Abstract
Food safety has emerged as a paramount concern in global health, prompting innovative approaches to ensure the safety of people's sustenance. In this study, a novel strategy was devised to fabricate Fe3O4-ZnO-MnO2 hybrid nanobiocatalysts, which exhibited remarkable enzymatic activity surpassing that of Horseradish peroxidase (HRP) catalysis. It demonstrated exceptional proficiency in decomposing 3,3′,5,5′-tetramethylbenzidine (TMB) without the need for harsh reaction conditions or the aid of H2O2. We established colorimetric detection systems based on Fe3O4-ZnO-MnO2-TMB both for nitrite (NO2−) and Listeria monocytogenes (LM) in food. Impressively, the detection limit of nitrite reached an astonishingly low level of 0.022 mg L−1, and the detection limit for LM was determined to be 3.5 cfu mL−1. These compelling results unequivocally validate the potential of these hybrid nanobiocatalysts to fortify food safety measures. Moreover, they serve as a valuable reference for the colorimetric detection of diverse analytes and the simultaneous detection of multiple targets.
Keywords: Fe3O4-ZnO-MnO2 nanocomposites, Food safety, Rapid detection, Nitrite, Listeria monocytogenes
Graphical abstract
Preparation procedure and enzymatic application of hybrid Fe3O4-ZnO-MnO2 nanocatalysts.
Highlights
-
•
Fe3O4-ZnO-MnO2 magnetic nanocomposites with various enzyme activities were successfully prepared.
-
•
Rapid and sensitive monitoring of nitrite and Listeria monocytogenes in food was realized based on TMB colorimetric system.
-
•
Nanomaterial-based solution for robust, rapid, and reliable food safety analysis.
1. Introduction
With the growing concern over food safety worldwide, it has become a major focus of global attention (Jiang et al., 2021). People are increasingly worried about the uncertainty surrounding food safety, leading to a sense of panic (He, Han, & Liu, 2019). Therefore, rapid identification of potential hazards in food is crucial to reassuring the public. As scientific advancements continue, food additives are not only used to enhance flavor but can also have adverse effects on consumers. Besides, research has shown that the contamination of food by pathogenic microorganisms can lead to foodborne illnesses, posing a serious threat to both physical and mental health (Kwon et al., 2021; Lefebvre et al., 2021; Y. Zhang et al., 2022). Therefore, accurately determining the presence of microorganisms and other additives in food is of utmost importance in ensuring dietary safety and reducing public anxiety.
To achieve rapid detection of food safety, two key factors must be considered: one is how to achieve signal amplification, and the other is how to quickly separate the target. In terms of realising signal amplification, natural enzymes, as a representative of fast catalysts, are widely used in various industrial and pharmaceutical fields (DiCosimo, McAuliffe, Poulose, & Bohlmann, 2013; Schmid et al., 2001). However, many factors, such as its high cost, cumbersome preparation procedure, harsh storage environment, and strict usage conditions, have limited its widespread application (Senko, Gladchenko, Maslova, & Efremenko, 2019; R. Zhang, Yan, & Fan, 2021). To address these issues, researchers explored functional nanocatalysts, which have been shown to have great potential to improve the catalytic function, efficiency, kinetic properties, and susceptibility to the diffusion of enzymes (Bilal et al., 2023; Kong et al., 2023; Liang, Fu, Yao, Li, & Li, 2022).
Currently, many research efforts have developed various nanobiocatalysts, including precious metals (Xu et al., 2019), metal oxides (Y. Li, Zhang, Qian, & Huang, 2022), metal sulphones (F. Li et al., 2023), carbon-based nanomaterials (Xia et al., 2022), metal-organic frameworks (MOFs) (Guo, Bao, Guo, Chen, & Wen, 2022), etc. Although the nanobiocatalysts disclosed above have been proven to have high catalytic efficiency and strong stability, most of the nanobiocatalysts are composed of expensive precious metals, which may make it difficult to control the cost of these nanobiocatalysts in applications. Therefore, further research on nanobiocatalysts with low prices and excellent performance is expected to achieve broad application prospects. Zinc‑manganese (ZnO-MnO2) dioxide aqueous batteries have the advantages of low cost, abundant resources, low toxicity, and high working potential (Sambandam et al., 2022; N. Zhang et al., 2023). Inspired by zinc‑manganese batteries, we aim to develop nanobiocatellites with high catalytic efficiency, low cost, and strong stability using zinc‑manganese as a raw material.
Magnetic metal oxides have exceptional superparamagnetic properties, making them ideal for rapid separation and purification of target substances in detection systems (H. Liu et al., 2020; Manigandan et al., 2019; Wang et al., 2019). The hybridization of magnetic metal oxides (such as Fe3O4) and nanobiocatalysts (such as ZnO-MnO2) will show strong advantages in analysis and detection through integrating separation and purification with signal amplification within the detection matrix. This innovative approach not only enhances the accuracy and sensitivity of food safety detection but also provides a reliable means to isolate and amplify signals, ensuring the reliable identification of hazardous substances, which holds immense potential for the rapid detection of food safety hazards.
Based on the activities of Fe3O4-ZnO-MnO2 hybrid nanobiocatalysts against various substrates, this study proposed an all-around improvement plan for food safety using NO2− and LM as examples. By utilizing the Fe3O4-ZnO-MnO2-TMB system, a rapid colorimetric detection system was developed. This research is of great significance for future studies on Fe3O4-ZnO-MnO2 hybrid nanocomplexes in the fields of food safety and analytical chemistry, as it establishes a standard and provides guidance for future research endeavors. Furthermore, the establishment of a reliable detection system for NO2− and LM holds promising prospects for the detection of food additives and foodborne pathogens. This advancement addresses the urgent need for accurate and efficient detection methods in the food industry. By utilizing the unique properties of Fe3O4-ZnO-MnO2 hybrid nano biocatalysts, this research opens up new avenues for enhancing food safety and ensuring the well-being of consumers. Continued research in this area will undoubtedly contribute to the development of innovative solutions for food safety monitoring and analysis.
