Abstract
Objective
To investigate changes in cardiometabolic risk factors after completion of cardiac rehabilitation (CR) for coronary heart disease (CHD) and ascertain whether the magnitude of improvement in cardiometabolic health differs between those with and without metabolic syndrome (MetS).
Methods
In this observational cohort study, data were analyzed from 1984 patients enrolled in CR at the University of Michigan between 2011-01-01 and 2020-02-29 for the indication of CHD. Patient characteristics were collected from standardized health questionnaires and during CR intake evaluations. Cardiometabolic biomarkers were recorded from baseline laboratory data and re-examined upon completion of CR. Differences in baseline patient characteristics by MetS status were compared using chi-square tests. Wilcoxon rank-sum tests were used to compare baseline differences, and signed-rank tests were used to evaluate the change in variables between baseline and completion of CR. The difference of change by MetS status was assessed using difference-in-differences regression models.
Results
Of the 1984 patients, 1070 (53.9%) met the criteria for MetS at baseline, of which 770 were male (72.0%). Those with MetS lost 1.43 pounds more (95% CI: 0.56, 2.31, P = 0.001), experienced a 0.21 larger drop in body mass index (95% CI: 0.03, 0.37, P = 0.02), and had a 0.31 greater reduction in waist circumference (95% CI: 0.08, 0.54, P = 0.008). Difference-in-differences regression models revealed those with MetS experienced a greater reduction in triglycerides and fasting glucose, with a difference of change of −8.70 for triglycerides (95% CI: −15.04, −2.37, P = 0.007) and −5.48 for glucose (95% CI: −10.44, −0.53, P = 0.03). There was no significant difference in the change in HDL-C or LDL-C for MetS status.
Conclusion
Compared to those without MetS, patients with MetS experienced a comparable or greater benefit from CR, particularly with respect to improvements in MetS components.
Keywords: chronic disease management, coronary heart disease, cardiometabolic risk factors, cardiovascular disease
Introduction
Metabolic syndrome (MetS) is characterized by a clustering of risk factors for cardiovascular disease (CVD) and diabetes mellitus (DM).1–3 Criteria for the clinical diagnosis of MetS commonly include the presence of at least three of the following five factors: abdominal obesity, elevated triglycerides, low high-density lipoprotein (HDL) cholesterol, hypertension and impaired fasting blood glucose.4,5 The overlapping of these conditions contribute substantially to morbidity and mortality, particularly due to increased risk of DM1 and CVDs such as coronary heart disease (CHD), heart failure (HF), and stroke.2,6
Cardiac rehabilitation (CR) is a comprehensive, evidence-based program designed primarily for secondary prevention of CVD. Robust literature supports the use of CR as a cost-effective intervention for individuals with a variety of cardiovascular conditions, with associated improvements in quality of life, morbidity, and mortality.3 Available evidence suggests that CR improves MetS, including each of its components.7 Previous research has indicated that it may be intrinsic factors, such as age, sex, or body mass index (BMI) that determine the level of functional improvement in patients undergoing CR,8 and other studies have found that CR is associated with smaller improvements in cardiometabolic health among those with DM (particularly type II) than among those without.9,10 However, whether MetS modifies the impact of CR on cardiometabolic health remains unclear.
In this study, we investigated changes in cardiometabolic risk factors from before to after the completion of CR in individuals with CHD and compared these changes amongst those with or without MetS identified at baseline. We hypothesized that cardiometabolic health would improve following CR with the indication related to CHD and that the magnitude of improvement would be greater in MetS.
