Abstract
Purpose
To evaluate the efficacy of the dexamethasone implant on the electrophysiological profile of Diabetic Macular Oedema (DMO) patients over six months.
Methods
In this prospective, single-center study 30 eyes of 22 patients were examined using comprehensive baseline assessments including best-corrected visual acuity (BCVA), central retinal thickness (CRT), contrast sensitivity (CS) and multifocal electroretinogram (mfERG), before and after 0.7mg dexamethasone implant injection, with follow-ups at months 1, 2, 4, and 6. The study employed mixed models to analyse within-subject and between-subject correlations, considering the complexities of multiple measurements per subject.
Results
At baseline, BCVA was 0.66 ± 0.104 logMAR, improving to 0.568 ± 0.104 logMAR by month 6 (P > 0.05). CRT significantly reduced from 521 ± 28.7 μm to 336 ± 28.7 μm (P < 0.05). CS slightly increased from 26.8 ± 1.23 letters to 28.5 ± 1.05 letters (P > 0.05). P wave amplitude saw a notable rise from 33.4 ± 5.66 μV to 47.9 ± 5.43 μV (P < 0.05). P wave implicit time changed minimally from 47.4 ± 0.503 seconds to 48.0 ± 0.503 seconds (P > 0.05). No severe adverse events were recorded.
Conclusion
These results underscore the 0.7mg dexamethasone implant’s potential in improving certain electrophysiological markers in DMO, while also highlighting the need for further investigation into its comprehensive impact on retinal function.
Keywords: dexamethasone implant, electroretinogram, diabetic macular oedema, visual function, macular thickness, visual acuity, contrast sensitivity
Introduction
Diabetic Macular Oedema (DMO) represents a principal cause of vision impairment in the diabetic population, characterised by the build-up of fluid in the macula due to the leaking of retinal vessels.1 This condition stems from a blood-retinal barrier defect, resulting in vascular leakage, fluid accumulation, and macular thickening, driven by the expression of inflammatory factors including vascular endothelial growth factor (VEGF), intercellular adhesion molecule-1, interleukin-6, and monocyte chemotactic protein-1. The pathogenesis of DMO, as described above, involves intricate pathways of inflammation and vascular leakage, directing the focus of therapeutic strategies towards these mechanisms.2 Standard care has evolved from medical management and laser photocoagulation to include advanced therapies targeting VEGF and corticosteroids, reflecting our deepened understanding of DMO’s pathogenesis.3,4
The introduction of intravitreal dexamethasone implants has emerged as a promising approach, showing significant efficacy in reducing macular thickness and improving visual acuity in DMO patients.5 In 2014, the intravitreal 0.7 mg dexamethasone implant (Ozurdex®) was approved for DMO treatment, offering a sustained-release mechanism that blocks the production of inflammatory mediators, showcasing not only an improvement in anatomical outcomes but highlighting a gap in functional assessments post-treatment.6
Multifocal electroretinogram (mfERG), a sophisticated technique for assessing retinal function, has been employed to evaluate the outcomes of various DMO treatments.7,8 MfERG offers an objective measure of retinal activity, providing crucial insights into the functional impact of therapeutic interventions.9 Despite the known anatomical benefits of dexamethasone implants, the specific effects on mfERG responses in DMO patients are not thoroughly understood. This study seeks to elucidate these effects, bridging the gap between anatomical improvements and functional outcomes in DMO treatment, with a particular focus on exploring changes in macular function over a 6-month period.
Methods
Study Design
This study is a prospective, single-centre investigation to evaluate the impact of the dexamethasone implant on the electrophysiological profile of patients with DMO. The study enrolled 30 eyes of 22 patients, divided into two groups: DMO naïve eyes and DMO refractory to previous treatments.
Ethics Approval
Ophthalmica Eye Institute review board approved the study protocol (Ref: 102020/004_OPH-Ozrdx). An informed consent was obtained from the study participants prior to study commencement. This study was conducted in accordance with the ethical standards laid down in the Declaration of Helsinki.
