ritonavir plus dexamethasone remain the standard of care for hospitalized patients with COVID-19 who require supplemental oxygen,34,35 although the efficacy of these treatments is modest.36 Adoptive immunotherapy holds potential as a complementary therapy to small-molecule antivirals in patients with severe COVID-19 or at high risk for progressive disease. Multiple recent trials employing various allogeneic cellular therapies, primarily T cell derived, also demonstrated the safety of these products in patients with COVID-19, some with promising signs of benefit, although the trials were all phase 1/2 and not powered for efficacy.17,20 Together, these data are laying the foundation for larger trials of cellular therapies for viral infections, particularly in immunocompromised patients at increased risk for severe infections.
In our study, we observed good initial clinical responses based on generally rapid improvements in oxygenation, improvement in pulmonary radiographic changes, and discharge from the hospital within a few days. However, all participants were concurrently treated with remdesivir, with or without dexamethasone and other therapies, and we did not have a control group in this pilot trial. A prior study reported a median time of 11 days to negative SARS-CoV-2 test from treatment initiation in immunosuppressed patients receiving combination antiviral and monoclonal antibody SARS-CoV-2 therapies.37 Our study found a median time to negative SARS-CoV-2 test of 7 days post-DVX201 infusion, but not all participants were immunosuppressed. We included two patients undergoing active therapy for ALL who demonstrated initial clinical improvement after infusion. However, both were subsequently readmitted 2–3 weeks later with recurrent COVID-19. These observations indicate that multiple doses are likely necessary in immunocompromised patients given an estimated half-life of infused NK cells of 1–2 weeks.38 Additional study is needed to understand the pharmacokinetics of allogeneic cellular therapy products.
We tested a panel of cytokines, chemokines, and inflammatory markers to investigate both the potential inflammatory and anti-inflammatory impacts of NK cell therapy that could contribute to both AEs (e.g., CRS) and efficacy. Similar to a trial using iNKT cells for COVID-19,20 we generally observed decreases or stability in IL-6, CRP, and ferritin after infusion. These markers are often associated with severe inflammation in COVID-19, and NK cell therapy could contribute to viral control and reduction in inflammation through killing of infected macrophages and other immune cells contributing to cytokine storm.29 Additionally, both our study and their study observed subtle increases in pro-inflammatory cytokines over the first week after infusion. Chemokines secreted by NK cells to recruit and activate other immune cell subsets include MCP-1, MIP1-α, IL-8, and IP-10,39 which were generally increased within 14 days after study infusion, especially for participants receiving the highest dose level and for DVX201-007, the participant with the most rapid decrease in SARS-CoV-2 viral load.
Our study adds to the sparse body of literature that is beginning to support the feasibility, safety, and possible efficacy of adoptive cellular therapies for COVID-19 and other viral infections. To the authors’ knowledge, this is the first report of adoptive immunotherapy with NK cell therapy for COVID-19, harnessing their unique innate immune function, and the only cellular therapy trial for SARS-CoV-2 that treated patients before they developed respiratory failure or required salvage treatment. Notably, it is one of the only studies of NK cell therapy for the treatment of any infection.31,35 Allogeneic NK cellular therapies allow for off-the-shelf access that may be more scalable and cost effective to produce than engineered or antigen-stimulated products, and they are associated with low rates of CRS and GVHD. The main limitation of this study was the relatively small number of enrolled participants and the absence of a control group (e.g., patients who received other SARS-CoV-2 therapies but not DVX-201) in the context of this phase 1 first-in-human trial focusing on safety. The use of concurrent treatments for COVID-19 also precludes an understanding of the potential direct efficacy of the NK cell therapy administered in this trial. We allowed participants to be eligible if receiving up to 0.5 mg/kg/day of prednisone (or equivalent), similar to other trials using cellular therapies for infectious diseases, given the concern that higher doses may suppress proliferation and cytotoxicity. Larger studies are needed to better determine to what extent corticosteroids impact the efficacy of cellular therapies for infectious diseases, in addition to their role in mitigating toxicities. We also note that the flow cytometric assay we used for this study did not differentiate NK cells based on CD56 intensity (i.e., dim versus bright). Future analyses of product-specific and endogenous NK cell immune reconstitution (e.g., expression levels of NKG2A) will be important to better understand potential efficacy.