Material and Methods
Patients
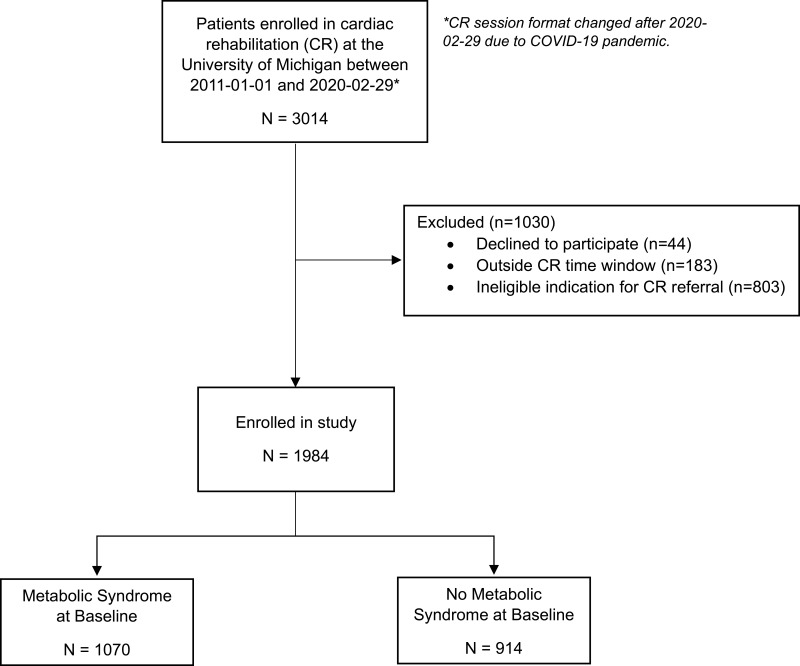
In this observational cohort study, we utilized the comprehensive data from 1984 patients enrolled in the University of Michigan CR program between 2011-01-01 and 2020-02-29 whose indication for CR was CHD-related. Eligible qualifying diagnoses included myocardial infarction (MI) with or without percutaneous coronary intervention (PCI), PCI, coronary artery bypass graft (CABG) surgery, and stable angina (Figure 1). The University of Michigan IRB approved this study (IRB#: HUM00045929, approved 2011–04-21). We obtained consent to participate in CR and use of patient data for research purposes from all participants at the time of CR enrollment.
Figure 1.
Study flow chart.
Data
The study data were analyzed retrospectively utilizing the same research methods and University of Michigan CR participant sample as the study conducted by Brandt et al (2023), which investigated the prevalence of factors among patients with CHD that may be considered low-risk for participation in alternative CR models.11 Self-reported patient characteristics, including age, sex, race, ethnicity, physical activity, smoking status, and history of peripheral arterial disease were collected from standardized health questionnaires and intake evaluation by an exercise physiologist. Comorbidities were derived from past diagnostic codes including stroke, heart failure, peripheral vascular disease, chronic obstructive pulmonary disease (COPD), diabetes, hypertension, atrial fibrillation or flutter, and presence of cardiac pacemaker or implantable cardioverter defibrillator. We defined angina based on the indication for referral validated by the exercise physiologist at the time of CR entry. Psychological distress was assessed by the Brief Symptom Inventory (BSI), consisting of 53 items that cover nine symptom dimensions of which we used four in our studies in CR (depression, hostility, anxiety, and global severity index).12
Metabolic biomarkers were obtained from participants’ health records from the date closest to baseline CR evaluation and collected again upon completion of CR. Resting blood pressure was measured via the American Heart Association guidelines using regularly calibrated oscillometric devices with patient sitting upright and forearm supported at the level of the heart after resting for 5 minutes.13 Upon CR entry and completion, body weight, height, and body fat percentage were measured using a bioelectrical impedance analysis (BIA) scale [Tanita, TBF-310, Tokyo, Japan], while waist circumference was measured at the mid-point between the lowest ribs and the iliac crest. MetS was defined using the standard American Heart Association criteria.4 Cardiorespiratory fitness (CRF) was measured at CR entry by peak oxygen consumption (VO2peak) using an electronic/motorized treadmill test. VO2peak was expressed as estimated metabolic equivalents (METs), and rating of perceived exertion was at peak. CRF was not measured at CR completion.
The current investigation was approved by and followed the recommendations of the Institutional Review Board of the University of Michigan Medical School (IRBMED) and was in accordance with the ethical standards as established by the 1964 Declaration of Helsinki and its later amendments.
Outcome
The primary outcome was the change in cardiometabolic risk factors from baseline to the completion of CR, as well as the difference of change in individuals with MetS compared to those without MetS.
Statistical Analysis
We compared baseline clinical and sociodemographic characteristics by MetS status using Wilcoxon rank-sum tests and Chi-square tests. Non-parametric Wilcoxon tests were used, not assuming normal distribution of continuous variables. The change in cardiometabolic parameters between baseline and completion of CR was assessed using non-parametric paired tests, or Wilcoxon signed-rank tests, as the same patients at baseline and at the end of CR were not independent. Patients with missing data for a specific measure, either at baseline or at the end of CR, were excluded when computing the statistics of the measure. To evaluate changes in cardiometabolic risk factors and measures of psychosocial health and cardiopulmonary fitness by MetS status, we conducted 17 difference-in-differences regression models, one for each measure, adjusting for age, sex, education, employment status, and indication for referral to CR. Patients who did not have MetS at baseline served as the reference group, as we hypothesized more improvement might be observed for patients having MetS at baseline. In difference-in-difference modeling, patients with missing data either at baseline or at the end of CR were excluded from modeling. An a-priori 2-tailed alpha of <0.05 was used to indicate statistical significance. All analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC).