Patient Selection
Patients were included if they had decreased vision due to co-existing DMO and clinically significant macular oedema (CSMO) according to the Early Treatment Diabetic Retinopathy Study (ETDRS) criteria.10 Exclusion criteria include type 1 diabetes, cataract or other media opacities at baseline, new onset of cataracts during the follow-up period, glaucoma, previous ocular surgery or retinal laser treatments, tractional retinal detachment, and macular ischaemia assessed with OCT-Angiography. Patients with neurodegenerative disorders such as Alzheimer and Parkinson and patients receiving retinotoxic medications were also excluded.
Procedures
Eligible patients underwent a comprehensive baseline examination, including best-corrected visual acuity (BCVA) measurement, contrast sensitivity assessment, slit-lamp examination, optical coherence tomography (OCT), and multifocal electroretinogram (mfERG). Following the baseline evaluation, patients received the dexamethasone 0.7mg implant (Ozurdex®). Follow-up examinations at months 1, 2, 4, and 6 will include full clinical evaluation (months 1, 2, 4 and 6) and mfERG (month 6 only). The dexamethasone implant was re-administered on a PRN regiment (VA decreases by ≤5 letters or retinal thickness ≥300 µm, recurrence intraretinal fluid (IRF) or subretinal fluid (SRF)) but not prior to month 4 post previous injection according to the local regulatory status.
Data Collection
Key parameters included central retinal thickness, presence of intraretinal (IRF) and/ or subretinal fluid (SRF) on OCT (Spectralis, Heidelberg Engineering GmbH, Heidelberg, Germany) and the presence of hard exudates. mfERG evaluations (RETI-port/scan 21, Roland Consult, Brandenburg an der Havel, Germany) focused on P wave amplitude (P1 R1) and implicit time (TP1 R1).
Endpoints
The co-primary endpoints are changes in BCVA, contrast sensitivity, and retinal thickness at month 6, and changes in mfERG in relation to baseline and final OCT characteristics. Secondary end point was the safety profile of the treatment.
Statistical Analysis
Statistical analysis was performed with Jamovi version 2.4 (The Jamovi project (2023), https://www.jamovi.org). Q-Q plots were used to assess data distribution. Data were presented as estimated marginal mean (EMM) ± standard error (SE). Since both eyes of some patients were included, a mixed model, accounting for within-subject and between-subject correlation, was used to compare data at different time points. P values below 0.05 were considered statistically significant.
Due to the complex hierarchical structure of our data and the lack of precise effect size and intraclass correlation coefficients from similar studies, we opted for a sample size informed by logistical constraints and clinical relevance, rather than traditional calculation methods.
Results
From the initially enrolled 30 eyes (22 patients), twenty-eight eyes (12 right, 16 left) of twenty patients (14 males, 6 females) were ultimately included and completed the study. This was after 2 patients (2 eyes) were excluded for non-compliance with the protocol. The mean age was 71.6 ± 1.8 years and the mean diabetes duration was 16.5 ± 6.1 years. Eleven eyes from 9 patients were naïve to previous treatment. Twenty eyes of 13 patients received a second Ozurdex injection at month 4 according to the PRN protocol criteria.
Best Corrected Visual Acuity (BCVA)
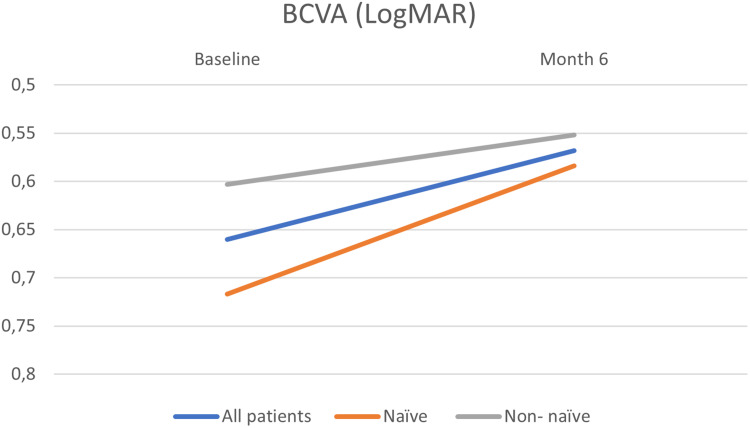
EMM BCVA at baseline was 0.66 ± 0.104 logmar, whereas EMM BCVA at month 6 was 0.568 ± 0.104 logmar (P = 0.413). Although eyes naïve to treatment showed a greater improvement compared to those refractory to previous treatments, this difference was not found to be statistically significant (P = 0.716) (Table 1, Figure 1).