In conclusion, this trial demonstrated the safety of adoptive immunotherapy with off-the-shelf NK cells for COVID-19 and provides the rationale and proof of concept for NK cell therapy for viral infections. Further study is needed to better understand the optimal dose, frequency of administration, and efficacy.
Materials and methods
Participants
We enrolled adults ≥18 years old requiring hospitalization for COVID-19 at the University of Washington from August 2021 to December 2022. Participants were required to have a peripheral blood oxygen saturation level of ≥93% on up to 6 L of supplemental oxygen by low-flow delivery. Microbiologic diagnosis of SARS-CoV-2 by a PCR-based test from the upper or lower respiratory tract was required. Participants had to meet two out of three criteria within 72 h from study consent, including IL-6 <150 pg/mL, CRP <10 mg/dL, and ferritin <1,000 ng/mL. Exclusion criteria included the following: (1) weight <40 kg; (2) ventilator dependent or diagnosed with ARDS or multisystem organ failure; (3) oxygen requirements exceeding 6 L of oxygen supplementation by nasal cannula; (4) expected intubation within 24 h; (5) expected discharge from the hospital within 72 h of planned DVX201 date of infusion; (6) known hypersensitivity to constituents of DVX201, such as DMSO, or antihistamine medications, which are required for pre-medication prior to infusion of DVX201; (7) history of baseline requirement of supplemental oxygen prior to COVID-19 diagnosis; (8) receipt of >0.5 mg/kg prednisone (or equivalent) daily, other than steroids administered for COVID-19; (9) pregnant or breastfeeding; and (10) inadequate organ function as defined by acute or chronic kidney injury requiring intermittent or continuous veno-venous hemodialysis or an estimated glomerular filtration rate (GFR) of <60 mL/min/1.73 m2 or abnormal liver function defined by AST (aspartate aminotransferase), ALT (alanine aminotransferase), or alkaline phosphatase ≥5 times the upper limit of normal.
The study was approved by the Fred Hutchinson Cancer Center (FH) Institutional Review Board (IRB). All participants provided written informed consent.
Manufacturing and characterization of the allogeneic NK cell product (DVX201)
DVX201 is a cryopreserved, allogeneic NK cell product developed by Deverra Therapeutics in Seattle, Washington, USA, and manufactured at the FH Cell Processing Facility. Manufacture of DVX201 is initiated by isolating CD34+ hematopoietic stem/progenitor cells from 8 pooled donor eligible umbilical cord blood units obtained from FDA-licensed public cord blood banks. The proprietary manufacturing process comprises two phases: an initial CD34+ stem/progenitor cell expansion phase followed by directed differentiation into NK cells. No feeder cells are required during the differentiation phase, and the process is animal component free. The final NK cell product was harvested and cryopreserved at fixed doses of 100 million, 300 million, or 900 million viable CD56+ NK cells per 20 mL dose. The final product consisted of a mixture of approximately 75% CD56+ NK cells and 24% CD56− dendritic cells, granulocytes, and other myeloid lineage derivatives (1% other cells) but, importantly, was devoid of donor T cells. The final drug product was manufactured, cryopreserved, and passed all clinical release criteria prior to subject enrollment.