2. Materials and methods
2.1. Reagents and apparatus
Ferric chloride hexahydrate (FeCl3.6H2O), trisodium citrate dihydrate (Na3Cit.2H2O), manganese chloride tetrahydrate (MnCl2. 4H2O), ethylene glycol (EG) was purchased from Aladdin reagents Co., Ltd. (Shanghai, China). Sodium hydroxide (NaOH), Zinc acetate (ZnAc) and sodium acetate (NaAc) were acquired from the National Pharmaceutical Group Chemical Reagents Co., Ltd. (Shanghai, China). Bovine serum albumin (BSA), TMB and HRP were received from Shanghai Macklin Biochemical Co., Ltd. (Shanghai, China). N-(3-(dimethylamino)-propyl)-N′-ethylcarbodiimide hydrochloride (EDC) and N-hydroxysuccinimide (NHS) were purchased from Sigma-Aldrich. (USA). As previously reported (L. Zhang et al., 2016), the 5′-amine-modified DNA aptamer (5’NH2-TTT TTT TTT TAT CCATGG GGC GGA GAT GAG GGG GAG GAG GGC GGG TAC CCG GTT GAT-3′) was selected for recognition of LM, which was provided by Sangon Biotech Co., Ltd. (Shanghai, China). All other chemicals and reagents used were of analytical grade and were used without further purification.
All the UV–Vis absorption spectra were acquired using Agilent Cary 60 UV–Vis (Agilent, USA). All fluorescence spectra were obtained using the Edinburgh spectrofluorometer-FS5 (Edinburgh, UK). Transmission electron microscopy (TEM) of nanoparticles was measured on a JEOL JEM-2100 F transmission electron microscope at an accelerating voltage of 200 kV (JEOL, Japan). Fourier Transform Infrared spectra (FTIR) from 500 to 4000 cm−1 were recorded using a Nicolet 6700 FTIR spectrometer (Thermo Inc., USA). Zetasizer Pro (Malvern Instruments, UK) performed the zeta potential measurements.
2.2. Synthesis of the Fe3O4-ZnO-MnO2 hybrid nanocomposites
The core magnetic nanospheres (Fe3O4) were prepared by solvothermal method described in the literature (N. Zhang et al., 2022). Simply put, 4.0 g FeCl3.6H2O and 1.0 g Na3Cit.2H2O were dissolved in 100.0 mL EG and stirred vigorously with magnetic force to form a homogeneous yellow solution. Then added 6.0 g of NaAc and stirred at room temperature for another 0.5 h. The mixture was transferred to a 100.0 mL stainless steel autoclave lined with polytetrafluoroethylene and then heated to 200 °C for 10 h. After cooling to room temperature, the sediment was washed with deionized water and ethanol to clear and dried overnight in a vacuum oven at 60 °C.
Inspired by previous research work, Zn-MnO2 was synthesized in a similar way (Y. Liu et al., 2023). Specifically, 0.2 mM MnCl2.4H2O and 50.0 mg BSA were dissolved in 10.0 mL of pure water and reacted with intense stirring in a beaker for 30 min. Added 3.0 g NaOH, 5 min later, then dropped 0.2 mM ZnAc solution, reaction for 12 h. The precipitation was collected by centrifugation at 12000 rpm for 15 min. The resulting sediment was dried overnight at 50 °C.
The Fe3O4-ZnO-MnO2 hybrid nanocomposites were prepared by electrostatic attraction reactions. 50.0 mg Fe3O4 nanoparticles and dissolved in 50.0 mL of ultrapure water, ultrasonic to a homogeneous state. Added 100.0 mg of ZnO-MnO2 nanocomposite powder and stirred vigorously at 50 °C for 3 h. The final product was magnetically separated, and dried at 50 °C, and finally stored at 4 °C for future use.
2.3. Various catalytic activity tests of Fe3O4-ZnO-MnO2 hybrid nanocomposites
Several enzyme reaction experiments were used to study the nano-simulated enzyme activity of Fe3O4-ZnO-MnO2 hybrid nanocomposites. Firstly, the peroxidase activity was tested, and the catalytic activities of HRP, Fe3O4-Ag-MnO2, and Fe3O4-ZnO-MnO2 hybrid nanocomposites were compared in the presence of H2O2 with TMB as the chromogenic substrate. Secondly, in the absence of H2O2, the oxidase-like activities of HRP, Fe3O4-Ag-MnO2, Zn-MnO2 and Fe3O4-ZnO-MnO2 hybrid nanocomposites were evaluated by TMB. Finally, the catalase-like activities of HRP, Fe3O4-Ag-MnO2 and Fe3O4-ZnO-MnO2 hybrid nanocomposites were studied for their ability to decompose oxygen generated by H2O2.
As a widely applicable commercial enzyme, HRP was used as a positive control in the enzyme activity assay below. The peroxidase activity was studied as below: 0.9 mL of Fe3O4-ZnO-MnO2/Fe3O4-Ag-MnO2 suspension (0.5 mg mL−1), 0.1 mL of TMB solution (0.1 g mL−1), and 1.0 μL of H2O2 (30 %) in sequence. The mixed liquor was incubated for 10 min at room temperature. The supernatant was sucked, and 0.1 mL of HCl (1.0 M) solution was added to terminate the reaction. The color and UV–Vis spectra ranging from 400 nm to 800 nm of the mixed solution were recorded.
The oxidase-like activity of various nanomaterials was evaluated by catalytic oxidation of TMB in the absence of H2O2. Simply put, 0.9 mL of nanomaterials water suspension (0.5 mg mL−1) and 0.1 mL of TMB water solution (0.1 g mL−1) were mixed well for 10 min at room temperature. The supernatant fluid was then measured by phone and UV–Vis's spectra.
The catalase-like activity of the nanocomplex was simply assessed by decomposing H2O2 to generate oxygen. In short, 1 mL Fe3O4-ZnO-MnO2/Fe3O4-Ag-MnO2 aqueous solution (0.5 mg mL−1) was mixed with 10.0 μL H2O2 (30 %), respectively. The number of bubbles produced by each experimental tube was observed after 5.0 min.