Results
Our final analytic sample (n = 1984) had a mean age of 63.4 years of age in those with and without the MetS; 74.9% were male and 82.6% were White (Table 1). Overall, 1070 individuals (53.9%) met the criteria for MetS. Those with MetS were more likely to be female (P = 0.001), had lower educational attainment (P < 0.0001), and were less likely to be actively employed (P < 0.0003). Those with MetS were more likely to have a past medical history of COPD (P = 0.006) and peripheral vascular disease (P = 0.001). At baseline, those with MetS had slightly greater psychological distress, as evident by higher scores on the psychosocial health indicator BSI-53, but both groups were average for age/gender.
Table 1.
Comparison of Baseline Sociodemographic and Clinical Characteristics of Participants, by Metabolic Syndrome Status
| Variable | Total, N = 1984a | Metabolic Syndrome, n = 1070 (53.9%) | No Metabolic Syndrome, n = 914 (46.1%) | Effect Size | Pb |
|---|---|---|---|---|---|
| Age, years (mean ± SD) | 63.4 ± 11.3 | 63.2 ± 10.7 | 63.6 ± 11.9 | 0.035 | 0.58 |
| Males, n (%) | 1486 (74.9) | 770 (72.0) | 716 (78.3) | 0.146 | 0.001 |
| White race/ethnicity, n (%) | 1624 (82.6) | 873 (82.1) | 751 (83.2) | 0.029 | 0.54 |
| Married, n (%) | 1433 (73.3) | 759 (72.1) | 674 (74.7) | 0.059 | 0.19 |
| Educational attainment, n (%) | |||||
| High school, some high school | 253 (13.1) | 158 (15.0) | 95 (10.9) | 0.122 | < 0.0001 |
| Associate’s degree, some college | 608 (31.5) | 383 (36.2) | 225 (25.8) | 0.226 | |
| Bachelor’s degree or higher | 360 (18.7) | 177 (16.8) | 183 (21.0) | 0.107 | |
| Post-graduate, professional degree | 606 (31.4) | 275 (26.0) | 331 (38.0) | 0.259 | |
| Other | 102 (5.3) | 64 (6.1) | 38 (4.4) | 0.076 | |
| Employment status, n (%) | |||||
| Active | 831 (41.9) | 417 (39.0) | 414 (45.3) | 0.128 | 0.0003 |
| Retired | 832 (41.9) | 472 (44.1) | 360 (39.4) | 0.095 | |
| Unemployed | 70 (3.5) | 41 (3.8) | 29 (3.2) | 0.033 | |
| Medically disabled | 116 (5.8) | 79 (7.4) | 37 (4.1) | 0.142 | |
| Unknown | 135 (6.8) | 61 (5.7) | 74 (8.1) | 0.095 | |
| Indication for referral to cardiac rehab program, n (%) | |||||
| MI | 516 (26.0) | 255 (23.8) | 261 (28.6) | 0.109 | 0.003 |
| MI/PCI | 355 (17.9) | 186 (17.4) | 169 (18.5) | 0.029 | |
| PCI/stent | 673 (33.9) | 376 (35.1) | 297 (32.5) | 0.055 | |
| CABG | 345 (17.4) | 186 (17.4) | 159 (17.4) | 0.000 | |
| Stable angina | 95 (4.8) | 67 (6.3) | 28 (3.1) | 0.152 | |
| Body composition, mean ± SD | |||||
| Weight, lbs | 195.4 ± 43.7 | 210.4 ± 42.7 | 177.9 ± 38.0 | 0.804 | < 0.0001 |
| Body mass index, kg/m2 | 29.8 ± 6.0 | 32.1 ± 5.8 | 27.1 ± 5.0 | 0.923 | < 0.0001 |
| Body fat percentage | 31.3 ± 9.4 | 34.8 ± 8.9 | 27.0 ± 8.1 | 0.917 | < 0.0001 |
| Metabolic syndrome components, mean ± SD | |||||
| Waist circumference, in | 40.9 ± 5.9 | 43.5 ± 5.4 | 37.8 ± 4.8 | 1.116 | < 0.0001 |
| HDL-C, mg/dL | 42.6 ± 12.6 | 38.3 ± 9.6 | 47.9 ± 13.8 | 0.808 | < 0.0001 |
| Triglycerides, mg/dL | 127.2 ± 73.2 | 152.7 ± 82.2 | 96.6 ± 44.5 | 0.849 | < 0.0001 |
