Abstract
The yields of H2O2 and H2 formed in the sonolysis of aqueous solution under noble gas are representative indexes for understanding the chemical effects of ultrasonic cavitation bubbles. In this study, the yields of H2O2 and H2 formed under Ar were evaluated as a function of the concentration of NaCl or KI. When these yields were analyzed by using a normalization technique, it was confirmed that the yields of H2 were more clearly related to Ar solubility than those of H2O2, suggesting that H2 is a more real probe to understand the chemical effects of cavitation bubbles in water. The effects of NaCl on sonochemical formation of oxidants were also compared with those of KI. When aqueous t-butanol solution was sonicated, the yields of H2 and the maximum temperature attained in a collapsing bubble (bubble temperature) decreased with increasing solution temperature and salt concentration, suggesting that these parameters affected the quantity related to the number (and/or size) of active bubbles as well as the quality related to the bubble temperatures.
Keywords: H2O2, H2, NaCl, KI, Butanol, Solution temperature, Bubble temperature
1. Introduction
When bubbles in water are adiabatically collapsed during ultrasonic irradiation, the inside of the bubbles reaches more than several thousand of degrees and several hundred of atmospheres. The bubbles of extremely high-temperature and high-pressure condition are called active bubbles and they are expected to be used in environmental purification technology, nanotechnology, medical treatments, etc. However, the chemical effects of active bubbles have not been fully clarified at present. To evaluate these details, a number of researchers have analyzed various reactions such as the formation of H2O2 and H2 from water sonolysis [1], [2], [3], oxidation of N2 [4], [5], [6], reduction of metal ions [7], [8], [9], degradation of organic compounds [10], [11], [12], degradation of polymers and microcapsules [13], [14], [15], and sonoluminescence and sonochemiluminescence [16], [17], [18]. Experiments have also been conducted under various irradiation conditions, such as the effects of the frequency and intensity of ultrasound, volume of solution, temperature of solution, and type of atmospheric gas. Furthermore, the effects of direct irradiation, indirect irradiation, standing wave formation, dual frequency ultrasound [19], high intensity focused ultrasound [20], and pulsed ultrasound [21] have been studied. So far, it is generally difficult to analyze the characteristics of active bubbles and its relation to chemical reactions, because the changes in the irradiation conditions alter the temperature, pressure, number, and size of active bubbles simultaneously. In addition, the characteristics of bubbles formed are quite diverse. For example, bubbles consist of the mixture of high temperature- and low temperature-bubbles and chemically active bubbles are not always attributed to high temperature-bubbles [22], [23]. Furthermore, the nature and dynamics of ultrasonic cavitation bubbles are influenced by multiple factors even when only one parameter is altered.
It is reported that the addition of inorganic ions or salts to sample solution is effective in enhancing sonochemical degradation rates of organic pollutants. For example, the sonochemical degradation rates of Acid Blue 40, methylene blue, and perfluorooctanoic acid are enhanced by the addition of HCO3– or CO32– [24], [25]. This is because OH radicals formed in the sonolysis of water react with HCO3− or CO32− to produce CO3− radicals, which are available for the degradation of these pollutants. Uddin and Okitsu investigated the effects of NaCl or Na2SO4 additives on the sonochemical degradation rates of several phenolic compounds under Ar during 200 kHz ultrasound irradiation [26]. They suggested that two phenomena are induced simultaneously by adding such salts to aqueous solution. One is that phenolic compounds tend to accumulate at the interface region of cavitation bubbles when such salts are added, resulting in the enhancement of the sonochemical degradation rate. The other is that the solubility of Ar gas in the aqueous solution decreases with increasing salts concentrations, resulting in a decreased sonochemical degradation rate because the number and/or size of active bubbles decreased and thus the rate of OH radical formation decreased. In the nature and dynamics of cavitation bubbles, it has been suggested that the addition of NaCl or Na2SO4 changes the quality (bubble temperature) and quantity (number and/or size) of active bubbles formed [26], [27], [28]. The effects of inorganic salts on active bubbles and sonochemical reactions are complex and thus not yet fully understood at present. Pflieger et al. reported the effect of NaCl on the intensity of sonoluminescence and the yield of H2O2 and H2 in the sonolysis of water under Ar and He, where the solubility of dissolved gas in water affected the global population of active bubbles and Na and Cl atoms influenced the yield of H2O2 and H2 [28]. The intensity of sonoluminescence is an important probe to understand the chemical effects of cavitation bubbles, however, its intensity is often dependent on the condition of collapsing bubble temperature. For example, its intensity may be not sensitive for low temperature bubbles, although such bubbles can influence the chemical reactions. Therefore, to understand the chemical effects of active bubbles, the discussion based on low temperature bubbles are also important.
In this study, the effects of the addition of inorganic salts (NaCl or KI) on the chemical reactions as well as quantity and quality of active bubbles were investigated in water and aqueous t-butanol solution under Ar by using indirect sonication system with 200 kHz ultrasound. NaCl and KI were chosen as additives. NaCl is a representative inorganic salt and is widely used in an inorganic additive in sonochemisty. On the other hand, KI is often used as a reactant to measure the yield of oxidants in sonochemistry, but the research of its salt effect is still limited. The reactions of anions with oxidants were also evaluated. The yield of H2O2 and H2 formed in the sonolysis of water and the yield of H2 formed in the sonolysis of t-butanol solutions at different salt concentration were analyzed in relation to the solubility of Ar gas. In addition, the bubble temperature was estimated from the kinetic analysis of the recombination reaction of methyl (CH3) radicals formed from the sonolysis of t-butanol. The temperature measured by the reaction kinetics could correspond to the average temperature of low temperature bubbles during sonication, but it should be noted that this temperature is very high to proceed the sonolysis of water and t-butanol: low temperature bubbles here could be considered as chemically active bubbles. The effects of solution temperature and addition of inorganic salts on the bubble temperature were discussed in connection with the chemical effects of ultrasonic cavitation.
2. Experimental
2.1. Ultrasound irradiation experiment
All the water used in the experiments was treated using a Millipore system (Milli-Q). For the irradiation of water, 120 mL of aqueous NaCl or KI solution was prepared in a cylindrical reaction vessel (inner diameter: 50 mm) and then bubbled with Ar in a water bath maintained at 20, 30, 40, or 50 °C using a water circulation system (TAITEC CL–150R). The reaction vessel was closed after gas bubbling and then the solution was sonicated in a temperature controlled water bath using a 65 mm ϕ oscillator (Kaijo; N0.91F3, Japan) and an ultrasonic generator (Kaijo 4021 type; No. 533, Japan; frequency: 200 kHz; nominal maximum power: 200 W). The cylindrical reaction vessel was mounted such that the flat bottom of the vessel was 4.0 mm away from the top of the oscillator [29]. The experimental set-up is shown in Fig.S1.