2.4. Rapid detection of NO2− in food by visual quantitative method
Based on the oxidation effect of TMB+ on NO2−, a rapid detection of NO2− in food was designed using the oxidase activity of Fe3O4-Ag-MnO2 hybrid nanocomposites to catalyze the generation of TMB+ from TMB. Under suitable conditions, the oxidation product of 0.2 mL TMB was color-reacted with NO2− at different concentrations of 0.5 mL. After 20 min, the solution of each experimental tube was recorded by camera and UV–Vis spectra.
2.5. Rapid detection of LM in food by colorimetric fluorescence dual-mode
Before the establishment of the detection system, the various bacteria required for the experiment were first cultured. All strains listed in Supporting Information Table S1 were preserved in liquid nitrogen and 15.0 % glycerol and revived by streaking on Luria-Bertani (LB) agar plates. Only Vibrio parahaemolyticus was cultured in tryptone soybean broth supplemented with 3.0 % NaCl. Other strains were routinely cultured in the LB common medium. The bacterial concentration was determined by the conventional plate counting method.
Capturing all the target bacteria as much as possible can effectively avoid false negative, so the capture efficiency of IMB nanoprobe was explored. Fe3O4-ZnO-MnO2 suspension 5.0 mg mL−1 was obtained, homogenized by ultrasonic dispersion, washed with PBS 3 times by magnetic adsorption, and then resuspended in 0.8 mL. 10 mg of EDC and NHS were added to activate surface carboxyl groups. 0.5 h later, washed with PBS by magnetic adsorption was performed again. The quantitative volume was 0.9 mL in PBS solution, and 0.1 mL of LM-specific aptamer (0.1 mM) was injected for an overnight reaction. Magnetic separation removes the unbound aptamers. The prepared Fe3O4-ZnO-MnO2-Apt nanoprobes were stored at 4 °C for later use. Different amounts of Fe3O4-ZnO-MnO2-Apt probes were co-incubated with 106cfu mL−1 concentrations of LM. The supernatant was taken after 0.5 h, diluted to a certain number, and coated for culture counting (Y. Liu et al., 2022).
In addition, a nanocomposite of Fe3O4-ZnO-MnO2 was created to find LM in food based on its oxidase and occupying effects. Under optimized experimental conditions, 0.1 mL Apt-Fe3O4-ZnO-MnO2 nanoprobe and 20.0 μL IgY-CV-CDs nanoprobe solutions were mixed with different concentrations of LM bacterial solution and incubated for 0.5 h. Magnetic separation was performed to remove the supernatant, and 1.0 mL of 80.0 μg mL−1 TMB was added for color development for 5 min. Magnetic separation was used to record the color of the supernatant in the test tube under sunlight and ultraviolet light, respectively.
3. Results and discussion
3.1. Characteristics of Fe3O4-ZnO-MnO2 hybrid nanocomposites
Graphic summary reveals the synthesis route of the Fe3O4-ZnO-MnO2 hybrid nanocomplex. In order to the separation efficiency, superparamagnetic Fe3O4 nanospheres were used as the magnetic core. The surface was then coated with ZnO-MnO2 nanocomposites to improve the catalytic efficiency. Through Zeta potential testing, the surface charge of Fe3O4 with a carboxyl group was −24.97 mV, and the surface charge of ZnO-MnO2 nanocomplex was 28.23 mV. It can be combined by electrostatic adsorption.
The morphology and microstructure of the as-prepared materials were investigated by TEM. As displayed in Fig. 1a, the spherical Fe3O4 nanoparticles composed of many smaller magnetite nanospheres exhibit good monodisperses, with an average diameter of 123.54 nm. Based on the experience of successfully preparing hybrid nanocomposites in the past (Y. Liu et al., 2023), the performance of the prepared Fe3O4-ZnO-MnO2 will be measured. The size of the hybrid composites increased by 164.41 nm due to the adhesion state of the Zn-MnO2 composites (Fig. 1b). TEM characterization was also performed on ZnO-MnO2 together, and the results showed a flaky morphology, which is due to the use of BSA as the template (Fig. S1a). The results of elemental analysis show that Zn and Mn elements exist in the ZnO-MnO2 structure (Fig. S1b). TEM-energy dispersive X-ray spectroscopy (EDS) elemental mapping also shows strong elemental signals for Zn and Mn (Fig. S1c).
Fig. 1.
A series of characterization of Fe3O4-ZnO-MnO2. a: TEM images of Fe3O4; b: TEM images of Fe3O4-ZnO-MnO2; c: HRTEM image of Fe3O4-ZnO-MnO2; d: TEM–EDS elemental mapping showing of Fe, Zn, Mn; e: Elemental analysis of Fe3O4-ZnO-MnO2.
Next, HRTEM results showed that the lattice distance within the (301) plane of MnO2 appearing on the surface of Fe3O4-ZnO-MnO2 composites was 0.213 nm (X. Liu et al., 2012). At the same time, a lattice distance of 0.260 nm that matched the (002) plane of ZnO was found (Fig. 1c) (Prasad et al., 2020). It was easy to see in Fig. 1d that the Fe elements were mostly in the middle of the nanosphere and the Zn and Mn elements were mostly on the outside and top of the sphere. This shows that the ZnO-MnO2 coating was successfully applied to the Fe3O4 nanosphere's surface. Moreover, the results of elemental analysis (Fig. 1d) showed that the peaks of elements Fe, Zn, and Mn were all present. The mass percentages of these elements were 92.18 % (Fe), 3.27 % (Zn), and 4.55 % (Mn), respectively.