| Fasting glucose, mg/dL | 112.6 ± 36.8 | 124.3 ± 42.1 | 98.3 ± 21.6 | 0.777 | < 0.0001 |
| Systolic BP, mm Hg | 117.2 ± 17.3 | 119.3 ± 17.3 | 114.7 ± 17.0 | 0.268 | < 0.0001 |
| Diastolic BP, mm Hg | 65.7 ± 9.9 | 66.4 ± 9.8 | 64.9 ± 10.0 | 0.152 | 0.0004 |
| Cardiometabolic risk factors, n (%) | |||||
| Type 1 diabetes | 54 (2.7) | 35 (3.3) | 19 (2.1) | 0.074 | 0.10 |
| Type 2 diabetes | 617 (31.1) | 516 (48.2) | 101 (11.1) | 0.889 | < 0.0001 |
| Hyperlipidemiac | 1852 (93.4) | 1025 (95.8) | 827 (90.5) | 0.211 | < 0.0001 |
| Low HDL-Cd | 616 (31.1) | 474 (44.3) | 142 (15.5) | 0.663 | < 0.0001 |
| Hypertensione | 1434 (72.3) | 935 (87.4) | 499 (54.6) | 0.775 | < 0.0001 |
| Physical inactivityf | 310 (15.6) | 179 (16.7) | 131 (14.3) | 0.066 | 0.14 |
| Current smoking | 106 (5.6) | 63 (6.2) | 43 (5.0) | 0.052 | 0.29 |
| Obesity | 810 (40.8) | 628 (58.7) | 182 (19.9) | 0.866 | < 0.0001 |
| Comorbidities, n (%) | |||||
| Stroke | 19 (1.0) | 11 (1.0) | 8 (0.9) | 0.010 | 0.819 |
| Heart failure | 27 (1.4) | 13 (1.2) | 14 (1.5) | 0.026 | 0.565 |
| Cardiac pacemaker or ICD | 127 (6.4) | 66 (6.2) | 61 (6.7) | 0.020 | 0.647 |
| COPD | 568 (28.6) | 334 (31.2) | 234 (25.6) | 0.124 | 0.006 |
| Peripheral vascular disease | 295 (14.9) | 186 (17.4) | 109 (11.9) | 0.156 | 0.001 |
| Atrial fibrillation/flutter | 453 (22.8) | 240 (22.4) | 213 (23.3) | 0.021 | 0.668 |
| Other lipids (mg/dL), mean ± SD | |||||
| Total cholesterol | 138.1 ± 36.9 | 137.9 ± 37.2 | 138.3 ± 36.6 | 0.011 | 0.55 |
| LDL-C | 70.3 ± 28.9 | 69.7 ± 29.0 | 71.1 ± 28.8 | 0.048 | 0.17 |
| Non-HDL-C | 95.5 ± 34.1 | 99.7 ± 34.6 | 90.5 ± 32.7 | 0.273 | < 0.0001 |
| Pharmacotherapy, n (%) | |||||
| Diabetes | 534 (26.9) | 463 (43.3) | 71 (7.8) | 0.892 | < 0.0001 |
| High Triglycerides | 36 (1.8) | 32 (3.0) | 4 (0.4) | 0.198 | 0.0003 |
| Low HDL-C | 33 (1.7) | 28 (2.6) | 5 (0.5) | 0.166 | < 0.0001 |
| Hypertension | 1825 (92.0) | 1036 (96.8) | 789 (86.3) | 0.385 | < 0.0001 |
| Statin | 1827 (92.1) | 1008 (94.2) | 819 (89.6) | 0.169 | 0.0002 |
| Psychosocial health, mean ± SD | |||||
| BSI-53 global severity index | 51.7 ± 10.3 | 52.2 ± 10.6 | 51.1 ± 10.0 | 0.107 | 0.02 |
| BSI-53 depression score | 51.5 ± 9.4 | 51.8 ± 9.7 | 51.2 ± 9.0 | 0.064 | 0.64 |
| BSI-53 anxiety score | 49.5 ± 10.0 | 49.6 ± 10.2 | 49.4 ± 9.7 | 0.020 | 0.80 |
| BSI-53 hostility score | 49.6 ± 9.0 | 50.1 ± 9.1 | 49.0 ± 8.7 | 0.124 | 0.01 |
| Cardiopulmonary fitness | |||||
| Estimated METs | 8.3 ± 3.1 | 7.4 ± 2.7 | 9.4 ± 3.3 | 0.663 | < 0.0001 |
| RPE | 15.8 ± 4.4 | 15.6 ± 2.2 | 16.1 ± 6.1 | 0.109 | 0.11 |
Notes: aPercentages for some variables reflect missing observations. bFrom Wilcoxon rank-sum tests and Chi-square tests. cTotal cholesterol ≥ 240 mg/dL, LDL-C ≥ 160 mg/dL, or treatment for elevated cholesterol. dHDL-C < 35 mg/dL for males, < 40 mg/dL for females, or treatment for low HDL. eSystolic blood pressure ≥ 130 mmHg, diastolic blood pressure ≥ 80 mmHg, or treatment for hypertension. fSelf-reported daily physical activity level of “sedentary”.