At each irradiation time, the gas sample was withdrawn from the head space of the reaction vessel and then analyzed using a gas chromatograph (GC). At the same time, the solution was withdrawn and then the yield of H2O2 formed was analyzed by a KI colorimetric method using a UV–visible absorption spectrophotometer (Shimadzu UV-2550, Japan) [30]: aqueous potassium hydrogen phthalate (0.10 mol/L) solution labeled as solution A and a mixture of aqueous KI (0.40 mol/L), NaOH (0.050 mol/L), and hexaammonium heptamolybdate (1.6 × 10-4 mol/L) solution labeled as solution B were prepared, respectively. 2.0 mL of solution A and 2.0 mL of solution B were added to 1.0 mL of the sample solution in this order, and then the absorption spectrum of the mixed solution was measured.
For the irradiation of aqueous t-butanol solution, 120 mL of aqueous NaCl or KI solution was prepared and then bubbled with Ar in a water bath at a predetermined temperature. Then, t-butanol was injected to this solution using a microsyringe from a septum to make 10 mmol L-1 t-butanol solution. The reason why t-butanol was added after Ar bubbling is to prevent volatilization of t-butanol during Ar bubbling. The irradiation was performed in the same manner as above. At each irradiation time, the gas sample and/or solution were withdrawn from the vessel and then analyzed using a GC or a UV–visible absorption spectrophotometer.
The yield of H2 was measured by a GC with a thermal conductivity detector (TCD). The yields of CH4, C2H6, C2H4, C2H2, CO, and CO2 were measured by a GC with a flame ionization detector (FID) and a methanizer. The following is the analysis condition for TCD-GC (Shimadzu GC-2014, Japan): column; Molecular sieve 5A, carrier gas; 40 mL min−1 of Ar, reference gas; 40 mL min−1 of Ar, column temperature; 50 ℃, injection volume; 0.5 mL. The following is the analysis condition for FID-GC (Shimadzu GC-14B, Japan): column; Shincarbon ST 50/80, carrier gas; 190 mL min−1 of N2, fuel gas; 100 mL min−1 of H2, combustion aid gas; 50 mL min−1 of air, column temperature; 190 ℃, injection volume; 0.5 mL.
2.2. Estimation of bubble temperature using reaction kinetics
When aqueous t-butanol solution was sonicated, t-butanol molecules undergo a thermal decomposition to form CH3 radicals. The CH3 radicals formed recombine to produce C2H6, C2H4, and C2H2 [31], [32], [33], [34]. The rate constant of the production of C2H6 (k1 = 2.4 × 1014 × T0.4 [M−1 s−1]) and C2H4 (k2 = 1.0 × 1016 × exp (−134000/RT) [M−1 s−1]) under CH3 radicals recombination are temperature dependent reactions. When C2H2 is assumed to be formed from dehydrogenation of C2H4, the bubble temperature can be estimated by analyzing the ratio of the yields of these compounds. In this study, the bubble temperature obtained at t min irradiation was defined as the average temperature of all the bubbles that generated from the start of irradiation (0 min irradiation) to t min irradiation.
2.3. Calculation of Ar solubility
When the solubility of Ar in pure water and in aqueous solution containing ions is α0 and α, respectively, the following equation has been reported [35]: log (α0/α) = h × I, where h represents the value specific to dissolved ions or gases, and I represents the ionic strength of the electrolyte. By using this equation, the solubility of Ar in aqueous NaCl solution of N (mol/L) can be calculated as α0/(100.1434N), and the solubility of Ar in aqueous KI solution of N’ (mol/L) can be calculated as α0/(100.1136N’) [36]. Details of calculation for the aqueous NaCl solution are described in Supplementary data.
3. Results and discussion
3.1. Effect of NaCl on H2O2 and H2 formation
When aqueous solution is sonicated under Ar, OH and H radicals are formed by the pyrolysis of water in high-temperature and high-pressure bubbles (active bubbles) and thus H2O2, H2 and H2O are formed by their recombination as follows.
| H2O → OH + H | (1) |
| 2OH → H2O2 | (2) |
| 2H → H2 | (3) |
| OH + H → H2O | (4) |
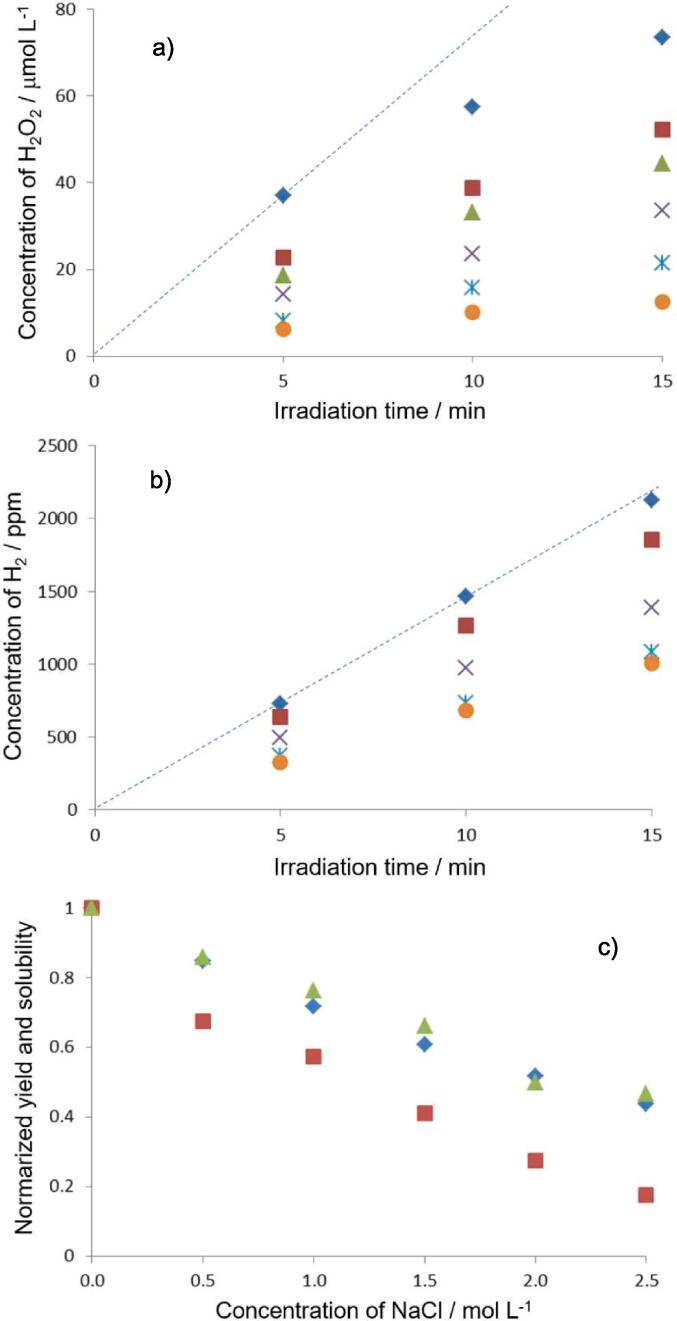
Figs. 1a and 1b show the yields of H2O2 and H2 versus ultrasound irradiation time for each NaCl concentration under Ar. Fig. S2a and S2b show the yields of H2O2 and H2 formed for each NaCl concentration at 10 min irradiation. From Fig. 1a and S1a, it was confirmed that the yield of H2O2 decreased as the NaCl concentration increased. Furthermore, Fig S2c and Fig.S2d show the formation rates of H2O2 and H2 at different irradiation time, respectively. In these figures, for the rate at 2.5 min, the average rate was calculated from the data of 0 min and 5 min irradiation. For the rate at 7.5 min, the average rate was calculated from 5 min to 10 min irradiation. Fig. 1a and Fig. S2c show that the yield of H2O2 increased with irradiation time, but the formation rate of H2O2 tended to decrease as the irradiation time increased. In contrast, from Figs. 1b and S2d, it was confirmed that the yield of H2 decreased as the NaCl concentration increased, but the formation rate of H2 remained almost constant regardless of the irradiation time: the behavior of the yield of H2 was different from that of H2O2.