The FTIR spectra of Fe3O4, Zn-MnO2, and Fe3O4-ZnO-MnO2 were demonstrated in Fig.S2a. Comparing the spectra results of Fe3O4 and Fe3O4-ZnO-MnO2, both showed a wide absorption band near 580 cm−1, which was caused by the vibration of the Fe − O bond, and a C O stretching vibration peak appeared at 1726 cm−1 (Y. Liu et al., 2019). Combined with the spectra curves of Zn-MnO2 and Fe3O4-ZnO-MnO2, the peaks observed in the region 475 cm−1 were a result of the Zn—O vibrations (Munir et al., 2023). The peaks at 1078 cm−1 and 638 cm−1 were related to the stretching of O–Mn–O vibrations (Hoseinpour & Ghaemi, 2018). This confirmed the presence of ZnO-MnO2 in the Fe3O4-ZnO-MnO2 again.
We further studied the magnetic properties of VSM. As shown in Fig.S2b, the magnetization curves of Fe3O4 and Fe3O4-ZnO-MnO2 hybrid composites show no remanence or coercivity at room temperature, indicating their superparamagnetic character. The saturation magnetization values of Fe3O4 and Fe3O4-ZnO-MnO2 were 56.50 emu g−1 and 50.03 emu g−1, respectively. Fe3O4-ZnO-MnO2, dispersed in a turbidized and uniform aqueous solution, can be separated within 1 min under the action of an external magnetic field to form a clear and transparent solution. This feature lays the foundation for subsequent rapid separation applications.
3.2. Catalytic performance test of Fe3O4-ZnO-MnO2
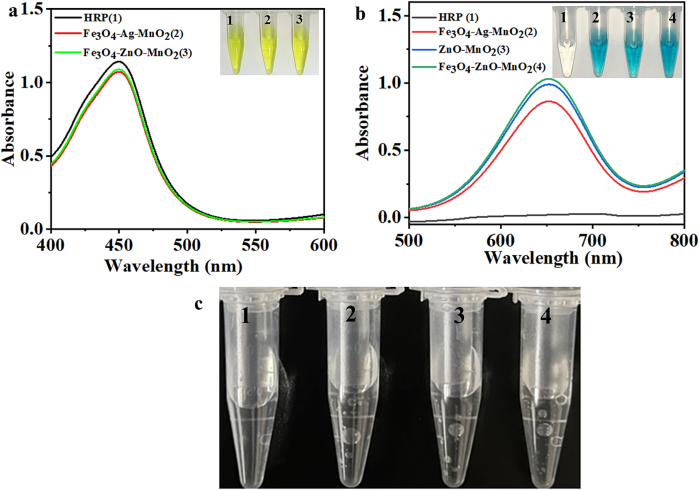
HRP was used as a positive control for enzyme activity. We performed color reaction with TMB and tested UV–Vis's spectra to verify the peroxidase and oxidase properties of Fe3O4-ZnO-MnO2. Meanwhile, the enzyme activity was compared with that of previously synthesized Fe3O4-Ag-MnO2 nanocomposites. From Fig. 2a, we can see that the two nanocomplexes have equal catalytic capacity. Even if HRP has the best catalytic effect, the absorbance value was not very different from that of the two nanocomposites. The color of the inserted test tubes was almost the same. These results indicate that the two nanocomplexes have the potential to replace HRP in peroxidase applications. Fig. 2b shows the results of TMB color development catalyzed by HRP and a variety of nanoparticles. Our previous experiments demonstrated that Fe3O4 cannot catalyze the oxidation of TMB in the absence of H2O2. Here, we further performed the experiment of HRP catalyzing TMB without the help of H2O2, and the results displayed that TMB could not be oxidized to appear blue. The other three nanomaterials can oxidize TMB from colorless to blue. The UV–vis spectra data showed almost no absorption at 652 nm (A652nm) in the HRP group, which was consistent with the colorlessness shown in the insert images. The other three nanomaterials have maximum absorption peaks at 652 nm. Both Fe3O4-Ag-MnO2 and ZnO-MnO2 had pretty much the same catalytic effects. This suggests that ZnO-MnO2 helped Fe3O4-Ag-MnO2's high oxidation effect. The absorbance values of the two nanomaterials were both higher than Fe3O4-Ag-MnO2, indicating that the catalytic effect of Fe3O4-ZnO-MnO2 on oxide TMB was stronger than that of Fe3O4-Ag-MnO2 in the absence of H2O2, prompting that the Fe3O4-ZnO-MnO2-TMB system has a good application prospect in colorimetric detection. Catalase test results show that H2O2 alone at room temperature produces no bubbles. HRP and two nanocomplexes could produce O2 from H2O2. The bubbles of Fe3O4-Ag-MnO2 were slightly lower than those of HRP and Fe3O4-ZnO-MnO2, suggesting that the catalase activity of Fe3O4-ZnO-MnO2 was not different from that of HRP and was higher than that of Fe3O4-Ag-MnO2(Fig. 2c).
Fig. 2.
a Visual and UV–Vis spectra of HRP(1), Fe3O4–Ag–MnO2 (2) and Fe3O4-ZnO-MnO2 (3) catalyzed the oxidation of chromogenic substrates in the presence of H2O2 to produce different color reactions; b Visual and UV–Vis spectra of HRP (1), Fe3O4–Ag–MnO2 (2), ZnO-MnO2 (3) and Fe3O4-ZnO-MnO2 (4) catalyzed the oxidation of chromogenic substrates in the absence of H2O2 to produce different color reactions; c Pictures of different substances catalyzing the decomposition of H2O2 to generate O2 (1: H2O2 alone, 2: HRP 3: Fe3O4–Ag–MnO2, 4: Fe3O4-ZnO-MnO2).
3.3. Feasibility test of visual detection of Fe3O4-ZnO-MnO2 hybrid nanocomposites
In order to explore the application of Fe3O4-ZnO-MnO2 in analytical detection, we utilized its efficient catalytic activity towards TMB and designed a colorimetric determination for nitrite in food. As shown in the illustrations in Fig. 3a, Fe3O4-ZnO-MnO2 oxidized colorless TMB to blue, and the color became green after mixing with a small amount of nitrite, and the color became brown-yellow after adding more amount. The UV–Vis spectra data shown that the A652nm decreases with an increase in the concentration of nitrite in the system. The results confirmed that the Fe3O4-ZnO-MnO2-TMB system could be used to measure the content of nitrite at different concentrations in food.