Table 1.
A Summary of BCVA (LogMAR) at Baseline and Month 6 in the Whole Cohort and Naïve and Non – Naïve Patients
| Timepoint | ||||||
| 95% Confidence Interval | ||||||
| Timepoint | Mean | SE | df | Lower | Upper | |
| Baseline BCVA | 0.660 | 0.104 | 33.8 | 0.449 | 0.870 | |
| Month 6 BCVA | 0.568 | 0.104 | 33.8 | 0.357 | 0.779 | |
| NAIVE: Timepoint | ||||||
| 95% Confidence Interval | ||||||
| NAIVE | Timepoint | Mean | SE | df | Lower | Upper |
| NO | Baseline BCVA | 0.603 | 0.134 | 30.3 | 0.330 | 0.876 |
| YES | Baseline BCVA | 0.717 | 0.158 | 36.5 | 0.396 | 1.037 |
| NO | Month 6 BCVA | 0.552 | 0.134 | 30.3 | 0.278 | 0.825 |
| YES | Month 6 BCVA | 0.584 | 0.158 | 36.5 | 0.263 | 0.905 |
Abbreviation: BCVA, Best Corrected Visual Acuity.
Figure 1.
Changes in BCVA (LogMAR) at Baseline and Month 6 for the whole cohort and naïve and non – naïve patients.
Central Retinal Thickness (CRT)
EMM CRT at baseline was 521 ± 28.7 μm, whereas EMM CRT at month 6 was 336 ± 28.7 μm (P < 0.01). Eyes naïve to treatment showed a statistically greater improvement compared to those refractory to previous treatment (P = 0.043) (Table 2).
Table 2.
A Summary of CRT (μm) at Baseline and Month 6 in the Whole Cohort and Naïve and Non – Naïve Patients
| Time Point | |||||||||
| 95% Confidence Interval | |||||||||
| Time Point | Mean | SE | df | Lower | Upper | ||||
| Baseline | 521 | 28.7 | 33.6 | 462 | 579 | ||||
| Month 6 | 336 | 28.7 | 33.6 | 278 | 395 | ||||
| NAÏVE:`Time Point` | |||||||||
| 95% Confidence Interval | |||||||||
| NAÏVE | Time Point | Mean | SE | df | Lower | Upper | |||
| NO | Baseline | 459 | 37.5 | 31.0 | 382 | 535 | |||
| YES | Baseline | 583 | 43.6 | 35.7 | 495 | 671 | |||
| NO | Month 6 | 340 | 37.5 | 31.0 | 264 | 417 | |||
| YES | Month 6 | 333 | 43.6 | 35.7 | 244 | 421 | |||
Abbreviation: CRT, Central Retinal Thickness.
Contrast Sensitivity (CS)
EMM CS at baseline was 26.8 ± 1.23 letters, whereas at month 6, it was 28.5 ± 1.05 letters (P = 0.294). The change in CS was statistically greater in eyes naïve to previous treatments, which showed improvement, compared to those refractory to previous treatments, where a slight reduction was observed (P = 0.032) (Table 3).
Table 3.
A Summary of CS (Letters) at Baseline and Month 6 in the Whole Cohort and Naïve and Non – Naïve Patients
| Time Point | |||||||
| 95% Confidence Interval | |||||||
| Time Point | Mean | SE | df | Lower | Upper | ||
| Baseline | 26.8 | 1.23 | 35.5 | 24.3 | 29.3 | ||
| Month 6 | 28.5 | 1.05 | 34.6 | 26.4 | 30.6 | ||
| NAÏVE:`Time Point` | |||||||
| 95% Confidence Interval | |||||||
| NAÏVE | Time Point | Mean | SE | df | Lower | Upper | |
| NO | Baseline | 31.3 | 1.54 | 33.7 | 28.2 | 34.4 | |
| YES | Baseline | 22.4 | 1.91 | 36.5 | 18.5 | 26.2 | |
| NO | Month 6 | 29.5 | 1.37 | 30.8 | 26.7 | 32.3 | |
| YES | Month 6 | 27.5 | 1.60 | 36.6 | 24.3 | 30.7 | |
Abbreviation: CS Contrast Sensitivity.