Fig. 1.
Yield of a) H2O2 and b) H2 formed by sonolysis of water at different NaCl concentration. ( ): 0 mol/L, (
): 0 mol/L, ( ): 0.5 mol/L, (
): 0.5 mol/L, ( ): 1.0 mol/L, (
): 1.0 mol/L, ( ): 1.5 mol/L, (
): 1.5 mol/L, ( ): 2.0 mol/L, (
): 2.0 mol/L, ( ): 2.5 mol/L. c) Normalized yield of H2O2 (
): 2.5 mol/L. c) Normalized yield of H2O2 ( ) and H2 (
) and H2 ( ) and normalized Ar solubility (
) and normalized Ar solubility ( ) at different NaCl concentration. Sonication time: 10 min.
) at different NaCl concentration. Sonication time: 10 min.
Fig. 2.
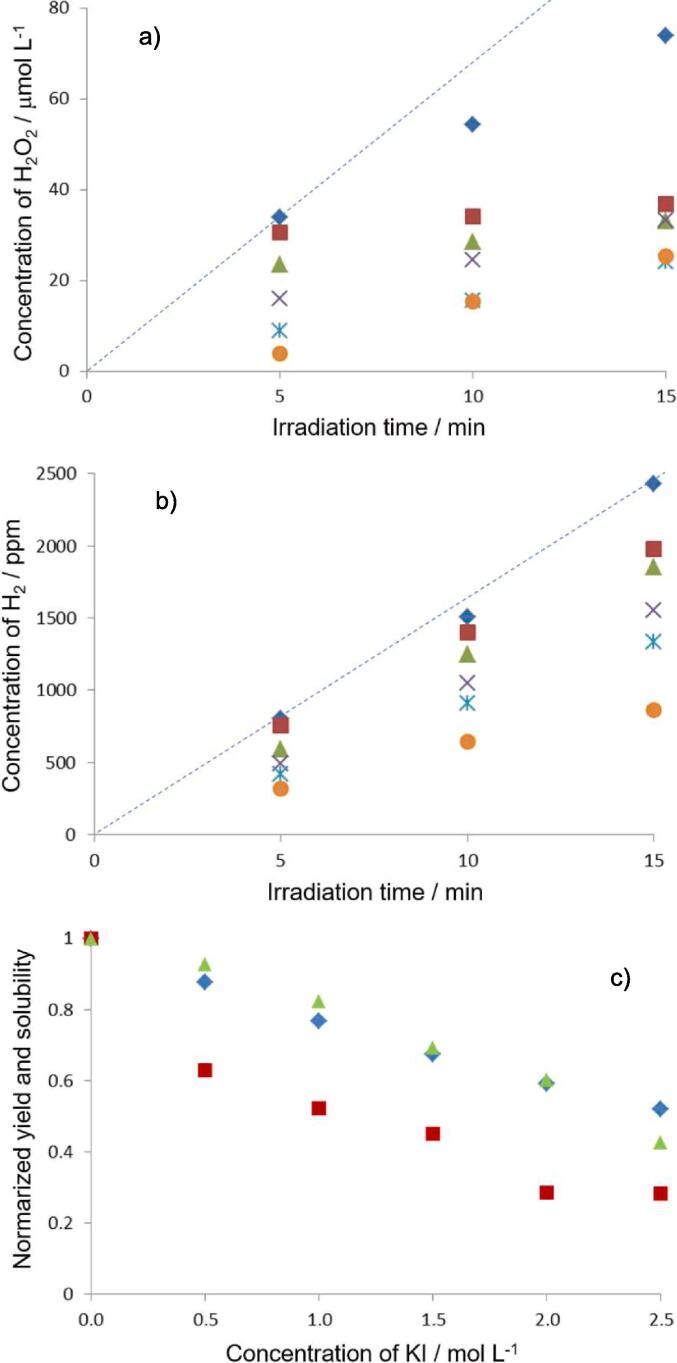
Yield of a) H2O2 and b) H2 formed by sonolysis of water at different KI concentration. ( ): 0 mol/L, (
): 0 mol/L, ( ): 0.5 mol/L, (
): 0.5 mol/L, ( ): 1.0 mol/L, (
): 1.0 mol/L, ( ): 1.5 mol/L, (
): 1.5 mol/L, ( ): 2.0 mol/L, (
): 2.0 mol/L, ( ): 2.5 mol/L. c) Normalized yield of H2O2 (
): 2.5 mol/L. c) Normalized yield of H2O2 ( ) and H2 (
) and H2 ( ) and normalized Ar solubility (
) and normalized Ar solubility ( ) at different KI concentration. Sonication time: 10 min.
) at different KI concentration. Sonication time: 10 min.
One possible reason for the decrease in the formation of H2O2 and H2 with increasing NaCl concentration would be the decrease in Ar solubility in solution due to the addition of NaCl. Previous studies have suggested that as the amount of dissolved gas in solution decreases, the number of chemically active bubbles formed decreases, resulting in a decrease in the progress of the pyrolysis of water [26], [28]. On the other hand, when the concentration of salts increased and the amount of dissolved gas decreased, the following phenomena would be induced [28], [37]: 1) bubble–bubble coalescence is retarded in a salt solution so that the smaller size of a bubble tends to exist in a salt solution, 2) the symmetrical collapse of a bubble occurs, and 3) stronger standing wave is formed. These phenomena could increase the formation of higher temperature bubbles which emit stronger sonoluminescence. However, it is also suggested that these phenomena cannot be linked to the chemical effects of active bubbles [28]. This would be because the chemical effects are induced by relatively lower temperature bubbles compared to higher temperature bubbles.