Fig. 3.
a: Feasibility verification of nitrite detection using UV–vis absorption spectra and photographs (Inset) (1: Oxidized TMB, 2: Oxidized TMB+ 10 mg L−1 NO2−, 3: Oxidized TMB+ 40 mg L−1 NO2−); b: Feasibility verification of LM detection using UV–vis absorption spectra and Photographs (Inset) (1: Blank, 2: with 103 cfu mL−1 LM).
In addition to the determination of small molecules, further exploration of the Fe3O4-ZnO-MnO2-TMB system is needed for the analysis of large-molecule substances. Taking the common foodborne pathogen LM as an example, the target bacteria were attached to the surface of the Fe3O4-ZnO-MnO2 nanoprobe through an immunological specific reaction, which covered the active site of catalytic oxidation of TMB, thus causing the catalytic ability of the target substance to be weakened. Fig. 3b confirms the feasibility of this application. When the target bacteria are present, the experimental color decreases, and the A652nm value is also lower than that of the blank control group. These results indicated that the inorganic small molecule nitrite and biological foodborne pathogens could be determined based on the Fe3O4-ZnO-MnO2-TMB system.
3.4. Optimizing the detection of NO2−
In the determination program of nitrite, several key factors must be considered, including TMB concentration, pH value of the detection system, temperature, TMB oxidation time, color development time, etc. The absorption at 652 nm was used as the basis for data processing here. The results in Fig. S3a show that with a catalyst content of 0.5 mg mL−1, the absorption at 652 nm increased with the increase in TMB concentration, and the increase rate decreased when the content was 0.4. Therefore, the influence of the TMB dosage of 0.4 mg mL−1. The influence of pH on catalysts cannot be ignored. Fig. S3b represents that with the increase in pH, the absorption at 652 nm increased successively and then gradually decreased. The catalytic effect was best when pH was 4, and the pH of the subsequent detection system was set to 4. The optimization of temperature was exhibited in Fig. S3c. It can be seen that both low and high temperatures have adverse effects on the catalytic effect, while the reaction was optimal at room temperature. Therefore, subsequent experiments were conducted at room temperature. The oxidation time of Fe3O4-ZnO-MnO2 on TMB showed that the A652nm rose slowly after 5 min. In order to save time, the subsequent oxidation time was set at 5 min. The oxidized blue TMB product was used for color reaction on 60 mg L−1 nitrite, and the A652nm did not increase significantly after 20 min, and the reaction was terminated after 20 min in the follow-up experiment.
3.5. Key applications of NO2− detection methods in food
Under optimized experimental conditions, colorimetric determination was performed on NO2− at a concentration of 0–60 mg L−1. The result is shown in Fig. 4. As can be seen from the inset, when the target substance was not present in the system, the color was blue. As the concentration of nitrite increased, the blue gradually deepened. When the concentration was 5 mg L−1, there was a significant color difference between the deepening of the blue degree of the experimental tube and the blank control. Therefore, the naked eye detection limit was determined to be 5 mg L−1. Established a linear regression curve with the absorbance at 652 nm as the vertical axis and the nitrite concentration as the horizontal axis. Within 0–15 mg L−1, Y = 0.0245× + 0.3012, R2 = 0.9569; Within 15–60 mg L−1, Y = -0.0113× + 0.7895, R2 = 0.9949. The detection limit for NO2− was calculated to be 0.022 mg L−1 according to the rule of 3δ/S (δ represents blank standard deviation, S stands for slope). The above results indicate that the Fe3O4-ZnO-MnO2-TMB system was suitable for rapid colorimetric detection of nitrite. The correlation comparison between the proposed detection system and other analytical methods for detecting NO2− was shown in Table S2. It can be seen that the detection system established by this research institute has unique advantages in many aspects such as detection range, operation process and detection time, and the detection limit was far lower than the China's national food safety standard and the World Health Organization (WHO) and the European Scientific Committee for Food (SCF) (Alsaiari et al., 2022). Therefore, it was reasonable to think that this system has the potential to be applied in the field of food safety rapid detection and screening.
Fig. 4.
a: The linear relationship between A652nm and NO2− concentrations from 0 to 60 mg L−1(Inset: photographs of the system with different concentrations of NO2−); b: Selectivity and interference studies (Inset: photographs, 110: blank, Cl−, P2O44−, Ca2+, Mg2+, K+, citric acid, glucose, alcohol, NO2−).
Precision and accuracy are two very important performance indexes in the analysis method. The precision and accuracy of the proposed method were measured by the standard recovery and Relative standard deviation (RSD). Several common interfering substances were selected to evaluate the anti-interference performance of the detection system, in which the concentration of NO2− was 10 mg L−1 and the concentration of interfering substances was 100 mg L−1. The results displayed that even if it was 10 times higher, it still did not affect the recognition of nitrite, hinting that the detection system can react specifically to nitrite. Taking fired mustard and sausage as examples, the application potential of detection systems in food was studied. The results exhibited that the spiked recovery rate was 83.71–102.95 % and the RSD was less than 16 %, which proved that the detection system could be used for the determination of nitrite content in food (Table 1).
Table 1.