Intraocular Pressure (IOP)
EMM IOP at baseline was 15 ± 1.09 mmHg, whereas at month 6, it increased to 19.3 ± 1.13 mmHg (P < 0.01). No statistically significant change was found between eyes naïve to previous treatments and those refractory to previous treatments (P = 0.088) (Table 4). In seven eyes from five patients, IOP exceeded 24 mmHg, necessitating the use of anti-glaucoma drops. No surgical interventions were required for any of the eyes.
Table 4.
A Summary of IOP (mmHg) at Baseline and Month 6 in the Whole Cohort and Naïve and Non – Naïve Patients
| Time Point | |||||||
| 95% Confidence Interval | |||||||
| Time Point | Mean | SE | df | Lower | Upper | ||
| Baseline | 15.0 | 1.09 | 30.3 | 12.8 | 17.2 | ||
| Month 6 | 19.3 | 1.13 | 33.6 | 17.0 | 21.6 | ||
| NAÏVE:`Time Point` | |||||||
| 95% Confidence Interval | |||||||
| NAÏVE | Time Point | Mean | SE | df | Lower | Upper | |
| NO | Baseline | 14.0 | 1.40 | 26.9 | 11.1 | 16.9 | |
| YES | Baseline | 15.9 | 1.66 | 32.9 | 12.6 | 19.3 | |
| NO | Month 6 | 20.5 | 1.40 | 26.9 | 17.6 | 23.4 | |
| YES | Month 6 | 18.2 | 1.77 | 38.2 | 14.6 | 21.8 | |
Abbreviation: IOP, Intraocular Pressure.
P Wave Amplitude (P1 R1)
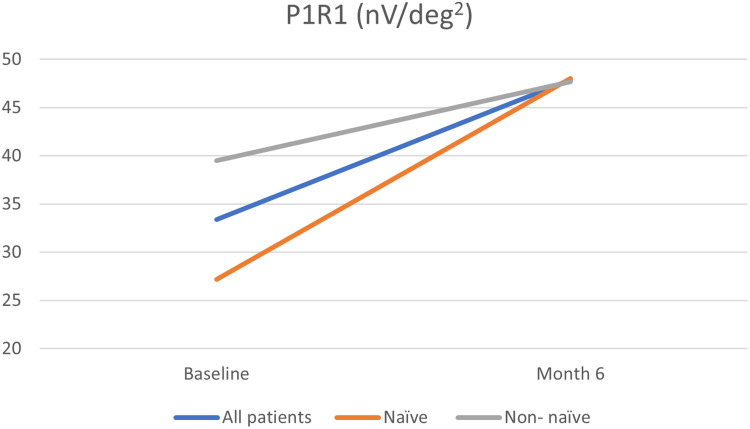
EMM P1 R1 at baseline was 33.4 ± 5.66 nV/deg2, whereas at month 6, it increased significantly to 47.9 ± 5.43 nV/deg2 (P = 0.011). Although eyes naïve to treatment showed a greater improvement compared to those refractory to previous treatments, this difference was not found to be statistically significant (P = 0.25) (Table 5, Figure 2).
Table 5.
A Summary of P Wave Amplitude (P1R1 (nV/deg2)) at Baseline and Month 6 in the Whole Cohort and Naïve and Non – Naïve Patients
| Time Point | ||||||
| 95% Confidence Interval | ||||||
| Time Point | Mean | SE | df | Lower | Upper | |
| Baseline | 33.4 | 5.66 | 30.2 | 21.8 | 44.9 | |
| Month 6 | 47.9 | 5.43 | 27.4 | 36.7 | 59.0 | |
| NAÏVE:`Time Point` | ||||||
| 95% Confidence Interval | ||||||
| NAÏVE | Time Point | Mean | SE | df | Lower | Upper |
| NO | Baseline | 39.5 | 7.34 | 25.8 | 24.42 | 54.6 |
| YES | Baseline | 27.2 | 8.63 | 33.7 | 9.67 | 44.7 |
| NO | Month 6 | 47.7 | 7.24 | 24.7 | 32.82 | 62.7 |
| YES | Month 6 | 48.0 | 8.10 | 29.7 | 31.49 | 64.6 |
Figure 2.