To discuss the chemical effects of active bubbles, the amount of Ar gas in each NaCl concentration was calculated and compared to the yield of H2O2 and H2 formed, respectively. Fig. 1c shows the Ar solubility in NaCl solution and the yield of H2O2 and H2 formed in NaCl solution, where these values are normalized to pure water. Fig.S2e and S2f also show the results obtained at 5 min and 15 min irradiation. It was observed that the yields of H2O2 were clearly lower than those of H2 and the relationship of the Ar solubility to the yield of H2 showed a better correlation than that to the yield of H2O2. Based on equations (2) and (3), the same amounts of H2O2 and H2 should be formed, but this was not the case in this study. For example, the results at 2.0 mol/L NaCl solution in Fig. S2a and S2b show that the yield of H2O2 was about 27 % of that in pure water, whereas the yield of H2 was about 50 % of that in pure water. Based on the report of Pfliegar et al. [28], it is probable that the reactions of Na and Cl atoms with H, H2, OH, H2O2, and H2O affect the yields of H2 and H2O2, where Na and Cl atoms could be formed in active bubbles under a high temperature condition. In this study, since it was difficult to discuss the effect of these atoms, we tried to discuss the reactions of anions with oxidants and the decomposition of oxidants.
First, we consider the reaction of OH radicals with Cl− ions in the presence of NaCl to discuss the reason why the yield of H2O2 was lower than that of H2. It is well known that the radiolysis exhibits similar chemical effects as the sonolysis. For example, OH radicals, H radicals, and solvated electrons are formed from the γ-ray radiolysis of water. When an aqueous NaCl solution was irradiated with γ-rays, the OH radicals formed could react with Cl− ions in the solution as equation (5) [38].
| (5) |
The reaction rate constants in equations (2) and (5) are 6.0 × 109 mol−1 s−1 and 4.3 × 109 mol−1 s−1, respectively. In the present study, the KI method was used for the colorimetric analysis of H2O2 (equation (6)). Therefore, during sonication, even if OH was converted to Cl as equation (5) or Cl was further reacted to Cl2 as equation (7), the reactions in equations (8), (9), (10), (11) would proceed: Cl and Cl2 would be always converted to I3- in the colorimetric analysis.
| (6) |
| (7) |
| (8) |
| (9) |
| (10) |
| (11) |
Taking into account equations (8), (9), (10), (11), the yield of I3− could include the yields of oxidants of Cl and Cl2. Therefore, it is difficult to explain the differences in the yields of H2O2 and H2 observed in Figs. 1 and S2 when using the KI colorimetric method. This is also discussed in the later section. One possible reason is that some of the formed H2O2 molecules could be decomposed during irradiation: the following equations (12), (13) could proceed based on the results of shock tube studies [39].
| (12) |
| (13) |
where M is the third body. In addition, OH radical and H2O2 may be consumed as equations (14), (15) [40], [41].
| (14) |
| (15) |
In addition, Yasui et al. suggested that the equation (16) would proceed inside a bubble and at the gas–liquid interface region [42].
| (16) |
Taking into account the lifetime of H2O2 in liquid water [43], the decomposition of H2O2 in liquid water could not occur naturally. On the other hand, it is reported that the equation (14) could proceed in aqueous H2O2 solution during hydrodynamic cavitation [44]. Therefore, it is probable that H2O2 formed in the solution could be also decomposed during ultrasonic cavitation.
In contrast, since the formed H2 molecules can escape from the solution to the gas phase in reaction vessel, they could not be consumed by chemical reactions in the solution phase.
3.2. Effect of KI on H2O2 and H2 formation
Next, the effect of KI instead of NaCl was investigated. Fig. 2a and 2b show the yields of H2O2 and H2 formed versus ultrasound irradiation time for each KI concentration under Ar. Figs. S3a and S3b show the yields of H2O2 and H2 formed for each KI concentration at 10 min irradiation. It was observed that the behaviors of the yields of H2O2 and H2 were the same tendency as those with NaCl.
In the experiment using NaCl solution, the progress of equations (7), (8), (9), (10), (11) was not able to be confirmed visually during sonication, because the KI colorimetric method was performed after sonication. In contrast, in the experiment using KI solution instead of NaCl solution, the change in color characteristic of I3− (progress of equations (17), (9), and (11)) was confirmed visually just during sonication, because I− existed already in the sonicated solution.
| (17) |
The Ar solubility in KI solution was calculated using the same method as in NaCl solution. Fig. 2c shows the Ar solubility and the yields of H2O2 and H2 for each KI concentration, where these values are normalized to pure water. It was confirmed that there was a good correlation between the Ar solubility and the yield of H2, but there was no good correlation with Ar solubility to the yield of H2O2. This result was the same trend as in NaCl solutions. Therefore, it can be considered that H2 could escape from the solution to the gas phase in reaction vessel and thus no further chemical reaction of H2 could proceed upon irradiation, while some H2O2 formed was consumed during ultrasonic irradiation as explained in equations (12), (13), (14), (15), (16). Based on the obtained results, the yield of H2 may be one of the indicators to understand the chemical effects of active Ar bubbles.
3.3. Effect of solution temperature on bubble temperature
The temperature attained in collapsing bubbles (bubble temperature) has been estimated by reaction kinetics. The bubble temperature should be the important information for understanding the chemical effects of active bubbles. Hart et al. sonicated Ar-CH4-dissolved water and estimated the bubble temperature to be 2000–2800 K based on the pyrolysis products of CH4, where the recombination kinetics of CH3 radicals were used [31]. Tauber et al. sonicated aqueous Ar-dissolved t-butanol solutions and estimated the bubble temperature to be 2300–3600 K based on the pyrolysis products of t-butanol [32]. Similarly, Rae et al. estimated the temperature to be 2300–4600 K using several alcohols [33]. In contrast, Suslick et al. estimated the temperature to be 5200 ± 650 K based on the kinetics of the reaction of metal carbonyl ligand substitution in organic solvents [45]. Although the studies which analyze the bubble temperature have been done so far, the causal relationship between the characteristics of bubbles and their chemical effects has not been clarified sufficiently [26], [27], [28].