The recoveries and RSD values of detection system in spiked food samples (, n = 3).
| Sample | Finded (mg L−1) | Added(mg L−1) | Calculated(mg L−1) | Recovery rate (%) | RSD (%) |
|---|---|---|---|---|---|
| Fuling mustard (mg L−1) | 5.00 | 5.72 ± 0.56 | 93.77 | 9.75 | |
| 1.10 | 10.00 | 10.59 ± 0.60 | 95.40 | 5.69 | |
| 5.00 | 6.28 ± 0.97 | 102.95 | 15.48 | ||
| 10.00 | 10.21 ± 0.88 | 91.98 | 8.65 | ||
| Sausage (mg L−1) | 5.00 | 6.7 ± 0.69 | 90.44 | 10.22 | |
| 2.43 | 10.00 | 11.58 ± 0.88 | 93.16 | 7.65 | |
| 5.00 | 6.22 ± 0.80 | 83.71 | 12.83 | ||
| 10.00 | 11.56 ± 0.96 | 93.00 | 8.35 | ||
| Milk (cfu mL−1) | 5 × 10 | (5.10 ± 0.62) × 10 | 102.02 | 12.16 | |
| 0 | 5 × 102 | (4.75 ± 0.56) × 102 | 95.01 | 11.79 | |
| 5 × 10 | (5.41 ± 0.49) × 10 | 108.25 | 9.06 | ||
| 5 × 102 | (4.98 ± 1.02) × 102 | 99.67 | 20.48 | ||
| Pork (cfu mL−1) | 5 × 10 | (5.23 ± 0.81) × 10 | 104.62 | 15.49 | |
| 0 | 5 × 102 | (4.67 ± 0.62) × 102 | 93.49 | 13.28 | |
| 5 × 10 | (5.05 ± 0.99) × 10 | 101.09 | 19.60 | ||
| 5 × 102 | (4.88 ± 0.91) × 102 | 97.68 | 18.65 |
3.6. Optimizing the detection of LM
In the determination procedure of LM, the content of the immunomagnetic nanoprobes in the detection process of LM was first optimized. Fig. S4a and S4b showed the number of bacteria in the positive control group and the number of bacteria in the supernatant when the immunomagnetic nanoprobe was 0.5 mg mL−1, respectively. It was calculated that the capture rate was 95.67 % at this time, suggesting that almost majority of the target bacteria could be captured. Fig. S4c showed that the target bacteria were attached to the surface by the Fe3O4-ZnO-MnO2-Apt nanoprobe. It was proved that the magnetic nanoprobe could have immune recognition reaction with the target bacteria, so the dosage of the immunomagnetic nanoprobe in the subsequent experiment was 0.5 mg mL−1. Besides, in order to better visualize the measurement, the TMB dosage was optimized. As shown in Fig. S5a, when the concentration of TMB was 0.08 mg mL−1, the increase rate slowed down, and the amount of TMB used in subsequent experiments was 0.08 mg mL−1. Similarly, the different color development time of TMB was measured, and the result was also stable after 5 min (Fig. S5b).
3.7. Applications of LM detection methods in food
Quantitative determination of 0–106 cfu mL−1 LM under optimal experimental conditions. The illustrations in Fig. 5a show that there was a color difference between the detection tube and the PBS blank tube when the target bacteria concentration was 103 cfu mL−1, and the naked-eye detection limit of the detection system was defined as 103 cfu mL−1. A linear regression curve was established with the A652nm for TMB oxidation products as the vertical axis and the log value for target bacterial concentration as the horizontal axis: Y = -0.0215lgC + 0.2057, R2 = 0.9825. The detection limit for LM was calculated to be 3.5 cfu mL−1 according to the rule of 3δ/S (δ represents the blank standard deviation and S represents the slope). Table S3 summarized the colorimetric detection methods of LM published in recent years. It can be seen that compared with other methods, the proposed method has the advantages of simple operation, sensitivity, and strong specificity. The superior performance of our colorimetric method was mainly attributed to the effective capture and separation effects of Fe3O4-ZnO-MnO2 hybrid magnetic nanoparticles and the TMB-mediated signal amplification system. Another huge advantage of our proposed approach is time savings, which is crucial for quickly controlling food safety and reducing economic losses.
Fig. 5.
a: The linear relationship between A652nm and logarithm of LM concentration from 0 to 106 cfu mL−1(Inset: photographs of the system with different concentrations of LM); b: Selectivity and interference studies of A652nm (Inset: photographs, 19: Blank, SA, O157:H7, Shigella, VP, S. typhimurium, mixture, mixture + LM, LM).
According to the similarity analysis of the bacterial survival matrix, 5 common foodborne pathogens were selected for anti-interference analysis. The illustration in Fig. 5c shows that the blue of the blank was similar to that of other interfering bacteria, while the blue of the test tube containing the target bacteria was obviously lighter. The A652nm corresponds to the color. It was proven that the detection system has an excellent ability to identify target bacteria. Meat and milk were selected as samples for the food simulation experiments. The results showed that the spiked recovery rate was 93.49–108.25 %, and the RSD was below 21 %, as illustrated in Table 1. This indicates that the detection system can be applied to the determination of LM content in food.
4. Conclusion
In conclusion, this study successfully synthesized the Fe3O4-ZnO-MnO2 hybrid nanocomplex with diverse enzyme activities. The peroxidase, catalase, and oxidase activities of the nanocomplex were comparable to those of HRP, demonstrating its excellent enzymatic properties. Specifically, using the Fe3O4-ZnO-MnO2-TMB system as a model, the nanoscale enzyme exhibited remarkable performance in detecting both small molecules like nitrite and large molecules like LM. This highlights the significant potential of the hybrid nanocomplex for applications in food detection. The advantages of the Fe3O4-ZnO-MnO2 hybrid nanocomplex over HRP lie in its unique properties, such as enhanced catalytic activity, stability, and potential for large-scale production. These features make it a promising alternative for various analytical applications, including food safety. The successful development of this nanocomposite paves the way for future research on its application in detecting other target substances. However, this study only discussed the detection of one target object, which could not meet the needs of the coexistence of multiple contaminants in food matrices, and did not fully apply the diverse enzyme activities of nanoenzymes. These deficiencies will prompt us to continue our in-depth study. In short, this study contributes to the advancement of nanobiocatalysts in the field of food safety and analytical chemistry, providing a solid foundation for further research and practical applications.
Funding
This study was supported by the Chinese National Natural Science Foundation (Grant No. 82003502 and 21904053), the Science and Technology Plan Project of Yantai (Grant No. 2022XDRH004), the Natural Science Foundation of Shandong Province (Grant No. ZR2023MB107), Shandong Key Laboratory of Biochemical Analysis (SKLBA2307) and the Youth Innovation Technology Project of Higher School in Shandong Province (Food Nanotechnology innovation team).