Changes in P wave amplitude (P1R1 (nV/deg2)) at Baseline and Month 6 for the whole cohort and naïve and non – naïve patients.
P Wave Implicit Time (TP1 R1)
EMM TP1 R1 at baseline was 47.4 ± 0.503 milliseconds, whereas at month 6, it increased slightly to 48.0 ± 0.503 milliseconds (P = 0.401). No statistically significant change was found between eyes naïve to previous treatments and those refractory to previous treatments (P = 0.088) (Table 6, Figure 3).
Table 6.
A Summary of P Wave Implicit Time (TP1 R1 (Msecs)) at Baseline and Month 6 in the Whole Cohort and Naïve and Non – Naïve Patients
| Time Point | ||||||
| 95% Confidence Interval | ||||||
| Time Point | Mean | SE | df | Lower | Upper | |
| Baseline | 47.4 | 0.503 | 46.1 | 46.4 | 48.5 | |
| Month 6 | 48.0 | 0.503 | 46.1 | 47.0 | 49.1 | |
| NAÏVE:`Time Point` | ||||||
| 95% Confidence Interval | ||||||
| NAÏVE | Time Point | Mean | SE | df | Lower | Upper |
| NO | Baseline | 48.2 | 0.633 | 42.0 | 46.9 | 49.4 |
| YES | Baseline | 46.7 | 0.783 | 48.4 | 45.2 | 48.3 |
| NO | Month 6 | 47.5 | 0.633 | 42.0 | 46.3 | 48.8 |
| YES | Month 6 | 48.5 | 0.783 | 48.4 | 47.0 | 50.1 |
Figure 3.
Changes in P wave implicit time (TP1 R1 (msecs)) at Baseline and Month 6 for the whole cohort and naïve and non – naïve patients.
No cases of endophthalmitis, severe inflammation, retinal detachment or other severe adverse events were recorded.
Discussion
In this study, we evaluated the effects of dexamethasone implant on patients with DMO over a six-month period, focusing on changes in BCVA, CRT, CS, IOP, and multifocal electroretinogram parameters (P wave amplitude and implicit time). Our results demonstrated significant improvement in CRT and multifocal electroretinogram P wave amplitude, suggesting enhanced retinal function and reduced macular thickness. Although BCVA and CS improved, these changes were not statistically significant. Notably, IOP increased, with some cases requiring management for elevated levels. These findings highlight the complex interplay between anatomical improvement and functional outcomes in DMO treatment with dexamethasone implants.
The significant reduction in CRT observed after dexamethasone implantation can be explained by dexamethasone’s potent anti-inflammatory effects. By mitigating inflammation, dexamethasone decreases vascular permeability, leading to reduced fluid accumulation within the macula, which is directly reflected in the decreased CRT.11 Additionally, the improvement in P wave amplitude, a measure of retinal function in multifocal electroretinogram, suggests that reducing macular oedema not only restores the retinal structure but may also improve the overall retinal function.12 This enhancement likely results from the alleviation of oedema-induced stress and damage to the retinal layers, particularly the photoreceptors and associated retinal elements, which are critical for signal transmission in visual processing.12,13
The lack of significant improvement in BCVA and CS despite anatomical improvements suggests that improvements in the physical structure of the retina, as indicated by a reduction in CRT and increased P wave amplitude, do not necessarily translate into better visual function, as measured by BCVA and CS. This discrepancy could be due to irreversible damage to the photoreceptors or other retinal components, which are crucial for vision.14,15 Such damage might not be repairable with anti-inflammatory treatments like dexamethasone, which primarily reduce swelling and inflammation but do not address the underlying cellular or molecular damage that affects visual function in diabetic patients.16
The disparity observed between the improvements in BCVA and multifocal electroretinography (mfERG) can be attributed to their distinct methods of assessing visual and retinal function. While BCVA is a subjective measure of visual performance that can be influenced by various factors beyond macular health, mfERG provides an objective quantification of retinal function by capturing the bioelectrical activity of the macula. Significant enhancement in mfERG amplitude suggests direct improvements in macular function, likely due to the resolution of macular oedema and enhanced retinal cell activity following treatment with dexamethasone. However, these cellular-level improvements may not immediately manifest as gains in visual acuity due to the complex integration and processing of visual signals by the brain, as well as potential irreversible structural changes within the retina that are not susceptible to current treatments.
