Abstract
Core fucosylation, catalyzed by α1,6-fucosyltransferase (FUT8), is an important N-glycosylation modification process that attaches a fucose residue via an α1,6-linkage to the core N-acetylglucosamine of N-glycans in mammals. Research over the past three decades has revealed the critical role of FUT8-mediated core fucosylation modification in various physiological and pathological processes, including cell growth, adhesion, receptor activation, antibody-dependent cellular cytotoxicity (ADCC), tumor metastasis and infections. This review discusses the immune system function involving FUT8 and the mechanisms by which core fucosylation regulates immunity and contributes to disease. A deeper understanding of these mechanisms can provide insights into cellular biology and suggest new therapeutic approaches and targets for related diseases.
Keywords: Core fucosylation, FUT8, Immune system, Disease
Protein glycosylation is a common and crucial post-translational modification that plays central roles in various physiological and pathological processes, including cell signaling and communication, tumor angiogenesis, immune system function, tumor cell migration and invasion, and cell-matrix interactions (Costa, 2017; Jones & Aplin, 2009; Moran, Gupta et al., 2011). Based on the glycosylation sites of peptides, protein glycosylation can be broadly classified into two main types in mammals: N-glycosylation (sequence: Asn-X-Ser/Thr, where X can be any amino acid except Pro) and O-glycosylation (sequence: Ser/Thr) (Dutta, Mandal et al., 2017). Fucosylation is an important subtype of N-glycosylation catalyzed by eleven fucosyltransferases (FUT1-11), which form α1,2-, α1,3-/4-, or α1,6-linked fucose residues, and two specialized protein O-fucosyltransferases (poFUT1-2) (Schneider, Al-Shareffi et al., 2017). Among these enzymes, mammalian α-1,6-fucosyltransferase (FUT8) is the sole enzyme responsible for core fucosylation, which adds α1,6-linked fucose to N-glycans (Wilson, Williams et al., 1976). Many glycoproteins undergo α1,6-fucosylation, and their biological functions are regulated by core fucosylation in numerous physiological processes and diseases. This review discusses the pivotal role of FUT8 in immune system and disease mechanisms, as well as its potential applications as a novel diagnostic marker and exploration of new therapeutic targets, aiming to advance host-directed therapies for relevant diseases.
1. Overview of FUT8 and core fucosylation
FUT8 is a type II membrane protein encoded by the FUT8 gene in humans (Wilson, Williams et al., 1976). It transfers a fucose residue from GDP-fucose to the inner GlcNAc portion of N-glycans, forming an α1-6 linkage (Wang, Inoue et al., 2005). Human FUT8 consists of 575 amino acids and is located on chromosome 14. Structurally and genetically, FUT8 differs from other α1,2, α1,3, and α1,4 fucosyltransferases (Yamaguchi, Fujii et al., 1999; Yamaguchi, Ikeda et al., 2000). The three-dimensional structure of human FUT8 was elucidated in 2007 using recombinant protein expressed in Sf21 cells infected with baculovirus (Ihara, Ikeda et al., 2007). According to their studies, FUT8 comprises three domains: an N-terminal helical domain, a catalytic domain, and a C-terminal SH3 domain.
FUT8 is biologically significant, and loss of core fucosylation modification can lead to growth retardation, emphysema, schizophrenia-like behaviors, and even death (Fukuda, Hashimoto et al., 2011; Wang, Inoue et al., 2005). Loss of core fucosylation in brain tissue may also contribute to cognitive deficits by affecting hippocampal long-term potentiation (LTP) (Gu, Fukuda et al., 2015). Furthermore, core fucosylation is involved in regulating the function of many glycoproteins. For example, loss of N-glycan core fucosylation in human IgG1 enhances ADCC (Ihara, Ikeda et al., 2006; Imai-Nishiya, Mori et al., 2007; Shields, Lai et al., 2002). Core fucosylation also modulates the function of immunoglobulins; for instance, it is essential for antigen recognition in humoral immune responses through IgG B-cell receptors (Li, Yu et al., 2015; Okazaki, Shoji-Hosaka et al., 2004), and stability of IgG (Sun, Xu et al., 2024).
2. The regulatory role of FUT8 and core fucosylation modification in the immune system
The immune system is a complex network of cells and proteins crucial for combating internal infections. Vertebrate immune defense systems consist of two subsystems-innate immunity and adaptive immunity. When antigens bind to specific receptors on immune system cells, a series of processes are triggered in the body. Immune receptors, typically located on cell membranes, recognize pathogens and activate immune responses. Major immune receptors include pattern recognition receptors (PRRs), Toll-like receptors (TLRs), killer activation and killer inhibition receptors (KARs and KIRs), complement receptors, Fc receptors, B-cell receptors (BCRs), and T-cell receptors (TCRs) (Williams;Wilkins 2007).
Cell surface glycans mediate many receptor-ligand interactions (Parker & Kohler, 2010). In the immune system, specific cell surface glycans are crucial for triggering signaling pathways that activate BCRs, TCRs, and TLRs (Dyken, Green et al., 2007; Grewal, Boton et al., 2006; Van; Leifer & Medvedev, 2016). Hereafter, we review the critical role of core fucosylation in these immune receptors and adhesion molecules.
2.1. Core fucosylation modification and BCR
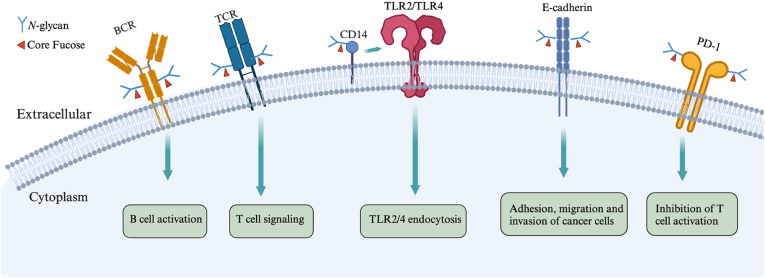
B cells are central to the adaptive humoral immune system, responsible for antigen (Ag) recognition and antibody (Ab) production. The BCR triggers signaling upon antigen recognition, ultimately leading to B-cell activation (Hagman, 2009). Blocking FUT8 has been reported to reduce pre-B cell generation in the bone marrow, particularly affecting responses to stromal cells (Li, Ishihara et al., 2008; Li, Liu et al., 2012), highlighting the critical biological function of FUT8 and core fucosylation in B-cell development. Further investigation is warranted to determine whether core fucosylation affects the biological functions of BCR (Fig. 1). Wen et al. found that core fucosylation mediates antigen recognition by IgG-BCR and its association with lipid raft drift, facilitating cellular signaling through BCR and antibody production (Li, Yu et al., 2015). FUT8 mediates early B cell development and functions since the B lineage genes, such as CD79a, CD79b, Ebf1, and Tcfe2a, were downregulated in Fut8KO pre-B cells (Li, Ishihara et al., 2008). It is well-known that IgG is highly core fucosylated, and loss of core fucosylation in IgG enhances affinity for Fcγ receptors in the Fc region, increasing ADCC mediated by NK cells (Fig. 1) (Shields, Lai et al., 2002). A recent study indicated that core fucosylated oligosaccharides in breast milk promote colonization by specific gut microbes, activating neonatal B cells via BCR-mediated signaling pathways, crucially supporting early gut microbiota formation and immune system development (Li, Bai et al., 2019). These studies suggest that core fucosylation regulates humoral immune responses mediated by BCR, offering new insights into immune system regulation and potential directions for developing interventions based on core fucosylation.
Fig. 1.
Regulation of immune system by FUT8 and core fucosylation. The diagram was created using BioRender.
2.2. Core fucosylation modification and TCR
T cells play a critical role in defending against intracellular pathogens such as viruses, parasites, and intracellular bacteria, as well as in assisting antibody responses against extracellular pathogens. The primary mediator of T-cell activation is the TCR, which consists of two distinct protein chains (α and β) and recognizes antigen peptides only when presented by MHC I or MHC II molecules on the cell surface.
Reduced N-glycosylation of TCR chains has been reported to enhance functional affinity (FA) and T-cell recognition capabilities (Kuball, Hauptrock et al., 2009), suggesting a unique role for TCR N-glycosylation in regulating T-cell activation. Notably, TCRs are highly core fucosylated glycoproteins (Fujii, Shinzaki et al., 2016), and core fucosylation of TCRs is crucial for T-cell signaling (Fig. 1). For instance, Fut8KO mice develop milder colitis compared to wild-type (WT) mice, with lower levels of T-helper cell 1/T-helper cell 2 (Th1/Th2) cytokines produced by Fut8KO T cells (Fujii, Shinzaki et al., 2016). Researchers have also observed that TCR complexes from Fut8KO mice fail to signal properly on CD4+ T cells and do not transfer to lipid raft drifts after activation (Fujii, Shinzaki et al., 2016). These findings indicate an increased core fucosylation of TCRs in T cells from intestinal tissues of patients with inflammatory bowel disease (IBD), suggesting that blocking this process could serve as a therapeutic strategy. Interestingly, core fucosylation of CD4+ T cells is significantly increased in patients with systemic lupus erythematosus (SLE), and Fut8KO OT-II CD4+ T cells show significantly reduced activation upon major histocompatibility complex II (pMHC-II) presentation of OVA323-339 on B cells compared to Fut8 WT OT-II CD4+ T cells (Liang, Mao et al., 2019). While this study suggests a role for core fucosylation of TCRs in the pathogenesis of SLE, further research is needed to determine whether core fucosylation serves as a potential novel biomarker with clinical and therapeutic implications for SLE patients.