CRediT authorship contribution statement
Shuyang Sun: Conceptualization. Wenteng Qiao: Formal analysis. Feng Yin: Methodology. Wei Mi: Formal analysis. LuliangWang: Funding acquisition. Yuhan Jia: Formal analysis. Mengjiao Wang: Software. Xinhui Liu: Software. Dacheng Wang: Conceptualization. Daotan Liu: Software. Chao Zhao: Conceptualization. Xiuling Song: Formal analysis. Yushen Liu: Writing – original draft, Funding acquisition. Yue Zhai: Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2024.101968.
Contributor Information
Yushen Liu, Email: yushenlys@163.com.
Yue Zhai, Email: zhaiyue@jlu.edu.cn.
Appendix A. Supplementary data
Supplementary material
Data availability
Data will be made available on request.
References
- Alsaiari M., Saleem A., Alsaiari R., Muhammad N., Latif U., Tariq M., et al. SiO2/Al2O3/C grafted 3-n propylpyridinium silsesquioxane chloride-based non-enzymatic electrochemical sensor for determination of carcinogenic nitrite in food products. Food Chemistry. 2022;369:130970–130977. doi: 10.1016/j.foodchem.2021.130970. [DOI] [PubMed] [Google Scholar]
- Bilal M., Rashid E.U., Munawar J., Iqbal H.M.N., Cui J., Zdarta J., et al. Magnetic metal-organic frameworks immobilized enzyme-based nano-biocatalytic systems for sustainable biotechnology. International Journal of Biological Macromolecules. 2023;237:123968–123985. doi: 10.1016/j.ijbiomac.2023.123968. [DOI] [PubMed] [Google Scholar]
- DiCosimo R., McAuliffe J., Poulose A.J., Bohlmann G. Industrial use of immobilized enzymes. Chemical Society Reviews. 2013;42(15):6437–6474. doi: 10.1039/c3cs35506c. [DOI] [PubMed] [Google Scholar]
- Guo T., Bao S., Guo J., Chen W., Wen L. Bimetallic au-Pd NPs embedded in MOF ultrathin nanosheets with tuned surface electronic properties for high-performance benzyl alcohol oxidation. Chemical Research in Chinese Universities. 2022;38(6):1344–1348. doi: 10.1007/s40242-022-2210-y. [DOI] [Google Scholar]
- He C., Han G., Liu Y. Food safety satisfaction in China and its influencing factors: Empirical study with a hierarchical linear model. Safety. 2019;5(1):1–17. doi: 10.3390/safety5010017. [DOI] [Google Scholar]
- Hoseinpour V., Ghaemi N. Novel ZnO-MnO2-Cu2O triple nanocomposite: Facial synthesis, characterization, antibacterial activity and visible light photocatalytic performance for dyes degradation-a comparative study. Materials Research Express. 2018;5(8):085012–085023. doi: 10.1088/2053-1591/aad2c6. [DOI] [Google Scholar]
- Jiang S., Wang F., Li Q., Sun H., Wang H., Yao Z. Environment and food safety: A novel integrative review. Environmental Science and Pollution Research. 2021;28(39):54511–54530. doi: 10.1007/s11356-021-16069-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong Y., Zhu Y., Song J., Liu Q., Song L., Fei X., Li X. A novel multimode biosensor for sensitive detection of AFB1 in food based on Mxenes nano enzymes. Food Chemistry. 2023;426:136645–136653. doi: 10.1016/j.foodchem.2023.136645. [DOI] [PubMed] [Google Scholar]
- Kwon K., Yoon T., Gwak H., Lee K., Hyun K.-A., Jung H.-I. Fully automated system for rapid enrichment and precise detection of enterobacteria using magneto-electrochemical impedance measurements. Biochip Journal. 2021;15(3):233–242. doi: 10.1007/s13206-021-00024-1. [DOI] [Google Scholar]
- Lefebvre D., Blanco-Valle K., Feraudet-Tarisse C., Merda D., Simon S., Fenaille F., et al. Quantitative determination of staphylococcus aureus enterotoxins types a to I and variants in dairy food products by multiplex immuno-LC-MS/MS. Journal of Agricultural and Food Chemistry. 2021;69(8):2603–2610. doi: 10.1021/acs.jafc.0c07545. [DOI] [PubMed] [Google Scholar]
- Li F., Wu H., Lv S., Ma Y., Wang B., Ren Y., et al. Two birds with one stone: Contemporaneously enhancing OER catalytic activity and stability for dual-phase medium-entropy metal sulfides. Small. 2023:2309025–2309037. doi: 10.1002/smll.202309025. [DOI] [PubMed] [Google Scholar]
- Li Y., Zhang Y., Qian K., Huang W. Metal-support interactions in metal/oxide catalysts and oxide-metal interactions in oxide/metal inverse catalysts. ACS Catalysis. 2022;12(2):1268–1287. doi: 10.1021/acscatal.1c04854. [DOI] [Google Scholar]
- Liang X., Fu N., Yao S., Li Z., Li Y. The progress and outlook of metal single-atom-site catalysis. Journal of the American Chemical Society. 2022;144(40):18155–18174. doi: 10.1021/jacs.1c12642. [DOI] [PubMed] [Google Scholar]
- Liu H., Chen Q., Cheng X., Wang Y., Zhang Y., Fan G. Sustainable and scalable in-situ fabrication of au nanoparticles and Fe3O4 hybrids as highly efficient electrocatalysts for the enzyme-free sensing of H2O2 in neutral and basic solutions. Sensors and Actuators B: Chemical. 2020;314:128067–128075. doi: 10.1016/j.snb.2020.128067. [DOI] [Google Scholar]
- Liu X., Wang Q., Zhao H., Zhang L., Su Y., Lv Y. BSA-templated MnO2 nanoparticles as both peroxidase and oxidase mimics. Analyst. 2012;137(19):4552–4558. doi: 10.1039/C2AN35700C. [DOI] [PubMed] [Google Scholar]