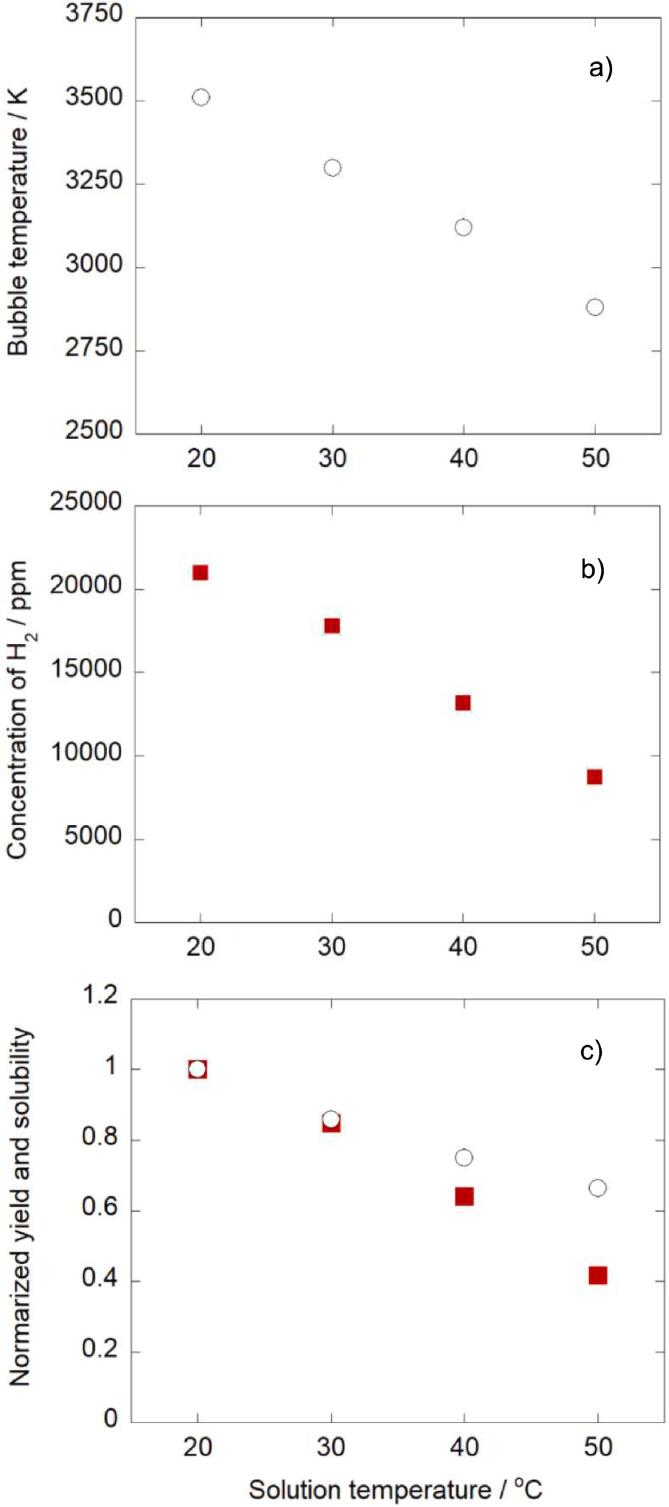
The solution temperature is the important parameter in sonochemistry, because it affects both of the amount of dissolved Ar gas in water and the amount of water vapor in bubbles. These amounts should affect simultaneously the quantity (number and/or size) and quality (high temperature and pressure) of the active bubbles formed. To discuss these ideas, 10 mmol L-1 t-butanol solution was sonicated at different solution temperature. The bubble temperature estimated is shown in Fig. 3a. Further data are also shown in Supplementary data (Fig.S4). It was clearly observed that the bubble temperature decreased as the solution temperature increased.
Fig. 3.
Effect of solution temperature on a) bubble temperature and b) yield of H2. c) Normalized yield of H2 ( ) and normalized Ar solubility (○) at different solution temperature. t-butanol concentration: 10 mmol/L, sonication time: 10 min.
) and normalized Ar solubility (○) at different solution temperature. t-butanol concentration: 10 mmol/L, sonication time: 10 min.
The yield of H2 is shown in Fig. 3b, where H2 is the main product in the sonolysis of aqueous t-butanol solution. From Fig. 3b, it was confirmed that the yield of H2 decreased with increasing solution temperature. The reasons for the decrease in the bubble temperature and the decrease in the H2 yield with increasing solution temperature would be because the increase in solution temperature decreased the Ar solubility in solution and increased the vapor pressure of water and t-butanol in Ar bubbles, which could decrease the quantity (number and/or size) and quality (temperature and pressure) of active bubbles simultaneously.
The maximum bubble temperature (Tmax) can be estimated using the following adiabatic compression equation:
| (18) |
where T0 is the initial temperature, R0 is the initial bubble radius, Rmin is the minimum bubble radius, and γ is the specific heat ratio. For example, the γ value of Ar (1.668 at 15 °C) is larger than that of H2O (1.324 at 100 °C) [46]. Considering these γ values, Tmax related to the quality of active bubbles will decrease with an increasing amount of H2O vapor in Ar bubbles. Since t-butanol is the molecule consisting of 15 atoms, it has the smaller γ value than H2O. Although the concentration of t-butanol is low, t-butanol molecules could affect Tmax in the same way as water molecules. In addition, the bubble temperature would be cooled when the endothermic reaction of water dissociation proceeds [47]. In this study, the effect of the endothermic reaction on the bubble temperature was unclear.
Fig. 3c shows the Ar solubility and the yield of H2, where these values are normalized to the values at 20 °C. Although both the Ar solubility and the yield of H2 decreased with increasing solution temperature, these values did not show the perfect correlation. This result cannot be explained only by the change in the quantity of bubbles, but also explained by the occurrence of the change in the quality of bubbles.