In the study by Mastropasqua et al, dexamethasone implant in patients with diabetic macular oedema showed early improvements in visual acuity and macular sensitivity, persisting for up to five months.8 However, multifocal electroretinogram and pattern electroretinogram values began to worsen after four months, indicating a temporary functional benefit. Our findings similarly indicate that while dexamethasone implants can improve structural and some functional aspects initially, the lasting recovery, particularly in visual function, may be limited due to the complex nature of diabetic macular oedema.
In Baget-Bernaldiz et al’s study on the impact of ranibizumab over one year for DMO patients, significant increases in mfERG response density were noted, particularly in spongiform DMO types.7 The research highlighted ranibizumab’s ability to boost macular electrophysiological activity where the ellipsoid zone and external limiting membrane (ELM) were well-preserved. However, hard exudates at the fovea impeded response density improvements. Our findings similarly demonstrate that while dexamethasone implants significantly reduce CRT and enhance P wave amplitude, these do not uniformly result in visual acuity or contrast sensitivity improvements, suggesting potential underlying photoreceptor damage not addressed by anti-inflammatory treatments alone. This comparison sheds light on the complex outcomes of different treatments on anatomical versus functional recovery in DMO management.
In a study investigating the impact of the dexamethasone implant on mfERG findings in patients with macular oedema due to central retinal vein occlusion (CRVO), Bulut et al found no significant change in mfERG measurements six months post-treatment.17 While there were non-significant improvements in retinal function as measured by mfERG, the study highlighted the complex nature of assessing functional recovery in CRVO-related macular oedema treated with dexamethasone implant. This study suggests that while Ozurdex may lead to anatomical improvements, its impact on functional mfERG outcomes at 6 months is not statistically significant, indicating a potential area for further research to understand the long-term effects of dexamethasone implant on retinal function in CRVO patients.
Our study’s limitations include a relatively small sample size and a single-centre design, which may limit the robustness of our analyses, especially in distinguishing the effects between naïve and non-naïve DMO eyes. This constrained sample size, shaped more by logistical and clinical constraints than precise statistical power calculations, may affect the generalisability of our findings. The lack of a control group is a major drawback, as it is essential to differentiate whether the changes observed in mfERG are due to the natural progression of the disease or the effects of the drug. Additionally, electrophysiological assessments were only conducted at the six-month mark, potentially missing significant changes that could have been observed at months 2 and 4, as well as overlooking later electrophysiological changes. The use of mixed models helped mitigate potential bias from including both eyes of some participants, but these factors collectively may restrict the broad applicability of our results.
In conclusion, our study demonstrates that the dexamethasone implant significantly influences the electrophysiological profile of patients with Diabetic Macular Oedema (DMO) over a six-month period, as evidenced by improvements in mfERG amplitude. These enhancements suggest both functional recovery and potential neuroprotective effects, underscoring the utility of mfERG as a sensitive and early biomarker of treatment efficacy. While limitations such as the relatively short follow-up period and the study’s modest cohort size necessitate caution in generalising the findings, these results strongly support further research and the potential integration of mfERG in routine clinical practice. This approach could enhance the evaluation and customisation of treatments for DMO patients, providing early indicators of therapeutic success and better long-term management strategies.
Funding Statement
This study was funded by Allergan.
Disclosure
The authors have no other conflict of interest to declare.