Abbreviations: SD, standard deviation; MI, myocardial infarction; PCI, percutaneous coronary intervention; CABG, coronary artery bypass graft; HDL-C, high-density lipoprotein cholesterol; ICD, implantable cardioverter defibrillator; COPD, chronic obstructive pulmonary disease; LDL, low-density lipoprotein; BSI, brief symptom inventory; METs, metabolic equivalents; RPE, rating of perceived exertion.
The most common qualifying diagnosis was PCI (33.9%), followed by MI (26.0%). There was no difference in CR sessions attended [MetS n = 1063, mean 23.6 ± 11.6, no MetS n = 905, mean 22.9 ± 11.6, P = 0.157], which was 2 or 3 sessions per week over 3 months. At baseline, those with MetS were more likely to be on medication for diabetes (ES = 0.892, P = <0.0001) and hypertension (ES = 0.385, P = <0.0001). While 92% of the total sample were being treated with cholesterol-lowering statins medication, less than 2.0% of patients were being treated with drugs specific for triglycerides and high-density lipoprotein cholesterol (HDL-C) across both groups.
At completion of CR, statistically significant improvement was observed in most measures of body composition and cardiometabolic risk factors as well as psychosocial health (Table 2). The change in biometric and cardiometabolic risk factors following participation in CR, by MetS status and components, is summarized in Table 2. After adjustment, both groups lost a significant amount of weight; however, compared to those without MetS, those with MetS lost an average of 1.43 pounds more (95% CI: 0.56, 2.31, P = 0.001), corresponding to a 0.21-point larger drop in body mass index (95% CI: 0.03, 0.37, P = 0.02), and also experienced a modestly greater reduction in waist circumference (−0.31 in [95% CI: −0.54, −0.08, P = 0.008]). A significant decrease in systolic blood pressure occurred in MetS which did not occur in the non-MetS group (−2.38 mmHg ± 17.68, P = 0.002), a difference that was not significant after adjustment (P = 0.08). As anticipated, there was a greater reduction in triglycerides and fasting glucose in those with MetS. The difference of change after adjustment for triglycerides was −8.70 mg/dL (95% CI: 15.04, −2.37, P = 0.007), and glucose −5.48 mg/dL (95% CI: −10.44, −0.53, P = 0.03). Both groups had an approximate 2 mg/dL increase in HDL-C. The mean baseline LDL-C was about 70 mg/dL in each group (Table 1) reflecting the use of statins, and both groups achieved an average of 25% further reduction in LDL-C. There was improvement in each of the variables of psychological distress in those with and without MetS with a greater improvement on adjusted difference in depression in those without MetS (P = 0.03).
Table 2.
