Highlights
-
•
p53 and p16 expression have prognostic importance in vulva cancer.
-
•
p53 vulva cancers have higher recurrence rates and lower survival than p16 positive.
-
•
While immunohistochemistry is important, it has not yet been fully integrated into all clinical practice.
Keyword: Vulva cancer, pre-invasive disease, immunohistochemistry, molecular pathology
Abstract
Vulvar squamous cell carcinomas (SCC) represent a heterogeneous group of patients with implications for prognosis and response to treatment. Human papillomavirus (HPV)-associated SCC is characterised by p16 positivity, whereas non-HPV SCC often shows aberrant p53 expression. We conducted a retrospective analysis involving 148 patients with vulvar SCC from two Gynecologic Oncology units from Sydney, Australia. Patients’ demographics, tumor characteristics, types of treatment and survival were analyzed and compared to p16 and p53 immunohistochemistry status. The p16-positive group was younger and included a higher prevalence of smokers, while the p53-positive group demonstrated greater comorbidity indices and was associated with tumor features that are independently related to poor prognosis. Compared to p16-positive patients our study has shown significantly higher recurrence rates and lower overall survival in the p53-positive group. Our findings support existing literature, emphasizing the prognostic significance of p16 and p53 in vulvar SCC. Despite the retrospective nature and variations in immunohistochemistry reporting, our study provides valuable insights into patient outcomes, particularly in a demographically diverse population. Future research, like the STRIVE trial, may determine if implementation of p16 and p53 stratified management algorithm will improve outcomes for women with vulvar SCC (McAlpine, 2024).
1. Introduction
Vulvar cancer accounts for 4 % of gynecological malignancies, of which the most common subtype is squamous cell carcinoma (SCC). Vulvar SCCs develop through two distinct pathways – one is associated with human papillomavirus and typically occurs in younger women (Höhn et al., 2021). p16INK4A (p16) expression is typically block positive in these cases, as it is a surrogate marker for HPV-driven disease. The other type, HPV-independent disease, occurs more frequently in older women and is often associated with vulvar dermatoses such as lichen sclerosis (Höhn et al., 2021). This phenotype is associated with aberrant p53 expression (Eva et al., 2022). TP53 is a tumor suppressor gene involved in maintaining genomic integrity by controlling cell cycle progression or inducing apoptosis. TP53 mutations are identified in 80 % of differentiated vulvar intraepithelial neoplasia (dVIN) and are deduced from p53 immunohistochemistry staining (Tessier-Cloutier et al., 2020). Because HPV-related and non-HPV related precursors to vulvar SCC cannot be reliably distinguished by routine microscopy, the International Society for the Vulvovaginal Disease recommends universal p16 and p53 staining in cases of suspected squamous neoplasia (Höhn et al., 2021).
These distinct profiles of vulvar SCC have been shown to correlate with treatment response and prognosis. p16 positive vulvar cancers demonstrate increased chemo-radiosensitivity (Proctor et al., 2020), and HPV-independent vulvar cancers have associated worse survival independent of stage and age at diagnosis (Eva et al., 2022). However, some studies have demonstrated no significant difference in prognosis between the two groups (Alonso et al., 2011).
Our objectives were 1. to determine whether p53 and p16 expression were associated with survival and recurrence outcomes in vulvar cancer, and 2. to determine whether they were independent prognostic factors when adjusted for patient, tumor and treatment factors.
2. Methods
Governance approval was obtained from South Western Sydney and Western Sydney Local Health Districts Human Research Ethics Committees (2019/ETH09967). A retrospective analysis of all patients diagnosed with vulvar cancer over a 10-year period at two Gynaecological Oncology units in Sydney, diagnosed from 1st January 2010 to 31st December 2019 was undertaken. Patients with non-SCC vulvar cancer were excluded from analysis. Patient factors (i.e. age, BMI, smoking status, Charlson Comorbidity Index, Index of Relative Socioeconomic Disadvantage), tumor pathology (i.e. cell type, immunohistochemistry, tumour size, depth of stromal invasion) and treatment factors (i.e. surgical management of primary tumour, node management, radiotherapy, chemotherapy, immunotherapy) were recorded for all patients. Treatment was determined by Gynaecological Oncology multi-disciplinary team meetings at each individual institution.