References
- 1.Leasher JL, Bourne RR, Flaxman SR, et al. Global estimates on the number of people blind or visually impaired by diabetic retinopathy: a meta-analysis from 1990 to 2010. Diabetes Care. 2016;39(9):1643–1649. doi: 10.2337/dc15-2171 [DOI] [PubMed] [Google Scholar]
- 2.Romero-Aroca P. Targeting the pathophysiology of diabetic macular edema. Diabetes Care. 2010;33(11):2484–2485. doi: 10.2337/dc10-1580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Browning DJ, Stewart MW, Lee C. Diabetic macular edema: evidence-based management. Indian J Ophthalmol. 2018;66(12):1736–1750. doi: 10.4103/ijo.IJO_1240_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Panos GD. Evolution of intravitreal therapy for retinal and macular disorders. J Int Med Res. 2020;48(1):300060518771411. doi: 10.1177/0300060518771411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grover D, Li TJ, Chong CC, Intravitreal steroids for macular edema in diabetes. Cochrane Database Syst Rev. 2008;1:CD005656. doi: 10.1002/14651858.CD005656.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schwartz SG, Scott IU, Stewart MW, Flynn Jr. HW Jr. Update on corticosteroids for diabetic macular edema. Clin Ophthalmol. 2016;10:1723–1730. doi: 10.2147/OPTH.S115546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baget-Bernaldiz M, Romero-Aroca P, Bautista-Perez A, Mercado J. Multifocal electroretinography changes at the 1-year follow-up in a cohort of diabetic macular edema patients treated with ranibizumab. Doc Ophthalmol. 2017;135(2):85–96. doi: 10.1007/s10633-017-9601-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mastropasqua R, Toto L, Borrelli E, et al. Morphology and function over a one-year follow up period after intravitreal dexamethasone implant (Ozurdex) in patients with diabetic macular edema. PLoS One. 2015;10(12):e0145663. doi: 10.1371/journal.pone.0145663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Creel DJ. Electroretinograms. Handb Clin Neurol. 2019;160:481–493. [DOI] [PubMed] [Google Scholar]
- 10.Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number 1. Early Treatment Diabetic Retinopathy Study research group. Arch Ophthalmol. 1985;103(12):1796–1806. doi: 10.1001/archopht.1985.01050120030015 [DOI] [PubMed] [Google Scholar]
- 11.Zur D, Iglicki M, Loewenstein A. The role of steroids in the management of diabetic macular edema. Ophthalmic Res. 2019;62(4):231–236. doi: 10.1159/000499540 [DOI] [PubMed] [Google Scholar]
- 12.Bearse MA Jr, Ozawa GY. Multifocal electroretinography in diabetic retinopathy and diabetic macular edema. Curr Diab Rep. 2014;14(9):526. doi: 10.1007/s11892-014-0526-9 [DOI] [PubMed] [Google Scholar]
- 13.Bearse MA Jr, Han Y, Schneck ME, Adams AJ. Retinal function in normal and diabetic eyes mapped with the slow flash multifocal electroretinogram. Invest Ophthalmol Vis Sci. 2004;45(1):296–304. doi: 10.1167/iovs.03-0424 [DOI] [PubMed] [Google Scholar]
- 14.Tonade D, Kern TS. Photoreceptor cells and RPE contribute to the development of diabetic retinopathy. Prog Retin Eye Res. 2021;83:100919. doi: 10.1016/j.preteyeres.2020.100919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang W, Lo ACY. Diabetic retinopathy: pathophysiology and treatments. Int J Mol Sci. 2018;19(6):1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Panos GD, Arruti N, Patra S. The long-term efficacy and safety of fluocinolone acetonide intravitreal implant 190 mug (ILUVIEN((R))) in diabetic macular oedema in a multi-ethnic inner-city population. Eur J Ophthalmol. 2021;31(2):620–629. doi: 10.1177/1120672119898414 [DOI] [PubMed] [Google Scholar]
- 17.Bulut MN, Calli U, Akcay G, Kivrak U, Bulut K, Ozerturk Y. Effects of dexamethasone implant on multifocal electroretinography in central retinal vein occlusion. J Ophthalmic Vis Res. 2018;13(1):23–28. doi: 10.4103/jovr.jovr_118_16 [DOI] [PMC free article] [PubMed] [Google Scholar]