The functionality of four unique batches of DVX201 NK cell product was assessed using an in vitro cytotoxicity assay in which a constant number of GFP-expressing Kasumi-1 target cells were co-cultured with DVX201 effector cells in a range of effector-to-target cell ratios for 24 h in a 37°C incubator. Cells were labeled with DAPI at the end of incubation and assessed by flow cytometry for the percentage of dead/dying DAPI+ GFP+ Kasumi-1 cells. Results from these assays were generated for information only as part of the batch records (Figure S6). Preclinical studies additionally demonstrated that DVX201 NK cells release IFN-γ and TNF-α in response to activation in the presence of transformed target cells and that they express the cell death receptor ligands TRAIL and FasL, which also play a role in eliminating stressed and diseased target cells. By RNA sequencing (RNA-seq) analysis, DVX201 NK cells display a cytotoxic NK cell signature (e.g., expression of perforin, granzymes A/B/H, granule proteins, NKG2D, CD161, CD94, cathepsin W) (data not shown).
The investigational product was cryopreserved prior to thawing at the bedside and administered as an intravenous infusion over 10–15 min within 90 min of thawing. Thawed DVX201 was held at 2°C–8°C in insulated coolers prior to infusion. Pre-medications included acetaminophen by mouth approximately 30 min prior to infusion and an antihistamine (intravenously) such as diphenhydramine approximately 15 min prior to infusion.
Trial design
This trial was an investigator-initiated, first-in-human, open-label, non-randomized, dose-escalation, phase 1 study to assess the safety and maximum tolerated dose (MTD) and/or recommended phase 2 dose (RP2D) of a cord-blood-derived allogeneic NK cell therapy for the treatment of COVID-19. Secondary objectives included an assessment of clinical and virological outcomes as well as evaluating changes in inflammatory markers in the blood.
Participants were enrolled on a 3+3 dose-escalation design. Each participant’s dose was determined by their consecutive enrollment sequence into the trial, without any predetermined criteria or choice based on individual patient characteristics. The first three enrolled patients received 100 million cells, the next three received 300 million cells, and the next three received 900 million cells. DVX201 was administered intravenously as a single dose on day 0. Participants were monitored for AEs for 28 days post-infusion or longer if experiencing related SAEs. All participants were followed daily via telehealth/telephone visits for 6 days post-discharge if discharged from the hospital prior to day 28. If no symptoms required an emergency room visit or in-person clinical evaluation, telehealth/telephone visits were reduced to twice a week until completion of study at day 28.
Sample collection and testing
Blood and NP swabs were collected prior to the infusion (baseline), twice on day 0 (infusion day; blood collected 8 h before and after infusion), and then once 1, 3, 7, 14, and 28 days after infusion. Laboratory assessments included a complete blood count with differential, basic metabolic panel, SARS-CoV-2 viral load of NP swabs, flow cytometry of peripheral blood mononuclear cells for CD3−/CD16+CD56+ NK cells, and serum levels of IL-6, CRP, and ferritin. Flow cytometry and SARS-CoV-2 viral load testing (with results provided as cycle threshold [CT] values) were performed at the University of Washington Department of Laboratory Medicine.
An additional panel of 19 cytokines, chemokines, and/or inflammatory markers was measured with a Luminex assay in the FH Immune Monitoring Core at the time points 8 h pre-infusion, 8 h post-infusion, and then days 1, 3, 7, 14, and 28 post-infusion. This panel was selected based on inflammatory markers associated with COVID-19 disease severity or NK cell function and consisted of Fas, granulocyte-colony-stimulating factor (G-CSF), granulocyte-macrophage CSF (GM-CSF), granzyme B, macrophage inflammatory protein-1 alpha (M1P-1α), IFN-γ, monocyte chemoattractant protein-1 (MCP-1/CCL2), TNF- α, IL-2, IL-4, IL-6, IL-7, IL-8, IL-10, IL-12, IL-15, IL-17, IL-18, and IP-10.40,41,42,43 Samples and cytokine standards were incubated overnight with Luminex microbeads (one unique bead population per cytokine) coated with cytokine-specific antibodies. Beads were washed and then incubated for 1 h with biotinylated anti-cytokine antibodies, followed by another wash and incubation for 30 min with a phycoerythrin-streptavidin conjugate. After a final wash, the assay was read on a Luminex instrument, which classifies each bead by its cytokine specificity and phycoerythrin fluorescence intensity. The phycoerythrin fluorescence of each bead is proportional to the cytokine concentration in the samples or standards, allowing the generation of a 5-parameter logistic standard curve for each cytokine, with sample concentrations calculated from these curves.