Pathology reports were analyzed from the location where they were performed at the time of diagnosis, which included reports by pathologists from two different units, as well as some private pathology providers. Immunohistochemistry results were retrospectively recorded from pathology reports where they had been tested. Patients were classified as within the p16 positive group if p16 was positive and p53 was either negative or no p53 result was available. Patients were classified as within the p53 group when p53 was reported as positive. The few patients who were positive for p16 and p53 (n = 5), were included in the p53 group. If patients were not positive for either p16 or p53 (either due to a returned negative test or because of a lack of testing), they were included in the ‘other’ group. Where p16 and p53 were reported as being variable or uncertain, they were included in the ‘other’ group. FIGO 2009 staging was used to classify stage as these patients were all diagnosed and treated prior to the 2021 FIGO staging revision.
Patients were followed up at their separate institutions to personalized schedules determined by their clinicians. Survival and recurrence outcomes were followed until the census date on 31 January 2024; the Australian My Health Record was used to determine patient status if death had not been recorded prior. Patient demographics were analysed with descriptive statistics (median, frequency). Chi-Square and Wilcoxon rank sum tests were used to determine the relationship of factors with IHC category. Adjusted Cox proportional hazard models were used to determine the impact of IHC on survival and recurrence, adjusting for significant possible confounding factors.
3. Results
There were 175 cases of vulvar cancer managed at these institutions over this 10-year period. All patients who had a diagnosis of FIGO Stage I-IV vulvar cancer with SCC subtype, who received treatment at these two sites were included, with 148 SCC cases included for analysis.
Patients had a median age of 69 years (Inter-quartile range (IQR) 55–78), with a median Body Mass Index of 29 kg/m2, and smoking rates of 25 % (current smokers). The Index of Relative Socio-economic Disadvantage (IRSD) included 43 % of patients in the lowest quintile. Median Charlson Comorbidity Index was 5. Of the included 148 cases, 81 had immunohistochemistry data available for analysis, representing 22 % (8/37) of patients from Site A and 66 % (73/111) of patients from Site B. Based on the FIGO 2009 staging, 56 % were stage 1 (Table 1).
Table 1.
Immunohistochemistry and relationship with patient and tumour factors.
| Characteristic | Total | p16 (n = 45) | p53 (n = 36) | Other (n = 67) | P |
|---|---|---|---|---|---|
| Site | LP: 25 % (37) WM: 75 % (111) |
LP: 7 % (3) WM: 93 % (42) |
LP: 14 % (5) WM: 86 % (31) |
LP: 43 % (29) WM: 57 % (38) |
<0.01 |
| Age (years) (IQR) | 69 (55–78) |
62 (50–71) |
71 (63–83) |
70 (58–81) |
<0.01 |
| BMI (kg/m2) (IQR) | 29 (24–37) |
30 (23–37) |
31 (24–38) |
28 (24–35) |
0.63 |
| Index of Relative Socio-economic Disadvantage (IQR) | 2 (1–4) |