Additionally, Wei et al. found that core fucosylation is significantly upregulated during thymic development from CD4−CD8− (double negative, DN) to CD4+CD8+ (double positive, DP) transition. Furthermore, they observed that loss of core fucosylation of TCRs in Fut8KO DP cells leads to reduced phosphorylation of ZAP70 (pZAP70), a cytoplasmic protein tyrosine kinase critical for initiating antigen receptor-mediated T-cell responses (Liang, Mao et al., 2019). These studies provide compelling evidence that core fucosylation regulates TCR development, response, and signaling in T cells.
2.3. Core fucosylation modification and TLRs
The innate immune system provides immediate and effective defense mechanisms against microbial infections. It promotes inflammatory responses and plays a role in activating the adaptive immune system to detect specific antigens. In vertebrates, TLRs are essential components of the innate immune response and belong to a type I transmembrane glycoprotein family (Gay & Gangloff, 2007). To date, 13 members of the mammalian TLR family have been described, categorized into six subfamilies based on functional and sequence characteristics (Roach, Glusman et al., 2005).
Fut8KO mice exhibit a lung emphysema-like phenotype similar to that seen in aged TLR4-deficient mice, suggesting that loss of core fucosylation affects the TLR4 signaling pathway (Iijima, Kobayashi et al., 2017). In vivo and in vitro experiments with Fut8KO macrophages stimulated with lipopolysaccharide (LPS) show inhibited production of inflammatory cytokines (Nakayama, Wakamatsu et al., 2019). The LPS receptor complex consists of two interacting receptors, CD14 and TLR4, along with an associated protein, MD-2 (Calvano, Agnese et al., 2003). As a member of the TLR family, TLR4 plays a crucial role in the innate immune response against microbial pathogens and serves as the receptor for LPS (Beutler, Du et al., 2001; Plociennikowska, Hromada-Judycka et al., 2015). CD14 is a binding protein for LPS (Wright, Ramos et al., 1990) and is essential for internalization of TLR4 (Zanoni, Ostuni et al., 2011). MD-2 binds to the extracellular domain of TLR4 and is considered part of the TLR4-MD-2 complex that interacts with LPS (Shimazu, Akashi et al., 1999). Studies indicate that core fucosylation levels of CD14 are elevated in this LPS receptor complex, and loss of core fucosylation leads to inadequate binding with the TLR4/MD-2 complex, resulting in impaired internalization (Iijima, Kobayashi et al., 2017; Nakayama, Wakamatsu et al., 2019). Since CD14 is essential for TLR2 signaling, immune responses mediated by TLR2 are also affected in Fut8KO macrophages (Nakayama, Wakamatsu et al., 2019). These findings suggest that core fucosylation is crucial for CD14-dependent TLR4 and TLR2 signaling in mouse macrophage activity (Fig. 1). However, the mechanisms by which core fucosylation regulates CD14-dependent membrane-bound receptor internalization need further clarification, and whether core fucosylation affects other TLR signaling pathways remains unclear.
2.4. Core fucosylation modification and other adhesion molecules
Cell surface antigens, also known as cell markers, serve as unique identifiers that help classify and identify cells. They are mostly molecules within the cell membrane. These molecules not only act as markers but also play crucial functional roles, such as in diagnosing diseases or guiding treatments. Glycosylation of cell surface proteins is associated with various important physiological processes, including cell proliferation, differentiation, migration, intercellular integrity and recognition, cell-matrix and host-pathogen interactions, immune regulation, and signal transduction (Varki, 1993).
Extensive research indicate that altered glycosylation patterns on cancer cell surfaces disrupt cell functions, ultimately leading to their metastatic and invasive behaviors (Hoja-Lukowicz, Link-Lenczowski et al., 2013; Julien, Ivetic et al., 2011; Kim, Ahn et al., 2012; Liu, Lin et al., 2010; Wei, Liu et al., 2012). Increased levels of core fucosylation and associated FUT8 gene expression appear to be critical factors in most cancers (Abbott, Nairn et al., 2008; Chrostek & Cylwik, 2011; Hutchinson, Du et al., 1991; Osumi, Takahashi et al., 2009; Potapenko, Haakensen et al., 2010). Various studies have identified the role of core fucosylation in E-cadherin-dependent adhesion, as both are increased in cancer tissues (Hanski, Klussmann et al., 1996; Osumi, Takahashi et al., 2009). E-cadherin is one of the most important molecules for intercellular adhesion in epithelial tissues. It is located on the surface of epithelial cells and is a crucial component of tight junctions essential for cell adhesion and maintaining epithelial phenotype (Gumbiner, 1996). Research by Hu et al. suggests that core fucosylation of E-cadherin regulates the accumulation of nuclear β-catenin in lung cancer cells (Hu, Shi et al., 2008). Nuclear accumulation of β-catenin increases the transcription of many oncogenes including c-Myc and cyclin D-1, leading to various diseases including cancer (Morin, 1999). Transfection of Fut8 into Fut8KO mouse renal epithelial cells restores the expression of E-cadherin and E-cadherin-dependent intercellular adhesion (Osumi, Takahashi et al., 2009), indicating that core fucosylation plays a crucial role in regulating intercellular adhesion in cancer (Fig. 1).
A recent study showed that FUT8 deficiency inhibited migration of MCF-7 cells through modulation of core fucosylation of E-cadherin and downstream FAK/integrin signaling pathways (Liu, Gao et al., 2019). FAK regulates breast cancer stem cells and breast cancer through integrin signaling (Guan, 2010). Thus, FUT8 serves as a potential biomarker for cancer detection and treatment. FUT8 deficiency not only inhibits adhesion, migration, and invasion of cancer cells but also enhances anti-tumor immune responses. For instance, loss of core fucosylation downregulates cell surface expression of PD-1 (programmed cell death 1) and promotes T cell activation, leading to more effective tumor clearance (Okada, Chikuma et al., 2017). PD-1 is highly expressed on exhausted T cells and inhibits T cell activation (Fig. 1) (Ahmadzadeh, Johnson et al., 2009). Core fucosylation of PD-1 is necessary for its proper surface expression, stability, and ligand binding (Okada, Chikuma et al., 2017), although the precise biochemical mechanisms by which core fucosylation affects PD-1 structure and function require further study for clarification.
2.5. Core fucosylation modification and IgG
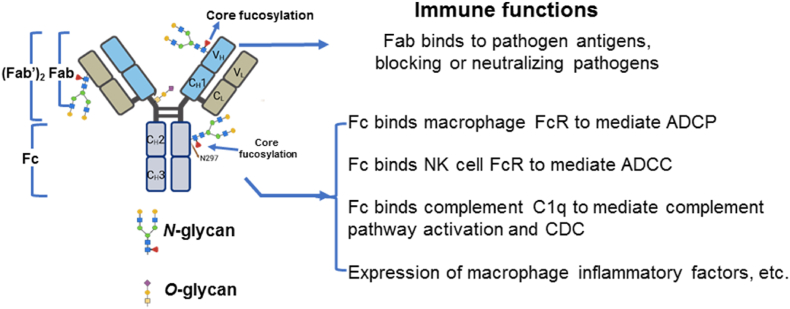
Recent advances in the study of glycosylation have highlighted the crucial role of IgG glycosylation (such as core fucosylation, galactosylation and sialylation) in modulating the function of IgG and its impact on immune responses, particularly in the context of viral and bacterial infections (Fig. 2). Core fucosylation is a key regulator of IgG-mediated immune responses, and its alteration has significant implications for both disease progression and therapeutic interventions. Several studies have demonstrated that the absence of core fucosylation (afucosylation) enhances IgG's binding affinity to the FcγRIIIa receptor, a key receptor involved in ADCC.
Fig. 2.
Role of IgG glycosylation (such as core fucosylation or galactosylation modification) in modulating the function of IgG and its impact on immune responses. ADCP: antibody-dependent cellular phagocytosis. ADCC: antibody-dependent cell-mediated cytotoxicity; CDC: complement-dependent cytotoxicity.
Wang et al. (2017) provided evidence linking afucosylated IgG with increased disease severity in Dengue viral infection, showing that enhanced FcγRIIIa binding leads to more robust immune activation and tissue damage (Wang, Sewatanon et al., 2017). Similarly, studies on COVID-19 have highlighted that the IgG antibodies from patients with severe COVID-19 exhibited proinflammatory Fc structures, with reduced fucosylation contributing to increased inflammation and disease severity (Chakraborty, Gonzalez et al., 2021; Hoepel, Chen et al., 2021). Larsen et al. further confirmed that afucosylated IgG is a marker of severe COVID-19, highlighting its role in modulating viral immune responses (Larsen, de Graaf et al., 2021). These findings underline the importance of core fucosylation in regulating the immune response during viral infections.
In the context of COVID-19, altered glycosylation at the time of diagnosis has been found to predict disease severity, suggesting a potential role of glycosylation as a biomarker for disease progression (Vicente, Alves et al., 2022). Furthermore, novel synthetic nanobodies have been developed to distinguish different IgG Fc glycoforms, providing a valuable tool for future research on antibody glycosylation (Kao, Gupta et al., 2022).
Mechanistic and structural insights are crucial for understanding how alterations in core-fucosylation impact protein functions, especially in the immune system. Continued research into the mechanistic and structural impacts of fucosylation on IgG function will be critical for developing targeted treatments and vaccines for viral infections.
