Abstract
Dicephalic parapagus conjoined twins, a rarely occurring form of conjoined twinning has poor prognosis and remains a significant cause of perinatal deaths. Since majority of cases of conjoined twins are not compatible with life, early and reliable detection with diagnostic medical imaging remains crucial for adequate patient counselling, medical and surgical management. We present a case of dicephalic parapagus twin gestation with associated congenital anomalies detected for the first time with ultrasound in the third trimester in a 29-year-old pregnant woman. An initial first trimester ultrasound at 14 weeks gestational age was unremarkable.
Ultrasound fetal anatomical survey in the third trimester remains reliable for the detection of rare fetal anomalies such as dicephalic parapagus twins that might have been missed in earlier scans. Continuous efforts should therefore be made for the inclusion of detailed anatomical survey in the early third trimester; particularly in women with unremarkable earlier ultrasound scans and in those reporting late for obstetric care.
Keywords: Conjoined twins, Ultrasound, Anomaly scan, Third trimester, Case report
Introduction
Conjoined twinning, resulting from abnormal cleavage of monozygotic twins [1] occurs in about 1 in 50,000 to 200,000 live births; with a small fraction of these cases identified as dicephalic parapagus twins [2].
Although the etiology of conjoined twinning remains unclear, 2 known theories (partial fission and fusion theories) have emerged in explaining its pathophysiology. The partial fission theory suggests that conjoined twins result from incomplete separation of the embryonic axis after 13 days post fertilization [3]. The fusion theory suggests that secondary fusion of embryo from separate fertilized ovum due to similarities in stem-cells characteristics leads to conjoined twins [4,5].
Conjoined twins are broadly categorized as either nondorsal (ventral, lateral, or caudal) or dorsal [5] depending on the body parts interconnected. Further description and classification according to parts of the body that are fused together have been proposed. These are thoracopagus, thoraco-omphalopagus, cephalopagus, omphalopagus, parapagus, craniopagus, ischiopagus, rachipagus, heteropagus and pygopagus [[5], [6], [7]].
Since conjoined twinning has poor prognosis; with approximately 60% of this anomaly resulting in stillbirth, and around 35% of cases dying within the first few hours of life [1,3], accurate and early detection with ultrasound or magnetic resonance imaging (MRI) at any stage of the pregnancy remains crucial. This ensures appropriate and adequate patient counseling and to allow for the consideration of various management strategies, including medical termination of pregnancy and surgical intervention where possible.
We therefore present a rare case of dicephalic parapagus conjoined twins with associated congenital anomalies detected with ultrasound fetal anatomical survey in the third trimester in a 29-year-old pregnant woman.
Case report
A 29-year-old gravida 3 para 2 pregnant woman with no previous history of spontaneous abortion or still birth was referred from a peripheral hospital to our hospital on account of severe lower abdominal pains, mild bleeding and loss of liquor per vaginum in a 28weeks gestation. She had no family history or previous births with congenital anomalies. The patient also indicated that even though a second trimester scan was requested from her previous health facility, she could not take this scan due to financial reasons.
Investigations and imaging findings
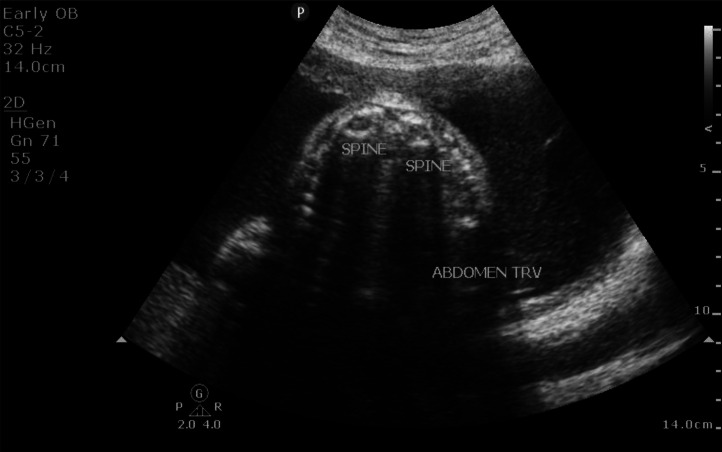
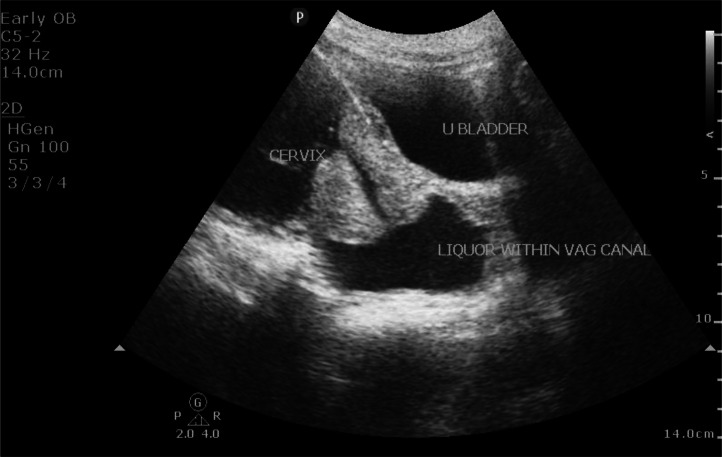