3 (2–4) |
2 (2–4) |
2 (1–4) |
0.51 |
| Charlson Comorbidity Index (IQR) | 5 (4–8) | 4 (3–6) |
6 (4–8) |
6 (5–8) |
0.02 |
| Smoker | 25 % (34) | 29 % (12) | 9 % (3) | 32 % (19) | 0.03 |
| Australian Born | 76 % (111) | 84 % (38) | 69 % (25) | 73 % (48) | 0.23 |
| Lymphovascular Invasion | 28 % (37) | 14 % (6) | 29 % (10) | 40 % (21) | 0.02 |
| Perineural Invasion | 14 % (18) | 0 % (0) | 24 % (8) | 20 % (10) | <0.01 |
| Largest Diameter (mm) (IQR) | 30 (13–45) |
22 (6–35) |
31 (13–40) |
35 (20–50) |
0.01 |
| Depth of Stromal Invasion (mm) (IQR) | 4.0 (2.0–7.8) |
2.1 (0.6–5.8) |
4.8 (3.4–7.5) |
5.0 (2.1–9.5) |
0.01 |
| Nodes Involvement | 35 % (49) | 23 % (10) | 31 % (11) | 45 % (28) | 0.06 |
| FIGO Stage | I: 56 % (79) II: 9 % (12) III: 29 % (40) IV: 6 % (9) |
I: 67 % (29) II: 9 % (4) III: 16 % (7) IV: 7 % (3) |
I: 57 % (20) II:11 % (4) III: 31 % (11) IV: 0 % (0) |
I: 48 % (30) II: 6 % (4) III: 35 % (22) IV: 10 % (6) |
0.14 |
| Palliated | 13 % (18) | 5 % (2) | 3 % (1) | 25 % (15) | 0.01 |
| Surgery Performed | 89 % (131) | 91 % (41) | 100 % (36) | 81 % (54) | 0.01 |
| Radiotherapy | 43 % (63) | 27 % (12) | 39 % (14) | 55 % (37) | 0.01 |
| Chemotherapy | 13 % (19) | 7 % (3) | 3 % (1) | 23 % (15) | 0.01 |
Comparing p16 vs p53 vs ‘other’, the p53 group were older than the p16 group at diagnosis. Patients in the p16 group were more likely to be smokers. Patients in the p53 group had greater Charlson Comorbidity Index than p16 (although this was not controlled for age). When comparing tumour factors, the p53 group were more likely to have lymphovascular and perineural invasion, had greater tumour diameters and greater depth of stromal invasion.
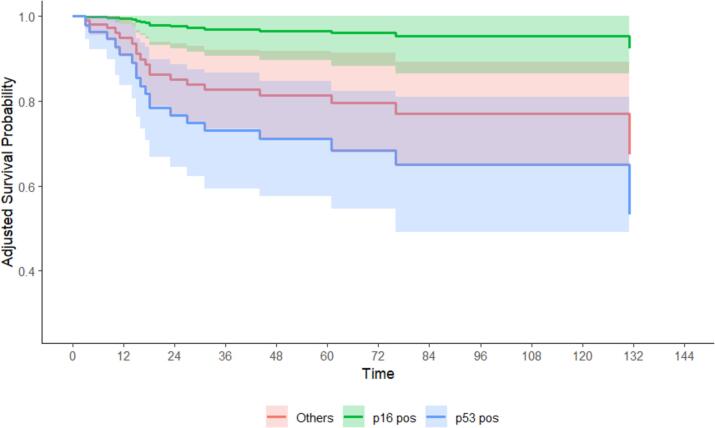
Patients were followed for a median of 51 months. By the census date in January 2024, 58 (39 %) of patients had died (17 % from tumour-specific mortality), and 53 (40 %) had developed recurrence. The p53 group had a greater hazard of tumor-specific mortality (14.14 [1.82–109.56], p < 0.01), all-cause mortality (3.85 [1.52–9.77], p < 0.01), and recurrence (5.84 [2.17–15.75], p < 0.01) compared to the p16 group. Cox regression models were adjusted for site, CCI, FIGO Stage (as an omnibus measure for node status, depth of stromal invasion, tumour diameter), palliative/curative intent, and whether each of surgery, radiotherapy, and chemotherapy were received. The p53 group had a greater hazard of tumor-specific mortality (27.48 [2.25–334.31], p = 0.03), all-cause mortality (3.42 [1.15–10.19], p = 0.01), and recurrence (5.19 [1.82–14.77], p = 0.01) compared to the p16 group. The estimated 5-year survival of the p53 group was significantly lower than the p16 group for tumor-specific mortality (68 % [55 %–82 %] vs 96 % [88 %–100 %]), and recurrence (57 % [44 %–69 %] vs 82 % [73 %–91 %]). A similar result for all-cause mortality (61 % [51 %–77 %] vs 81 % [68 %–93 %]) was not statistically significant. Fig. 1. Table 2.
Fig. 1.
Tumor-specific mortality, by p16, p53 or ‘other’ immunohistochemistry status.
Table 2.