3. Core fucosylation modification and diseases
3.1. Core fucosylation modification and cancer
Aberrant fucosylation is one of the most important oligosaccharide modifications in cancer and inflammation, often dysregulated by altered expression of fucosyltransferases (Dube & Bertozzi, 2005; Miyoshi, Moriwaki et al., 2008; Pinho & Reis, 2015; Reily, Stewart et al., 2019; Scott, Elliott et al., 2020). FUT8 has been shown to be upregulated in various cancers, including lung cancer (Chen, Jan et al., 2013; Wang, Inoue et al., 2005), hepatocellular carcinoma (Cheng, Gao et al., 2016; Noda, Miyoshi et al., 1998; Noda, Okayama et al., 2018), colorectal cancer (Osumi, Takahashi et al., 2009), ovarian cancer (Lv, Song et al., 2019), prostate cancer (Wang, Chen et al., 2014), breast cancer (Tu, Wu et al., 2017), melanoma (Agrawal, Fontanals-Cirera et al., 2017), thyroid cancer (Ito, Miyauchi et al., 2003), and pancreatic cancer (Tada, Ohta et al., 2020). Moreover, FUT8 correlates with patient prognosis and is considered a prognostic biomarker for lung cancer (Honma, Kinoshita et al., 2015), colorectal cancer (Noda, Okayama et al., 2018), and prostate cancer (Scott & Munkley, 2019). Regulatory mechanisms for the upregulation of FUT8 across multiple cancer types include several pathways: (1) modulation of PD-1 expression (Okada, Chikuma et al., 2017), (2) alteration of ADCC (Pereira, Chan et al., 2018), and (3) regulation of transforming growth factor β1 receptor (TGF-β), epidermal growth factor receptor (EGFR) (Matsumoto, Yokote et al., 2008), α3β1 integrin (Zhao, Itoh et al., 2006), and E-cadherin (Hu, Shi et al., 2008). Based on these findings, FUT8 is considered a promising drug target for various cancer types. Additionally, highly core fucosylated glycoproteins such as α-fetoprotein show significant potential as cancer biomarkers but are less pronounced in other liver diseases (Aoyagi, Isokawa et al., 1998; Aoyagi, Suzuki et al., 1988; Miyoshi, Moriwaki et al., 2012). Other core fucosylated glycoproteins, such as E-cadherin and Cancer antigen 125 (CA-125), have also been identified as potential cancer biomarkers based on their levels of core fucosylation (Geng, Shi et al., 2004; Shao, Sokolik et al., 1994).
3.2. Core fucosylation modification and infectious diseases
As intracellular parasites dependent on host cell protein synthesis machinery, viruses' survival is closely linked to host cell processes essential for regulating virus replication. It has been reported that infections from various virus families lead to modifications in host cell glycosylation profiles, involving mechanisms that activate host cell glycosyltransferase transcription (Cebulla, Miller et al., 2000; Norden, Nystrom et al., 2013; Nystrom, Grahn et al., 2007; Nystrom, Norden et al., 2009; Papic, Maxwell et al., 2012). Increasing evidence suggests that various virus infections alter the glycosylation profile of infected cells by upregulating specific host cell FUT8 expression, such as hepatitis C virus (HCV) (Xiang, Yang et al., 2017, Li, Liu et al., 2019), hepatitis B virus (HBV) (Takamatsu, Shimomura et al., 2016), and human papillomavirus (HPV) (Xu et al., 2024a, Xu et al., 2024b). Recently we found that HCV-induced FUT8 causes 5-FU drug resistance in human hepatoma Huh7.5.1 cells (Li, Liu, et al., 2019), and EGFR core fucosylation, induced by HCV, promotes TRIM40-mediated-RIG-I ubiquitination and suppresses interferon-I antiviral defenses (Pan, Xie et al., 2024). Furthermore, elevated FUT8 expression enhances cellular susceptibility to HBV, sustaining long-term infection states (Takamatsu, Shimomura et al., 2016). A genetic variation (SNP rs4131564) in FUT8 is associated with accelerated HIV-1 disease progression, indicating the significance of N-glycan fucosylation despite no clear in vitro effects on the immune system or HIV-1 replication (van Pul, Maurer et al., 2023).
These findings provide new perspectives on the mechanisms of viral infection, and FUT8 may serve as a potential target for developing novel therapeutic strategies against viral infections. Understanding the interaction between viral infection and host cell FUT8 could improve our understanding of viral pathogenic mechanisms and potentially lead to new interventions to control the occurrence and progression of viral infections.
3.3. FUT8 and neurological disorders
Mutations in human FUT8 lead to a rare genetic metabolic disorder known as FUT8-CDG (FUT8-Congenital Disorder of Glycosylation) (Ng, Xu et al., 2018). FUT8-CDG is a severe disease primarily affecting the nervous system: common symptoms include seizures, hypotonia, developmental delay, and intellectual disabilities. Difficulty feeding may result in failure to thrive, and patients often exhibit slow growth rates (Ng, Xu et al., 2018). Some patients are severely affected by the disease and may die in early childhood, similar to symptoms observed in Fut8 gene knockout mice (Ng, Xu et al., 2018). To date, there are no FDA-approved treatment options for FUT8-CDG. Treatment aims to manage symptoms and prevent complications. Oral supplementation with dietary sugar L-fucose has led to clinical symptom improvement in severely affected twins with FUT8-CDG, although this has not been formally studied in clinical trials (Park, Reunert et al., 2020). Recent study also showed that exogenous L-fucose suppressed neuroinflammation (Xu et al., 2024a, Xu et al., 2024b), which might be closely related to mental disorder. Studies have shown that core fucosylation and FUT8 expression are significantly increased in serum samples from epilepsy and refractory epilepsy patients. Serum FUT8 has been found to have potential value in predicting refractory epilepsy (Tudor, Nedic Erjavec et al., 2023). Additionally, research has found that Fut8 deficiency affects the proliferation and neuronal differentiation of adult neural stem/progenitor cells (aNSPCs) and impairs learning and memory in mice. Furthermore, Fut8 regulates adult neurogenesis through the PI3K/Akt signaling pathway (Guo, Sun et al., 2024). Elevated serum FUT8 levels are significantly associated with an increased risk of early Parkinson's disease-related symptoms, suggesting that serum FUT8 could serve as a potential biomarker for the early detection of Parkinson's disease (Wang, Yu et al., 2024). These findings provide new perspectives for understanding adult neurogenesis.
3.4. FUT8 and autoimmune diseases
Systemic lupus erythematosus (SLE) is a chronic autoimmune disease affecting various organs, particularly the skin, joints, blood, kidneys, and central nervous system. Studies have found significantly increased core fucosylation of CD4+ T cells in SLE patients (Liang, Mao et al., 2018). Through Fut8 deficiency, CD4+ T cell activation is reduced, and experimental autoimmune encephalomyelitis induction syndrome in Fut8KO mice improves (Liang, Mao et al., 2018). Moreover, Fut8KO CD4+ T cells exhibit significantly reduced T cell activation, and phosphorylation of ZAP-70 in Fut8 WT CD4+ T cells is influenced by core fucosylation levels (Liang, Mao et al., 2018). These results suggest that core fucosylation is crucial for efficient TCR-pMHC-II contact in CD4+ T cell activation and excessive core fucosylation may serve as a potential new biomarker in serum of SLE patients.
Ulcerative colitis (UC) is considered an autoimmune and inflammatory bowel disease. Recent research indicates that core fucosylation of mucins increases mucin viscosity and resistance to shear stress, with elevated mucin levels and FUT8 levels in UC patients (Cantero-Recasens, Burballa et al., 2022). The study demonstrates that FUT8 overexpression increases secretion of specific mucins (MUC1, MUC2, and MUC5AC) and makes these mucins more difficult to remove from the cell surface. Fut8 deficiency leads to intracellular accumulation of MUC1 and alters the secretion ratio of MUC2 to MUC5AC (Cantero-Recasens, Burballa et al., 2022). These findings align with phenotypes observed in Fut8KO mice, which are protected from UC effects (Fujii, Shinzaki et al., 2016). The study reveals that FUT8 changes the biophysical properties of mucin by regulating cell surface levels of MUC1 and secretion of MUC2 and MUC5AC, suggesting these changes may promote interactions between bacteria and epithelial cells, leading to inflammation and progression of UC.
4. FUT8 inhibitors
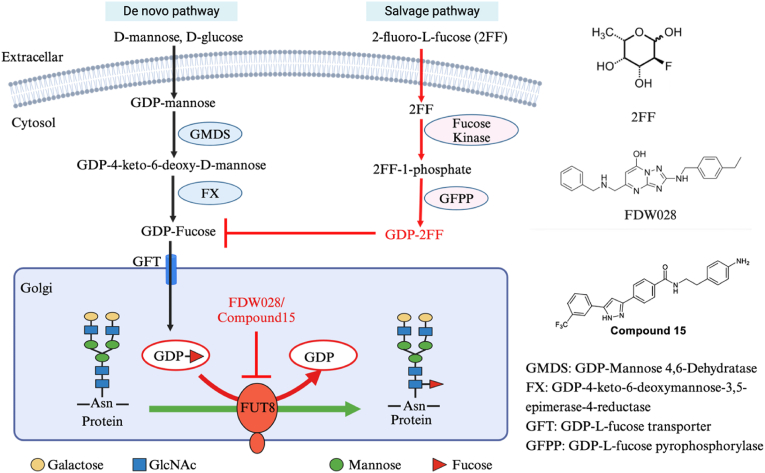
2-Fluoro-L-fucose (2FF) is a specific fucose analog that acts by disrupting fucosylation processes (Fig. 3) (Okeley, Alley et al., 2013). It can easily enter cells and is metabolically converted into the corresponding donor substrate mimic GDP-2FF. Accumulation of GDP-2FF in cells inhibits the formation of natural GDP-Fucose, thereby blocking the de novo pathway of GDP-Fucose synthesis (Rillahan, Antonopoulos et al., 2012). Due to the accumulation of GDP-2FF, fucosylation on the cell surface is significantly inhibited (Dumont, Lehner et al., 2015). Therefore, 2FF has been used to reduce cell surface fucosylation, such as Lewis antigens in colon cancer cells and core fucosylation blockade in HL-60 cells.