Prior to the current ultrasound scan, her earlier scan from a different health facility which revealed a 14 weeks gestation was unremarkable and the accompanying ultrasound report did not indicate any anomaly. The current ultrasound performed for the patient in our facility revealed an abnormal-looking fetal head with absent brain tissues and cranium above the orbits that was seen just adjacent to a well-formed head with intact brain tissues and cranium (Fig. 1). The well-formed head however depicted a cleft lip and palate. Careful evaluation of the spine also revealed 2 well-formed spines lying side-by-side that were fused at the sacral region (Fig. 2). No evidence of spina bifida was detected. Also noted was mild polyhydramnios and a mildly dilated maternal cervix with noticeable fluid (possibly amniotic fluid) within the vaginal canal (Fig. 3). A single trunk with 2 normal appearing upper limbs and 2 lower limbs noted. Normal appearance of a single liver, 2 kidneys, stomach and urinary bladder noted. The heart demonstrated bradycardia and lungs were unremarkable. Normal umbilical cord insertion noted.
Fig. 1.
Sonograms revealing 2 fetal heads; with 1 head having intact cranium and brain tissues (A) and the other showing absent cranial vault and brain tissue above the orbits (B).
Fig. 2.
Sonogram of the fetal mid abdomen (axial view) shows 2 spines (with posterior shadowing) lying side-by-side.
Fig. 3.
Sonogram of the maternal lower uterine segment (sagittal view) shows mildly dilated cervix with liquor within vaginal canal.
After the ultrasound scan, a diagnosis of dicephalic parapagus conjoined twins with associated gross anomalies (anencephaly and cleft lip and palate) was arrived at.
Follow-up outcomes
An MRI could not be performed for this patient because she could not afford the cost of the examination. Hence, based on the ultrasound findings, the patient was counselled on the potential risks and prognosis associated with the anomalies detected. As prognosis of conjoined twins; particularly dicephalic parapagus twins with the associated detected anomalies is very poor, medical termination of pregnancy was agreed upon after thorough counselling of the patient by the medical team. However, the patient had a spontaneous abortion about 6 hours after the ultrasound scan and the conjoined twins died few hours after delivery.
Gross examination after delivery revealed 2 fetal heads (lying side-by-side) on a single trunk; with 1 head demonstrating anencephaly and the other head showing cleft lip and palate (Fig. 4). A postdelivery ultrasound scan that was performed did not reveal any retained products of conception and the patient did not present with any complication postdelivery while receiving treatment in the hospital and after discharge from the hospital.
Fig. 4.
Postdelivery images of the dicephalic parapagus conjoined twins showing 2 heads lying side-by-side on a single trunk; with 1 head showing cleft lip and palate and the other showing features of anencephaly.
Discussion
Conjoined twinning, also referred to as Siamese twins, occurs in about 1 in 50,000 to 200,000 live births [5]. It has been seen to affect more females than males with a ratio of 3:1 [1].
The etiology of conjoined twins remains unclear; with no clear maternal, environmental or genetic predisposing factors associated with this anomaly [2]. However, 2 theories namely the fission and fusion theories have been proposed in explaining the pathophysiology of conjoined twinning. The fission theory suggests incomplete division after 13th day of an otherwise normal monozygotic twins during embryogenesis as the cause of conjoined twins [3]. This incomplete division often times leads to fused organs and body parts. The second theory suggests that conjoined twinning occur whenever there is secondary fusion of embryo from separate fertilized ovum due to similarities in stem-cells characteristics [4,5].
Depending on the body part interconnected, conjoined twins are broadly classified as dorsally connected or nondorsally connected (ventral, lateral, or caudal) [5]. Nondorsal twins usually share a single umbilical cord and organs, while dorsal twins have separate umbilical cords and internal organs. Further classifications have also been proposed in describing conjoined twins based on the specific body parts that are fused together. These are craniopagus (cranium), thoracopagus (thorax), thoraco-omphalopagus (abdomen and thorax), omphalopagus (abdomen), cephalopagus (head and umbilical region), ischiopagus (pelvis and lower abdomen), parapagus (sides), rachipagus (vertebral column), pygopagus (perineum and sacrum) and heteropagus (parasitic twin) [6,7].