- Liu Y., Sun M., Qiao W., Cong S., Zhang Y., Wang L., et al. Multicolor colorimetric visual detection of Staphylococcus aureus based on Fe3O4-ag-MnO2 composites nano-oxidative mimetic enzyme. Analytica Chimica Acta. 2023;1239:340654–340661. doi: 10.1016/j.aca.2022.340654. [DOI] [PubMed] [Google Scholar]
- Liu Y., Wang J., Zhao C., Guo X., Song X., Zhao W., et al. A multicolorimetric assay for rapid detection of listeria monocytogenes based on the etching of gold nanorods. Analytica Chimica Acta. 2019;1048:154–160. doi: 10.1016/j.aca.2018.10.020. [DOI] [PubMed] [Google Scholar]
- Liu Y., Wang X., Shi X., Sun M., Wang L., Hu Z., et al. A colorimetric sensor for Staphylococcus aureus detection based on controlled click chemical-induced aggregation of gold nanoparticles and immunomagnetic separation. Microchimica Acta. 2022;189(3):104–112. doi: 10.1007/s00604-022-05211-x. [DOI] [PubMed] [Google Scholar]
- Manigandan R., Dhanasekaran T., Padmanaban A., Giribabu K., Suresh R., Narayanan V. Bifunctional hexagonal Ni/NiO nanostructures: Influence of the core-shell phase on magnetism, electrochemical sensing of serotonin, and catalytic reduction of 4-nitrophenol. Nanoscale Advances. 2019;1(4):1531–1540. doi: 10.1039/c8na00342d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munir R., Ali K., Naqvi S.A.Z., Maqsood M.A., Bashir M.Z., Noreen S. Biosynthesis of Leucaena Leucocephala leaf mediated ZnO, CuO, MnO2, and MgO based nano-adsorbents for reactive golden yellow-145 (RY-145) and direct Red-31 (DR-31) dye removal from textile wastewater to reuse in agricultural purpose. Separation and Purification Technology. 2023;306 doi: 10.1016/j.seppur.2022.122527. [DOI] [Google Scholar]
- Prasad K.S., Prasad S.K., Ansari M.A., Alzohairy M.A., Alomary M.N., AlYahya S., et al. Tumoricidal and bactericidal properties of ZnONPs synthesized using Cassia auriculata leaf extract. Biomolecules. 2020;10(7) doi: 10.3390/biom10070982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambandam B., Mathew V., Kim S., Lee S., Kim S., Hwang J.Y., et al. An analysis of the electrochemical mechanism of manganese oxides in aqueous zinc batteries. Chem. 2022;8(4):924–946. doi: 10.1016/j.chempr.2022.03.019. [DOI] [Google Scholar]
- Schmid A., Dordick J.S., Hauer B., Kiener A., Wubbolts M., Witholt B. Industrial biocatalysis today and tomorrow. Nature. 2001;409(6817):258–268. doi: 10.1038/35051736. [DOI] [PubMed] [Google Scholar]
- Senko O., Gladchenko M., Maslova O., Efremenko E. Long-term storage and use of artificially immobilized anaerobic sludge as a powerful biocatalyst for conversion of various wastes including those containing xenobiotics to biogas. Catalysts. 2019;9(4):326–345. doi: 10.3390/catal9040326. [DOI] [Google Scholar]
- Wang Y., Zhao M., Hou C., Yang X., Li Z., Meng Q., Liang C. Graphene-based magnetic metal organic framework nanocomposite for sensitive colorimetric detection and facile degradation of phenol. Journal of the Taiwan Institute of Chemical Engineers. 2019;102:312–320. doi: 10.1016/j.jtice.2019.06.019. [DOI] [Google Scholar]
- Xia D., Yu H., Li H., Huang P., Li Q., Wang Y. Carbon-based and carbon-supported nanomaterials for the catalytic conversion of biomass: A review. Environmental Chemistry Letters. 2022;20(3):1719–1744. doi: 10.1007/s10311-022-01402-3. [DOI] [Google Scholar]
- Xu H., Luo X., Wang J., Su Y., Zhao X., Li Y. Spherical sandwich au@Pd@UIO-67/Pt@UIO-n (n=66, 67, 69) core-shell catalysts: Zr-based metal-organic frameworks for effectively regulating the reverse water-gas shift reaction. ACS Applied Materials & Interfaces. 2019;11(22):20291–20297. doi: 10.1021/acsami.9b04748. [DOI] [PubMed] [Google Scholar]
- Zhang L., Huang R., Liu W., Liu H., Zhou X., Xing D. Rapid and visual detection of listeria monocytogenes based on nanoparticle cluster catalyzed signal amplification. Biosensors & Bioelectronics. 2016;86:1–7. doi: 10.1016/j.bios.2016.05.100. [DOI] [PubMed] [Google Scholar]
- Zhang N., Huang T., Xie P., Yang Z., Zhang L., Wu X., Cai Z. Epitaxial growth of guanidyl-functionalized magnetic metal-organic frameworks with multiaffinity sites for selective capture of global phosphopeptides. ACS Applied Materials & Interfaces. 2022;14(34):39364–39374. doi: 10.1021/acsami.2c10353. [DOI] [PubMed] [Google Scholar]
- Zhang N., Wang J., Liu X., Wang P.-F., Liu Y.-G., Xie Y., Yi T.-F. Towards high-performance aqueous Zn-MnO2 batteries: Formation mechanism and alleviation strategies of irreversible inert phases. Composites Part B-Engineering. 2023;260:110770–110790. doi: 10.1016/j.compositesb.2023.110770. [DOI] [Google Scholar]
- Zhang R., Yan X., Fan K. Nanozymes inspired by natural enzymes. Accounts of Materials Research. 2021;2(7):534–547. doi: 10.1021/accountsmr.1c00074. [DOI] [Google Scholar]
- Zhang Y., Zhang J., Chang X., Qin S., Song Y., Tian J., Ma A. Analysis of 90 listeria monocytogenes contaminated in poultry and livestock meat through whole-genome sequencing. Food Research International. 2022;159:11641–11649. doi: 10.1016/j.foodres.2022.111641. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Data Availability Statement
Data will be made available on request.