Fig. 3.
GDP-Fucose biosynthesis, FUT8 catalytic reaction and inhibition of core fucosylation by 2FF, FDW028 and compound 15. This figure illustrates the mechanisms by which 2FF, FDW028, and Compound 15 inhibit core fucosylation. The figure also shows the biosynthetic pathways of GDP-fucose through the de novo and salvage pathways, as well as the catalytic reaction of FUT8. 2FF interferes with the salvage pathway, while FDW028 and Compound 15 block the catalytic activity of FUT8. The chemical structures of the inhibitors are displayed, highlighting their roles in disrupting core fucosylation. The diagram was created using BioRender.
FDW028 is a potent and selective inhibitor targeting FUT8 (Fig. 3) (Wang, Zhang et al., 2023). It interacts with specific sites on the FUT8 protein through hydrogen bonds and π-cation/π-anion interactions, thereby inhibiting FUT8 activity. In vitro experiments have demonstrated that FDW028 interacts with FUT8 in a dose-dependent manner with high ligand binding affinity, showing promising inhibitory effects (Wang, Zhang et al., 2023). Further research indicates that FDW028 stabilizes the structure of the FUT8 protein, increases its melting temperature, and thereby sustainably inhibits FUT8 activity in cells (Wang, Zhang et al., 2023). Pull-down experiments have confirmed the specific binding capacity of FDW028 to FUT8, highlighting its selective action on FUT8 (Wang, Zhang et al., 2023).
Recently, researchers used virtual screening and chemical optimization to identify novel FUT8 inhibitors, leading to the discovery of compound 15, which has a unique chemical structure and demonstrated in vitro antitumor activity (Lv, Zhang et al., 2024). Chemical pulldown experiments and binding assays confirmed that compound 15 selectively binds to FUT8. In vivo studies showed that compound 15 has promising anti-colorectal cancer (CRC) effects in SW480 xenografts, supporting its potential for CRC treatment (Lv, Zhang et al., 2024).
In summary, small molecule inhibitors of core fucosylation such as 2FF, FDW028 and compound 15 have been shown to inhibit proliferation of tumor cells in liver cancer (Zhou, Fukuda et al., 2017), pancreatic cancer (Liang, Fukuda et al., 2021), colorectal cancer (Lv, Zhang et al., 2024)., and others (Wang, Zhang et al., 2023). However, further research and clinical validation are needed to determine their successful application in the treatment of FUT8-related diseases. As understanding of their mechanisms of action in vivo deepens and comprehensive evaluations of their safety and efficacy are conducted, their potential uses in FUT8-related therapies can be established.
5. Conclusion and perspectives
Core fucosylation is an essential form of glycosylation that involves the addition and modification of sugar chains, playing a crucial role in the function and stability of proteins. Within cells, core fucosylation functions by regulating the activation of various signaling pathways and the binding affinity of glycoprotein ligands.
This article explores the role of core fucosylation in the regulation of immune system function, disease progression, and treatment. However, the precise structural changes induced by the presence or absence of core fucosylation, and how these changes affect downstream processes, are still not fully understood. Gaining a deeper understanding of these structural and mechanistic insights will be crucial for developing therapeutic strategies targeting core fucosylation. For example, therapies that modulate core fucosylation in glycoproteins could potentially be used to enhance immune responses in diseases like cancer and viral infections. Additionally, investigating how core fucosylation affects glycoprotein stability, trafficking, and degradation could provide valuable information for designing new biopharmaceuticals.
In summary, core fucosylation plays an important role in cellular signal regulation and immune responses. Through in-depth research and exploration, we can better understand the mechanisms of core fucosylation, providing new ideas and methods for disease prevention, diagnosis, and treatment, thereby contributing more significantly to human health.
CRediT authorship contribution statement
Qiu Pan: Writing – original draft, Visualization, Validation, Software, Methodology, Investigation, Formal analysis, Data curation. Xiao-Lian Zhang: Writing – review & editing, Validation, Supervision, Resources, Project administration, Funding acquisition, Conceptualization.
Funding
This work was supported by: National Natural Science Foundation of China grants 82230078, 22077097, 91740120, and 21572173; National Outstanding Youth Foundation of China grant 81025008; National Key R&D Program of China grants 2022YFA1303500, 2018YFA0507603.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Abbott K.L., Nairn A.V., Hall E.M., Horton M.B., McDonald J.F., Moremen K.W., Dinulescu D.M., Pierce M. "Focused glycomic analysis of the N-linked glycan biosynthetic pathway in ovarian cancer.". Proteomics. 2008;8(16):3210–3220. doi: 10.1002/pmic.200800157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal P., Fontanals-Cirera B., Sokolova E., Jacob S., Vaiana C.A., Argibay D., Davalos V., McDermott M., Nayak S., Darvishian F., Castillo M., Ueberheide B., Osman I., Fenyö D., Mahal L.K., Hernando E. "A systems biology approach identifies FUT8 as a driver of melanoma metastasis.". Cancer Cell. 2017;31(6):804–819. doi: 10.1016/j.ccell.2017.05.007. e807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmadzadeh M., Johnson L.A., Heemskerk B., Wunderlich J.R., Dudley M.E., White D.E., Rosenberg S.A. "Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired.". Blood. 2009;114(8):1537–1544. doi: 10.1182/blood-2008-12-195792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoyagi Y., Isokawa O., Suda T., Watanabe M., Suzuki Y., Asakura H. "The fucosylation index of alpha-fetoprotein as a possible prognostic indicator for patients with hepatocellular carcinoma.". Cancer. 1998;83(10):2076–2082. [PubMed] [Google Scholar]
- Aoyagi Y., Suzuki Y., Isemura M., Nomoto M., Sekine C., Igarashi K., Ichida F. "The fucosylation index of alpha-fetoprotein and its usefulness in the early diagnosis of hepatocellular carcinoma.". Cancer. 1988;61(4):769–774. doi: 10.1002/1097-0142(19880215)61:4<769::aid-cncr2820610422>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Beutler B., Du X., Poltorak A. Identification of toll-like receptor 4 (Tlr4) as the sole conduit for LPS signal transduction: Genetic and evolutionary studies. Journal of Endotoxin Research. 2001;7(4):277–280. [PubMed] [Google Scholar]
- Calvano J.E., Agnese D.M., Um J.Y., Goshima M., Singhal R., Coyle S.M., Reddell M.T., Kumar A., Calvano S.E., Lowry S.F. Modulation of the lipopolysaccharide receptor complex (CD14, TLR4, MD-2) and toll-like receptor 2 in systemic inflammatory response syndrome-positive patients with and without infection: Relationship to tolerance. Shock. 2003;20(5):415–419. doi: 10.1097/01.shk.0000092269.01859.44. [DOI] [PubMed] [Google Scholar]
- Cantero-Recasens G., Burballa C., Ohkawa Y., Fukuda T., Harada Y., Curwin A.J., Brouwers N., Thun G.A., Gu J., Gut I., Taniguchi N., Malhotra V., I. C. Consortium "The ulcerative colitis-associated gene FUT8 regulates the quantity and quality of secreted mucins.". Proceedings of the National Academy of Sciences of the U S A. 2022;119(43) doi: 10.1073/pnas.2205277119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cebulla C.M., Miller D.M., Knight D.A., Briggs B.R., McGaughy V., Sedmak D.D. "Cytomegalovirus induces sialyl Lewis(x) and Lewis(x) on human endothelial cells.". Transplantation. 2000;69(6):1202–1209. doi: 10.1097/00007890-200003270-00027. [DOI] [PubMed] [Google Scholar]
- Chakraborty S., Gonzalez J., Edwards K., Mallajosyula V., Buzzanco A.S., Sherwood R., Buffone C., Kathale N., Providenza S., Xie M.M., Andrews J.R., Blish C.A., Singh U., Dugan H., Wilson P.C., Pham T.D., Boyd S.D., Nadeau K.C., Pinsky B.A.…Wang T.T. "Proinflammatory IgG Fc structures in patients with severe COVID-19.". Nature Immunology. 2021;22(1):67–73. doi: 10.1038/s41590-020-00828-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C.Y., Jan Y.H., Juan Y.H., Yang C.J., Huang M.S., Yu C.J., Yang P.C., Hsiao M., Hsu T.L., Wong C.H. "Fucosyltransferase 8 as a functional regulator of nonsmall cell lung cancer.". Proceedings of the National Academy of Sciences of the U S A. 2013;110(2):630–635. doi: 10.1073/pnas.1220425110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng L., Gao S., Song X., Dong W., Zhou H., Zhao L., Jia L. "Comprehensive N-glycan profiles of hepatocellular carcinoma reveal association of fucosylation with tumor progression and regulation of FUT8 by microRNAs.". Oncotarget. 2016;7(38):61199–61214. doi: 10.18632/oncotarget.11284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrostek L., Cylwik B. "[The alteration of proteins glycosylation in liver diseases].". Polski Merkuriusz Lekarski. 2011;31(181):60–64. [PubMed] [Google Scholar]