Safety monitoring
Patients were monitored for AEs and DLTs. AEs were reported according to the NCI Common Terminology Criteria for Adverse Events (CTCAE) v.5.0. Dose-limiting toxicities were defined as any grade ≥3 infusion-related reaction within 24 h (immediate toxicity) or any treatment emergent toxicity grade ≥3 within 7 days (days 0–6) after infusion not otherwise attributed to complications from COVID-19.
Statistical methods
Descriptive statistics were used to summarize baseline characteristics, AEs, and outcomes. Changes in inflammatory markers were calculated by measuring the log2 fold change between each post-infusion time point divided by the pre-infusion baseline sample per participant. Analyses were performed using Rstudio 4.3.1 software.
Data and code availability
The datasets generated and analyzed for this study are available from the corresponding author after publication upon reasonable request, with investigator financial support, and with appropriate documentation of IRB approval and/or data access agreements as applicable.
Acknowledgments
The authors thank Drs. Filippo Milano, Surabhi (Sarah) Vora, and Guang-Shing Cheng for serving on the data safety and monitoring board. We also thank the patients for participating in this trial. This trial was funded by a grant to J.A.H. from Deverra Therapeutics.
Author contributions
J.A.H. and C.S.D. designed the study. J.A.H. enrolled the participants. J.A.H., W.L.L., E.K., M.K.S., D.F., A.T., J.K.B., L.H.A., G.R.R., F.R., and N.T.R. collected data, performed data analysis, and/or interpreted results. J.A.H., W.L.L., and E.K. wrote the paper. C.S., J.C., J.B., and C.S.D. designed and managed the study product. All authors reviewed the final manuscript.
Declaration of interests
A.T., C.S., J.C., J.B., and C.S.D. are employees of and have equity in Deverra Therapeutics. C.S.D. is also an employee of Coeptis Therapeutics. J.A.H. received research funding from Deverra Therapeutics for the conduct of this trial, has received research funding from Allovir, Gilead, Takeda, and Merck, and served as a consultant for Moderna, Allovir, Gilead, SentiBio, Modulus, Takeda, and CSL Behring.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.omtm.2024.101361.
Supplemental information
References
- 1.Huang I., Pranata R. Lymphopenia in severe coronavirus disease-2019 (COVID-19): systematic review and meta-analysis. J. Intensive Care. 2020;8:36. doi: 10.1186/s40560-020-00453-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moore J.B., June C.H. Cytokine release syndrome in severe COVID-19. Science. 2020;368:473–474. doi: 10.1126/science.abb8925. [DOI] [PubMed] [Google Scholar]
- 3.Davis H.E., McCorkell L., Vogel J.M., Topol E.J. Long COVID: major findings, mechanisms and recommendations. Nat. Rev. Microbiol. 2023;21:133–146. doi: 10.1038/s41579-022-00846-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beigel J.H., Tomashek K.M., Dodd L.E., Mehta A.K., Zingman B.S., Kalil A.C., Hohmann E., Chu H.Y., Luetkemeyer A., Kline S., et al. Remdesivir for the Treatment of Covid-19 — Final Report. N. Engl. J. Med. 2020;383:1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldman J.D., Lye D.C.B., Hui D.S., Marks K.M., Bruno R., Montejano R., Spinner C.D., Galli M., Ahn M.