Comparison of Changes in Cardiometabolic Risk Factors and Cardiopulmonary Fitness Outcomes Following Participation in Cardiac Rehabilitation, by Metabolic Syndrome Status
| Variable | Metabolic Syndrome, N = 1070 (53.9%) | No Metabolic Syndrome, N = 914 (46.1%) | Difference of Changec (95% CI) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| na | Change from Baselineb (mean ± SD) | P | na | Change from Baselineb (mean ± SD) | P | Unadjusted | P | Adjustedd | P | |
| Body composition | ||||||||||
| Weight, lbs | 716 | −4.68 ± 8.68 | < 0.0001 | 636 | −3.58 ± 7.37 | < 0.0001 | −1.10 (−1.96, −0.24) | 0.01 | −1.43 (−2.31, −0.56) | 0.001 |
| Body mass index, kg/m2 | 714 | −0.74 ± 1.82 | < 0.0001 | 635 | −0.56 ± 1.43 | < 0.0001 | −0.18 (−0.35, −0.01) | 0.04 | −0.21 (−0.37, −0.03) | 0.02 |
| Body fat percentage | 619 | −0.82 ± 3.23 | < 0.0001 | 553 | −0.57 ± 2.28 | < 0.0001 | −0.24 (−0.56, 0.07) | 0.13 | −0.31 (−0.63, 0.01) | 0.06 |
| Metabolic syndrome components | ||||||||||
| Waist circumference, in | 628 | −0.81 ± 1.99 | < 0.0001 | 545 | −0.57 ± 1.94 | < 0.0001 | −0.24 (−0.46, −0.01) | 0.04 | −0.31 (−0.54, −0.08) | 0.008 |
| HDL-C, mg/dL | 578 | 2.14 ± 6.73 | < 0.0001 | 487 | 2.68 ± 8.15 | < 0.0001 | −0.54 (−1.45, 0.37) | 0.24 | −0.20 (−1.13, 0.73) | 0.67 |
| Triglycerides, mg/dL | 578 | −14.41 ± 61.05 | < 0.0001 | 487 | −5.09 ± 43.63 | 0.0001 | −9.32 (−15.62, −3.02) | 0.004 | −8.70 (−15.04, −2.37) | 0.007 |
| Fasting glucose, mg/dL | 375 | −3.78 ± 37.03 | 0.001 | 167 | 1.74 ± 20.03 | 0.42 | −5.52 (−10.33, −0.70) | 0.02 | −5.48 (−10.44, −0.53) | 0.03 |
| Systolic BP, mm Hg | 715 | −2.38 ± 17.68 | 0.002 | 630 | −0.37 ± 16.49 | 0.70 | −2.01 (−3.83, −0.18) | 0.03 | −1.68 (−3.55, 0.18) | 0.08 |
| Diastolic BP, mm Hg | 715 | −1.11 ± 10.59 | 0.008 | 628 | −0.98 ± 10.27 | 0.05 | −0.13 (−1.25, 0.99) | 0.82 | −0.26 (−1.41, 0.89) | 0.66 |
| Other lipids, mg/dL | ||||||||||
| Total cholesterol | 578 | −0.08 ± 31.97 | 0.62 | 487 | −1.20 ± 28.59 | 0.84 | 1.12 (−2.51, 4.76) | 0.54 | 0.94 (−2.81, 4.69) | 0.62 |
| LDL-C | 577 | −19.59 ± 29.06 | < 0.0001 | 486 | −16.97 ± 28.85 | < 0.0001 | −2.62 (−6.11, 0.87) | 0.14 | −1.50 (−5.06, 2.05) | 0.41 |
| Non-HDL-C | 578 | −2.21 ± 30.33 | 0.11 | 487 | −3.82 ± 26.54 | 0.002 | 1.61 (−1.81, 5.02) | 0.36 | 1.08 (−2.43, 4.60) | 0.55 |
| Psychosocial health, mean ± SD | ||||||||||
| BSI-53 global severity index | 654 | −3.66 ± 7.64 | < 0.0001 | 572 | −4.50 ± 7.76 | < 0.0001 | 0.84 (−0.02, 1.71) | 0.06 | 0.82 (−0.06, 1.71) | 0.07 |
| BSI-53 depression score | 654 | −1.67 ± 7.75 | < 0.0001 | 572 | −2.51 ± 7.14 | < 0.0001 | 0.84 (0.00, 1.67) | 0.05 | 0.92 (0.08, 1.76) | 0.03 |
| BSI-53 anxiety score | 654 | −3.32 ± 8.66 | < 0.0001 | 572 | −3.99 ± 8.59 | < 0.0001 | 0.67 (−0.30, 1.63) | 0.18 | 0.60 (−0.37, 1.57) | 0.23 |
| BSI-53 hostility score | 654 | −2.79 ± 8.00 | < 0.0001 | 572 | −2.26 ± 7.99 | < 0.0001 | −0.54 (−1.43, 0.36) | 0.24 | −0.39 (−1.32, 0.54) | 0.41 |
| Cardiopulmonary fitness | ||||||||||
| RPE | 485 | −3.02 ± 3.04 | < 0.0001 | 447 | −3.53 ± 6.77 | < 0.0001 | 0.51 (−0.17, 1.19) | 0.14 | 0.34 (−0.26, 0.93) | 0.27 |
Notes: aNumber of patients with data at both baseline and end of program. bFrom Wilcoxon signed-rank tests. cFrom difference-in-differences regression models specifying robust estimates of variance. Patients without metabolic syndrome are treated as the reference. dDerived from difference-in-differences models adjusted for age, sex, education, employment status, and indication for referral to cardiac rehab.