- Costa J. Glycoconjugates from extracellular vesicles: Structures, functions and emerging potential as cancer biomarkers. Biochimica et Biophysica Acta (BBA) - Reviews on Cancer. 2017;1868(1):157–166. doi: 10.1016/j.bbcan.2017.03.007. [DOI] [PubMed] [Google Scholar]
- Dube D.H., Bertozzi C.R. "Glycans in cancer and inflammation–potential for therapeutics and diagnostics.". Nature Reviews Drug Discovery. 2005;4(6):477–488. doi: 10.1038/nrd1751. [DOI] [PubMed] [Google Scholar]
- Dumont M., Lehner A., Bardor M., Burel C., Vauzeilles B., Lerouxel O., Anderson C.T., Mollet J.C., Lerouge P. "Inhibition of fucosylation of cell wall components by 2-fluoro 2-deoxy-L-fucose induces defects in root cell elongation.". The Plant Journal. 2015;84(6):1137–1151. doi: 10.1111/tpj.13071. [DOI] [PubMed] [Google Scholar]
- Dutta D., Mandal C., Mandal C. Unusual glycosylation of proteins: Beyond the universal sequon and other amino acids. Biochimica et Biophysica Acta (BBA) - General Subjects. 2017;1861(12):3096–3108. doi: 10.1016/j.bbagen.2017.08.025. [DOI] [PubMed] [Google Scholar]
- Dyken Van, J S., Green R.S., Marth J.D. "Structural and mechanistic features of protein O glycosylation linked to CD8+ T-cell apoptosis.". Molecular and Cellular Biology. 2007;27(3):1096–1111. doi: 10.1128/MCB.01750-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii H., Shinzaki S., Iijima H., Wakamatsu K., Iwamoto C., Sobajima T., Kuwahara R., Hiyama S., Hayashi Y., Takamatsu S., Uozumi N., Kamada Y., Tsujii M., Taniguchi N., Takehara T., Miyoshi E. Core fucosylation on T cells, required for activation of T-cell receptor signaling and induction of colitis in mice, is increased in patients with inflammatory bowel disease. Gastroenterology. 2016;150(7):1620–1632. doi: 10.1053/j.gastro.2016.03.002. [DOI] [PubMed] [Google Scholar]
- Fukuda T., Hashimoto H., Okayasu N., Kameyama A., Onogi H., Nakagawasai O., Nakazawa T., Kurosawa T., Hao Y., Isaji T., Tadano T., Narimatsu H., Taniguchi N., Gu J. "Alpha1,6-fucosyltransferase-deficient mice exhibit multiple behavioral abnormalities associated with a schizophrenia-like phenotype: Importance of the balance between the dopamine and serotonin systems.". Journal of Biological Chemistry. 2011;286(21):18434–18443. doi: 10.1074/jbc.M110.172536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gay N.J., Gangloff M. "Structure and function of Toll receptors and their ligands.". Annual Review of Biochemistry. 2007;76:141–165. doi: 10.1146/annurev.biochem.76.060305.151318. [DOI] [PubMed] [Google Scholar]
- Geng F., Shi B.Z., Yuan Y.F., Wu X.Z. "The expression of core fucosylated E-cadherin in cancer cells and lung cancer patients: Prognostic implications.". Cell Research. 2004;14(5):423–433. doi: 10.1038/sj.cr.7290243. [DOI] [PubMed] [Google Scholar]
- Grewal P.K., Boton M., Ramirez K., Collins B.E., Saito A., Green R.S., Ohtsubo K., Chui D., Marth J.D. "ST6Gal-I restrains CD22-dependent antigen receptor endocytosis and Shp-1 recruitment in normal and pathogenic immune signaling.". Molecular and Cellular Biology. 2006;26(13):4970–4981. doi: 10.1128/MCB.00308-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu W., Fukuda T., Isaji T., Hang Q., Lee H.H., Sakai S., Morise J., Mitoma J., Higashi H., Taniguchi N., Yawo H., Oka S., Gu J. Loss of α1,6-fucosyltransferase decreases hippocampal long term potentiation: Implications for core fucosylation in the regulation of ampa receptor heteromerization and cellular signaling. Journal of Biological Chemistry. 2015;290(28):17566–17575. doi: 10.1074/jbc.M114.579938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan J.L. "Integrin signaling through FAK in the regulation of mammary stem cells and breast cancer.". IUBMB Life. 2010;62(4):268–276. doi: 10.1002/iub.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumbiner B.M. Cell adhesion: The molecular basis of tissue architecture and morphogenesis. Cell. 1996;84(3):345–357. doi: 10.1016/s0092-8674(00)81279-9. [DOI] [PubMed] [Google Scholar]
- Guo H., Sun Q., Huang X., Wang X., Zhang F., Qu W., Liu J., Cheng X., Zhu Q., Yi W., Shu Q., Li X. "Fucosyltransferase 8 regulates adult neurogenesis and cognition of mice by modulating the Itga6-PI3K/Akt signaling pathway.". Science China Life Sciences. 2024;67(7):1427–1440. doi: 10.1007/s11427-023-2510-0. 2024 Jul. [DOI] [PubMed] [Google Scholar]
- Hagman J. "Conveying the message: Identification of ig-alpha and ig-beta as components of the B cell receptor complex.". The Journal of Immunology. 2009;183(3):1503–1504. doi: 10.4049/jimmunol.0990055. [DOI] [PubMed] [Google Scholar]
- Hanski C., Klussmann E., Wang J., Bohm C., Ogorek D., Hanski M.L., Kruger-Krasagakes S., Eberle J., Schmitt-Graff A., Riecken E.O. "Fucosyltransferase III and sialyl-Le(x) expression correlate in cultured colon carcinoma cells but not in colon carcinoma tissue.". Glycoconjugate Journal. 1996;13(5):727–733. doi: 10.1007/BF00702336. [DOI] [PubMed] [Google Scholar]
- Hoepel W., Chen H.J., Geyer C.E., Allahverdiyeva S., Manz X.D., de Taeye S.W., Aman J., Mes L., Steenhuis M., Griffith G.R., Bonta P.I., Brouwer P.J.M., Caniels T.G., van der Straten K., Golebski K., Jonkers R.E., Larsen M.D., Linty F., Nouta J.…den Dunnen J. "High titers and low fucosylation of early human anti-SARS-CoV-2 IgG promote inflammation by alveolar macrophages.". Science Translational Medicine. 2021;13(596) doi: 10.1126/scitranslmed.abf8654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoja-Lukowicz D., Link-Lenczowski P., Carpentieri A., Amoresano A., Pochec E., Artemenko K.A., Bergquist J., Litynska A. L1CAM from human melanoma carries a novel type of N-glycan with Galbeta1-4Galbeta1- motif. Involvement of N-linked glycans in migratory and invasive behaviour of melanoma cells. Glycoconjugate Journal. 2013;30(3):205–225. doi: 10.1007/s10719-012-9374-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honma R., Kinoshita I., Miyoshi E., Tomaru U., Matsuno Y., Shimizu Y., Takeuchi S., Kobayashi Y., Kaga K., Taniguchi N., Dosaka-Akita H. "Expression of fucosyltransferase 8 is associated with an unfavorable clinical outcome in non-small cell lung cancers.". Oncology. 2015;88(5):298–308. doi: 10.1159/000369495. [DOI] [PubMed] [Google Scholar]
- Hu P., Shi B., Geng F., Zhang C., Wu W., Wu X.Z. "E-cadherin core fucosylation regulates nuclear beta-catenin accumulation in lung cancer cells.". Glycoconjugate Journal. 2008;25(9):843–850. doi: 10.1007/s10719-008-9144-6. [DOI] [PubMed] [Google Scholar]
- Hutchinson W.L., Du M.Q., Johnson P.J., Williams R. Fucosyltransferases: Differential plasma and tissue alterations in hepatocellular carcinoma and cirrhosis. Hepatology. 1991;13(4):683–688. [PubMed] [Google Scholar]
- Ihara H., Ikeda Y., Taniguchi N. "Reaction mechanism and substrate specificity for nucleotide sugar of mammalian alpha1,6-fucosyltransferase–a large-scale preparation and characterization of recombinant human FUT8.". Glycobiology. 2006;16(4):333–342. doi: 10.1093/glycob/cwj068. [DOI] [PubMed] [Google Scholar]
- Ihara H., Ikeda Y., Toma S., Wang X., Suzuki T., Gu J., Miyoshi E., Tsukihara T., Honke K., Matsumoto A., Nakagawa A., Taniguchi N. "Crystal structure of mammalian alpha1,6-fucosyltransferase, FUT8.". Glycobiology. 2007;17(5):455–466. doi: 10.1093/glycob/cwl079. [DOI] [PubMed] [Google Scholar]
- Iijima J., Kobayashi S., Kitazume S., Kizuka Y., Fujinawa R., Korekane H., Shibata T., Saitoh S.I., Akashi-Takamura S., Miyake K., Miyoshi E., Taniguchi N. "Core fucose is critical for CD14-dependent Toll-like receptor 4 signaling.". Glycobiology. 2017;27(11):1006–1015. doi: 10.1093/glycob/cwx075. [DOI] [PubMed] [Google Scholar]