-Y., Nahass R.G., et al. Remdesivir for 5 or 10 Days in Patients with Severe Covid-19. N. Engl. J. Med. 2020;383:1827–1837. doi: 10.1056/NEJMoa2015301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gupte V., Hegde R., Sawant S., Kalathingal K., Jadhav S., Malabade R., Gogtay J. Safety and clinical outcomes of remdesivir in hospitalised COVID-19 patients: a retrospective analysis of active surveillance database. BMC Infect. Dis. 2022;22 doi: 10.1186/s12879-021-07004-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li L., Zhang W., Hu Y., Tong X., Zheng S., Yang J., Kong Y., Ren L., Wei Q., Mei H., et al. Effect of Convalescent Plasma Therapy on Time to Clinical Improvement in Patients With Severe and Life-threatening COVID-19: A Randomized Clinical Trial. JAMA. 2020;324:460–470. doi: 10.1001/jama.2020.10044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ye M., Fu D., Ren Y., Wang F., Wang D., Zhang F., Xia X., Lv T. Treatment with convalescent plasma for COVID-19 patients in Wuhan, China. J. Med. Virol. 2020;92:1890–1901. doi: 10.1002/jmv.25882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guaraldi G., Meschiari M., Cozzi-Lepri A., Milic J., Tonelli R., Menozzi M., Franceschini E., Cuomo G., Orlando G., Borghi V., et al. Tocilizumab in patients with severe COVID-19: a retrospective cohort study. Lancet Rheumatol. 2020;2:e474–e484. doi: 10.1016/S2665-9913(20)30173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.The RECOVERY Collaborative Group Dexamethasone in Hospitalized Patients with Covid-19. N. Engl. J. Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tang L., Yin Z., Hu Y., Mei H. Controlling Cytokine Storm Is Vital in COVID-19. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.570993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu X., Han M., Li T., Sun W., Wang D., Fu B., Zhou Y., Zheng X., Yang Y., Li X., et al. Effective treatment of severe COVID-19 patients with tocilizumab. Proc. Natl. Acad. Sci. USA. 2020;117:10970–10975. doi: 10.1073/pnas.2005615117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhong J., Tang J., Ye C., Dong L. The immunology of COVID-19: is immune modulation an option for treatment? Lancet Rheumatol. 2020;2:e428–e436. doi: 10.1016/S2665-9913(20)30120-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Helleberg M., Niemann C.U., Moestrup K.S., Kirk O., Lebech A.-M., Lane C., Lundgren J. Persistent COVID-19 in an Immunocompromised Patient Temporarily Responsive to Two Courses of Remdesivir Therapy. J. Infect. Dis. 2020;222:1103–1107. doi: 10.1093/infdis/jiaa446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kampouri E., Hill J.A., Dioverti V. COVID-19 after hematopoietic cell transplantation and chimeric antigen receptor (CAR)-T-cell therapy. Transpl. Infect. Dis. 2023;25 doi: 10.1111/tid.14144. [DOI] [PubMed] [Google Scholar]
- 16.Langerbeins P., Hallek M. COVID-19 in patients with hematologic malignancy. Blood. 2022;140:236–252. doi: 10.1182/blood.2021012251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haidar G., Jacobs J.L., Kramer K.H., Naqvi A., Heaps A., Parikh U., McCormick K.D., Sobolewski M.D., Agha M., Bogdanovich T., et al. Therapy With Allogeneic Severe Acute Respiratory Syndrome Coronavirus-2-Specific T Cells for Persistent Coronavirus Disease 2019 in Immunocompromised Patients. Clin. Infect. Dis. 2023;77:696–702. doi: 10.1093/cid/ciad233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gladstone D.E., D’Alessio F., Howard C., Lyu M.