3.4. Effects of inorganic salts on the sonolysis of aqueous t-butanol solution and bubble temperature
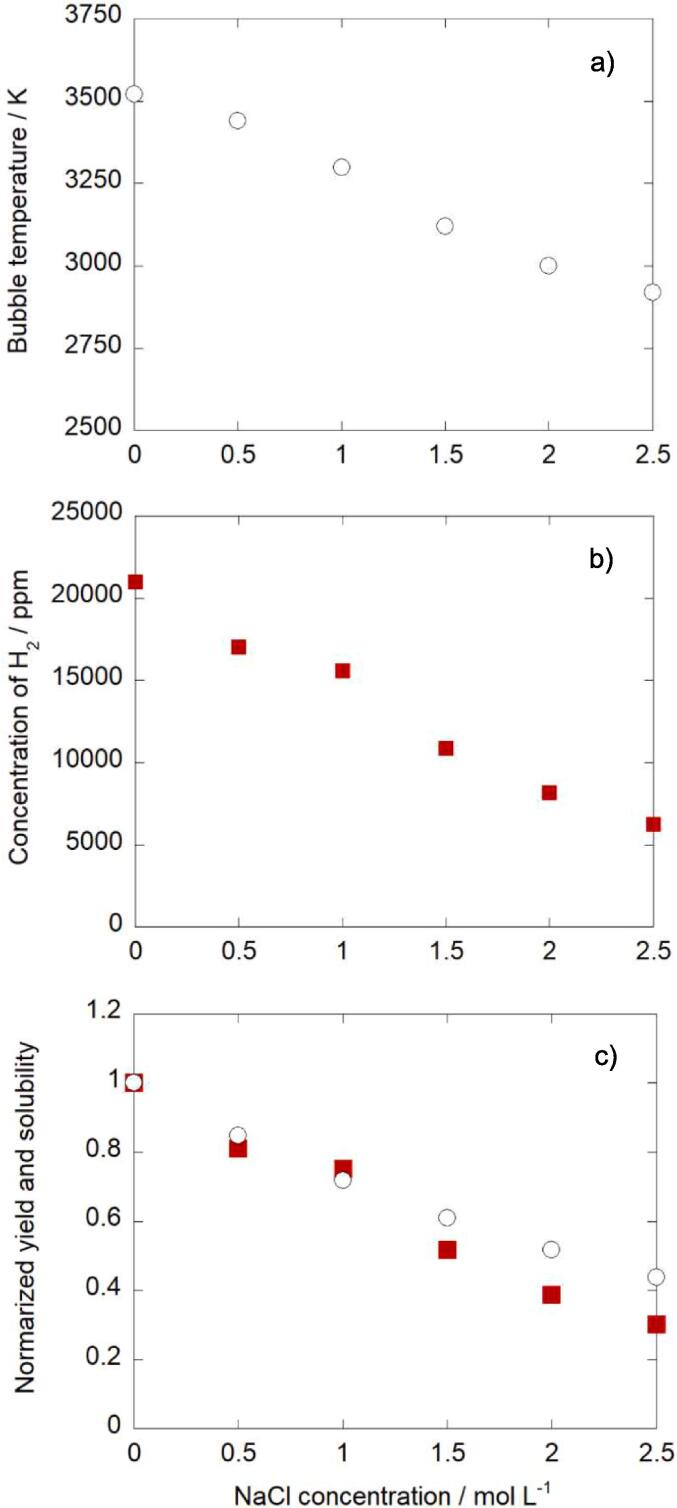
The effects of NaCl addition on the sonolysis of aqueous t-butanol solution and the bubble temperature were investigated. Fig. 4a shows the bubble temperature at 10 min irradiation versus NaCl concentration. Further data are also shown in Supplementary data (Fig.S4). It was confirmed that the bubble temperature decreased with increasing NaCl concentration. Fig. 4b shows the yield of H2 formed from the sonolysis of aqueous t-butanol solution. In the absence of NaCl, it should be noted that the yield of H2 from aqueous t-butanol solution was about 14 times higher than that of H2 from water (Fig. 1b): the sonolysis of t-butanol occurred much more effectively than that of water. This should be because the bond energy of C-H in butanol is lower than that of O-H in water. In addition, from Fig. 4b, the yield of H2 decreased with increasing NaCl concentration. Fig. 4c shows the Ar solubility and the yield of H2 for each NaCl concentration. Although both the Ar solubility and the yield of H2 decreased with increasing NaCl concentration, no perfect correlation was observed between them. This result corresponded well with the results of the effect of solution temperature (Fig. 3c), where the changes in the quality (temperature and pressure) of bubbles occurred in addition to the changes in the quantity (number and/or size) of bubbles. The same phenomenon was observed when KI was added instead of NaCl. The results using KI are shown in Supplementary Data (Figs. S4–S7).
Fig. 4.
Effect of NaCl concentration on a) bubble temperature and b) yield of H2. c) Normalized yield of H2 ( ) and normalized Ar solubility (○) at different NaCl concentration. t-butanol concentration: 10 mmol/L, sonication time: 10 min.
) and normalized Ar solubility (○) at different NaCl concentration. t-butanol concentration: 10 mmol/L, sonication time: 10 min.
Next, we consider the progress of the pyrolysis reactions of water and t-butanol. When aqueous t-butanol solution is sonicated, both water and t-butanol in and/or at the interface region of active bubbles are pyrolyzed as equations (1) and (19).
| (19) |
Water and t-butanol have the vapor pressures of 2.33 kPa and 5.52 kPa at 20 °C, respectively [48]. Firstly, we considered that the pyrolysis reactions of t-butanol would occur as equation (19) so that no CO2 would be formed. However, the experimental results showed that CO2 was formed as much as 5 vol% of CO, suggesting that OH radicals formed in equation (1) react with t-butanol and its degradation products, producing CO2. To confirm whether OH radicals react with t-butanol and its degradation products, aqueous t-butanol solution was sonicated and then a KI colorimetric method was conducted to analyze the formation of H2O2. Since no formation of H2O2 was confirmed, OH radicals reacted quickly with t-butanol and its degradation products.
Previous studies have reported that the amount of dissolved gas in solution was closely related to the chemical effects of active bubbles: a good correlation between the amount of dissolved gas and the yield of H2O2 was observed, when water was sonicated under He, Ne, Ar, Kr, and Xe [34]. In addition, the amount of the dissolved gas affected the rate of 1-hexanol degradation and rate of Au(III) reduction [49]. In the present study, it was clear that the bubble temperature decreases with increasing solution temperature and inorganic salt (NaCl or KI) concentration and these parameters affect not only the quantity (number and/or size) of active bubbles, but also the quality (temperature and pressure) of the bubbles. Taking into account that a large number of active bubbles with different size are formed transiently and repeatedly in a sonicated solution [2], [50] and the bubbles consist of the mixture of high temperature- and low temperature-bubbles [22], [23], [28], the characteristics of ultrasonic cavitation bubbles are very complex. Further studies related to the quantity and quality of active bubbles are needed to clarify the real of ultrasonic cavitation bubbles.
4. Conclusion
The chemical effects of active Ar bubbles in water were evaluated by analyzing the yields of H2 and H2O2. These yields decreased with increasing solution temperature and salt concentration of NaCl and KI. It was suggested that the yield of H2 was more reasonable compared with that of H2O2, because OH radical and H2O2 could be consumed by further reactions. Therefore, the yield of H2 could be a precise indicator to understand the chemical effects of active Ar bubbles. A normalization technique for the yields of H2O2 and H2 and the amount of Ar gas in solution was also effective to discuss the change in the quantity and quality of active bubbles. The reaction of OH with I− was more clearly confirmed in comparison with that of OH with Cl−. In the sonolysis of aqueous t-butanol solution, the temperatures attained in collapsing bubbles and the yields of H2 were evaluated under different solution temperature and salt concentration. It was suggested that the temperature (related to the quality) of active bubbles and the number and/or size (related to the quantity) of active bubbles decreased with increasing inorganic salts concentration. Since the quantity and quality of active bubbles often change simultaneously and complexly, it is important to develop an effective analytical probe to evaluate both of those changes in the future.