Abbreviations: SD, standard deviation; CI, confidence interval; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; BSI, brief symptom inventory; METs, metabolic equivalents; RPE, rating of perceived exertion.
Discussion
In this observational study, we found that participation in CR was associated with improved cardiometabolic risk profile, including reductions in systolic BP, weight, body fatness, lipids, and components of the MetS, as well as improved psychosocial health. Overall, compared to patients without MetS, those with MetS experienced a similar or greater improvement in cardiometabolic health.
Our findings are in line with evidence that CR improves clinical outcomes broadly3 and MetS specifically.7 However, other studies have found smaller associations of CR with improvement in metabolic parameters among those with DM,9,10 which is not consistent with the results we found for MetS. It is not surprising that education and monitoring during CR would result in greater improvement in modifiable CHD risk factors such as triglycerides, waist circumference, weight, and blood pressure. In a study surveying patients’ awareness of CVD risk factors at CR entry, the results showed that many patients are not aware of their risk factors and may even underestimate the significant risk factors such as a sedentary lifestyle, cigarette smoking and DM have on CVD.14 These findings further support the use of CR programs, with targeted educational components, for improvement in cardiometabolic health, including each of the MetS criteria.
Differences in patient population, study design, skills of CR staff, and the specific CR intervention may also explain these discrepancies.15 Furthermore, although MetS and DM often share a similar underlying pathology, it is possible that those with MetS may respond differently to CR than those with DM. In one study that specifically examined CHD patients with MetS, a 6-month CR program improved body composition, metabolic health, inflammation, and cardiopulmonary fitness.16 However, that study only included patients with CABG and did not compare those with and without MetS.
The reduction in recurrent CV events and death following CR is well established. To what degree the positive benefits have long-term value in persons with and without MetS remains to be seen.17 Given the increasing burden of cardiometabolic diseases, the prevalence of MetS in CHD, and the lack of long-term benefit of CR-like tailored programs focused on promoting metabolic fitness, longer-duration chronic disease management than CR may be necessary to sustain the benefits.18
Our study has important strengths. We collected detailed cardiometabolic data both at baseline and at the completion of CR, allowing us to assess prospective changes in these variables. These data were collected as part of a comprehensive and ongoing preventive cardiology research database encompassing a range of CV risk factors and secondary prevention programs for individuals with established CVD. In addition, the data collection was conducted by trained staff using standardized protocols, minimizing reliance on patient recall and enhancing data accuracy.
Several limitations should also be considered. The study utilized medical records to identify relevant data, some of which were not originally collected for research purposes. As a result, the number of patients with sufficient data to specify MetS at CR exit is notably smaller compared to CR entry. Consequently, this reduced patient group may not be representative of the entire study cohort. Furthermore, we cannot rule out residual confounding, particularly due to unmeasured variables including fasting insulin, and inflammatory markers such as high sensitivity c-reactive protein. Finally, our sample consisted of predominantly very well-educated White patients at a major academic medical center; therefore, generalizability may be limited to populations with similar sociodemographic and cardiometabolic characteristics.