-A., Mock J.R., Gibbs K.W., Abrams D., Huang M., Zeng K., Herlihy J.P., et al. Randomized, double-blinded, placebo-controlled trial of allogeneic cord blood T-regulatory cells for treatment of COVID-19 ARDS. Blood Adv. 2023;7:3075–3079. doi: 10.1182/bloodadvances.2022009619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bowdish M.E., Barkauskas C.E., Overbey J.R., Gottlieb R.L., Osman K., Duggal A., Marks M.E., Hupf J., Fernandes E., Leshnower B.G., et al. A Randomized Trial of Mesenchymal Stromal Cells for Moderate to Severe Acute Respiratory Distress Syndrome from COVID-19. Am. J. Respir. Crit. Care Med. 2023;207:261–270. doi: 10.1164/rccm.202201-0157OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hammond T.C., Purbhoo M.A., Kadel S., Ritz J., Nikiforow S., Daley H., Shaw K., van Besien K., Gomez-Arteaga A., Stevens D., et al. A phase 1/2 clinical trial of invariant natural killer T cell therapy in moderate-severe acute respiratory distress syndrome. Nat. Commun. 2024;15:974. doi: 10.1038/s41467-024-44905-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brandstadter J.D., Yang Y. Natural killer cell responses to viral infection. J. Innate Immun. 2011;3:274–279. doi: 10.1159/000324176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu J., Lanier L.L. Natural killer cells and cancer. Adv. Cancer Res. 2003;90:127–156. doi: 10.1016/s0065-230x(03)90004-2. [DOI] [PubMed] [Google Scholar]
- 23.Cook K.D., Waggoner S.N., Whitmire J.K. NK Cells and Their Ability to Modulate T Cells during Virus Infections. Crit. Rev. Immunol. 2014;34:359–388. doi: 10.1615/critrevimmunol.2014010604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Loh J., Chu D.T., O’Guin A.K., Yokoyama W.M., Virgin H.W. Natural killer cells utilize both perforin and gamma interferon to regulate murine cytomegalovirus infection in the spleen and liver. J. Virol. 2005;79:661–667. doi: 10.1128/JVI.79.1.661-667.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marischen L., Englert A., Schmitt A.-L., Einsele H., Loeffler J. Human NK cells adapt their immune response towards increasing multiplicities of infection of Aspergillus fumigatus. BMC Immunol. 2018;19:39. doi: 10.1186/s12865-018-0276-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paul S., Lal G. The Molecular Mechanism of Natural Killer Cells Function and Its Importance in Cancer Immunotherapy. Front. Immunol. 2017;8:1124. doi: 10.3389/fimmu.2017.01124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Market M., Angka L., Martel A.B., Bastin D., Olanubi O., Tennakoon G., Boucher D.M., Ng J., Ardolino M., Auer R.C. Flattening the COVID-19 Curve With Natural Killer Cell Based Immunotherapies. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.01512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zafarani A., Razizadeh M.H., Pashangzadeh S., Amirzargar M.R., Taghavi-Farahabadi M., Mahmoudi M. Natural killer cells in COVID-19: from infection, to vaccination and therapy. Future Virol. 2023;18:177–191. doi: 10.2217/fvl-2022-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ghasemzadeh M., Ghasemzadeh A., Hosseini E. Exhausted NK cells and cytokine storms in COVID-19: Whether NK cell therapy could be a therapeutic choice. Hum. Immunol. 2022;83:86–98. doi: 10.1016/j.humimm.2021.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wilk A.J., Rustagi A., Zhao N.Q., Roque J., Martínez-Colón G.J., McKechnie J.L., Ivison G.T., Ranganath T., Vergara R., Hollis T., et al. A single-cell atlas of the peripheral immune response in patients with severe COVID-19. Nat. Med. 2020;26:1070–1076. doi: 10.1038/s41591-020-0944-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith D.M., Schafer J.R., Tullius B., Witkam L., Paust S. Natural killer cells for antiviral therapy. Sci. Transl. Med. 2023;15 doi: 10.1126/scitranslmed.abl5278. [DOI] [PubMed] [Google Scholar]