Dicephalic parapagus conjoined twinning is a very rare form of nondorsal conjoined twinning [8] with very few reported cases in the literature. Clinical presentation varies depending on the extent of organ sharing. Typically, they are interconnected laterally or anterolaterally; with 2 distinct heads and necks, fused at the thorax and abdomen. They may have shared limbs and a single torso, while the internal organ systems (heart, lungs, gastrointestinal tract) may be partially or fully shared [8]. This is seen in our case report where the conjoined twins demonstrated 2 distinct heads, a common torso and internal organs as well as common upper and lower limbs. Furthermore, several congenital anomalies such as facial, gastrointestinal tract, cranial, neural tube, musculoskeletal, genitourinary tract and limb defects have been reported in more than 60% of cases of conjoined twins [2,9]. This is relatable to our case report where cleft lip and palate was detected in one of the twins while anencephaly was detected in the other twin. Also, polyhydramnios is identified in about 50%-60% of cases of conjoined twins [10] and this is relatable to our case report where there was evidence of polyhydramnios.
Ultrasound and magnetic resonance imaging (MRI) are useful nonionizing radiological examinations for both the perinatal diagnosis and surgical intervention planning in conjoined twins [11]. In the first trimester, ultrasound detectable features such as absence of separating membrane, inseparable body parts (head or torso) with changes in fetal position, bifid appearance of fetal pole, fetal spine hyperextension, the presence of umbilical cord with more than 3 vessels and increased nuchal translucency [10,12,13] have been seen to be diagnostic for conjoined twins. Despite the feasibility of first trimester ultrasound scan in the diagnosing of conjoined twins, it is however worth noting that certain features such as limited fetal movements before 10weeks gestation may lead to false-positives whereby monoamniotic twins may be mistaken for conjoined twins [14]. Follow-up scan after 10weeks is therefore warranted in such instances where definite diagnosis is required for decision making by both clinicians and expectant parents.
Detailed fetal anatomy scan in the early third trimester continues to gain attention in recent times since it allows for the detection of fetal anomalies that might have been missed earlier on in the pregnancy and for anomalies that become visible in the later stages of pregnancy [15]. Particularly in low resource settings where there is inadequate periconceptional care, pregnant women reporting late for antenatal care and inadequate skilled ultrasound operators, the third trimester anomaly scan is of relevance for the detection of fetal anomalies of significant perinatal mortality and morbidity [16]. This is relatable to our case where previous ultrasound from a peripheral health facility could not detect conjoined twinning but was diagnosed for the first time in the third trimester in our facility. It is however worth noting that adequate liquor volume and polyhydramnios enhances the survey of fetal anatomy in the third trimester. Hence the presence of polyhydramnios occurring in more than 50% of conjoined twins cases [10] makes the diagnosis in the third trimester feasible.
In situations where severe oligohydramnios and increased maternal adiposity hinders better visualization and evaluation of conjoined twins with ultrasound, fetal MRI provides a better alternative for the assessment of conjoined twins [17]. Also, the multiplanar capability of MRI allows for improved anatomical detailing as well as accurate detection of anomalies and positions of organs.
Since most cases of conjoined twins result in stillbirth and death within few hours of birth [1,3], management of this condition involves adequate counselling of the expectant parents. In cases where there is severe organ sharing which is incompatible with life, medical termination of pregnancy is recommended. In the event where surgical intervention is considered, it requires the surgical planning team to establish the degree of organ sharing and sites of fusion between the conjoined twins. Both MRI and 3-dimensional (3D) simulations and modelling have also been seen as useful in recent times during the surgical separation planning phase of conjoined twins [5].
Conclusion
Antenatal ultrasound imaging, even for those performed in the third trimester remains crucial for the detection of rare and lethal fetal anomalies such as dicephalic parapagus conjoined twinning and related congenital anomalies. Continuous efforts should therefore be made for the inclusion of detailed anatomical survey in the early third trimester; particularly in women with unremarkable earlier ultrasound scans and in those reporting late for obstetric care. This will allow for appropriate intervention and well-informed delivery options.
Patient consent
Written informed consent for the publication of this case report was obtained from the patient.
Footnotes
Competing Interests: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this article.