CRediT authorship contribution statement
Yuki Nakata: Writing – review & editing, Writing – original draft, Investigation. Yoshiteru Mizukoshi: Writing – review & editing, Investigation. Kenji Okitsu: Writing – review & editing, Writing – original draft, Supervision, Investigation, Funding acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Kenji Okitsu acknowledges the support of JSPS KAKENHI Grant Number 17K06908.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ultsonch.2024.107146.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Son Y., Seo J. Effects of gas saturation and sparging on sonochemical oxidation activity in open and closed systems, Part I: H2O2 generation. Ultrason. Sonochem. 2022;90 doi: 10.1016/j.ultsonch.2022.106214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dehane A., Merouani S., Hamdaoui O., Yasui K., Ashokkumar M. A hydrogen-based technique for determining the number density of acoustic microreactors (actives bubbles) in sonicated solutionsInt. J. Hydrogen Energy. 2023;46:13430–13441. [Google Scholar]
- 3.Asakura Y., Yasuda K. Frequency and power dependence of the sonochemical reaction. Ultrason. Sonochem. 2021;81 doi: 10.1016/j.ultsonch.2021.105858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wakeford C.A., Blackburn R., Lickiss P.D. Effect of ionic strength on the acoustic generation of nitrite, nitrate and hydrogen peroxide. Ultrason. Sonochem. 1999;6:141–148. [Google Scholar]
- 5.Misik V., Riesz P. Nitric oxide formation by ultrasound in aqueous solution. J. Phys. Chem. 1996;100:17986–17994. [Google Scholar]
- 6.Okitsu K., Kunichika R., Asada S. Quantitation and evaluation of NO2−, NO3−, and H2O2 in the sonolysis of aqueous NaOH solution under air and air-Ar mixture: effects of solution temperature, ultrasonic power, and ratio of gas mixture. Ultrason. Sonochem. 2023;100 doi: 10.1016/j.ultsonch.2023.106612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Okitsu K., Kurisaka I., Nanzai B., Takenaka N., Bandow H. Mechanism for sonochemical reduction of Au(III) in aqueous butanol solution under Ar based on the analysis of gaseous and water-soluble products. Ultrason. Sonochem. 2020;69 doi: 10.1016/j.ultsonch.2020.105241. [DOI] [PubMed] [Google Scholar]
- 8.Yasuda K., Iwata T., Mizuno Y., Yamamoto Y. Synthesis of Au@Pd core–shell nanoparticles by ultrafine bubbles and ultrasound without capping and reducing agents. Chem. Lett. 2024;53:145. [Google Scholar]
- 9.Yasui K. The Reducing Agents in Sonochemical Reactions without Any Additives. Molecules. 2023;28:4198. doi: 10.3390/molecules28104198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hamdaoui O. General analytical solution expressions for analyzing Langmuir-type kinetics of sonochemical degradation of nonvolatile organic contaminants in water. Ultrason. Sonochem. 2023;98 doi: 10.1016/j.ultsonch.2023.106536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fagan W.P., Villamena F.A., Zweier J.L., Weavers L.K. In situ EPR spin trapping and competition kinetics demonstrate temperature-dependent mechanisms of synergistic radical production by ultrasonically activated persulfate. Environ. Sci. Technol. 2022;56:3729–3738. doi: 10.1021/acs.est.1c08562. [DOI] [PubMed] [Google Scholar]
- 12.Ryu B., Wong K.T., Choong C.E., Kim J.-R., Kim H., Kim S.-H., Jeon B.-H., Yoon Y., Snyder S.A., Jang M. Degradation synergism between sonolysis and photocatalysis for organic pollutants with different hydrophobicity: A perspective of mechanism and application for high mineralization efficiency. J. Hazard. Mater. 2021;416 doi: 10.1016/j.jhazmat.2021.125787. [DOI] [PubMed] [Google Scholar]
- 13.Henglein A., Gutierrez M. Sonolysis of polymers in aqueous solution. New observations on pyrolysis and mechanical degradation. J. Phys. Chem. 1988;92:3705–3707. [Google Scholar]
- 14.Price G.J., Smith P.F. Ultrasonic degradation of polymer solutions: 2. The effect of temperature, ultrasound intensity and dissolved gases on polystyrene in toluene. Polymer. 1993;34:4111. [Google Scholar]
- 15.Inui A., Honda A., Yamanaka S., Ikeno T., Yamamoto K. Effect of ultrasonic frequency and surfactant addition on microcapsule destruction. Ultrason. Sonochem. 2021;70:10538. doi: 10.1016/j.ultsonch.2020.105308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Didenko Y.T., McNamara W.B., Suslick K.S. Temperature of multibubble sonoluminescence in water. J. Phys. Chem. A. 1999;103:10783–10788. [Google Scholar]
- 17.Yusof N.S.M., Anandan S., Sivashanmugam P., Flores E.M.M., Ashokkumar M. A correlation between cavitation bubble temperature, sonoluminescence and interfacial chemistry – A minireview. Ultrason. Sonochem. 2022;85 doi: 10.1016/j.ultsonch.2022.105988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yasui K., Tuziuti T., Kozuka T., Towata A., Iida Y. Relationship between the bubble temperature and main oxidant created inside an air bubble under ultrasound. J. Chem. Phys. 2007;127 doi: 10.1063/1.2790420. [DOI] [PubMed] [Google Scholar]
- 19.Zare M., Alfonso-Muniozguren P., Bussemaker M.J., Sears P., Serna-Galvis E.A., Torres-Palma R.A., Lee J. A fundamental study on the degradation of paracetamol under single- and dual-frequency ultrasound. Ultrason. Sonochem. 2023;94 doi: 10.1016/j.ultsonch.2023.106320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yusof N.S.M., Ashokkumar M. Sonochemical synthesis of gold nanoparticles by using high intensity focused ultrasound. Chem. Phys. Chem. 2015;16:775–781. doi: 10.1002/cphc.201402697. [DOI] [PubMed] [Google Scholar]
- 21.Deojay D.M., Sostaric J.Z., Weavers L.K. Exploring the effects of pulsed ultrasound at 205 and 616 kHz on the sonochemical degradation of octylbenzene sulfonate. Ultrason. Sonochem. 2011;18:801–809. doi: 10.1016/j.ultsonch.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 22.Abe S., Choi P.-K. Spatiotemporal Separation of Na-Atom Emission from Continuum Emission in Sonoluminescence. Jpn. J. Appl. Phys. 2009;48:07GH02. [Google Scholar]