Oncological outcomes for vulvar SCC patients, by p16, p53 or ‘other’ immunohistochemistry status.
| Characteristic | p16 (n = 45) | p53 (n = 36) | Others (n = 67) | P | Total |
|---|---|---|---|---|---|
| Duration of Follow-Up (months) | 74 (43–111) | 68 (27–104) | 39 (12–68) | <0.01 | 58 (21–92) |
| Palliative intent | 5 % (2) | 3 % (1) | 25 % (15) | 0.01 | 13 % (18) |
| All-Cause Mortality | 13 % (6) | 47 % (17) | 52 % (35) | <0.01 | 39 % (58) |
| Tumour-Specific Mortality | 2 % (1) | 31 % (11) | 19 % (13) | <0.01 | 17 % (25) |
| Recurrence | 15 % (6) | 64 % (21) | 43 % (26) | <0.01 | 40 % (53) |
4. Discussion
In this retrospective analysis, we identified a correlation between p16/p53 immunohistochemical profile of vulvar SCCs and oncological outcomes, when controlling for other patient, tumour and treatment factors. Of 148 vulvar SCCs diagnosed over a 10-year period, patients with p16 positive vulvar cancers had lower disease-specific mortality and lower risk of recurrence compared to p53 positive vulvar cancers. p53 positive vulvar cancers had more aggressive pathological features at diagnosis (greater depth of stromal invasion, greater tumour diameter, higher LVSI), but the association with oncological outcomes was present even when adjusting for these and demographic factors in a multivariate model.
This dataset encompasses a decade of patient data from 2010 to 2019, with follow-up through January 2024, validating previous literature findings. A meta-analysis of studies evaluating p16 and p53 immunohistochemistry in vulvar cancer outcomes where 475 cases of vulvar SCC were tested for p16 expression (38 % p16 positive) and 310 cases were tested for p53 expression (54 % p53 positive) found that when p16 positive vs negative patients are compared, women with p16 positive vulvar SCC had a significantly more favourable overall survival compared to p16 negative. When p53 positive vs negative patients were compared, women with p53 positive vulvar SCC had a significantly worse overall survival compared to p53 negative with an 80 % higher risk (Sand et al., 2019).
Our project allowed for integration of patient, tumour and treatment factors to investigate independence of p16 and p53 as prognostic markers. While conducting the study over two sites allowed for a better understanding of the impact of patterns of care in treatment and outcomes, there were significant differences in the number of cases where immunohistochemistry staining was performed, and different pathologists conducting and interpreting the results of this testing. There have been calls in the pathology community to standardize p53 immunohistochemistry interpretation, and as such, the variation in our sample is a limitation (Tessier-Cloutier et al., 2020). In addition, the lack of universal immunohistochemical testing was a weakness of this study. We included patients for whom immunohistochemical testing data was not available, not performed or those who tested negative to one or both of p16 or p53, and labelled these as a separate group, ‘other’. The oncological outcomes for this group were between those of the known p16 and p53 cases, representing a group that likely contains patients from both p16 and p53 groups. Whilst this study captured data from patients at two large centres over a long period of time, the sample size was still limited, meaning that confidence intervals were relatively large and sophisticated statistical techniques for handling competing risks could not be employed. Further subgroup analysis into the impact of positive margins and presence of p53 positivity at margins was not possible due to case numbers.
This sample represents a select population from a community with high comorbidities and low socio-economic status. However, when compared to data published by an institution with a different demographic subset of Sydney, these patients were comparable in age, FIGO stage distribution and node involvement (Barlow et al., 2020). There were differences in recurrence rates, although this may be reflective of a longer follow-up time in our study. While FIGO stage data were comparable with this study also conducted in New South Wales, our group included a lower proportion of Stage I and higher numbers of Stage II patients than those from another state in Australia (Tan et al., 2012).
While this study is retrospective in nature, it confirms previous findings of the impact of p16 and p53 in determining prognosis for vulvar SCC patients. The difference in outcomes between p16 and p53 patients may be used to tailor surgical management in the future – this is being investigated through the STRIVE trial, which will use HPV and p53 to stratify surgical management (McAlpine, 2024). In the STRIVE trial, the authors aim for >85 % reporting of p16 and p53 status within 21 days, in order to inform these surgical decisions – our experience was of lower immunohistochemistry reporting rates, particularly at one institution. In addition, a clear difference in risk of recurrence rates seen in our data may be used to justify a future trial comparing different follow-up plans for these groups of patients.