Conclusion
In conclusion, this study provides evidence to support the use of CR as a means to promote cardiometabolic health among patients with CHD. Compared to people without MetS, those with MetS experienced a comparable or greater benefit from CR, particularly with respect to improvements in MetS components. CR should be recommended for all eligible patients with CHD, including those with MetS.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Alberti KGMM, Eckel RH, Grundy SM. et al. Harmonizing the Metabolic Syndrome. Circulation. 2009;120(16):1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644 [DOI] [PubMed] [Google Scholar]
- 2.Vassallo P, Driver SL, Stone NJ. Metabolic Syndrome: an Evolving Clinical Construct. Prog Cardiovasc Dis. 2016;59(2):172–177. doi: 10.1016/j.pcad.2016.07.012 [DOI] [PubMed] [Google Scholar]
- 3.Taylor RS, Dalal HM, McDonagh STJ. The role of cardiac rehabilitation in improving cardiovascular outcomes. Nat Rev Cardiol. 2022;19(3):180–194. doi: 10.1038/s41569-021-00611-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grundy SM, Brewer Jr HB, Cleeman JI, Smith Jr SC, Lenfant C. Definition of metabolic syndrome: report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109(3):433–438. doi: 10.1161/01.CIR.0000111245.75752.C6 [DOI] [PubMed] [Google Scholar]
- 5.Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365(9468):1415–1428. doi: 10.1016/S0140-6736(05)66378-7 [DOI] [PubMed] [Google Scholar]
- 6.Einarson TR, Acs A, Ludwig C, Panton UH. Prevalence of cardiovascular disease in type 2 diabetes: a systematic literature review of scientific evidence from across the world in 2007-2017. Cardiovasc Diabetol. 2018;17(1):83. doi: 10.1186/s12933-018-0728-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sadeghi M, Salehi-Abargouei A, Kasaei Z, Sajjadieh-Khajooie H, Heidari R, Roohafza H. Effect of cardiac rehabilitation on metabolic syndrome and its components: a systematic review and meta-analysis. J Res Med Sci. 2016;21(1):18. doi: 10.4103/1735-1995.178757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bianchi S, Maloberti A, Peretti A, et al. Determinants of Functional Improvement After Cardiac Rehabilitation in Acute Coronary Syndrome. High Blood Press Cardiovasc Prev. 2021;28(6):579–587. doi: 10.1007/s40292-021-00473-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khadanga S, Savage PD, Ades PA. Insulin Resistance and Diabetes Mellitus in Contemporary Cardiac Rehabilitation. J Cardiopulm Rehabil Prev. 2016;36(5):331–338. doi: 10.1097/HCR.0000000000000187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Laddu DR, Ozemek C, Hauer TL, et al. Cardiometabolic responses to cardiac rehabilitation in people with and without diabetes. Int J Cardiol. 2020;301:156–162. doi: 10.1016/j.ijcard.2019.11.134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brandt EJ, Garfein J, Pai CW, et al. Identifying Factors for Low-Risk Participation in Alternative Cardiac Rehabilitation Models for Patients with Coronary Heart Disease Using MI’S SCOREPAD. Cardiovasc Ther. 2023;2023:7230325. doi: 10.1155/2023/7230325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kolman L, Shin NM, Krishnan SM, et al. Psychological distress in cardiac rehabilitation participants. J Cardiopulm Rehabil Prev. 2011;31(2):81–86. doi: 10.1097/HCR.0b013e3181f688e1 [DOI] [PubMed] [Google Scholar]
- 13.Muntner P, Shimbo D, Carey RM, et al. Measurement of Blood Pressure in Humans: a Scientific Statement From the American Heart Association. Hypertension. 2019;73(5):e35–e66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maloberti A, Monticelli M, Bassi I, Riccobono S, Giannattasio C. Low Awareness of Cardiovascular Risk Factor Among Patients Admitted in Cardiac Rehabilitation: new Data for Further Implementation of Cardiovascular Rehabilitation Program. High Blood Press Cardiovasc Prev. 2021;28(3):253–254. doi: 10.1007/s40292-021-00451-z [DOI] [PubMed] [Google Scholar]
- 15.Walden P, Jiang Q, Jackson EA, Oral EA, Weintraub MS, Rubenfire M. Assessing the incremental benefit of an extended duration lifestyle intervention for the components of the metabolic syndrome. Diabetes Metab Syndr Obes. 2016;9:177–184. doi: 10.2147/DMSO.S94772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Onishi T, Shimada K, Sunayama S, et al. Effects of cardiac rehabilitation in patients with metabolic syndrome after coronary artery bypass grafting. J Cardiol. 2009;53(3):381–387. doi: 10.1016/j.jjcc.2009.01.004 [DOI] [PubMed] [Google Scholar]
- 17.Joseph MS, Konerman MA, Zhang M, et al. Long-term outcomes following completion of a structured nutrition and exercise lifestyle intervention program for patients with metabolic syndrome. Diabetes Metab Syndr Obes. 2018;11:753–759. doi: 10.2147/DMSO.S175858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hamm LF, Kavanagh T, Campbell RB, et al. Timeline for peak improvements during 52 weeks of outpatient cardiac rehabilitation. J Cardiopulm Rehabil. 2004;24(6):374–80;quiz381–2. doi: 10.1097/00008483-200411000-00002 [DOI] [PubMed] [Google Scholar]