- Imai-Nishiya H., Mori K., Inoue M., Wakitani M., Iida S., Shitara K., Satoh M. "Double knockdown of alpha1,6-fucosyltransferase (FUT8) and GDP-mannose 4,6-dehydratase (GMD) in antibody-producing cells: A new strategy for generating fully non-fucosylated therapeutic antibodies with enhanced ADCC.". BMC Biotechnology. 2007;7:84. doi: 10.1186/1472-6750-7-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito Y., Miyauchi A., Yoshida H., Uruno T., Nakano K., Takamura Y., Miya A., Kobayashi K., Yokozawa T., Matsuzuka F., Taniguchi N., Matsuura N., Kuma K., Miyoshi E. Expression of alpha1,6-fucosyltransferase (FUT8) in papillary carcinoma of the thyroid: Its linkage to biological aggressiveness and anaplastic transformation. Cancer Letters. 2003;200(2):167–172. doi: 10.1016/s0304-3835(03)00383-5. [DOI] [PubMed] [Google Scholar]
- Jones C.J., Aplin J.D. Glycosylation at the fetomaternal interface: Does the glycocode play a critical role in implantation? Glycoconjugate Journal. 2009;26(3):359–366. doi: 10.1007/s10719-008-9152-6. [DOI] [PubMed] [Google Scholar]
- Julien S., Ivetic A., Grigoriadis A., QiZe D., Burford B., Sproviero D., Picco G., Gillett C., Papp S.L., Schaffer L., Tutt A., Taylor-Papadimitriou J., Pinder S.E., Burchell J.M. "Selectin ligand sialyl-Lewis x antigen drives metastasis of hormone-dependent breast cancers.". Cancer Research. 2011;71(24):7683–7693. doi: 10.1158/0008-5472.CAN-11-1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao K.S., Gupta A., Zong G., Li C., Kerschbaumer I., Borghi S., Achkar J.M., Bournazos S., Wang L.X., Ravetch J.V. "Synthetic nanobodies as tools to distinguish IgG Fc glycoforms.". Proceedings of the National Academy of Sciences of the U S A. 2022;119(48) doi: 10.1073/pnas.2212658119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y.S., Ahn Y.H., Song K.J., Kang J.G., Lee J.H., Jeon S.K., Kim H.C., Yoo J.S., Ko J.H. "Overexpression and beta-1,6-N-acetylglucosaminylation-initiated aberrant glycosylation of TIMP-1: A "double whammy" strategy in colon cancer progression.". Journal of Biological Chemistry. 2012;287(39):32467–32478. doi: 10.1074/jbc.M112.370064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuball J., Hauptrock B., Malina V., Antunes E., Voss R.H., Wolfl M., Strong R., Theobald M., Greenberg P.D. "Increasing functional avidity of TCR-redirected T cells by removing defined N-glycosylation sites in the TCR constant domain.". Journal of Experimental Medicine. 2009;206(2):463–475. doi: 10.1084/jem.20082487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen M.D., de Graaf E.L., Sonneveld M.E., Plomp H.R., Nouta J., Hoepel W., Chen H.J., Linty F., Visser R., Brinkhaus M., Šuštić T., de Taeye S.W., Bentlage A.E.H., Toivonen S., Koeleman C.A.M., Sainio S., Kootstra N.A., Brouwer P.J.M., Geyer C.E.…Vidarsson A.U., COVID-19 and b. s. group "Afucosylated IgG characterizes enveloped viral responses and correlates with COVID-19 severity.". Science. 2021;371(6532) doi: 10.1126/science.abc8378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leifer C.A., Medvedev A.E. "Molecular mechanisms of regulation of Toll-like receptor signaling.". Journal of Leukocyte Biology. 2016;100(5):927–941. doi: 10.1189/jlb.2MR0316-117RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M., Bai Y., Zhou J., Huang W., Yan J., Tao J., Fan Q., Liu Y., Mei D., Yan Q., Yuan J., Malard P., Wang Z., Gu J., Tanigchi N., Li W. Core fucosylation of maternal milk N-glycan evokes B cell activation by selectively promoting the l-fucose metabolism of gut bifidobacterium spp. and lactobacillus spp. mBio. 2019;10(2) doi: 10.1128/mBio.00128-19. 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Ishihara K., Yokota T., Nakagawa T., Koyama N., Jin J., Mizuno-Horikawa Y., Wang X., Miyoshi E., Taniguchi N., Kondo A. "Reduced alpha4beta1 integrin/VCAM-1 interactions lead to impaired pre-B cell repopulation in alpha 1,6-fucosyltransferase deficient mice.". Glycobiology. 2008;18(1):114–124. doi: 10.1093/glycob/cwm107. [DOI] [PubMed] [Google Scholar]
- Li S., Liu X.Y., Pan Q., Wu J., Liu Z.H., Wang Y., Liu M., Zhang X.L. "Hepatitis C virus-induced FUT8 causes 5-FU drug resistance in human hepatoma Huh7.5.1 cells.". Viruses. 2019;11(4):378. doi: 10.3390/v11040378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Liu Q., Pang Y., Jin J., Wang H., Cao H., Li Z., Wang X., Ma B., Chi Y., Wang R., Kondo A., Gu J., Taniguchi N. "Core fucosylation of mu heavy chains regulates assembly and intracellular signaling of precursor B cell receptors.". Journal of Biological Chemistry. 2012;287(4):2500–2508. doi: 10.1074/jbc.M111.303123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Yu R., Ma B., Yang Y., Jiao X., Liu Y., Cao H., Dong W., Liu L., Ma K., Fukuda T., Liu Q., Ma T., Wang Z., Gu J., Zhang J., Taniguchi N. Core fucosylation of IgG B cell receptor is required for antigen recognition and antibody production. The Journal of Immunology. 2015;194(6):2596–2606. doi: 10.4049/jimmunol.1402678. [DOI] [PubMed] [Google Scholar]
- Liang C., Fukuda T., Isaji T., Duan C., Song W., Wang Y., Gu J. "α1,6-Fucosyltransferase contributes to cell migration and proliferation as well as to cancer stemness features in pancreatic carcinoma.". Biochimica et Biophysica Acta (BBA) - General Subjects. 2021;1865(6) doi: 10.1016/j.bbagen.2021.129870. [DOI] [PubMed] [Google Scholar]
- Liang W., Mao S., Li M., Zhang N., Sun S., Fang H., Zhang J., Gu J., Wang J., Li W. "Ablation of core fucosylation attenuates the signal transduction via T cell receptor to suppress the T cell development.". Molecular Immunology. 2019;112:312–321. doi: 10.1016/j.molimm.2019.06.011. [DOI] [PubMed] [Google Scholar]
- Liang W., Mao S., Sun S., Li M., Li Z., Yu R., Ma T., Gu J., Zhang J., Taniguchi N., Li W. "Core fucosylation of the T cell receptor is required for T cell activation.". Frontiers in Immunology. 2018;9:78. doi: 10.3389/fimmu.2018.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D., Gao Z., Yue L. "Fucosyltransferase 8 deficiency suppresses breast cancer cell migration by interference of the FAK/integrin pathway.". Cancer Biomarkers. 2019;25(4):303–311. doi: 10.3233/CBM-190209. [DOI] [PubMed] [Google Scholar]
- Liu J.J., Lin B., Hao Y.Y., Li F.F., Liu D.W., Qi Y., Zhu L.C., Zhang S.L., Iwamori M. "Lewis(y) antigen stimulates the growth of ovarian cancer cells via regulation of the epidermal growth factor receptor pathway.". Oncology Reports. 2010;23(3):833–841. [PubMed] [Google Scholar]
- Lv X., Song J., Xue K., Li Z., Li M., Zahid D., Cao H., Wang L., Song W., Ma T., Gu J., Li W. "Core fucosylation of copper transporter 1 plays a crucial role in cisplatin-resistance of epithelial ovarian cancer by regulating drug uptake.". Molecular Carcinogenesis. 2019;58(5):794–807. doi: 10.1002/mc.22971. [DOI] [PubMed] [Google Scholar]
- Lv Y., Zhang Z., Wang M., Wang Y., Chen M., Jia J., Guo Y., Wang K., Li Z., Wang W., Li H. "Discovery of novel FUT8 inhibitors with promising affinity and in vivo efficacy for colorectal cancer therapy.". Bioorganic Chemistry. 2024;149 doi: 10.1016/j.bioorg.2024.107492. [DOI] [PubMed] [Google Scholar]
- Matsumoto K., Yokote H., Arao T., Maegawa M., Tanaka K., Fujita Y., Shimizu C., Hanafusa T., Fujiwara Y., Nishio K. "N-Glycan fucosylation of epidermal growth factor receptor modulates receptor activity and sensitivity to epidermal growth factor receptor tyrosine kinase inhibitor.". Cancer Science. 2008;99(8):1611–1617. doi: 10.1111/j.1349-7006.2008.00847.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi E., Moriwaki K., Nakagawa T. "Biological function of fucosylation in cancer biology.". Journal of Biochemistry. 2008;143(6):725–729. doi: 10.1093/jb/mvn011. [DOI] [PubMed] [Google Scholar]
- Miyoshi E., Moriwaki K., Terao N., Tan C.C., Terao M., Nakagawa T., Matsumoto H., Shinzaki S., Kamada Y. "Fucosylation is a promising target for cancer diagnosis and therapy.". Biomolecules. 2012;2(1):34–45. doi: 10.3390/biom2010034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran A.P., Gupta A., Joshi L. Sweet-talk: Role of host glycosylation in bacterial pathogenesis of the gastrointestinal tract. Gut. 2011;60(10):1412–1425. doi: 10.1136/gut.2010.212704. [DOI] [PubMed] [Google Scholar]
- Morin P.J. beta-catenin signaling and cancer. BioEssays. 1999;21(12):1021–1030. doi: 10.1002/(SICI)1521-1878(199912)22:1<1021::AID-BIES6>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Nakayama K., Wakamatsu K., Fujii H., Shinzaki S., Takamatsu S., Kitazume S., Kamada Y., Takehara T., Taniguchi N., Miyoshi E. "Core fucose is essential glycosylation for CD14-dependent Toll-like receptor 4 and Toll-like receptor 2 signalling in macrophages.". Journal of Biochemistry. 2019;165(3):227–237. doi: 10.1093/jb/mvy098. [DOI] [PubMed] [Google Scholar]