Funding statement
H.O. was awarded a Royal Australian and New Zealand College of Obstetricians and Gynaecologists Women's Health Foundation Research Grant for this project.
CRediT authorship contribution statement
Helena M Obermair: Data curation, Funding acquisition, Investigation, Project administration, Writing – original draft. James Elhindi: Data curation, Software, Validation, Visualization, Writing – review & editing. Alison Brand: Methodology, Resources, Supervision, Writing – review & editing. Unine Herbst: Conceptualization, Funding acquisition, Supervision, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
-
•Westmead Team.
-
•Dr Unine Herbst, Gynaecological Oncologist, Westmead Hospital.
-
•Prof Alison Brand, Gynaecological Oncologist, Westmead Hospital.
-
•James Elhindi, WSLHD Research and Education Network.
-
•
-
•Liverpool Team.
-
•Dr Michelle Harrison (Medical Oncologist) + Dr Karen Lim (Radiation Oncologist), Liverpool Hospital.
-
•Prof Felix Chan + Dr Murad Al-Aker.
-
•
-
•
Pathologists involved in IHC testing.
-
•
RANZCOG NSW State Committee Trainee Research Grant
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.gore.2024.101544.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Alonso I., Fusté V., del Pino M., Castillo P., Torné A., Fusté P., et al. Does human papillomavirus infection imply A different prognosis in vulvar squamous cell carcinoma? Gynecol. Oncol. 2011;122(3):509–514. doi: 10.1016/j.ygyno.2011.05.016. [DOI] [PubMed] [Google Scholar]
- Barlow E.L., Lambie N., Donoghoe M.W., Naing Z., Hacker N.F. The clinical relevance of p16 and p53 status in patients with squamous cell carcinoma of the vulva. J. Oncol. 2020;2020:3739075. doi: 10.1155/2020/3739075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eva L., Sadler L., Thompson J.M., Sahota S., Fong K.L., Jones R.W., et al. HPV-independent and HPV-associated vulvar squamous cell carcinoma: Two different cancers. Int. J. Gynecol. Cancer. 2022 doi: 10.1136/ijgc-2022-003616. [DOI] [PubMed] [Google Scholar]
- Höhn A.K., Brambs C.E., Hiller G.G.R., May D., Schmoeckel E., Horn L.C. 2020 WHO classification of female genital tumors. Geburtshilfe. Frauenheilkd. 2021;81(10):1145–1153. doi: 10.1055/a-1545-4279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAlpine, J.,2024. STRatIfication of Vulvar Squamous Cell Carcinoma by HPV and p53 Status to Guide Excision (STRIVE) ClinicalTrials.gov: ClinicalTrials.gov; [updated 19/08/2024; cited 2024 September 7]. Available from: https://clinicaltrials.gov/study/NCT05576831.
- Proctor L., Hoang L., Moore J., Thompson E., Leung S., Natesan D., et al. Association of human papilloma virus status and response to radiotherapy in vulvar squamous cell carcinoma. Int. J Gynecol. Cancer. 2020;30(1):100–106. doi: 10.1136/ijgc-2019-000793. [DOI] [PubMed] [Google Scholar]
- Sand F.L., Nielsen D.M.B., Frederiksen M.H., Rasmussen C.L., Kjaer S.K. The prognostic value of p16 and p53 expression for survival after vulvar cancer: A systematic review and meta-analysis. Gynecol. Oncol. 2019;152(1):208–217. doi: 10.1016/j.ygyno.2018.10.015. [DOI] [PubMed] [Google Scholar]
- Tan J., Chetty N., Kondalsamy-Chennakesavan S., Crandon A., Garrett A., Land R., et al. Validation of the FIGO 2009 staging system for carcinoma of the vulva. Int. J. Gynecol. Cancer. 2012;22(3):498–502. doi: 10.1097/IGC.0b013e318241d994. [DOI] [PubMed] [Google Scholar]
- Tessier-Cloutier B., Kortekaas K.E., Thompson E., Pors J., Chen J., Ho J., et al. Major p53 immunohistochemical patterns in in situ and invasive squamous cell carcinomas of the vulva and correlation with TP53 mutation status. Mod. Pathol. 2020;33(8):1595–1605. doi: 10.1038/s41379-020-0524-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.