References
- 1.Mian A., Gabra N.I., Sharma T., Topale N., Gielecki J., Tubbs R.S., Loukas M., et al. Conjoined twins: From conception to separation, a review. Clin Anat. 2017;30(3):385–396. doi: 10.1002/ca.22839. [DOI] [PubMed] [Google Scholar]
- 2.Mutchinick O.M., Luna-Muñoz L., Amar E., Bakker M.K., Clementi M., Cocchi G., et al. Conjoined twins: a worldwide collaborative epidemiological study of the international clearinghouse for birth defects surveillance and research. Am J Med Genet C Semin Med Genet. 2011;0(4):274–287. doi: 10.1002/ajmg.c.30321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spitz L., Kiely E.M. Conjoined twins. JAMA. 2003;289(10):1307–1310. doi: 10.1001/jama.289.10.1307. [DOI] [PubMed] [Google Scholar]
- 4.Melo Â., Dinis R., Portugal A., Sousa A.I., Cerveira I. Early prenatal diagnosis of parapagus conjoined twins. Clin Pract. 2018;8(2):1039. doi: 10.4081/cp.2018.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Afzal A.R., Montero F.J. StatPearls. StatPearls Publishing; Treasure Island (FL): 2024. Conjoined twins.http://www.ncbi.nlm.nih.gov/books/NBK560839/ Accessed October 11, 2024Available: [Google Scholar]
- 6.Spencer R. Anatomic description of conjoined twins: a plea for standardized terminology. J Pediatr Surg. 1996;31(7):941–944. doi: 10.1016/s0022-3468(96)90417-0. [DOI] [PubMed] [Google Scholar]
- 7.Baken L., Rousian M., Kompanje E.J.O., Koning A.H.J., van der Spek P.J., Steegers E.A.P., et al. Diagnostic techniques and criteria for first-trimester conjoined twin documentation: a review of the literature illustrated by three recent cases. Obstet Gynecol Surv. 2013;68(11):743–752. doi: 10.1097/OGX.0000000000000000. [DOI] [PubMed] [Google Scholar]
- 8.Chen C.-P., Hsu C.-Y., Su J.-W., Cindy Chen H.-E., Hwa-Ruey Hsieh A., Hwa-Jiun Hsieh A., et al. Conjoined twins detected in the first trimester: a review. Taiwanese J Obstetr Gynecol. 2011;50(4):424–431. doi: 10.1016/j.tjog.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 9.Arnold J., Luton A., Davies J. Introduction: unique challenges in the care of conjoined twins. Sem Perinatol. 2018;42(6):319–320. doi: 10.1053/j.semperi.2018.07.007. [DOI] [PubMed] [Google Scholar]
- 10.van den Brand S.F., Nijhuis J.G., van Dongen P.W. Prenatal ultrasound diagnosis of conjoined twins. Obstet Gynecol Surv. 1994;49(9):656–662. [PubMed] [Google Scholar]
- 11.Mehollin-Ray A.R. Prenatal and postnatal radiologic evaluation of conjoined twins. Sem Perinatol. 2018;42(6):369–380. doi: 10.1053/j.semperi.2018.07.015. [DOI] [PubMed] [Google Scholar]
- 12.Tongsong T., Chanprapaph P., Pongsatha S. First-trimester diagnosis of conjoined twins: a report of three cases. Ultrasound in Obstet Gyne. 1999;14(6):434–437. doi: 10.1046/j.1469-0705.1999.14060434.x. [DOI] [PubMed] [Google Scholar]
- 13.Sebire N.J., Souka A., Skentou H., Geerts L., Nicolaides K.H. First trimester diagnosis of monoamniotic twin pregnancies. Ultrasound Obstet Gynecol. 2000;16(3):223–225. doi: 10.1046/j.1469-0705.2000.00229.x. [DOI] [PubMed] [Google Scholar]
- 14.E P., E J. First-trimester diagnosis of conjoined twins. Prenatal diagn. 2005;25(9):820–826. doi: 10.1002/pd.1267. [DOI] [PubMed] [Google Scholar]
- 15.Ficara A., Syngelaki A., Hammami A., Akolekar R., Nicolaides K.H. Value of routine ultrasound examination at 35–37 weeks’ gestation in diagnosis of fetal abnormalities. Ultrasound in Obstetr Gynecol. 2020;55(1):75–80. doi: 10.1002/uog.20857. [DOI] [PubMed] [Google Scholar]
- 16.Arkorful J., Ackom S., Fiagbedzi E., Obour E., Nyamson J., Ofori I.N. Antenatally diagnosed myelomeningocele with associated chiari ii malformation in the third trimester: a case report. Radiol Case Rep. 2024;19(12):5990–5994. doi: 10.1016/j.radcr.2024.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abdullah H., Wahab N.A., Bakar K.A. Fetal MRI of thoraco-omphalopagus conjoined twins. Case Rep. 2017;2017 doi: 10.1136/bcr-2017-219793. [DOI] [PMC free article] [PubMed] [Google Scholar]