- Ng B.G., Xu G., Chandy N., Steyermark J., Shinde D.N., Radtke K., Raymond K., Lebrilla C.B., AlAsmari A., Suchy S.F., Powis Z., Faqeih E.A., Berry S.A., Kronn D.F., Freeze H.H. "Biallelic mutations in FUT8 cause a congenital disorder of glycosylation with defective fucosylation.". The American Journal of Human Genetics. 2018;102(1):188–195. doi: 10.1016/j.ajhg.2017.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda K., Miyoshi E., Uozumi N., Yanagidani S., Ikeda Y., Gao C., Suzuki K., Yoshihara H., Yoshikawa K., Kawano K., Hayashi N., Hori M., Taniguchi N. "Gene expression of alpha1-6 fucosyltransferase in human hepatoma tissues: A possible implication for increased fucosylation of alpha-fetoprotein.". Hepatology. 1998;28(4):944–952. doi: 10.1002/hep.510280408. [DOI] [PubMed] [Google Scholar]
- Noda M., Okayama H., Kofunato Y., Chida S., Saito K., Tada T., Ashizawa M., Nakajima T., Aoto K., Kikuchi T., Sakamoto W., Endo H., Fujita S., Saito M., Momma T., Ohki S., Kono K. "Prognostic role of FUT8 expression in relation to p53 status in stage II and III colorectal cancer.". PLoS One. 2018;13(7) doi: 10.1371/journal.pone.0200315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norden R., Nystrom K., Aurelius J., Brisslert M., Olofsson S. "Virus-induced appearance of the selectin ligand sLeX in herpes simplex virus type 1-infected T-cells: Involvement of host and viral factors.". Glycobiology. 2013;23(3):310–321. doi: 10.1093/glycob/cws160. [DOI] [PubMed] [Google Scholar]
- Nystrom K., Grahn A., Lindh M., Brytting M., Mandel U., Larson G., Olofsson S. "Virus-induced transcriptional activation of host FUT genes associated with neo-expression of Ley in cytomegalovirus-infected and sialyl-Lex in varicella-zoster virus-infected diploid human cells.". Glycobiology. 2007;17(4):355–366. doi: 10.1093/glycob/cwl083. [DOI] [PubMed] [Google Scholar]
- Nystrom K., Norden R., Muylaert I., Elias P., Larson G., Olofsson S. "Induction of sialyl-Lex expression by herpes simplex virus type 1 is dependent on viral immediate early RNA-activated transcription of host fucosyltransferase genes.". Glycobiology. 2009;19(8):847–859. doi: 10.1093/glycob/cwp057. [DOI] [PubMed] [Google Scholar]
- Okada M., Chikuma S., Kondo T., Hibino S., Machiyama H., Yokosuka T., Nakano M., Yoshimura A. "Blockage of core fucosylation reduces cell-surface expression of PD-1 and promotes anti-tumor immune responses of T cells.". Cell Reports. 2017;20(5):1017–1028. doi: 10.1016/j.celrep.2017.07.027. [DOI] [PubMed] [Google Scholar]
- Okazaki A., Shoji-Hosaka E., Nakamura K., Wakitani M., Uchida K., Kakita S., Tsumoto K., Kumagai I., Shitara K. "Fucose depletion from human IgG1 oligosaccharide enhances binding enthalpy and association rate between IgG1 and FcgammaRIIIa.". Journal of Molecular Biology. 2004;336(5):1239–1249. doi: 10.1016/j.jmb.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Okeley N.M., Alley S.C., Anderson M.E., Boursalian T.E., Burke P.J., Emmerton K.M., Jeffrey S.C., Klussman K., Law C.L., Sussman D., Toki B.E., Westendorf L., Zeng W., Zhang X., Benjamin D.R., Senter P.D. "Development of orally active inhibitors of protein and cellular fucosylation.". Proceedings of the National Academy of Sciences of the U S A. 2013;110(14):5404–5409. doi: 10.1073/pnas.1222263110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osumi D., Takahashi M., Miyoshi E., Yokoe S., Lee S.H., Noda K., Nakamori S., Gu J., Ikeda Y., Kuroki Y., Sengoku K., Ishikawa M., Taniguchi N. "Core fucosylation of E-cadherin enhances cell-cell adhesion in human colon carcinoma WiDr cells.". Cancer Science. 2009;100(5):888–895. doi: 10.1111/j.1349-7006.2009.01125.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Q., Xie Y., Zhang Y., Guo X., Wang J., Liu M., Zhang X.L. "EGFR core fucosylation, induced by hepatitis C virus, promotes TRIM40-mediated-RIG-I ubiquitination and suppresses interferon-I antiviral defenses.". Nature Communications. 2024;15(1):652. doi: 10.1038/s41467-024-44960-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papic N., Maxwell C.I., Delker D.A., Liu S., Heale B.S., Hagedorn C.H. "RNA-sequencing analysis of 5' capped RNAs identifies many new differentially expressed genes in acute hepatitis C virus infection.". Viruses. 2012;4(4):581–612. doi: 10.3390/v4040581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J.H., Reunert J., He M., Mealer R.G., Noel M., Wada Y., Grüneberg M., Horváth J., Cummings R.D., Schwartz O., Marquardt T. "L-Fucose treatment of FUT8-CDG.". Mol Genet Metab Rep. 2020;25:100680. doi: 10.1016/j.ymgmr.2020.100680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker R.B., Kohler J.J. "Regulation of intracellular signaling by extracellular glycan remodeling.". ACS Chemical Biology. 2010;5(1):35–46. doi: 10.1021/cb9002514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira N.A., Chan K.F., Lin P.C., Song Z. "The "less-is-more" in therapeutic antibodies: Afucosylated anti-cancer antibodies with enhanced antibody-dependent cellular cytotoxicity.". mAbs. 2018;10(5):693–711. doi: 10.1080/19420862.2018.1466767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinho S.S., Reis C.A. Glycosylation in cancer: Mechanisms and clinical implications. Nature Reviews Cancer. 2015;15(9):540–555. doi: 10.1038/nrc3982. [DOI] [PubMed] [Google Scholar]
- Plociennikowska A., Hromada-Judycka A., Borzecka K., Kwiatkowska K. "Co-operation of TLR4 and raft proteins in LPS-induced pro-inflammatory signaling.". Cellular and Molecular Life Sciences. 2015;72(3):557–581. doi: 10.1007/s00018-014-1762-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potapenko I.O., Haakensen V.D., Luders T., Helland A., Bukholm I., Sorlie T., Kristensen V.N., Lingjaerde O.C., Borresen-Dale A.L. "Glycan gene expression signatures in normal and malignant breast tissue; possible role in diagnosis and progression.". Molecular Oncology. 2010;4(2):98–118. doi: 10.1016/j.molonc.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reily C., Stewart T.J., Renfrow M.B., Novak J. "Glycosylation in health and disease.". Nature Reviews Nephrology. 2019;15(6):346–366. doi: 10.1038/s41581-019-0129-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rillahan C.D., Antonopoulos A., Lefort C.T., Sonon R., Azadi P., Ley K., Dell A., Haslam S.M., Paulson J.C. "Global metabolic inhibitors of sialyl- and fucosyltransferases remodel the glycome.". Nature Chemical Biology. 2012;8(7):661–668. doi: 10.1038/nchembio.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roach J.C., Glusman G., Rowen L., Kaur A., Purcell M.K., Smith K.D., Hood L.E., Aderem A. "The evolution of vertebrate Toll-like receptors.". Proceedings of the National Academy of Sciences of the U S A. 2005;102(27):9577–9582. doi: 10.1073/pnas.0502272102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider M., Al-Shareffi E., Haltiwanger R.S. "Biological functions of fucose in mammals.". Glycobiology. 2017;27(7):601–618. doi: 10.1093/glycob/cwx034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott E., Elliott D.J., Munkley J. "Tumour associated glycans: A route to boost immunotherapy?". Clinica Chimica Acta. 2020;502:167–173. doi: 10.1016/j.cca.2019.12.015. [DOI] [PubMed] [Google Scholar]
- Scott E., Munkley J. "Glycans as biomarkers in prostate cancer.". International Journal of Molecular Sciences. 2019;20(6):1389. doi: 10.3390/ijms20061389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao M.C., Sokolik C.W., Wold F. Specificity studies of the GDP-[L]-fucose: 2-acetamido-2-deoxy-beta-[D]-glucoside (Fuc-->Asn-linked GlcNAc) 6-alpha-[L]-fucosyltransferase from rat-liver golgi membranes. Carbohydrate Research. 1994;251:163–173. doi: 10.1016/0008-6215(94)84283-3. [DOI] [PubMed] [Google Scholar]
- Shields R.L., Lai J., Keck R., O'Connell L.Y., Hong K., Meng Y.G., Weikert S.H., Presta L.G. "Lack of fucose on human IgG1 N-linked oligosaccharide improves binding to human Fcgamma RIII and antibody-dependent cellular toxicity.". Journal of Biological Chemistry. 2002;277(30):26733–26740. doi: 10.1074/jbc.M202069200. [DOI] [PubMed] [Google Scholar]