- 32.Ciurea S.O., Schafer J.R., Bassett R., Denman C.J., Cao K., Willis D., Rondon G., Chen J., Soebbing D., Kaur I., et al. Phase 1 clinical trial using mbIL21 ex vivo–expanded donor-derived NK cells after haploidentical transplantation. Blood. 2017;130:1857–1868. doi: 10.1182/blood-2017-05-785659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Myers J.A., Miller J.S. Exploring the NK cell platform for cancer immunotherapy. Nat. Rev. Clin. Oncol. 2021;18:85–100. doi: 10.1038/s41571-020-0426-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.COVID-19 Treatment Guidelines Panel Hospitalized Adults: Therapeutic Management. COVID-19 Treat. Guidel. 2024. https://www.covid19treatmentguidelines.nih.gov/management/clinical-management-of-adults/hospitalized-adults--therapeutic-management/
- 35.Gasior M., Ferreras C., de Paz R., Bueno D., Mozo Y., Sisinni L., Canizales J.T., González B., Olivas-Mazón R., Marcos A., et al. The role of early natural killer cell adoptive infusion before engraftment in protecting against human herpesvirus-6B encephalitis after naïve T-cell-depleted allogeneic stem cell transplantation. Transfusion (Paris) 2021;61:1505–1517. doi: 10.1111/trf.16354. [DOI] [PubMed] [Google Scholar]
- 36.Razzack A.A., Hassan S.A., Pasya S.K.R., Erasani G., Kumar S., Rocha-Castellanos D.M., Lopez-Mendez A., Razzack S.A. A Meta-Analysis of Association between Remdesivir and Mortality among Critically-Ill COVID-19 Patients. Infect. Chemother. 2021;53:512–518. doi: 10.3947/ic.2021.0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rotundo S., Berardelli L., Gullì S., La Gamba V., Lionello R., Russo A., Trecarichi E.M., Torti C. Early initiation of combined therapy in severely immunocompromised patients with COVID-19: a retrospective cohort study. BMC Infect. Dis. 2024;24:564. doi: 10.1186/s12879-024-09466-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lowry L.E., Zehring W.A. Potentiation of Natural Killer Cells for Cancer Immunotherapy: A Review of Literature. Front. Immunol. 2017;8:1061. doi: 10.3389/fimmu.2017.01061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Walzer T., Dalod M., Robbins S.H., Zitvogel L., Vivier E. Natural-killer cells and dendritic cells: “l’union fait la force”. Blood. 2005;106:2252–2258. doi: 10.1182/blood-2005-03-1154. [DOI] [PubMed] [Google Scholar]
- 40.Abel A.M., Yang C., Thakar M.S., Malarkannan S. Natural Killer Cells: Development, Maturation, and Clinical Utilization. Front. Immunol. 2018;9 doi: 10.3389/fimmu.2018.01869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Coomes E.A., Haghbayan H. Interleukin-6 in Covid-19: A systematic review and meta-analysis. Rev. Med. Virol. 2020;30:1–9. doi: 10.1002/rmv.2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Han H., Ma Q., Li C., Liu R., Zhao L., Wang W., Zhang P., Liu X., Gao G., Liu F., et al. Profiling serum cytokines in COVID-19 patients reveals IL-6 and IL-10 are disease severity predictors. Emerg. Microb. Infect. 2020;9:1123–1130. doi: 10.1080/22221751.2020.1770129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pan D.Z., Odorizzi P.M., Schoenichen A., Abdelghany M., Chen S., Osinusi A., Patterson S.D., Downie B., Juneja K., Wallin J.J. Remdesivir improves biomarkers associated with disease severity in COVID-19 patients treated in an outpatient setting. Commun. Med. 2023;3:1–6. doi: 10.1038/s43856-022-00232-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and analyzed for this study are available from the corresponding author after publication upon reasonable request, with investigator financial support, and with appropriate documentation of IRB approval and/or data access agreements as applicable.