- 23.Ashokkumar M., Lee J., Iida Y., Yasui K., Kozuka T., Tuziuti T., Towata A. Spatial distribution of acoustic cavitation bubbles at different ultrasound frequencies. Chem. Phys. Chem. 2010;11:1680–1684. doi: 10.1002/cphc.200901037. [DOI] [PubMed] [Google Scholar]
- 24.Minero C., Pellizzari P., Maurino V., Pelizzetti E., Vione D. Enhancement of dye sonochemical degradation by some inorganic anions present in natural waters. Appl. Catal. B Environ. 2008;77:308–316. [Google Scholar]
- 25.Thi L.-A.-P., Do H.-T., Lo S.-L. Enhancing decomposition rate of perfluorooctanoic acid by carbonate radical assisted sonochemical treatment. Ultrason. Sonochem. 2014;21:1875–1880. doi: 10.1016/j.ultsonch.2014.03.027. [DOI] [PubMed] [Google Scholar]
- 26.Uddin H.M., Nanzai B., Okitsu K. Effects of Na2SO4 or NaCl on sonochemical degradation of phenolic compounds in an aqueous solution under Ar: positive and negative effects induced by the presence of salts. Ultrason. Sonochem. 2016;28:144–149. doi: 10.1016/j.ultsonch.2015.06.028. [DOI] [PubMed] [Google Scholar]
- 27.Pflieger R., Lee J., Nikitenko S.I., Ashokkumar M. Influence of He and Ar Flow Rates and NaCl Concentration on the Size Distribution of Bubbles Generated by Power Ultrasound. J. Phys. Chem. B. 2015;119:12682–12688. doi: 10.1021/acs.jpcb.5b08723. [DOI] [PubMed] [Google Scholar]
- 28.Pflieger R., Nikitenko S.I., Ashokkumar M. Effect of NaCl salt on sonochemistry and sonoluminescence in aqueous solutions. Ultrason. Sonochem. 2019;59 doi: 10.1016/j.ultsonch.2019.104753. [DOI] [PubMed] [Google Scholar]
- 29.Okitsu K., Yue A., Tanabe S., Matsumoto H., Yobiko Y., Yoo Y. Sonolytic Control of Rate of Gold(III) Reduction and Size of Formed Gold Nanoparticles in an Aqueous Solution: Relation Between Reduction Rates and Sizes of Formed Nanoparticles. Bull. Chem. Soc. Jpn. 2002;75:2289–2296. [Google Scholar]
- 30.Alegria A.E., Lion Y., Kondo T., Riesz P. Sonolysis of aqueous surfactant solutions. Probing the interfacial region of cavitation bubbles by spin trapping. J. Phys, Chem. 1989;93:4908–4913. [Google Scholar]
- 31.Hart E.J., Fischer C.-H., Henglein A. Sonolysis of hydrocarbons in aqueous solution. Radiat. Phys. Chem. 1990;36:511–516. [Google Scholar]
- 32.Tauber A., Mark G., Schuchmann H.-P., von Sonntag C. Sonolysis of tert-butyl alcohol in aqueous solution. J. Chem. Soc., Perkin Trans. 1999;2:1129–1135. [Google Scholar]
- 33.Rae J., Ashokkumar M., Eulaerts O., von Sonntag C., Reisse J., Grieser F. Estimation of ultrasound induced cavitation bubble temperatures in aqueous solutions. Ultrason. Sonochem. 2005;12:325–329. doi: 10.1016/j.ultsonch.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 34.Okitsu K., Suzuki T., Takenaka N., Bandow H., Nishimura R., Maeda Y. Acoustic Multibubble Cavitation in Water: A New Aspect of the Effect of a Rare Gas Atmosphere on Bubble Temperature and Its Relevance to Sonochemistry. J. Phys. Chem. b. 2006;110:20081–20084. doi: 10.1021/jp064598u. [DOI] [PubMed] [Google Scholar]
- 35.Clever H.L. Sechenov salt-effect parameter. J. Chem. Eng. Data. 1983;28:340–343. [Google Scholar]
- 36.Lang W., Zander R. Salting-out of oxygen from aqueous electrolyte solutions: Prediction and measurement. Ind. Eng. Chem. Fundam. 1986;25:785. [Google Scholar]
- 37.Yasui K. Springer; Cham, Switzerland: 2018. Acoustic Cavitation and Bubble Dynamics. [Google Scholar]
- 38.Jayson G.G., Parsons B.J., Swallow A.J. Some simple, highly reactive, inorganic chlorine derivatives in aqueous solution. Their formation using pulses of radiation and their role in the mechanism of the Fricke dosimeter, J. Chem. Soc. Faraday Transactions. 1973;1(69):1597–1607. [Google Scholar]
- 39.Hong Z., Farooq A., Barbour E.A., Davidson D.F., Hanson R.K. Hydrogen Peroxide Decomposition Rate: A Shock Tube Study Using Tunable Laser Absorption of H2O near 2.5 μm. J. Phys. Chem. A. 2009;113:12919–12925. doi: 10.1021/jp907219f. [DOI] [PubMed] [Google Scholar]
- 40.Hong Z., Cook R.D., Davidson D.F., Hanson R.K. A Shock Tube Study of OH + H2O2 → H2O + HO2 and H2O2 + M → OH + M using Laser Absorption of H2O and OH. J. Phys. Chem. A. 2010;114:5718–5727. doi: 10.1021/jp100204z. [DOI] [PubMed] [Google Scholar]
- 41.Hong Z., Vasu S.S., Davidson D.F., Hanson R.K. Experimental Study of the Rate of OH + HO → H2O + O2 at High Temperatures Using the Reverse Reaction. J. Phys. Chem. A. 2010;114:5520–5525. doi: 10.1021/jp100739t. [DOI] [PubMed] [Google Scholar]
- 42.Yasui K., Tuziuti T., Sivakumar M., Iida Y. Theoretical study of single-bubble sonochemistry. J. Chem. Phys. 2005;122 doi: 10.1063/1.1925607. [DOI] [PubMed] [Google Scholar]
- 43.Yasui K. Production of O Radicals from Cavitation Bubbles under Ultrasound. Molecules. 2022;27:4788. doi: 10.3390/molecules27154788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang X., Wang J., Guo P., Guo W., Wang C. Degradation of rhodamine B in aqueous solution by using swirling jet-induced cavitation combined with H2O2. J. Hazard. Mater. 2009;169:486–491. doi: 10.1016/j.jhazmat.2009.03.122. [DOI] [PubMed] [Google Scholar]
- 45.Suslick K.S., Hammerton D.A., Cline R.E., Jr. Sonochemical hot spot. J. Am. Chem. Soc. 1986;108:5641–5642. [Google Scholar]
- 46.Kagaku-binran (1993), II-234-235. Ed by The Chemical Society of Japan, Maruzen, Japan.
- 47.Yasui K. Effect of liquid temperature on sonoluminescence. Phys. Rev. E. 2001;64 doi: 10.1103/PhysRevE.64.016310. [DOI] [PubMed] [Google Scholar]
- 48.Kagaku Binran (1993), II-132, Ed by The Chemical Society of Japan, Maruzen, Japan. Kagaku Binran, II-123 . Maruzen; Japan: 1993. Ed by The Chemical Society of Japan. [Google Scholar]
- 49.Okitsu K., Kurisaka I., Nanzai B., Takenaka N., Bandow H. Sonochemistry of Aqueous NaAuCl4 Solutions with C3–C6 Alcohols Under a Noble Gas Atmosphere. Ultrason. Sonochem. 2018;41:397–403. doi: 10.1016/j.ultsonch.2017.09.040. [DOI] [PubMed] [Google Scholar]
- 50.Brotchie A., Grieser F., Ashokkumar M. Effect of power and frequency on bubble-size distributions in acoustic cavitation. Phys. Rev. Lett. 2009;102 doi: 10.1103/PhysRevLett.102.084302. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.