- Shimazu R., Akashi S., Ogata H., Nagai Y., Fukudome K., Miyake K., Kimoto M. MD-2, a molecule that confers lipopolysaccharide responsiveness on Toll-like receptor 4. Journal of Experimental Medicine. 1999;189(11):1777–1782. doi: 10.1084/jem.189.11.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y., Xu X., Wu T., Fukuda T., Isaji T., Morii S., Nakano M., Gu J. "Core fucosylation within the Fc-FcγR degradation pathway promotes enhanced IgG levels via exogenous L-fucose.". Journal of Biological Chemistry. 2024;300(8) doi: 10.1016/j.jbc.2024.107558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada K., Ohta M., Hidano S., Watanabe K., Hirashita T., Oshima Y., Fujnaga A., Nakanuma H., Masuda T., Endo Y., Takeuchi Y., Iwashita Y., Kobayashi T., Inomata M. "Fucosyltransferase 8 plays a crucial role in the invasion and metastasis of pancreatic ductal adenocarcinoma.". Surgery Today. 2020;50(7):767–777. doi: 10.1007/s00595-019-01953-z. [DOI] [PubMed] [Google Scholar]
- Takamatsu S., Shimomura M., Kamada Y., Maeda H., Sobajima T., Hikita H., Iijima M., Okamoto Y., Misaki R., Fujiyama K., Nagamori S., Kanai Y., Takehara T., Ueda K., Kuroda S., Miyoshi E. "Core-fucosylation plays a pivotal role in hepatitis B pseudo virus infection: A possible implication for HBV glycotherapy.". Glycobiology. 2016;26(11):1180–1189. doi: 10.1093/glycob/cww067. [DOI] [PubMed] [Google Scholar]
- Tu C.F., Wu M.Y., Lin Y.C., Kannagi R., Yang R.B. "FUT8 promotes breast cancer cell invasiveness by remodeling TGF-beta receptor core fucosylation.". Breast Cancer Research. 2017;19(1):111. doi: 10.1186/s13058-017-0904-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tudor L., Nedic Erjavec G., Nikolac Perkovic M., Konjevod M., Uzun S., Kozumplik O., Mimica N., Lauc G., Svob Strac D., Pivac N. The association of the polymorphisms in the FUT8-related locus with the plasma glycosylation in post-traumatic stress disorder.". International Journal of Molecular Sciences. 2023;24(6):5706. doi: 10.3390/ijms24065706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Pul L., Maurer I., Boeser-Nunnink B.D.M., Harskamp A.M., van Dort K.A., Kootstra N.A. "A genetic variation in fucosyltransferase 8 accelerates HIV-1 disease progression indicating a role for N-glycan fucosylation.". AIDS. 2023;37(13):1959–1969. doi: 10.1097/QAD.0000000000003689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varki A. "Biological roles of oligosaccharides: All of the theories are correct.". Glycobiology. 1993;3(2):97–130. doi: 10.1093/glycob/3.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicente M.M., Alves I., Gaifem J., Rodrigues C.S., Fernandes Â., Dias A.M., Štambuk J., Petrović T., Oliveira P., Ferreira-da-Silva F., Soares A., Seixas N., Teixeira T., Malheiro L., Abreu M.M., Lauc G., Sarmento E Castro R., Pinho S.S. "Altered IgG glycosylation at COVID-19 diagnosis predicts disease severity.". European Journal of Immunology. 2022;52(6):946–957. doi: 10.1002/eji.202149491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Chen J., Li Q.K., Peskoe S.B., Zhang B., Choi C., Platz E.A., Zhang H. Overexpression of α (1,6) fucosyltransferase associated with aggressive prostate cancer. Glycobiology. 2014;24(10):935–944. doi: 10.1093/glycob/cwu051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Inoue S., Gu J., Miyoshi E., Noda K., Li W., Mizuno-Horikawa Y., Nakano M., Asahi M., Takahashi M., Uozumi N., Ihara S., Lee S.H., Ikeda Y., Yamaguchi Y., Aze Y., Tomiyama Y., Fujii J., Suzuki K.…Taniguchi N. "Dysregulation of TGF-beta1 receptor activation leads to abnormal lung development and emphysema-like phenotype in core fucose-deficient mice.". Proceedings of the National Academy of Sciences of the U S A. 2005;102(44):15791–15796. doi: 10.1073/pnas.0507375102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T.T., Sewatanon J., Memoli M.J., Wrammert J., Bournazos S., Bhaumik S.K., Pinsky B.A., Chokephaibulkit K., Onlamoon N., Pattanapanyasat K., Taubenberger J.K., Ahmed R., Ravetch J.V. "IgG antibodies to dengue enhanced for FcγRIIIA binding determine disease severity.". Science. 2017;355(6323):395–398. doi: 10.1126/science.aai8128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q.R., Yu X., Li Y., Zhu M.Z. "Correlations among serum alpha-(1,6)-fucosyltransferase and early symptoms associated with Parkinson's disease: A cross-sectional retrospective study.". Brain Research Bulletin. 2024;212 doi: 10.1016/j.brainresbull.2024.110959. [DOI] [PubMed] [Google Scholar]
- Wang M., Zhang Z., Chen M., Lv Y., Tian S., Meng F., Zhang Y., Guo X., Chen Y., Yang M., Li J., Qiu T., Xu F., Li Z., Zhang Q., Yang J., Sun J., Zhang H., Li H., Wang W. "FDW028, a novel FUT8 inhibitor, impels lysosomal proteolysis of B7-H3 via chaperone-mediated autophagy pathway and exhibits potent efficacy against metastatic colorectal cancer.". Cell Death & Disease. 2023;14(8):495. doi: 10.1038/s41419-023-06027-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei T., Liu Q., He F., Zhu W., Hu L., Guo L., Zhang J. "The role of N-acetylglucosaminyltransferases V in the malignancy of human hepatocellular carcinoma.". Experimental and Molecular Pathology. 2012;93(1):8–17. doi: 10.1016/j.yexmp.2012.01.011. [DOI] [PubMed] [Google Scholar]
- Williams, Wilkins L. Immunology. Lippincott's Illustrated Reviews. 2007;20 [Google Scholar]
- Wilson J.R., Williams D., Schachter H. "The control of glycoprotein synthesis: N-Acetylglucosamine linkage to a mannose residue as a signal for the attachment of L-fucose to the asparagine-linked N-acetylglucosamine residue of glycopeptide from alpha1-acid glycoprotein.". Biochemical and Biophysical Research Communications. 1976;72(3):909–916. doi: 10.1016/s0006-291x(76)80218-5. [DOI] [PubMed] [Google Scholar]
- Wright S.D., Ramos R.A., Tobias P.S., Ulevitch R.J., Mathison J.C. "CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein.". Science. 1990;249(4975):1431–1433. doi: 10.1126/science.1698311. [DOI] [PubMed] [Google Scholar]
- Xiang T., Yang G., Liu X., Zhou Y., Fu Z., Lu F., Gu J., Taniguchi N., Tan Z., Chen X., Xie Y., Guan F., Zhang X.L. "Alteration of N-glycan expression profile and glycan pattern of glycoproteins in human hepatoma cells after HCV infection.". Biochimica et Biophysica Acta (BBA) - General Subjects. 2017;1861(5 Pt A):1036–1045. doi: 10.1016/j.bbagen.2017.02.014. [DOI] [PubMed] [Google Scholar]
- Xu X., Fukuda T., Takai J., Morii S., Sun Y., Liu J., Ohno S., Isaji T., Yamaguchi Y., Nakano M., Moriguchi T., Gu J. "Exogenous l-fucose attenuates neuroinflammation induced by lipopolysaccharide.". Journal of Biological Chemistry. 2024;300(1) doi: 10.1016/j.jbc.2023.105513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S., Shi C., Zhou R., Han Y., Li N., Qu C., Xia R., Zhang C., Hu Y., Tian Z., Liu S., Wang L., Li J., Zhang Z. "Mapping the landscape of HPV integration and characterising virus and host genome interactions in HPV-positive oropharyngeal squamous cell carcinoma.". Clinical and Translational Medicine. 2024;14(1):e1556. doi: 10.1002/ctm2.1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi Y., Fujii J., Inoue S., Uozumi N., Yanagidani S., Ikeda Y., Egashira M., Miyoshi O., Niikawa N., Taniguchi N. "Mapping of the alpha-1,6-fucosyltransferase gene, FUT8, to human chromosome 14q24.3.". Cytogenetics and Cell Genetics. 1999;84(1–2):58–60. doi: 10.1159/000015215. [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y., Ikeda Y., Takahashi T., Ihara H., Tanaka T., Sasho C., Uozumi N., Yanagidani S., Inoue S., Fujii J., Taniguchi N. Genomic structure and promoter analysis of the human alpha1, 6-fucosyltransferase gene (FUT8) Glycobiology. 2000;10(6):637–643. doi: 10.1093/glycob/10.6.637. [DOI] [PubMed] [Google Scholar]
- Zanoni I., Ostuni R., Marek L.R., Barresi S., Barbalat R., Barton G.M., Granucci F., Kagan J.C. "CD14 controls the LPS-induced endocytosis of Toll-like receptor 4.". Cell. 2011;147(4):868–880. doi: 10.1016/j.cell.2011.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Itoh S., Wang X., Isaji T., Miyoshi E., Kariya Y., Miyazaki K., Kawasaki N., Taniguchi N., Gu J. "Deletion of core fucosylation on alpha3beta1 integrin down-regulates its functions.". Journal of Biological Chemistry. 2006;281(50):38343–38350. doi: 10.1074/jbc.M608764200. [DOI] [PubMed] [Google Scholar]
- Zhou Y., Fukuda T., Hang Q., Hou S., Isaji T., Kameyama A., Gu J. "Inhibition of fucosylation by 2-fluorofucose suppresses human liver cancer HepG2 cell proliferation and migration as well as tumor formation.". Scientific Reports. 2017;7(1):11563. doi: 10.1038/s41598-017-11911-9. [DOI] [PMC free article] [PubMed] [Google Scholar]